Abstract
Developing instant detection systems with disease diagnostic capabilities holds immense importance for remote or resource-limited areas. However, the task of creating these systems—which are simultaneously easy to operate, rapid in detection, and cost-effective—remains a challenge. In this study, we present a compact highly sensitive photothermal reverse transcriptase–loop-mediated isothermal amplification (RT-LAMP) chip (SPRC) designed for the detection of multiple diseases. The nucleic acid (NA) amplification on the chip is achieved through LAMP driven by either LED illumination or simple sunlight focusing. SPRC performs sample addition and amplification within a limited volume and autonomous enrichment of NA during the sample addition process, achieving a limit of detection (LOD) as low as 0.2 copies per microliter. Through 120 clinical samples, we achieved an accuracy of 95%, with a specificity exceeding 97.5%. Overall, SPRC has achieved promising progress in the application of point-of-care testing (POCT) by using light energy to simultaneously detect multiple diseases.
A photothermal compact chip capable of sample processing and amplification enables simultaneous detection of multiple diseases.
INTRODUCTION
Early, rapid, and precise disease diagnosis in resource-limited environments is pivotal for curbing virus transmission and enhancing treatment efficacy (1). The 2019 coronavirus (COVID-19) pandemic underscored the constraints of centralized diagnostic systems, characterized by prolonged testing times and excessive reliance on specialized facilities and personnel, particularly in regions lacking sophisticated medical infrastructure (2, 3). Consequently, there arises a pressing imperative to develop a point-of-care testing (POCT) platform that embodies simplicity in operation, rapidity in detection, affordability, as well as high sensitivity and specificity (4, 5). Regrettably, these comprehensive diagnostic solutions remain scarce. While lateral flow immunoassays offer rapid and economical testing, they often compromise on sensitivity and specificity (6, 7). Polymerase chain reaction (PCR), hailed as the benchmark for virus diagnosis (8), demands extensive analytical apparatus and skilled personnel, thereby limiting its accessibility (9, 10). As alternatives, various nucleic acid (NA) amplification methods for disease diagnosis have emerged, including loop-mediated isothermal amplification (LAMP) (11–14), recombinase polymerase amplification (15–17), and rolling circle amplification (RCA) (18, 19). Among these, LAMP stands out for its utilization of six primers, robust impurity tolerance, independence from costly detection instruments, and shorter detection times (20, 21). However, rapid response also comes with some drawbacks, necessitating careful attention to avoid contamination during DNA preparation and mixing of reaction components to prevent false-positive results (22). In addition, for nasopharyngeal swab samples, which contain abundant DNA from oral microbiota or food, nonspecific amplification can occur. This can be mitigated by adding an acid-enhanced enzyme, namely, Tte UvrD helicase, to the system to optimize reaction outcomes (23, 24).
NA amplification conventionally takes place in thermal cyclers, posing a challenge for integrating LAMP into POCT devices due to their high-power consumption. Therefore, photothermal heating systems have emerged, replacing Peltier elements with light sources such as lasers or light-emitting diodes (LEDs) for heating purposes (25). Recently, several plasmonic photothermal systems leveraging gold nanostructures have surfaced, generating heat via the coherent oscillation of electrons, thereby enabling swift photothermal heating (26–29). Similarly, the envelopment of particles or films with carbon-based materials can induce heating through electron-phonon coupling-induced atomic lattice vibration (30–32). Nonetheless, the intricate preparation procedures of these materials, coupled with the necessity for high-power laser equipment and challenges related to nanoparticle aggregation, pose substantial hurdles. As a viable alternative to nanoparticles, gold (Au) thin films offer a straightforward preparation process and rapid photothermal conversion when coupled with LEDs, making them a convenient choice for POCT applications (33–35).
Despite the advantages of LAMP, there remain several areas requiring refinement for its application in POCT (36). To enhance user-friendliness, it is imperative to streamline sample processing and detection procedures (8), minimizing the need for complex operations such as NA extraction and multiplex sampling (37). Traditional NA extraction methods, including magnetic beads and adsorption columns, inherently compromise sensitivity due to limitations in elution volume (38–40). By optimizing methods such as surface modification with oligonucleotides (23) or adhering porous structures (41), capture efficiency can be greatly improved. Recent studies on NA enrichment have focused on using electrostatic forces between charges to attract NA molecules (42–44). Chitosan, renowned for its biocompatibility and cost effectiveness, has garnered widespread utilization in this regard (45, 46). Under acidic conditions, the primary amines of chitosan [pKa (where Ka is the acid dissociation constant) = 6.2 to 7.0] become positively charged, facilitating their binding with negatively charged NA molecules (47, 48). In addition, chitosan oligosaccharide, a degradation product of chitosan, has gained prominence due to its facile water solubility and low-molecular weight. The modification of filter paper with chitosan oligosaccharide increases the surface area to volume ratio, thereby enhancing NA adsorption capacity (49). Another formidable challenge in the development of “sample-in, result-out” POCT devices is multiplex detection (50), given the intricacies of the sampling process. Consequently, simultaneous detection of multiple pathogenic targets within a sample holds paramount importance (51). Recent studies have unveiled various multiplex detection platforms capable of discerning between two or more target genes in a single reaction (52, 53). However, these methodologies often require additional equipment (table S1) and impose rigorous demands on primer design. Hence, there exists an urgent need to devise multitarget detection strategies tailored for POCT applications (54).
Here, we introduce a compact highly sensitive photothermal reverse transcriptase–LAMP (RT-LAMP) chip (SPRC) designed for the concurrent detection of multiple diseases. SPRC facilitates on-chip NA amplification via LED-driven photothermal conversion or straightforward sunlight focusing, making it highly adaptable for deployment in resource-limited regions. Combining all essential features of the POCT system, SPRC aligns with the stringent requirements outlined by the World Health Organization (table S2). Within the confined space of the chip, SPRC achieves regional division of sample addition and amplification simultaneously, facilitating efficient NA enrichment (approximately 350×) and sample aliquots during the loading process. With a rapid detection time of just 30 min, SPRC offers the capability to identify multiple diseases affordably and with high sensitivity. The per-reaction detection cost stands at approximately $1.62, achieving a limit of detection (LOD) as low as 0.2 copies/μl. To enhance usability, we have developed a compact device explicitly tailored for LED illumination and sunlight focusing, further streamlining SPRC’s operational workflow. Using λ DNA, we have characterized the fundamental properties of SPRC and successfully demonstrated its ability to simultaneously detect hepatitis B virus (HBV), hepatitis C virus (HCV), influenza A virus (IAV), and HIV on one single chip across 80 clinical serum samples (320 tests in total). This comprehensive evaluation yielded exceptional accuracy (95%) and specificity (98.75%). Iqn addition, we conducted nasopharyngeal swab testing to demonstrate the multi-type sample handling capability of SPRC. In summary, SPRC integrates principles of optics, chemistry, biology, and thermodynamics, harnessing light energy for the concurrent detection of multiple diseases. Its versatility and efficacy render SPRC well-suited for widespread adoption in the POCT domain.
RESULTS
Structure and function of SPRC
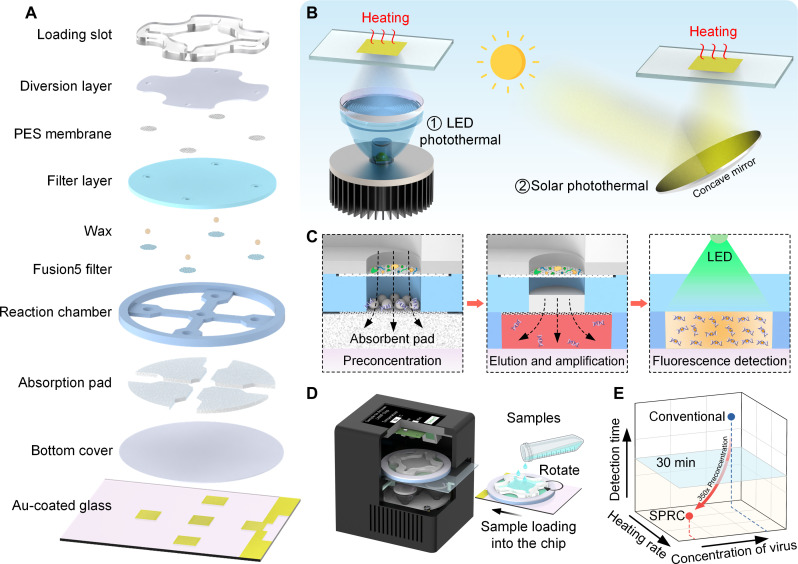
Figure 1A displays an exploded view of the SPRC. The diversion layer is used to evenly distribute the solution from the loading slot to each reaction chamber, enabling multiplex detection. The filter layer, made up of two different types of filter paper [polyethersulfone (PES) membrane and Fusion5 filter paper] and paraffin particles, is primarily used to filter impurities and capture NAs in the solution. After passing through the diversion layer and filter layer, the remaining solution is absorbed by the absorption pad. Then, by manually rotating the loading slot, the filter papers enriched with NAs can be moved directly above the respective reaction chamber for on-chip elution and amplification by bottom heating.
Fig. 1. Schematic illustrations and working principle of sensitive photothermal RT-LAMP chip (SPRC).
(A) Structural explosion view of SPRC. (B) Heating methods using LED irradiation and sunlight focusing. (C) Workflow of SPRC. (D) Compact unit for easy use of SPRC. (E) Comparison of our work with conventional PCR methods. PES, polyethersulfone.
The heating function is achieved by patterned Au-plated glass, allowing each reaction chamber to be heated individually during the heating process rather than heating the entire glass, thereby improving heating efficiency and avoiding resource waste (fig. S1). Because the interaction between the Au film and DNA polymerase significantly affects the amplification process (55), we separate them by adding a bottom cover plate, facilitating the reutilization of Au-plated glass, and providing an economic approach. When photons irradiate the Au film surface, localized surface plasmon resonance is excited, simultaneously elevating the film surface electrons to a high-energy state, resulting in the generation of hot electrons. The whole transition is typically completed within femtoseconds (56). Subsequently, the heat from the hot electrons rapidly disperses within the Au film, ultimately achieving a uniform heat distribution. Figure 1B shows the photothermal conversion process driven by LED and sunlight. The working principle of the SPRC is illustrated in Fig. 1C. The pre-enrichment of NAs is achieved while loading the sample, followed by on-chip NA elution and LAMP. Last, the amplified products are detected by fluorescence detection. For the operability of the SPRC, we developed a compact device (Fig. 1D), which can automatically extract, amplify, and detect NA, only requires transferring the lysed sample to the loading slot. Compared to conventional LAMP, the SPRC can achieve about 350× preconcentration, allowing for accurate detection results within 30 min even for low-concentration samples (Fig. 1E).
Photothermal and filter paper modification
The plasmonic photothermal conversion of the Au, which is determined by the thickness of the film, was calculated through numerical simulations. We calculated the electromagnetic field distribution of Au films with thicknesses of 10, 80, and 150 nm on a glass substrate. When the wavelength of the incident light is 465 nm, the 10-nm-thick Au film transmits a large amount of electromagnetic energy (Fig. 2A, a), and its resistive heating saturates along the thickness direction of the film (fig. S2A). As the thickness of the film increases, more and more incident light is absorbed (Fig. 2A, b). When the film thickness reaches 150 nm, almost no electromagnetic waves can pass through the film (Fig. 2A, c), generating a large amount of heat inside the film (fig. S2B). From a macroscopic perspective, the Au film with a thickness of 120 nm is almost opaque, and the absorbed light energy is emitted in the form of heat (fig. S2C). As the increase in thickness of the Au film, the absorption rate of incident light also increases. When the film thickness reaches 120 nm, the absorption of incident light saturates (fig. S3). On the other hand, the maximum absorbance is at 496 nm, but due to the limitations of commercial LEDs, we chose a blue LED with a wavelength of 465 nm as the light source for SPRC.
Fig. 2. Au film photothermal and filter paper modification.
(A) Electromagnetic field distribution of the Au film with a thickness from 10 to 120 nm. (B) Highest temperatures of Au films with different thicknesses (10 to 150 nm) after being irradiated by LEDs of different powers (characterizing by current: 300 to 1500 mA for 3 min). (C) Heating rate and resistance change rate of a combination of the Au film with different sizes and planar condensers with different angles. (D) Partial enlarged images of sampling and amplification positions to show the working process. (E) Process of modifying chitosan oligosaccharides on the surface of Fusion5. (F) Capture efficiency of the DNA with the input amounts of 0.5, 1, 2, 4, and 8 ng. (G) Fluorescence images of the particle filtration for each filter layer. (H) Adsorption efficiency of DNA samples with concentrations of 1, 100, and 10 pM on the surface of Fusion5. a.u., arbitrary units.
To verify the impact of the light source on the heating performance, we conducted related experiments (fig. S4), and the results also showed that the wavelength of 465 nm provides the best heating effect on the Au thin films. For the testing experiment analyzing the effect of light intensity and thickness of the Au layer on heat absorption, Fig. 2B summarizes the highest temperature as the evaluation results. Increasing the thickness beyond 120 nm will not yield significant enhancement in the photothermal effect. In addition, heat dissipation from the LED emerges as a notable issue with the escalation of its driving current. Consequently, we opted to use a 120-nm Au film for photothermal conversion under 1000-mA LED irradiation. Because of the rapid internal heat conduction in Au film, the generated heat quickly dissipates into the surrounding environment when the film area is very large. Therefore, it is also necessary to explore the effect of the Au film area on the heating performance. To monitor the surface temperature during the heating process, we patterned the Au thin film into an “S” shape layout (fig. S5, A and B). Because the resistance of most metals increases linearly with temperature, thin film resistors can reflect the surface temperature without the need for additional thermocouples or other temperature sensors. To focus the light on the Au film, a lens is typically used to focus the light. We explored the impact of commercial lenses and combined lenses on the heating effect (fig. S6), and the results proved that our combination method has a better focusing effect and can achieve faster heating. For a faster heating rate and higher resistance change rate of the designed Au film, we compared the impact of different sizes of Au film and different angles of planar condensers on the heating effect (Fig. 2C and fig. S7). On the basis of the comprehensive heating rate and resistance change rate, we ultimately selected a size of L = 8 mm and S = 20 μm for the Au film pattern in the SPRC.
There are two different modes for the SPRC, namely, the sampling mode and the amplification mode (Fig. 2D). To better facilitate NA adsorption, we adopted a dual-layer filter paper scheme. The upper layer uses a PES membrane to filter out impurities from the sample while controlling the flow rate. The lower layer is made of chitosan oligosaccharide-modified Fusion5 filter paper, used for adsorbing NAs (Fig. 2E). Chitosan oligosaccharides are degradation products of chitosan, which are much cheaper and have similar pKa values (approximately 6.3 to 6.5) to chitosan. The results in figs. S8 and S9 show that chitosan oligosaccharide is successfully modified onto Fusion5 and can achieve the adsorption of NAs. We compared the effects of different conditions and chitosan concentrations on DNA capture efficiency (fig. S10), and the results showed that filter paper modified with chitosan oligosaccharides after silanization had better capture capability, and the concentration of chitosan oligosaccharides had little effect on DNA adsorption. The captured DNA on the filter paper gradually tends to saturate as the DNA content in the sample increases while still maintaining a capture efficiency of more than 70% for an 8 ng of sample input (Fig. 2F). Figure 2G displays the fluorescence images of each layer, proving that larger impurity particles are filtered out by the upper PES membrane, while NAs are captured only in the lower layer, and no impurity particles or NAs were observed in the absorption pad. The modified Fusion5 filter paper can capture up to 1 ml of NAs at a concentration of 1 nM, which is sufficient for various clinical samples (Fig. 2H).
On-chip elution and performance testing
The flow rate of the sample is closely related to the pore size of the PES membrane, and their relations were tested in Fig. 3A. It can be seen that the flow rate of the solution gradually increases with the increase of the membrane pore size, and the flow rate significantly affects the DNA capture efficiency (fig. S11A). Because chitosan oligosaccharides can only adsorb NAs under acidic conditions, which can inhibit the LAMP reaction, it is necessary to rinse with deionized (DI) water after NA adsorption. We found that compared to the flow rate, the volume of rinsing has a greater impact on the DNA capture efficiency (Fig. 3B and fig. S11B). Within a certain range, as the volume of rinsing increases, the inhibitory impurities remaining on Fusion5 markedly decrease without notably affecting the adsorption of NAs. However, the capacity of the absorbent pad on the chip is limited, with each pad able to absorb about 500 μl of liquid, making the volume ratio of the sample solution and rinse solution particularly important. To adsorb as many NAs as possible without overly affecting the amplification effect, we treated each filter paper with 350 μl of sample solution and rinsed with 150 μl of DI water. Integrating the above experimental results, we explored the flow rate and DNA capture efficiency under different pore sizes (Fig. 3C). Despite the 0.45-μm pore size membrane having a capture efficiency of more than 95%, we ultimately chose a 3-μm PES membrane for upper layer filtration considering the time for sample processing.
Fig. 3. On-chip elution and performance of SPRC.
(A) Time required for samples to flow through filter membranes with different pore sizes. (B) Effects of different volumes of DI water flushing on amplification curves. (C) Flow rate and DNA capture efficiency of filter membranes with different pore sizes. (D) Uniformity of solution flowing into each chamber. (E) Diffusion time of NA from Fusion5 to solution. (F) LAMP amplification curve for different contact times between Fusion5 and the gel reagents. (G) The sealing effect of paraffin particles on the reaction system. (H) Effect of agarose and paraffin on LAMP. Fluorescence images were captured by a camera, gray-scale calculations were performed to obtain fluorescence values, and the detection threshold was set to five times the SD of the blank. (I) Detection limit of HBV and HCV.
To achieve multiplex detection, we should evaluate the uniformity of the sample solution flowing into each chamber. Figure 3D shows that the volume of solution flowing to the four absorbent pads is basically consistent, and the deviation remains within 10% within 60 s. This ensures that each Fusion5 filter paper adsorbs the same amount of NAs, which is conducive to multiplex detection. Besides this, for ease of operation, the reagents in the reaction chamber were pre-added. We used 0.5% low melting point agarose to pregelatinize the reaction system to extend the storage time. Experimental results showed that even at a concentration of 1.25% low melting point agarose will not notably reduce the amplification effect of LAMP (fig. S11, C to E). When the light irradiates the Au film, the NAs on Fusion5 will be washed off because the agarose melts. This process can be simulated numerically, showing that the NAs on the membrane can be completely washed off within 100 s (Fig. 3E). In addition, we compared the impact of different contact times between Fusion5 and the gel reagents on LAMP (Fig. 3F). It is evident that longer contact with the reagent ensures a better amplification effect on Fusion5 filter paper. For integrated chips, the sealing of the reaction system is also an important issue. Figure 3G shows the importance of sealing with paraffin particles. Without the sealing of paraffin, NAs in the solution may diffuse with the evaporation of the solution, which is not conducive to biosafety. Our experiments demonstrated that agarose and paraffin have almost no effect on LAMP reactions (Fig. 3H). Under various conditions, the LOD of SPRC is 0.2 copies/μl for λ DNA [48,000 base pairs (bp)] and 0.46 copies/μl for hepatitis B DNA (3200 bp) and hepatitis C RNA (9600 bp) (Fig. 3I). Fusion5 filter paper is primarily composed of glass fibers, which are more prone to entangling with longer NA molecules, thereby resulting in different LOD.
Heating and preservation of SPRC
On the basis of the experimental results, patterned Au-coated glass was fabricated (fig. S12A). Under the illumination from a light source of 3 W, the surface of the Au thin film could be heated to 65°C within 20 s (Fig. 4A). To minimize the impact of temperature differences on amplification results, we increased the bottom area of the cylindrical chambers while reducing their height. Each chamber owns a bottom diameter of 4 mm and a height of 1.7 mm, accommodating a reaction volume of 21 μl. Figure 4B shows that each chamber can heat 20 μl of solution to 65°C within 120 s, and the temperature distribution inside the chamber is uniform during the heating process. Simulation results showed that the maximum temperature difference between the top and bottom of the chamber was 1.6°C, which did not affect the LAMP reaction, and nonspecific amplification was not observed during the experiments. Because of the inevitable electric heating interfere from the applied voltage in the long-term monitoring process of the patterned Au film resistance, it is necessary to test the appropriate detection voltage. On the basis of the results shown in Fig. 4C, we found that during the synergistic effect of photothermal and input voltage, the overall temperature fluctuation on the surface of the Au film gradually decreased as the input voltage increased. Considering both factors, we chose an input voltage of 2 V to measure the resistance of the Au film. This sensor exhibits good linearity and facilitates integrated temperature measurement (fig. S12, B and C). We used a microcontroller to control the temperature on the Au-coated glass via proportional integral derivative (PID), thereby ensuring excellent heating uniformity between each chamber (Fig. 4D). Besides this, the SPRC can flexibly change the size and number of chambers according to actual needs (fig. S13A). Each type of chip is divided into two parts: the upper disposable reactor and the lower Au-coated glass substrate, which need to be combined for use. The entire chip can be sealed in a zip-lock bag for long-term storage (Fig. 4E). We confirmed that the performance of the SPRC does not noticeably change over up to 4 weeks (Fig. 4F).
Fig. 4. On-chip temperature sensor and chip performance.
(A) Thermal imaging of Au-plated glass under the illumination form five light sources of 3 W. (B) Numerical simulation of a single chamber heating process. (C) Heating performance under the input voltage of an amplitude no more than 5 V. (D) Temperature variations of the test solution within five chambers during LAMP reaction. (E) Schematic diagram of SPRC assembly and storage. (F) Time threshold (Tt) value of the SPRC after the storage for 4 weeks. (G) Explosion view of the compact device. (H) Operating principle of the compact device.
To facilitate the operability of the SPRC, we have designed a compact device that consists of a lower LED light source and an upper fluorescence detection system (Fig. 4G). It also can theoretically be divided into three parts: the power module, the LED photothermal module, and the sunlight focusing photothermal module according to its operating principle (Fig. 4H). A microcontroller was used for temperature control and fluorescence detection, it can be adjusted via a human-machine interface, and the detection results of each chamber were displayed on the screen (fig. S13, B to D). In addition, by setting a concave mirror and solar panels, sunlight can be used in the compact device for the simultaneous detection of multiple diseases. This device operates without the need for external power sources or batteries, relying solely on sunlight for heating and detection functions, making it highly suitable for on-site testing in outdoor environments. Table S3 provides a cost analysis of the SPRC system.
Multitarget clinical serum testing
To evaluate the clinical applicability of SPRC, we conducted tests on hepatitis B, hepatitis C, influenza A, and AIDS (see table S4 for patient information). For each disease, venous blood was collected from 80 volunteers (Fig. 5A). The infection status of hepatitis B, hepatitis C, and influenza A was independently confirmed by the First Affiliated Hospital of Harbin Medical University (Harbin, Heilongjiang), and that of AIDS was confirmed by the Center for Disease Control and Prevention (Harbin, Heilongjiang). The research protocol was approved by the ethics committee of the First Affiliated Hospital of Harbin Medical University. Taking the quality control samples of HBV and HCV as examples, SPRC demonstrated excellent repeatability in 35 groups of control experiments (Fig. 5B).
Fig. 5. Testing of Serum samples with SPRC for various diseases diagnoses.
(A) Clinical trial protocol. A total of 320 tests were analyzed by SPRC and conventional reverse transcription PCR (RT-PCR), including 30 patients with hepatitis B, 15 patients with hepatitis C, 15 patients with influenza A, 10 patients with AIDS, and 10 healthy individuals. (B) Repeatability test of HBV and HCV based on the SPRC (****P < 0.0001). (C) Normalized fluorescence signal levels in 320 tests. The four vertical color blocks represent blood test results from the same volunteer. (D) Fluorescence value of most positive samples (+) is significantly higher than that of negative (−) (**P < 0.01 and ****P < 0.0001). (E) Evaluation of the consistency between SPRC and RT-PCR analyses showed positive correlations (Pearson’s r values: rHBV = 0.82; rHCV = 0.86; rIAV = 0.80; rHIV = 0.78). (F) Receiver operating characteristic (ROC) curves. The dashed line represents the threshold estimated by ROC analysis. The area under the curve of all pathogens is close to 1.
Each sample was separately analyzed using our designed SPRC or a desktop PCR system with the same reagents and primers for clinical sample testing, and the result of normalized fluorescence intensity and evaluated accuracy of the SPRC test was shown in Fig. 5C. We detected 29 of 30 HBV patients, 15 of 15 HCV patients, 8 of 10 influenza A patients, and 9 of 10 HIV patients, successfully identified all negatives. Thus, among the 80 samples tested, we successfully detected 76 (95%). In the accurately detected samples, the fluorescence intensity of positive samples was significantly higher than that of negatives (Fig. 5D). Next, we compared fluorescence intensity (from the result of SPRC) with the cycle threshold (Ct) of conventional reverse transcription PCR (RT-PCR) and the result was shown in Fig. 5E. To better represent the linear relationship between them, we performed logarithmic processing on it [in a form of “−log2(Ct)”] and observed a strong consistency between these two methods. SPRC and RT-PCR demonstrate a robust positive correlation with Pearson’s r values of 0.82 (HBV), 0.86 (HCV), 0.80 (IAV), and 0.78 (HIV). Furthermore, we constructed receiver operating characteristic (ROC) curves for the four diseases (Fig. 5F), the area under the curve is 1 for all except HIV. Since the fact that the fluorescence value of an undetected positive sample crosses the negative, resulting in reduced specificity (98.75%).
We compared the SPRC results with the medical PCR detection system of the First Affiliated Hospital of Harbin Medical University (table S5). The four undetected samples had Ct values above 34. On the basis of the instructions of the commercial test kit, the original viral load was calculated to be 2 × 102 to 4 × 102 copies/ml, which is below the LOD of the SPRC. The serum separation and lysis were achieved by conventional method, which is not essential in our study because we have designed a manual centrifuge device and a corresponding photothermal chip to replace this process (movie S1). We compared our simplified method with traditional methods in terms of their impact on detection results (figs. S14 and S15). The results indicate that using the manual centrifuge device and photothermal chip completely replaces complex instruments for sample processing without affecting detection outcomes.
Nasopharyngeal swabs and sunlight-driven clinical testing
To avoid nonspecific amplification, we added Tte UvrD helicase to the pregelation reagent. We collected nasopharyngeal swabs from 20 patients with adenovirus (ADV) and 20 patients with COVID-19 (patient information is provided in table S4) and prefiltered the samples by using additional filters to remove larger particulate impurities such as food residues (fig. S16).
The detected fluorescence values were normalized (Fig. 6A), similar to the serum detection method, the four vertical color blocks represent test results from the same volunteer. For adenovirus, all 20 positive samples and 20 negative samples were detected, with the fluorescence intensity of all positive samples significantly higher than that of the other samples (Fig. 6B). A strong positive correlation was observed between the fluorescence intensity of the detection results and the Ct values of RT-PCR (Fig. 6C). For severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), we reliably detected 18 of 20 patients with COVID-19, identifying the N, E, and O genes. The results for two samples were uncertain, as only two of the three target genes were detected (Fig. 6D). The Ct values for the two missed samples were both above 34 cycles (table S7), corresponding to an original viral load of 2 × 102 to 4 × 102 copies/ml, which is below the SPRC’s LOD. We also compared the RT-PCR Ct values with the SPRC detection results, finding good positive correlations for all three genes (Fig. 6E). We further constructed the ROC curve, demonstrating SPRC’s high specificity (97.5%) (fig. S17). This indicates that SPRC can be used for nasopharyngeal swab detection and meets the current needs.
Fig. 6. Detecting nasopharyngeal swab samples with SPRC.
(A) Standardized fluorescence levels of 20 patients with adenovirus (ADV) and 20 patients with COVID-19 (N: nucleocapsid gene, E: envelope gene, O: open reading frame 1a gene). (B) Fluorescence detection results of ADV showing significant differences between positive and negative samples. The threshold for positive results was set at five times the SD of negative results (****P < 0.0001). (C) Comparison of detection results from SPRC and RT-PCR Ct values, with a Pearson’s r value of 0.82. (D) Fluorescence values of patient with COVID-19 were significantly higher than those of the negative group. (E) Evaluation of analytical concordance between SPRC and RT-PCR. The results showed a positive correlation (Pearson’s r values: rN = 0.84; rE = 0.77; rO = 0.71). (F) Appearance of sunlight-driven device. (G) Using sunlight-driven devices for the detection of ADV, successfully detecting 28 of 30 samples (93.33%). (H) Fluorescence detection results of ADV under sunlight driving. ****P < 0.0001. (I) Comparison of sunlight detection results with RT-PCR Ct values.
Benefiting from the low power consumption of the control system, it can be also powered by solar panels to break the dependence on external electric power sources. Therefore, we have designed a portable device for solar photothermal applications (Fig. 6F), and its heating performance under the impact of light intensity was measured over three consecutive days (fig. S18). During sunny days, the device can operate effectively throughout most daylight hours and reach 65°C within 25 s. We conducted 30 control experiments using sunlight-driven POCT devices on sunny days (Fig. 6G). Compared to LED-driven devices above, the sunlight-driven detection showed slightly lower accuracy, detecting 28 of 30 samples (93.33%). Raw fluorescence intensities measured using photodiodes indicated that positive samples generally exhibited higher fluorescence than negatives (Fig. 6H). Figure 6I illustrates a positive correlation between fluorescence intensity and RT-PCR Ct values (Pearson’s r value is 0.7). The nasopharyngeal swab testing demonstrated SPRC’s capability to handle multiple types of samples, and even in areas where LED lighting is inconvenient, SPRC can still perform rapid NA testing.
DISCUSSION
In summary, SPRC integrates multiple advanced features to address challenges in molecular diagnostics, particularly in remote or resource-limited settings, offering several advantages: (i) Efficient enrichment of NAs (approximately 350×) and reagent aliquots are simultaneously completed during the sample addition process. Elution and amplification of NAs on the chip are achieved through photothermal conversion driven by either LED irradiation or simple sunlight focusing, thereby providing a suitable solution for remote areas. (ii) The per-reaction detection cost stands at approximately $1.62, achieving a LOD of 0.2 copies/μl for λ DNA. Compared to conventional RT-PCR methods, SPRC substantially reduces costs while ensuring detection sensitivity. (iii) Combined with the compact device, SPRC can achieve low-cost, highly sensitive detection of four diseases within 30 min (movie S2), while the traditional RT-PCR method takes about 2 hours. Therefore, SPRC can achieve infectious disease screening before emergency surgery or rapid blood transfusion. (iv) Up to four diseases of hepatitis B, hepatitis C, influenza A, and AIDS can be simultaneously detected in serum on a chip, with a diagnostic accuracy of 95% and specificity of 98.75%. SPRC can also be used for the detection of nasopharyngeal swab samples, achieving a diagnostic accuracy of 95% and specificity of 97.5%.
In conjunction with a compact device, SPRC enables the rapid detection of HBV, HCV, IAV, and HIV, with a LOD of 0.46 copies/μl. Clinical testing across 80 venous blood samples has demonstrated good concordance between SPRC and standard RT-PCR. In addition to serum samples, SPRC can also perform testing on whole blood and nasopharyngeal swabs, demonstrating excellent multi-type sample handling capability. SPRC integrates sample processing, elution, amplification, and detection processes into one step, making it suitable for the simultaneous detection of various pathogens while maintaining high sensitivity. Compared with other detection methods, SPRC does not require sample extraction or purification steps, only simple heating lysis; therefore, it does not require professional operators and complex instruments.
SPRC harbors great development potential, allowing flexible alternations according to the amount of target pathogens. It can achieve rapid on-site testing at a lower cost and can conduct early, rapid, and accurate disease diagnosis in resource-limited areas. By connecting the compact device to the internet, test results can synchronize with medical systems, helping to build a more comprehensive and accurate testing platform for the global health system (fig. S19). This integration presents a promising future for disease surveillance, outbreak response, and patient management worldwide.
MATERIALS AND METHODS
Materials
GPTMS [(3-glycidyloxypropyl) trimethoxysilane], acetone, methanol, isopropanol, acetic acid, and NaOH were purchased from Sigma-Aldrich (Missouri, USA). Chitosan oligosaccharide (molecular weight ≤ 2000), low melting point agarose, and MES (2-(N-morpholino) ethanesulfonic acid) were purchased from Aladdin (Shanghai, China). Fusion5 filter paper was from Whatman (Cytiva, Utah, USA). The PES membrane was from Delvstlab (Zhejiang, China). The polydimethylsiloxane (PDMS) prepolymer and curing agent kit (Sylgard 184) were obtained from Dow Corning (Michigan, USA).
Computational simulation
We use COMSOL for photothermal simulation and analysis. The material properties are shown in table S8. The Au thin film was placed on a glass substrate and covered with water. Au thin films of different thicknesses (10, 40, 80, 120, and 150 nm) were applied to simulations to calculate the absorption and resistive heating of the Au film. The Au dielectric constant used in the simulation process is referenced from Johnson and Christy (57), and the dielectric constants of glass and water are 5 and 1.77, respectively.
Preparation of patterned Au Thin Films
The patterned Au thin film is processed using the soft lithography method. The mask and processing flow are shown in fig. S5. Briefly, the positive photoresist was first spin-coated on the surface of a clean glass substrate and then used the prepared mask for photolithography. After developing and baking, different thicknesses of Au films are deposited by vacuum evaporation (ZHD300, Beijing Tecno Technology Co. Ltd., China). Then, the entire glass was soaked in acetone solution for 2 hours, cleaned thoroughly, and dried with nitrogen.
Modification of Fusion5 filter paper
The Fusion5 filter paper was first treated in an oxygen plasma machine (Diener Electronics, ZEPTO, Germany) for treatment (100 W for 1 min, 20 sccm of O2 flow, and 0.65-mbar pressure), then soaked in GPTMS (2.5% methanol solution), and incubated on a shaker for 1 hour. This process is to add rich epoxy groups to the filter paper. After taking it out, it was carefully cleaned with DI water, transferred it to a 0.2% (w/v) chitosan oligosaccharide solution (prepared with 0.1% acetic acid solution and adjusted to pH 6.0 with 1 M NaOH solution), and then incubated overnight on a shaker. The modified Fusion5 filter paper was washed three times in DI water and then dried in a vacuum drying oven (50°C for 2 hours). A hole punch was used to cut into rounds, and it was placed in a sealed bag for the next experiment.
Fluorescence characteristics of filtration systems
We use 10-μm polystyrene fluorescent microspheres to represent impurity particles in the sample solution. A mixture of NAs and fluorescent microspheres was added to the filtration system, and after the solution was completely absorbed by the absorption pad, fluorescence images of each layer were captured under a fluorescence stereomicroscope (AXIO Zoom.V16, Zeiss, Germany). Characterize the enrichment efficiency of NAs by measuring the fluorescence intensity of Fusion5 filter paper.
Characterization by XPS and SEM
The electron binding energy of surface elements in Fusion5 filter paper was analyzed using an x-ray photoelectron spectroscopy (ESCALAB 250Xi, Thermo Fisher Scientific, USA). Microscopic photographs of Fusion5 filter paper were taken using a scanning electron microscope (SU5000, Hitachi, Japan).
Reaction conditions
λ DNA and DNA Marker were purchased from Takara (Beijing, China). HeLa cells were purchased from Procell (Wuhan, China) and purified using the Cell DNA Isolation Kit (DC102–01, Vazyme, China). The HBV and HCV quality control samples were purchased from Kangchesitan Biotechnology (Beijing, China). The primers used in this work are listed in table S9. All primers were synthesized from Sangon Biotechnology (Shanghai, China). Because of the temperature increase caused by blue light on the Au thin film, which may affect fluorescence intensity (fig. S4A), we used SYTO-82 as a fluorescent dye in the reaction system excited by green light (58). At the same time, UDG enzyme and deoxyuridine triphosphate (dUTP) were introduced into this system for to degrades uracil-containing amplification products before amplification starts for preventing cross-contamination (59, 60).
For the evaluation of DNA capture efficiency, DNA was diluted with a 10 mM MES (adjusted to pH 5.0 with 1 M NaOH) solution, and then 350 μl of sample solution was aspirated onto Fusion5 filter paper at a flow rate of 270 μl/min, and then 150 μl of DI water was used in the same manner. Place the filter paper into an Eppendorf tube containing 25 μl of the mixture for real-time fluorescence analysis. The mixture consisted of 12.5 μl of Taq PCR Mix (2X, dye-free, Sangon Biotechnology, Shanghai, China), 0.5 μl of SYTO 82 (50 μM, Thermo Fisher Scientific, USA), 2 μl of primer mix, and 10 μl of ribonuclease (RNase)–free water. The thermal cycling protocol included an initialization at 95°C for 3 min, followed by 30 cycles between 95°C for 15 s, 58°C for 20 s, and 72°C for 20 s, and lastly a further extension at 72°C for 10 min.
For RT-LAMP, we used 0.5% low melting point agarose to pregelatinize the reaction system, including 10 μl of WarmStart LAMP 2X Master Mix (E1700L, New England Biolabs, USA), 0.14 μl of dUTP (100 mM), 0. 4 μl of UDG (1000 U/ml), 0.5 μl of SYTO 82 (50 μM; Thermo Fisher Scientific, USA), 2 μl of 10x Primer Mix (For conventional RT-LAMP, 1.6 μM each of forward inner primer (FIP) and backward inner primer (BIP), 0.2 μM each of F3 and B3, and 0.4 μM each of loop F (LF) and loop B (LB). For RT-LAMP of HIV, 0.2 μM each of AceIN-F3, AceIN-B3a, and AceIN-B3b primers; 0.8 μM each of AceIN-FIPe, AceIN-FIPf, AceIN-LF, and AceIN-LB primers; and 1.6 μM AceIN-BIP), 1 μl of 10% low melting point agarose, and 5.96 μl of RNase-free water. For nasopharyngeal swab testing, an additional 0.5 μl of Tte UvrD Helicase (M1202S, New England Biolabs, USA) is required to reduce nonspecific amplification. According to the manufacturer’s recommendation, the RT-LAMP reaction was incubated at 65°C for 30 min.
Fabrication of the SPRC
SPRC is divided into upper and lower parts for on-chip sample processing and amplification. The solution in the sample addition area flows through the loading slot, diversion layer, PES membrane, paraffin, and Fusion5 filter paper and is lastly absorbed by the absorbent pad. After spinning the chip, the Fusion5 filter paper is aligned with the gel reagents in the reaction chamber for on-chip elution and amplification reactions. We explored the required amount of paraffin particles for sealing by heating 20 μl of DI water (fig. S20A). Considering the limited space between the two filter papers, we ultimately chose 6 mg of paraffin particles to seal each filter chamber. The reaction chamber is made of PDMS. Because PDMS has a porous structure, to solve the problem of water absorption during the heating process, we added 15% mineral oil during the configuration of PDMS (fig. S20, B and C). Because of the addition of mineral oil, oil droplets will precipitate on the surface of PDMS, which just provides sealing conditions for subsequent reactions. Except for the reaction chamber and Au-coated glass, the other layers are made of PMMA by laser cutting. In the process of making the chip, it is assembled from bottom to top according to Fig. 1A. The PMMA boards are bonded by vacuum hot pressing at 5 MPa and 110°C for 1 hour.
Design and assembly of the compact device
We used SolidWorks to design the 3D structure of the device, and the 3D printing technology to process the shell of the device. The device adopts the STM32H750V8T6 microcontroller for overall control, and the printed circuit board (PCB) was produced by Jialichuang Company. For the light source system, LEDs were purchased from Lumileds (PR02, USA), plane condensers were purchased from Youjing Optoelectronics (Shenzhen, China), and Fresnel lenses were purchased from Meiying Technology (Shenzhen, China). For the fluorescence detection system, four green LEDs were used for fluorescence excitation, and an OV5640 camera was used to capture images. The excitation filter (passing light of 460 to 540 nm) and emission filter (passing light of wavelength above 590 nm) were purchased from PHTODE (Beijing, China).
Virus detection in clinical samples
All procedures involving clinical trials were conducted in accordance with the guidance of the ethics committee of the First Affiliated Hospital of Harbin Medical University (license number 2023IIT333). For all participants, research and clinical staff wore personal protective equipment. On-site clinical venous blood samples were collected from adults undergoing preoperative infectious disease examinations at the First Affiliated Hospital of Harbin Medical University. During testing, after which a 10 mM MES (pH 5.0) solution is added, the serum was first heated in a photothermal lysis chip for 8 min to lyse the virus. Subsequently, the solution was added into the loading slot for autonomous enrichment and amplification. Eventually, the fluorescence intensity in each chamber is analyzed after the reaction is completed. For comparison with RT-PCR results, NA extraction was performed using the Pre-NAT II (PerkinElmer, USA), and amplification along with real-time fluorescence quantification was conducted using the ABI7500 (Thermo Fisher Scientific, USA). The viral load in the samples was determined using NA quantitative detection kits for HBV, HCV, IAV, and HIV (Sansure, China).
Nasopharyngeal swab samples were collected on-site from patients undergoing respiratory virus testing at the First Affiliated Hospital of Harbin Medical University and preserved in inactivated virus sampling tubes. During the detection process, a 10 mM MES solution (pH 5.0) was first added to the samples. The solution was then passed through a 0.22-μm PES membrane and loaded into the loading slot for autonomous enrichment and amplification. Last, the fluorescence intensity of each chamber was analyzed after the reaction was completed. To compare with the RT-PCR results, NA extraction was carried out with the SSNP-9600A (Bioperfectus, China), while amplification and real-time fluorescence quantification were performed using the SLAN-96P (Hongshitech, China). The viral load in the samples was determined using NA quantitative detection kits for six respiratory pathogens (IAV, influenza B virus, respiratory syncytial virus, human rhinovirus, adenovirus, and Mycoplasma pneumoniae) and novel coronavirus (SARS-CoV-2) (Sansure, China).
Statistical analysis
Data were analyzed and plotted using Origin software and reported as means ± SDs. One-way analysis of variance (ANOVA) was used to analyze the data with IBM SPSS Statistics software, followed by Tukey’s post hoc test for multiple group comparisons. The P values were used to indicate the level of statistical significance (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001).
Acknowledgments
We would like to express our sincere gratitude to Topsure Biotechnology Co. Ltd. (Heilongjiang, China) for the invaluable support and collaboration during this research.
Funding: This work is financially supported by the National Natural Science Foundation of China (nos. 12072096 and 12372260), the Self-Planned Task of State Key Laboratory of Robotics and System (HIT) (no. SKLRS202405B), and the Fundamental Research Funds for the Central Universities (no. HITDZIJ2023073).
Author contributions: Conceptualization: W.G., Haizhou Zhou, and Y.R. Methodology: W.G. and R.Y. Investigation: K.M., C.S., and X.L. Visualization: K.M. and M.X. Supervision: Z.G. and R.X. Writing—original draft: W.G., Hongwei Zhou, and T.S. Writing—review and editing: W.G., Y.T., and Y.R.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
The PDF file includes:
Figs. S1 to S20
Tables S1 to S9
Legends for movies S1 and S2
References
Other Supplementary Material for this manuscript includes the following:
Movies S1 and S2
REFERENCES AND NOTES
- 1.Natalia A., Zhang L., Sundah N. R., Zhang Y., Shao H., Analytical device miniaturization for the detection of circulating biomarkers. Nat. Rev. Bioeng. 1, 481–498 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang F., Karamagi H., Nsenga N., Nanyunja M., Karinja M., Amanfo S., Chase-Topping M., Calder-Gerver G., McGibbon M., Huber A., Wagner-Gamble T., Guo C.-G., Haynes S., Morrison A., Ferguson M., Awandare G. A., Mutapi F., Yoti Z., Cabore J., Moeti M. R., Woolhouse M. E. J., Predictors of COVID-19 epidemics in countries of the World Health Organization African Region. Nat. Med. 27, 2041–2047 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faust L., Zimmer A. J., Kohli M., Saha S., Boffa J., Bayot M. L., Nsofor I., Campos L., Mashamba-Thompson T., Herrera R., Emeka E., Shrestha S., Ugarte-Gil C., Katamba A., Pambudi I., Bichara D., Calderon R. I., Ahmadzada N., Safdar M. A., Nikam C., dos Santos Lázari C., Hussain H., Win M. M., Than K. Z., Ahumibe A., Waning B., Pai M., SARS-CoV-2 testing in low- and middle-income countries: Availability and affordability in the private health sector. Microbes Infect. 22, 511–514 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.W. H. Organization, (WHO), “Laboratory and point-of-care diagnostic testing for sexually transmitted infections, including HIV” (Publication 978-92-4-007708-9, WHO, 2023; https://iris.who.int/bitstream/handle/10665/374252/9789240077089-eng.pdf).
- 5.Najjar D., Rainbow J., Sharma Timilsina S., Jolly P., de Puig H., Yafia M., Durr N., Sallum H., Alter G., Li J. Z., Yu X. G., Walt D. R., Paradiso J. A., Estrela P., Collins J. J., Ingber D. E., A lab-on-a-chip for the concurrent electrochemical detection of SARS-CoV-2 RNA and anti-SARS-CoV-2 antibodies in saliva and plasma. Nat. Biomed. Eng. 6, 968–978 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krammer F., Simon V., Serology assays to manage COVID-19. Science 368, 1060–1061 (2020). [DOI] [PubMed] [Google Scholar]
- 7.National SARS-CoV-2 Serology Assay Evaluation Group , Performance characteristics of five immunoassays for SARS-CoV-2: A head-to-head benchmark comparison. Lancet Infect. Dis. 20, 1390–1400 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song M., Hong S., Lee L. P., Multiplexed ultrasensitive sample-to-answer RT-LAMP chip for the identification of SARS-CoV-2 and influenza viruses. Adv. Mater. 35, e2207138 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Teymouri M., Mollazadeh S., Mortazavi H., Naderi Ghale-noie Z., Keyvani V., Aghababaei F., Hamblin M. R., Abbaszadeh-Goudarzi G., Pourghadamyari H., Hashemian S. M. R., Mirzaei H., Recent advances and challenges of RT-PCR tests for the diagnosis of COVID-19. Pathol. Res. Pract. 221, 153443 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang C., Yang Z., Jiang H., A film-lever actuated switch technology for multifunctional, on-demand, and robust manipulation of liquids. Nat. Commun. 13, 4902 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin H., Yu W., Sabet K. A., Bogumil M., Zhao Y., Hambalek J., Lin S., Chandrasekaran S., Garner O., Di Carlo D., Emaminejad S., Ferrobotic swarms enable accessible and adaptable automated viral testing. Nature 611, 570–577 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snodgrass R., Gardner A., Semeere A., Kopparthy V. L., Duru J., Maurer T., Martin J., Cesarman E., Erickson D., A portable device for nucleic acid quantification powered by sunlight, a flame or electricity. Nat. Biomed. Eng. 2, 657–665 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AbdElFatah T., Jalali M., Yedire S. G., Hosseini I. I., del Real Mata C., Khan H., Hamidi S. V., Jeanne O., Siavash Moakhar R., McLean M., Patel D., Wang Z., McKay G., Yousefi M., Nguyen D., Vidal S. M., Liang C., Mahshid S., Nanoplasmonic amplification in microfluidics enables accelerated colorimetric quantification of nucleic acid biomarkers from pathogens. Nat. Nanotechnol. 18, 922–932 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Chandrasekaran S. S., Agrawal S., Fanton A., Jangid A. R., Charrez B., Escajeda A. M., Son S., Mcintosh R., Tran H., Bhuiya A., de León Derby M. D., Switz N. A., Armstrong M., Harris A. R., Prywes N., Lukarska M., Biering S. B., Smock D. C. J., Mok A., Knott G. J., Dang Q., Van Dis E., Dugan E., Kim S., Liu T. Y.; IGI Testing Consortium, Moehle E. A., Kogut K., Eskenazi B., Harris E., Stanley S. A., Lareau L. F., Tan M. X., Fletcher D. A., Doudna J. A., Savage D. F., Hsu P. D., Rapid detection of SARS-CoV-2 RNA in saliva via Cas13. Nat. Biomed. Eng. 6, 944–956 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niebling L., Nitzsche R., Sieksmeyer T., Haskamp V., Kissenkötter J., Abd El Wahed A., Teufel T., Hermann H., Paust N., Homann A. R., Fast and on-site animal species identification in processed meat via centrifugal microfluidics and isothermal amplification. Lab Chip 24, 975–984 (2024). [DOI] [PubMed] [Google Scholar]
- 16.Ma Y., Wei H., Wang Y., Cheng X., Chen H., Yang X., Zhang H., Rong Z., Wang S., Efficient magnetic enrichment cascade single-step RPA-CRISPR/Cas12a assay for rapid and ultrasensitive detection of Staphylococcus aureus in food samples. J. Hazard. Mater. 465, 133494 (2024). [DOI] [PubMed] [Google Scholar]
- 17.Liu Y., Yang Y., Wang G., Wang D., Shao P.-L., Tang J., He T., Zheng J., Hu R., Liu Y., Xu Z., Niu D., Lv J., Yang J., Xiao H., Wu S., He S., Tang Z., Liu Y., Tang M., Jiang X., Yuan J., Dai H., Zhang B., Multiplexed discrimination of SARS-CoV-2 variants via plasmonic-enhanced fluorescence in a portable and automated device. Nat. Biomed. Eng. 7, 1636–1648 (2023). [DOI] [PubMed] [Google Scholar]
- 18.Xu H., Wu X., Liu Q., Yang C., Shen M., Wang Y., Liu S., Zhao S., Xiao T., Sun M., Ding Z., Bao J., Chen M., Gao M., A universal strategy for enhancing the circulating Mirnas’ detection performance of rolling circle amplification by using a dual-terminal stem-loop padlock. ACS Nano 18, 436–450 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali M. M., Li F., Zhang Z., Zhang K., Kang D.-K., Ankrum J. A., Le X. C., Zhao W., Rolling circle amplification: A versatile tool for chemical biology, materials science and medicine. Chem. Soc. Rev. 43, 3324–3341 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Ganguli A., Mostafa A., Berger J., Aydin M. Y., Sun F., de Ramirez S. A. S., Valera E., Cunningham B. T., King W. P., Bashir R., Rapid isothermal amplification and portable detection system for SARS-CoV-2. Proc. Natl. Acad. Sci. U.S.A. 117, 22727–22735 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xun G., Lane S. T., Petrov V. A., Pepa B. E., Zhao H., A rapid, accurate, scalable, and portable testing system for COVID-19 diagnosis. Nat. Commun. 12, 2905 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbasi I., Kirstein O. D., Hailu A., Warburg A., Optimization of loop-mediated isothermal amplification (LAMP) assays for the detection of Leishmania DNA in human blood samples. Acta Trop. 162, 20–26 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bokelmann L., Nickel O., Maricic T., Pääbo S., Meyer M., Borte S., Riesenberg S., Point-of-care bulk testing for SARS-CoV-2 by combining hybridization capture with improved colorimetric LAMP. Nat. Commun. 12, 1467 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paul R., Ostermann E., Wei Q., Advances in point-of-care nucleic acid extraction technologies for rapid diagnosis of human and plant diseases. Biosens. Bioelectron. 169, 112592 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim B. K., Lee S.-A., Park M., Jeon E. J., Kim M. J., Kim J. M., Kim H., Jung S., Kim S. K., Ultrafast real-time PCR in photothermal microparticles. ACS Nano 16, 20533–20544 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.You M., Jia P., He X., Wang Z., Feng S., Ren Y., Li Z., Cao L., Gao B., Yao C., Singamaneni S., Xu F., Quantifying and adjusting plasmon-driven nano-localized temperature field around gold nanorods for nucleic acids amplification. Small Methods 5, e2001254 (2021). [DOI] [PubMed] [Google Scholar]
- 27.Lee J.-H., Cheglakov Z., Yi J., Cronin T. M., Gibson K. J., Tian B., Weizmann Y., Plasmonic photothermal gold bipyramid nanoreactors for ultrafast real-time bioassays. J. Am. Chem. Soc. 139, 8054–8057 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Blumenfeld N. R., Bolene M. A. E., Jaspan M., Ayers A. G., Zarrandikoetxea S., Freudman J., Shah N., Tolwani A. M., Hu Y., Chern T. L., Rogot J., Behnam V., Sekhar A., Liu X., Onalir B., Kasumi R., Sanogo A., Human K., Murakami K., Totapally G. S., Fasciano M., Sia S. K., Multiplexed reverse-transcriptase quantitative polymerase chain reaction using plasmonic nanoparticles for point-of-care COVID-19 diagnosis. Nat. Nanotechnol. 17, 984–992 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Cheong J., Yu H., Lee C. Y., Lee J.-U., Choi H.-J., Lee J.-H., Lee H., Cheon J., Fast detection of SARS-CoV-2 RNA via the integration of plasmonic thermocycling and fluorescence detection in a portable device. Nat. Biomed. Eng. 4, 1159–1167 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shrestha K., Kim S., Han J., Florez G. M., Truong H., Hoang T., Parajuli S., Am T., Kim B., Jung Y., Abafogi A. T., Lee Y., Song S. H., Lee J., Park S., Kang M., Huh H. J., Cho G., Lee L. P., Mobile efficient diagnostics of infectious diseases via On-Chip RT-qPCR: MEDIC-PCR. Adv. Sci. 10, 2302072 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parvin R., Zhang L., Zu Y., Ye F., Photothermal responsive digital polymerase chain reaction resolving exosomal micrornas expression in liver cancer. Small 19, e2207672 (2023). [DOI] [PubMed] [Google Scholar]
- 32.Zhang L., Li W., Parvin R., Wang X., Fan Q., Ye F., Screening prostate cancer cell-derived exosomal microRNA expression with photothermal-driven digital PCR. Adv. Funct. Mater. 32, 2207879 (2022). [Google Scholar]
- 33.Ahrberg C. D., Choi J. W., Lee J. M., Lee K. G., Lee S. J., Manz A., Chung B. G., Plasmonic heating-based portable digital PCR system. Lab Chip 20, 3560–3568 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Son J. H., Cho B., Hong S., Lee S. H., Hoxha O., Haack A. J., Lee L. P., Ultrafast photonic PCR. Light Sci. Appl. 4, e280 (2015). [Google Scholar]
- 35.Cui X., Ruan Q., Zhuo X., Xia X., Hu J., Fu R., Li Y., Wang J., Xu H., Photothermal nanomaterials: A powerful light-to-heat converter. Chem. Rev. 123, 6891–6952 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Oliveira Coelho B., Sanchuki H. B. S., Zanette D. L., Nardin J. M., Morales H. M. P., Fornazari B., Aoki M. N., Blanes L., Essential properties and pitfalls of colorimetric reverse transcription loop-mediated isothermal amplification as a point-of-care test for SARS-CoV-2 diagnosis. Mol. Med. 27, 30 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gu Y., Zhuang B., Han J., Li Y., Song X., Zhou X., Wang L., Liu P., Modular-based integrated microsystem with multiple sample preparation modules for automated forensic DNA typing from reference to challenging samples. Anal. Chem. 91, 7435–7443 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Pérez-Cataluña A., Cuevas-Ferrando E., Randazzo W., Falcó I., Allende A., Sánchez G., Comparing analytical methods to detect SARS-CoV-2 in wastewater. Sci. Total Environ. 758, 143870 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adams N. M., Bordelon H., Wang K.-K. A., Albert L. E., Wright D. W., Haselton F. R., Comparison of three magnetic bead surface functionalities for RNA extraction and detection. ACS Appl. Mater. Interfaces 7, 6062–6069 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Reinholt S. J., Baeumner A. J., Microfluidic isolation of nucleic acids. Angew. Chem. Int. Ed. Engl. 53, 13988–14001 (2014). [DOI] [PubMed] [Google Scholar]
- 41.He B., Wang L., Jin X., Zhang X., Sha R., Liang Y., Wang Y., Xie W., Shi J., Peng H., Porous agarose layered magnetic graphene oxide nanocomposite for virus RNA monitoring in wastewater. Anal. Chem. 96, 9167–9176 (2024). [DOI] [PubMed] [Google Scholar]
- 42.Li S., Han B., Zhou D., Gu Y., Li B., Ma J., Fu R., Qi X., Liu P., One-stop extraction and in situ RT-qPCR for ultrasensitive detection of highly diluted SARS-CoV-2 in large-volume samples from aquatic environments. Anal. Chem. 95, 2339–2347 (2023). [DOI] [PubMed] [Google Scholar]
- 43.Koo B., Kim M. G., Lee K., Kim J. Y., Lee S., Kim S.-H., Shin Y., Automated sample-to-answer system for rapid and accurate diagnosis of emerging infectious diseases. Sens. Actuators B Chem. 380, 133382 (2023). [Google Scholar]
- 44.Liu F., Yang Y., Wan X., Gao H., Wang Y., Lu J., Xu L.-P., Wang S., Space-confinment-enhanced fluorescence detection of DNA on hydrogel particles array. ACS Nano 16, 6266–6273 (2022). [DOI] [PubMed] [Google Scholar]
- 45.Wang S., Duan H., Lin J., A centrifugal microfluidic platform for rapid detection of pathogens based on chitosan nucleic acid extraction and RAA-T7-CRISPR/Cas13a nucleic acid detection. Sens. Actuators B Chem. 393, 134223 (2023). [Google Scholar]
- 46.Li S., Li B., Li X., Liu C., Qi X., Gu Y., Lin B., Sun L., Chen L., Han B., Guo J., Huang Y., Wu S., Ren L., Wang J., Bai J., Ma J., Yao M., Liu P., An ultrasensitive and rapid “sample-to-answer” microsystem for on-site monitoring of SARS-CoV-2 in aerosols using “in situ” tetra-primer recombinase polymerase amplification. Biosens. Bioelectron. 219, 114816 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka T., Sakai R., Kobayashi R., Hatakeyama K., Matsunaga T., contributions of phosphate to DNA adsorption/desorption behaviors on aminosilane-modified magnetic nanoparticles. Langmuir 25, 2956–2961 (2009). [DOI] [PubMed] [Google Scholar]
- 48.Mao H.-Q., Roy K., Troung-Le V. L., Janes K. A., Lin K. Y., Wang Y., August J. T., Leong K. W., Chitosan-DNA nanoparticles as gene carriers: Synthesis, characterization and transfection efficiency. J. Control. Release 70, 399–421 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Yang K., Pan J., Deng G., Hua C., Zhu C., Liu Y., Zhu L., Mkit: A mobile nucleic acid assay based on a chitosan-modified minimalistic microfluidic chip (CM3-chip) and smartphone. Anal. Chim. Acta 1253, 341030 (2023). [DOI] [PubMed] [Google Scholar]
- 50.Ackerman C. M., Myhrvold C., Thakku S. G., Freije C. A., Metsky H. C., Yang D. K., Ye S. H., Boehm C. K., Kosoko-Thoroddsen T.-S. F., Kehe J., Nguyen T. G., Carter A., Kulesa A., Barnes J. R., Dugan V. G., Hung D. T., Blainey P. C., Sabeti P. C., Massively multiplexed nucleic acid detection with Cas13. Nature 582, 277–282 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao Z., Wang J., Zhang P., Zhang Y., Miao Y., Gao S., Deng Y., Geng L., Recent advances in microfluidic chip integrated electronic biosensors for multiplexed detection. Biosens. Bioelectron. 121, 272–280 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Sun Y., Zhang S., Qi L., Zhang X., Yang M., Guo Z., Wang Z., Du Y., Advancing multiple detection in RT-LAMP with a specific probe assembled from plural three-way-junction structures. Anal. Chem. 95, 17808–17817 (2023). [DOI] [PubMed] [Google Scholar]
- 53.Chen F., Lyu C., Li Z., Xiu L., Li H., Xie Y., Cao R., Hu Q., Yin K., Fully integrated microfluidic platform for multiplexed detection of hunov by a dynamic confined-space-implemented one-pot Rpa-lamp system. Adv. Sci. 11, e2306612 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ludwig K. U., Schmithausen R. M., Li D., Jacobs M. L., Hollstein R., Blumenstock K., Liebing J., Słabicki M., Ben-Shmuel A., Israeli O., Weiss S., Ebert T. S., Paran N., Rüdiger W., Wilbring G., Feldman D., Lippke B., Ishorst N., Hochfeld L. M., Beins E. C., Kaltheuner I. H., Schmitz M., Wöhler A., Döhla M., Sib E., Jentzsch M., Moench E.-M. C., Borrajo J. D., Strecker J., Reinhardt J., Cleary B., Geyer M., Hölzel M., Macrae R., Nöthen M. M., Hoffmann P., Exner M., Regev A., Zhang F., Schmid-Burgk J. L., LAMP-seq enables sensitive, multiplexed COVID-19 diagnostics using molecular barcoding. Nat. Biotechnol. 39, 1556–1562 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roche P. J. R., Beitel L. K., Khan R., Lumbroso R., Najih M., Cheung M. C. K., Thiemann J., Veerasubramanian V., Trifiro M., Chodavarapu V. P., Kirk A. G., Demonstration of a plasmonic thermocycler for the amplification of human androgen receptor DNA. Analyst 137, 4475–4481 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Clavero C., Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photonics 8, 95–103 (2014). [Google Scholar]
- 57.Johnson P. B., Christy R. W., Optical constants of the noble metals. Phys. Rev. B 6, 4370–4379 (1972). [Google Scholar]
- 58.Haydar T. F., Ang E., Rakic P., Mitotic spindle rotation and mode of cell division in the developing telencephalon. Proc. Natl. Acad. Sci. U.S.A. 100, 2890–2895 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lai M. Y., Bukhari F. D. M., Zulkefli N. Z., Ismail I., Mustapa N. I., Soh T. S. T., Hassan A. H., Peariasamy K. M., Lee Y. L., Suppiah J., Thayan R., Lau Y. L., Colorimetric detection of SARS-CoV-2 by uracil-DNA glycosylase (UDG) reverse transcription loop-mediated isothermal amplification (RT-LAMP). Int. J. Infect. Dis. 120, 132–134 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qian C., Wang R., Wu H., Zhang F., Wu J., Wang L., Uracil-mediated new photospacer-adjacent motif of Cas12a To realize visualized DNA detection at the single-copy level free from contamination. Anal. Chem. 91, 11362–11366 (2019). [DOI] [PubMed] [Google Scholar]
- 61.Bhamla M. S., Benson B., Chai C., Katsikis G., Johri A., Prakash M., Hand-powered ultralow-cost paper centrifuge. Nat. Biomed. Eng. 1, 0009 (2017). [Google Scholar]
- 62.Li B., Qi J., Fu L., Han J., Choo J., deMello A. J., Lin B., Chen L., Integrated hand-powered centrifugation and paper-based diagnosis with blood-in/answer-out capabilities. Biosens. Bioelectron. 165, 112282 (2020). [DOI] [PubMed] [Google Scholar]
- 63.Franco J. J., Nagata T., Okamoto T., Mukai S., An ultralow-cost portable centrifuge from discarded materials for medical applications. Sci. Rep. 13, 3081 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang B.-H., Jang K.-W., Yu E.-S., Na H., Lee Y.-J., Ko W.-Y., Bae N., Rho D., Jeong K.-H., Ultrafast plasmonic nucleic acid amplification and real-time quantification for decentralized molecular diagnostics. ACS Nano 17, 6507–6518 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jayanath N. Y., Nguyen L. T., Vu T. T., Tran L. D., Development of a portable electrochemical loop mediated isothermal amplification (LAMP) device for detection of hepatitis B virus. RSC Adv. 8, 34954–34959 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharma S., Thomas E., Caputi M., Asghar W., RT-LAMP-based molecular diagnostic set-up for rapid hepatitis C virus testing. Biosensors 12, 298 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ocwieja K. E., Sherrill-Mix S., Liu C., Song J., Bau H., Bushman F. D., A Reverse transcription loop-mediated isothermal amplification assay optimized to detect multiple HIV subtypes. PLOS ONE 10, e0117852 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heithoff D. M., Barnes L. V, Mahan S. P., Fox G. N., Arn K. E., Ettinger S. J., Bishop A. M., Fitzgibbons L. N., Fried J. C., Low D. A., Samuel C. E., Mahan M. J., Assessment of a smartphone-based loop-mediated isothermal amplification assay for detection of SARS-CoV-2 and influenza viruses. JAMA Netw. Open 5, e2145669 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li F., Zhao L.-q., Deng J., Zhu R.-n., Sun Y., Liu L.-y., Li Y.-y., Qian Y., Detecting human adenoviruses in respiratory samples collected from children with acute respiratory infections by loop-mediated isothermal amplification. Zhonghua Er Ke Za Zhi 51, 52–57 (2013). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S20
Tables S1 to S9
Legends for movies S1 and S2
References
Movies S1 and S2