Abstract
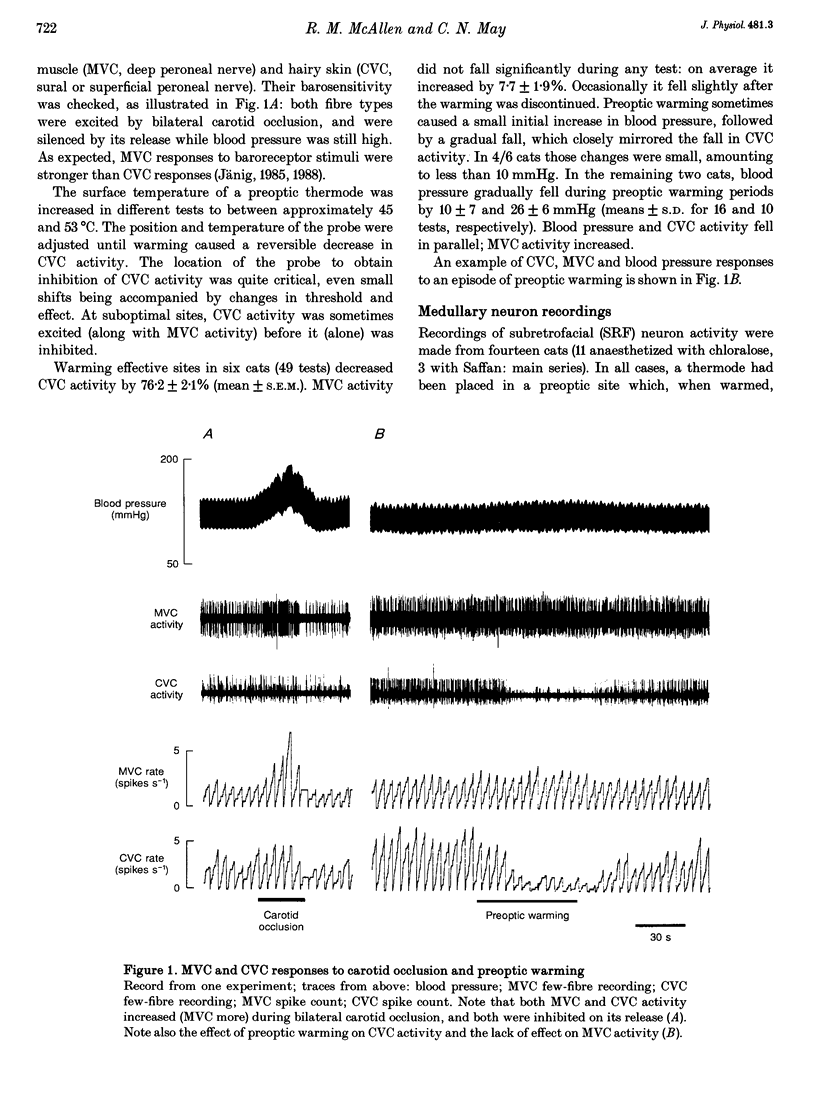
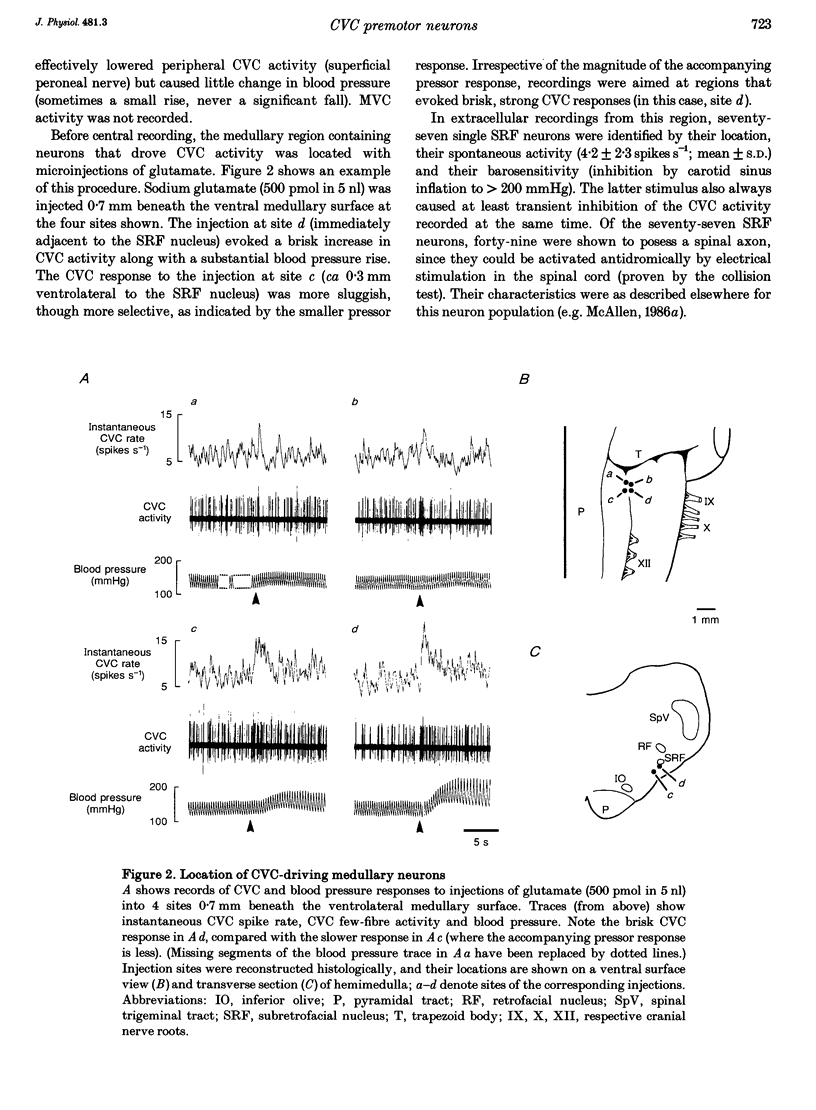
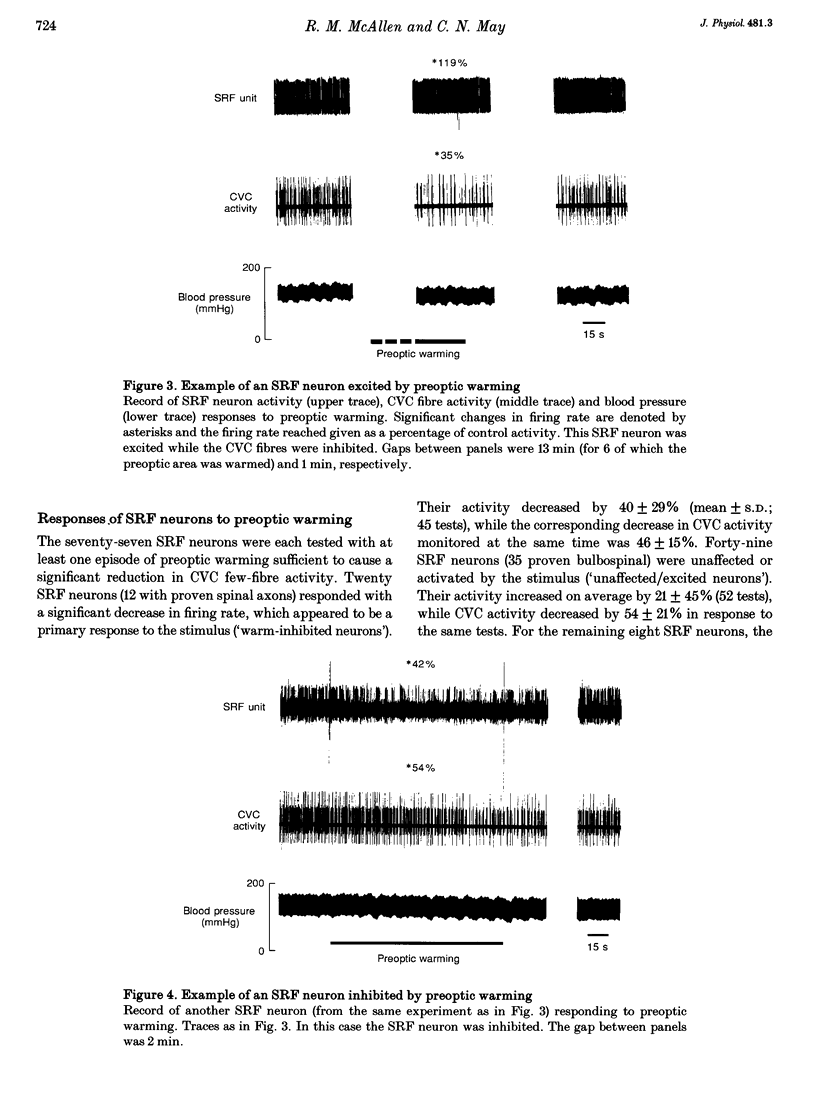
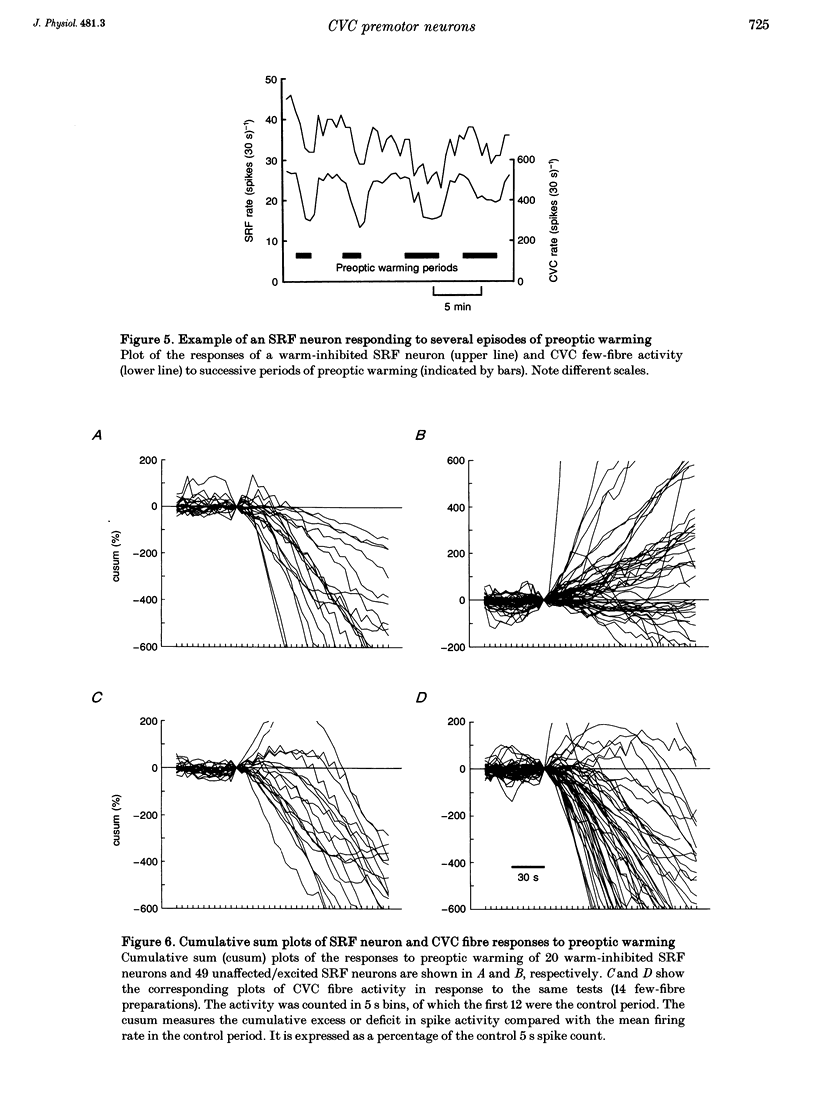
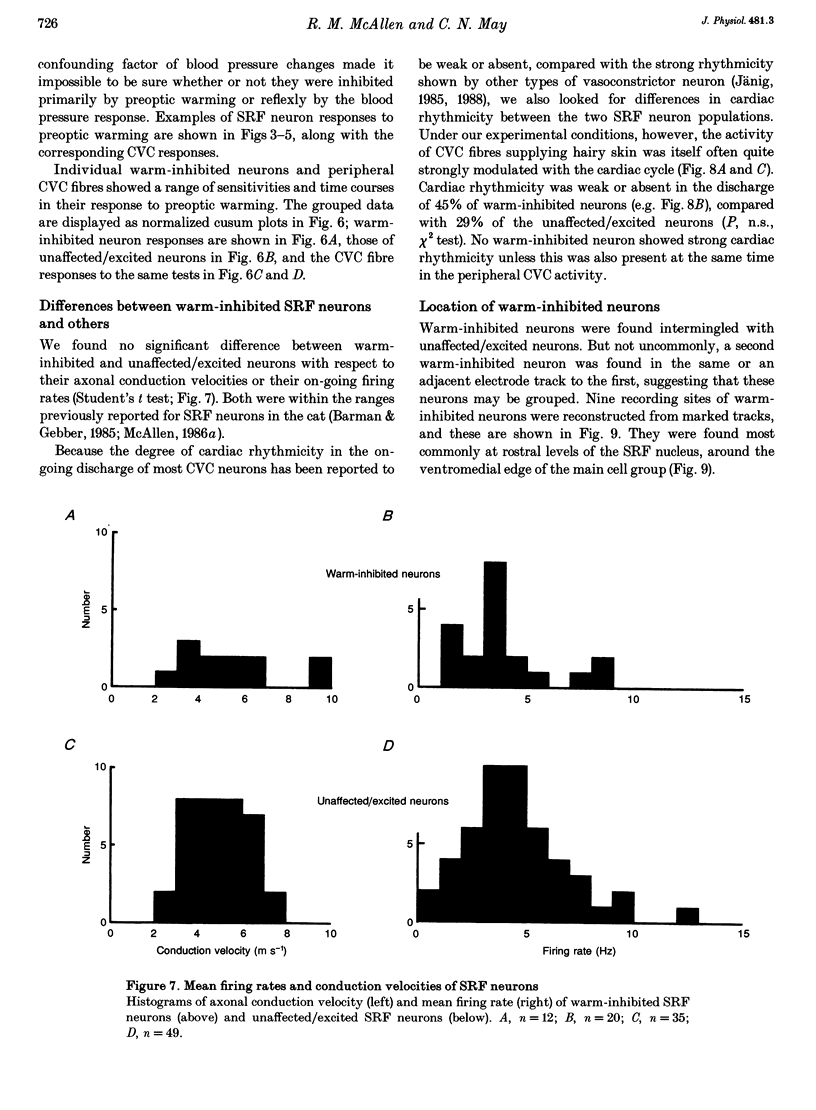
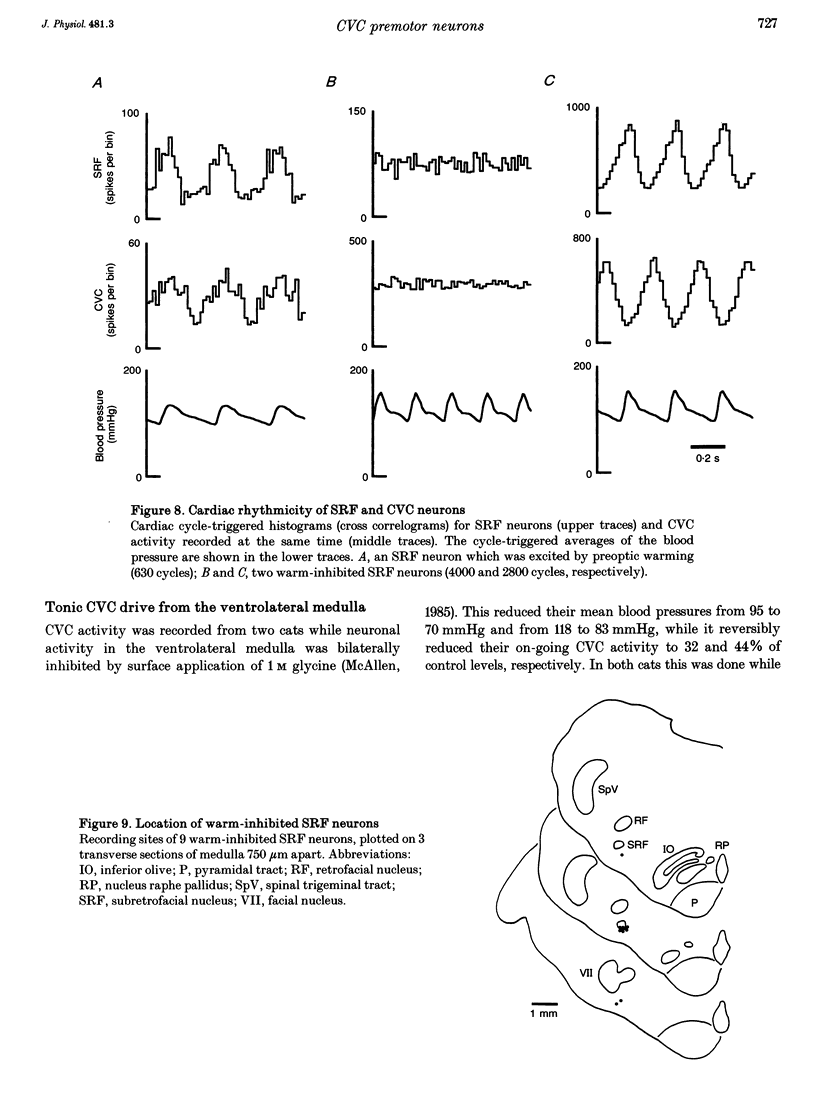
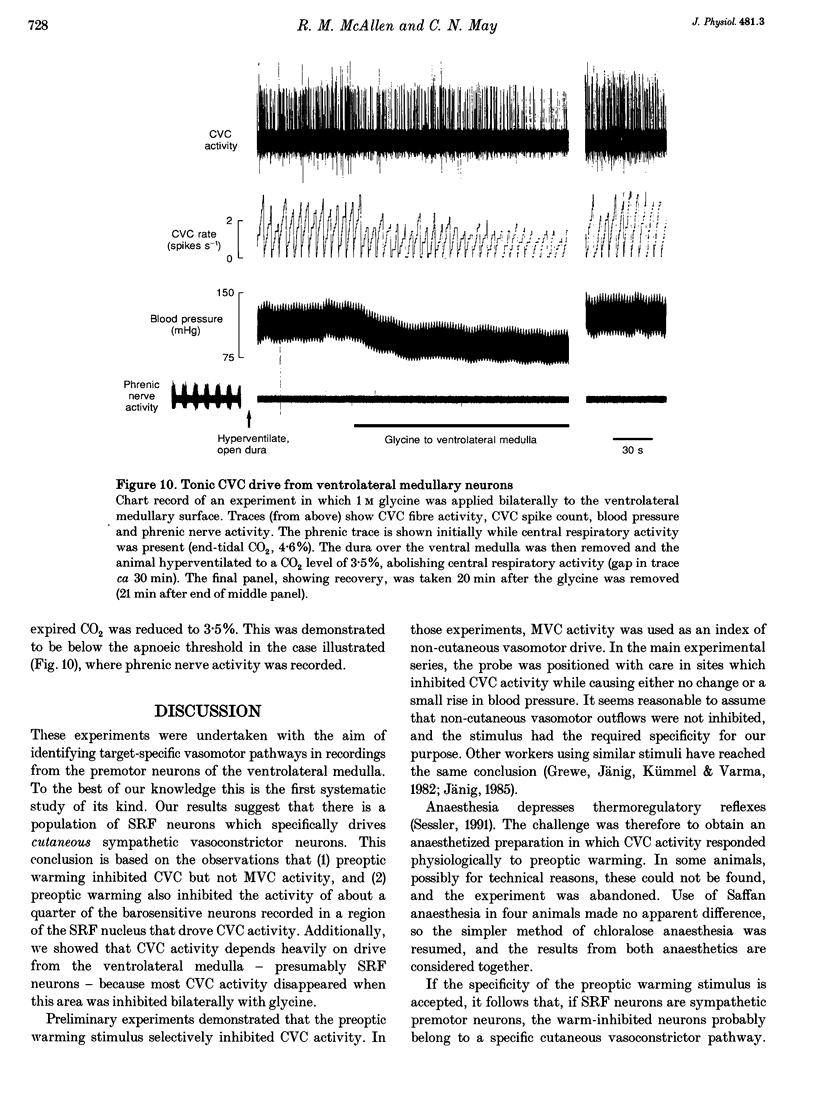
1. Sympathetic and subretrofacial neuron responses to preoptic warming were studied in chloralose- or Saffan-anaesthetized, paralysed cats. 2. Warming a thermode in the preoptic region inhibited the activity of cutaneous vasoconstrictor fibres supplying hairy skin. Muscle vasoconstrictor fibre activity recorded at the same time was either unaffected or raised. 3. Small injections of sodium glutamate (5 nl, 0.1 M) were made into the region of the subretrofacial nucleus in the ventrolateral medulla. The part of that region where glutamate injections evoked brisk increases in cutaneous vasoconstrictor fibre activity was chosen for further study. 4. Extracellular single unit recordings were made in that area from seventy-seven subretrofacial neurons, which were identified by their barosensitivity (inhibition by carotid blind sac inflation). Forty-seven of them were antidromically activated by stimulation in the spinal cord. 5. The activity of twenty subretrofacial neurons (twelve proven bulbospinal) was significantly reduced by periods of preoptic warming. Cutaneous vasoconstrictor activity recorded at the same time also fell. Forty-nine subretrofacial neurons (thirty-five proven bulbospinal) were unaffected or excited by periods of preoptic warming that inhibited cutaneous vasoconstrictor fibres. The response of eight neurons was unclear. 6. No difference in either mean firing rate or axonal conduction velocity was found between neurons inhibited by preoptic warming and other subretrofacial neurons. 7. The subretrofacial neurons inhibited by warming were found intermingled with those unaffected or excited. Marked recording sites of warm-inhibited neurons were clustered around the ventromedial border of the subretrofacial nucleus. 8. In two cats, bilateral inhibition of subretrofacial neurons by surface application of 1 M glycine reduced cutaneous vasoconstrictor fibre activity to 32 and 44% of control levels. 9. The results suggest that specific cutaneous vasoconstrictor premotor neurons exist in the subretrofacial nucleus. These apparently provide most of the background excitatory drive to cutaneous vasomotor neurons. Central warming stimuli may act, at least in part, by withdrawing that drive.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barman S. M., Gebber G. L. Axonal projection patterns of ventrolateral medullospinal sympathoexcitatory neurons. J Neurophysiol. 1985 Jun;53(6):1551–1566. doi: 10.1152/jn.1985.53.6.1551. [DOI] [PubMed] [Google Scholar]
- Beluli D. J., Weaver L. C. Differential control of renal and splenic nerves without medullary topography. Am J Physiol. 1991 Apr;260(4 Pt 2):H1072–H1079. doi: 10.1152/ajpheart.1991.260.4.H1072. [DOI] [PubMed] [Google Scholar]
- Boczek-Funcke A., Dembowsky K., Häbler H. J., Jänig W., McAllen R. M., Michaelis M. Classification of preganglionic neurones projecting into the cat cervical sympathetic trunk. J Physiol. 1992;453:319–339. doi: 10.1113/jphysiol.1992.sp019231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calaresu F. R., Yardley C. P. Medullary basal sympathetic tone. Annu Rev Physiol. 1988;50:511–524. doi: 10.1146/annurev.ph.50.030188.002455. [DOI] [PubMed] [Google Scholar]
- Dampney R. A., Czachurski J., Dembowsky K., Goodchild A. K., Seller H. Afferent connections and spinal projections of the pressor region in the rostral ventrolateral medulla of the cat. J Auton Nerv Syst. 1987 Jul;20(1):73–86. doi: 10.1016/0165-1838(87)90083-x. [DOI] [PubMed] [Google Scholar]
- Dampney R. A., McAllen R. M. Differential control of sympathetic fibres supplying hindlimb skin and muscle by subretrofacial neurones in the cat. J Physiol. 1988 Jan;395:41–56. doi: 10.1113/jphysiol.1988.sp016907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Seagard J. L., Hopp F. A., Kampine J. P. Differential control of sympathetic activity to kidney and skeletal muscle by ventral medullary neurons. J Auton Nerv Syst. 1992 Jan;37(1):1–10. doi: 10.1016/0165-1838(92)90139-8. [DOI] [PubMed] [Google Scholar]
- Gibbins I. L. Vasoconstrictor, vasodilator and pilomotor pathways in sympathetic ganglia of guinea-pigs. Neuroscience. 1992;47(3):657–672. doi: 10.1016/0306-4522(92)90174-z. [DOI] [PubMed] [Google Scholar]
- Gilbey M. P., Stein R. D. Characteristics of sympathetic preganglionic neurones in the lumbar spinal cord of the cat. J Physiol. 1991 Jan;432:427–443. doi: 10.1113/jphysiol.1991.sp018392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild A. K., Dampney R. A., Bandler R. A method for evoking physiological responses by stimulation of cell bodies, but not axons of passage, within localized regions of the central nervous system. J Neurosci Methods. 1982 Nov;6(4):351–363. doi: 10.1016/0165-0270(82)90036-x. [DOI] [PubMed] [Google Scholar]
- Guyenet P. G., Haselton J. R., Sun M. K. Sympathoexcitatory neurons of the rostroventrolateral medulla and the origin of the sympathetic vasomotor tone. Prog Brain Res. 1989;81:105–116. doi: 10.1016/s0079-6123(08)62002-6. [DOI] [PubMed] [Google Scholar]
- Imamura K., Onoda N. The cumulative sum analysis of spike discharges in a small sample. Jpn J Physiol. 1983;33(1):135–138. doi: 10.2170/jjphysiol.33.135. [DOI] [PubMed] [Google Scholar]
- Jänig W. Organization of the lumbar sympathetic outflow to skeletal muscle and skin of the cat hindlimb and tail. Rev Physiol Biochem Pharmacol. 1985;102:119–213. doi: 10.1007/BFb0034086. [DOI] [PubMed] [Google Scholar]
- Jänig W., Szulczyk P. The organization of lumbar preganglionic neurons. J Auton Nerv Syst. 1981 Apr;3(2-4):177–191. doi: 10.1016/0165-1838(81)90062-x. [DOI] [PubMed] [Google Scholar]
- Lipski J. Antidromic activation of neurones as an analytic tool in the study of the central nervous system. J Neurosci Methods. 1981 Jun;4(1):1–32. doi: 10.1016/0165-0270(81)90015-7. [DOI] [PubMed] [Google Scholar]
- Lovick T. A. Differential control of cardiac and vasomotor activity by neurones in nucleus paragigantocellularis lateralis in the cat. J Physiol. 1987 Aug;389:23–35. doi: 10.1113/jphysiol.1987.sp016644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen R. M. Action and specificity of ventral medullary vasopressor neurones in the cat. Neuroscience. 1986 May;18(1):51–59. doi: 10.1016/0306-4522(86)90178-8. [DOI] [PubMed] [Google Scholar]
- McAllen R. M., Dampney R. A. Vasomotor neurons in the rostral ventrolateral medulla are organized topographically with respect to type of vascular bed but not body region. Neurosci Lett. 1990 Mar 2;110(1-2):91–96. doi: 10.1016/0304-3940(90)90793-9. [DOI] [PubMed] [Google Scholar]
- McAllen R. M. Identification and properties of sub-retrofacial bulbospinal neurones: a descending cardiovascular pathway in the cat. J Auton Nerv Syst. 1986 Oct;17(2):151–164. doi: 10.1016/0165-1838(86)90090-1. [DOI] [PubMed] [Google Scholar]
- McAllen R. M. Mediation of the fastigial pressor response and a somatosympathetic reflex by ventral medullary neurones in the cat. J Physiol. 1985 Nov;368:423–433. doi: 10.1113/jphysiol.1985.sp015866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllen R. M., Neil J. J., Loewy A. D. Effects of kainic acid applied to the ventral surface of the medulla oblongata on vasomotor tone, the baroreceptor reflex and hypothalamic autonomic responses. Brain Res. 1982 Apr 22;238(1):65–76. doi: 10.1016/0006-8993(82)90771-5. [DOI] [PubMed] [Google Scholar]
- Polson J. W., Halliday G. M., McAllen R. M., Coleman M. J., Dampney R. A. Rostrocaudal differences in morphology and neurotransmitter content of cells in the subretrofacial vasomotor nucleus. J Auton Nerv Syst. 1992 May 1;38(2):117–137. doi: 10.1016/0165-1838(92)90232-6. [DOI] [PubMed] [Google Scholar]
- Sessler D. I. Central thermoregulatory inhibition by general anesthesia. Anesthesiology. 1991 Oct;75(4):557–559. [PubMed] [Google Scholar]
- Shafton A. D., Oldfield B. J., McAllen R. M. CRF-like immunoreactivity selectively labels preganglionic sudomotor neurons in cat. Brain Res. 1992 Dec 25;599(2):253–260. doi: 10.1016/0006-8993(92)90399-t. [DOI] [PubMed] [Google Scholar]


