Abstract
Background
Intermittent fasting (IF) holds promise for enhancing metabolic health. However, the optimum IF forms and their superiority over continuous energy restriction (CER) remain unclear due to disconnected findings.
Methods
We systematically searched PubMed, Embase, and the Cochrane databases for meta-analyses of randomized controlled trials (RCTs) investigating the association between IF and metabolic health outcomes. Subsequently, we performed an umbrella review and network meta-analysis (NMA) to evaluate the efficacy of different forms of IF (time-restricted eating (TRE), alternate-day fasting (ADF), and 5:2 diet (regular eating for 5 days and energy restriction for 2 days per week)) compared to CER and usual diets on metabolic health outcomes. To assess the certainty of both direct and indirect estimates, we employed the Confidence in Network Meta-Analysis (CINeMA) approach. Additionally, we calculated the surface under the cumulative ranking curve (SUCRA) for each dietary strategy to determine their ranking in terms of metabolic health benefits.
Results
Ten of the best and non-redundant meta-analysis studies, involving 153 original studies and 9846 participants, were included. When considering direct evidence only, all IF forms significantly reduced body weight compared to usual diets. In NMA incorporating indirect evidence, all IF regimens also significantly reduced body weight compared to usual diets. In the SUCRA of NMA, IF ranked higher than usual diets or CER in 85.4% and 56.1% of the outcomes, respectively. ADF had the highest overall ranking for improving metabolic health (ranked first: 64.3%, ranked second: 14.3%).
Conclusions
Overall, all IF forms demonstrate potentials to improve metabolic health, with ADF appearing to produce better outcomes across investigated outcomes. Further high-quality trials are warranted to confirm the (relative) efficacy of IF on metabolic health.
Trial registration
PROSPERO (record no: CRD42022302690).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-024-03716-1.
Keywords: Intermittent fasting, Alternate-day fasting, Time-restricted eating, Metabolic health, Continuous energy restriction
Background
The widespread occurrence of metabolic syndrome, ranging from 12.5 to 31.4% depending on the region, poses a significant health risk [1], leading to heightened risks of type 2 diabetes [2], cardiovascular diseases [3], non-alcoholic fatty liver disease [4], reproductive system disorders [5], and certain types of cancers [6, 7]. Therefore, addressing metabolic health is a global priority.
Multiple lines of evidence suggest that optimizing lifestyle factors, particularly dietary patterns, can effectively promote metabolic well-being [8–11]. Continuous energy restriction (CER), which aims to reduce overall energy intake per day, is a common dietary strategy for weight loss and subsequent improvement in metabolic parameters. However, CER can be challenging for many individuals to maintain [12, 13]. An alternative dietary approach, known as intermittent fasting (IF), has gained attention. IF is an eating pattern in which individuals alternate between periods of eating and defined extended fasting phases, which may span several hours to days, with energy intake during non-fasting periods either remaining normal or reduced [14]. Common IF regimens include (1) the 5:2 diet, which involves adopting a very low-energy diet for 2 days each week while eating without restrictions for the remaining 5 days [15]; (2) alternate-day fasting (ADF), which entails alternating between very low-energy intake and unrestricted eating every 24 h [14]; and (3) time-restricted eating (TRE), in which individuals typically fast for 14 to 16 h daily, with caloric intake not necessarily restricted [13].
While several meta-analyses have examined the effects of IF compared to CER or usual diets on metabolic health, limited research has investigated the relative efficacy of specific IF formats (5:2, ADF, TRE) and CER in enhancing metabolic parameters. To address this gap, we conducted an umbrella review and utilized network meta-analysis (NMA) to compare the effects of different IF forms and CER on metabolic health.
Methods
This study was pre-registered with PROSPERO (Registration No.: CRD42022302690). We conducted searches on PubMed, Embase, and Cochrane from their inception to November 29, 2022, using synonyms of IF (see Additional File 1: Table S1 for the detailed search strategy).
Eligibility criteria
We included meta-analyses of randomized controlled trial (RCT) investigating the association between IF and metabolic health outcomes in humans (details in Additional File 1: Table S2). We excluded the study that (1) was not a meta-analysis, (2) did not investigate IF in relation to metabolic health in humans, and (3) only pooled estimates from an observational design.
Study screening, data extraction, and the best study selection
Two reviewers, Y.-E.C. and H.-L.T., independently screened articles and extracted data. In cases where disagreements could not be resolved through consensus, a third researcher, L.-W.C., acted as an arbitrator during the negotiation process. For each eligible article, we extracted effect estimates for outcome measures related to metabolic health, including anthropometry measurements, blood pressure, glycemic parameters, and lipid profile.
To prevent duplication of original papers across multiple meta-analyses, we employed a selection process to identify the “best study” for each unique comparison of exposure and outcome [16, 17] based on several criteria: (1) largest number of original studies, (2) results from the Assessment of Multiple Systematic Reviews 2 (AMSTAR2) tool, and (3) largest number of participants in the same comparisons (see Additional File 1: Text S1 for the details of the study selection process) [16, 17].
Assessment of methodological quality and rating evidence quality
We employed the AMSTAR2 tool [18, 19] to evaluate the methodological quality of all included meta-analyses [14, 15, 20–56] and the established Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach (criteria and results provided in Additional File 1: Table S3) [37, 57–64] to evaluate the evidence quality for each outcome comparison within the selected best studies [21, 25, 26, 33, 37, 50, 53–56]. Based on these assessments, we classified the result into categories of high, moderate, low, or critically low (very low) quality/certainty.
The Cochrane Risk of Bias tool (RoB 2.0) was used to evaluate the RCT studies included in the NMA, categorizing each study’s overall risk of bias as high, some, or low [65].
Finally, we applied the Confidence in Network Meta-Analysis (CINeMA) framework to assess the confidence in the NMA results and assigned an overall confidence rating of very low, low, moderate, or high (see Additional File 1: Table S4) [61, 66–83].
Statistical methods
We extracted mean changes, standard deviations (SDs), and sample sizes from the original studies included in the best study. We converted 95% confidence intervals (CIs), standard errors, or interquartile ranges into SDs using the methods described in the Cochrane Handbook [84]. Subsequently, we performed random-effects NMA [85] on each outcome separately using the Stata software (version 16, Stata, College Station, TX, USA). We used the contrast-based model to conduct NMA, and estimated the ranking of different diets in terms of their efficacy on metabolic parameters using the SUCRA (surface under the cumulative ranking curve) analysis. We employed meta-regressions to assess interactions between IF and baseline BMI, age, gender, and intervention duration, using likelihood ratio tests. Sensitivity analyses were conducted by restricting the NMA analyses in healthy populations.
Summarizing the effectiveness of different dietary strategies on metabolic outcomes
For direct evidence, we organized all pairwise comparisons of IF with usual or CER diets, and color-coded them based on the certainty of our GRADE assessment.
In NMA, which incorporates indirect evidence, we categorized the certainty of the results based on the CINeMA assessment into two groups: (1) moderate to high certainty and (2) very low to low certainty. We then examined the impacts of dietary interventions on metabolic health. If a dietary intervention was significantly better than the reference diet (usual or CER diet) and any other non-reference diet (that was also significantly better than the reference diet), we classified it as having a “major effect” on metabolic health. However, if the dietary intervention was only significantly better than the reference diet, we classified it as having a “minor effect”. If a dietary intervention is not significantly different from the reference diet, it was categorized as having “no effect” [10].
Results
Out of the initial 302 articles identified across three online databases, 39 were included after a thorough selection process (Fig. 1). To avoid redundancy on the same research question, we further selected 10 best meta-analyses for each unique comparison, as mentioned earlier.
Fig. 1.
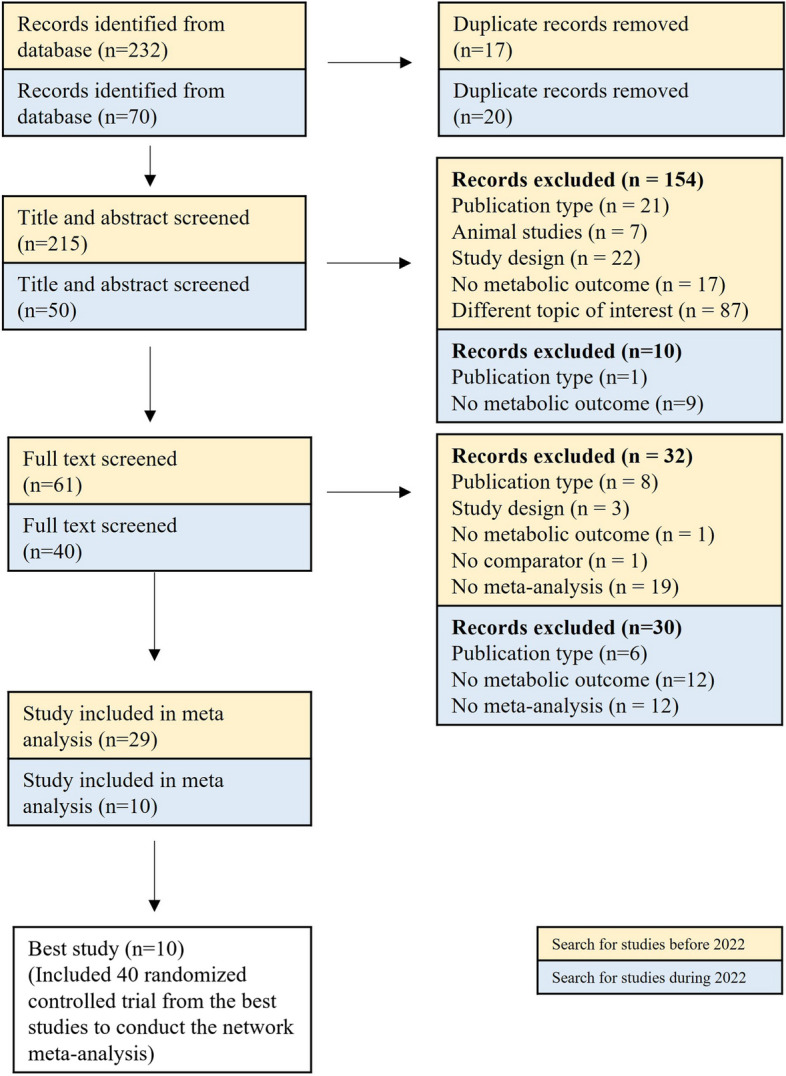
Flowchart for study selection
Study characteristics
Of the 10 best meta-analyses included, five were published in 2022 [50, 53–56]. Each encompassed a range of original studies, ranging from 5 to 43, conducted in countries in North and South America, Asia, Europe, Africa, and Australia/Oceania. The number of included participants in these studies ranged from 131 to 2483, with ages spanning 18 and 79 years old. While three of the meta-analysis studies focused on overweight or obese participants, the others did not impose any weight restrictions. The types of IF examined in these studies included TRE [21, 26, 50, 54, 55], ADF [25, 26, 53–55], 5:2/4:3 diet (days with usual diet: days with very low-energy diet per week) [37, 54, 56], and short-term intermittent energy restriction (STIER) (weekly intermittent energy restriction; fasting lasting more than 7 days) [33]. The duration of the dietary intervention ranged from 2 weeks to 24 months. When assessing methodological quality using AMSTAR2, five studies were rated as critically low quality [21, 25, 26, 33, 53], and the remaining five studies were rated as low quality [37, 50, 54–56] (Table 1).
Table 1.
Characteristics of included studies
| First author (year) | Search start and end dates | No. of included studies | Country of original studies | Total no. of participants | Age (year) | Population | Type of IF | Type of comparator | Diet Duration (months) | AMSTAR2 | Unique comparisons and available outcome measurements | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comparisons | Outcomes | |||||||||||
| Harris (2018) [33] |
Database inception~ 10/2016 |
5 | Canada, Sweden, USA | 376 | 21~69 | Overweight / obese adults | STIER | CER | 3.5~12 | Critically low quality | [STIER] - [CER] | BW |
| Cui (2020) [25] |
Database inception~ 3/1/2020 |
7 | USA, Korea, Canada, Malaysia, France | 269 | 18~70 | Healthy / overweight / obese adults | ADF | Usual diet | 1~12 | Critically low quality | [ADF] - [Usual] | BW, FM, SBP, DBP, FBG, TC, LDL-C, HDL-C, TG |
| Park (2020 [26] | Database inception~ ~12/31/2019 | 8 | USA, Austria, Korea, China, UK, Iran, Australia | 728 | 18~65 | Healthy / overweight / obese adults | ADF, TRE | CER, usual diet | 1~6 | Critically low quality | [TRE] - [ADF] | BW, BMI, FM, WC, FBG, TG, TC, LDL-C, HDL-C |
| [ADF] - [Usual] | BMI, WC, FI | |||||||||||
| [ADF] - [CER] | FI, TC, TG, FBG | |||||||||||
| Pellegrini (2020) [21] |
Database inception~ 1/31/2019 |
5 | USA, Iran, Turkey, Italy, Canada, Denmark, UK | 131 | 22~56 (mean) | Healthy / overweight / obese adults, one with prediabetes | TRE | Usual diet | 1~2 | Critically low quality | [TRE] - [Usual] | BW, FM, FFM, SBP, DBP, FBG, FI, TG, TC, LDL-C, HDL-C |
| Schwingshackl (2021) [37] | Database inception~ 3/25/2019 | 17 | USA, Australia, UK, Serbia, Germany, Norway, Malaysia | 1328 | 32~68 (mean) | Healthy / overweight / obese adults, some with MetS, T2DM | 5:2; 4:3 | CER, usual diet | 3~13 | Low quality | [5:2/4:3] - [Usual] | BW, FM, WC, SBP, FBG, HbA1c, LDL-C, TG |
| [5:2/4:3] - [CER] | BW, FM, WC, SBP, FBG, HbA1c, LDL-C, TG | |||||||||||
| Elortegui Pascual (2022) [53] |
Database inception~ 6/7/2021 |
24 | NAa | 1768 | 23~68 (mean) | Healthy / overweight / obese adults, without MetS, prediabetes, or T2DM | ADF, 5:2, TRE | CER, usual diet | 0.5~6.5 | Critically low quality | [ADF] - [CER] | BW |
| Gu (2022) [55] | Database inception~ 6/2021 | 43 | Brazil, China, Germany, Iran, Italy, Korea, Malaysia, New Zealand, Norway, Spain, Tunisia, Turkey, the UK, USA | 2483 | 18~79 | Healthy / overweight / obese adults | IER, 5:2, ADF, TRE, RDIF | CER, usual diet | 1~16 | Low quality | [TRE] - [CER] | BW, BMI, FM, FFM, WC, SBP, DBP |
| [ADF] - [CER] | BMI, FM, WC, SBP | |||||||||||
| Kim (2022) [54] |
2011~ 12/31/2021 |
16 | NAa | 1074 | 18~70 | Overweight / obese adults, some with MetS, T2DM | ADF, 5:2, TRE | CER | 3~13 | Low quality | [5:2] - [CER] | BW, BMI, WC, SBP, DBP, FBG, FI, HbA1c, TC, LDL-C, HDL-C, TG |
| [ADF] - [CER] | FFM, DBP, LDL-C, HDL-C | |||||||||||
| [TRE] - [CER] | FBG, FI, HbA1c, TC, LDL-C, HDL-C, TG | |||||||||||
| Liu (2022) [50] | Database inception~ 2/26/2022 | 17 | China, Switzerland, USA, Germany, Brazil, Italy | 899 | 19~54 (mean) | Healthy / overweight / obese adults, some with MetS, NAFLD, T2DM | TRE | CER, usual diet | 1~12 | Low quality | [TRE] - [Usual/CER] | BW, BMI, FM, WC, SBP, DBP, FBG, HbA1c, TC, LDL-C, HDL-C, TG |
| Wang (2022) [56] | Database inception~ 12/2021 | 11 | NAa | 790 | 18~75 | Overweight / obese adults, some with MetS, T2DM | 5:2, ADF | CER | 1~24 | Low quality | [5:2] - [CER] | FM, FFM |
4:3 Regular eating for four days and energy restriction for three days per week, 5:2 Regular eating for five days and energy restriction for two days per week, ADF Alternate day fasting, BMI Body mass index, BW Body weight, CER Continuous energy restriction, DBP Diastolic blood pressure, FBG Fasting blood glucose, FFM Fat free mass, FI Fasting insulin, FM Fat mass, HbA1c Glycosylated hemoglobin, HDL-C High density lipoprotein cholesterol, IF Intermittent fasting, LDL-C Low density lipoprotein cholesterol, MetS Metabolic syndrome, NAFLD Non-alcoholic fatty liver disease, RDIF Ramadan diurnal intermittent fasting, SBP Systolic blood pressure, STIER Short-term intermittent energy restriction, T2DM Type 2 Diabetes Mellitus, TC Total cholesterol, TG Triglycerides, TRE Time-restricted eating, UK United Kingdom, USA United States of America, WC Waist circumference
aThe systematic review did not include information about the countries where the original studies were conducted
Effects of IF on metabolic health: a qualitative analysis of direct evidence
The summary of the comparison of different dietary interventions is presented in Table 2, and the forest plot is displayed in Additional File 1: Fig. S1.
Table 2.
Summary of the direct evidence on effects of IF on metabolic outcomes in included meta-analyses
| Comparison | No. of studies |
No. of population | Population characteristics | Diet duration (months) |
Mean difference (95% CI) |
I2 | AMSTAR2 | GRADE | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IF form | Reference diet |
BMI | Disease | |||||||||
| Normal | Overweight | Obesity | ||||||||||
| Anthropometric measures | ||||||||||||
| BW, kg | ||||||||||||
| TRE | Usual | 5 | 131 | ✓ | ✓ | ✓ | preDM | 1~2 | -0.38 (-0.71, -0.05) | 0 | Critically low | Low |
| ADF | 7 | 269 | ✓ | ✓ | ✓ | - | 1~12 | -4.30 (-5.54, -3.05) | 96 | Critically low | Very low | |
| 5:2/4:3 | 6 | 348 | ✓ | ✓ | ✓ | - | 3~6 | -4.83 (-5.46, -4.21) | 3 | Low | Low | |
| TRE | CER | 8 | - | ✓ | ✓ | ✓ | - | 1~16 | -2.41 (-5.14, 0.33) | 42 | Low | Moderate |
| ADF | 8 | 393 | ✓ | ✓ | ✓ | - | 0.75~6.5 | -0.17 (-1.03, 0.69) | 76.2 | Critically low | Very low | |
| 5:2 | 8 | 700 | - | ✓ | ✓ | - | 3~13 | -0.02 (-0.17, 0.13) | 0 | Low | High | |
| 5:2/4:3 | 13 | 991 | ✓ | ✓ | ✓ | T2DM | 3~13 | -0.55 (-1.01, -0.09) | 0 | Low | Moderate | |
| STIER | 5 | 317 | - | ✓ | ✓ | - | 3.5~12 | -1.36 (-3.23, 0.51) | 37.6 | Critically low | Low | |
| TRE | Usual/CER | 17 | 899 | ✓ | ✓ | ✓ | MetS, NAFLD, T2DM | 1.3~12 | -1.60 (-2.27, -0.93) | 90.2 | Low | Low |
| TRE | ADF | 1 | 185 | - | ✓ | ✓ | - | 3 | 0.39 (-1.68, 2.46) | - | Critically low | Very low |
| BMI, kg/m2 | ||||||||||||
| ADF | Usual | 4 | 293 | ✓ | ✓ | ✓ | - | 1~3 | -0.96 (-1.42, -0.50) | 52 | Critically low | Low |
| TRE | CER | 4 | - | ✓ | ✓ | ✓ | - | 1~16 | -0.66 (-2.22, 0.91) | 51 | Low | Moderate |
| ADF | 5 | - | ✓ | ✓ | ✓ | - | 1~16 | -0.66 (-1.57, 0.24) | 45 | Low | Moderate | |
| 5:2 | 5 | 436 | - | ✓ | ✓ | T2DM | 3~13 | 0.08 (-0.11, 0.27) | 0 | Low | High | |
| TRE | Usual/CER | 8 | 616 | ✓ | ✓ | ✓ | MetS, NAFLD, T2DM | 2~12 | -0.53 (-1.10, 0.04) | 84.5 | Low | Low |
| TRE | ADF | 1 | 185 | - | ✓ | ✓ | - | 3 | 0.45 (0.01, 0.89) | - | Critically low | Very low |
| FM, kg | ||||||||||||
| TRE | Usual | 4 | 100 | ✓ | - | - | - | 1.3~2 | -0.83 (-1.89, 0.24) | 31 | Critically low | Low |
| ADF | 6 | 226 | ✓ | ✓ | ✓ | - | 1~12 | -4.96 (-8.08, 1.85) | 99 | Critically low | Very low | |
| 5:2/4:3 | 6 | 279 | ✓ | ✓ | ✓ | - | 3~6 | -2.54 (-3.78, -1.31) | 62 | Low | Low | |
| TRE | CER | 5 | - | ✓ | ✓ | ✓ | - | 1~16 | -2.58 (-4.55, 0.61) | 45 | Low | Moderate |
| ADF | 6 | - | ✓ | ✓ | ✓ | - | 1~16 | -2.80 (-6.34, 0.74) | 78 | Low | Low | |
| 5:2 | 5 | 438 | - | ✓ | ✓ | T2DM | 3~24 | 0.06 (-1.02, 1.14) | 0 | Low | High | |
| 5:2/4:3 | 10 | 743 | - | ✓ | ✓ | T2DM | 3~13 | -0.66 (-1.14, -0.19) | 0 | Low | High | |
| TRE | Usual/CER | 13 | 629 | ✓ | ✓ | ✓ | NAFLD | 1.3~12 | -1.48 (-1.59, -1.38) | 32 | Low | High |
| TRE | ADF | 1 | 185 | ✓ | ✓ | ✓ | - | 3 | 0.55 (-0.30, 1.40) | - | Critically low | Very low |
| FFM, kg | ||||||||||||
| TRE | Usual | 4 | 100 | ✓ | - | - | - | 1.5~2 | 0.00 (-0.78, 0.79) | 0 | Critically low | Low |
| TRE | CER | 5 | - | ✓ | ✓ | ✓ | - | 1~16 | -1.27 (-2.82, 0.29) | 2 | Low | High |
| ADF | 5 | 342 | - | ✓ | ✓ | - | 3~6.5 | 0.08 (-0.13, 0.30) | 0 | Low | Moderate | |
| 5:2 | 5 | 438 | - | ✓ | ✓ | T2DM | 1~24 | -0.23 (-0.97, 0.51) | 29 | Low | High | |
| WC, cm | ||||||||||||
| ADF | Usual | 2 | 201 | - | ✓ | ✓ | - | 3 | -2.41 (-4.95, 0.13) | 63.1 | Critically low | Low |
| 5:2/4:3 | 2 | 142 | - | ✓ | ✓ | - | 3 | -1.73 (-3.69, 0.24) | 0 | Low | Moderate | |
| TRE | CER | 6 | - | ✓ | ✓ | ✓ | - | 1~16 | -3.66 (-6.08, 1.24) | 38 | Low | High |
| ADF | 3 | - | ✓ | ✓ | ✓ | - | 1~16 | -1.37 (-4.08, 1.34) | 28 | Low | High | |
| 5:2 | 5 | 393 | - | ✓ | ✓ | - | 3~6.5 | -0.06 (-0.26, 0.14) | 0 | Low | Moderate | |
| 5:2/4:3 | 8 | 641 | - | ✓ | ✓ | T2DM | 3~10.5 | -0.57 (-1.56, 0.41) | 0 | Low | High | |
| TRE | Usual/CER | 7 | 512 | ✓ | ✓ | ✓ | MetS, NAFLD | 2~12 | -0.07 (-0.98, 0.83) | 0 | Low | High |
| TRE | ADF | 1 | 185 | - | ✓ | ✓ | 3 | 0.39 (-0.95, 1.73) | - | Critically low | Very low | |
| Blood pressure | ||||||||||||
| SBP, mmHg | ||||||||||||
| TRE | Usual | 2 | 33 | ✓ | - | - | - | 2 | -2.27 (-19.52, 14.98) | 88.9 | Critically low | Very low |
| ADF | 4 | 175 | ✓ | ✓ | ✓ | - | 1~12 | -4.42 (-7.35, -1.49) | 84 | Critically low | Very low | |
| 5:2/4:3 | 5 | 254 | ✓ | ✓ | ✓ | - | 3 | -6.11 (-9.59, -2.64) | 0 | Low | Moderate | |
| TRE | CER | 4 | - | ✓ | ✓ | ✓ | - | 1~16 | -3.19 (-7.63, 1.26) | 0 | Low | High |
| ADF | 3 | - | ✓ | ✓ | ✓ | - | 1~16 | -4.34 (-14.50, 5.83) | 79 | Low | Low | |
| 5:2 | 4 | 301 | - | ✓ | ✓ | - | 3~6.5 | 0.20 (-0.03, 0.42) | 0 | Low | Moderate | |
| 5:2/4:3 | 8 | 572 | - | ✓ | ✓ | T2DM | 3~6.5 | 0.24 (-2.08, 2.55) | 0 | Low | High | |
| TRE | Usual/CER | 8 | 454 | ✓ | ✓ | ✓ | MetS | 1.3~12 | -2.46 (-6.43, 1.51) | 77.4 | Low | Low |
| DBP, mmHg | ||||||||||||
| TRE | Usual | 2 | 33 | ✓ | - | - | - | 2 | -2.76 (-19.52, 14.98) | 88.1 | Critically low | Very low |
| ADF | 4 | 175 | ✓ | ✓ | ✓ | - | 1~12 | -3.41 (-5.91, -0.92) | 80 | Critically low | Very low | |
| TRE | CER | 4 | - | ✓ | ✓ | ✓ | - | 1~16 | -3.47 (-7.00, 0.06) | 0 | Low | Moderate |
| ADF | 3 | 239 | - | ✓ | ✓ | - | 3~6.5 | -0.04 (-0.29, 0.22) | 0 | Low | Moderate | |
| 5:2 | 3 | 224 | - | ✓ | ✓ | - | 3~6.5 | -0.04 (-0.30, 0.22) | 0 | Low | Moderate | |
| TRE | Usual/CER | 8 | 454 | ✓ | ✓ | ✓ | MetS | 1.3~12 | -1.78 (-4.72, 1.16) | 72.2 | Low | Low |
| Glycemic parameters | ||||||||||||
| FBG, mg/dl | ||||||||||||
| TRE | Usual | 4 | 90 | ✓ | ✓ | ✓ | preDM | 1.3~2 | -2.45 (-4.72, -0.17) | 0 | Critically low | Low |
| ADF | 4 | 144 | ✓ | ✓ | ✓ | - | 2~6 | -3.02 (-6.52, 0.48) | 89 | Critically low | Very low | |
| 5:2/4:3 | 4 | 224 | - | ✓ | ✓ | - | 3 | -2.16 (-7.38, 3.24) | 43 | Low | Low | |
| TRE | CER | 1 | 65 | - | ✓ | ✓ | MetS | 3 | -0.09 (-0.57, 0.40) | - | Low | Very low |
| ADF | 3 | 225 | ✓ | ✓ | ✓ | - | 1~6 | -1.34 (-4.74, 2.06) | 55.6 | Critically low | Low | |
| 5:2 | 7 | 545 | - | ✓ | ✓ | - | 3~13 | 0.15 (-0.02, 0.33) | 89 | Low | Low | |
| 5:2/4:3 | 8 | 584 | - | ✓ | ✓ | T2DM | 3~10.5 | 1.08 (-0.54, 2.70) | 5 | Low | High | |
| TRE | Usual/CER | 12 | 676 | ✓ | ✓ | ✓ | NAFLD, T2DM | 1~6 | -4.08 (-7.74, -0.42) | 96.6 | Low | Low |
| TRE | ADF | 1 | 185 | - | ✓ | ✓ | - | 3 | -2.52 (-8.01, 2.97) | - | Critically low | Very low |
| FI, μIU/ml | ||||||||||||
| TRE | Usual | 4 | 90 | ✓ | ✓ | ✓ | preDM | 1.3~2 | -0.69 (-1.64, 0.26) | 48.9 | Critically low | Very low |
| ADF | 2 | 67 | - | ✓ | ✓ | - | 2~3 | -1.50 (-10.11, 7.11) | 0 | Critically low | Moderate | |
| TRE | CER | 1 | 65 | - | ✓ | ✓ | MetS | 3 | 0.02 (-0.46, 0.51) | - | Low | Very low |
| ADF | 3 | 226 | - | ✓ | ✓ | - | 2~6 | 0.12 (-2.34, 2.59) | 55.8 | Critically low | Low | |
| 5:2 | 4 | 315 | - | ✓ | ✓ | - | 3~13 | -0.09 (-0.31, 0.14) | 81 | Low | Very low | |
| HbA1c, % | ||||||||||||
| 5:2/4:3 | Usual | 1 | 101 | - | ✓ | ✓ | - | 3 | 0.00 (-0.08, 0.08) | - | Low | Very low |
| TRE | CER | 1 | 65 | - | ✓ | ✓ | MetS | 3 | -0.01 (-0.50, 0.47) | - | Low | Very low |
| 5:2 | 4 | 388 | - | ✓ | ✓ | GDM, T2DM | 3~13 | 0.01 (-0.19, 0.21) | 65 | Low | Moderate | |
| 5:2/4:3 | 6 | 570 | - | ✓ | ✓ | MetS | 3~13 | -0.01 (-0.07, 0.06) | 0 | Low | High | |
| TRE | Usual/CER | 6 | 355 | - | ✓ | ✓ | MetS, T2DM | 2~6 | -0.11 (-0.50, 0.27) | 98.8 | Low | Very low |
| Lipid profile | ||||||||||||
| TG, mg/dl | ||||||||||||
| TRE | Usual | 4 | 90 | ✓ | ✓ | ✓ | preDM | 1.3~2 | 6.24 (-13.40, 25.88) | 86.8 | Critically low | Very low |
| ADF | 5 | 208 | ✓ | ✓ | ✓ | - | 2~12 | -11.27 (-20.53, -2.00) | 96 | Critically low | Very low | |
| 5:2/4:3 | 5 | 254 | ✓ | ✓ | ✓ | - | 3 | -17.71 (-33.66, -2.66) | 52 | Low | Low | |
| TRE | CER | 1 | 65 | - | ✓ | ✓ | MetS | 3 | -0.02 (-0.51, 0.47) | - | Low | Very low |
| ADF | 3 | 225 | ✓ | ✓ | ✓ | - | 2~6 | -5.87 (-32.06, 20.32) | 50.5 | Critically low | Low | |
| 5:2 | 6 | 494 | ✓ | ✓ | ✓ | - | 3~13 | 0.06 (-0.12, 0.24) | 25 | Low | High | |
| 5:2/4:3 | 8 | 587 | - | ✓ | ✓ | T2DM | 3~10.5 | -3.54 (-11.51, 5.31) | 0 | Low | High | |
| TRE | Usual/CER | 13 | 767 | ✓ | ✓ | ✓ | MetS, NAFLD, T2DM | 1.3~12 | -8.64 (-18.01, 0.73) | 97 | Low | Low |
| TRE | ADF | 1 | 185 | - | ✓ | ✓ | - | 3 | 16.81 (-29.84, 63.47) | - | Critically low | Very low |
| TC, mg/dl | ||||||||||||
| TRE | Usual | 4 | 90 | ✓ | ✓ | ✓ | preDM | 1.3~2 | 9.14 (-3.69, 21.96) | 80.9 | Critically low | Very low |
| ADF | 5 | 174 | ✓ | ✓ | ✓ | - | 2~12 | -11.32 (-18.20, -4.44) | 96 | Critically low | Very low | |
| TRE | CER | 1 | 65 | - | ✓ | ✓ | MetS | 3 | 0.00 (-0.48, 0.49) | - | Low | Very low |
| ADF | 3 | 225 | - | ✓ | ✓ | - | 2~6 | -2.79 (-11.31, 5.74) | 0 | Critically low | Moderate | |
| 5:2 | 6 | 494 | - | ✓ | ✓ | - | 3~13 | -0.2 (-0.37, -0.02) | 51 | Low | Low | |
| TRE | Usual/CER | 10 | 657 | ✓ | ✓ | ✓ | NAFLD, T2DM | 1~12 | -6.10 (-7.86, -4.34) | 4.6 | Low | High |
| TRE | ADF | 1 | 185 | - | ✓ | ✓ | - | 3 | 8.49 (-6.09, 23.08) | - | Critically low | Very low |
| LDL-C, mg/dl | ||||||||||||
| TRE | Usual | 4 | 90 | ✓ | ✓ | ✓ | preDM | 1.3~2 | 3.99 (-6.41, 14.39) | 69.3 | Critically low | Very low |
| ADF | 4 | 151 | ✓ | ✓ | ✓ | - | 2~12 | -5.82 (-10.45, -1.19) | 92 | Critically low | Very low | |
| 5:2/4:3 | 5 | 254 | ✓ | ✓ | ✓ | - | 3 | -1.44 (-5.40, 2.70) | 67 | Low | Very low | |
| TRE | CER | 1 | 65 | - | ✓ | ✓ | MetS | 3 | -0.07 (-0.55, 0.42) | - | Low | Very low |
| ADF | 3 | 229 | - | ✓ | ✓ | - | 3~6.5 | 0.00 (-0.26, 0.26) | 0 | Low | Moderate | |
| 5:2 | 6 | 494 | - | ✓ | ✓ | - | 3~13 | -0.20 (-0.38, -0.02) | 0 | Low | High | |
| 5:2/4:3 | 8 | 579 | - | ✓ | ✓ | T2DM | 3~10.5 | 0.00 (-1.98, 1.98) | 19 | Critically low | Moderate | |
| TRE | Usual/CER | 10 | 689 | ✓ | ✓ | ✓ | NAFLD, T2DM | 1.3~12 | -3.63 (-8.05, 0.78) | 81.3 | Low | Low |
| TRE | ADF | 1 | 185 | - | ✓ | ✓ | - | 3 | -3.09 (-12.55, 6.37) | - | Critically low | Very low |
| HDL-C, mg/dl | ||||||||||||
| TRE | Usual | 4 | 90 | ✓ | ✓ | ✓ | preDM | 1.3~2 | 1.36 (-1.43, 4.14) | 53.9 | Critically low | Very low |
| ADF | 5 | 174 | ✓ | ✓ | ✓ | - | 2~12 | -1.05 (-2.92, 0.83) | 71 | Critically low | Low | |
| TRE | CER | 1 | 65 | - | ✓ | ✓ | MetS | 3 | 0.18 (-0.31, 0.66) | - | Low | Very low |
| ADF | 3 | 229 | - | ✓ | ✓ | - | 3~6.5 | -0.34 (-0.61, -0.08) | 79 | Low | Moderate | |
| 5:2 | 6 | 494 | - | ✓ | ✓ | - | 3~13 | 0.05 (-0.12, 0.23) | 0 | Low | High | |
| TRE | Usual/CER | 12 | 755 | ✓ | ✓ | ✓ | NAFLD, T2DM | 1~12 | 0.75 (-0.73, 2.24) | 73.6 | Low | Moderate |
| TRE | ADF | 1 | 185 | - | ✓ | ✓ | - | 3 | -0.77 (-5.40, 3.85) | - | Critically low | Very low |
Bold font: statistically significant associations
4:3 Regular eating for four days and energy restriction for three days per week, 5:2 Regular eating for five days and energy restriction for two days per week, ADF Alternate day fasting, BMI Body mass index, BW Body weight, CER Continuous energy restriction, DBP Diastolic blood pressure, FBG Fasting blood glucose, GDM Gestational diabetes mellitus, FFM Fat free mass, FI Fasting insulin, FM Fat mass, HbA1c Hemoglobin A1c, HDL-C High density lipoprotein cholesterol, IF Intermittent fasting, LDL-C Low density lipoprotein cholesterol, MetS Metabolic syndrome, NAFLD Nonalcoholic fatty liver disease, preDM Prediabetes mellitus, SBP Systolic blood pressure, STIER Short-term intermittent energy restriction, T2DM Type 2 diabetes mellitus, TC Total cholesterol, TG Triglycerides, TRE Time-restricted eating, WC Waist circumference
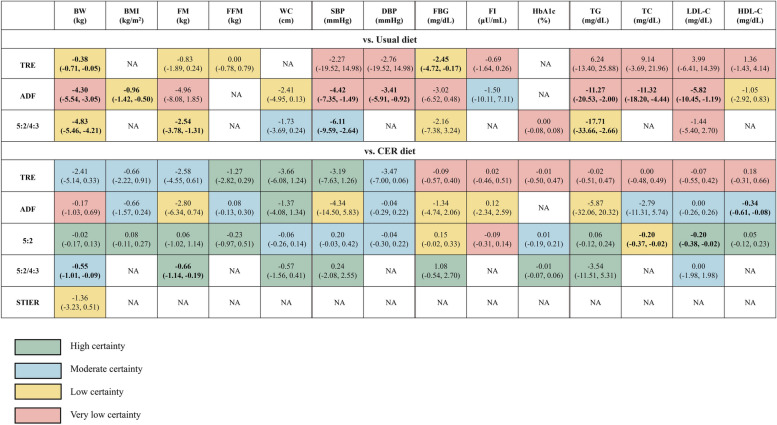
Anthropometric measures
There were ten unique meta-analysis comparisons for body weight (BW) [21, 25, 26, 33, 37, 50, 53–55] (Table 2). When comparing IF (TRE [21], ADF [25], 5:2/4:3 [37]) to usual diets, all showed a significant reduction in body weight (range of mean difference: − 0.38 to − 4.83 kg), but with low or very low certainty. When compared to CER, only the 5:2/4:3 diet demonstrated a significant effect on reducing body weight (difference in mean: − 0.55 kg; 95% CI: − 1.01 to − 0.09) with moderate certainty [37]. TRE approach also reduced body weight compared to usual/CER diets (− 1.60 kg; − 2.27 to − 0.93), with low certainty [50].
There were six unique meta-analysis comparisons for body mass index (BMI) [26, 50, 54, 55] (Table 2). When comparing different diets, only ADF demonstrated a significant reduction in BMI compared to usual diets [26] (− 0.96 kg/m2; − 1.42 to − 0.50) and TRE (− 0.45 kg/m2; − 0.89 to − 0.01) [26], with low and very low certainty [26].
There were nine unique meta-analysis comparisons for fat mass (FM) [21, 25, 26, 37, 50, 55, 56] (Table 2). The 5:2/4:3 diet significantly reduced FM with low (compared to usual diets) (− 2.54 kg; − 3.78 to − 1.31) and high certainty (compared to CER) (− 0.66 kg; − 1.14 to − 0.19) [37]. Additionally, TRE also significantly reduced FM (− 1.48 kg; − 1.59 to − 1.38) compared to usual/CER diets with high certainty [50].
However, no significant differences were observed between any form of IF and the reference diets in fat-free mass (FFM) and waist circumference (WC) outcomes (Table 2).
Blood pressure
There were eight unique meta-analysis comparisons for systolic blood pressure (SBP) [21, 25, 37, 50, 54, 55] (Table 2). Only ADF (− 4.42 mmHg; − 7.35 to − 1.49; very low certainty) [25] and 5:2/4:3 diet (− 6.11 mmHg; − 9.59 to − 2.64; moderate certainty) [37] significantly reduced SBP compared to usual diets.
There were six unique meta-analysis comparisons for diastolic blood pressure (DBP) [21, 25, 50, 54, 55] (Table 2). Only ADF significantly reduced DBP compared to usual diets (− 3.41 mmHg; − 5.91 to − 0.92) [25], albeit with very low certainty.
Glycemic parameters
There were nine unique meta-analysis comparisons for fasting blood glucose (FBG) [21, 25, 26, 37, 50, 54] (Table 2). Only TRE significantly reduced FBG compared to usual diets (− 2.45 mg/dl; − 4.72 to − 0.17) [21] or usual/CER diets (− 4.08 mg/dl; − 7.74 to − 0.42) [50], with low certainty for both comparisons.
However, no significant differences were observed between any IF form and the reference diets in fasting insulin (FI) and glycosylated hemoglobin (HbA1c) outcomes (Table 2).
Lipid profile
There were nine unique meta-analysis comparisons for triglycerides (TG) [21, 25, 26, 37, 50, 54] (Table 2). Only ADF (− 11.27 mg/dl; − 20.53 to − 2.00) [25] and 5:2/4:3 diet (− 17.71 mg/dl; − 33.66 to − 2.66) [37] significantly reduced TG levels compared to usual diets, with very low and low certainty.
There were seven unique meta-analysis comparisons for total cholesterol (TC) [21, 25, 26, 50, 54] (Table 2). TRE significantly reduced TC levels compared to usual/CER diets (− 6.10 mg/dl; − 7.86 to − 4.34) with high certainty [50]. Additionally, the 5:2 diet significantly reduced TC levels compared to CER (− 0.2 mg/dl; − 0.37 to − 0.02) [54] with low certainty and ADF significantly reduced TC levels compared to usual diets (− 11.32 mg/dl; − 18.20 to − 4.44) [25] with very low certainty.
There were nine unique meta-analysis comparisons for low density lipoprotein cholesterol (LDL-C) [21, 25, 26, 37, 50, 54] (Table 2). When compared to CER, only the 5:2 diet significantly reduced LDL-C levels (− 0.20 mg/dl; − 0.38 to − 0.02), with high certainty [54]. ADF significantly reduced LDL-C levels compared to usual diets (− 5.82 mg/dl; − 10.45 to − 1.19) with very low certainty [25].
There were seven unique meta-analysis comparisons for high density lipoprotein cholesterol (HDL-C) [21, 25, 26, 50, 54] (Table 2). Among all comparisons, only the ADF diet significantly reduced HDL-C levels compared to CER (− 0.34 mg/dl; − 0.61 to − 0.08), with moderate certainty [54].
Summary of the direct evidence with GRADE assessment
The summary of the direct evidence with our GRADE assessment is presented in Fig. 2. IF significantly improved metabolic health outcomes in 42% (13 out of 31) of the comparisons with usual diets, with efficacy shown across all IF forms for body weight reduction, albeit with low or very low certainty. Among IF regimens, ADF generally demonstrated superior performance across metabolic health outcomes, but most of the evidence associated with ADF had very low certainty. The only significant association supported by moderate certainty is the reduction in SBP associated with the 5:2/4:3 diet.
Fig. 2.
All comparisons of direct evidence. Effect estimates are mean differences (95% CI). Bold font: statistically significant associations. 4:3: regular eating for 4 days and energy restriction for 3 days per week, 5:2: regular eating for 5 days and energy restriction for 2 days per week. ADF, alternate day fasting; BMI, body mass index; BW, body weight; CER, continuous energy restriction; DBP, diastolic blood pressure; FBG, fasting bloodglucose; FFM, fat-free mass; FI, fasting insulin; FM, fat mass; HbA1c, glycosylated hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; STIER, short-term intermittent energy restriction; TC, total cholesterol; TG, triglycerides; TRE, time-restricted eating; WC, waist circumference
When compared to CER, only 8% (4 out of 53) of the comparisons indicated that IF significantly improved metabolic health. Notably, the 5:2 diet performed significantly better than CER for LDL-C levels, while the 5:2/4:3 diet exhibited significant improvements in BW and FM, all with moderate and high certainty.
Effects of IF on metabolic health: results from quantitative network meta-analysis
Supplementary Additional File 1: Table S5 [86–125] and Fig. S2 presents the characteristics and risk of bias of the original studies included in the best studies, respectively. Additional File 1: Table S4 provides a detailed assessment of the certainty of evidence using the CINeMA approach, while Additional File 1: Table S6 offers network evidence for pairwise comparisons. Additional File 1: Fig. S3 displays the network plots, and Additional File 1: Fig. S4 details the SUCRA. Below, we summarize the key findings based on the criteria outlined in the Methods section.
To determine the specific impact of a specific dietary strategy on metabolic outcomes in the quantitative NMA, we further excluded comparisons involving mixed strategies (5:2/4:3) and STIER (as it deviated from the standard regimen of IF and provided only BW outcome data compared to CER).
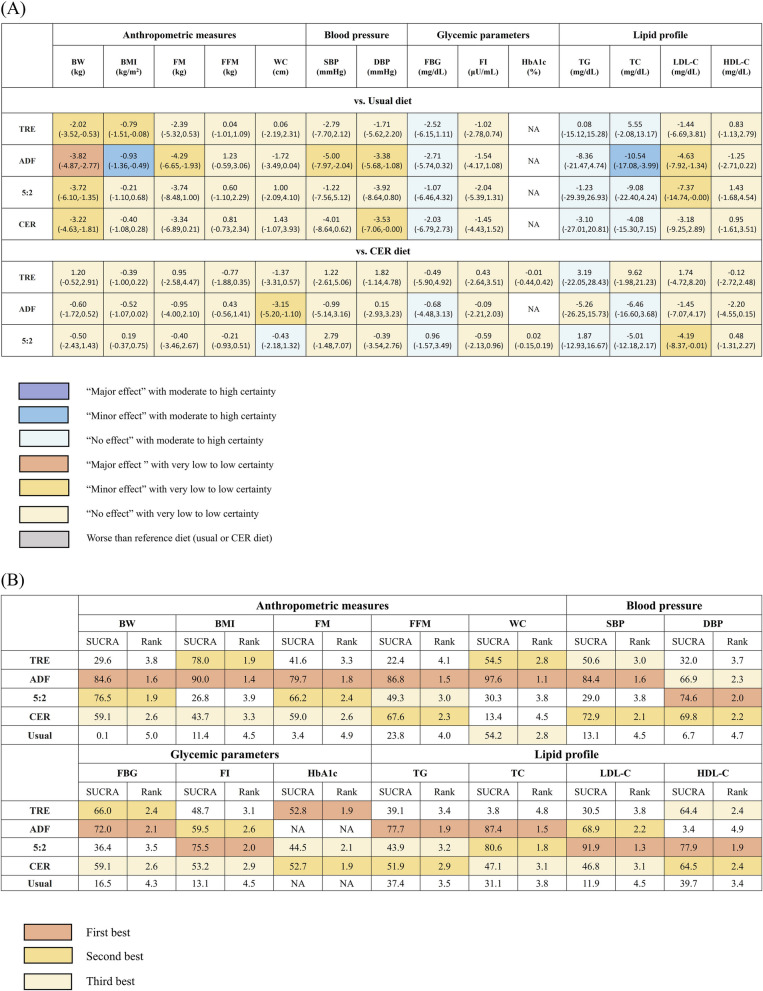
NMA results for anthropometric measures
When compared to usual diet, ADF showed major effect on BW, albeit with low certainty. TRE and the 5:2 diet demonstrated minor effects on BW, with low-very low certainty. ADF and TRE had minor effects on BMI, with ADF showing moderate certainty and TRE showing very low certainty. ADF exhibited a minor effect on FM, with very low certainty (Fig. 3A).
Fig. 3.
Summary results of network meta-analysis. A Different diet compared to usual or CER diet. B Ranking by SUCRA analysis. Effect estimates for panel A are mean differences (95% CI). Numbers presented in panel B are SUCRA values and mean rank; greater SUCRA values or lower mean rank values indicate superior dietary strategies. 5:2: regular eating for 5 days and energy restriction for 2 days per week. ADF, alternate day fasting; BMI, body mass index; BW, body weight; CER, continuous energy restriction; DBP, diastolic blood pressure; FBG, fasting blood sugar; FFM, fat-free mass; FI, fasting insulin; FM, fat mass; HbA1c, glycosylated hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; SUCRA, surface under the cumulative ranking curve; TC, total cholesterol; TG, triglycerides; TRE, time-restricted eating; WC, waist circumference. Mixed strategy comparisons (5:2/4:3) and STIER (as it deviated from the standard regimen of IF) were excluded in this quantitative network meta-analysis
When compared to CER, only ADF demonstrated minor effects on WC with very low certainty (Fig. 3A).
When compared to TRE, ADF (− 1.79 kg; − 3.38 to − 0.21) significantly decreased BW, albeit with low certainty (Additional File 1: Table S6). According to the SUCRA analysis, ADF consistently ranked first for anthropometric measures (Fig. 3B).
NMA results for blood pressure
When compared to usual diets, ADF demonstrated a minor effect on both SBP and DBP with low-very low certainty (Fig. 3A).
However, no significant differences were observed when comparing IF to CER or within the IF group itself (Additional File 1: Table S6). In terms of SUCRA rankings, ADF ranked first and third for SBP and DBP, respectively (Fig. 3B).
NMA results for glycemic parameters
When compared to usual diets, all forms of IF showed no significant effect on FBG and FI, with moderate-high certainty for FBG and low-very low certainty for FI.
No significant differences were observed when comparing IF to CER or within the IF group itself (Additional File 1: Table S6). In the SUCRA analysis, ADF ranked first or second for FBG and FI, while the rankings of other IF forms were inconsistent (Fig. 3B).
NMA results for lipid profile
When compared to usual diets, ADF had a minor effect on TC and LDL-C with moderate and low certainty, respectively. 5:2 diet also had a minor effect on LDL-C with low certainty.
When compared to CER, 5:2 diet showed a minor effect on LDL-C levels with low certainty. (Fig. 3A).
When compared to TRE, both the 5:2 diet (− 14.63 mg/dl; − 28.27 to − 0.99) with moderate certainty and ADF (− 16.09 mg/dl; − 25.06 to − 7.11) with low certainty significantly decreased TC (Additional File 1: Table S6). Based on SUCRA analysis, ADF ranked first for TG and TC, while the 5:2 diet ranked first for LDL-C and HDL-C (Fig. 3B).
In our network meta-analysis, across all studied outcomes, IF in general ranked better than usual diets (85.4% of the comparisons) and CER (56.1% of the comparisons).
Meta-regression and sensitivity analyses
Several significant interactions were identified between IF and baseline BMI, age, gender, and duration of intervention in the NMA meta-regression analyses (Additional File 1: Table S7). Specifically, a higher baseline BMI decreased the treatment effects of both ADF and TRE on BW and FM, of ADF on WC, but increased the treatment effect of the 5:2 diet on WC compared to a usual diet. Longer intervention duration decreased the treatment effects of both ADF and TRE on BW, and of both ADF and the 5:2 diet on FM. Lastly, the treatment effect of ADF on WC decreased with age. However, these results should be interpreted with caution due to the limited number of studies available for each treatment comparison. In sensitivity analyses restricted to healthy populations, ADF still consistently displayed higher rankings for most outcomes (except for glycemic parameters), aligned with the main analyses (Additional File 1: Fig. S5).
Discussion
To our knowledge, this umbrella review is the first to comprehensively elucidate the impact of IF diets on metabolic outcomes, utilizing both direct and indirect evidence, and employing NMA to rank the efficacy of various IF forms. Our findings indicate that all IF regimens significantly reduced BW compared to a usual diet, although their effects were less pronounced when compared to CER diet. When considering direct evidence across all metabolic outcomes, IF showed significant improvements in 42% of the comparisons with usual diets and 8% when compared to CER. Whether from direct evidence or NMA, ADF consistently exhibited more favorable outcomes overall. Moreover, ADF emerged as the top-ranked IF approach for enhancing metabolic health, being ranked first in 64.3% of the comparisons and second in 14.3%. These findings suggest that IF may serve as suitable and non-inferior, and, in some cases, superior alternative to improve metabolic health compared to both usual diets and CER.
IF can promote metabolic benefits by shifting metabolism from glucose reliance to using ketone bodies from fatty acids, reducing lipid synthesis and fat storage, thus potentially improving body composition [126]. In addition, the reduction in blood pressure might be attributed to the activation of the parasympathetic nervous system, driven by increased activity of cholinergic neurons in the brainstem [127]. IF can also reduce insulin resistance by lowering body fat, leading to improved insulin sensitivity and glucose metabolism [128]. During fasting, the liver upregulates peroxisome proliferator-activated receptor alpha (PPARα) and peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC-1α), thus enhancing fatty acid oxidation and reducing triglyceride accumulation. This process lowers very low-density lipoprotein cholesterol (VLDL) production and blood LDL-C levels [129].
Previous studies have shown that adherence to CER may decline over time, leading to outcomes that may not align with expectations [13]. Among the meta-analyses we included, two studies investigated adherence to both IF and CER. In the study by Elortegui Pascual et al., adherence percentages ranged from 71.7 to 98% for ADF, 73.5 to 98% for the 5:2 diet, 83 to 89% for TRE, and 80 to 90% for CER [53]. In contrast, Schwingshackl et al. reported an average adherence of 76% (range: 44% to 98%) for 5:2/4:3 diets, while CER had a lower average adherence of 62% (range: 32% to 78%) [37].
In most studies, both IF and CER did not result in significant adverse events, and the probability of side effects was low [15]. Among individuals adhering to a CER diet, 2% to 7% reported mild occurrences of mild nausea, dizziness, feeling cold, constipation, lack of concentration, being preoccupied with food, mood swings or bad temper, mild headache, and decreased energy levels [15]. The implementation of IF diets for most participants resulted in symptoms such as constipation [15, 130], diarrhea [130], dizziness [15, 130], reported hunger [15], mild headaches [15, 25, 130], temporary sleep disturbances [15], and bad breath [15]. Nevertheless, studies have shown that these symptoms tends to improve over time for the majority of participants following an IF eating pattern [15, 25, 130].
We conducted a comprehensive investigation into the effects of IF diets, including TRE, ADF, 5:2/4:3, and STIER diets, on metabolic outcomes including anthropometric measures, blood pressure, glycemic parameters, and lipid profile. Notably, the majority of the best studies we included were published after 2022. Therefore, our umbrella review offers a current and comprehensive synthesis of the evidence on this topic. Furthermore, we undertook a comprehensive comparison between various IF regimens and two reference diets, namely the usual diet and CER. Additionally, we utilized network meta-analysis, which enabled us to evaluate both direct and indirect evidence, to assess the relative efficacy of different IF regimens on metabolic health.
However, it is noteworthy that 63% of the direct evidence available was classified as low or very low quality. Furthermore, the intervention durations varied in studies included in the original meta-analyses, ranging from as short as half a month to as long as 24 months. Therefore, further high-quality research is needed to investigate the effects of different intervention durations and the long-term sustainability of these effects.
Conclusions
All IF diets demonstrated significant reductions in body weight compared to a usual diet, although their effects were less prominent for other metabolic outcomes and when compared to CER. Among the IF regimens, ADF seemed to yield better results overall across the investigated outcomes, except for HDL-C. However, the majority of direct evidence available had low or very low certainty, underscoring the need for further well-conducted RCTs to strengthen these findings. Nonetheless, considering the available evidence as a whole, IF appears to be a promising dietary strategy that is at least comparable to CER in terms of improving metabolic health.
Supplementary Information
Additional file 1. Text S1. Details of the study selection process. Table S1. Search strategies. Table S2. List of discrepancies between the initial protocol and the final analysis. Table S3. Criteria and results of the GRADE assessment. Table S4. Criteria and results of the CINeMA assessment. Table S5. Characteristics of original studies within included studies. Table S6. Network evidence of pair-wise comparison with CINeMA. Table S7. Results for meta-regression. Figure S1. Summary of the direct evidence. Figure S2. Risk of bias in the original studies included in the systematic review. Figure S3. Network plots. Figure S4. Results for surface under the cumulative ranking (SUCRA) analaysis. Figure S5. Results for the sensitivity analysis restricted to healthy people with surface under the cumulative ranking (SUCRA).
Acknowledgements
Not applicable.
Abbreviations
- ADF
Alternate-day fasting
- AMSTAR2
Assessment of Multiple Systematic Reviews 2
- BMI
Body mass index
- BW
Body weight
- CER
Continuous energy restriction
- CIs
Confidence intervals
- CINeMA
Confidence in Network Meta-Analysis
- DBP
Diastolic blood pressure
- FBG
Fasting blood glucose
- FFM
Fat-free mass
- FI
Fasting insulin
- FM
Fat mass
- GRADE
Grading of Recommendations, Assessment, Development and Evaluation
- HbA1c
Glycosylated hemoglobin
- HDL-C
High-density lipoprotein cholesterol
- IF
Intermittent fasting
- LDL-C
Low-density lipoprotein cholesterol
- NMA
Network meta-analysis
- PGC-1α
Peroxisome proliferator-activated receptor-gamma coactivator 1-alpha
- PPARα
Peroxisome proliferator-activated receptor alpha
- RCT
Randomized controlled trial
- SBP
Systolic blood pressure
- SEs
Standard errors
- SUCRA
Surface-under-the-cumulative-ranking-curve analysis
- TC
Total cholesterol
- TG
Triglycerides
- TRE
Time-restricted eating
- VLDL
Very low-density lipoprotein cholesterol
- WC
Waist circumference
Authors’ contributions
L-WC conceptualized the research. YEC and HLT conducted the literature search and literature screening. YEC and HLT extracted the data. YEC analyzed the data and wrote the first draft of the paper. YKT provided essential methodology and statistical consultations. All authors interpreted the data, read the manuscript, and approved the final version.
Funding
This study is supported by grants awarded to L-WC (MOST110-2314-B-002-290-MY2, P110A290; NSTC112-2314-B-002-322-MY3) from the Ministry of Science and Technology (now the National Science and Technology Council). L-WC receives further financial support from the National Taiwan University Higher Education Sprout Project (111L7304, 111L7306, 110L7418, 110L881002) within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Noubiap JJ, Nansseu JR, Lontchi-Yimagou E, Nkeck JR, Nyaga UF, Ngouo AT, et al. Geographic distribution of metabolic syndrome and its components in the general adult population: a meta-analysis of global data from 28 million individuals. Diabetes Res Clin Pract. 2022;188:109924. [DOI] [PubMed] [Google Scholar]
- 2.Regufe VM, Pinto CM, Perez PM. Metabolic syndrome in type 2 diabetic patients: a review of current evidence. Porto Biomed J. 2020;5(6):e101. [DOI] [PMC free article] [PubMed]
- 3.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49(4):403–14. [DOI] [PubMed] [Google Scholar]
- 4.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65(8):1038–48. [DOI] [PubMed] [Google Scholar]
- 5.Leisegang K, Henkel R, Agarwal A. Obesity and metabolic syndrome associated with systemic inflammation and the impact on the male reproductive system. Am J Reprod Immunol. 2019;82(5):e13178. [DOI] [PubMed] [Google Scholar]
- 6.Bishehsari F, Voigt RM, Keshavarzian A. Circadian rhythms and the gut microbiota: from the metabolic syndrome to cancer. Nat Rev Endocrinol. 2020;16(12):731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, Zheng X, Zong X, Li Z, Li N, Hur J, et al. Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer. Gut. 2021;70(6):1147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwingshackl L, Chaimani A, Hoffmann G, Schwedhelm C, Boeing H. A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus. Eur J Epidemiol. 2018;33:157–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwingshackl L, Chaimani A, Schwedhelm C, Toledo E, Pünsch M, Hoffmann G, Boeing H. Comparative effects of different dietary approaches on blood pressure in hypertensive and pre-hypertensive patients: a systematic review and network meta-analysis. Crit Rev Food Sci Nutr. 2019;59(16):2674–87. [DOI] [PubMed] [Google Scholar]
- 10.Ge L, Sadeghirad B, Ball GD, da Costa BR, Hitchcock CL, Svendrovski A, et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: systematic review and network meta-analysis of randomised trials. BMJ. 2020;369:m696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neuenschwander M, Hoffmann G, Schwingshackl L, Schlesinger S. Impact of different dietary approaches on blood lipid control in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Eur J Epidemiol. 2019;34:837–52. [DOI] [PubMed] [Google Scholar]
- 12.Varady K. Intermittent versus daily calorie restriction: which diet regimen is more effective for weight loss? Obes Rev. 2011;12(7):e593–601. [DOI] [PubMed] [Google Scholar]
- 13.Rynders CA, Thomas EA, Zaman A, Pan Z, Catenacci VA, Melanson EL. Effectiveness of intermittent fasting and time-restricted feeding compared to continuous energy restriction for weight loss. Nutrients. 2019;11(10):2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alhamdan B, Garcia-Alvarez A, Alzahrnai A, Karanxha J, Stretchberry D, Contrera K, et al. Alternate-day versus daily energy restriction diets: which is more effective for weight loss? A systematic review and meta-analysis. Obes Sci Pract. 2016;2(3):293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He S, Wang J, Zhang J, Xu J. Intermittent versus continuous energy restriction for weight loss and metabolic improvement: a meta-analysis and systematic review. Obesity. 2021;29(1):108–15. [DOI] [PubMed] [Google Scholar]
- 16.Veettil SK, Wong TY, Loo YS, Playdon MC, Lai NM, Giovannucci EL, Chaiyakunapruk N. Role of diet in colorectal cancer incidence: umbrella review of meta-analyses of prospective observational studies. JAMA Net Open. 2021;4(2):e2037341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neuenschwander M, Ballon A, Weber KS, Norat T, Aune D, Schwingshackl L, Schlesinger S. Role of diet in type 2 diabetes incidence: umbrella review of meta-analyses of prospective observational studies. BMJ. 2019;366:l2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [DOI] [PMC free article] [PubMed]
- 19.Baker WL, Bennetts M, Coleman CI, Cappelleri JC. Appraising Evidence. In: Biondi-Zoccai, G, editor. Umbrella reviews: evidence synthesis with overviews of reviews and meta-epidemiologic studies. Cham: Springer; 2016. 10.1007/978-3-319-25655-9_9.
- 20.Harris L, Hamilton S, Azevedo LB, Olajide J, De Brún C, Waller G, et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: a systematic review and meta-analysis. JBI Evidence Synthesis. 2018;16(2):507–47. [DOI] [PubMed] [Google Scholar]
- 21.Pellegrini M, Cioffi I, Evangelista A, Ponzo V, Goitre I, Ciccone G, et al. Effects of time-restricted feeding on body weight and metabolism. A systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21:17–33. [DOI] [PubMed] [Google Scholar]
- 22.Correia JM, Santos I, Pezarat-Correia P, Minderico C, Mendonca GV. Effects of intermittent fasting on specific exercise performance outcomes: a systematic review including meta-analysis. Nutrients. 2020;12(5):1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moon S, Kang J, Kim SH, Chung HS, Kim YJ, Yu JM, et al. Beneficial effects of time-restricted eating on metabolic diseases: a systemic review and meta-analysis. Nutrients. 2020;12(5):1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng H, Zhu L, Kord-Varkaneh H, Santos HO, Tinsley GM, Fu P. Effects of intermittent fasting and energy-restricted diets on lipid profile: a systematic review and meta-analysis. Nutrition. 2020;77:110801. [DOI] [PubMed] [Google Scholar]
- 25.Cui Y, Cai T, Zhou Z, Mu Y, Lu Y, Gao Z, et al. Health effects of alternate-day fasting in adults: a systematic review and meta-analysis. Front Nutr. 2020;7:586036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park J, Seo Y-G, Paek Y-J, Song HJ, Park KH, Noh H-M. Effect of alternate-day fasting on obesity and cardiometabolic risk: a systematic review and meta-analysis. Metabolism. 2020;111:154336. [DOI] [PubMed] [Google Scholar]
- 27.Borgundvaag E, Mak J, Kramer CK. Metabolic impact of intermittent fasting in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of interventional studies. J Clin Endocrinol Metab. 2021;106(3):902–11. [DOI] [PubMed] [Google Scholar]
- 28.Mirmiran P, Bahadoran Z, Gaeini Z, Moslehi N, Azizi F. Effects of Ramadan intermittent fasting on lipid and lipoprotein parameters: An updated meta-analysis. Nutr Metab Cardiovasc Dis. 2019;29(9):906–15. [DOI] [PubMed] [Google Scholar]
- 29.Cioffi I, Evangelista A, Ponzo V, Ciccone G, Soldati L, Santarpia L, et al. Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: a systematic review and meta-analysis of randomized controlled trials. J Transl Med. 2018;16(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashtary-Larky D, Bagheri R, Tinsley GM, Asbaghi O, Paoli A, Moro T. Effects of intermittent fasting combined with resistance training on body composition: a systematic review and meta-analysis. Physiol Behav. 2021;237:113453. [DOI] [PubMed] [Google Scholar]
- 31.Fernando HA, Zibellini J, Harris RA, Seimon RV, Sainsbury A. Effect of Ramadan fasting on weight and body composition in healthy non-athlete adults: a systematic review and meta-analysis. Nutrients. 2019;11(2):478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Li Q, Liu Y, Jiang H, Chen W. Intermittent fasting versus continuous energy-restricted diet for patients with type 2 diabetes mellitus and metabolic syndrome for glycemic control: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2021;179:109003. [DOI] [PubMed] [Google Scholar]
- 33.Harris L, McGarty A, Hutchison L, Ells L, Hankey C. Short-term intermittent energy restriction interventions for weight management: a systematic review and meta-analysis. Obes Rev. 2018;19(1):1–13. [DOI] [PubMed] [Google Scholar]
- 34.Yin C, Li Z, Xiang Y, Peng H, Yang P, Yuan S, et al. Effect of intermittent fasting on non-alcoholic fatty liver disease: systematic review and meta-analysis. Front Nutr. 2021;8:709683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang F, Liu C, Liu X, Pan X, Li X, Tian L, et al. Effect of epidemic intermittent fasting on cardiometabolic risk factors: a systematic review and meta-analysis of randomized controlled trials. Front Nutr. 2021;8:69325. [DOI] [PMC free article] [PubMed]
- 36.Chen J-H, Lu LW, Ge Q, Feng D, Yu J, Liu B, et al. Missing puzzle pieces of time-restricted-eating (TRE) as a long-term weight-loss strategy in overweight and obese people? A systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2023;63(15):2331–47. [DOI] [PubMed] [Google Scholar]
- 37.Schwingshackl L, Zähringer J, Nitschke K, Torbahn G, Lohner S, Kühn T, et al. Impact of intermittent energy restriction on anthropometric outcomes and intermediate disease markers in patients with overweight and obesity: systematic review and meta-analyses. Crit Rev Food Sci Nutr. 2021;61(8):1293–304. [DOI] [PubMed] [Google Scholar]
- 38.Jahrami HA, Faris ME, Janahi AI, Janahi MI, Abdelrahim DN, Madkour MI, et al. Does four-week consecutive, dawn-to-sunset intermittent fasting during Ramadan affect cardiometabolic risk factors in healthy adults? A systematic review, meta-analysis, and meta-regression. Nutr Metab Cardiovasc Dis. 2021;31(8):2273–301. [DOI] [PubMed] [Google Scholar]
- 39.Pureza IRDOM, de Lima MM, da Silva AE Jr, Praxedes DRS, Vasconcelos LGL, Bueno NB. Effect of early time-restricted feeding on the metabolic profile of adults with excess weight: a systematic review with meta-analysis. Clin Nutr. 2021;40(4):1788–99. [DOI] [PubMed] [Google Scholar]
- 40.Jahrami HA, Alsibai J, Clark CC, Faris ME. A systematic review, meta-analysis, and meta-regression of the impact of diurnal intermittent fasting during Ramadan on body weight in healthy subjects aged 16 years and above. Eur J Nutr. 2020;59(6):2291–316. [DOI] [PubMed] [Google Scholar]
- 41.Jahrami HA, Alsibai J, Obaideen AA. Impact of Ramadan diurnal intermittent fasting on the metabolic syndrome components in healthy, non-athletic Muslim people aged over 15 years: a systematic review and meta-analysis. Br J Nutr. 2020;123(1):1–22. [DOI] [PubMed] [Google Scholar]
- 42.Roman YM, Dominguez MC, Easow TM, Pasupuleti V, White CM, Hernandez AV. Effects of intermittent versus continuous dieting on weight and body composition in obese and overweight people: a systematic review and meta-analysis of randomized controlled trials. Int J Obes. 2019;43(10):2017–27. [DOI] [PubMed] [Google Scholar]
- 43.Jahrami H, BaHammam A, Kalaji Z, Madkour M, Hassanein M. A systematic review, meta-analysis, and meta-regression of the impact of diurnal intermittent fasting during Ramadan on glucometabolic markers in healthy subjects. Diabetes Res Clin Pract. 2020;165:108226. [DOI] [PubMed] [Google Scholar]
- 44.Cho Y, Hong N, Kim KW, Cho SJ, Lee M, Lee YH, et al. The effectiveness of intermittent fasting to reduce body mass index and glucose metabolism: a systematic review and meta-analysis. J Clin Med. 2019;8(10):1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Headland M, Clifton PM, Carter S, Keogh JB. Weight-loss outcomes: a systematic review and meta-analysis of intermittent energy restriction trials lasting a minimum of 6 months. Nutrients. 2016;8(6):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Correia JM, Santos I, Pezarat-Correia P, Silva AM, Mendonca GV. Effects of ramadan and non-ramadan intermittent fasting on body composition: a systematic review and meta-analysis. Front Nutr. 2021;7:625240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Q, Zhang C, Wang H, Ma Z, Liu D, Guan X, et al. Intermittent fasting versus continuous calorie restriction: which is better for weight loss? Nutrients. 2022;14(9):1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi JH, Cho YJ, Kim H-J, Ko S-H, Chon S, Kang J-H, et al. Effect of carbohydrate-restricted diets and intermittent fasting on obesity, type 2 diabetes mellitus, and hypertension management: consensus statement of the Korean Society for the Study of Obesity, Korean Diabetes Association, and Korean Society of Hypertension. Clin Hypertens. 2022;28(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan X, Wang J, Yang S, Gao M, Cao L, Li X, et al. Effect of intermittent fasting diet on glucose and lipid metabolism and insulin resistance in patients with impaired glucose and lipid metabolism: a systematic review and meta-analysis. Int J Endocrinol. 2022;2022:6999907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu L, Chen W, Wu D, Hu F. Metabolic efficacy of time-restricted eating in adults: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2022;107(12):3428–41. [DOI] [PubMed] [Google Scholar]
- 51.Wang W, Wei R, Pan Q, Guo L. Beneficial effect of time-restricted eating on blood pressure: a systematic meta-analysis and meta-regression analysis. Nutr Metab. 2022;19(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trabelsi K, Ammar A, Boukhris O, Glenn JM, Clark CC, Stannard SR, et al. Dietary intake and body composition during Ramadan in athletes: a systematic review and meta-analysis with meta-regression. J Am Nutr Assoc. 2023;42(1):101–22. [DOI] [PubMed] [Google Scholar]
- 53.Elortegui Pascual P, Rolands MR, Eldridge AL, Kassis A, Mainardi F, Lê KA, et al. A meta-analysis comparing the effectiveness of alternate day fasting, the 5: 2 diet, and time-restricted eating for weight loss. Obesity. 2023;31:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim K-K, Kang J-H, Kim EM. Updated meta-analysis of studies from 2011 to 2021 comparing the effectiveness of intermittent energy restriction and continuous energy restriction. Journal of Obesity & Metabolic Syndrome. 2022;31(3):230–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gu L, Fu R, Hong J, Ni H, Yu K, Lou H. Effects of intermittent fasting in human compared to a non-intervention diet and caloric restriction: a meta-analysis of randomized controlled trials. Front Nutr. 2022;9:871682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J, Wang F, Chen H, Liu L, Zhang S, Luo W, et al. Comparison of the effects of intermittent energy restriction and continuous energy restriction among adults with overweight or obesity: an overview of systematic reviews and meta-analyses. Nutrients. 2022;14(11):2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. [DOI] [PubMed] [Google Scholar]
- 58.Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. 2011;64(4):407–15. [DOI] [PubMed] [Google Scholar]
- 59.Viguiliouk E, Glenn AJ, Nishi SK, Chiavaroli L, Seider M, Khan T, et al. Associations between dietary pulses alone or with other legumes and cardiometabolic disease outcomes: an umbrella review and updated systematic review and meta-analysis of prospective cohort studies. Advances. Nutrition. 2019;10(Supplement_4):S308–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64(12):1294–302. [DOI] [PubMed] [Google Scholar]
- 61.Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol. 2011;64(12):1303–10. [DOI] [PubMed] [Google Scholar]
- 62.Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol. 2011;64(12):1283–93. [DOI] [PubMed] [Google Scholar]
- 63.Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. Journal of clinical epidemiology. 2011;64(12):1277–82. [DOI] [PubMed] [Google Scholar]
- 64.Pandis N, Fleming PS, Worthington H, Salanti G. The quality of the evidence according to GRADE is predominantly low or very low in oral health systematic reviews. PLoS ONE. 2015;10(7):e0131644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Higgins JP, Sterne JA, Savovic J, Page MJ, Hróbjartsson A, Boutron I, et al. A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev. 2016;10(Suppl 1):29–31. [Google Scholar]
- 66.Nikolakopoulou A, Higgins JP, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, Salanti G. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17(4):e1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 68.Noetel M, Sanders T, Gallardo-Gómez D, Taylor P, del Pozo Cruz B, Van Den Hoek D, et al. Effect of exercise for depression: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2024;384:e075847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swift DL, Johannsen NM, Lavie CJ, Earnest CP, Blair SN, Church TS. Effects of clinically significant weight loss with exercise training on insulin resistance and cardiometabolic adaptations. Obesity. 2016;24(4):812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kernan WN, Inzucchi SE, Sawan C, Macko RF, Furie KL. Obesity: a stubbornly obvious target for stroke prevention. Stroke. 2013;44(1):278–86. [DOI] [PubMed] [Google Scholar]
- 71.Gao M, Piernas C, Astbury NM, Hippisley-Cox J, O’Rahilly S, Aveyard P, Jebb SA. Associations between body-mass index and COVID-19 severity in 6· 9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol. 2021;9(6):350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heymsfield SB, Gonzalez MC, Shen W, Redman L, Thomas D. Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes Rev. 2014;15(4):310–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goodwin S. The practical guide to the identification, evaluation and treatment of overweight and obesity in adults. Clin Nurse Spec. 2002;16(3):164. [Google Scholar]
- 74.Stamler J. The INTERSALT study: background, methods, findings, and implications. Am J Clin Nutr. 1997;65(2):626S-S642. [DOI] [PubMed] [Google Scholar]
- 75.Group UPDS. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). The lancet. 1998;352(9131):837–53. [PubMed] [Google Scholar]
- 76.Jayedi A, Zeraattalab-Motlagh S, Jabbarzadeh B, Hosseini Y, Jibril AT, Shahinfar H, et al. Dose-dependent effect of carbohydrate restriction for type 2 diabetes management: a systematic review and dose-response meta-analysis of randomized controlled trials. Am J Clin Nutr. 2022;116(1):40–56. [DOI] [PubMed] [Google Scholar]
- 77.Lenters-Westra E, Schindhelm R, Bilo H, Groenier K, Slingerland R. Differences in interpretation of haemoglobin A1c values among diabetes care professionals. Neth J Med. 2014;72(9):462–6. [PubMed] [Google Scholar]
- 78.Mason SA, Keske MA, Wadley GD. Effects of vitamin C supplementation on glycemic control and cardiovascular risk factors in people with type 2 diabetes: a GRADE-assessed systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2021;44(2):618–30. [DOI] [PubMed] [Google Scholar]
- 79.Expert Panel on Detection E. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–97. [DOI] [PubMed] [Google Scholar]
- 80.Rahilly-Tierney CR, Lawler EV, Scranton RE, Gaziano JM. Cardiovascular benefit of magnitude of low-density lipoprotein cholesterol reduction: a comparison of subgroups by age. Circulation. 2009;120(15):1491–7. [DOI] [PubMed] [Google Scholar]
- 81.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, et al. High-density lipoprotein cholesterol and cardiovascular disease Four prospective American studies. Circulation. 1989;79(1):8–15. [DOI] [PubMed] [Google Scholar]
- 82.Grasgruber P, Hrazdíra E. Nutritional and socio-economic predictors of adult height in 152 world populations. Econ Hum Biol. 2020;37:100848. [DOI] [PubMed] [Google Scholar]
- 83.Ryan DH, Yockey SR. Weight loss and improvement in comorbidity: differences at 5%, 10%, 15%, and over. Curr Obes Rep. 2017;6:187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chandler J, Cumpston M, Li T, Page MJ, Welch VJ. Cochrane handbook for systematic reviews of interventions. Hoboken: Wiley; 2019.
- 85.Chen TT, Tu YK. Statistical models for overviews of reviews. In: Biondi-Zoccai G, editor. Umbrella reviews: evidence synthesis with overviews of reviews and meta-epidemiologic studies. Cham: Springer; 2016. 10.1007/978-3-319-25655-9_10.
- 86.Beaulieu K, Casanova N, Oustric P, Hopkins M, Varady K, Finlayson G, Gibbons C. An exploratory investigation of the impact of “fast” and “feed” days during intermittent energy restriction on free-living energy balance behaviours and subjective states in women with overweight/obesity. Eur J Clin Nutr. 2021;75(3):430–7. [DOI] [PubMed] [Google Scholar]
- 87.Gray KL, Clifton PM, Keogh JB. The effect of intermittent energy restriction on weight loss and diabetes risk markers in women with a history of gestational diabetes: a 12-month randomized control trial. Am J Clin Nutr. 2021;114(2):794–803. [DOI] [PubMed] [Google Scholar]
- 88.Pureza IR, da Silva Junior AE, Praxedes DRS, Vasconcelos LGL, de Lima Macena M, de Melo ISV, et al. Effects of time-restricted feeding on body weight, body composition and vital signs in low-income women with obesity: a 12-month randomized clinical trial. Clin Nutr. 2021;40(3):759–66. [DOI] [PubMed] [Google Scholar]
- 89.Steger FL, Donnelly JE, Hull HR, Li X, Hu J, Sullivan DK. Intermittent and continuous energy restriction result in similar weight loss, weight loss maintenance, and body composition changes in a 6 month randomized pilot study. Clin Obes. 2021;11(2):e12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Templeman I, Smith HA, Chowdhury E, Chen YC, Carroll H, Johnson-Bonson D, et al. A randomized controlled trial to isolate the effects of fasting and energy restriction on weight loss and metabolic health in lean adults. Sci Transl Med. 2021;13(598):eabd8034. [DOI] [PubMed] [Google Scholar]
- 91.Beaulieu K, Casanova N, Oustric P, Turicchi J, Gibbons C, Hopkins M, et al. Matched weight loss through intermittent or continuous energy restriction does not lead to compensatory increases in appetite and eating behavior in a randomized controlled trial in women with overweight and obesity. J Nutr. 2020;150(3):623–33. [DOI] [PubMed] [Google Scholar]
- 92.Kunduraci YE, Ozbek H. Does the energy restriction intermittent fasting diet alleviate metabolic syndrome biomarkers? A randomized controlled trial. Nutrients. 2020;12(10):3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the treat randomized clinical trial. JAMA Intern Med. 2020;180(11):1491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pureza I, Melo ISV, Macena ML, Praxedes DRS, Vasconcelos LGL, Silva-Júnior AE, et al. Acute effects of time-restricted feeding in low-income women with obesity placed on hypoenergetic diets: randomized trial. Nutrition (Burbank, Los Angeles County, Calif). 2020;77:110796. [DOI] [PubMed] [Google Scholar]
- 95.Razavi R, Parvaresh A, Abbasi B, Yaghoobloo K, Hassanzadeh A, Mohammadifard N, et al. The alternate-day fasting diet is a more effective approach than a calorie restriction diet on weight loss and hs-CRP levels. Int J Vitam Nutr Res. 2021;91(3-4):242–50. [DOI] [PubMed]
- 96.Gabel K, Kroeger CM, Trepanowski JF, Hoddy KK, Cienfuegos S, Kalam F, Varady KA. Differential effects of alternate-day fasting versus daily calorie restriction on insulin resistance. Obesity. 2019;27(9):1443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Headland ML, Clifton PM, Keogh JB. Effect of intermittent compared to continuous energy restriction on weight loss and weight maintenance after 12 months in healthy overweight or obese adults. Int J Obesity (2005). 2019;43(10):2028–36. [DOI] [PubMed] [Google Scholar]
- 98.Cai H, Qin YL, Shi ZY, Chen JH, Zeng MJ, Zhou W, et al. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: a randomised controlled trial. BMC Gastroenterol. 2019;19(1):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carter S, Clifton P, Keogh J. The effect of intermittent compared with continuous energy restriction on glycaemic control in patients with type 2 diabetes: 24-month follow-up of a randomised noninferiority trial. Diabetes Res Clin Pract. 2019;151:11–9. [DOI] [PubMed] [Google Scholar]
- 100.Cho A-R, Moon J-Y, Kim S, An K-Y, Oh M, Jeon JY, et al. Effects of alternate day fasting and exercise on cholesterol metabolism in overweight or obese adults: a pilot randomized controlled trial. Metabolism. 2019;93:52–60. [DOI] [PubMed] [Google Scholar]
- 101.Hutchison AT, Liu B, Wood RE, Vincent AD, Thompson CH, O’Callaghan NJ, et al. Effects of intermittent versus continuous energy intakes on insulin sensitivity and metabolic risk in women with overweight. Obesity (Silver Spring, Md). 2019;27(1):50–8. [DOI] [PubMed] [Google Scholar]
- 102.Johari MI, Yusoff K, Haron J, Nadarajan C, Ibrahim KN, Wong MS, et al. A randomised controlled trial on the effectiveness and adherence of modified alternate-day calorie restriction in improving activity of non-alcoholic fatty liver disease. Sci Rep. 2019;9(1):11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Parvaresh A, Razavi R, Abbasi B, Yaghoobloo K, Hassanzadeh A, Mohammadifard N, et al. Modified alternate-day fasting vs. calorie restriction in the treatment of patients with metabolic syndrome: a randomized clinical trial. Complement Ther Med. 2019;47:102187. [DOI] [PubMed] [Google Scholar]
- 104.Stekovic S, Hofer SJ, Tripolt N, Aon MA, Royer P, Pein L, et al. Alternate day fasting improves physiological and molecular markers of aging in healthy. Non-obese Hum Cell Metab. 2019;30(3):462-76.e6. [DOI] [PubMed] [Google Scholar]
- 105.Bowen J, Brindal E, James-Martin G, Noakes M. Randomized trial of a high protein, partial meal replacement program with or without alternate day fasting: similar effects on weight loss, retention status, nutritional, metabolic, and behavioral outcomes. Nutrients. 2018;10(9):1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carter S, Clifton PM, Keogh JB. Effect of intermittent compared with continuous energy restricted diet on glycemic control in patients with type 2 diabetes: a randomized noninferiority trial. JAMA Netw Open. 2018;1(3):e180756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Conley M, Le Fevre L, Haywood C, Proietto J. Is two days of intermittent energy restriction per week a feasible weight loss approach in obese males? A randomised pilot study. Nutr Diet. 2018;75(1):65–72. [DOI] [PubMed] [Google Scholar]
- 108.Coutinho SR, Halset EH, Gåsbakk S, Rehfeld JF, Kulseng B, Truby H, Martins C. Compensatory mechanisms activated with intermittent energy restriction: a randomized control trial. Clinical nutrition (Edinburgh, Scotland). 2018;37(3):815–23. [DOI] [PubMed] [Google Scholar]
- 109.Oh M, Kim S, An K-Y, Min J, Yang HI, Lee J, et al. Effects of alternate day calorie restriction and exercise on cardio-metabolic risk factors in overweight and obese adults: an exploratory randomized controlled study. BMC Public Health. 2018;18:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schübel R, Nattenmüller J, Sookthai D, Nonnenmacher T, Graf ME, Riedl L, et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: a randomized controlled trial. Am J Clin Nutr. 2018;108(5):933–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sundfør TM, Svendsen M, Tonstad S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: a randomized 1-year trial. Nutr Metab Cardiovasc Dis. 2018;28(7):698–706. [DOI] [PubMed] [Google Scholar]
- 112.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27(6):1212-21.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barnosky A, Kroeger CM, Trepanowski JF, Klempel MC, Bhutani S, Hoddy KK, et al. Effect of alternate day fasting on markers of bone metabolism: an exploratory analysis of a 6-month randomized controlled trial. Nutrition and healthy aging. 2017;4(3):255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tinsley GM, Forsse JS, Butler NK, Paoli A, Bane AA, La Bounty PM, et al. Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur J Sport Sci. 2017;17(2):200–7. [DOI] [PubMed] [Google Scholar]
- 115.Trepanowski JF, Kroeger CM, Barnosky A, Klempel MC, Bhutani S, Hoddy KK, et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: a randomized clinical trial. JAMA Intern Med. 2017;177(7):930–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Carter S, Clifton PM, Keogh JB. The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Res Clin Pract. 2016;122:106–12. [DOI] [PubMed] [Google Scholar]
- 117.Catenacci VA, Pan Z, Ostendorf D, Brannon S, Gozansky WS, Mattson MP, et al. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity. 2016;24(9):1874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moro T, Tinsley G, Bianco A, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Trans Med. 2016;14(1):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Betts JA, Richardson JD, Chowdhury EA, Holman GD, Tsintzas K, Thompson D. The causal role of breakfast in energy balance and health: a randomized controlled trial in lean adults. Am J Clin Nutr. 2014;100(2):539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Varady KA. Alternate day fasting and endurance exercise combine to reduce body weight and favorably alter plasma lipids in obese humans. Obesity. 2013;21(7):1370–9. [DOI] [PubMed] [Google Scholar]
- 121.Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr. 2013;110(8):1534–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Varady KA, Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Haus JM, et al. Alternate day fasting for weight loss in normal weight and overweight subjects: a randomized controlled trial. Nutr J. 2013;12:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes. 2011;35(5):714–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Varady KA, Bhutani S, Klempel MC, Kroeger CM. Comparison of effects of diet versus exercise weight loss regimens on LDL and HDL particle size in obese adults. Lipids Health Dis. 2011;10:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85(4):981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vasim I, Majeed CN, DeBoer MD. Intermittent fasting and metabolic health. Nutrients. 2022;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Malinowski B, Zalewska K, Węsierska A, Sokołowska MM, Socha M, Liczner G, et al. Intermittent fasting in cardiovascular disorders—an overview. Nutrients. 2019;11(3):673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Albosta M, Bakke J. Intermittent fasting: is there a role in the treatment of diabetes? A review of the literature and guide for primary care physicians. Clin Diabetes Endocrinol. 2021;7(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Santos HO, Macedo RC. Impact of intermittent fasting on the lipid profile: assessment associated with diet and weight loss. Clin Nutr ESPEN. 2018;24:14–21. [DOI] [PubMed] [Google Scholar]
- 130.Termannsen AD, Varming A, van Elst C, Bjerre N, Nørgaard O, Hempler NF, et al. Feasibility of time-restricted eating in individuals with overweight, obesity, prediabetes, or type 2 diabetes: a systematic scoping review. Obesity. 2023;31(6):1463–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Text S1. Details of the study selection process. Table S1. Search strategies. Table S2. List of discrepancies between the initial protocol and the final analysis. Table S3. Criteria and results of the GRADE assessment. Table S4. Criteria and results of the CINeMA assessment. Table S5. Characteristics of original studies within included studies. Table S6. Network evidence of pair-wise comparison with CINeMA. Table S7. Results for meta-regression. Figure S1. Summary of the direct evidence. Figure S2. Risk of bias in the original studies included in the systematic review. Figure S3. Network plots. Figure S4. Results for surface under the cumulative ranking (SUCRA) analaysis. Figure S5. Results for the sensitivity analysis restricted to healthy people with surface under the cumulative ranking (SUCRA).
Data Availability Statement
No datasets were generated or analysed during the current study.