Abstract
Purpose
To evaluate the therapeutic effect of oral administration of Lactiplantibacillus plantarum P101 (P101) on skeletal injury in young rats exposed to titanium dioxide nanoparticles (TiO2 NPs), and explore the potential mechanism.
Methods
Four-week-old male rats were orally administration to TiO2 NPs and supplemented with P101 2 hours later for 4 weeks. The growth and development, food intake, bone metabolism and serum inflammatory markers of the rats were evaluated. Their tibias were observed and evaluated using microcomputed tomography (micro-CT), tartrate-resistant acid phosphatase (TRAP) staining, immunohistochemistry (IHC) and real-time quantitative PCR (RT-qPCR). We observed the tibia growth plate using safranin and fast green staining. 16S rDNA sequence analysis of fecal samples was performed to observe changes in the gut microbiota.
Results
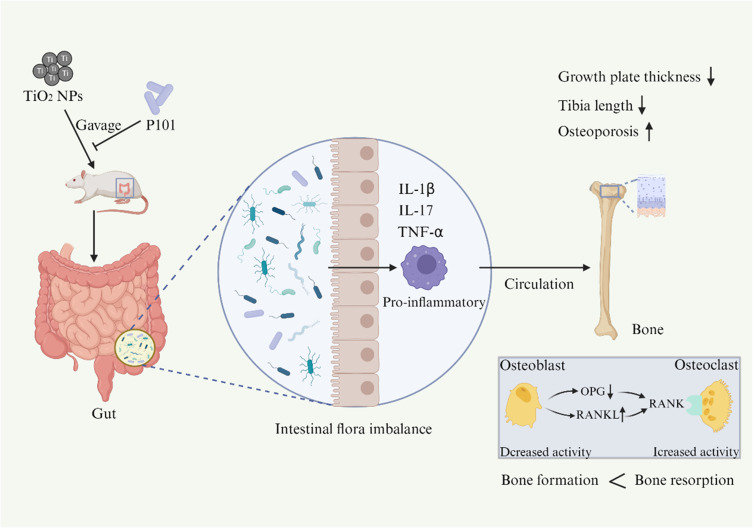
Our results showed that TiO2 NPs can lead to bone growth inhibition and osteoporosis, induce intestinal flora imbalance, and induce inflammation in young rats. Further mechanistic studies suggested that TiO2 NPs disrupts intestinal flora and increases serum IL-1β levels, which increased the expression of RANKL in bone, thereby enhancing osteoclast differentiation and function, leading to bone loss. Through a P101 supplementation experiment, we found that P101 ameliorated the inflammation and osteoporosis on bone caused by TiO2 NPs.
Conclusion
This study showed that the mechanism by which P101 alleviates bone damage caused by TiO2 NPs may be through restoring intestinal microbial homeostasis and inhibiting inflammatory response.
Keywords: TiO2 NPs, P101, young rats, intestinal flora, bone injury, IL-1β
Graphical Abstract
Introduction
With continual advances in nanotechnology, TiO2 NPs have been widely used in daily life.1 Because of their superior physical, chemical, and biological characteristics, TiO2 NPs are utilized in cosmetics, paints, medicine, and food additives.2–4 TiO2 NPs are added as whitening agents to white chocolate, candy, chewing gum and other products.5 Children in Europe are exposed to TiO2 NPs of at least 2 mg/kg body weight daily, with a greater intake than adults. A survey study showed that American children consume at least 1 mg/kg body weight TiO2 NPs daily.6 The easy access to food containing TiO2 NPs has raised great concern about their potential health effects, especially in children. Some studies have shown that orally administration to TiO2 NPs can affect the intestinal and liver function of young rats.7,8 Our team has demonstrated that TiO2 NPs disrupt normal skeletal structure in young rats.9 However, there is almost no research on alleviating bone damage caused by TiO2 NPs.
Normal growth of bones, especially long bones, is important for children’s final height. In China, parents are paying increasing attention to the height of their children. Children whose height falls below the third percentile are defined as having short stature compared to people of the same age, gender, and race. In the same age group, the proportion of children with short stature in China is 3.7%, which is not a small proportion.10 The main cause of short stature in children is poor skeletal development.11 One study showed that nursing from rats exposed to TiO2 NPs can affect the body growth of their offspring.12 It is particularly urgent to find a way to alleviate the damage caused by TiO2 NPs in children.
As an important digestive organ of the human body, the gut is an important defense line against damage caused by oral exposure to nanomaterials through food. Normal gut structure and function and intestinal microbial homeostasis can regulate immune balance to maintain health.13 There are at least 1013 microorganisms in the human gut.14 Firmicutes and Bacteroidetes are the predominant phyla of the gut microbiota, accounting for 90% of the gut microbiota. Actinobacteria and Proteobacteria also account for a certain proportion of the gut microbiota.15 Reports have indicated that oral administration of TiO2 NPs induces intestinal inflammatory injury in young rats and has a great impact on the quantity of the intestinal microbiota, particularly Lactobacillus, Firmicutes, and Proteobacteria.8,16 Notably, these gut-residing Lactobacillus strains can not only balance gut barrier integrity but also ameliorate the host immune response.17 Lactobacillus rhamnosus GG, a type of Lactobacillus, has been studied several times, and its anti-inflammatory effects have been well demonstrated. Moreover, it can alleviate the intestinal flora imbalance caused by TiO2 NPs. Additionally, mounting evidence supports the role of Lactobacillus plantarum species in gut microbiota dysbiosis, the modulation of intestinal homeostasis, and the alleviation of inflammation.18 It is good for treating ulcerative colitis, cardiovascular diseases, osteoporosis, hyperuricemia, and other diseases.19–22 You et al showed that P101 has superior anti-inflammatory properties compared with Lactobacillus rhamnosus GG.23 Therefore, P101 was used in this study.
Accordingly, utilizing Lactobacillus to alleviate the negative effects of TiO2 NPs may be a potential therapy that deserves further investigation. In this study, we explored the adverse effects of TiO2 NPs on bones in juvenile rats. We also investigated whether P101 had a protective effect against TiO2 NP-induced bone damage in young rats.
Materials and Methods
Preparation and Characterization of TiO2 NPs
TiO2 NPs were purchased from Aladdin Industrial Corporation (Shanghai, China). The morphology of the TiO2 NPs was observed by transmission electron microscopy (TEM). The suspension was prepared by mixing powdered TiO2 NPs with 1% phosphate buffered saline (PBS, pH 7.4). The mixture was ultrasonicated for half an hour, and then vortexed through a simulated vortex mixer to ensure uniform dispersion of the TiO2 NPs in the aqueous solution.
Preparation of Probiotics
P101 (CCTCC M 2021108) was dissolved in sufficient sterile Man–Rogosa–Sharpe (MRS) purchased from a company based in Beijing, China (Solarbio Science and Technology Co. Ltd). The mixtures were then incubated under an anaerobic environment at 37 °C for 16 h. Then, the bacterial mixture was centrifuged to remove the supernatant and retain the precipitates. The bacteria were resuspended twice with 1% PBS to adjust their concentration to 108 colony-forming units/mL (CFU/mL) in a final volume of 200 μL.
Animal and Experimental Design
Purchased pregnant Sprague–Dawley (SD) rats from the Jiangxi University of Chinese Medicine. All animal experiments were approved by the Animal Ethics Committee of Nanchang University, which complies with the Guide for the Care and Use of Laboratory Animals published by the USA National Institutes of Health. This study received approval from the Animal Care Review Board of Nanchang University, Jiangxi, China (approval number: NCULAE-20221031015).
The offspring received a 3-week period of breastfeeding after birth. After one week of adaptation after weaning, 20 male SD rats (4 weeks old, 57.78 ± 2.28 g) were selected as experimental subjects for this study. The rats were randomly assigned into four groups: control group, TiO2 NPs group, TiO2 NPs + P101 group, and P101 group. The rats in the TiO2 NPs + P101 group were orally administered TiO2 NPs and supplemented with P101 2 hours later. The dose of TiO2 NPs was 100 mg/kg and the dose of P101 was 200 µL. The rats were provided with ad libitum access to sufficient food and purified water, housed in standard plastic cages, and placed in constant temperature (23 ± 1 °C) dedicated animal rooms with 12 hours of darkness and 12 hours of light.
The rats were treated for 28 days throughout the entire experimental period. Feces were collected in sterile centrifuge tubes on the last day, after which the young rats were anesthetized by inhalation of carbon dioxide. Blood samples were immediately collected from the ophthalmic vein after eyeball extraction. The serum and feces were stored at −80 °C. The left tibias were fixed with 4% paraformaldehyde.
Growth and Development Evaluation
The food intakes and weights of the young rats were recorded at the same time every day. The length of the body from the nose to the anus was measured and recorded biweekly. Finally, the body mass index (BMI = body weight/body length2) was calculated from the weight and height as the root weight. The tibia length was measured on the last day.
Serum Biochemical Indicator Analysis
Bone metabolism was analyzed by measuring serum levels of alkaline phosphatase (ALP), calcium (Ca), and phosphorus (P) levels. Calculate the Ca/P ratio based on the detected Ca and P values. These indices were detected by an automatic biochemical analyzer (Chemray 800, Rayto Life and Analytical Sciences Co., Ltd). purchased from a company in Shanghai, China.
Determination of Serum Inflammatory Factor Levels
Inflammatory cytokine levels, such as interleukin-17 (IL-17) (Multisciences, EK317/3-96), interleukin-1β (IL-1β) (Servicebio, GER0002-96T), and tumor necrosis factor-α (TNF-α) (Thermo Fisher Scientific, 88–7340-88) were assessed using rat enzyme-linked immunosorbent assay (ELISA) kits. In addition, all procedures were performed according to the instructions in the kit.
Safranin and Fast Green Staining of Bone Tissue Samples
The tibia was removed from the fixative and immersed in a solution of 10% formic acid–formalin for decalcification. After that, embed the decalcified tibia in paraffin and then section it. The bone tissue sections were placed in fast green staining solution for 1–5 min, and the excess staining solution was removed by washing with water until the cartilage became colorless. The samples were soaked in 1% hydrochloric acid alcohol for 10s, and then gently cleaned with tap water. Bone tissue sections were subjected to 1–5s of safranin staining solution and rapidly dehydrated in three cylinders of absolute ethanol for 5s, 2s, and 10s, and then placed in a fourth cylinder containing absolute ethanol. Then, the slide in the fourth cylinder was inspected under a microscope (E100, Nikon). The altitudes of the growth plates subjected to safranin and fast green staining were analyzed using ImageJ software.
Tartrate-Resistant Acid Phosphatase Staining of Bone Tissue Samples
After dewaxing and washing, the paraffin sections of tibia were placed in a wet box and incubated with distilled water at 37 °C for 2 h. After that, the slices were incubated with the prepared TRAP incubator solution at 37 °C for 20 min. The nuclei were re-stained with hematoxylin solution for 15s, then differentiation fluid and ammonia were added. Finally, seal the slices with neutral gum. The stained osteoclast envelope was wine red and the nucleus was light blue. The number of osteoclasts was counted at 200x microscope (E100, Nikon).
Evaluation of Bone Microstructure in Micro-CT
Micro-CT (SkyScan 1275, Bruker, Germany) was used to evaluate the microstructure and bone metabolism of the tibias in each group. The datasets were reconstructed with NRecon software (version 1.7.4.2) to obtain three-dimensional (3D) images. Threshold segmentation was performed on the region of interest (ROI), and the influence of soft tissue and fluid in the medullary cavity was excluded to obtain trabecular data for further analysis. Direct or indirect indicators of bone mass include the bone surface area (BS), surface area/bone volume (BS/BV), bone volume fraction (BV/TV), and bone surface density (BS/TV). The indices used for evaluating the morphology and structure of the trabecular bone included the trabecular separation (Tb.Sp), trabecular pattern factor (Tb.Pf), trabecular number (Tb.N), structure model index (SMI), and connectivity density (Conn.Dn). Bone mineral density (BMD) can assess bone quality and strength.
Immunohistochemical Analysis of Bone Tissue
After the paraffin sections were deparaffinized in water, circles were drawn with a histochemical pen, and pepsinase was added to the circles until the tissue was completely covered. The sections were then incubated in an oven for half an hour at 37 °C for antigen repair. The slides were placed in 3% hydrogen peroxide. Incubate cells at 25 °C for 25 min in the dark to block endogenous peroxidase activity. The slides were then incubated with 3% BSA and blocked for half an hour at 25 °C. Primary antibodies named osteoprotegerin (OPG) and nuclear factor-κB ligand (RANKL) were incubated with the sections overnight at low temperature (4 °C) in a wet box. The next day, secondary antibodies against the corresponding species were added to the bone tissue, which was then incubated at room temperature for 50 min. The nuclei were counterstained with hematoxylin after staining with DAB. Collect slice images using a light microscope (E100, Nikon). The nuclei were stained blue with hematoxylin, and DAB positive cells were stained brown-yellow. In the whole field of view, the integrated optical density (IOD) was quantified using ImageJ software at 200× magnification.
Real-Time Quantitative PCR Analysis of Bone Tissue
Total RNA in tibia tissue was obtained with an RNA extraction kit. The absorbance of the extracted total RNA was determined with a software (Nanodrop 2000) to measure the purity of total RNA. RNA integrity was determined using agarose gel electrophoresis. Then, a cDNA synthesis kit (Servicebio, G3337) was used to reverse transcribe 1 μg of extracted RNA to obtain cDNA. Finally, the amplification was completed by fluorescence quantitative PCR (Bio-rad, CFX Connect). The gene transcription level was calculated using the 2−ΔΔCt method and the primers are shown in Table S1.
16S rDNA Sequencing and Analysis of the Fecal Microbiota
According to the manufacturers’ instructions, DNA was extracted from each rat’s sample using the Feces Genomic DNA Purification Kit (BSC48S1E, Hang Zhou, China). Genomic DNA was used as a template for PCR amplification using specific primers with barcodes according to the selection of the sequencing region. Amplify the V3-V4 region of the small-subunit (16S) ribosomal RNA gene in prokaryotes (bacteria). The primers used are 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The constructed amplicon library was subsequently sequenced at Guangdong Magigene Biotechnology Co., Ltd. (Guangzhou, China). The date was analyzed on a platform (Microeco Tech Co., Ltd. Shenzhen, China). Linear discriminant analysis effect size (LEfSe) method was used to identify microbial taxa with significant differences. A linear discriminant analysis (LDA) >3 was set for the analysis.
Statistical Analysis
SPSS v25.0 software was used for one-way ANOVA. Data were expressed as means ± SD. GraphPad (GraphPad Software, USA) was used for plotting. *P < 0.05, **P < 0.01, ***P < 0.001 compared with the control group. #P < 0.05, ##P < 0.01, ### P < 0.001 compared with the TiO2 NPs group. A significance level of P < 0.05 was considered indicative of statistical significance between groups in this experiment.
Results
Characterization of TiO2 NPs
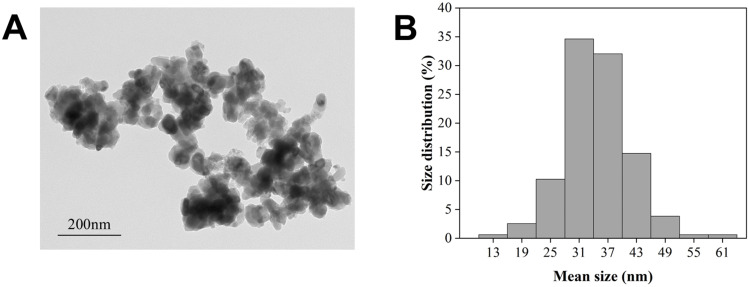
The TiO2 NPs particles were rod-shaped and had a measured average diameter of 34.81 ± 6.8 nm (Figure 1A and B).
Figure 1.
Morphology and size of TiO2 NPs. (A) TEM image. (B) Percent size distribution.
Changes in Growth and Development
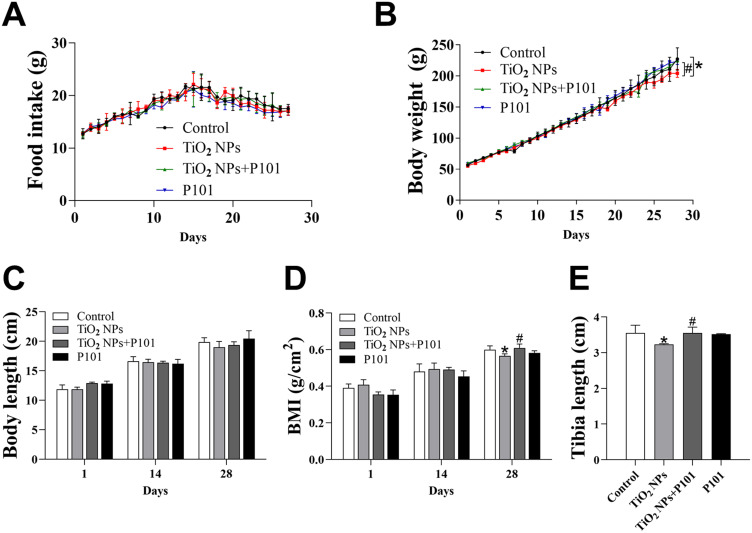
We observed that the body weight in the TiO2 NPs group was significantly decreased compared to the control group (Figure 2B). Moreover, there was a notable increase in weight gain observed in the TiO2 NPs + P101 group compared to the TiO2 NPs group. At the same time, there was no statistical difference in food intake among the groups (Figure 2A). In this experiment, no difference in body length and BMI on the 14th day was observed among the four groups (Figure 2C and D). However, on the 28th day after gavage, the BMI and tibia length were significantly lower in the TiO2 NPs group than those in the control group, while the BMI and tibia length were significantly greater in the TiO2 NPs + P101 group than in the TiO2 NPs group (Figure 2D and E). The changes in these indicators demonstrated that the TiO2 NPs inhibited the body weight gain of young rats and impaired the growth of their long bones. P101 can alleviate the developmental toxicity of young rats exposed to TiO2 NPs.
Figure 2.
Characterization of growth. (A) Food intake. (B)Body weight. (C) Body length. (D) BMI. (E) Length of the tibia. *P<0.05, vs control. #P<0.05, vs TiO2 NPs.
Serum Biochemistry and Inflammation Indicators
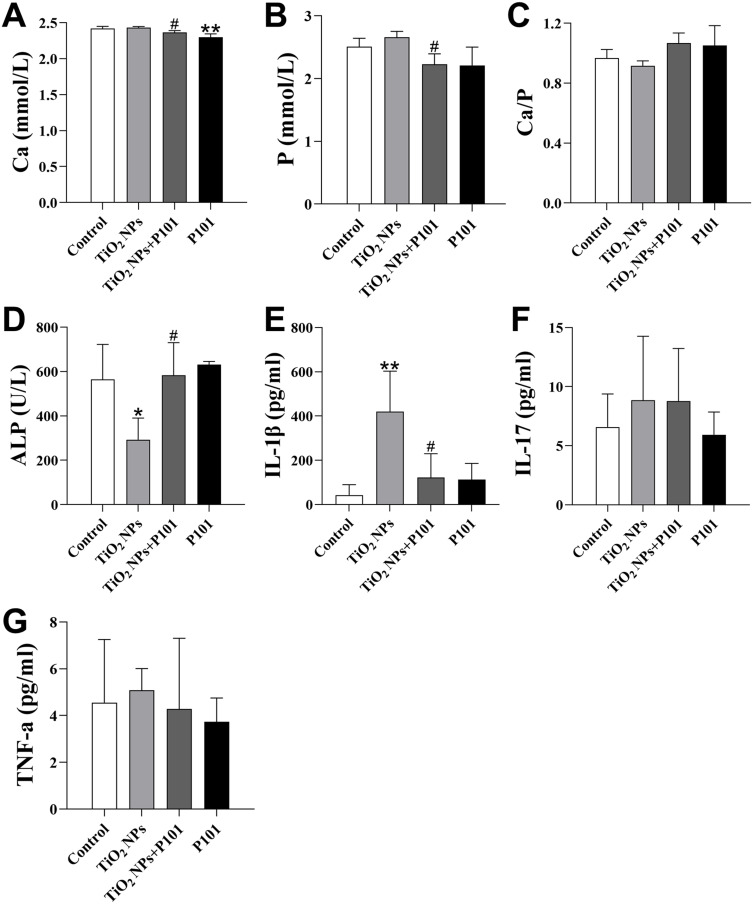
Ca, P and ALP can be used to examine bone metabolism. These data are shown in Figure 3A–D. The Ca and P levels were greater and the Ca/P ratio was lower in the TiO2 NPs group than in the control group, but these differences were not statistically significant. Moreover, the TiO2 NPs + P101 group exhibited a significantly lower levels of Ca and P compared to the TiO2 NPs group (Figure 3A and B). Although there was an increasing trend in the Ca/P ratio in the TiO2 NPs + P101 group, the difference was not significant (Figure 3C). In the TiO2 NPs group, the ALP level was significantly lower, while the IL-1β level were significantly greater compared to the control group (Figure 3D and E). After P101 administration, the TiO2 NPs + P101 group exhibited a significant increase in ALP and a significant decrease in IL-1β levels compared to the TiO2 NPs group. The serum levels of TNF-α and IL-17 were not different among the groups (Figure 3F and G). These data indicate that oral exposure to TiO2 NPs can interfere with bone metabolism. The IL-1β level indicated that TiO2 NPs could cause systemic inflammation in young rats, and P101 could alleviate the increased levels of inflammatory factors induced by TiO2 NPs.
Figure 3.
Serum indices. (A) Ca. (B) P. (C) Ca/P. (D) ALP. (E) IL-1β. (F) IL-17. (G) TNF-α. *P<0.05, **P<0.01, vs control. #P<0.05, vs TiO2 NPs.
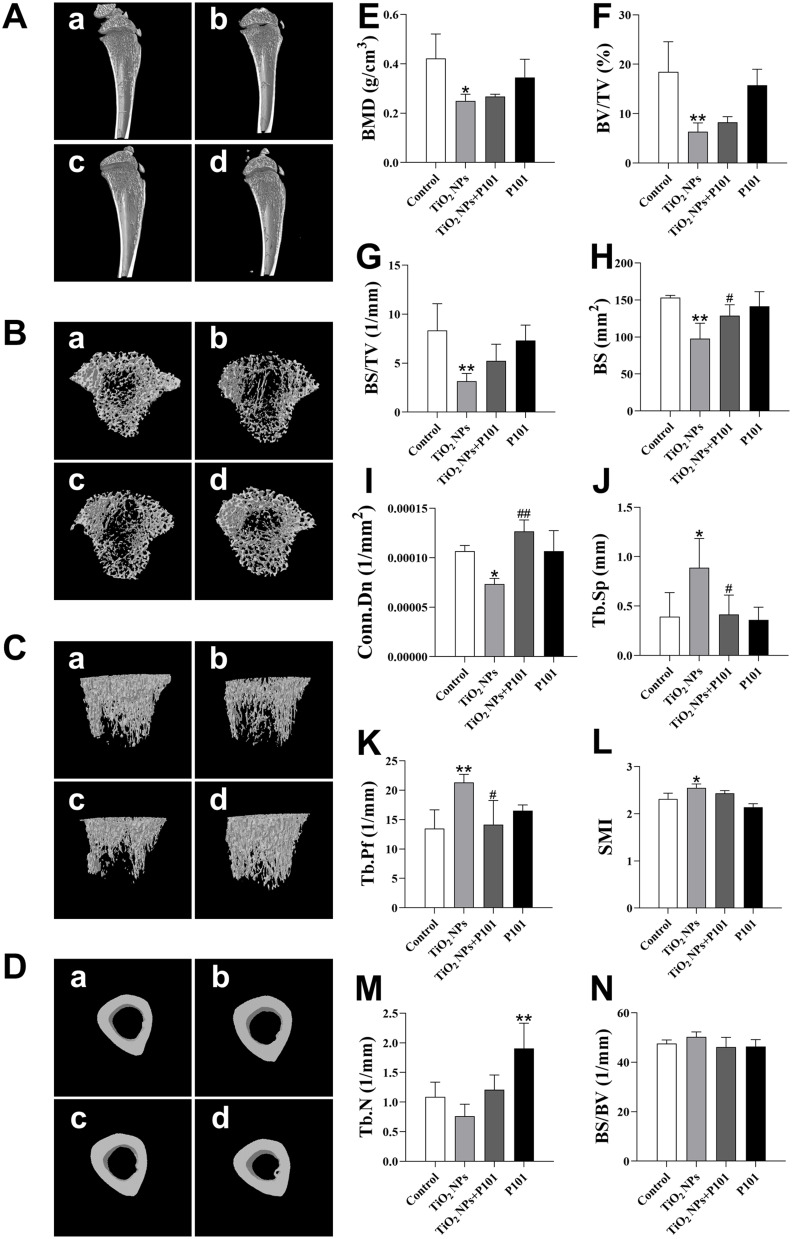
Bone-Structure Observation by Micro-CT and Data Analysis
To examine whether the bone microstructure of the tibia of the rats in the TiO2 NPs group was altered and whether P101 could protect it, the structure and morphology of the rat bones were evaluated by micro-CT. As shown in Figure 4A–D, there were no apparent differences observed in the cortical bone of each group according to the three-dimensional images. However, the TiO2 NPs group exhibited sparse tibial trabeculae and decreased bone density. After P101 supplementation, the bone trabeculae were denser, and the bone density rose in the young rats.
Figure 4.
3D images of tibia and morphological data of the trabecular. (A) Sagittal image of the tibia bone. (B) Transverse section image of the bone trabecula. (C) Overall image of the bone trabecula. (D) Transverse section image of the cortical bone. In which, a) Control; b) TiO2 NPs; c) TiO2 NPs + P101; d) P101. (E) BMD. (F) BV/TV. (G) BS/TV. (H) BS. (I) Conn.Dn. (J) Tb.Sp. (K) Tb.Pf. (L) SMI. (M) Tb.N. (N) BS/BV. *P<0.05, **P<0.01, vs control. #P<0.05, ##P<0.01, vs TiO2 NPs.
We further analyzed the trabecular bone data of these groups. The obtained trabecular bone morphological data are shown in Figure 4E–N. The data are consistent with the corresponding images. Compared to the control group, we observed that the BMD, Conn.Dn, BS, BV/TV, and BS/TV decreased significantly, while the Tb.Pf, Tb.Sp, and SMI metrics were significantly increased in the TiO2 NPs group (Figure 4E–L). Although the BS/BV increased and the Tb.N decreased in the TiO2 NPs group, the difference was not statistically significant (Figure 4M and N).
After P101 supplementation, in the TiO2 NPs + P101 group, the BS and Conn.Dn were significantly increased, while Tb.Sp and Tb.Pf were significantly decreased compared to the TiO2 NPs group (Figure 4H–K). The BMD, BV/TV, and BS/TV tended to increase, while the SMI tended to decrease compared to the TiO2 NPs group, but the differences were not significant (Figure 4E–G, and L). Moreover, the Tb.N was significantly greater in the P101 group compared to the control group (Figure 4M). These images and data indicate that TiO2 NPs can decrease bone mass, damage the microstructure of bone tissue, and cause osteoporosis in rats. P101 can alleviate bone damage caused by TiO2 NPs and confer certain benefits to the skeleton of normal rats.
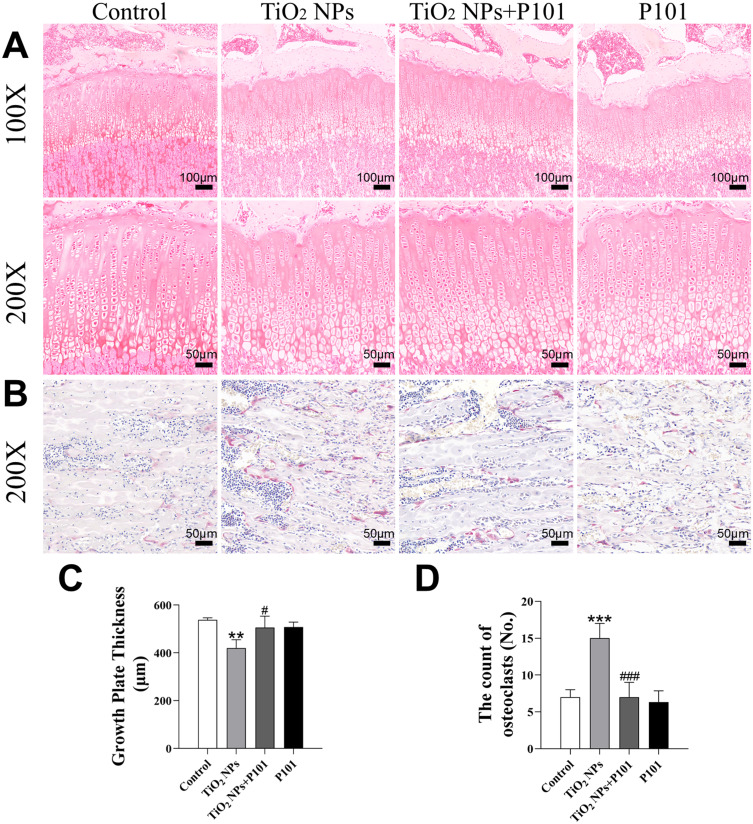
Morphology and Data Analysis of Growth Plates and Osteoclasts
To further observe the growth plates and osteoclasts of rats, safranin and fast green staining and TRAP staining were used to stain the tibial sections of the rats (Figure 5A and B). Compared to that of the control group, the growth plate altitude of the group exposed to TiO2 NPs was significantly lower (Figure 5C). In comparison to the TiO2 NPs group, the TiO2 NPs + P101 group showed a notable elevation in growth plate thickness. The number of osteoclasts in the TiO2 NPs group was significantly higher than that in control group, and the number of osteoclasts in the TiO2 NPs + P101 group was significantly lower than that in the TiO2 NPs group (Figure 5D). These results indicated that oral exposure to TiO2 NPs caused growth plate thinning and osteoclast proliferation in young rats, while P101 administration could alleviate this damage to some extent.
Figure 5.
Morphology and data analysis of growth plates and osteoclasts. (A) Image of tibia growth plate (100X, 200X). (B) Image of tibia stained with TRAP (200×). (C) Growth plate thickness. (D) The count of osteoclasts. **P<0.01, ***P<0.001, vs control. #P<0.05, ###P<0.001, vs TiO2 NPs.
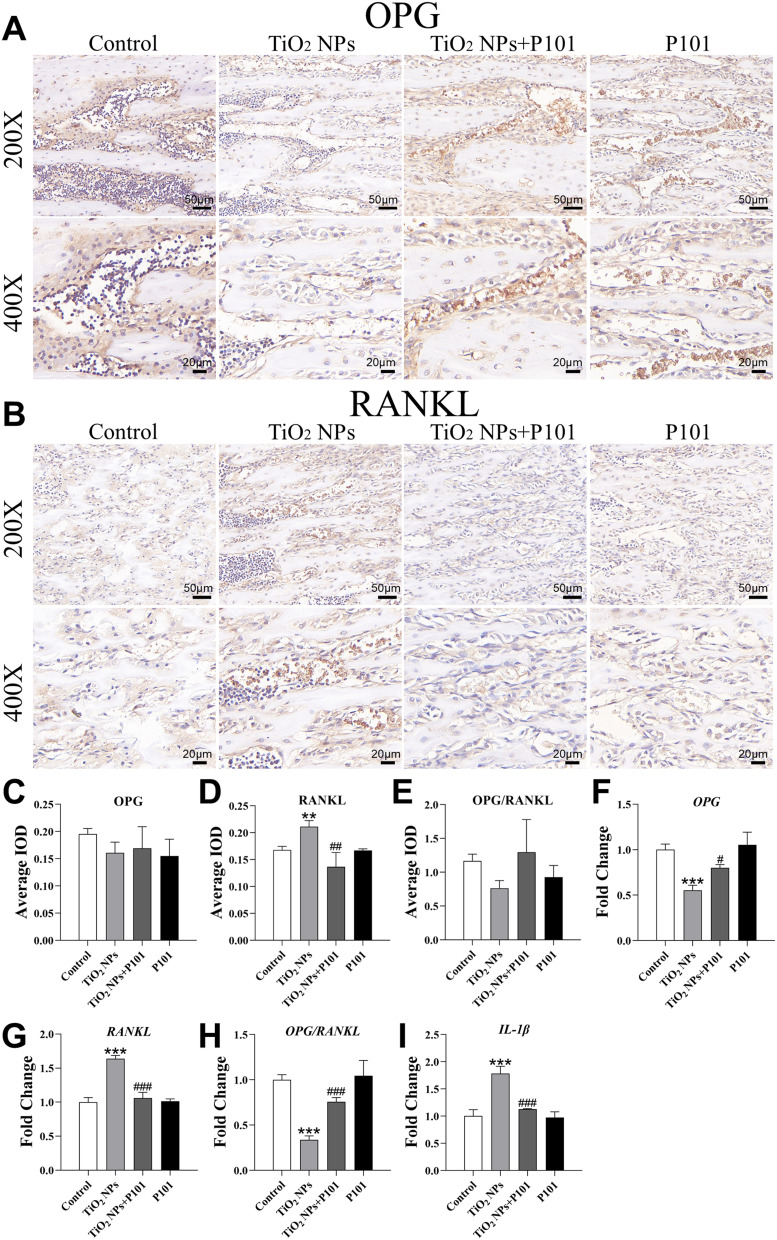
IL-1β, OPG and RANKL Expression Levels in the Tibia
Figure 6A and B show OPG and RANKL protein staining. IHC revealed that OPG expression and OPG/RANKL ratio did not significantly differ among the four groups (Figure 6C and E). The expression of RANKL was significantly greater in the TiO2 NPs group than in the control group (Figure 6D). Additionally, there was a notable decrease in the expression of RANKL in the TiO2 NPs + P101 group compared to the TiO2 NPs group. RT-qPCR results showed that compared with those in the control group, the expression levels of OPG and OPG/RANKL ratio in the TiO2 NPs group were significantly decreased, while the expression levels of RANKL and IL-1β were significantly up-regulated (Figure 6F-I). In the TiO2 NPs + P101 group, the expression levels of OPG and OPG/RANKL ratio were significantly increased, while expression levels of RANKL and IL-1β were significantly decreased compared to the TiO2 NPs group. These results demonstrated that TiO2 NPs can induce inflammatory response in bone and enhance osteoclast activity and bone resorption. P101 could alleviate the adverse reactions caused by TiO2 NPs.
Figure 6.
IL-1β, OPG and RANKL expression levels in the tibia. IHC Image of bone tissue (A) OPG (200×, 400×), (B) RANKL (200×, 400×). (C) Mean integrated optical density (IOD) of OPG expression. (D) Mean IOD of RANKL expression. (E) Mean IOD of OPG/RANKL ratio. RT-qPCR results of (F) OPG, (G) RANKL, (H) OPG/ RANKL, (I) IL-1β. **P<0.01, ***P<0.001, vs control. #P<0.05, ##P<0.01, ###P<0.001, vs TiO2 NPs.
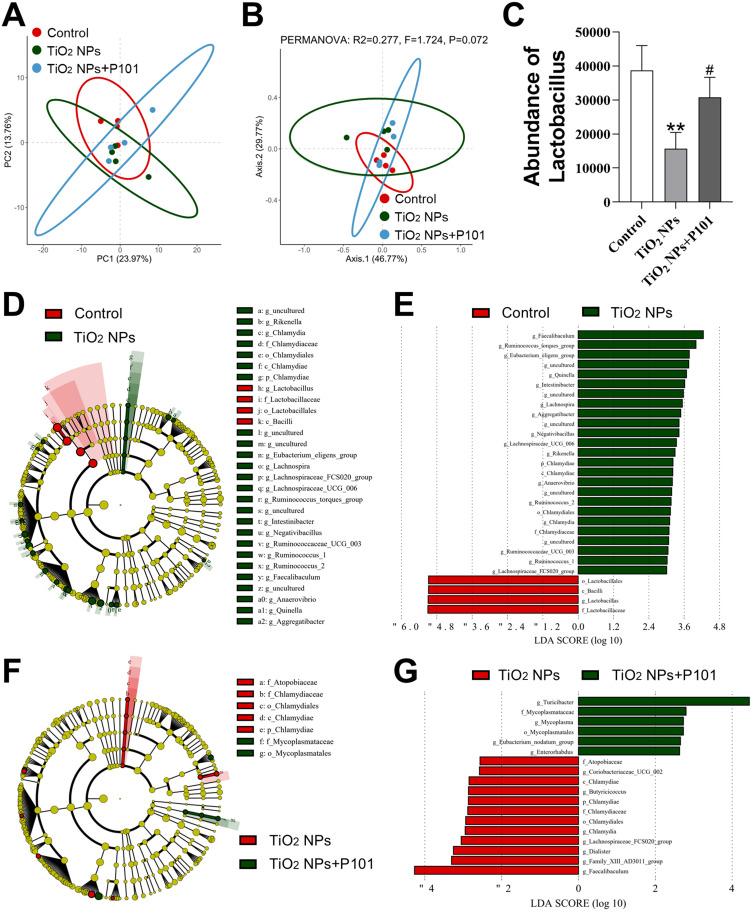
Analysis of the Gut Microbiota
The intestine contains a large number of microbiota. Principal component analysis (PCA) was utilized to assess the differences in microbial composition among the control group, TiO2 NPs group, and TiO2 NPs + P101 group (Figure 7A). Principal coordinates analysis (PCoA) was also employed for comparison (Figure 7B). Compared to the control group, the results of LEfSe results indicated a significant change in the gut microbiota in the TiO2 NPs group (Figure 7D and E). In particular, the abundances of the class Bacilli, order Lactobacillales, family Lactobacillaceae, and genus Lactobacillus were significantly lower in the TiO2 NPs exposed group (Figure 7C-E). The addition of P101 significantly attenuated the gut microbial alterations caused by TiO2 NPs and increased the abundance of Lactobacillus in the intestinal flora of young rats (Figure 7C, F and G). These studies showed that oral exposure to TiO2 NPs altered the gut microbiota composition and significantly reduced the abundance of Lactobacillus. P101 supplementation alleviated the effect of TiO2 NPs on the gut microbiota.
Figure 7.
Changes in gut microbiota in young rats. (A) PCA. (B) PCoA. (C) Abundance of Lactobacillus. (D and E) LEfSe analysis of the control group and TiO2 NPs group. (F and G) LEfSe analysis of the TiO2 NPs group and TiO2 NPs + P101 group. **P<0.01, vs control. #P<0.05, vs TiO2 NPs.
Discussion
Many TiO2 NPs are produced and widely used in everyday products associated with children, and the average estimated intake of TiO2 NPs in children is greater than that in adults. Children’s exposure to environmental pollutants may pose greater health risks than adults’ due to their growth and development.24 A prior study demonstrated that TiO2 NPs can damage bone and lead to osteoporosis. Research has shown that oral supplementation with probiotics can reduce intestinal inflammation, reverse gut microbial imbalances, and reduce bone loss in rats.25 In this study, we orally administered TiO2 NPs and P101 to young rats to investigate whether P101 can alleviate the bone injury caused by TiO2 NPs in young rats.
Macroscopic growth and development were assessed with commonly used indicators such as body weight, body length, BMI, and tibia length. The body weight and BMI of the TiO2 NPs group were significantly lower, while the weight and BMI of the TiO2 NPs + P101 group rats returned to the control level after P101 intake (Figure 2B and D). During the experiment, we observed a decreasing trend in body length in the TiO2 NPs groups (Figure 2C). Moreover, the TiO2 NPs group had significantly shorter tibial lengths (Figure 2E). After supplementation with P101, the tibia length of rats exposed to TiO2 NPs returned to control levels. In the case of no difference in food intake among the groups of rats, our results showed that TiO2 NPs had a negative influence on the body growth of young rats, especially on body weight. This result differs from the conclusions of Cheng et al, who did not find that TiO2 NPs were significantly toxic to the weight and tibial length of young rats.9 The different results observed may be due to the different ages of the rats used in this study. Exposure to TiO2 NPs may have different effects at different ages.
Bone length is strongly correlated with growth plate height. The growth plate is a layer of hyaline cartilage tissue between the distal epiphysis and metaphysis of long bones, that has the ability to grow longitudinally. Our study showed that the height of the growth plates in the TiO2 NPs group was shorter compared to the control group, while the growth plate height returns to the level of the control group after P101 treatment (Figure 5C). Thus, the growth plates of young rats were highly damaged by TiO2 NPs and supplementation with P101 attenuated this adverse effect.
The structural changes of the tibia can be visualized by micro-CT. According to these 3D images, the microstructure of the tibia, especially the cancellous bone, in juvenile rats exposed to TiO2 NPs was disrupted (Figure 4A–D). The extent of destruction was mitigated by P101 supplementation. The data were used to further evaluate the degree of microstructural damage to the trabecular bone in the four groups (Figure 4E–N). These data also confirmed bone tissue damage after exposure to TiO2 NPs, characterized by decreased bone mineral density, reduced trabecular number, and disruption of trabecular structure. This result showed that TiO2 NPs caused osteoporosis, which is consistent with the findings of Cheng et al.9 Moreover, after P101 intake, the bone density increased. In this study, we found that this tibial bone damage was relieved after supplementation with P101.
Ca is the main mineral of bone. Serum Ca and P levels can evaluate the effects of bone metabolism on the different groups. The serum Ca and P levels showed an upward trend, and the Ca to P ratio tended to decrease after exposure to TiO2 NPs (Figure 3A–C). After P101 supplementation, the changes in these indicators were opposite to those in the group exposed to TiO2 NPs. In this study, the serum ALP concentration was lower in the TiO2 NPs group and greater in the TiO2 NPs + P101 group (Figure 3D). These results are inconsistent with previous research studies, possibly because the young rats were in the somatic growth phase. Circulating ALP activity changes at different stages of life and development.26 Additionally, it has been shown that the highest level of ALP is detected during periods of rapid growth in childhood, such as infancy and adolescence.27
Bone osteoblast and osteoclast activity were assessed by immunohistochemistry and RT-qPCR. The results showed that the RANKL expression was increased in young rats orally exposed to TiO2 NPs and decreased with P101 supplementation (Figure 6B, D and G). As shown in Figure 5D, the count of osteoclasts increased in the TiO2 NPs group. RANKL produced by osteoblasts reflects the activity of osteoclasts. The binding of receptor activator of nuclear factor-kappa B (RANK) to RANKL on the surface of osteoclasts leads to increased bone resorption. This result showed that TiO2 NPs increased bone resorption, which is consistent with the findings of Cheng et al.9 Additionally, we found that P101 can inhibit osteoclast activity.
Our results suggested that TiO2 NPs had adverse effects on the growth and development, and bone damage of young rats, and that these effects could be alleviated by P101 supplementation. The underlying mechanism is worthy of further exploration. Zhao et al reported that oral exposure to TiO2 NPs caused gut inflammation and barrier damage in young rats, which was associated with an imbalance in intestinal microbial homeostasis.8 Many studies have confirmed that host homeostasis may be disrupted when the gut microbiota is altered or disrupted, causing obesity, inflammatory bowel disease, rheumatoid arthritis, and many other diseases.28–30 Currently, intestinal microbiota dysregulation is recognized as one of the key factors causing osteoporosis in the host. Therefore, the toxic effects of TiO2 NPs on skeletal growth and development in young rats may be mediated by dysbiosis of the gut microbiota.
In this study, we orally administered TiO2 NPs and P101 to young rats and further assessed alterations in the gut microbiota. After 4 weeks, notable alterations were observed in both the quantity and predominant species within the intestinal microbiota among groups (Figure 7A–E). LEfSE analysis revealed that the species composition of the gut microbiota in the TiO2 NPs group changed. The abundances of Chlamydiae, Chlamydiales, Chlamydiaceae and Chlamydia increased in the TiO2 NPs group. Furthermore, the abundance of Lactobacillus was significantly lower in the TiO2 NPs group (Figure 7C–E). In this study, P101 supplementation alleviated the intestinal dysbiosis (Figure 7A and B). Our results suggest that oral administration of P101 reduced TiO2 NP-induced alterations in the gut microbial composition. Our results are in agreement with those of Zhao et al, who reported that TiO2 NPs can reduce the abundance of Lactobacillus in young rats, while probiotic supplementation alleviated the gut microbiota imbalance and increased the abundance of Lactobacillus.8
Alterations in the gut microbial composition contribute to systemic inflammation.31 Dysregulation of the gut microbiota may lead to disruption of the integrity of the epithelial barrier, allowing LPS (an outer membrane component of gram-negative bacteria) and bacterial peptides to enter the bloodstream, which triggers the activation of immune cells and leads to increased production of proinflammatory cytokines, including IL-1β.32,33 Probiotics influence many physiological processes in the host, including development and intestinal homeostasis. Nie et al reported that P101 can reduce inflammatory responses in rats.34
We evaluated the serum levels of inflammatory cytokines in young rats. The results showed that the TiO2 NPs group exhibited elevated levels of the pro-inflammatory cytokine IL-1β (Figure 3G). IL-1β, which is mainly expressed by macrophages and dendritic cells is involved in regulating systemic diseases.35 In the TiO2 NPs group, we found that the expression of IL-1β was up-regulated in the bone of young rats (Figure 6I). Extensive evidence has shown that IL-1β can promote bone resorption in alveolar bone by stimulating osteoclast formation.36–38 The OPG/RANK/RANKL system is influenced by IL-1β and is associated with the balance between osteoblasts and osteoclasts.39 This study showed that TiO2 NPs disrupted intestinal flora and increased serum IL-1β levels, which increased the expression of RANKL in bone, thereby enhancing osteoclast differentiation and function, leading to bone loss.
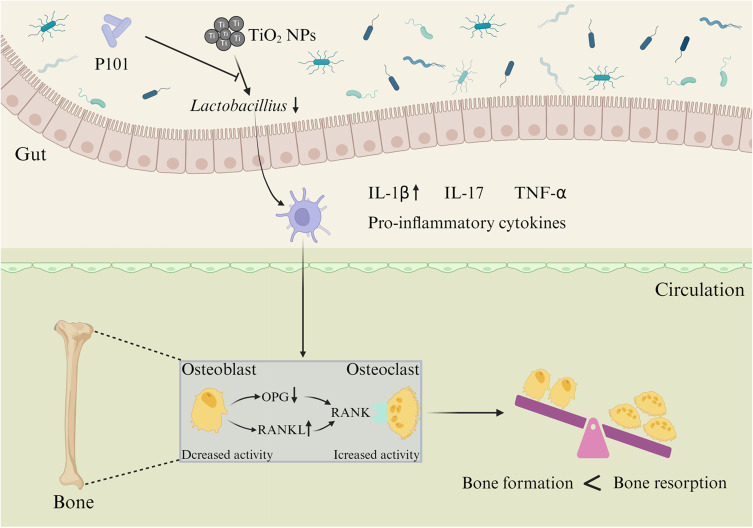
Studies have shown that probiotic therapy can inhibit the activity of osteoclasts in ovariectomy-induced mice by inhibiting IL-6 secretion and intestinal inflammatory response, thereby enhancing bone mass.40,41 In this study, we found that the number of osteoclasts was reduced in the young rats treated with P101 (Figure 5D). In addition, the results of IHC and RT-qPCR showed that OPG expression was up-regulated, while IL-1β and RANKL expression were down-regulated in the TiO2 NPs + P101 group (Figure 6C-I). Thus, our study showed that P101 treatment can reduce the expression of pro-inflammatory cytokines and inhibit osteoclast activity. In addition, we concluded that P101 alleviates the adverse effects of TiO2 NPs on bone growth and osteoporosis in young rats by restoring intestinal microbial homeostasis and inhibiting inflammation (Figure 8).
Figure 8.
P101 can alleviate the adverse effects of TiO2 NPs on bone growth and osteoporosis in young rats by restoring intestinal microbial homeostasis and inhibiting inflammation. (“↑” increase, “↓” decrease).
Conclusions
The present study investigated the effects of TiO2 NPs on skeletal system in young rats and to investigated whether P101 had a therapeutic effect on bone damage. In summary, our results indicate that TiO2 NPs reduced body weight, decreased tibia length, and damaged the bone structure in young rats. This may be due to an imbalance in the intestinal flora and an increase in systemic proinflammatory factors such as IL-1β. Moreover, P101 supplementation alleviated the bone injury and gut microbial disturbance induced by TiO2 NPs.
Funding Statement
This work was supported by the National Natural Science Foundation of China (Grant No. 82360173); and Jiangxi Province Natural Science Foundation of China-Key Program (Grant No. 20224ACB206013).
Disclosure
Lixin Xie and Lihua Feng are co-first authors for this study. The authors report no conflicts of interest in this work.
References
- 1.Peters RJB, Bouwmeester H, Gottardo S, et al. Nanomaterials for products and application in agriculture, feed and food. Trends Food Sci Technol. 2016;54:155–164. doi: 10.1016/j.tifs.2016.06.008 [DOI] [Google Scholar]
- 2.Dréno B, Alexis A, Chuberre B, Marinovich M. Safety of titanium dioxide nanoparticles in cosmetics. J Eur Acad Dermatol Venereol. 2019;33(S7):34–46. doi: 10.1111/jdv.15943 [DOI] [PubMed] [Google Scholar]
- 3.Yemmireddy VK, Hung Y-C. Selection of photocatalytic bactericidal titanium dioxide (TiO2) nanoparticles for food safety applications. LWT Food Sci Technol. 2015;61(1):1–6. doi: 10.1016/j.lwt.2014.11.043 [DOI] [Google Scholar]
- 4.Ziental D, Czarczynska-Goslinska B, Mlynarczyk DT, et al. Titanium dioxide nanoparticles: prospects and applications in medicine. Nanomaterials. 2020;10(2):387. doi: 10.3390/nano10020387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iavicoli I, Leso V, Bergamaschi A. Toxicological effects of titanium dioxide nanoparticles: a review of in vivo studies. J Nanomater. 2012;2012(1):964381. doi: 10.1155/2012/964381 [DOI] [PubMed] [Google Scholar]
- 6.Weir A, Westerhoff P, Fabricius L, Hristovski K, von Goetz N. Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol 2012;46(4):2242–2250. doi: 10.1021/es204168d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia X, Wang S, Zhou L, Sun L. The potential liver, brain, and embryo toxicity of titanium dioxide nanoparticles on mice. Nanoscale Res Lett. 2017;12(1):478. doi: 10.1186/s11671-017-2242-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y, Tang Y, Chen L, et al. Restraining the TiO(2) nanoparticles-induced intestinal inflammation mediated by gut microbiota in juvenile rats via ingestion of Lactobacillus rhamnosus GG. Ecotoxicol Environ Saf. 2020;206:111393. doi: 10.1016/j.ecoenv.2020.111393 [DOI] [PubMed] [Google Scholar]
- 9.Cheng W, Xu X, Lang Y, et al. Anatase and rutile TiO(2) nanoparticles lead effective bone damage in young rat model via the IGF-1 signaling pathway. Int J Nanomed. 2021;16:7233–7247. doi: 10.2147/ijn.S333632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma J, Zhang Z, Niu W, et al. Education, altitude, and humidity can interactively explain spatial discrepancy and predict short stature in 213,795 Chinese school children. Front Pediatr. 2019;7:425. doi: 10.3389/fped.2019.00425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranke MB. Growth and development. Bone dysplasia--a frequent cause of short stature in children. Nat Rev Endocrinol. 2014;10(6):317–318. doi: 10.1038/nrendo.2014.49 [DOI] [PubMed] [Google Scholar]
- 12.Yao L, Chen L, Chen B, et al. Toxic effects of TiO(2) NPs in the blood-milk barrier of the maternal dams and growth of offspring. Ecotoxicol Environ Saf. 2021;208:111762. doi: 10.1016/j.ecoenv.2020.111762 [DOI] [PubMed] [Google Scholar]
- 13.Olszak T, An D, Zeissig S, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336(6080):489–493. doi: 10.1126/science.1219328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill SR, Pop M, Deboy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355–1359. doi: 10.1126/science.1124234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rinninella E, Raoul P, Cintoni M, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14. doi: 10.3390/microorganisms7010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan J, Wang D, Li K, et al. Toxic effects of the food additives titanium dioxide and silica on the murine intestinal tract: mechanisms related to intestinal barrier dysfunction involved by gut microbiota. Environ Toxicol Pharmacol. 2020;80:103485. doi: 10.1016/j.etap.2020.103485 [DOI] [PubMed] [Google Scholar]
- 17.Rastogi S, Singh A. Gut microbiome and human health: exploring how the probiotic genus Lactobacillus modulate immune responses. Front Pharmacol. 2022;13:1042189. doi: 10.3389/fphar.2022.1042189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi R, Ye J, Fan H, et al. Lactobacillus plantarum LLY-606 supplementation ameliorates hyperuricemia via modulating intestinal homeostasis and relieving inflammation. Food Funct. 2023;14(12):5663–5677. doi: 10.1039/d2fo03411e [DOI] [PubMed] [Google Scholar]
- 19.Hao H, Zhang X, Tong L, et al. Effect of extracellular vesicles derived from lactobacillus plantarum Q7 on gut microbiota and ulcerative colitis in mice. Front Immunol. 2021;12:777147. doi: 10.3389/fimmu.2021.777147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y, Wang X, Wang J, et al. Lactobacillus plantarum strains as potential probiotic cultures with cholesterol-lowering activity. J Dairy Sci. 2013;96(5):2746–2753. doi: 10.3168/jds.2012-6123 [DOI] [PubMed] [Google Scholar]
- 21.Li S, Han X, Liu N, Chang J, Liu G, Hu S. Lactobacillus plantarum attenuates glucoco-rticoid-induced osteoporosis by altering the composition of rat gut microbiota and serum metabolic profile. Front Immunol. 2023;14:1285442. doi: 10.3389/fimmu.2023.1285442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chien CY, Chien YJ, Lin YH, et al. Supplementation of lactobacillus plantarum (TCI227) prevented potassium-oxonate-induced hyperuricemia in rats. Nutrients. 2022;14(22):4832. doi: 10.3390/nu14224832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.You T, Zhao Y, Liu S, Xu H. Lactiplantibacillus plantarum P101 attenuated cyclophosphamide-induced liver injury in mice by regulating the Nrf2/ARE signaling pathway. Int J Mol Sci. 2023;24(17):13424. doi: 10.3390/ijms241713424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landrigan PJ, Fuller R, Fisher S, et al. Pollution and children’s health. Sci Total Environ. 2019;650(Pt 2):2389–2394. doi: 10.1016/j.scitotenv.2018.09.375 [DOI] [PubMed] [Google Scholar]
- 25.Guo M, Liu H, Yu Y, et al. Lactobacillus rhamnosus GG ameliorates osteoporosis in ovariectomized rats by regulating the Th17/Treg balance and gut microbiota structure. Gut Microbes. 2023;15(1):2190304. doi: 10.1080/19490976.2023.2190304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crofton PM. Wheat-germ lectin affinity electrophoresis for alkaline phosphatase isoforms in children: age-dependent reference ranges and changes in liver and bone disease. Clin Chem. 1992;38(5):663–670. doi: 10.1093/clinchem/38.5.663 [DOI] [PubMed] [Google Scholar]
- 27.Fleisher GA, Eickelberg ES, Elveback LR. Alkaline phosphatase activity in the plasma of children and adolescents. Clin Chem. 1977;23(3):469–472. doi: 10.1093/clinchem/23.3.469 [DOI] [PubMed] [Google Scholar]
- 28.Greiner T, Bäckhed F. Effects of the gut microbiota on obesity and glucose homeostasis. Trends Endocrinol Metab. 2011;22(4):117–123. doi: 10.1016/j.tem.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 29.Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146(6):1489–1499. doi: 10.1053/j.gastro.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breban M. Gut microbiota and inflammatory joint diseases. Joint Bone Spine. 2016;83(6):645–649. doi: 10.1016/j.jbspin.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 31.Jamar G, Ribeiro DA, Pisani LP. High-fat or high-sugar diets as trigger inflammation in the microbiota-gut-brain axis. Crit Rev Food Sci Nutr. 2021;61(5):836–854. doi: 10.1080/10408398.2020.1747046 [DOI] [PubMed] [Google Scholar]
- 32.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–141. doi: 10.1016/j.cell.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo JY, Groer M, Dutra SVO, Sarkar A, McSkimming DI. Gut microbiota and immune system interactions. Microorganisms. 2020;8(10):1587. doi: 10.3390/microorganisms8101587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nie P, Wang M, Zhao Y, Liu S, Chen L, Xu H. Protective effect of lactobacillus rhamnosus GG on TiO(2) nanoparticles-induced oxidative stress damage in the liver of young rats. Nanomaterials. 2021;11(3):803. doi: 10.3390/nano11030803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cardoso EM, Reis C, Manzanares-Céspedes MC. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad Med. 2018;130(1):98–104. doi: 10.1080/00325481.2018.1396876 [DOI] [PubMed] [Google Scholar]
- 36.Garlet GP. Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res. 2010;89(12):1349–1363. doi: 10.1177/0022034510376402 [DOI] [PubMed] [Google Scholar]
- 37.Stashenko P, Dewhirst FE, Peros WJ, Kent RL, Ago JM. Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J Immunol. 1987;138(5):1464–1468. doi: 10.4049/jimmunol.138.5.1464 [DOI] [PubMed] [Google Scholar]
- 38.Huynh NC, Everts V, Pavasant P, Ampornaramveth RS. Interleukin-1β induces human cementoblasts to support osteoclastogenesis. Int J Oral Sci. 2017;9(12):e5. doi: 10.1038/ijos.2017.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takegami N, Akeda K, Yamada J, et al. RANK/RANKL/OPG system in the intervertebral disc. Arthritis Res Ther. 2017;19(1):121. doi: 10.1186/s13075-017-1332-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyu Z, Hu Y, Guo Y, Liu D. Modulation of bone remodeling by the gut microbiota: a new therapy for osteoporosis. Bone Res. 2023;11(1):31. doi: 10.1038/s41413-023-00264-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee CS, Kim JY, Kim BK, Lee IO, Park NH, Kim SH. Lactobacillus-fermented milk products attenuate bone loss in an experimental rat model of ovariectomy-induced post-menopausal primary osteoporosis. J Appl Microbiol. 2021;130(6):2041–2062. doi: 10.1111/jam.14852 [DOI] [PubMed] [Google Scholar]