Abstract
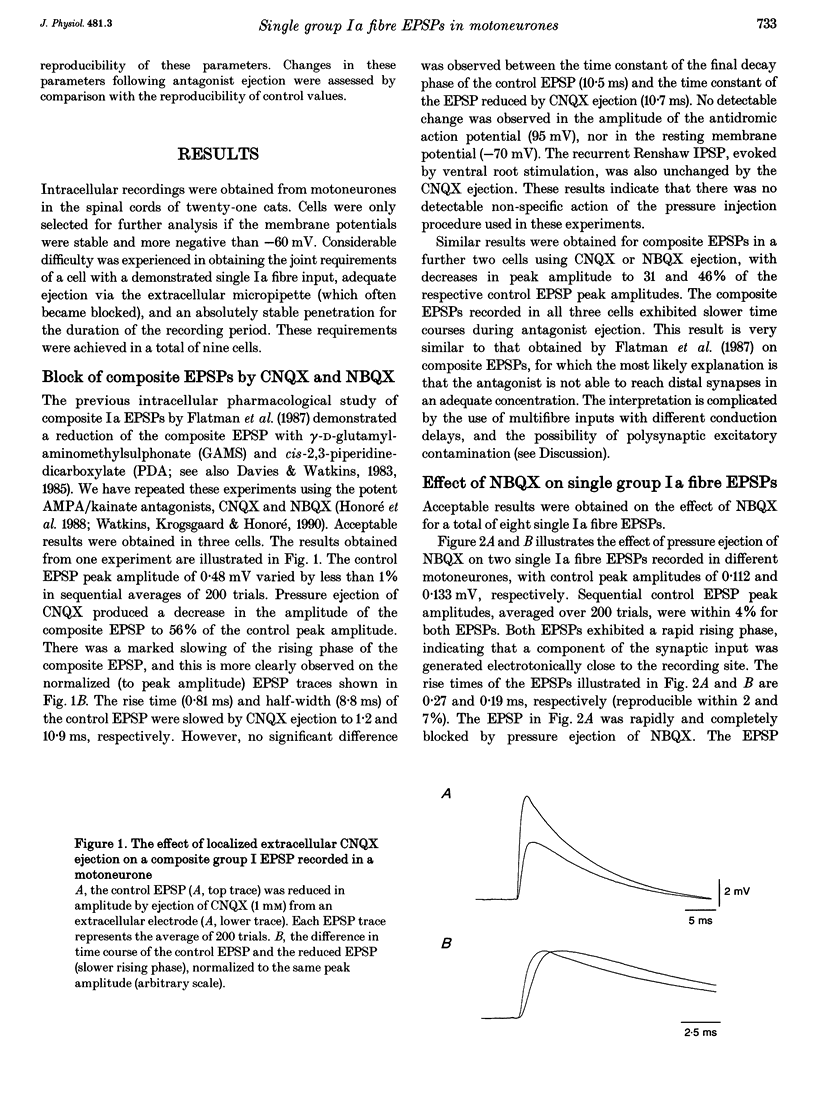
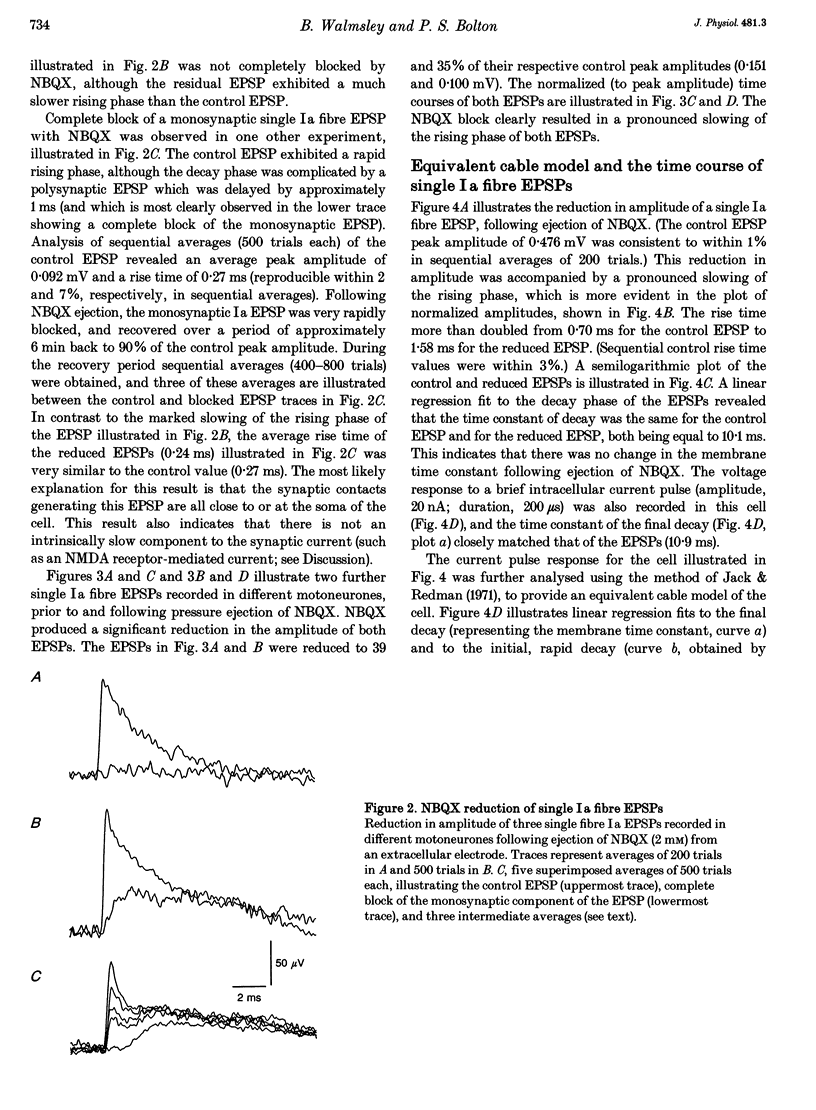
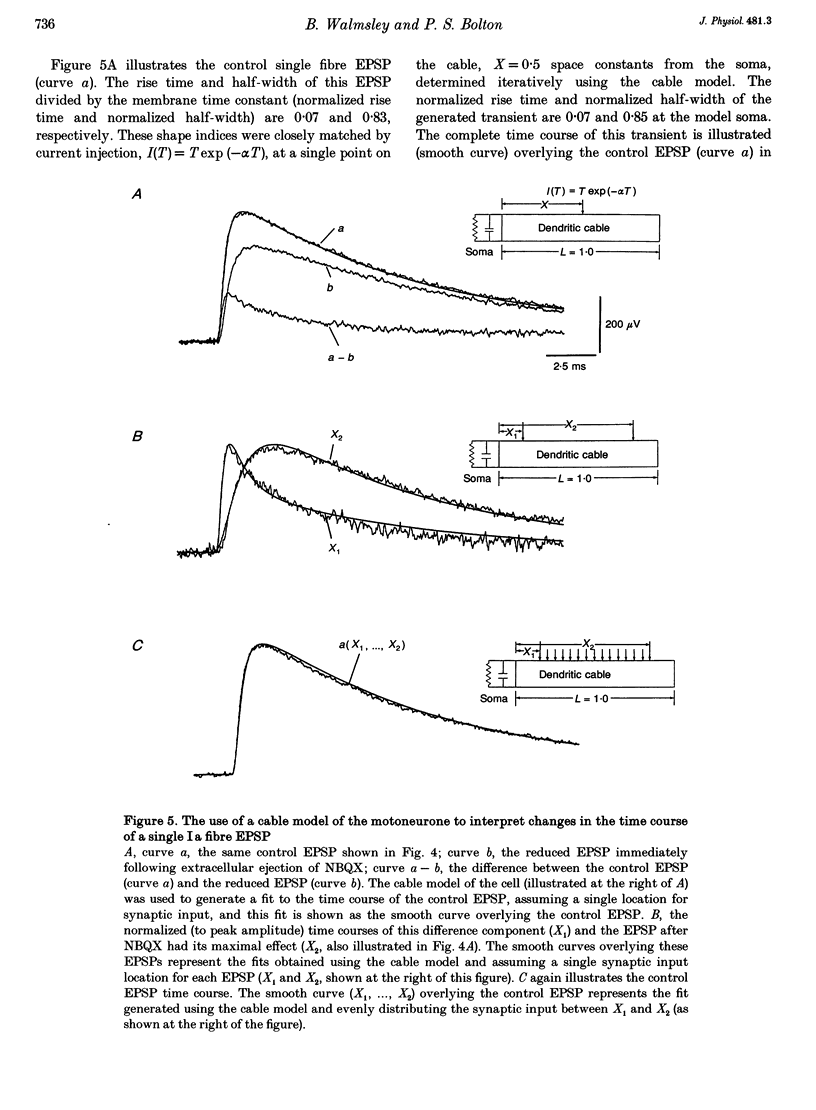
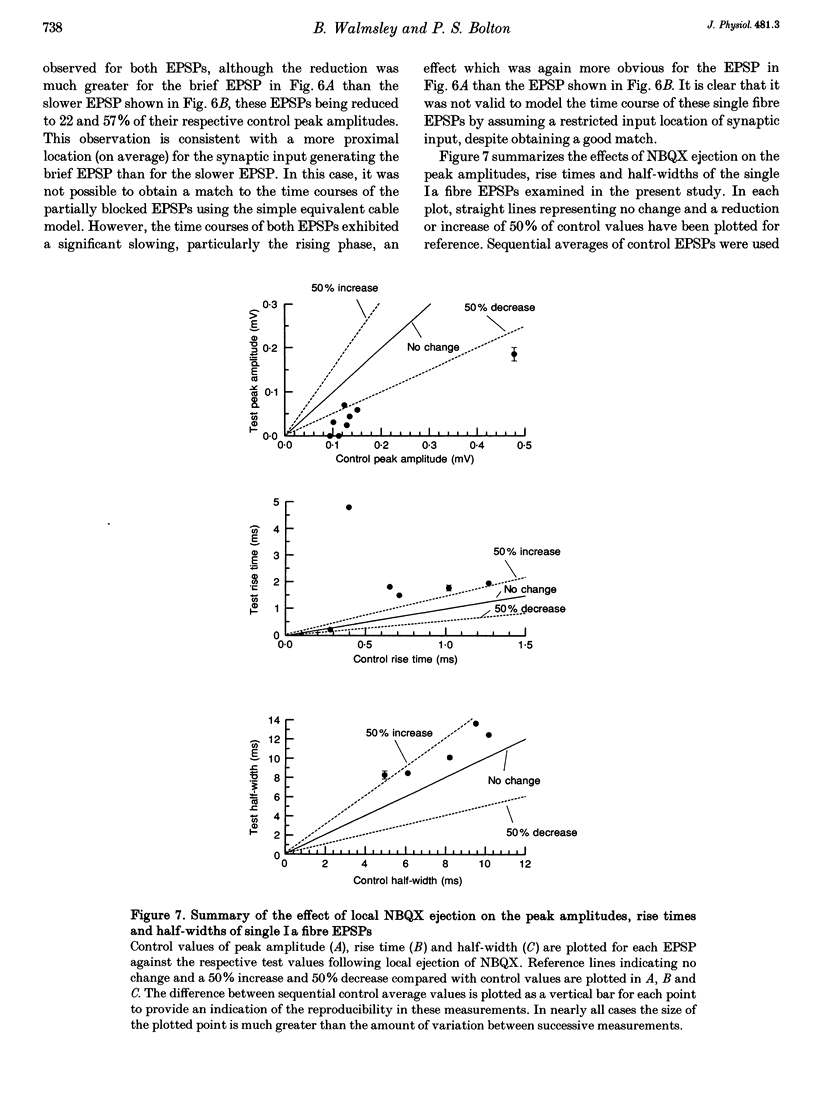
1. Direct experimental evidence was obtained on the spatial distribution of active synaptic contacts from single Ia muscle afferents on the dendrites of lumbosacral motoneurones in anaesthetized cats. 2. An extracellular micropipette was used to pressure eject the AMPA/kainate receptor antagonists 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) or 2,3-dihydroxy-6-nitro-7-sulphamoyl-benzo(F)quinoxaline (NBQX) in close proximity to the intracellular recording site, in order to create an extracellular concentration gradient of the antagonist. The effect of antagonist ejection on the time course and amplitude of excitatory postsynaptic potentials (EPSPs) evoked in motoneurones by impulses in single group Ia fibres was examined. 3. Pressure ejection of NBQX resulted in a complete block of the monosynaptic group Ia EPSP in two cells, and a significant reduction to 23-57% of control EPSP peak amplitudes in a further six cells (mean, 27%; n = 8). These effects were not associated with changes in membrane potential or membrane time constant. 4. The reduction in amplitude of these single group Ia fibre EPSPs following ejection of NBQX was usually accompanied by a pronounced slowing in the time course of the EPSPs. On average, the EPSP rise times and half-widths were increased by 269 and 37%, respectively. This is most probably due to a considerable spatial spread of the synaptic contacts along the dendrites of motoneurones, with the most proximal synaptic contacts (producing the briefest synaptic potentials) subjected to a greater reduction in amplitude due to a higher local antagonist concentration. 5. An equivalent dendritic cable model of the motoneurone was used to interpret the observed changes in the time course of single fibre EPSPs. The time course of control single fibre EPSPs examined in the present study could be well matched using the cable model and assuming a single location for synaptic input. The observation of a slowed EPSP time course following antagonist ejection indicated that this assumption was not correct and that there was in fact considerable spatial spread in the synaptic contacts arising from these single afferent fibres. These results provide direct evidence that spatial spread of synaptic input may not be detected using the time course of a synaptic potential in conjunction with a neuronal cable model of the postsynaptic cell.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. G., Fyffe R. E. The morphology of group Ia afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1978 Jan;274:111–127. doi: 10.1113/jphysiol.1978.sp012137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Walmsley B., Hodgson J. A. HRP anatomy of group Ia afferent contacts on alpha motoneurones. Brain Res. 1979 Jan 12;160(2):347–352. doi: 10.1016/0006-8993(79)90430-x. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Depressant actions of gamma-D-glutamylaminomethyl sulfonate (GAMS) on amino acid-induced and synaptic excitation in the cat spinal cord. Brain Res. 1985 Feb 18;327(1-2):113–120. doi: 10.1016/0006-8993(85)91505-7. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Role of excitatory amino acid receptors in mono- and polysynaptic excitation in the cat spinal cord. Exp Brain Res. 1983;49(2):280–290. doi: 10.1007/BF00238587. [DOI] [PubMed] [Google Scholar]
- Finkel A. S., Redman S. J. The synaptic current evoked in cat spinal motoneurones by impulses in single group 1a axons. J Physiol. 1983 Sep;342:615–632. doi: 10.1113/jphysiol.1983.sp014872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Iansek R., Redman S. J. An analysis of the cable properties of spinal motoneurones using a brief intracellular current pulse. J Physiol. 1973 Nov;234(3):613–636. doi: 10.1113/jphysiol.1973.sp010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iansek R., Redman S. J. The amplitude, time course and charge of unitary excitatory post-synaptic potentials evoked in spinal motoneurone dendrites. J Physiol. 1973 Nov;234(3):665–688. doi: 10.1113/jphysiol.1973.sp010366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Miller S., Porter R., Redman S. J. The time course of minimal excitory post-synaptic potentials evoked in spinal motoneurones by group Ia afferent fibres. J Physiol. 1971 Jun;215(2):353–380. doi: 10.1113/jphysiol.1971.sp009474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J. An electrical description of the motoneurone, and its application to the analysis of synaptic potentials. J Physiol. 1971 Jun;215(2):321–352. doi: 10.1113/jphysiol.1971.sp009473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E., Yoshioka K. Ia afferent excitation of motoneurones in the in vitro new-born rat spinal cord is selectively antagonized by kynurenate. J Physiol. 1986 Jan;370:515–530. doi: 10.1113/jphysiol.1986.sp015948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W., Burke R. E., Holmes W. R., Jack J. J., Redman S. J., Segev I. Matching dendritic neuron models to experimental data. Physiol Rev. 1992 Oct;72(4 Suppl):S159–S186. doi: 10.1152/physrev.1992.72.suppl_4.S159. [DOI] [PubMed] [Google Scholar]
- Rall W., Burke R. E., Smith T. G., Nelson P. G., Frank K. Dendritic location of synapses and possible mechanisms for the monosynaptic EPSP in motoneurons. J Neurophysiol. 1967 Sep;30(5):1169–1193. doi: 10.1152/jn.1967.30.5.1169. [DOI] [PubMed] [Google Scholar]
- Redman S. Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiol Rev. 1990 Jan;70(1):165–198. doi: 10.1152/physrev.1990.70.1.165. [DOI] [PubMed] [Google Scholar]
- Redman S., Walmsley B. Amplitude fluctuations in synaptic potentials evoked in cat spinal motoneurones at identified group Ia synapses. J Physiol. 1983 Oct;343:135–145. doi: 10.1113/jphysiol.1983.sp014885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheardown M. J., Nielsen E. O., Hansen A. J., Jacobsen P., Honoré T. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline: a neuroprotectant for cerebral ischemia. Science. 1990 Feb 2;247(4942):571–574. doi: 10.1126/science.2154034. [DOI] [PubMed] [Google Scholar]
- Tracey D. J., Walmsley B. Synaptic input from identified muscle afferents to neurones of the dorsal spinocerebellar tract in the cat. J Physiol. 1984 May;350:599–614. doi: 10.1113/jphysiol.1984.sp015220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley B., Nicol M. J. Calcium facilitation of group Ia EPSPs evoked in cat spinal motoneurones in vivo. Neurosci Lett. 1991 May 27;126(2):184–186. doi: 10.1016/0304-3940(91)90549-9. [DOI] [PubMed] [Google Scholar]
- Walmsley B., Nicol M. J. The effects of Ca2+, Mg2+ and kynurenate on primary afferent synaptic potentials evoked in cat spinal cord neurones in vivo. J Physiol. 1991 Feb;433:409–420. doi: 10.1113/jphysiol.1991.sp018434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley B., Stuklis R. Effects of spatial and temporal dispersion of synaptic input on the time course of synaptic potentials. J Neurophysiol. 1989 Apr;61(4):681–687. doi: 10.1152/jn.1989.61.4.681. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Krogsgaard-Larsen P., Honoré T. Structure-activity relationships in the development of excitatory amino acid receptor agonists and competitive antagonists. Trends Pharmacol Sci. 1990 Jan;11(1):25–33. doi: 10.1016/0165-6147(90)90038-a. [DOI] [PubMed] [Google Scholar]


