Abstract
Background
Despite advancements in the treatment landscape for psoriasis (PsO) and psoriatic arthritis (PsA), some patients may not achieve the desired disease improvement due to undertreatment. Understanding patient perspectives on treatment expectations can inform patient-centered decisions and enhance treatment satisfaction.
Objective
To describe patient-identified treatment goals and expectations for managing psoriatic disease.
Methods
A cross-sectional study was conducted using a survey through MyPsoriasisTeam, an online social community. The survey was available to its US-based patients aged ≥21 years with self-reported diagnoses of PsO and/or PsA. The study assessed patients’ treatment goals, satisfaction with treatment outcomes, and satisfaction with health care providers (HCPs). Responses were summarized using descriptive statistics.
Results
This analysis included 386 patients (PsO, n = 130; PsA with/without PsO, n = 256). Treatment goals varied by psoriatic disease type. The top 3 treatment goals for PsO were reduce itching (73.1%), reduction in size/thickness (68.5%), and reduction in the number of plaques (63.1), and for PsA, were reducing joint pain (77.7%), lessening fatigue (64.8%), and reducing joint stiffness (62.1%). Patient satisfaction with treatment outcomes was low (extremely/very satisfied: PsO, 7.5%/8.5% and PsA, 9.2%/20.2%). Overall, 73.1% with PsO were treated by a dermatologist, and a dermatologist or rheumatologist treated 74.6% with PsA. Overall, patient satisfaction with HCPs who treated their disease was lacking (PsO, 19.3% and 19.3%; PsA, 27.3% and 33.6% were extremely and very satisfied, respectively).
Conclusion
These findings suggest the need for enhanced communication between patients and HCPs to align treatment goals and expectations and to improve treatment satisfaction and disease management.
Keywords: patient perspectives, psoriatic disease, treatment goals, burden of disease, satisfaction with health care providers
Introduction
Psoriatic disease (PsD), including psoriasis (PsO) and psoriatic arthritis (PsA), is an immune-mediated chronic condition that often requires life-long management and is characterized by systemic inflammation.1,2 Both PsO and PsA have a range of manifestations and are associated with high individual and societal burdens that lead to cumulative quality of life impairment for patients. 2
Despite available effective treatments, many patients with PsO are treated suboptimally, resulting in treatment discontinuations, poor treatment satisfaction, and an increased disease burden, causing cumulative life-course impairment.3,4 Although existing evidence-based treatment guidelines aim for higher treatment targets in PsD, there is clinical inertia among some clinicians that delays the initiation of biologic therapies in eligible patients. 5 Research has noted widespread patient dissatisfaction with current treatment options as well as lack of health care provider (HCP) visits and delay in initiating or continuing therapies because of concerns with long-term safety, administration challenges, and cost.5,6
Shared decision-making involves patients and HCPs working together to make treatment decisions based on the best available evidence and the values and preferences of patients. It also includes individualized clinical factors relating to psoriatic disease, medical comorbidities, and concomitant medication use. 7 Recent guidelines for PsO management emphasize the importance of an ongoing conversation between HCPs and patients. This approach is crucial in promoting comprehensive care, empowering patients, and enhancing their quality of life. 8 However, it is known that HCPs often have limited time to spend with their patients to discuss their disease extensively, primarily relating to comorbidities and emotional well-being associated with PsD, and develop a shared treatment plan. 9 The American Academy of Dermatology guidelines recommend tailoring treatment choices to the individual patient. 10 Therefore, educating and supporting patients with PsD is essential to communicate effectively with HCPs. This will allow them to collaboratively set treatment goals and make decisions about their care plans.11,12
MyHealthTeam online social communities provide people with specific chronic conditions a place to view educational resources, share personal stories, and converse with others suffering from the same condition. MyPsoriasisTeam, one of over 50 online communities within MyHealthTeam, is for people with PsO and/or PsA. 13
Given the significant impacts of PsD on patients’ lives and the noted dissatisfaction with current treatment options, understanding patient-identified treatment goals and expectations is crucial for better management of PsD.3,4 This study aimed to understand patients’ perspectives on managing their PsD, their satisfaction with treatment, and satisfaction with their HCPs by surveying eligible members of the MyPsoriasisTeam community.
Methods
Study Design and Participants
A non-interventional, cross-sectional study was conducted using a quantitative online survey of MyPsoriasisTeam online social community members. Members of the MyPsoriasisTeam community who were aged 21 years or older, lived in the United States, self-reported their diagnoses of PsO and/or PsA, and consented to participate in the survey were recruited. Any member who did not meet the above criteria was excluded.
The survey was programmed using Qualtrics and was developed by the sponsor and MyHealthTeam’s research team. It was comprised of 41 questions. Members of the MyPsoriasisTeam community were invited to participate in the survey via email. The email contained a link to a survey that first asked for consent to participate using IRB-approved language. Upon qualification, members were allowed to complete the survey. The questions appeared in a standard sequence, allowing members to think through and recall their treatment journey. All questions required respondents to provide an answer, and only fully completed surveys were included in the analysis. All questions included prepopulated answer choice selections and no free text option. The answers within the prepopulated choices were rotated to eliminate bias. All responses were anonymized. The study was conducted under the sponsor’s oversight, and ethical guidelines were followed. Ethical approval for this study was obtained from Advarra (MOD01420007).
Objectives
The primary objective was to describe patients’ perspectives regarding treatment expectations and goals for their PsD. Secondary objectives were to (1) describe patient perspectives regarding satisfaction with their treatments, (2) describe the burden of disease and treatment benefits, and (3) demonstrate satisfaction with their HCPs.
Additionally, patient satisfaction and shared decision-making with their treating HCPs were assessed based on the type of treatment provider (dermatologist or rheumatologist vs other HCPs).
Statistical Analyses
Descriptive statistics were used to analyze the survey responses. All data were presented as percentages of patients, and key differentiators were noted with 95% confidence intervals (CIs). When the cell count was ≤5 (either n ≤ 5 or N-n ≤ 5), the Clopper-Pearson interval was used to determine the 95% CIs for response rates. If the cell count was n > 5 and N-n > 5, CIs were calculated using the normal approximation to the binomial distribution. The findings were interpreted descriptively, as the study was not designed for hypothesis testing.
Results
Patient Characteristics
In total, 386 patients were included in the study, of which 18.1% were male, 81.9% were female, and 86.3% were non-Hispanic White/Caucasian. Among them, 130 (33.7%) reported having PsO, and 256 (66.3%) reported having PsA with or without concomitant PsO (Table 1). Most patients reported having moderate (PsO, 43.8%; PsA, 42.2%) or severe (PsO, 31.5%; PsA, 46.5%) disease.
Table 1.
Patient Characteristics.
| Psoriasis (N = 130) | Psoriatic arthritis (N = 256) | |
|---|---|---|
| Sex, n (%) | ||
| Male | 26 (20.0) | 44 (17.2) |
| Female | 104 (80.0) | 212 (82.8) |
| Age, years, n (%) | ||
| 30 to 39 | 4 (3.1) | 8 (3.1) |
| 40 to 49 | 11 (8.5) | 25 (9.8) |
| 50 to 64 | 45 (34.6) | 143 (55.9) |
| 65 to 79 | 64 (49.2) | 74 (28.9) |
| ≥80 | 6 (4.6) | 6 (2.3) |
| Self-reported disease severity, n (%) | ||
| Mild | 32 (24.6) | 29 (11.3) |
| Moderate | 57 (43.8) | 108 (42.2) |
| Severe | 41 (31.5) | 119 (46.5) |
| Ethnicity, n (%) | ||
| White/Caucasian (non-hispanic) | 107 (82.3) | 226 (88.3) |
| Hispanic or latino | 6 (4.6) | 8 (3.1) |
| Black/African/Caribbean | 9 (6.9) | 8 (3.1) |
| Native/Indigenous | 1 (0.8) | 4 (1.6) |
| Asian/Pacific islander | 3 (2.3) | 0 (0.0) |
| Other | 2 (1.5) | 5 (2.0) |
| Prefer not to answer | 2 (1.5) | 5 (2.0) |
| Education level, n (%) | ||
| ≤ High school or equivalent | 18 (13.9) | 46 (18.0) |
| Some college or associate degree | 53 (40.8) | 106 (41.4) |
| ≥ College degree | 52 (40.0) | 99 (38.7) |
| Prefer not to answer | 7 (5.4) | 5 (2.0) |
| Treated by a specialist, n (%) | ||
| Dermatologist | 95 (73.1) | 48 (18.8) |
| Rheumatologist | 4 (3.1) | 143 (55.9) |
| Primary care provider/family doctor | 13 (10.0) | 25 (9.8) |
| Internal medicine specialist | 1 (0.8) | 3 (1.2) |
| Nurse practitioner | 4 (3.1) | 5 (2.0) |
| Physician assistant | 1 (0.8) | 5 (2.0) |
| Other | 1 (0.8) | 9 (3.5) |
| I do not see a doctor for my psoriatic disease | 11 (8.5) | 18 (7.0) |
| Current treatment for psoriasis/psoriatic arthritis (select all that apply), n (%) | ||
| Topical cream (eg, corticosteroids, retinoids) | 76 (58.5) | 131 (51.2) |
| Oral steroids or immunosuppressant medications (eg, prednisone, methotrexate, cyclosporin) | 6 (4.6) | 76 (29.7) |
| Advanced oral medication | 12 (9.2) | 35 (13.7) |
| Use of biologic medication | 27 (20.8) | 142 (55.5) |
| UV Light therapy | 11 (8.5) | 13 (5.1) |
| Other | 13 (10.0) | 24 (9.4) |
| Currently not treating disease, but have in the past | 20 (15.4) | 23 (9.0) |
| I have never treated my psoriasis/psoriatic arthritis | 4 (3.1) | 5 (2.0) |
| Duration of treatment with current biologic/advanced oral medication a | N = 37 | N = 167 |
| Less than or equal to 1 year | 22 (59.5) | 81 (48.5) |
| 2 to 4 years | 8 (21.6) | 62 (37.1) |
| 5 to 7 years | 1 (2.7) | 15 (9.0) |
| 8 to 10 years | 2 (5.4) | 4 (2.4) |
| More than 10 years | 4 (10.8) | 5 (3.0) |
| Satisfaction with the appearance of skin (number, thickness, or scaling of plaques), n (%) | ||
| Extremely satisfied | 9 (6.9) | n/a |
| Very satisfied | 8 (6.2) | |
| Somewhat satisfied | 23 (17.7) | |
| Not very satisfied | 49 (37.7) | |
| Not at all satisfied | 41 (31.5) | |
| Impact of psoriasis on emotional well-being, n (%) | ||
| 5 = High impact | 44 (33.8) | 102 (39.8) |
| 4 | 47 (36.2) | 83 (32.4) |
| 3 = Neutral | 22 (16.9) | 48 (18.8) |
| 2 | 6 (4.6) | 15 (5.9) |
| 1 = No impact | 11 (8.5) | 8 (3.1) |
| Physical well-being, n (%) | ||
| 5 = High impact | 38 (29.2) | 123 (48.0) |
| 4 | 50 (38.5) | 93 (36.3) |
| 3 = Neutral | 26 (20.0) | 26 (10.2) |
| 2 | 7 (5.4) | 9 (3.5) |
| 1 = No impact | 9 (6.9) | 5 (2.0) |
| Family relationships, n (%) | ||
| 5 = High impact | 14 (10.8) | 53 (20.7) |
| 4 | 13 (10.0) | 67 (26.2) |
| 3 = Neutral | 46 (35.4) | 73 (28.5) |
| 2 | 12 (9.2) | 22 (8.6) |
| 1 = No impact | 45 (34.6) | 41 (16.0) |
| Social interactions, n (%) | ||
| 5 = High impact | 38 (29.2) | 79 (30.9) |
| 4 | 30 (23.1) | 78 (30.5) |
| 3 = Neutral | 30 (23.1) | 64 (25.0) |
| 2 | 13 (10.0) | 16 (6.3) |
| 1 = No impact | 19 (14.6) | 19 (7.4) |
aTwo patients with psoriasis and 10 patients with psoriatic arthritis were currently being treated with both biologic and advanced oral medication.
A dermatologist treated 73.1% of patients with PsO. Among patients with PsA, 18.8% were treated by a dermatologist, and 55.9% were treated by a rheumatologist.
Among the PsO population, only 9.2% used advanced oral medication, and 20.8% used a biologic medication. Of those treated with an advanced oral or biologic (n = 37), 59.5% were treated for ≤1 year. Among patients with PsA, 13.7% and 55.5% used advanced oral medication and a biologic, respectively, and of those (n = 167), 48.5% used it for ≤1 year.
Among patients with PsO, 69.2% were not very satisfied with the appearance of their skin, and 33.8% reported a high impact of PsO on their emotional well-being. Among patients with PsA, 39.8% and 48.0% reported a high impact of the disease on their emotional and physical well-being, indicating a high disease burden.
Patient Perspectives Regarding Treatment Expectations and Treatment Goals
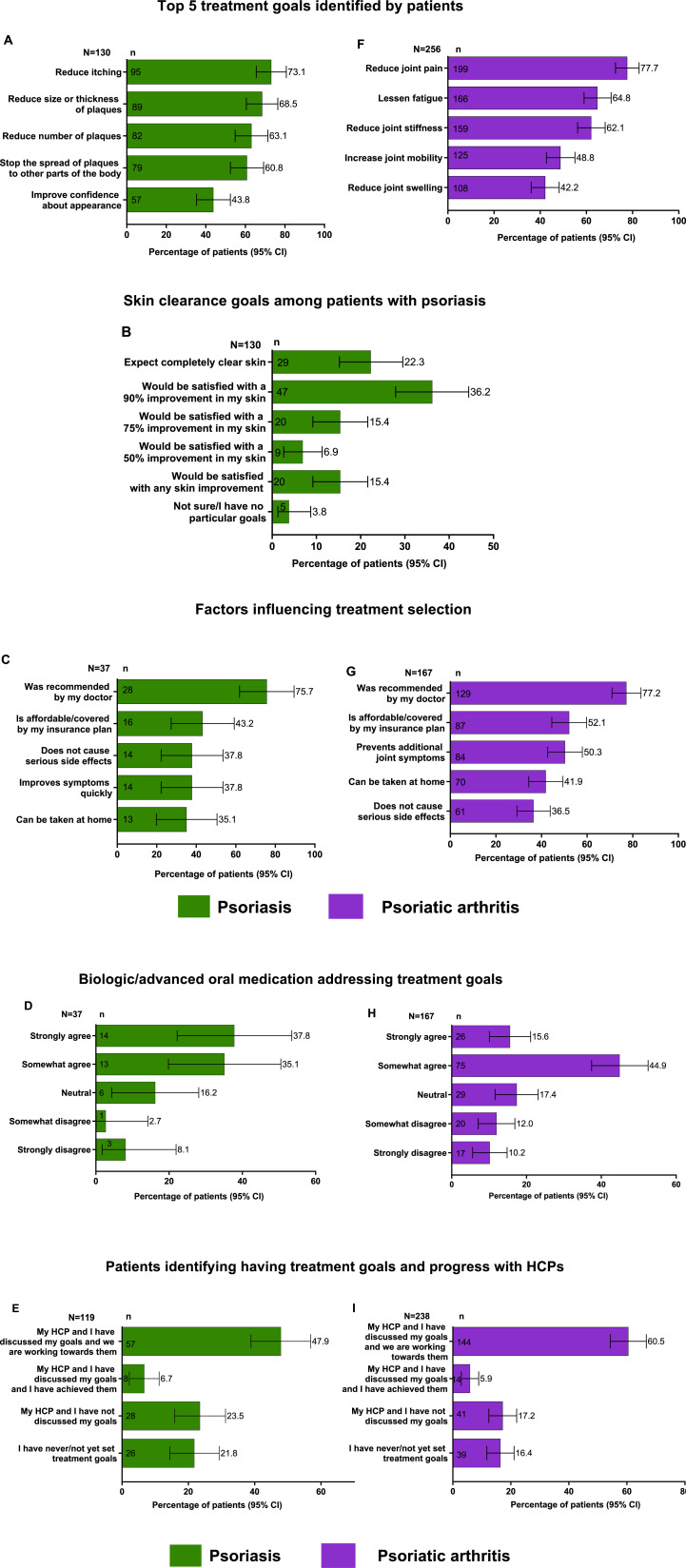
The patient’s perspectives regarding treatment goals varied by the type of PsD. Among patients with PsO, the top 3 treatment goals (Figure 1(A)) identified by patients were reducing itching (73.1%), reduction in size and thickness of plaques (68.5%), and reduction in the Number of plaques (63.1%). Notably, only 22.3% expected completely clear skin, and an additional 36.2% expected 90% clear skin as their skin clearance goals (Figure 1(B)). In the PsO population, only 37 patients were treated with an advanced oral or biologic. These patients identified the following as the top 5 factors that affected their treatment selection: recommendation from the doctor (75.7%), affordability and insurance coverage of the treatment (43.2%), potential to cause serious side effects and quick symptom relief (37.8% each), and if the treatment could be taken at home (35.1%, Figure 1(C)). Of them, 14 (37.8%) strongly agreed, and 13 (35.1%) somewhat agreed that the treatment was addressing their treatment goals (Figure 1(D)). Among patients who were being treated for PsO by an HCP (n = 119), less than half (47.9%) reported that they discussed their treatment goals with their HCPs and are working toward them, while 23.5% reported that they did not discuss their treatment goals with their HCPs, and 21.8% had never/not yet set any treatment goals (Figure 1(E)).
Figure 1.
Patient perspectives regarding treatment expectations and goals of their psoriatic disease N = number of responses; N = number of respondents; PsA, psoriatic arthritis; PsO, psoriasis (A) Top five treatment goals identified by patients with PsO; (B) Skin clearance goals in patients with PsO; (C) Factors influencing treatment selection among patients with PsO; (D) Biologic/advanced oral medications addressing treatment goals in patients with PsO; (E) Patients with PsO identifying having discussed treatment goals and progress with HCPs; (F) Top five treatment goals identified by patients with PsA; (G) Factors influencing treatment selection among patients with PsA; (H) Biologic/advanced orals addressing treatment goals in patients with PsA; (I) Patients with PsA identifying having discussed treatment goals and progress with HCPs.
Among patients with PsA, the top three treatment goals (Figure 1(F)) were to reduce joint pain (77.7%), lessen fatigue (64.8%), and reduce joint stiffness (62.1%). Among patients who were treated with an advanced oral/biologic (n = 167), the decision to start a biologic or advanced oral medication was recommended mainly by their doctor (77.2%), was affordable/covered by an insurance plan (52.1%), or if it prevented additional joint symptoms (50.3%, Figure 1(G)). Among these patients, 15.6% strongly agreed, and 44.9% somewhat agreed that their treatment addressed their goals (Figure 1(H)). Among patients who were being treated for PsA by an HCP (n = 238), 60.5% reported that they discussed their treatment goals with their HCPs and are working toward them, while 17.2% reported that they did not discuss their treatment goals with their HCPs, and 16.4% had never/not yet set any treatment goals (Figure 1(J)).
Few patients with PsO were aware that they could target nearly or completely clear skin and indicated it as their treatment goal. Among patients with PsO who were treated by a dermatologist, 36.8% were aware that they could achieve nearly clear or completely clear skin, while only 4.2% of patients treated by other HCPs were aware of the same (Figure S1A). Notably, only 26.3% of patients treated by a dermatologist expected completely clear skin as their treatment goal, while 11.4% treated by other HCPs reported that they desired this goal (Figure S1B).
Patient Satisfaction with Treatment Outcomes
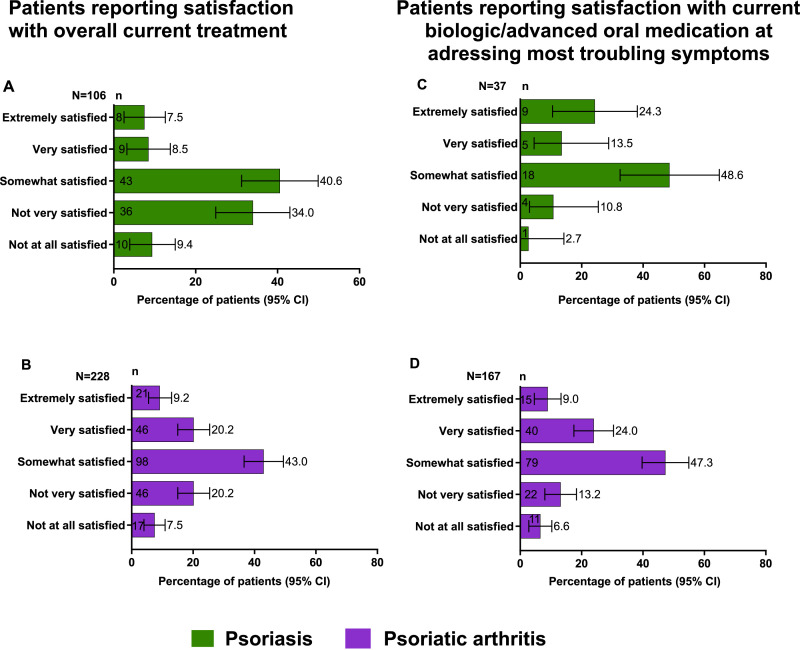
Patient satisfaction with treatment was low among both groups. Overall, 106 patients with PsO were currently being treated for their disease. Of these patients, 40.6% were somewhat satisfied with their current treatment regimen, and only 7.5% and 8.5% were either extremely satisfied and very satisfied, respectively (Figure 2(A)). Among patients who were treated with an advanced oral medication/biologic (n = 37), 24.3% and 13.5% reported that they were extremely satisfied and very satisfied, respectively (Figure 2(B)).
Figure 2.
Patient satisfaction with treatment outcomes n = number of responses; N = number of respondents; PsA, psoriatic arthritis; PsO, psoriasis (A) Patients with PsO reporting satisfaction with overall current treatment; (B) Patients with PsA reporting satisfaction with overall current treatment; (C) Patients with PsO reporting satisfaction with current biologic/advanced oral treatments at addressing most troubling symptoms; (D) Patients with PsA reporting satisfaction with current biologic/advanced oral treatments at addressing most troubling symptoms.
Among patients with PsA, 228 patients were currently treated for their disease. Of them, 9.2% were extremely satisfied, 20.2% were very satisfied, and 43.0% were somewhat satisfied with their current treatment (Figure 2(C)). Among those who received an advanced treatment (n = 167), 9.0% were extremely satisfied, and 24.0% were very satisfied with their current treatment addressing their most troubling symptoms (Figure 2(D)).
The Burden of Disease and Treatment Benefits
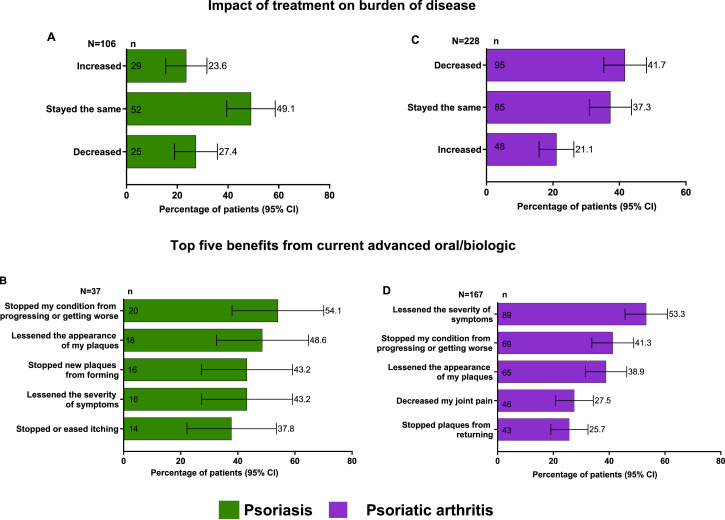
The disease burden was reported to be high in the studied population. In the PsO population, 49.1% reported that the burden of disease, ie, the impact of psoriasis on their quality of life, had remained the same since starting their current treatment. In addition, 27.4% reported a decrease in their disease burden (Figure 3(A)). Among patients who were currently being treated with biologic/advanced oral medications, the common benefits were noted as follows: stopping my condition from progressing or getting worse (54.1%), lessening the appearance of my plaques (48.6%), lessening the severity of symptoms and stopped new plaques from forming (43.2% each), and stopped or eased itching (37.8%); 64.9% reported that the treatment relieved their symptoms and has continued to work and 21.6% reported that the treatment initially relieved their symptoms but has stopped working over time (Figure 3(B)). Additionally, most patients (56.8%) did not experience any side effects. Among patients who required a change in treatment (n = 12), four patients (33.3%) reported that there were no barriers to switching treatments, while 2 patients (16.7%) reported that they did not want to deal with insurance approvals. Additionally, 10 patients (83.3%) reported that they would decide on the next medication together with their doctor.
Figure 3.
Burden of disease and treatment benefits n = number of responses; N = number of respondents; PsA, psoriatic arthritis; PsO, psoriasis (A) Impact of treatment on burden of disease among patients with PsO; (B) Top 5 benefits from current advanced oral/biologic treatments among patients with PsO; (C) Impact of treatment on burden of disease among patients with PsA; (D) Top 5 benefits from current advanced oral/biologic treatments among patients with PsA.
Among patients with PsA, 41.7% reported a decrease in disease burden with the current treatment, 37.3% reported that it remained the same, and 21.1% reported an increase in their disease burden (Figure 3(C)). Among patients who were currently being treated with biologic/advanced oral medication, the common benefits were noted as lessened disease severity (53.3%), stopped my condition from progressing or getting worse (41.3%), lessened the appearance of my plaques (38.9%), decreased joint pain (27.5%), and stopped plaques from returning (25.7%, Figure 3(D)). Almost half of the population (47.9%) did not experience any side effects. Among patients who required a change in treatment (n = 75), 45.3% reported no barriers when switching. However, 26.7% reported that they did not want to deal with getting insurance approval, 21.3% reported that they did not like the potential side effects from other medications, and 74.7% (n = 56) reported that they would decide on the next medication together with their doctor.
Patient Satisfaction With HCPs
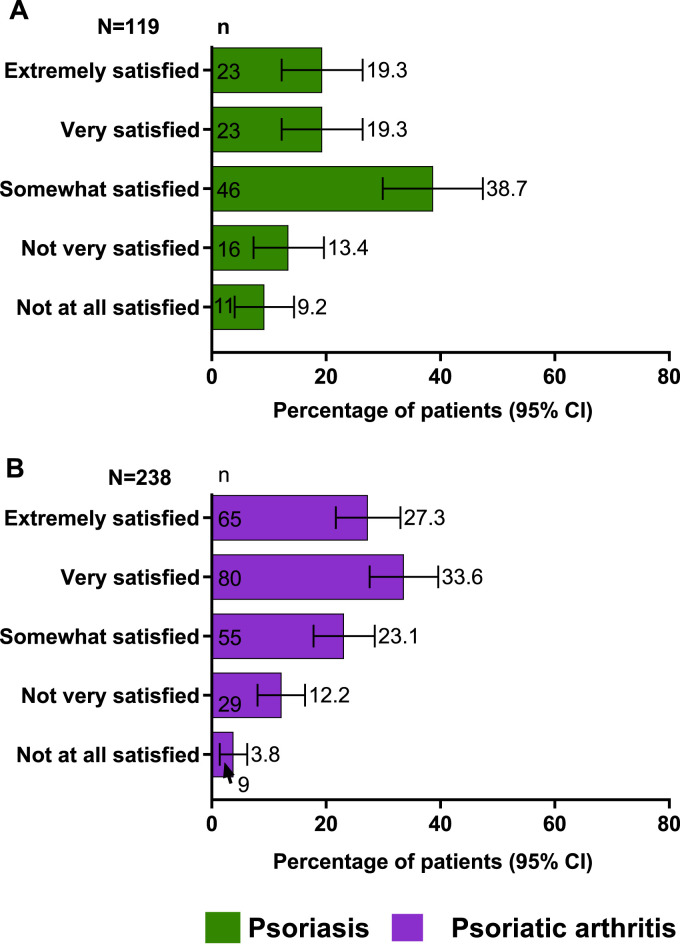
Overall, patient satisfaction with their HCPs was lacking. Among patients with PsO, only 19.3% were extremely satisfied, and another 19.3% were very satisfied with their care (Figure 4(A)). Among patients with PsA, 27.3% were extremely satisfied, and 33.6% were very satisfied with the care they received from their treating HCPs (Figure 4(B)).
Figure 4.
Patient satisfaction with HCPs: (A) among patients with PsO; (B) among patients with PsA n = number of responses; N = number of respondents; PsA, psoriatic arthritis; PsO, psoriasis.
When evaluated by the type of treatment provider, among patients with PsO, a greater proportion treated by a dermatologist were extremely satisfied and very satisfied (22.1% each, Figure S1C) with their provider in contrast to those treated by other HCPs (8.3% each, respectively). The patients treated by a dermatologist were also more likely to have discussed their treatment goals (55.8%) than those treated by other HCPs (50.0%, Figure S1D). Similarly, among patients with PsA, a greater proportion was extremely and very satisfied (29.3% and 35.1%) with their rheumatologist or dermatologist than those who were treated by other HCPs (19.1% and 27.7%, respectively, Figure S2A) and had discussed their treatment goals with their rheumatologist or dermatologist (70.2 %) than those who were treated by other HCPs (51.1%, Figure S2B).
Discussion
This cross-sectional study sought to examine patients’ treatment goals and expectations with PsO and PsA and assess their satisfaction with their current treatments and HCPs. The importance of this research lies in achieving a deep understanding of patient attitudes and preferences, which is key to achieving greater satisfaction with treatment, better medication adherence, and improved health outcomes.5,9,14
The results from our study showed that patients with PsO and PsA had different treatment goals based in part on their disease type. The National Psoriasis Foundation recommends treating to achieve complete skin clearance with less than 1% of the body covered. 10 A survey of dermatologists and rheumatologists in North America and Europe revealed that their primary goal was keeping PsO symptoms at bay, improving normal activities and patient self-esteem. However, patients surveyed prioritized reducing itching, achieving clear skin, and improving appearance. The study found that patients frequently aimed to be free of itching, consistent with previous reports.14–16 Thus, while clearance of skin lesions, important to clinicians, often correlates with symptoms of itch, the patients’ prioritization of itch was higher than that of the physical appearance of lesions, which is novel and should be noted by the clinicians. As reported in our survey, the top goals among patients with PsA were mostly similar to those in prior reports. 17 However, patients with PsA in our study population prioritized fatigue as one of their top concerns, which is often overlooked by clinician-led treatment targets.
Regarding medication use, more than half of the patients with PsO and PsA were treated with a topical agent. Overall, 30.0% of patients with PsO and 69.2% with PsA used an advanced oral or biologic medication; among those, most had been on these advanced medications for less than a year. In addition, among those taking an advanced oral/biologic treatment, few agreed that the medication was meeting their treatment goals. The treatment options for PsO and PsA have been greatly expanded in the past two decades with the introduction of numerous biologic agents in the market. The safety and efficacy of these agents have been established, and clinical practice guidelines have been advocating their use in eligible patients who do not meet treatment goals or fail prior treatments. Switching biologics is recommended if the initial treatment does not effectively clear the skin or improve joint symptoms.10,18,19
The study population experienced a substantial burden of disease. The data revealed a lack of awareness among patients with PsO regarding the possibility of achieving clear skin through treatment. Patients treated by dermatologists were more informed and had higher treatment expectations compared to those treated by other HCPs. However, only 36.8% of patients treated by dermatologists were aware of the possibility of achieving clear skin. This highlights the need for enhanced patient education and communication about attainable treatment results.
In our study, many patients did not discuss or establish treatment goals with their providers. However, more patients treated by a dermatologist for PsO and a rheumatologist or dermatologist for PsA reported having discussed and established treatment goals. This highlighted a disparity between patients treated by a specialist and those treated by other HCPs. Patients receiving care from specialist HCPs such as dermatologists or rheumatologists reported a more favorable health care experience, highlighting the importance of specialized care in effectively managing PsO and PsA, as these specialized professionals may possess a deeper understanding of the conditions and the various treatment options available.
Additionally, the study examined the factors influencing the decision to start biologic or oral therapy and found that an HCP recommendation played a critical role, reinforcing the requirement for these patients to consult a specialist: a dermatologist for PsO, and a dermatologist and/or a rheumatologist for PsA. Furthermore, most patients reported no barriers to switching treatments, further emphasizing the necessity for enhanced communication between patients and HCPs regarding treatment choices.
Overall satisfaction with HCPs was lacking in our surveyed study population, similar to other reports indicating room for improvement in the care provided to patients with PsD.4,15 Standardized consultations have been shown to be inadequate for addressing the individual challenges and health care needs of patients with PsD. To adopt a more patient-centered approach, health professionals should make minor structural changes to their clinical services, discuss emotional well-being and concerns beyond biomedical factors, and offer personalized health education. 20
The limitations of this study include convenience sampling from social media. There is a potential for selection bias in this study, as patients who are dissatisfied with their treatment outcomes are more likely to seek support in online groups, while patients with clear skin may not see the need or benefits of joining such groups. The findings may not be generalizable to the broader patient population and recall bias cannot be excluded. The study is descriptive and was not for hypothesis testing, so the findings should be interpreted accordingly and within the context of the overall literature.
In conclusion, the study highlighted the need for improved communication between patients and HCPs to improve patient awareness of available treatment options and align management decisions to self-identified treatment goals. Enhanced communication and shared decision-making strategies may be used to improve treatment satisfaction and disease management. Patients with PsO and PsA should optimally seek care from a trained specialist HCP: a dermatologist for PsO, and a dermatologist or rheumatologist for PsA. Future research should focus on developing and evaluating these strategies and exploring potential barriers to their implementation in clinical practice.
Supplemental Material
Supplemental Material for Patient-Identified Treatment Goals for Psoriatic Disease: Results From a US Patient Survey by April Armstrong, Noah A. Levit, MD, Beth Schneider, Richard Seiden, Linyu Shi, Blair Kaplan, and Jashin J. Wu in Journal of Psoriasis and Psoriatic Arthritis®
Acknowledgements
Medical writing support was provided by Dalia Majumdar, PhD, and editorial support by Angela T. Hadsell, BA, both employees of AbbVie.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: has received research grants and personal fees from BMS, Eli Lilly, Janssen, Leo Pharma, and Novartis; personal fees from Boehringer Ingelheim/Parexel, Celgene, Dermavant, Genentech, GlaxoSmithKline, Menlo Therapeutics, Merck, Modernizing Medicine, Ortho Dermatologics, Pfizer, Regeneron, Sanofi Genzyme, Science 37, Sun Pharma, and Valeant; and grants from Dermira, Kyowa Hakko Kirin, and UCB.
NL has received honoraria from AbbVie, Eli Lilly, Incyte, Pfizer, Bristol Myers Squibb, Arcutis, Regeneron, and Sanofi and participated in a speakers’ bureau for Eli Lilly, Regeneron, and Sanofi.
BS is an employee of MyHealthTeam and has received research grants from AbbVie.
RS: has been diagnosed with PsO and PsA and has served on the Board of Directors of the National Psoriasis Foundation.
BK and LS are full-time employees of AbbVie Inc. And may hold AbbVie stock.
JJW is or has been an investigator, consultant, or speaker for AbbVie, Almirall, Amgen, Arcutis, Aristea Therapeutics, Bausch Health (Ortho Dermatologics), Boehringer Ingelheim, BMS, Dermavant, Dr Reddy’s Laboratories, Eli Lilly, Galderma, Janssen, LEO Pharma, Mindera, Novartis, Regeneron, Sanofi Genzyme, Solius, Sun Pharmaceutical, UCB, and Zerigo Health Funding The author(s) received no financial support for the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the AbbVie provided financial support for the study and participated in the design, study conduct, analysis, and interpretation of the data, as well as in the writing, review, and approval of this publication. All authors had access to relevant data and participated in the drafting, review, and approval of this manuscript. No honoraria or payments were made for authorship.
Supplemental Material: Supplemental material for this article is available online.
Ethical Statement
Ethical Approval
Ethical approval for this study was obtained from Advarra (MOD01420007).
Consent
All participants provided written informed consent.
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal, Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://vivli.org/ourmember/abbvie/ then select “Home”.*
References
- 1.Augustin M. Cumulative life course impairment: identifying patients at risk. Curr Probl Dermatol. 2013;44:74-81. doi: 10.1159/000350555 [DOI] [PubMed] [Google Scholar]
- 2.Boehncke WH, Menter A. Burden of disease: psoriasis and psoriatic arthritis. Am J Clin Dermatol. 2013;14(5):377-388. doi: 10.1007/s40257-013-0032-x [DOI] [PubMed] [Google Scholar]
- 3.Armstrong AW, Robertson AD, Wu J, Schupp C, Lebwohl MG. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the national psoriasis foundation surveys, 2003-2011. Jama Dermatol. 2013;149(10):1180-1185. doi: 10.1001/jamadermatol.2013.5264 [DOI] [PubMed] [Google Scholar]
- 4.Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based multinational assessment of psoriasis and psoriatic arthritis survey. J Am Acad Dermatol. 2014;70(5):871-881. doi: 10.1016/j.jaad.2013.12.018 [DOI] [PubMed] [Google Scholar]
- 5.Strober BE, Walt JM, Armstrong AW, et al. Clinical goals and barriers to effective psoriasis care. Dermatol Ther. 2019;9(1):5-18. doi: 10.1007/s13555-018-0279-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lebwohl MG, Kavanaugh A, Armstrong AW, Van Voorhees AS. US perspectives in the management of psoriasis and psoriatic arthritis: patient and physician results from the population-based multinational assessment of psoriasis and psoriatic arthritis (MAPP) survey. Am J Clin Dermatol. 2016;17(1):87-97. doi: 10.1007/s40257-015-0169-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Légaré F, Adekpedjou R, Stacey D, et al. Interventions for increasing the use of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2018;7(7):CD006732. doi: 10.1002/14651858.cd006732.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80(4):1073-1113. doi: 10.1016/j.jaad.2018.11.058 [DOI] [PubMed] [Google Scholar]
- 9.Lebwohl M, Langley RG, Paul C, et al. Evolution of patient perceptions of psoriatic disease: results from the understanding psoriatic disease leveraging insights for treatment (UPLIFT) survey. Dermatol Ther. 2022;12(1):61-78. doi: 10.1007/s13555-021-00635-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029-1072. doi: 10.1016/j.jaad.2018.11.057 [DOI] [PubMed] [Google Scholar]
- 11.Sommer R, Augustin M. How to improve people-centred healthcare in dermatology? - medical Conferences. In: Proceedings of the 6th IFPA WPPA Conference; 2023. https://conferences.medicom-publishers.com/proceedings/33600/. Accessed 7 December 2023. [Google Scholar]
- 12.Vermeulen FM, Kraaij GEVD, Tupker RA, et al. Towards more shared decision making in dermatology: development of evidence-based decision cards for psoriasis and atopic eczema treatments. Acta Derm Venereol. 2020;100(19):5863. doi: 10.2340/00015555-3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Psoriasis and Psoriatic Arthritis Support Online . Psoriasis and psoriatic arthritis social network - MyPsoriasisTeam. https://www.mypsoriasisteam.com/. Accessed 11 December 2023.
- 14.Armstrong A, Edson-Heredia E, Zhu B, et al. Treatment goals for psoriasis as measured by patient benefit index: results of a national psoriasis foundation survey. Adv Ther. 2022;39(6):2657-2667. doi: 10.1007/s12325-022-02124-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong AW, Bohannan B, Mburu S, et al. Patient perspectives on psoriatic disease burden: results from the global psoriasis and beyond survey. Dermatology. 2023;239(4):621-634. doi: 10.1159/000528945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorelick J, Shrom D, Sikand K, et al. Understanding treatment preferences in patients with moderate to severe plaque psoriasis in the USA: results from a cross-sectional patient survey. Dermatol Ther. 2019;9(4):785-797. doi: 10.1007/s13555-019-00334-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogdie A, Husni ME, Bush K, et al. AB0769 Patient identified treatment goals in psoriatic arthritis: decreasing pain and increasing activity level are high priorities | Annals of the Rheumatic Diseases. ARD (Ann Rheum Dis). 2019. https://ard.bmj.com/content/78/Suppl_2/1853 [Google Scholar]
- 18.Brownstone ND, Hong J, Mosca M, et al. Biologic treatments of psoriasis: an update for the clinician. Biologics. 2021;15:39-51. doi: 10.2147/btt.s252578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merola JF, Lockshin B, Mody EA. Switching biologics in the treatment of psoriatic arthritis. Semin Arthritis Rheum. 2017;47(1):29-37. doi: 10.1016/j.semarthrit.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 20.Khoury LR, Skov L, Møller T. Facing the dilemma of patient‐centred psoriasis care: a qualitative study identifying patient needs in dermatological outpatient clinics. Br J Dermatol. 2017;177(2):436-444. doi: 10.1111/bjd.15292 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Patient-Identified Treatment Goals for Psoriatic Disease: Results From a US Patient Survey by April Armstrong, Noah A. Levit, MD, Beth Schneider, Richard Seiden, Linyu Shi, Blair Kaplan, and Jashin J. Wu in Journal of Psoriasis and Psoriatic Arthritis®
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal, Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://vivli.org/ourmember/abbvie/ then select “Home”.*