Abstract
Background
Asthma is a health condition with worldwide relevance, evaluated based on the necessary treatment to control symptoms and exacerbations. Severe asthma is uncontrolled despite high doses of ICS-LABA and treatment for triggering factors. Severe eosinophilic asthma is characterized by an increase in eosinophils in the peripheral circulation, walls, and passages of the respiratory tract. Biologic treatments such as benralizumab have demonstrated effectiveness as aids in decreasing respiratory tract inflammation and improving the management of symptoms in patients living with asthma.
Objective
To assess the efficacy and safety of benralizumab as an add-on therapy for patients with severe, uncontrolled asthma and elevated blood eosinophil counts.
Methods
Observational, analytic and ambispective study in 21 patients diagnosed with severe eosinophilic asthma treated with benralizumab, to determine the treatment’s effectiveness through the change in estimated respiratory function by spirometry through the forced expiratory volume in one second (FEV1) value, reduction in second controlling treatment, serum eosinophil reduction, change in the Asthma Control Test score and the Asthma Control Questionnaire test at 6 and 16 months of treatment.
Results
An average difference of 241.43 mL (±461.43) in FEV1 at 6 months was found, as well as an average FeNO reduction of 49.8 ppm and eosinophil reduction of 612.78 cells at 12 months of treatment, additionally, CSI requirements were reduced in 95% of patients.
Conclusion
Benralizumab improved respiratory function as well as key biomarkers such as eosinophil count, exhaled nitric oxide fraction (FeNO), which reflected in a decreased requirement of inhaled corticosteroids and improved symptom control.
Keywords: asthma, eosinophils, benralizumab, biologics, CSI-LABA therapy
Background
Asthma is a chronic inflammatory disease of the respiratory tract that affects over 300 million people around the world. Despite pharmacological advances, it remains a relevant condition worldwide that requires a complex and challenging management. Asthma severity is evaluated based on the necessary treatment to control symptoms and exacerbations. Severe asthma is characterized by an uncontrolled condition despite the use of high-dose optimized combinations of inhaled corticosteroids and long-acting beta-agonists (ICS-LABA) or high doses of ICS-LABA are required to avoid exacerbations.1
Severe eosinophilic asthma is characterized by an increase in eosinophil count in peripheral circulation, the walls and passages of the respiratory tract.2 Eosinophils are a white blood cell subtype that migrate to defend tissues against infections, mediate allergic response and contribute to tissue inflammation. They contain cytoplasmic granules that, when activated, they drive proinflammatory cytokine production.2,3 Activation and degranulation of eosinophils results in respiratory tract tissue inflammation and contributes to mucus hypersecretion and bronchospasm in patients with the disease.1,4
Severe eosinophilic asthma is associated with a higher risk of exacerbation and worse respiratory function.2,3 According to reports by the Global Initiative for Asthma (GINA), it represents 50 to 60% of all severe asthma cases. Recent estimations of a cohort in the International Severe Asthma Registry suggest that severe eosinophilic asthma constitutes the most prevalent asthma phenotype with up to 84% of cases.1
The lack of adequate disease control in this population is established by the use of different evaluation tools, such as the Asthma Control Test (ACT) and the Asthma Control Questionnaire (ACQ); with scores ranging from <20 or >1.5, respectively.5 When adequate disease control is not achieved despite the use of optimized therapy and control of aggravating factors and/or comorbidities, the diagnosis of severe asthma can be established and aided by biomarkers, and physicians can determine a type 2 phenotype, which can be a candidate for biologic management with one of the four options currently available in Mexico. For the case of severe eosinophilic asthma, according to GINA guidelines, patients can be managed with benralizumab, an anti-interleukin-5.1
Benralizumab is a monoclonal antibody that acts through a union with the alpha subunit of the IL-5 (IL-5Rα) expressed on the surface of eosinophil and basophil cells. The absence of fucose in the Fc domain of benralizumab determines its affinity for Fc-RIII receptors in effector cells, such as natural killer lymphocytes (NK cells). This causes the apoptosis of eosinophils and basophils through the reinforcement of antibody-dependent cellular cytotoxicity (ADCC), which reduces eosinophilic inflammation.2,3 Several clinical trials have corroborated the safety and efficacy of benralizumab in patients with severe eosinophilic asthma with eosinophil predominance above 300 cell/μL unresponsive to other therapeutic approaches.2,6 Therefore, patients with severe eosinophilic asthma have benefited from the use of benralizumab in disease control, exacerbations, drug dosage reduction, continuous use of ICS and in a reduced need of maintenance treatment, which can help reduce the economic implications of the use of biologic treatment and the presentation of adverse events.7,8
The use of benralizumab was evaluated in a Phase 4 trial including 22 centers in four countries, where 110 (92%) of patients reduced the dosage of ICS-formoterol: 18 (15%) to a medium dosage, 20 (17%) to a low dosage and 72% (61%) to utilization only on a necessary basis. In 113 (96%) patients, reductions were sustained up to 48 weeks; 114 (91%) patients in the dose reduction group did not present exacerbations during the gradual reduction period. These findings show that patients managed with benralizumab can have significant reductions in ICS therapy while maintaining disease control.9
In Mexico, studies reporting the effectiveness of benralizumab based on clinical control and respiratory function improvement are scarce. Therefore, the main objective of the present study was to evaluate the effect of benralizumab on the reduction of ICS-LABA utilization in patients with severe eosinophilic asthma maintaining control. We also set out to evaluate the effectiveness of benralizumab in respiratory function improvement through the analysis of FEV1 prior to bronchodilator, FeNO and serum eosinophil count as well as symptom improvement evaluated through clinical questionnaires (ACT and ACQ).
Methods
An analytic, ambispective, longitudinal study was conducted at the Regional Hospital “General Ignacio Zaragoza” within the Clinical Immunology and Allergy Department in Mexico City during 2023. The sample size was not determined and the population was selected by convenience, through clinical records that met the inclusion criteria: patients aged 18 years or older, diagnosed with severe uncontrolled eosinophilic asthma, treated with subcutaneous benralizumab 30 mg every 8 weeks, with an ACQ5 score greater than 1.5, ACT less than 19 points, and who had agreed to participate in the study by signing a written consent form. Patients who refused to sign the consent form, those with treatment adherence of less than 80% (5 applications), those who required a change in treatment to the biologic agent and all deceased patients were eliminated from the study.
To describe the study population and assess treatment efficacy, sociodemographic data were analyzed as well as history of benralizumab treatment utilization at 6 and 12 months.
To describe the study population and evaluate treatment effectiveness, we analyzed sociodemographic data as well as treatment utilization history with benralizumab at 6 and 12 months. The primary variables selected were dose reduction of the second control treatment in patients with clinical improvement evaluated every 2 months through a comprehensive review, no exacerbations with the need for systemic corticosteroids and improvement in lung function. FEV1, serum eosinophil count. Treatment reduction was evaluated by therapeutic group since the use of the specific drug depended on the patient’s needs and the availability of the drug in the institution. Secondary variables were Asthma Control Test (ACT) score, Asthma Control Questionnaire (ACQ score) and FeNO. Quantitative variables were expressed in averages and standard deviations (SD). Categoric variables were analyzed through frequencies and percentages. Variables were compared against the basal measurements of FEV1, ACT, ACQ, serum eosinophil count and FeNO at 6 and 12 months, through a paired-sample t test, with a significance level of α=0.05 and a confidence interval of 95%.
Ethical Declaration
The conduct of this research study was approved by the teaching and research coordination of the Regional Hospital ‘General Ignacio Zaragoza. It was carried out according to Helsinki’s statement.
Results
We included 21 patients diagnosed with severe and uncontrolled eosinophilic asthma, 13 women (61.9%) and 8 men (38.1%), with an age range of 37 to 70 years, with all but one patient, presenting with at least one comorbidity. On the subject of biologic treatment history prior to the start of benralizumab, 33.3% (n = 7) had received a different biologic before benralizumab. The analysis by Body Mass Index (BMI) and pulmonary obstruction severity estimated by FEV1 are described in Table 1.
Table 1.
Population Characteristics at Baseline
| Age (Years) | Frequency (n=21) | % |
|---|---|---|
| 31–40 | 1 | 4.76% |
| 41–50 | 5 | 23.81% |
| 51–60 | 9 | 42.86% |
| 61–70 | 6 | 28.57% |
| Gender | ||
| Female | 13 | 61.90% |
| Male | 8 | 38.10% |
| Body Mass Index (kg/m2) | ||
| Ideal weight 18.5 to 24.9 kg/m2 | 2 | 9.52% |
| Overweight 25 to 29.9 kg/m2 | 10 | 47.62% |
| Grade I obesity 30 to 34.9 kg/m2 | 8 | 38.10% |
| Grade II obesity 35 to 35.9 kg/m2 | 1 | 4.76% |
| Comorbidities | ||
| Aspirin-exacerbated respiratory disease | 5 | 23.81% |
| Allergic rhinitis (AR) | 9 | 42.86% |
| AR + CRSwNP | 3 | 14.29% |
| Other | 4 | 19.05% |
| Prior biologic | ||
| Yes | 7 | 33.33% |
| Omalizumab | 7 | 100% |
| No | 14 | 66.67% |
| Pulmonary obstruction severity by FEV1 | ||
| Mild | 14 | 66.67% |
| Moderate | 1 | 4.76% |
| Moderately severe | 4 | 19.05% |
| Severe | 2 | 9.52% |
Abbreviations: CRSwNP, Chronic rhinosinusitis associated with nasal polyp; AR, Allergic rhinitis; kg, Kilograms; m2, square meter; FEV1, Forced expiratory volume.
Table 2 displays the summary measures for the primary and secondary variables, where we observed an increase in FEV1 throughout follow-up and a statistically significant difference of 241.43 mL (±461.43) after 6 months of treatment and 369.44 mL (±519.28) at 12 months.
Table 2.
Respiratory Function, Quality of Life and Biomarkers at Baseline, 6 and 12 Months of Treatment
| Evaluation | Basal | 6 months | 12 months |
|---|---|---|---|
FeNO (ppm) 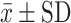 |
n=13 | n=19 | n=17 |
| 84.77±43.90 | 40.79±37.01 | 35.00±18.67 | |
FEV1 (mL) 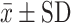 |
n=21 | n=21 | n=18 |
| 1937.61±505.01 | 2179.04±536.06* | 2256.66±471.56 * | |
ACT (score) 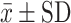 |
n=21 | n=21 | n=18 |
| 16±2.86 | 22±2.97 | 23±1.95 | |
ACQ-5 (score) 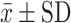 |
n=21 | n=21 | n=18 |
| 2.90±0.83 | 0.68±0.64 | 0.38±0.47 | |
Eosinophil count (cell/μL)  |
n=21 | n=18 | n=18 |
| 590.48±553.75 | 145.00±313.11 | 23.33±47.52 |
Notes: *P = 0.02 by t test.
Abbreviations: FeNO, Exhaled nitric oxide fraction; ppm, Parts per million; mL, Milliliters; cel/μL, Cells per microliter; 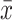 , average; SD, Standard deviation; FEV1, Forced expiratory volume; ACT, Asthma control test; ACQ-5, Asthma control questionnaire.
, average; SD, Standard deviation; FEV1, Forced expiratory volume; ACT, Asthma control test; ACQ-5, Asthma control questionnaire.
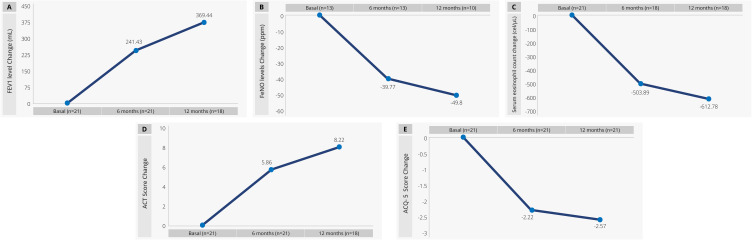
In all main response evaluations, we estimated the average of the difference at 6 and 12 months of treatment in comparison to the average obtained at baseline for each patient, and those patients with no data available for this comparison were excluded from the graphic analysis. The graphic shows a progressive improvement in all response variables. The most significant change was observed for FEV1, where at 6 months of treatment with benralizumab, there was an increase of 241.43 mL (±461.44) (Figure 1A), and similarly, for FeNO, where a decrease of 39.77 ppm (±50.12) can be seen in Figure 1B. The eosinophil count change was considerable, with a depletion of 612.78 cell/μL (± 549.11) at 12 months of treatment (Figure 1C). For the case of the ACT and ACQ-5 tests, both scores showed improvement with an increase for the ACT score and a −2.57 for the case of ACQ-5 (see Figure 1D and E).
Figure 1.
Respiratory function, quality of life and biomarkers at baseline, 6 and 12 months of treatment. (A) FEV1. (B) FeNO. (C) Eosinophil count (D) ACT score (E) ACQ-5.
Abbreviations: FeNO, Exhaled nitric oxide fraction; ppm, Parts per million; mL, Milliliters; cell/μL, Cells per microliter; FEV1, Forced expiratory volume; ACT, Asthma control test; ACQ-5, Asthma control questionnaire.
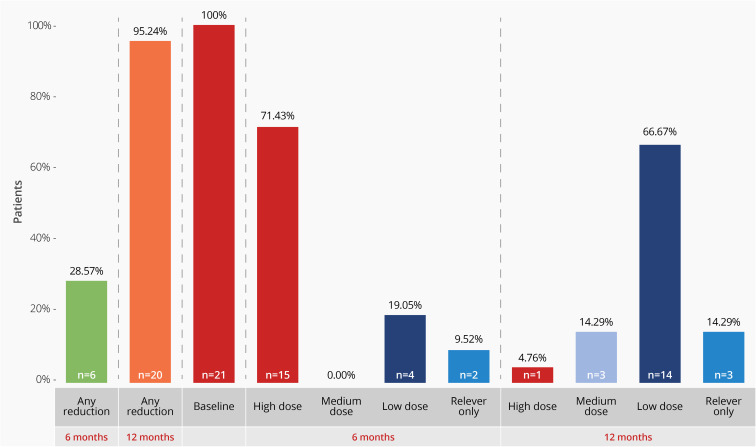
Out of the total population starting the study with high doses of ICS, 28.6% of patients were on a lower dose for asthma control after 6 months of treatment with benralizumab, with 19.1% (n = 4) requiring low-dose and 9.5% (n = 2) suspended ICS treatment. At 12 months, 95.2% (n = 20) were on a lower dosage of ICS or had suspended treatment (14.3%, n = 3) (Figure 2).
Figure 2.
Inhaled corticosteroids dose and dose reduction at baseline, 6 and 4 months of treatment with benralizumab.
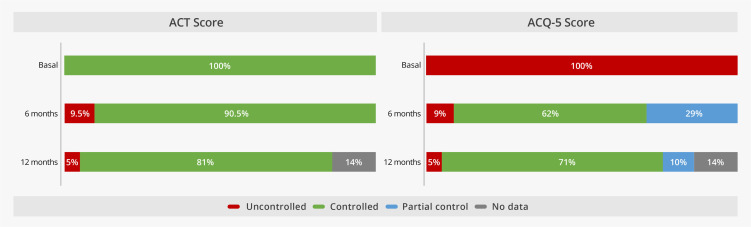
On the subject of patient control scores, at the start of the study, 100% of patients were uncontrolled in both the ACT and ACQ-5 test, while at 6 months of treatment with benralizumab, 19 patients (90.5%) had achieved disease control according to the ACT. For the case of ACQ-5, 62% (n = 13) had achieved good control at 6 months of treatment, and 29% (n = 6) had achieved partial control of the disease. At 12 months of treatment, 71% (n = 15) had achieved good disease control (Figure 3).
Figure 3.
Test scores by level of disease control at baseline, 6 and 12 months of treatment with benralizumab.
Abbreviations: ACT, Asthma control test; ACQ-5, Asthma control questionnaire.
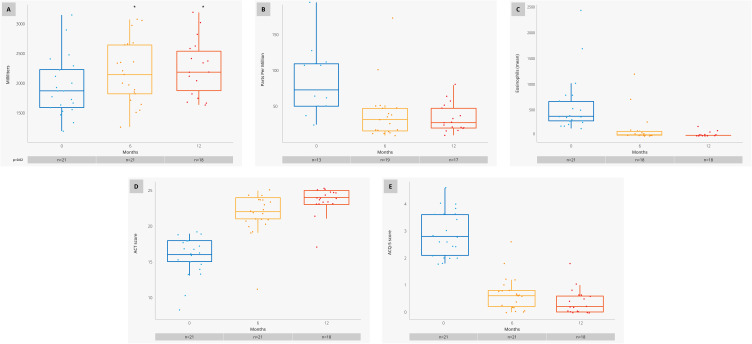
A dispersion graphic analysis was performed for the response variables (Figure 4), showing a noticeable improvement at 6 months of treatment with benralizumab for all variables, where FEV1 reported a statistically significant difference of 241.43 mL (±461.43) at 6 months of treatment and 369.44 mL (±519.28) at 12 months, through a comparative analysis by an unequal group to test against the basal evaluation, with a significance level of α=0.05 (Figure 4A).
Figure 4.
Dispersion graphics for response variables at baseline, 6 and 12 months. (A) FEV1. (B) FeNO. (C) Eosinophil count. (D) ACT score (E) ACQ-5.
Notes: * Statistically significant.
Abbreviations: FeNO, Exhaled nitric oxide fraction; FEV1, Forced expiratory volume; ACT, Asthma control test; ACQ-5, Asthma control questionnaire.
FeNO dispersion showed a clear reduction versus basal levels, reaching levels below 50 ppm (Figure 4B). For the case of serum eosinophil count, we reported a large difference, where the baseline average was over 500 cell/μL going down to 20 cell/μL; however, this comparison was only performed in 8 individuals where data was reported, therefore these results must be analyzed with caution (Figure 4C). For the ACT and ACQ-5 test scores, improvements were reported where the basal averages started at 16 ± 2.86 and 2.90±0.83, respectively, and reaching scores of 23 ± 1.95 and 0.38 ± 0.47 at 12 months (Figure 4D and E).
No patient reported adverse events while using benralizumab during the follow-up period, at 12 months, only 3 patients had had an exacerbation secondary to an infectious disease.
Discussion
Since marketing release in 2017, benralizumab response has been evaluated in different populations, corroborating the rapid onset of action and disease control observed in the drug pivotal Phase III clinical trials.10
Data from the SIROCCO and CALIMA clinical trials provided evidence that benralizumab was capable of reducing the asthma exacerbation rates in 51% and 28%, respectively, when compared to placebo in the subgroup of patients with high eosinophil levels (≥300 cell/μL),10–12 coinciding with the findings of the present study, where we report an average eosinophil reduction of 612.78 cell/μL (± 549.11) at 12 months of treatment. Similarly, FeNO evaluations below 45 ppb are associated with clinical and spirometric improvement as well as bronchial hyperreactivity with ICS use, highlighting the average change in FeNO of 39.8 ppb as early as 6 months of treatment.
The SIROCCO and CALIMA reported that patients treated with benralizumab showed improvements in the total scores in asthma symptom tests, such as ACQ when compared to the placebo group, in patients aged 12 to 18 years old. These differences were lower than the established minimal clinically relevant difference of 0.5 points,10–12 Published evidence has established that this is a difficult endpoint to achieve, especially for additional treatments in patients managed with ICS or ICS-LABA regimens. Our analysis showed and increased the score average for both the ACT and ACQ-5 tests, showing that for the case of ACT, at 6 months of treatment with benralizumab, 90.5% of patients had achieved control. Patients improved their scores above the required 0.5 that is the minimum necessary improvement to evaluate a response in the ACQ questionnaire. At 12 months, with a 71% of control and 29% reporting a partial level of control, it showed a reduction in exacerbations that is consistent with the evidence reported in the pivotal studies.10
The quest for reducing the use and dosage of therapies such as systemic oral corticosteroids (SOC) has been one of the main objectives to demonstrate the expected benefit of the use of novel biologic therapies in asthma management. For the case of benralizumab, this was analyzed in the ZONDA and PONENTE studies.7,8
The primary objective in ZONDA was to evaluate the capacity of benralizumab to reduce SOC dosages in patients with severe refractory asthma who depended on SOC and the reduction of eosinophil levels. The probability of SOC dose reduction was 4 times greater for the benralizumab group when compared to placebo. Up to two-thirds of patients treated with benralizumab were able to reduce their SOC dose by ≥50%. Additionally, 50% of eligible patients (baseline prednisone dose ≤12,5 mg) who received benralizumab, suspended SOC use entirely.
The ADNHI in practice study provided the first analysis showing a reduction in the use of inhaled therapies including ICS-LABA in patients treated with benralizumab; showing a successful focus on the decreased use of inhaled ICS-LABA in patients treated with the novel biologic, where 76% of patients with controlled asthma met the base medication reduction criteria.
Management of severe asthma that includes the use of biologic therapies allows for a reduction in exacerbation rates and improves symptom control, respiratory function, and quality of life while providing the potential of suspending oral corticosteroid use and reducing the exposure of patients to high dosages of ICS-LABA, therefore potentially decreasing the adverse effects related to the use of these treatments.
The results obtained in ADNHI-IP add up to the evidence supporting the efficacy of benralizumab for the control of severe eosinophilic asthma, allowing for dose reductions of base inhaled therapies while setting up the stage for additional studies evaluating the level of dose reductions in ICS-LABA therapies.13
The SHAMAL multicentric, randomized, open-label, parallel-groups clinical trial developed to evaluate the percentage of patients able to reduce dosage of their maintenance treatment with ICS-LABA toward anti-inflammatory relief in patients with severe eosinophilic asthma without losing clinical control of the disease during dose reduction. They provided evidence that 92% of patients were able to successfully reduce the high-dose ICS-LABA treatment, with over 60% of patients reducing the management to by demand regimens without compromising disease control.14 The present study results confirm the evidence on the subject, where 100% of study subjects were managed with high doses of ICS; treatment with benralizumab allowed for 95.2% of patients to reduce ICS dosage by 12 months of treatment including 5 patients (23.8%) who were able to suspend ICS-LABA management. These results suggest that benralizumab has a positive impact on the use and dependance on inhaled corticosteroids for the majority of patients, although individual variations in treatment response are to be expected.
Similarly, the CALIMA study showed a reduction in exacerbation frequency and respiratory function improvement and the ZEPHYR 2 trial reported a solid long-term reduction in the rate of exacerbations after treatment with benralizumab.4,5,15 The SHAMAL study reported that 92% of patients with severe eosinophilic asthma were able to reach effective control of the disease with the administration of benralizumab, reducing corticosteroid use by 73% in comparison with the control group, in accordance with the results of the present study, where 95% of patients were able to reduce CSI-LABA dosage at 12 months of treatment. Likewise, they reported a reduction in FEV1 in some patients, as evidenced both a correlation of the FeNO level variations and serum eosinophil count. It must be noted that the exacerbation rates remained low (3 events) throughout the follow-up period, adding up to the evidence on the efficacy and positive impact of benralizumab.6
The present study, as well as the results published from the SIROCCO, CALIMA, ANDHI IP, SHAMAL, and ZEPHYR trials, confirms the efficacy of benralizumab at approximately 46 weeks with a dose administered every eight weeks, differing from mepolizumab and reslizumab treatments, which require application every 4 weeks. The reduction of ICS-LABA requirements in patients treated with benralizumab has the potential implication of reduced economic burden for patients and health institutions.10–12,14
Study Limitations
There are no data on the agent used as the second control treatment, as the aim of the study was to identify general ICS dose reductions. Treatment selection and dose adjustment were carefully evaluated every 2 months. Although the sample size is limited and the study design does not allow for the use of placebo, the change in biomarkers, the clinical improvement in patients, the improvement in lung function and the consistency with previous studies with benralizumab minimize the potential confounding bias caused by the effect of patient participation in the study. This study did not collect data to evaluate clinical remission, so a conclusion in this regard is premature; however, the results show that benralizumab may be effective in inducing remission in patients with uncontrolled severe eosinophilic asthma.
Conclusions
Benralizumab is an anti-IL-5Rα monoclonal antibody with increasing evidence supporting its use as an effective treatment option for patients diagnosed with severe eosinophilic asthma. The results of the present study indicate that patients treated with this biologic can achieve a notable reduction of relevant parameters including an average reduction of 39.8 ppb for FeNO at 6 months and 612.78 cell/μL (± 549.11) for the case of serum eosinophil count at 12 months, proving the efficacy of benralizumab in eosinophilic inflammation control. Additionally, at 12 months of treatment, we observed a significant and progressive improvement of respiratory function through FEV1 prior to bronchodilator with gains of 24,143 mL, indicating an improved lung capacity in evaluated patients.
The use of specific instruments such as ACT and ACQ-5 helped confirm clinical control improvement and significant symptom reduction, where 100% of patients were uncontrolled at baseline, 90.5% of patients reported improvement in ACT scores at 6 months, and 71% on ACQ-5 scores, which translates into an improved quality of life for patients treated with benralizumab. It must also be noted that benralizumab allowed for a significant reduction in ICS-LABA utilization with 61.9% of patients requiring lower doses at 12 months of treatment, which represents direct benefit towards patient’s health and a potential reduction to the secondary effects associated with ICS-LABA management.
These findings support the crucial role of novel effective management in this medical condition and the potential contributions to better prognosis. The key point for successful management with benralizumab and other monoclonal antibodies is adequate selection according to the profile of the target patient, considering the predominant phenotype and the presence of basal factors that can help clinicians discern the patient group that will benefit fully from treatment selection.
Funding Statement
HS Estudios Farmacoeconómicos provided support for medical writing, following Good Publication Practices (GPP3). This work is funded by AstraZeneca. The sponsor does not play any role in the study design, data collection and analysis or decision to publish.
Social Media Text
A study in patients with severe eosinophilic asthma confirms the effectiveness of benralizumab by improvements in clinical and symptomatic control in FEV1, FeNO, eosinophil count and ICS-LABA dose reduction starting as early as 6 months.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Global Initiative For Asthma. 2023. Available from: https://ginasthma.org/pocket-guide-for-asthma-management-and-prevention/. Accessed Nov 1, 2024.
- 2.Menezies-Glow A, Hoyte F, Price B, et al. Clinical Remission in Severe Asthma: a Pooled Post Hoc Analysis of the Patient Journey with Benralizumab. Adv Ther. 2022;39(5):2065–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skolnik N, Carnahan S. Primary care of asthma: new options for severe eosinophilic asthma. Curr Med Res Opin. 2019;35(7):1309–1318. doi: 10.1080/03007995.2019.1595966 [DOI] [PubMed] [Google Scholar]
- 4.Cushen B, Menzies-Gow A. Benralizumab: an updated treatment of eosinophilic asthma. Expert Rev Respir Med. 2020;14(5):435–444. doi: 10.1080/17476348.2020.1739526 [DOI] [PubMed] [Google Scholar]
- 5.Venancio-Hernández M, Mendieta-Flores E, Mendiola-Marín J, Alaniz-Flores A, Reyes-Arellano M. Abordaje diagnóstico del asma difícil de tratar y asma grave. Rev Alerg Mex. 2022;69(Supl1):s94–s111. doi: 10.29262/ram.v69iSupl1.1046 [DOI] [PubMed] [Google Scholar]
- 6.Liaqat A, Mason M, Foster B, et al. Evidence-Based Approach of Biologic Therapy in Bronchial Asthma. J Clin Med. 2023;12(13):4321. doi: 10.3390/jcm12134321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menzies-Gow A, Corren J, Bel E, et al. Corticosteroid tapering with benralizumab treatment for eosinophilic asthma: PONENTE Trial. ERJ Open Res. 2019;5(3):00009–2019. doi: 10.1183/23120541.00009-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nair P, Wenzel S, Rabe K, et al. Oral Glucocorticoid-Sparing Effect of Benralizumab in Severe Asthma. N Engl J Med. 2017;376(25):2448–2458. doi: 10.1056/NEJMoa1703501 [DOI] [PubMed] [Google Scholar]
- 9.Jackson D, Heaney L, Humbert M, et al. Reduction of daily maintenance inhaled corticosteroids in patients with severe eosinophilic asthma treated with benralizumab (SHAMAL): a randomised, multicentre, open-label, phase 4 study. Lancet. 2023;403:271–281. [DOI] [PubMed] [Google Scholar]
- 10.Pelaia C, Vatrella A, Bruni A, Terracciano R, Pelaia G. Benralizumab in the treatment of severe asthma: design, development and potential place in therapy. Drug Des Devel Ther. 2018;21:619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FitzGerald J, Bleecker E, Nair P, Korn S, Ohta K, Lommatzsch M. CALIMA study investigators. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled Phase 3 trial. Lancet. 2016;388:2128. [DOI] [PubMed] [Google Scholar]
- 12.Bleecker E, Fitzgerald J, Chanez P, et al. SIROCCO study investigators Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomized, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115–2127. [DOI] [PubMed] [Google Scholar]
- 13.Louis R, Harrison T, Chanez P, et al. Severe Asthma Standard-of-Care Background Medication Reduction With Benralizumab: ANDHI in Practice Substudy. J Allergy Clin Immunol Pract. 2023;11(6):1759–1770.e7. doi: 10.1016/j.jaip.2023.03.009 [DOI] [PubMed] [Google Scholar]
- 14.Jackson D, Heaney L, Humbert M, et al. SHAMAL: reduction of maintenance inhaled corticosteroids in patients with severe eosinophilic asthma treated with benralizumab: a randomised phase 4 study. ERJ Open Res. 2023;403:271–281. [DOI] [PubMed] [Google Scholar]
- 15.de Salud S. 2022. Available from: https://www.gob.mx/salud/prensa/331-en-mexico-8-5-millones-de-personas-viven-con-asma-iner?idiom=es. Accessed Nov 1, 2024.