Abstract
Introduction
Chronic kidney disease (CKD) is a pervasive disease of the current century that usually affects the adult population, especially people with diabetes and hypertension. According to the recent studies, inflammation, oxidative stress, apoptosis, and mitochondrial dysfunction are determining risk factors in the pathogenesis of CKD. Melatonin as a strong antioxidant is produced in various tissues including the kidneys. The present clinical trial aims to examine the efficacy of melatonin supplementation on metabolic parameters, oxidative stress, and inflammatory biomarkers in diabetic patients with CKD.
Methods
This is a double-blind, randomized, placebo-controlled clinical study that will be investigated the impacts of melatonin supplementation in diabetic patients with CKD. Laboratory findings will be applied to diagnose diabetic CKD. Forty-eight eligible diabetic subjects with CKD will be selected and randomly assigned to receive 5 mg melatonin tablets or identical placebo twice daily for 10 weeks. Participants will be asked to remain on their usual diet and physical activity. The primary outcome of this study is changes in oxidative stress and inflammatory biomarkers. The secondary outcomes include changes in lipid profile, renal function indicators, fasting blood sugar and serum insulin, systolic and diastolic blood pressure (SBP and DBP), serum phosphorous concentration, sleep quality, body weight, body mass index (BMI), and waist circumference (WC). Statistical analysis will be conducted using the SPSS software (version 25).
Discussion
We hypothesize that melatonin administration may be useful for treating diabetic CKD by modulating oxidative stress, inflammation, regulating lipid metabolism, and increasing insulin sensitivity through different mechanisms. The current trial will exhibit the effects of melatonin, whether negative or positive, on diabetic CKD status.
Ethical aspects
The current trial received approval from Medical Ethics Committee of Tehran University of Medical Sciences, Tehran, Iran (IR.TUMS.SHARIATI.REC.1402.072).
Trial registration
This study had been registered in Iranian Registry of Clinical Trials. Registration number: IRCT20170202032367N9 on 11 August 2023. https://www.irct.ir/trial/70709.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13063-024-08584-x.
Keywords: Chronic kidney disease, Melatonin, Metabolic parameters, Oxidative stress, Inflammatory biomarkers
Introduction
CKD is a pervasive disease of the current century that usually affects the adult population, especially people with diabetes and hypertension [1, 2]. Almost 40% of diabetic patients develop CKD as a typical microvascular complication [3, 4]. Epidemiological studies have shown that more than 700 million adults (9% of global population) suffer from CKD worldwide [5]. In Iran, 27.5% of the population (24% in male and 30.3% in female) are involved in this clinical pathological condition [6]. Since the prevalence of CKD is increasing, cardiovascular morbidity and mortality risk is growing rapidly, emphasizing early prevention and treatment of the CKD [7].
Due to the great importance of finding an appropriate strategy to manage this condition, several studies have been performed [8]. Recent studies have shown that inflammation, oxidative stress, apoptosis, and mitochondrial dysfunction are determining risk factors in the pathogenesis of CKD [9]. Considering the major role of oxidative stress in the progression of CKD, treatment using antioxidants can be effective in this field. Melatonin as a strong antioxidant is produced in various tissues including the kidneys and other tissues like pineal gland. Also, melatonin could exert intracrine, autocrine, or paracrine functions [10]. The results of the CREAM study indicate a direct relationship between disruption of melatonin secretion rhythm and kidney dysfunction [11]. Melatonin can play its antioxidant role directly by scavenging free radicals or indirectly by increasing the activity of antioxidant enzymes (SOD, catalase, GPX) and decreasing the activity of oxidant ones [12]. There are several studies that administered melatonin supplements as a strategy for modulating oxidative stress and inflammation and reported favorable effects [13–17].
Also, lipid profile abnormalities are common in CKD patients. In the early stages of kidney failure, changes in lipoprotein metabolism and activity of some key enzymes are the causes of dyslipidemia. In the final stages, metabolic abnormalities may further progress and negatively impact kidney function [18]. Since the most common lipid-lowering medications (statins and fibrates) may cause adverse effects including hepatotoxicity and myopathy, identification of supplemental agents with the ability to modulate lipid profile has attracted much attention. Melatonin is also effective in regulating lipid metabolism and can improve dyslipidemia by increasing insulin sensitivity, elevating lipoprotein lipase (LPL) activity, reducing fat tissue lipolysis, increasing LDL receptor activity, inhibiting cholesterol absorption from the intestine, and converting cholesterol into bile acids. In a systematic review and meta-analysis of randomized controlled trials, Mohammadi et al. concluded that melatonin supplementation can induce dramatic favorable effects on triglyceride and total cholesterol levels [19].
As mentioned above, the positive effects of melatonin in various metabolic disorders have been proven. However, there is no study about CKD patients exclusively. Therefore, we designed this randomized, placebo-controlled, parallel clinical trial to examine the effects of melatonin supplementation on oxidative stress, inflammatory biomarkers, and lipid profile in diabetic patients with CKD.
Patients and methods
Participants
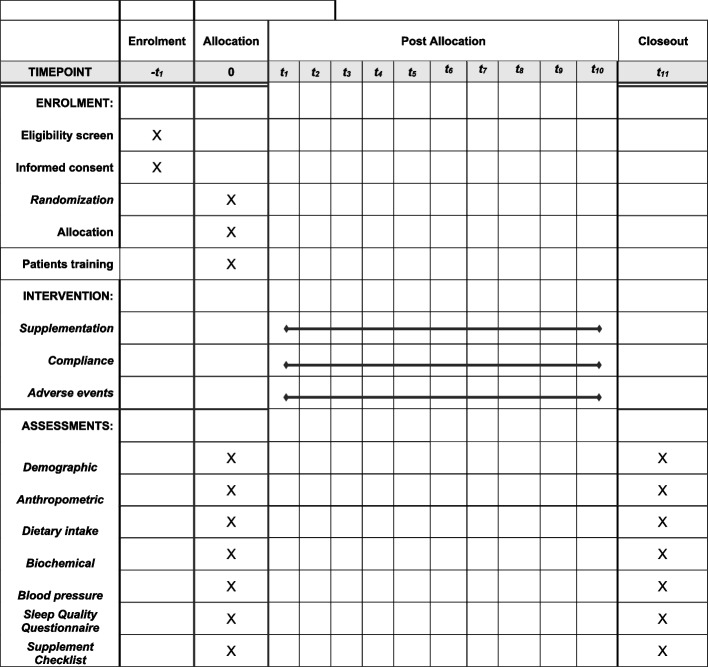
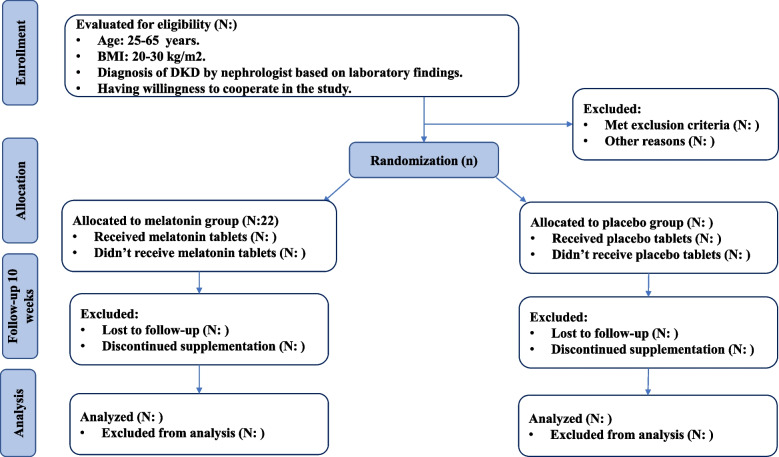
The present study is a phase III parallel randomized double-blind placebo-controlled clinical trial (RCT) among diabetic patients with CKD in the stages before dialysis (stage 3–4). This clinical trial is registered at the Iranian Registry of Clinical Trials (ID: IRCT20170202032367N9). CKD will be confirmed by a nephrologist through the use of laboratory findings. This study is designed to be conducted at Shariati Hospital, affiliated with Tehran University of Medical Sciences, Tehran, Iran. All participants will read and sign the informed consent. We will request consent for review of participants’ medical records and for the collection of blood samples to assess oxidative stress, inflammatory markers, lipid profile, renal function indicators, fasting blood sugar and serum insulin, and serum phosphorous concentration. This study has already been approved by the ethics committee of Tehran University of Medical Sciences (IR.TUMS.SHARIATI.REC.1402.072). We developed the study protocol based on Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) 2013 checklist (Supplemental File 1). Timeline of the trial and study flow chart of enrolment, allocation, intervention, and assessment are presented in Table 1 and Figs. 1 and 2, respectively. Before the study is conducted, any changes to the current protocol that have to do with patient safety and welfare, protocol deviations, inadvertent changes that do not impact subject rights, study risk or benefit, data integrity, safety, or welfare must be approved by the Department of Clinical Nutrition and the Medical Ethics Committee of Tehran University of Medical Sciences, Tehran, Iran. All protocol modifications will be reported to the Trials journal (www.trialsjournal.biomedcentral.com). The significant number of exclusion criteria should be underlined as a reason for the researchers to stay longer at Shariati Hospital to recruit enough participants and achieve the desired sample size.
Table 1.
Timeline of the trial
| Explanation of the trial activities | Time (month) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
| Material preparation | * | * | * | * | ||||||||||||
| Recruitment | * | * | * | * | ||||||||||||
| Clinical assessments at baseline | * | * | * | * | ||||||||||||
| Nutritional assessments at baseline | * | * | * | * | ||||||||||||
| Biochemical assessments at baseline | * | * | * | * | ||||||||||||
| Intervention | * | * | * | * | * | |||||||||||
| Clinical assessments after intervention | * | * | * | * | * | |||||||||||
| Nutritional assessments after intervention | * | * | * | * | * | |||||||||||
| Biochemical assessments after intervention | * | * | * | * | * | |||||||||||
| Data analysis | * | * | ||||||||||||||
| Writing the final report of the trial | * | |||||||||||||||
| The expected time | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * | * |
Fig. 1.
Study flowchart
Fig. 2.
Timeline of the trial
Inclusion criteria
In this study, we will recruit diabetic patients with CKD in the stages before dialysis (stage 3-4) which is confirmed by a nephrologist through the use of laboratory findings; aged 25-65 years with a body mass index (BMI) > 20 and < 30 kg/m2; and who have willingness to cooperate in the study.
Non-inclusion criteria
Patients will not be included if they (1) have autoimmune kidney disease or glomerulonephritis; (2) have blood pressure (BP) > 160/100 mmHg; (3) are pregnant or lactating women or have a plan to get pregnant in the next 6 months; (4) have infectious, inflammatory diseases, thyroid gland disorders, and thrombocytopenia; (5) are under enteral and parenteral nutritional support; (6) have a history of taking omega-3 and antioxidant supplements (vitamin E, vitamin C, vitamin B6, selenium, zinc, and beta-carotene separately) from 3 months before entering the study; (7) are taking glucocorticoids with a dose of more than 5 mg, antibiotics, fluvoxamine, non-steroidal anti-inflammatory drugs (NSAIDs), and warfarin; (8) are smokers; and (9) have night shift jobs.
Exclusion criteria
We will exclude the following patients: (1) those who do not want to continue taking supplements, (2) those who get pregnant throughout the study; (3) those who did not consume more than 10% of supplements in each follow-up; and (4) those who enter the stage of dialysis or kidney transplantation.
Study design
Individuals who meet the inclusion criteria will be enrolled. After recruitment, participants will pass a 2-week run-in period, during which three 24-h food recalls (2 working days and 1 weekend day) and two 1-day physical activity records will be completed for each patient to collect demographic and dietary information. To measure biochemical parameters, 10-mL overnight fasting venous blood samples will be obtained from each patient. At the end of run-in period, anthropometric measures, oxidative stress and inflammatory biomarkers, lipid profile, glycemic status, renal function, blood pressure, serum phosphorous level, and sleep quality will be evaluated.
Randomization
After recruiting participants, we will use stratified block randomization, in which participants will be stratified based on age (under 30 and above 30) and sex (male/female) into different blocks. For each patient in a certain block, a matched person in terms of age and sex would be placed in other block. Then, the two patients in a single block would be randomly assigned into the intervention and control groups. We will employ a 1:1 allocation ratio to ensure equal representation in both groups. Furthermore, our study is designed as a superiority trial. A third person who is not aware of the study’s aim will randomly allocate participants using a computer-generated random sequence (sequentially numbered).
Blinding
All patients, researchers, nephrologist, and laboratory staff will all be blind to the intervention. The supplements used in both groups are identical in shape, color, and smell. The supplements will be given to both groups packing in similar containers. The supplement cans separated by the letters A or B. The blinding code will be unknown until the end of the study except in emergency cases. Study participants will take their bottles in two times: at their first visit and in the middle of the trial, at week 5.
Study intervention
Patients in the experimental group (N = 24) will receive 5 mg melatonin supplements twice a day for 10 weeks. Patients in the control group (N = 24) will consume placebo tablets containing starch. Participants in both groups will be requested to take the tablets before bedtime for 10 weeks. Weight, size, shape, taste, color, smell, and lot number are the same in melatonin and placebo capsules. Even the patients in the placebo group will not deprived of their main medical treatment during the study. There is no anticipated harm and compensation for trial participation; if any of the participants have a problem, necessary treatments will be done for them. Safety of melatonin supplementation was confirmed by study pharmacist. All subjects will be asked not to change their routine daily diet, physical activity, and medicines. There will be no special criteria for discontinuing or modifying allocated interventions. Melatonin or placebo will not require alteration to usual care pathways (including use of any medication) and these will continue for both trial arms. If any of the participants does not consume 10% of their capsules, this state is considered as a research dropout and is excluded from the study.
Outcomes
Primary and secondary outcomes
The main primary outcome in the current study would be oxidative stress biomarkers including total antioxidant capacity (TAC), total oxidative stress (TOS), and malondialdehyde (MDA) as well as inflammatory markers including interleukin-6 (IL-6) and highly sensitive C reactive protein (hs-CRP). The study secondary outcome variables would be lipid profile (total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL)), renal function indicators (blood urea nitrogen (BUN), creatinine, uric acid), fasting blood sugar and serum insulin, systolic and diastolic blood pressure (SBP and DBP), serum phosphorous concentration, sleep quality, weight, BMI, WC, energy intake, and consumption of energy-contributing nutrients including carbohydrates, fat, and protein.
Measurements and assessments
Dietary intake and physical activity
To assess dietary intakes throughout the study, they will be asked to fill three 1-day dietary recalls at the beginning of the study, weeks 5 and 10 of intervention. Dietary recall format is presented in the online supplemental file. We will compute food and nutrient intake of study participants based on the examination of these dietary recalls. The Nutritionist 4 software, which was modified for Iranian foods, will be used to perform this calculation.
In addition, two 1-day physical activity records will be gathered from all participants to examine the difference in activity between two groups. In order to analyze physical activity records, we will use MET-h/day values for each type of physical activity, based on published guidelines [20], considering the time spent by each participant.
Anthropometric measures
Data on anthropometric indices including body weight, height, WC, and BMI will be collected at study baseline and end of the trial. Participants will be weighed in a fasting state, without shoes with minimal clothing to the nearest 0.1 kg accuracy, using a digital scale. Standing height will be measured using a standard stadiometer without shoes with an accuracy of 0.5 cm. WC will be measured to the nearest 0.1 cm accuracy by non-stretching tape measure around the abdomen at the distance between the suprailiac bone and the last rib. BMI will be calculated by the measured height and weight (weight (kg)/height (m2)).
Clinical outcomes
Systolic and diastolic blood pressures will be measured twice at the right arm using a mercury barometer calibrated by the Institute of Standardization and Industrial Research, with a 15-min interval in between measurements, while the patient is sitting quietly for 5 min. The average of the two measurements will be analyzed to calculate the systolic and diastolic blood pressures. Before starting the intervention and at the end of trial, the sleep quality of the patients will be evaluated using a self-rated questionnaire which assesses sleep quality and disturbances over a 1-month time interval. Nineteen individual items generate seven “component” scores. The sum of scores for these seven components yields one global score: Pittsburgh Sleep Quality Index (PSQI) [21].
Other biochemical variables
At the study baseline and after the intervention, a 10-mL venous blood sample will be taken from each person after 12-h fasting. Then, serum will be isolated from whole blood by centrifugation for 10 min at 3500 rpm and for further analysis serum samples will be stored at − 80 °C. The process of accessing serum concentrations of inflammatory factors and oxidative stress biomarkers (TAC, TOS, MDA, IL-6, hs-CRP) will be done by the enzyme-linked immunosorbent assay (ELISA) commercial kits.
Sample size calculation
Considering the type I error of 5% (α = 0.05) and type II error of 20% (β = 0.20, power = 80%) and total antioxidant capacity (TAC) as the key variable [13], we manually, without the use of any software, calculated required sample size using the following formula:
![]()
n = sample size in each group
α = type 1 error = 0.05
β = power = 80%
S 1 2 = variance of the intervention group = (173)2
S 2 2 = variance of the control group = (108)2
∆2 = minimal clinically important difference = (86)2
Based on this formula, we reached a sample size of 22 people in each group. Considering a 10% likelihood of dropping out, the number of participants in each group increased to 24 people.
Data management and monitoring
A clinical trial monitor occasionally supervises the study progress, ensures patient rights and well-being are safeguarded, the protocol, ethical requirements, standards, and regulations are being followed, the essential documentation is available, and collected data are accurate as there were recorded. One of the investigators will check the coding, security, and storage of data. In addition, he/she will evaluate data entry and data values twice. If any participant reports the occurrence of adverse events, more information is required to make decision about excluding the participants from the trial. Unblinding is permissible in this situation based on the Medical Ethics Committee criteria.
Adherence to the intervention
The study progress will be pursued by calling the patients once a week to ensure that they regularly consume the tablets. Adherence to the intervention will be checked by counting the returned tablets at the half and end of the trial visits. Compliance rate will be computed according to the following formula and poor compliance will be considered as less than 90% [22].
Statistical analysis
Statistical analysis will be conducted using the SPSS software (version 25, SPSS Inc., Chicago, IL, USA). The Kolmogorov–Smirnov test will be applied to examine the normality of data. Moreover, we will use chi-square test and Fisher’s exact test to compare categorical variables. Also, independent sample t-test and Wilcoxon rank-sum test will be applied to compare continuous variables within-group, whereas we will use paired sample t-test and Mann–Whitney U test for between group comparisons. Normally distributed variables will be reported as the mean and standard deviation, while the median and interquartile range (IQR) will be used for reporting non-normally distributed variables. To compare the differences in primary and secondary outcomes between the two study groups at the end of the trial and also adjust the final findings for potential confounders, we will apply the analysis of covariance (ANCOVA) test. Subgroup analysis or adjusted analysis will not be applicable. A p value less than 0.05 will be regarded as statistically significant. If we have missing data, statistical analysis will be done using the intention to treat (ITT) method by imputation.
Discussion
DM as the leading cause of diabetic kidney disease (DKD) is one of the most common diseases of the present century [2, 23]. People with DKD may develop ESRD more rapidly, compared to people with non-diabetic CKD [24, 25]. And since there is no definitive treatment for ESRD, preventing the disease from progressing to end stage can improve the patient’s quality of life, relatively [26]. Treatment with antioxidant agents is one of the main topics of interest for the management of DKD [27].
Melatonin or N-acetyl-5-methoxytryptamine is a natural antioxidant of the body, which belongs to the indolamines group [28]. A large number of experimental studies have investigated the favorable effects of melatonin on human health. Melatonin supplementation may protect the body against several pathophysiological conditions by scavenging free radicals and modulating apoptosis and autophagy [29, 30]. Moreover, a growing body of evidence from animal studies found that melatonin supplementation not only increases the levels of SOD and catalase activity and prevents increasing MDA and MPO levels, but also reduces the excessive penetration of immune system cells into the kidney [31–34]. As a result, subsequent activation of inflammatory mediators such as TNF-α and IL-1β occurs less [35]. Furthermore, many experimental studies concluded that melatonin has some properties that make it able to prevent lipid peroxidation. For example, it has high fat solubility and easily passes through cell membranes [36], scavenges the hydroxyl and peroxyl free radicals that play a major role in the initiation and spreading lipid peroxidation, respectively [37, 38]. There are some other studies that have reported the role of melatonin in improving mitochondrial morphology and function, reducing angiotensin 2 induced apoptosis [39], blocking the NF-ĸB and iNOS pathway in kidney tissue, reducing proteinuria [40], and downregulating the activity of peroxidase enzymes such as lipoxygenase [41]. Although many experimental studies have investigated the effects of melatonin on various metabolic disorders, there are not enough clinical trials assessing the efficacy of melatonin in patients with DKD. This gap can be addressed through conduction of relevant clinical trials with human subjects.
Regarding to the DKD pathophysiology, we hypothesize that melatonin may improve metabolic parameters, oxidative stress, and inflammatory biomarkers in diabetic patients with CKD. According to aforementioned hypothesis, the present trial is designed. One of the novel characteristics of the current clinical study is suggesting an endogenous antioxidant, which has less side effects than other exogenous antioxidants [42]. Furthermore, melatonin has not pro-oxidant properties and unlike other antioxidants, it does not participate in the oxidation–reduction cycles after scavenging free radicals [43].
Trial status
The date of registration was 11 August 2023 (protocol version: 1.0). The recruitment started on 20 October 2023 and will be almost finished on 5 May 2024. The study will finish approximately on 3 May 2024.
Supplementary Information
Acknowledgements
We sincerely thank the Karen company, Yazd, Iran and the cooperation of the study participants.
Code availability
Not applicable.
Abbreviations
- BMI
Body mass index
- BUN
Blood urea nitrogen
- CKD
Chronic kidney disease
- CREAM
Centre for Research and Education in Art and Media
- DKD
Diabetic kidney disease
- DM
Diabetes mellitus
- DBP
Diastolic blood pressure
- ELISA
Enzyme-linked immunosorbent assay
- ESRD
End stage renal disease
- GPX
Glutathione peroxidase
- HDL
High-density lipoprotein
- hs-CRP
Highly sensitive C reactive protein
- IL-6
Interleukin-6
- iNOS
Inducible nitric oxide synthase
- ITT
Intention to treat
- LDL
Low-density lipoprotein
- LPL
Lipoprotein lipase
- MDA
Malondialdehyde
- MET
Metabolic equivalents
- MPO
Myeloperoxidase
- NF-ĸB
Nuclear factor kappa B
- NSAIDs
Non-steroidal anti-inflammatory drugs
- PSQI
Pittsburgh Sleep Quality Index
- RCT
Randomized controlled trial
- SES
Socio-economic status
- SBP
Systolic blood pressure
- SOD
Superoxide dismutase
- SPIRIT
Standard Protocol Items: Recommendations for Interventional Trials
- TAC
Total antioxidant capacity
- TC
Total cholesterol
- TG
Triglyceride
- TNF-α
Tumor necrosis factor alpha
- TOS
Total oxidative stress
- WC
Waist circumference
Authors’ contributions
SS, FN, MH, FP, HM, and HI conceived and developed the idea for the study. SS and MH contributed to data collection. SS and FN wrote drafts of the manuscript. HM advised on statistical analysis. HM and HI contributed to the final revision of the manuscript. The manuscript has been read and approved by all authors.
Funding
The trial funding was used by Tehran University of Medical Science (TUMS), Grant number: 68738. The funder is not involved in the study design, collection, management, analysis and interpretation, writing of the manuscript, and the decision to submit the report for publication, including whether they will have ultimate authority over any of these activities.
Data availability
The first and corresponding authors will have access to interim results and make the final decision to terminate the trial. The non-identifiable individual patients’ data will become available to other researchers in academic institutions. The datasets analyzed during the current study and statistical code are available from the corresponding author on reasonable request, as is the full protocol.
Declarations
Ethics approval and consent to participate
The current trial received approval from Medical Ethics Committee of Tehran University of Medical Sciences, Tehran, Iran (IR.TUMS.SHARIATI.REC.1402.072).
Written informed consent will be obtained from participants before participation in the research project.
Consent for publication
Not applicable-no identifying images or other personal or clinical details of participants are presented here or will be presented in reports of the trial results. The participant information materials and informed consent form are available from the corresponding author on request.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kalantar-Zadeh K, Jafar TH, Nitsch D, Neuen BL, Perkovic V. Chronic kidney disease. Lancet. 2021;398(10302):786–802. [DOI] [PubMed] [Google Scholar]
- 2.Rahmani A, Maleki V, Niknafs B, Tavakoli-Rouzbehani OM, Tarighat-Esfanjani A. Effect of Nigella sativa supplementation on kidney function, glycemic control, oxidative stress, inflammation, quality of life, and depression in diabetic hemodialysis patients: study protocol for a double-blind, randomized controlled trial. Trials. 2022;23(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordheim E, Jenssen TG. Chronic kidney disease in patients with diabetes mellitus. Endocr Connect. 2021;10(5):R151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oshima M, Toyama T, Haneda M, Furuichi K, Babazono T, Yokoyama H, et al. Estimated glomerular filtration rate decline and risk of end-stage renal disease in type 2 diabetes. PLoS One. 2018;13(8):e0201535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoogeveen EK. The epidemiology of diabetic kidney disease. Kidney Dialysis. 2022;2(3):433–42. [Google Scholar]
- 6.Dehghani A, Alishavandi S, Nourimajalan N, Fallahzadeh H, Rahmanian V. Prevalence of chronic kidney diseases and its determinants among Iranian adults: results of the first phase of Shahedieh cohort study. BMC Nephrol. 2022;23(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tonelli M, Muntner P, Lloyd A, Manns BJ, Klarenbach S, Pannu N, et al. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet. 2012;380(9844):807–14. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Kim D, Oh YS, Jun H-S. Lysophosphatidic acid signaling in diabetic nephropathy. Int J Mol Sci. 2019;20(11):2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Promsan S, Lungkaphin A. The roles of melatonin on kidney injury in obese and diabetic conditions. BioFactors. 2020;46(4):531–49. [DOI] [PubMed] [Google Scholar]
- 10.Kvetnoy I. Extrapineal melatonin in pathology: new perspectives. Neuroendocrinol Lett. 2002;23(1):92–6. [PubMed] [Google Scholar]
- 11.Koch BC, van der Putten K, Van Someren EJ, Wielders JP, Ter Wee PM, Nagtegaal JE, et al. Impairment of endogenous melatonin rhythm is related to the degree of chronic kidney disease (CREAM study). Nephrol Dial Transplant. 2010;25(2):513–9. [DOI] [PubMed] [Google Scholar]
- 12.Sampaio RV, Conceição S, Miranda MS, Sampaio Lde F, Ohashi OM. MT3 melatonin binding site, MT1 and MT2 melatonin receptors are present in oocyte, but only MT1 is present in bovine blastocyst produced in vitro. Reprod Biol Endocrinol. 2012;10:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satari M, Bahmani F, Reiner Z, Soleimani A, Aghadavod E, Kheiripour N, et al. Metabolic and anti-inflammatory response to melatonin administration in patients with diabetic nephropathy. Iran J Kidney Dis. 2021;1(1):22. [PubMed] [Google Scholar]
- 14.Koziróg M, Poliwczak AR, Duchnowicz P, Koter-Michalak M, Sikora J, Broncel M. Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J Pineal Res. 2011;50(3):261–6. [DOI] [PubMed] [Google Scholar]
- 15.Raygan F, Ostadmohammadi V, Bahmani F, Reiter RJ, Asemi Z. Melatonin administration lowers biomarkers of oxidative stress and cardio-metabolic risk in type 2 diabetic patients with coronary heart disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38(1):191–6. [DOI] [PubMed] [Google Scholar]
- 16.Alamdari NM, Mahdavi R, Roshanravan N, Yaghin NL, Ostadrahimi A, Faramarzi E. A double-blind, placebo-controlled trial related to the effects of melatonin on oxidative stress and inflammatory parameters of obese women. Horm Metab Res. 2015;47(07):504–8. [DOI] [PubMed] [Google Scholar]
- 17.Celinski K, Konturek PC, Slomka M, Cichoz-Lach H, Brzozowski T, Konturek SJ, et al. Effects of treatment with melatonin and tryptophan on liver enzymes, parameters of fat metabolism and plasma levels of cytokines in patients with non-alcoholic fatty liver disease—14 months follow up. J Physiol Pharmacol. 2014;65(1):75–82. [PubMed] [Google Scholar]
- 18.Mikolasevic I, Žutelija M, Mavrinac V, Orlic L. Dyslipidemia in patients with chronic kidney disease: etiology and management. Int J Nephrol Renovasc Dis. 2017;10:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammadi-Sartang M, Ghorbani M, Mazloom Z. Effects of melatonin supplementation on blood lipid concentrations: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2018;37(6 Pt A):1943–54. [DOI] [PubMed] [Google Scholar]
- 20.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498-504. [DOI] [PubMed] [Google Scholar]
- 21.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 22.Carrier M, Abou-Nassar K, Mallick R, Tagalakis V, Shivakumar S, Schattner A, et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380(8):711–9. [DOI] [PubMed] [Google Scholar]
- 23.Ansari ZM, Nasiruddin M, Khan RA, Haque SF. Protective role of Nigella sativa in diabetic nephropathy: a randomized clinical trial. Saudi J Kidney Dis Transpl. 2017;28(1):9–14. [DOI] [PubMed] [Google Scholar]
- 24.Piccoli GB, Grassi G, Cabiddu G, Nazha M, Roggero S, Capizzi I, et al. Diabetic kidney disease: a syndrome rather than a single disease. Rev Diabet Stud. 2015;12(1–2):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Wang Z, Zhou J, Sun W, Wang Y, Han M, et al. Effects of traditional Chinese herbal medicine in patients with diabetic kidney disease: study protocol for a randomized controlled trial. Trials. 2018;19(1):389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldstein SL, Graham N, Burwinkle T, Warady B, Farrah R, Varni JW. Health-related quality of life in pediatric patients with ESRD. Pediatr Nephrol. 2006;21:846–50. [DOI] [PubMed] [Google Scholar]
- 27.Kvetnoy I. Extrapineal melatonin in pathology: new perspectives for diagnosis, prognosis and treatment of illness. Neuro Endocrinol Lett. 2002;23(Suppl 1):92–6. [PubMed] [Google Scholar]
- 28.Reiter RJ, Tan D-X, Mayo JC, Sainz RM, Leon J, Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim Pol. 2003;50(4):1129–46. [PubMed] [Google Scholar]
- 29.Fernández A, Ordóñez R, Reiter RJ, González-Gallego J, Mauriz JL. Melatonin and endoplasmic reticulum stress: relation to autophagy and apoptosis. J Pineal Res. 2015;59(3):292–307. [DOI] [PubMed] [Google Scholar]
- 30.Sampaio RV, Conceição DSB, Miranda MS, Sampaio LDFS, Ohashi OM. MT3 melatonin binding site, MT1 and MT2 melatonin receptors are present in oocyte, but only MT1 is present in bovine blastocyst produced in vitro. Reprod Biol Endocrinol. 2012;10(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motawi TK, Ahmed SA, Hamed MA, El-Maraghy SA, Aziz WM. Combination of melatonin and certain drugs for treatment of diabetic nephropathy in streptozotocin-induced diabetes in rats. Diabetol Int. 2016;7:413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elbe H, Vardi N, Esrefoglu M, Ates B, Yologlu S, Taskapan C. Amelioration of streptozotocin-induced diabetic nephropathy by melatonin, quercetin, and resveratrol in rats. Hum Exp Toxicol. 2015;34(1):100–13. [DOI] [PubMed] [Google Scholar]
- 33.Erşahin M, Şehirli Ö, Toklu HZ, Süleymanoglu S, Emekli-Alturfan E, Yarat A, et al. Melatonin improves cardiovascular function and ameliorates renal, cardiac and cerebral damage in rats with renovascular hypertension. J Pineal Res. 2009;47(1):97–106. [DOI] [PubMed] [Google Scholar]
- 34.Rodríguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chávez M, Herrera-Acosta J, et al. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol. 2002;282(2):F191–201. [DOI] [PubMed] [Google Scholar]
- 35.Omurtag GZ, Tozan A, Şehirli AÖ, Şener G. Melatonin protects against endosulfan-induced oxidative tissue damage in rats. J Pineal Res. 2008;44(4):432–8. [DOI] [PubMed] [Google Scholar]
- 36.Retter RJ, Pablos ML, Agapito TT, Guerrero JM. Melatonin in the context of the free radical theory of aging. Ann N Y Acad Sci. 1996;786(1):362–78. [DOI] [PubMed] [Google Scholar]
- 37.Reiter RJ, Melchiorri D, Sewerynek E, Poeggeler B, Barlow-Walden L, Chuang J, et al. A review of the evidence supporting melatonin’s role as an antioxidant. J Pineal Res. 1995;18(1):1–11. [DOI] [PubMed] [Google Scholar]
- 38.Reiter RJ. Oxidative damage in the central nervous system: protection by melatonin. Prog Neurobiol. 1998;56(3):359–84. [DOI] [PubMed] [Google Scholar]
- 39.Stacchiotti A, Favero G, Giugno L, Lavazza A, Reiter RJ, Rodella LF, et al. Mitochondrial and metabolic dysfunction in renal convoluted tubules of obese mice: protective role of melatonin. PLoS One. 2014;9(10):e111141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee I-C, Kim S-H, Lee S-M, Baek H-S, Moon C, Kim S-H, et al. Melatonin attenuates gentamicin-induced nephrotoxicity and oxidative stress in rats. Arch Toxicol. 2012;86:1527–36. [DOI] [PubMed] [Google Scholar]
- 41.Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR. Melatonin—a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93(3):350–84. [DOI] [PubMed] [Google Scholar]
- 42.Abraham P, Kolli VK, Rabi S. Melatonin attenuates methotrexate-induced oxidative stress and renal damage in rats. Cell Biochem Funct. 2010;28(5):426–33. [DOI] [PubMed] [Google Scholar]
- 43.Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44(3):280–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The first and corresponding authors will have access to interim results and make the final decision to terminate the trial. The non-identifiable individual patients’ data will become available to other researchers in academic institutions. The datasets analyzed during the current study and statistical code are available from the corresponding author on reasonable request, as is the full protocol.