Abstract
Background
Anxiety disorders are commonly associated with a higher risk of fatal cardiovascular diseases (CVD). Anxiety disorders lead to dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, thus weakening the key neuronal components of the autonomic nervous system (ANS) that are involved in cardiovascular functions, leading to increased cardiovascular risk.
Purpose
Impaired ANS activity, as reduced parasympathetic tone is strongly associated with an increased risk of CVD in anxiety disorders. Slow pranayama influences the ANS by activating the parasympathetic tone and deactivating the sympathetic tone in healthy volunteers and various diseased conditions. Therefore, we aimed to study the effects of slow pranayama and savasana on cardiac autonomic function tests in anxiety disorder patients.
Methods
Anxiety disorder patients (N = 140) of either sex between the age group 18 and 40 years attending the psychiatry outpatient department (OPD) in JIPMER were recruited for the study and were randomly assigned into the pranayama group and control group. The Pranayama group practised slow pranayama and savasana for 8 weeks along with routine psychiatric care, while the control group continued with routine psychiatric care only. Outcome measures were heart rate variability (HRV), baroreflex sensitivity (BRS), 30:15 ratio during lying to standing, E: I ratio during deep breathing, and ∇DBP during isometric handgrip, which were assessed before and after the intervention period.
Results
After 8 weeks in the Pranayama group, the HRV parameters showed significant improvement towards the parasympathetic domain. Also, there was a significant increase in parasympathetic reactivity with a decrease in sympathetic reactivity and significant improvement in BRS.
Conclusion
Slow pranayama and savasana practice in anxiety disorder patients as an adjunct to routine psychiatric care effectively improves cardiac autonomic function with a shift towards parasympathetic predominance, with significant improvements in cardiovascular parameters. Slow pranayamas with savasana may be incorporated into the routine care of these patients to enhance their cardiovascular health.
Keywords: Anxiety disorder, pranayama, heart rate variability, baroreflex sensitivity
Introduction
Anxiety disorders are important mood disorders commonly associated with a higher risk of fatal cardiovascular diseases (CVD), including coronary heart disease, sudden cardiac death and cerebrovascular diseases, 1 with a global prevalence of 0.9%–28.3%. 2
They are characterised by autonomic symptoms such as hot flushes, palpitations, perspirations, and tremors, in addition to emotional and cognitive symptoms. Anxiety disorders lead to dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, thus weakening the key neuronal components of the autonomic nervous system (ANS) that are involved in cardiovascular functions, leading to increased cardiovascular risk. 3 As per the polyvagal theory, the vagus nerve connects ANS function and social involvement. Increased vagal activity encourages social participation and reduces sympathetic activity that influences emotional components regulating altruistic behaviours. 4
Cardiovascular autonomic function tests are a helpful tool for diagnosing cardiac autonomic neuropathy. Heart rate variability (HRV) and baroreflex sensitivity (BRS) serve as noninvasive measures of ANS activity. Optimal cardiac health is characterised by increased variability, with lower HRV linked to CVD and mortality. Impaired ANS activity, as reduced parasympathetic tone, reflected in decreased HRV and BRS values, is strongly associated with increased risk of CVD in both psychotropic medicated and non-medicated anxiety disorders.5–9
Vagal nerve stimulation is an effective treatment for various conditions, including anxiety disorders. Slow pranayama influences the ANS by activating the parasympathetic tone and deactivating the sympathetic tone in healthy volunteers,10, 11 resulting in reduced heart rate (HR), improved mood, cognition and stress perception. However, there are a limited number of studies showing the effect of slow pranayama and savasana therapy on cardiac autonomic function in anxiety disorder. Therefore, we evaluated the effects of slow pranayama and savasana on cardiac autonomic function tests in anxiety disorder patients.
Methods
Study Design
This randomised control trial was conducted after receiving approval from the JIPMER Postgraduate Research Monitoring Committee (PGRMC) and Institute Ethics Committee (IEC) for human studies (IEC approval number: JIP/IEC/2019/402). Registration in the Clinical Trials Registry of India was done (CTRI/2021/04/033247). This study was conducted in the autonomic function testing (AFT) laboratory and cardiovascular research laboratory (CVRL) Laboratory at the Department of Physiology, JIPMER, Puducherry.
Sample Size
The sample size was calculated to be 65 in each group using the statistical procedure for comparing the two independent means in PS software version 3.1.2, with a 5% level of significance and 80% power with the unadjusted mean difference (95%CI) in LF: HF ratio in HRV between the groups is 0.124 (-0.406 to 0.653). 12 With a 20% dropout rate, it was projected to be 78 in each group.
Study Participants
A total of 140 anxiety disorder patients of either sex between the age group 18 and 40 years attending the psychiatry outpatient department (OPD) in JIPMER were recruited for the study (Figure 1) and randomly assigned into two groups. Participants were recruited from February 2019 to January 2022. Out of 67 anxiety disorder patients in the control group, 53 completed the study; 59 out of 73 anxiety disorder patients in the Pranayama group completed the study.
Figure 1. Schematic Representation of Recruitment of Cases.
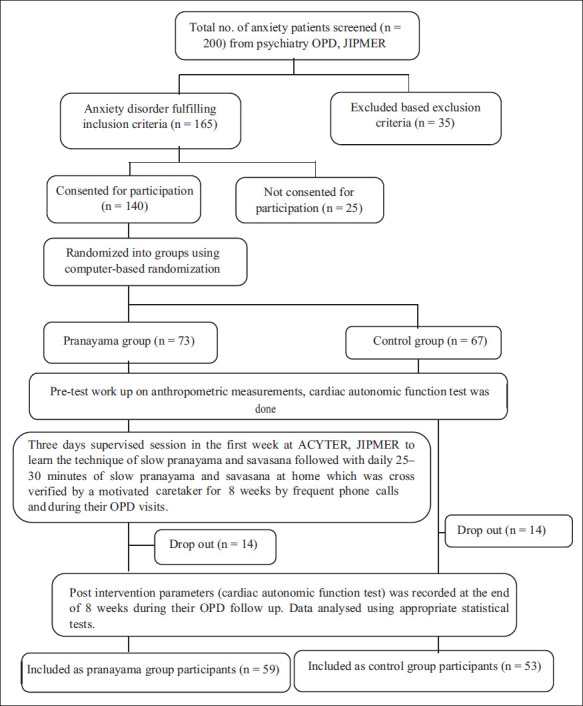
Group Identification
Two groups were studied
Pranayama group (n = 59): Patients with anxiety disorder on routine psychiatric care with slow pranayama and savasana therapy.
Control group (n = 53): Patients with anxiety disorder on routine psychiatric care.
Inclusion Criteria
Patients diagnosed with any anxiety disorders (generalised anxiety disorder, panic disorder, specific phobia, agoraphobia, unspecified anxiety disorder, other specified anxiety disorder, social anxiety disorder, anxiety disorder due to other medical conditions, substance or medication-induced anxiety disorder) based on DSM-5 criteria, who were stable with standard routine treatment for anxiety disorders from psychiatry OPD.
Age: 18–40 years.
Able to read and understand English and/or Tamil.
Willing to practice pranayama.
Both male and female.
Exclusion Criteria
Known cases of diabetes, hypertension or cardiac diseases, renal disease, other psychiatric illness, autonomic dysfunction, and pulmonary disorder.
Patients who underwent yoga therapy in the past.
Recording Procedure
After obtaining written and informed consent, participants were advised to attend the Physiology Department’s autonomic function test (AFT) laboratory with a light breakfast. They were instructed to refrain from vigorous physical activity for 12 hours; to avoid tea, caffeine products, tobacco, and alcohol for 24 hours; and to withhold anticholinergics, antihistaminics, sympathomimetic and parasympathomimetic agents if any for 12 hours before the recording. The recordings were obtained after 2 hours of light breakfast between 09:00 a.m. and 10:00 a.m. During the recordings, the temperature of the lab was maintained at 25°C. Also, external noise and disturbances were avoided to ensure a calm and quiet environment during the recording. The following parameters were recorded before and after intervention in both groups. The schematic representation of the study procedure and case recruitment is represented in Figure 1.
Recording of Anthropometric and Basal Cardiovascular Parameters
Height in centimetres was measured using a wall-mounted stadiometer (Easy careTM, NO: 26 SM), and body weight was recorded using an automated weighing machine (Charder Electronics, Taichung, Taiwan 2013). BMI was calculated using Quetelet’s index.
Heart rate (HR), systolic blood pressure (SBP), and diastolic blood pressure (DBP) were recorded after 10 minutes of rest in a supine position on a couch in the lab (room temperature maintained at 25°C) using an automated sphygmomanometer–AccuSureTM (Model no: TMB-1490-A). The arm cuff of the automatic sphygmomanometer was 12 cm in width, 44 cm in length, and the cuff tube length was 60 cm. The participants were made to sit in an upright position in an armed chair. The BP cuff was tied on the arm approximately 2 cm above the cubital fossa, with the cuff being neither too tight nor too loose. SBP, DBP and HR were recorded. Later the mean arterial pressure (MAP) and rate pressure product (RPP) were calculated.
Recording of Short-term HRV
The study participants were instructed to void before the recording and were given supine rest in the laboratory for 15 minutes. ECG electrodes were fixed on the subject and connected to the machine for lead II ECG recording for 5–10 minutes in a silent room with a room temperature of 25°C to record HRV using European Task Force guidelines. 13 During the recording, the participants were instructed to reduce body movements to a minimum.
BIOPAC MP 36 (MP36E1203002693) was used to record ECG signals during rest. BIOPAC data was transported to Windows-based BIOPAC Student Lab 4.0.0 software. The ECG signals were digitalised and saved for offline analysis on the computer. Artefacts and ectopics, which were filtered with bandpass filters, were deleted. The artefact correction was kept below 5% of total beats. From the 360-second ECG data, the programme recognised all R waves and calculated the R-R interval.
HRV Analysis
The R-R intervals for the entire recording were taken and saved in text format. The R-R interval data was analysed using Kubios version 2.0. (HRV analysis software). The software was based on Matlab and was compiled into a standalone C program using Matlab.
For HRV analysis, 360-second data from the R-R tachogram was utilised. The RR tachogram was interpolated at 2 Hz, and the fast Fourier transformation (FFT) was computed with the help of the Welch periodogram, which was used for power spectral analysis. Mean RR, Mean HR, RMSSD, SDNN, NN50, and pNN50 were time domain parameters, whereas Total Power (TP), a normalised unit of LF (LF nu), a normalised unit of HF (HF nu), and the ratio of low-frequency to high-frequency are frequency domain parameters.
Recording of HR Response to Standing
ECG electrodes were connected from the subject to the polygraph for 5 minutes in a silent room with a room temperature of 25°C after 10 minutes of supine rest. NIBP cuff was tied around the arm of the participant. The continuous ECG was obtained and monitored using BIOPAC MP-36 throughout the procedure. Resting HR and blood pressure (BP) were measured. The participants were then told to stand without assistance in 3 seconds for 5 minutes. HR response was continually obtained using the ECG. The data from the BIOPAC MP-36 system was digitalised and analysed using BIOPAC Student Lab 4.0.0 software, which allowed us to extract the values from the R-R tachogram. The extracted values were plotted on a line graph using Microsoft Excel 2007. Finally, when standing, the 30:15 ratio was computed by dividing the largest R-R interval at the 30th beat by the smallest R-R interval at the 15th beat.
Recording of HR Response to Deep Breathing
The study participants were asked to sit comfortably under controlled laboratory conditions. ECG electrodes were connected from the subject to the polygraph. The NIBP cuff was tied around the arm of the participant. The continuous ECG was obtained and monitored using BIOPAC MP-36 throughout the procedure. Resting HR and BP were measured. The participants were then instructed to take deep breaths at a rate of six breaths/minute (5 seconds for inspiration and expiration for each breath). They were instructed to give maximum effort, and not to hold their breath between inspiration and expiration. The ECG obtained during the procedure was analysed using BIOPAC Student Lab 4.0.0 software. Maximum and minimum RR interval with each cycle were recorded and plotted on a line graph using Microsoft Excel 2007, and the average was taken for six cycles. The average value of expiration and inspiration was used to calculate the E: I ratio.
Recording of BP Response to Isometric Handgrip
The study participants were instructed and demonstrated how to use a handgrip dynamometer. The participants were made to sit comfortably under controlled laboratory conditions. Maximum voluntary contraction (MVC) was determined 20 minutes before the actual procedure. The participants were requested to hold the dynamometer with their dominant hand with full strength for a few seconds, and the value was recorded. Baseline BP and HR were measured. The participants were instructed to hold the dynamometer at 30% of MVC for 3–5 minutes and inform the investigator when they could not maintain the handgrip pressure. BP and HR were measured at the 1-minute interval for 4 minutes, just before the release of handgrip pressure, and two minutes after the grip was released. ∆DBP is noted as the difference between the maximum DBP during the procedure and the resting DBP.
Recording of BRS
BRS was recorded at rest using a noninvasive, continuous finger arterial BP monitor Finapres (Finometer Pro, Finapres Medical Systems BV, Amsterdam, The Netherlands). The measurement of finger arterial pressure is developed based on the volume clamp technique of Penaz and the Physiocal criteria of Wesseling. 14 In the technique, the finger arterial pressure is reconstructed to represent brachial arterial pressure using generalised waveform inverse modelling, generalised level correction and individual height correction for the two arteries. The analysis was performed using this reconstructed brachial arterial waveform. The spontaneous oscillations of BP and interbeat intervals were used for time-domain cross-correlation analysis for determining BRS. 15 This technique of cross-correlation was validated against the gold standard technique for measuring BRS, that is, drug challenge with phenylephrine and nitroprusside. 16 The recording was taken continuously for 10 minutes in a supine posture after 10 minutes of rest in the same position. The BRS values obtained over the time interval were averaged and expressed in ms/mmHg.
Intervention
Yoga (Slow Pranayama and Savasana)
The participants of the Pranayama group were given slow pranayama and savasana along with routine psychiatric care. They were trained in the Advanced Centre for Yoga Therapy Education and Research (ACYTER) by a qualified yoga instructor for 3 days in the first week to learn the breathing techniques. Then, the participants were asked to practice those slow pranayamas and savasana at home for 30–35 minutes every day for 8 weeks, monitored by a motivated caretaker at home by maintaining a patient diary, and this was cross-checked through frequent phone calls. They were encouraged to do the same during their visit to OPD. The following schedule of yoga intervention (slow pranayama and savasana), as listed in Table 1, was given, following which post-interventional recordings were made. Slow pranayama and savasana were continued according to the participant’s wish even after the completion of the study period.
Table 1. Yoga Module for Anxiety Disorder Patients in Pranayama Group.
| S.NO | Yoga Technique | Duration | |
| 1 | Free hand exercises | 2 minutes | |
| 2 | Slow pranayama | Anulom-vilom | 5 minutes |
| Chandranadi | 5 minutes | ||
| Bhramari | 5 minutes | ||
| Sheetali | 5 minutes | ||
| 3 | Savasana | 10 minutes | |
| Total | 32 minutes | ||
Dropouts
Dropouts were not included in the analysis; only individuals who finished the study were included in the analysis. Some causes for dropouts included: social isolation during COVID, lockdown during COVID, patients whose medication was altered over these 8 weeks, and inconsistent yoga practice at home.
Statistical Analysis of Data
Continuous variables were represented as mean and standard deviation or median and range depending on the distribution of data. A normality test was carried out for continuous variables using the Kolmogorov-Smirnov (KS) sample test. The independent Student’s t-test for normally distributed variables and the Mann-Whitney U-test for non-normally distributed variables were used to compare continuous data between two groups (control and Pranayama group). Also, analysis of covariance (ANCOVA) was used to compare continuous data between two groups (control and pranayama group) by controlling the linear effect of covariate variables such as gender and BMI at the end of 8 weeks. The paired Student’s t-test for normally distributed variables and Wilcoxon’s signed-rank sum test for non-normally distributed variables were used to evaluate changes in continuous data over time (i.e., pre and post-intervention in the Pranayama group and basal and after 8 weeks recordings in the control group). All statistical analysis were done using a 5% level of significance, two-tailed significance, and a P value of <.05 was considered significant. IBM SPSS (Statistical Package for Social Sciences) version 22.0 was used for statistical analysis.
Results
The study included 140 anxiety disorder patients of both genders, with a mean age of 32.79 ± 7.28 years, randomly assigned to one of two groups: Control or Pranayama group. Out of 140 participants, 112 (control group n = 53; Pranayama group n = 59) completed the study, and their data are stated in the following Tables 2 and 3.
Table 2. Comparison of Anthropometric and Cardiovascular Parameters Between the Control and Pranayama Groups.
| Variables | Control Group(n = 53) | Pranayama Group (n = 59) | ||
| Pre | Post | Pre | Post | |
| Age | 33.87 ± 7.67 | 31.92 ± 6.86 | ||
| Height (cm) | 161.92 ± 9.415 | 162.54 ± 9.081 | ||
| Weight (kg) | 66.11 ± 15.44 | 66.55 ± 15.36 | 65.38 ± 11.55 | 63.58 ± 11.55***,↓↓↓ |
| BMI | 25.23 ± 5.73 | 25.55 ± 5.63* | 24.74 ± 4.41 | 24.19 ± 4.44***,↓↓↓ |
| HR (bpm) | 70.55 ± 7.66 | 73.38 ± 10.82* | 69.32 ± 10.38 | 64.86 ± 7.91**,↓↓↓ |
| SBP (mmHg) | 121.39 ± 13.00 | 123.81 ± 16.07 | 123.22 ± 10.08 | 116.02 ± 8.65***,↓ |
| DBP (mmHg) | 70.59 ± 8.30 | 71.18 ± 9.98 | 73.23 ± 8.10 | 68.36 ± 5.30***,↓ |
| MAP (mmHg) | 90.78 ± 9.28 | 92.26 ± 11.75 | 93.69 ± 8.28 | 87.47 ± 5.93***,↓ |
| RPP (mmHg/min) | 85.40 ± 11.28 | 90.97 ± 18.72** | 85.62 ± 15.91 | 75.39 ± 11.77***,↓↓↓ |
The data are presented as mean ± SD.
The statistical analysis was performed using the paired student t-test for intragroup analysis (pre vs. post), and the ANCOVA test for intergroup analysis (post vs. post).
The P < .05 was statistically considered significant.
***P < .001; **P < .01; *P < .05, for intragroup analysis, before and after 8 weeks.
↓- decrease. ↑↑/↓↓: P < .01, ↑↑↑/↓↓↓: P < .001 for intergroup analysis at the end of 8 weeks between the control and Pranayama groups.
BMI: body mass index; HR: heart rate; SBP: systolic blood pressure; DBP: diastolic blood pressure; MAP: mean arterial pressure; RPP: rate pressure product.
Table 3. Comparison of Cardiovascular Autonomic Function Test and BRS Values Between the Control and Pranayama Groups.
| Variables | Control Group (n = 53) | Pranayama Group (n = 59) | ||
| Pre | Post | Pre | Post | |
| Time domain indices of HRV | ||||
| Mean RR (ms)$ | 839.45 ± 123.54 | 809.87 ± 118.45 | 851.20 ± 143.08 | 885.97 ± 133.69*,↑↑ |
| SDNN (ms)# | 35.4 (22.8–47.1) | 31.4 (23.4–44.2) | 40.1 (27.1–55.0) | 51.6 (32.8–63.5)**,↑↑ |
| RMSSD (ms)# | 26.1 (13.35–38.45) | 22.6 (15.95–39.55) | 33.1 (20.3– 46.6) | 40.6 (24.6–51.9)* |
| NN50 (count)# | 25.8 (3.0–68.0) | 13.0 (3.0–68.5) | 30.0 (5.0–102.0) | 63.0 (13.0–97.0) |
| pNN50 (%)# | 8.0 (0.9–21.5) | 4.1 (1.0–19.15) | 5.7 (1.2–28.8) | 22.1 (3.5–29.8)* |
| Frequency domain indices of HRV | ||||
| Total power (ms 2 )# | 1051.0 (484.5–2138.0) | 1231.0 (537.5–1602.5) | 1553.0 (604.0–2375.0) | 2416.0 (856.0–3124.0)*,↑ |
| LF (nu)$ | 55.25 ± 20.15 | 58. 29 ± 19.83 | 53.07 ± 17.85 | 46.36 ± 18.32*,↓↓ |
| HF (nu)$ | 44.75 ± 20.15 | 41.28 ± 19.57 | 46.28 ± 17.73 | 53.65 ± 18.32*,↑↑↑ |
| LF:HF# | 1.41 (0.66–2.70) | 1.59 (0.68–2.90) | 1.2 (0.64–1.93) | 0.64 (0.43–1.19)**,↓↓↓ |
| Autonomic reactivity tests | ||||
| 30:15# | 1.17 (1.09–1.30) | 1.15 (1.07–1.35) | 1.19 (1.11–1.52) | 1.40 (1.17–1.50)**,↑↑ |
| E:I # | 1.25 (1.13–1.33) | 1.24 (1.14–1.40) | 1.25 (1.19–1.39) | 1.41 (1.23–1.48)*** |
| ∆DBP (mmHg)# | 14.0 (5.50–18.00) | 15.0 (10.0-16.0) | 15.0 (10.0–19.0) | 11.00 (4.00–15.00)***,↓ |
| BRS | ||||
| BRS | 14.12 ± 9.22 | 12.67 ± 7.15 | 13.89 ± 6.16 | 18.15 ± 6.65***,↑↑↑ |
$ - The parametric data are presented as mean± SD.
# - The non-parametric data are presented as median (range).
The statistical analysis was performed using the paired student t-test, Wilcoxon signed-rank sum test for intragroup analysis (pre vs post), and ANCOVA test for intergroup analysis (post vs post).
The P < .05 was statistically considered significant.
***P < .001; **P < .01; *P < .05, for intragroup analysis, before and after 8 weeks.
↑- increase, ↑- decrease. ↑↑↑/↓↓↓: P < .001; ↑↑/↓↓: P < .01; ↑/↓: P < .05 for intergroup analysis at the end of 8 weeks between the control group and Pranayama group.
∆DBP: diastolic blood pressure difference in isometric handgrip test; 30:15: heart rate response to standing from lying to standing; BRS: baroreflex sensitivity; E:I: expiration: inspiration; HF: high-frequency; HF nu: high-frequency in normative units; LF: low-frequency; LF: HF ratio: low-frequency: high-frequency ratio; LF nu: low-frequency in normative units; Mean HR: mean heart rate; Mean RR: mean RR interval; pNN50: percentage of NN50 intervals; NN50: consecutive NN intervals with difference more than 50 ms; RMSSD: root mean square of standard deviation; SDNN: standard deviation of NN intervals; TP: total power.
The baseline data of the control group (n = 53) and Pranayama group (n = 59) were similar in all aspects of anthropometric measurements and cardiovascular measures like HRV and CAFT without showing any significant difference (Table 2). Following 8 weeks, there was a significant decrease in SBP (P < .001), DBP (P < .001), MAP (P < .001), RPP (P < .001) and HR (P < .01) in the Pranayama group, whereas in the control group HR (P < .05) and RPP (P < .01) increased significantly.
The change in HRV, autonomic reactivity tests, and BRS values in the Pranayama group over 8 weeks is shown in Table 3. In the time domain indices of HRV, there was a significant increase in the mean RR (P < .05), SDNN (P < .01), RMSSD (P < .05), and pNN50 (P < .05) in the Pranayama group. In the frequency domain indices of HRV, there was a significant improvement in HF nu (P < .05) and TP (P < .05), with a significant reduction in LF nu (P < .05) and LF: HF ratio (P < .01) A significant rise in BRS (P < .001) was observed in the Pranayama group over 8 weeks, with no significant change in the control group.
After 8 weeks of slow Pranayama and Savasana therapy, the 30:15 ratio (P < .01) and E:I ratio (P < .001) had significantly increased, while ∆DBP (P < .001) had decreased considerably.
Tables 2 and 3 show the comparison of parameters between the Control and Pranayama groups after 8 weeks. In the time domain indices, the Pranayama group showed a significantly high mean RR (P < .01), SDNN (P < .001), RMSSD (P < .001), NN50 (P < .05), pNN50 (P < .001), whereas in the frequency domain indices, the Pranayama group showed a significantly high HF (P < .001) and TP (P < .001) with a significantly less LF (P < .01) and LF:HF ratio (P < .001). After 8 weeks, BRS (P < .001) in the Pranayama group was significantly greater than in the control group. We also observed a substantial rise in the 30:15 ratio (P < .001) and E: I ratio (P < .01), as well as a significant reduction in ∆DBP (P < .001) in autonomic reactivity tests.
To calculate the effect size, partial eta squared of ANCOVA analysis was used where eta=0.01 (small effect), eta=0.06 (medium effect) and eta=0.14 (large effect). We obtained a large effect for the parameters such as weight, BMI, HR, RPP, SDNN, LF (nu), HF (nu), LF:HF, 30:15 and BRS.
Discussion
SBP and DBP depend on the sympathetic nervous system (SNS), whereas resting HR is mainly determined by the parasympathetic nervous system (PNS). The MAP is derived as DBP+1/3 (SBP - DBP) and thus, it is determined by SNS. RPP is calculated as a product of SBP and HR divided by 100 and represents myocardial stress. Increased SBP, DBP, MAP and RPP indicate an increase in sympathetic activity17, 18 and are well-known risk factors associated with cardiovascular morbidity and mortality. 19 Following 8 weeks, the Pranayama group showed a significant fall in resting HR reflecting an increase in parasympathetic tone. Also, they showed a reduction in SBP, DBP, MAP and RPP (Table 2), indicating a decrease in sympathetic activity, which lessens the workload on the heart and increases tissue perfusion resulting in improved cardiovascular functions. Our findings were comparable with a study where slow Pranayama significantly reduced cardiovascular parameters such as HR and DBP in young healthy volunteers. 20
Resting parasympathetic activity is represented by time-domain HRV indices. 21 In the Pranayama group, there was a significant increase in mean RR, SDNN, RMSSD, and pNN50 after 8 weeks, indicating an enhanced cardiac vagal modulation. This was more apparent with the mean RR and SDNN being significantly more (P < .01) in the Pranayama group compared to the control group after 8 weeks (Table 3). Among the frequency domain indices of HRV, HF indicates resting parasympathetic activity and TP represents total vagal modulation of the heart. After 8 weeks, there was a significant rise in TP and HFnu in the Pranayama group; moreover, TP (P < .05) and HFnu (P < .001) were significantly higher in the Pranayama group compared to the control group. Thus, 8 weeks of slow pranayama and savasana therapy appears to improve the vagal drive to the heart in anxiety disorder patients and help to reduce cardiovascular risks in them as higher cardiac morbidity is linked to reduced cardiac vagal modulation. 22 Our findings are comparable to the study conducted on the effect of breathing practices on HRV among healthy adolescents and depressive women.23, 24 In addition, the Pranayama group had a significant decline in LF (P < .05) reflecting lowered resting sympathetic activity in these participants and LF was significantly less in the Pranayama group compared to the control group (P < .01). Thus, after 8 weeks of slow pranayama and savasana therapy added to routine psychiatric care, an improvement in vagal tone with attenuation in sympathetic tone was observed. The simultaneous alteration of sympathetic and vagal tone changed the sympathovagal balance, favouring a stronger parasympathetic tone, as evident from the reduced LF:HF ratio (P < .01). This was more apparent with the LF: HF being significantly less (P = .001) in the Pranayama group compared to the control group following 8 weeks. A previous study also showed that slow pranayama and savasana increased vagal activity, and reduced sympathetic activation and LF:HF in healthy subjects. 25
The 30:15 ratio and E:I ratio represent vagal reactivity; a higher ratio indicates a high vagal tone. In the isometric handgrip test, ∇DBP indicates sympathetic reactivity. 26 In the Pranayama group, there was a significant increase in the 30:15 ratio and E:I ratio with a significant decrease in ∇DBP. This was further apparent as the 30:15 ratio was more (P < .01) and ∇DBP was less (P < .05) in the Pranayama group compared to the control group after 8 weeks. Thus, 8 weeks of slow pranayama and savasana treatment in anxiety disorder patients appear to improve vagal reactivity and reduce sympathetic reactivity. Practice of savasana has been shown to decrease ∇DBP in young healthy adults. 27 Also, 3 months of yoga training was effective in improving 30:15 ratio and E: I ratio in healthy adults. 28 BRS specifies the strength of the baroreflex arc in maintaining cardiovascular homeostasis and is an indicator of vagal tone. Following 8 weeks, the Pranayama group showed a significant increase in BRS (P < .001) and it was significantly higher than the control group (P < .001) (Table 3). Our finding is in line with a study where slow breathing techniques significantly improved BRS in yoga-naive young healthy volunteers. 29 Thus, regular practice of slow pranayama and savasana strengthened the BRS and might decrease the cardiovascular risk in anxiety disorder patients as reduced BRS is an indicator of increased CV risk. 17
Slow Pranayama breathing generates inhibitory signals and hyperpolarising current by mechanically stretching tissues that initiate the synchronisation of neural elements in the central nervous system causing shifts in the autonomic balance towards parasympathetic dominance. 30 Also during the slow pranayamic breathing, the asymmetrical vector forces generated by the mechanical activity of the heart, lungs, and blood travelling throughout the circulatory system, could have a lateralisation impact on the autonomic balance augmenting the vagal tone. 31 During savasana, the complete relaxation could markedly influence cardiac autonomic modulation by changes in respiratory frequency. Thus, Savasana, by increasing the parasympathetic discharge could be decreasing the HR; and by decreasing the sympathetic discharge could be decreasing the HR and force of contraction, thereby reducing the stroke volume and cardiac output, resulting in decrease in SBP. Also, the decrease in DBP might be due to the reduced vasomotor tone that decreases peripheral resistance. 27 Thus, Slow pranayama and savasana, by improving the vagal tone and reducing the sympathetic tone, cause decreased activation of the HPA axis, thereby improving the cardiovascular parameters, including HRV. Complete relaxation during savasana could also help to bring down anxiety levels. As anxiety through activation of the limbic system-HPA axis alters these parameters, pranayama and savasana therapy works to restore the homeostasis.
Thus, the anxiety disorder patients after 8 weeks of slow pranayama and savasana practice in addition to routine psychiatric care, showed significant improvement in parasympathetic activity and reactivity, decreased sympathetic activity and reactivity, and improved sympathovagal balance.
Strength of this Study
This study included homogenous groups of anxiety disorder patients, and they were compared with no distribution bias. Also, our study could cast light on the effectiveness of slow Pranayama and Savasana therapy as an adjuvant treatment modality for anxiety disorder patients.
Limitations to this Study
A double-blinded study would have added more value. However, it was not possible to blind the participants from the yoga intervention. Also, assessment of the study’s outcome at multiple time points within the 8 weeks could have been more informative for the inference.
Conclusion
Our study demonstrates that slow pranayama and savasana practice in anxiety disorder patients as an adjunct to routine psychiatric care effectively improves cardiac autonomic function with a positive shift towards parasympathetic predominance, with significant improvements in cardiovascular parameters. Slow Pranayama with Savasana may be incorporated into the routine care of these patients to enhance their cardiovascular health.
Abbreviations
ANS, autonomic nervous system; BMI, body mass index; BRS, baroreflex sensitivity; CVD, cardiovascular disease; DBP, diastolic blood pressure; HPA, hypothalamic-pituitary axis; HRV, heart rate variability; HR, heart rate; MAP, mean arterial pressure; PNS, parasympathetic nervous system; RPP, rate pressure product; SBP: systolic blood pressure; SNS, sympathetic nervous system.
Acknowledgement
Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, for providing financial assistance in the form of an intramural MD research grant and Mr. Swaroop Ramanan, Yoga Instructor of ACYTER, JIPMER, Puducherry for providing supervised sessions of pranayama and Savasana for the study participants.
Authors Contributions
All authors are accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Dr. Pravati Pal, Dr. Gopal Krushna Pal, Dr. Balaji Bhardwaj, and Dr. Nivedita Nanda were responsible for conceptualising the research and deciding on the methodology to be followed. Dr Kavitha Natarajan was responsible for the acquisition of data, data analyses, interpretation and manuscript drafting. Dr. Pravati Pal, Dr. Gopal Krushna Pal, Dr. Balaji Bhardwaj, and Dr. Nivedita Nanda were involved in critically reviewing it for important intellectual content. Overall mentorship and approval of the final version were done by Dr. Pravati Pal.
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, for providing financial assistance through an intramural MD research grant. (JIP/ Res/ Intramural/ Sub Com/2020-21 dated 11.09.2020).
ORCID iDs: Natarajan Kavitha  https://orcid.org/0000-0003-0396-8212
https://orcid.org/0000-0003-0396-8212
Pravati Pal  https://orcid.org/0009-0003-4061-0241
https://orcid.org/0009-0003-4061-0241
Authors’ Note
During the research study, Dr. Gopal Krushna Pal held the position of Professor (Senior Scale) in the Department of Physiology at JIPMER, Puducherry. Subsequently, he transitioned to the role of Executive Director at All India Institute of Medical Sciences, Patna, in July 2022.
Data Availability Statement
The datasets generated during and/or analysed during the current study and full study protocol are/will be available upon request from the corresponding author.
Institutional Ethical Committee Approval Number
This randomised control trial was conducted after receiving approval from the JIPMER Postgraduate Research Monitoring Committee (PGRMC) and JIPMER Institutional Ethics Committee (IEC) for human studies (IEC approval number: JIP/IEC/2019/402) dated 27/01/2020. Registration in the Clinical Trials Registry of India was done (CTRI/2021/04/033247). The informed consent was obtained from all participants of the study. All methods were carried out in accordance with relevant guidelines and regulations.
ICJME Statement
The clinical trial was registered under the Clinical Trials Registry of India was done. CTRI registration number: CTRI/2021/04/033247. URL: https://ctri.nic.in/Clinicaltrials/main1.php?EncHid=73598.54201
References
- 1.Allgulander C. Anxiety as a risk factor in cardiovascular disease. Curr Opin Psychiatry 2016; 29(1): 13–17. DOI:10.1097/YCO.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 2.Baxter AJ, Scott KM, Vos T, et al. The global prevalence of anxiety disorders: a systematic review and meta-regression. Psychol Med 2013; 43: 897–910. DOI:10.1017/S003329171200147X. [DOI] [PubMed] [Google Scholar]
- 3.Hinds JA, Sanchez ER.. The role of the hypothalamus–pituitary–adrenal (HPA) axis in test-induced anxiety: assessments, physiological responses, and molecular details. Stresses 2022; 2(1):146–155. DOI:10.3390/stresses2010011. [Google Scholar]
- 4.Porges SW. Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology . 1995; 32(4): 301–318. DOI:10.1111/j.1469- 8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim K, Lee S, Kim JH.. Diminished autonomic neurocardiac function in patients with generalized anxiety disorder. Neuropsychiatr Dis Treat 2016; 12: 3111–3118. DOI:10.2147/NDT.S121533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson AA, Keren N, Lilja A, et al. Utility of baroreflex sensitivity as a marker of stress. J Cogn Eng Decis Mak 2016; 10: 167–177. DOI:10.1177/1555343416653887. [Google Scholar]
- 7.Yeragani VK, Meiri PC, Pohl R, et al. Heart rate and blood pressure changes during postural change and isometric handgrip exercise in patients with panic disorder and normal controls. Acta Psychiatr Scand 1990; 81: 9–13. DOI:10.1111/j.1600-0447. 1990.tb06441. [DOI] [PubMed] [Google Scholar]
- 8.Singh LN, Singh YG, Oinam P, et al. A study of parasympathetic function in Manipuri patients with generalized anxiety disorder. J Med Soc 2014; 28: 22–24. DOI:10.4103/0972-4958. 135220. [Google Scholar]
- 9.Chalmers JA, Quintana DS, Abbott MJ, et al. Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Front Psychiatry 2014; 5: 80. DOI:10.3389/fpsyt.2014. 00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pal GK, Agarwal A, Karthik S, et al. Slow yogic breathing through right and left nostril influences sympathovagal balance, heart rate variability, and cardiovascular risks in young adults. North Am J Med Sci 2014; 6: 145–151. DOI:10.4103/1947- 2714.128477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tyagi A, Cohen M.. Yoga and heart rate variability: a comprehensive review of the literature. Int J Yoga 2016; 9: 97–113. DOI:10.4103/0973-6131.183712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hewett ZL, Pumpa KL, Smith CA, et al. Effect of a 16-week Bikram yoga program on heart rate variability and associated cardiovascular disease risk factors in stressed and sedentary adults: a randomized controlled trial. BMC Complement Altern Med 2017; 17: 226. DOI:10.1186/s12906-017- 1740-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996; 93(5): 1043–1065. PMID: 8598068. [PubMed] [Google Scholar]
- 14.Imholz BP, Wieling W, van Montfrans GA, et al. Fifteen years experience with finger arterial pressure monitoring: assessment of the technology. Cardiovasc Res 1998; 38(3): 605–616. DOI:10.1016/s0008-6363(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 15.Westerhof BE, Gisolf J, Stok WJ, et al. Time-domain cross-correlation baroreflex sensitivity: performance on the EUROBAVAR data set. J Hypertens . 2004; 22(7): 1371-1380. DOI:10.1097/01.hjh.0000125439.28861.ed. [DOI] [PubMed] [Google Scholar]
- 16.Wesseling KH, Karemaker JM, Castiglioni P, et al. Validity and variability of xBRS: instantaneous cardiac baroreflex sensitivity. Physiol Rep 2017; 5(22): e13509. DOI:10.14814/phy2.13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pal GK, Pal P.. Autonomic nervous system. In: Comprehensive textbook of medical physiology . 2nd ed. Volume 1: New Delhi; Philadelphia: Jaypee Brothers Medical Publishers, 2016, 285–324. DOI:10.5005/jp/books/12960. [Google Scholar]
- 18.Ganong WF. Cardiovascular regulatory mechanisms. In: Review of medical physiology . 22nd ed. Singapore: The McGraw-Hill Companies, 2005, pp. 597–610. [Google Scholar]
- 19.Sesso HD, Stampfer MJ, Rosner B, et al. Systolic and diastolic blood pressure, pulse pressure, and mean arterial pressure as predictors of cardiovascular disease risk in men. Hypertension 2000; 36(5): 801–807. DOI:10.1161/01.hyp.36.5.801. [DOI] [PubMed] [Google Scholar]
- 20.Sharma VK, Trakroo M, Subramaniam V, et al. Effect of fast and slow pranayama on perceived stress and cardiovascular parameters in young health-care students. Int J Yoga 2013; 6(2): 104–110. DOI: 10.4103/0973-6131.113400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malik M, Camm AJ.. Heart rate variability and clinical cardiology. Br Heart J 1994; 71: 3–6. DOI:10.1136/hrt.71.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jandackova VK, Scholes S, Britton A, et al. Healthy lifestyle and cardiac vagal modulation over 10 years: Whitehall II cohort study. J Am Heart Assoc 2019; 8(19): e012420. DOI:10.1161/JAHA.119.012420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuppusamy M, Kamaldeen D, Pitani R, et al. Effects of yoga breathing practice on heart rate variability in healthy adolescents: a randomized controlled trial. Integr Med Res 2020; 9(1): 28–32. DOI:10.1016/j.imr.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu IH, Wu WL, Lin IM, et al. Effects of yoga on heart rate variability and depressive symptoms in women: a randomized controlled trial. J Altern Complement Med . 2017; 23: 310–316. DOI:10.1089/acm.2016.0135. [DOI] [PubMed] [Google Scholar]
- 25.Nivethitha L, Mooventhan A, Manjunath NK.. Effects of various prāṇāyāma on cardiovascular and autonomic variables. Anc Sci Life 2016; 36(2): 72–77. DOI:10.4103/asl.ASL_178_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathias CJ, Bannister R.. Autonomic failure: a text book of clinical disorders of the autonomic nervous system. In: Bannister R and Mathias CJ (eds) Investigation of autonomic disorders . Oxford: Oxford University Press, 1992, p. 266. [Google Scholar]
- 27.Sardessai SR, Pandarbale SS.. Study of effect of shavasana on handgrip and cold pressor test on heart rate and blood pressure in young adults. J Evolution Med Dent Sci 2020; 9(18): 1480–1483. DOI: 10.14260/jemds/2020/32. [Google Scholar]
- 28.Amadawala T, Rukadikar C, Deshpande D, et al. Effectiveness of yoga on Ewing’s battery autonomic function test: cross-sectional study. Int J Physiol Pathophysiol Pharmacol 2023; 15(2): 21–30. PMID: 37216172; PMCID: PMC10195213. [PMC free article] [PubMed] [Google Scholar]
- 29.Mason H, Vandoni M, Debarbieri G, et al. Cardiovascular and respiratory effect of yogic slow breathing in the yoga beginner: what is the best approach. Evid Based Complement Alternat Med 2013; 2013: 743504. DOI:10.1155/2013/74350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jerath R, Edry JW, Barnes VA, et al. Physiology of long pranayamic breathing: neural respiratory elements may provide a mechanism that explains how slow deep breathing shifts the autonomic nervous system. Med Hypotheses 2006; 67(3): 566–571. DOI:10.1016/j.mehy.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 31.Kullok S, Mayer C, Backon J, et al. Interactions between non-symmetric mechanical vector forces in the body and the autonomic nervous system: basic requirements for any mechanical technique to engender long-term improvements in autonomic function as well as in the functional efficiency of the respiratory, cardiovascular, and brain systems. Med Hypotheses 1990; 31: 91–97. DOI:10.1016/0306-9877(90)90120-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study and full study protocol are/will be available upon request from the corresponding author.


