Abstract
Background
Heart transplantation has become a valuable and well‐accepted treatment option for end‐stage heart failure. Rejection of the transplanted heart by the recipient's body is a risk to the success of the procedure, and life‐long immunosuppression is necessary to avoid this. Clear evidence is required to identify the best, safest and most effective immunosuppressive treatment strategy for heart transplant recipients. To date, there is no consensus on the use of immunosuppressive antibodies against T‐cells for induction after heart transplantation.
Objectives
To review the benefits, harms, feasibility and tolerability of immunosuppressive T‐cell antibody induction versus placebo, or no antibody induction, or another kind of antibody induction for heart transplant recipients.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 11, 2012), MEDLINE (Ovid) (1946 to November Week 1 2012), EMBASE (Ovid) (1946 to 2012 Week 45), ISI Web of Science (14 November 2012); we also searched two clinical trial registers and checked reference lists in November 2012.
Selection criteria
We included all randomised clinical trials (RCTs) assessing immunosuppressive T‐cell antibody induction for heart transplant recipients. Within individual trials, we required all participants to receive the same maintenance immunosuppressive therapy.
Data collection and analysis
Two authors extracted data independently. RevMan analysis was used for statistical analysis of dichotomous data with risk ratio (RR), and of continuous data with mean difference (MD), both with 95% confidence intervals (CI). Methodological components were used to assess risks of systematic errors (bias). Trial sequential analysis was used to assess the risks of random errors (play of chance). We assessed mortality, acute rejection, infection, Cytomegalovirus (CMV) infection, post‐transplantation lymphoproliferative disorder, cancer, adverse events, chronic allograft vasculopathy, renal function, hypertension, diabetes mellitus, and hyperlipidaemia.
Main results
In this review, we included 22 RCTs that investigated the use of T‐cell antibody induction, with a total of 1427 heart‐transplant recipients. All trials were judged to be at a high risk of bias. Five trials, with a total of 606 participants, compared any kind of T‐cell antibody induction versus no antibody induction; four trials, with a total of 576 participants, compared interleukin‐2 receptor antagonist (IL‐2 RA) versus no induction; one trial, with 30 participants, compared monoclonal antibody (other than IL‐2 RA) versus no antibody induction; two trials, with a total of 159 participants, compared IL‐2 RA versus monoclonal antibody (other than IL‐2 RA) induction; four trials, with a total of 185 participants, compared IL‐2 RA versus polyclonal antibody induction; seven trials, with a total of 315 participants, compared monoclonal antibody (other than IL‐2 RA) versus polyclonal antibody induction; and four trials, with a total of 162 participants, compared polyclonal antibody induction versus another kind, or dose of polyclonal antibodies.
No significant differences were found for any of the comparisons for the outcomes of mortality, infection, CMV infection, post‐transplantation lymphoproliferative disorder, cancer, adverse events, chronic allograft vasculopathy, renal function, hypertension, diabetes mellitus, or hyperlipidaemia. Acute rejection occurred significantly less frequently when IL‐2 RA induction was compared with no induction (93/284 (33%) versus 132/292 (45%); RR 0.73; 95% CI 0.59 to 0.90; I2 57%) applying the fixed‐effect model. No significant difference was found when the random‐effects model was applied (RR 0.73; 95% CI 0.46 to 1.17; I2 57%). In addition, acute rejection occurred more often statistically when IL‐2 RA induction was compared with polyclonal antibody induction (24/90 (27%) versus 10/95 (11%); RR 2.43; 95% CI 1.01 to 5.86; I2 28%). For all of these differences in acute rejection, trial sequential alpha‐spending boundaries were not crossed and the required information sizes were not reached when trial sequential analysis was performed, indicating that we cannot exclude random errors.
We observed some occasional significant differences in adverse events in some of the comparisons, however definitions of adverse events varied between trials, and numbers of participants and events in these outcomes were too small to allow definitive conclusions to be drawn.
Authors' conclusions
This review shows that acute rejection might be reduced by IL‐2 RA compared with no induction, and by polyclonal antibody induction compared with IL‐2 RA, though trial sequential analyses cannot exclude random errors, and the significance of our observations depended on the statistical model used. Furthermore, this review does not show other clear benefits or harms associated with the use of any kind of T‐cell antibody induction compared with no induction, or when one type of T‐cell antibody is compared with another type of antibody. The number of trials investigating the use of antibodies against T‐cells for induction after heart transplantation is small, and the number of participants and outcomes in these RCTs is limited. Furthermore, the included trials are at a high risk of bias. Hence, more RCTs are needed to assess the benefits and harms of T‐cell antibody induction for heart‐transplant recipients. Such trials ought to be conducted with low risks of systematic and random error.
Keywords: Humans; Heart Transplantation; Antibodies, Monoclonal; Antibodies, Monoclonal/immunology; Antibodies, Monoclonal/therapeutic use; Antibodies, Monoclonal, Humanized; Antibodies, Monoclonal, Humanized/therapeutic use; Antilymphocyte Serum; Antilymphocyte Serum/immunology; Basiliximab; Daclizumab; Graft Rejection; Graft Rejection/immunology; Graft Rejection/prevention & control; Immunoglobulin G; Immunoglobulin G/therapeutic use; Immunosuppression Therapy; Immunosuppression Therapy/methods; Muromonab‐CD3; Muromonab‐CD3/therapeutic use; Randomized Controlled Trials as Topic; Receptors, Interleukin‐2; Receptors, Interleukin‐2/antagonists & inhibitors; Receptors, Interleukin‐2/immunology; Recombinant Fusion Proteins; Recombinant Fusion Proteins/therapeutic use; T‐Lymphocytes; T‐Lymphocytes/immunology
Plain language summary
T‐cell antibodies for immunosuppression after heart transplantation
Heart transplantation is sometimes possible for people with failing hearts who otherwise would die. Immunosuppressive treatment is necessary after heart transplantation to prevent rejection of the transplanted heart, and has two phases. The first phase is induction treatment, which is given at the time of transplantation, and only for a very short period. The second phase is maintenance treatment, which often starts at the time of the operation, but is given for much longer ‐ often for life.
Antibodies are molecules that combat specific targets. Antibodies against T‐cells (a type of white blood cell) ‐ known as T‐cell antibodies ‐ are used as a form of induction treatment in the first two weeks after heart transplantation. Different types of antibodies are used, but the benefits and harms of each type is unclear.
We reviewed the evidence about the effect of T‐cell antibodies in people who had had a heart transplant. We hoped to establish whether there is a role for antibodies against T‐cells after heart transplantation, and, if so, which antibody preparation works best and causes the least harm. We found 22 studies that included 1427 people who had received a heart‐transplant. Most of the trial participants received T‐cell antibodies only in the first two weeks after surgery, but treatment for some continued for 10 weeks.
All these trials had high risk of bias (that is risk of overestimation of benefits and underestimation of harms). We compared any kind of T‐cell antibody induction versus no induction. Furthermore, we compared interleukin‐2 receptor antagonists versus no induction, monoclonal T‐cell antibody versus no induction, interleukin‐2 receptor antagonists versus monoclonal antibody (other than IL‐2 RA) induction, interleukin‐2 receptor antagonists versus polyclonal antibody induction, and monoclonal antibody (other than IL‐2 RA) induction versus polyclonal antibody induction. We found no significant differences in incidence of survival, and we found no significant difference in adverse effects (e.g. infection, cytomegalovirus infection, post‐transplantation lymphoproliferative disorder, cancer, chronic allograft vasculopathy, renal function, hypertension, diabetes mellitus, or hypertension) for any of the comparisons. The incidence of acute rejection may occur less frequently in patients treated with interleukin‐2 receptor antagonist induction compared with no induction , and in patients treated with polyclonal antibody induction compared with interleukin‐2 receptor antagonist induction. However, systematic errors and random errors cannot be excluded, and our findings were dependent upon choice of statistical model. Accordingly, our observations are not robust and more trials are needed to confirm or reject these findings.
Summary of findings
Summary of findings for the main comparison. Antibody induction for heart transplant recipients.
| Antibody induction for heart transplant recipients | ||||||
| Patient or population: heart transplant recipients Settings: hospital Intervention: antibody induction Comparison: no induction | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE)1 | Comments | |
| Assumed risk | Corresponding risk | |||||
| No induction | Antibody induction | |||||
| Mortality Mortality at latest follow‐up Follow‐up: 6‐12 months | Study population | RR 1.53 (0.85 to 2.76) | 606 (5 studies) | ⊕⊕⊝⊝ low | ||
| 59 per 1000 | 90 per 1000 (50 to 162) | |||||
| Moderate | ||||||
| 55 per 1000 | 84 per 1000 (47 to 152) | |||||
|
Acute rejection
Acute rejection grade 3A or more verified by biopsy Follow‐up: 12 months |
Study population | RR 0.73 (0.46 to 1.17) | 576 (4 studies) | ⊕⊕⊝⊝ low | ||
| 452 per 1000 | 330 per 1000 (208 to 529) | |||||
| Moderate | ||||||
| 409 per 1000 | 299 per 1000 (188 to 479) | |||||
| Infection Number of participants diagnosed with infection Follow‐up: 12 months | Study population | RR 0.99 (0.79 to 1.24) | 545 (3 studies) | ⊕⊕⊝⊝ low | ||
| 395 per 1000 | 391 per 1000 (312 to 490) | |||||
| Moderate | ||||||
| 367 per 1000 | 363 per 1000 (290 to 455) | |||||
| Cytomegalovirus infection Number of patients diagnosed with CMV infection Follow‐up: 12 months | Study population | RR 0.86 (0.63 to 1.19) | 606 (5 studies) | ⊕⊕⊝⊝ low | ||
| 221 per 1000 | 190 per 1000 (140 to 264) | |||||
| Moderate | ||||||
| 226 per 1000 | 194 per 1000 (142 to 269) | |||||
| Post‐transplantation lymphoproliferative disorder Number of participants diagnosed with PTLD Follow‐up: 12 months | Study population | RR 0.74 (0.14 to 3.82) | 606 (5 studies) | ⊕⊕⊝⊝ low | ||
| 10 per 1000 | 7 per 1000 (1 to 37) | |||||
| Moderate | ||||||
| 5 per 1000 | 4 per 1000 (1 to 19) | |||||
| Cancer Number of participants diagnosed with cancer Follow‐up: 12 months | Study population | RR 1.01 (0.45 to 2.28) | 576 (4 studies) | ⊕⊕⊝⊝ low | ||
| 38 per 1000 | 38 per 1000 (17 to 86) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
1All trials with high risk of bias according to the Cochrane risk of bias assessment tool
Abbreviations: CMV = Cytomegalovirus; PTLD = post‐transplantation lymphoproliferative disorder
Summary of findings 2. Interleukin‐2 RA compared to no induction for heart transplant recipients.
| Interleukin‐2 RA compared to no induction for heart transplant recipients | ||||||
| Patient or population: heart transplant recipients Settings: hospital Intervention: interleukin‐2 RA Comparison: no induction | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE)1 | Comments | |
| Assumed risk | Corresponding risk | |||||
| No induction | Interleukin‐2 RA | |||||
| Mortality Mortality at latest follow‐up Follow‐up: 12 months | Study population | RR 1.53 (0.85 to 2.76) | 576 (4 studies) | ⊕⊕⊝⊝ low | ||
| 62 per 1000 | 94 per 1000 (52 to 170) | |||||
| Moderate | ||||||
| 59 per 1000 | 90 per 1000 (50 to 163) | |||||
| Acute rejection Acute rejection grade 3A or more verified by biopsy Follow‐up: 12 months | Study population | RR 0.73 (0.46 to 1.17) | 576 (4 studies) | ⊕⊕⊝⊝ low | ||
| 452 per 1000 | 330 per 1000 (208 to 529) | |||||
| Moderate | ||||||
| 409 per 1000 | 299 per 1000 (188 to 479) | |||||
| Infection Number of participants diagnosed with infection Follow‐up: 12 months | Study population | RR 0.99 (0.79 to 1.24) | 545 (3 studies) | ⊕⊕⊝⊝ low | ||
| 395 per 1000 | 391 per 1000 (312 to 490) | |||||
| Moderate | ||||||
| 367 per 1000 | 363 per 1000 (290 to 455) | |||||
| Cytomegalovirus infection Number of participants diagnosed with CMV infection Follow‐up: 12 months | Study population | RR 0.86 (0.62 to 1.19) | 576 (4 studies) | ⊕⊕⊝⊝ low | ||
| 229 per 1000 | 197 per 1000 (142 to 273) | |||||
| Moderate | ||||||
| 228 per 1000 | 196 per 1000 (141 to 271) | |||||
| Post‐transplantation lymphoproliferative disorder Number of participants diagnosed with PTLD Follow‐up: 12 months | Study population | RR 0.61 (0.08 to 4.92) | 576 (4 studies) | ⊕⊕⊝⊝ low | ||
| 7 per 1000 | 4 per 1000 (1 to 34) | |||||
| Moderate | ||||||
| 2 per 1000 | 1 per 1000 (0 to 10) | |||||
| Cancer Number of participants diagnosed with cancer Follow‐up: 12 months | Study population | RR 1.01 (0.45 to 2.28) | 576 (4 studies) | ⊕⊕⊝⊝ low | ||
| 38 per 1000 | 38 per 1000 (17 to 86) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
1All trials with high risk of bias according to the Cochrane risk of bias assessment tool
Abbreviations: CMV = Cytomegalovirus; PTLD = post‐transplantation lymphoproliferative disorder
Summary of findings 3. Interleukin‐2 RA induction compared to monoclonal antibody induction for heart transplant recipients.
| Interleukin‐2 RA induction compared to monoclonal antibody induction for heart transplant recipients | ||||||
| Patient or population: heart transplant recipients Settings: hospital Intervention: interleukin‐2 RA induction Comparison: monoclonal antibody induction | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE)1 | Comments | |
| Assumed risk | Corresponding risk | |||||
| Monoclonal antibody (other than IL‐2 RA)induction | Interleukin‐2 RA induction | |||||
| Mortality Mortality at latest follow‐up Follow‐up: 1‐10 years | Study population | RR 0.8 (0.19 to 3.41) | 159 (2 studies) | ⊕⊕⊝⊝ low1 | ||
| 212 per 1000 | 170 per 1000 (40 to 725) | |||||
| Moderate | ||||||
| 226 per 1000 | 181 per 1000 (43 to 771) | |||||
| Acute rejection Acute rejection grade 3A or more verified by biopsy Follow‐up: 1‐10 years | Study population | RR 0.97 (0.77 to 1.23) | 159 (2 studies) | ⊕⊕⊝⊝ low | ||
| 550 per 1000 | 534 per 1000 (423 to 677) | |||||
| Moderate | ||||||
| 602 per 1000 | 584 per 1000 (464 to 740) | |||||
| Infection Number of participants diagnosed with infection Follow‐up: 1‐10 years | Study population | RR 0.91 (0.54 to 1.53) | 159 (2 studies) | ⊕⊕⊝⊝ low | ||
| 638 per 1000 | 580 per 1000 (344 to 975) | |||||
| Moderate | ||||||
| 589 per 1000 | 536 per 1000 (318 to 901) | |||||
| Cytomegalovirus infection Number of participants diagnosed with CMV infection Follow‐up: 1‐10 years | Study population | RR 0.94 (0.43 to 2.06) | 159 (2 studies) | ⊕⊕⊝⊝ low | ||
| 288 per 1000 | 270 per 1000 (124 to 592) | |||||
| Moderate | ||||||
| 233 per 1000 | 219 per 1000 (100 to 480) | |||||
| Post‐transplantation lymphoproliferative disorder Number of participants diagnosed with PTLD Follow‐up: 1‐10 years | Study population | RR 0.31 (0.01 to 7.38) | 159 (2 studies) | ⊕⊕⊝⊝ low | ||
| 12 per 1000 | 4 per 1000 (0 to 92) | |||||
| Moderate | ||||||
| 17 per 1000 | 5 per 1000 (0 to 125) | |||||
| Cancer Number of participants diagnosed with cancer Follow‐up: 1‐10 years | Study population | RR 0.84 (0.4 to 1.77) | 159 (2 studies) | ⊕⊕⊝⊝ low1 | ||
| 125 per 1000 | 105 per 1000 (50 to 221) | |||||
| Moderate | ||||||
| 172 per 1000 | 144 per 1000 (69 to 304) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
1 All trials with high risk of bias according to the Cochrane risk of bias assessment tool
Abbreviations: CMV = Cytomegalovirus; PTLD = post‐transplantation lymphoproliferative disorder
Summary of findings 4. Interleukin‐2 RA versus polyclonal antibody induction for heart transplant recipients.
| Interleukin‐2 RA versus polyclonal antibody induction for heart transplant recipients | ||||||
| Patient or population: heart transplant recipients Settings: hospital Intervention: interleukin‐2 RA Comparison: polyclonal antibody | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE)1 | Comments | |
| Assumed risk | Corresponding risk | |||||
| Polyclonal antibody | Interleukin‐2 RA | |||||
| Mortality Mortality at latest follow‐up Follow‐up: 1‐10 years | Study population | RR 1.1 (0.65 to 1.88) | 185 (4 studies) | ⊕⊕⊝⊝ low1 | ||
| 221 per 1000 | 243 per 1000 (144 to 416) | |||||
| Moderate | ||||||
| 218 per 1000 | 240 per 1000 (142 to 410) | |||||
| Acute rejection Acute rejection grade 3A or more verified by biopsy Follow‐up: 1‐10 years | Study population | RR 2.43 (1.01 to 5.86) | 185 (4 studies) | ⊕⊕⊝⊝ low | ||
| 105 per 1000 | 256 per 1000 (106 to 617) | |||||
| Moderate | ||||||
| 117 per 1000 | 284 per 1000 (118 to 686) | |||||
| Infection Number of participants diagnosed with infection Follow‐up: 1‐10 years | Study population | RR 0.85 (0.71 to 1.03) | 155 (3 studies) | ⊕⊕⊝⊝ low | ||
| 800 per 1000 | 680 per 1000 (568 to 824) | |||||
| Moderate | ||||||
| 778 per 1000 | 661 per 1000 (552 to 801) | |||||
| Cytomegalovirus infection Number of participants diagnosed with CMV infection Follow‐up: 1‐10 years | Study population | RR 0.97 (0.53 to 1.75) | 185 (4 studies) | ⊕⊕⊝⊝ low | ||
| 200 per 1000 | 194 per 1000 (106 to 350) | |||||
| Moderate | ||||||
| 186 per 1000 | 180 per 1000 (99 to 326) | |||||
| Post‐transplantation lymphoproliferative disorder Number of participants diagnosed with PTLD Follow‐up: 1‐10 years | See comment | See comment | Not estimable | 185 (4 studies) | ⊕⊕⊝⊝ low | |
| Cancer Number of participants diagnosed with cancer Follow‐up: 1‐10 years | Study population | RR 9 (0.52 to 156.91) | 185 (4 studies) | ⊕⊕⊝⊝ low | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
1 All trials with high risk of bias according to the Cochrane risk of bias assessment tool
Abbreviations: CMV = Cytomegalovirus; PTLD = post‐transplantation lymphoproliferative disorder
Summary of findings 5. Monoclonal antibody compared to polyclonal antibody for heart transplant recipients.
| Monoclonal antibody compared to polyclonal antibody for heart transplant recipients | ||||||
| Patient or population: heart transplant recipients Settings: hospital Intervention: monoclonal antibody Comparison: polyclonal antibody | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE)1 | Comments | |
| Assumed risk | Corresponding risk | |||||
| Polyclonal antibody | Monoclonal antibody | |||||
| Mortality Mortality at latest follow‐up Follow‐up: 6‐15 months | Study population | RR 1 (0.9 to 1.1) | 315 (7 studies) | ⊕⊕⊝⊝ low | ||
| 277 per 1000 | 277 per 1000 (250 to 305) | |||||
| Moderate | ||||||
| 182 per 1000 | 182 per 1000 (164 to 200) | |||||
| Acute rejection Acute rejection grade 3A or more verified by biopsy Follow‐up: 12 months | Study population | RR 0.96 (0.67 to 1.37) | 146 (3 studies) | ⊕⊕⊝⊝ low | ||
| 648 per 1000 | 622 per 1000 (434 to 888) | |||||
| Moderate | ||||||
| 762 per 1000 | 732 per 1000 (511 to 1000) | |||||
| Infection Number of participants diagnosed with infection Follow‐up: 6‐12 months | Study population | RR 1.12 (0.91 to 1.39) | 214 (5 studies) | ⊕⊕⊝⊝ low1 | ||
| 462 per 1000 | 517 per 1000 (420 to 642) | |||||
| Moderate | ||||||
| 526 per 1000 | 589 per 1000 (479 to 731) | |||||
| Cytomegalovirus infection Number of participants diagnosed with CMV infection Follow‐up: 12‐15 months | Study population | RR 1.32 (0.77 to 2.28) | 201 (4 studies) | ⊕⊕⊝⊝ low | ||
| 162 per 1000 | 213 per 1000 (124 to 368) | |||||
| Moderate | ||||||
| 162 per 1000 | 214 per 1000 (125 to 369) | |||||
| Post‐transplantation lymphoproliferative disorder Number of participants diagnosed with PTLD Follow‐up: mean 6‐15 months | Study population | RR 3.84 (0.45 to 32.96) | 157 (4 studies) | ⊕⊕⊝⊝ low | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Cancer Number of participants diagnosed with cancer Follow‐up: mean 6‐15 months | Study population | RR 2.19 (0.51 to 9.43) | 157 (4 studies) | ⊕⊕⊝⊝ low1 | ||
| 26 per 1000 | 56 per 1000 (13 to 242) | |||||
| Moderate | ||||||
| 18 per 1000 | 39 per 1000 (9 to 170) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
1 All trials with high risk of bias according to the Cochrane risk of bias assessment tool
Abbreviations: CMV = Cytomegalovirus; PTLD = post‐transplantation lymphoproliferative disorder
Background
Description of the condition
Heart transplantation is an established treatment option for end‐stage heart failure in selected patients. Heart transplantation is performed in people with end‐stage heart failure caused by a wide range of conditions such as, ischaemic heart disease and valvular heart disease in adult recipients, and cardiomyopathies and congenital heart disease in children. To date, more than 100,000 heart transplantations worldwide have been reported to the International Society for Heart and Lung Transplantation (ISHLT) (Stehlik 2012), and more than 5000 heart transplantations are performed annually. More than 85% of recipients survive for a year, and more than 75% survive for five years (Taylor 2009; Stehlik 2012).
Heart transplant recipients are at risk of significant morbidity and mortality due to their body's natural immunological rejection of the new heart, which necessitates lifelong immunosuppressive treatment (Lechler 2005). Ten years after transplantation 34% of all heart transplant recipients have had a malignancy (cancer). Furthermore, 97% of surviving recipients have hypertension, 93% have hyperlipidaemia (high levels of fats in the blood), 52% have angiographic chronic allograft vasculopathy (plaques that obstruct blood flow through the heart vessels), 39% have diabetes mellitus, and 14% have severe renal insufficiency (Taylor 2009).
Description of the intervention
Immunosuppressive treatment is necessary after heart transplantation to prevent rejection of the transplanted heart. Immunosuppressive treatment consists of induction treatment and maintenance treatment. Induction treatment is given at the time of transplantation ‐ only for a very short period ‐ while maintenance treatment, which also often starts at the time of transplantation, is given for a very long period, and often for life. Maintenance immunosuppressive therapy in heart transplantation often involves three types of drugs directed against the T‐cell activation and proliferation cascade, namely: antiproliferative agents (mycophenolate mofetil or azathioprine), calcineurin inhibitors (tacrolimus or cyclosporine), and steroids (prednisolone) (Flechner 2008). Mammalian target of rapamycin inhibitors can also be used as part of the immunosuppressive treatment (Flechner 2008). The right combination and dose of these drugs has been the focus of much debate, especially as calcineurin inhibitors are nephrotoxic (harm the kidneys) and the prolonged use of steroids can cause several complications (Oaks 2001; Cantarovich 2007; Flechner 2008; Penninga 2010). No combination of these maintenance immunosuppressive agents is able to prevent acute and chronic rejection and graft failure completely without causing adverse reactions (O'Neill 2004; Stevens 2004; Hauptman 2005; Kobashigawa 2006).
Induction treatment consisting of antibodies specific for T‐lymphocytes has also been used to prevent rejection (Kobashigawa 2006; Uber 2007). The aim of T‐cell‐specific antibody induction is to deplete the circulating T‐lymphocytes in the first days after transplantation before the full effect of maintenance immunosuppressive treatment is reached, and thus to reduce the number of acute rejections in the first months after transplantation. In addition, this temporary manipulation of the immune system by the use of antibody induction to promote acceptance of the graft, might allow for long‐term reduction of maintenance immunosuppressive treatment (Chatenoud 2008). However, T‐cell‐specific antibody induction causes profound immunosuppression and might increase the risk of infections, sepsis, post‐transplant lymphoproliferative disorder, and malignancies (Swinnen 1990; Uber 2007).
Antibody induction is usually started before, or at the same time as, maintenance immunosuppressive therapy, and is typically used for a short, defined period. Use of antibody induction may allow for delayed introduction, or a reduced dose, of calcineurin inhibitors (Potter 2005; Uber 2007).
To date, several T‐cell‐specific antibody induction agents have been used. These include polyclonal antibodies of horse or rabbit, specifically: anti‐thymocyte globulin (ATG) or anti‐lymphocyte globulin (ALG), or one of the monoclonal antibodies (other than interleukin‐2 receptor antagonists (IL‐2 RA)) specific for the CD3 receptor (muromonab‐CD3), the interleukin‐2 receptor (BT563 (Inolimomab), daclizumab or basiliximab), or the CD52 surface protein (alemtuzumab) (Uber 2007).
How the intervention might work
ATG, ALG, muromonab‐CD3 and alemtuzumab all tend to remove the functional T‐cell population from circulation, thereby causing profound immunosuppression. IL‐2 RAs have been developed to increase the specificity of immunosuppression, thereby avoiding the toxicity associated with over‐immunosuppression. These antagonists exert their effect through binding to the alpha subunit of the interleukin‐2 receptor found only on activated T‐cells. Blockade of the interleukin‐2 receptor results in prevention of interleukin‐2‐stimulated clonal expression of the T‐cell (Mueller 2004; Uber 2007).
T‐cell‐specific antibody induction is expected to reduce the risk of rejection, nevertheless data from the ISHLT Registry show that between 2004 and 2008, patients with one‐year follow‐up who received antibody induction were more likely to experience a treated rejection episode (24% to 30%) than those not treated with antibody induction (21%). It is possible, though, that patients at a higher risk for rejection could have been more likely to have received antibody induction (Taylor 2009).
Why it is important to do this review
In order to reduce morbidity and increase survival in heart transplant patients it is essential to identify the optimally safe and effective immunosuppressive treatment strategy (Kobashigawa 2006). At best, the recipient should develop immunological tolerance for the graft, and general immunity should not be interfered with (Chen 2006). In addition, the adverse effects of the different immunosuppressive agents should be avoided, so that these do not limit the patient's survival from complications related to renal and cardiovascular disease, and cancer (Groetzner 2005; Flechner 2008).
The ISHLT report that antibody induction is used in 47% of heart transplant recipients (Stehlik 2012). Patients received IL‐2 RAs (28% of all heart transplant recipients), polyclonal antibodies (18% of all heart transplant recipients), or the monoclonal antibody alemtuzumab (2% of all heart transplant recipients) (Stehlik 2012). No patients received muromonab‐CD3 (Stehlik 2012). The current pattern of practice for antibody induction in most centres is to give antibody induction to all patients, or to no patients. However, some centres give antibody induction only to patients with renal dysfunction, to allow for a reduction in the dose of calcineurin inhibitor, or only to patients with an increased risk of rejection. To date, there is no consensus on the use of immunosuppressive antibody induction after heart transplantation (Uber 2007).
Objectives
To review the benefits, harms, feasibility and tolerability of immunosuppressive T‐cell antibody induction versus placebo, or no antibody induction, or another kind of antibody induction for heart transplant recipients.
Methods
Criteria for considering studies for this review
Types of studies
All randomised clinical trials (RCTs) assessing immunosuppressive T‐cell antibody induction for heart transplant recipients.
Types of participants
First‐time heart transplant recipients, irrespective of age and sex. We analysed and reported data from adult and paediatric (child) patients separately, if possible, as differences in immunology between these populations might be expected (Zuppan 2009).
Types of interventions
Trials that compared any dose and duration of immunosuppressive antibody induction with:
interleukin‐2 receptor antagonists (IL‐2 RAs) (e.g. BT563 (Inolimomab), daclizumab, basiliximab);
polyclonal antibodies (anti‐thymocyte globulin (ATG), anti‐lymphocyte globulin (ALG));
monoclonal antibodies (other than IL‐2 RA, including alemtuzumab and muromonab‐CD3).
We analysed the following comparisons:
any kind of antibody induction versus no induction;
IL‐2 RA induction versus no induction;
monoclonal antibody (other than IL‐2 RA) versus no induction;
IL‐2 RA induction versus monoclonal antibody (other than IL‐2 RA) induction;
IL‐2 RA induction versus polyclonal antibody induction;
monoclonal antibody (other than IL‐2 RA) versus polyclonal antibody induction;
ATG versus ALG induction;
high‐dose ATG versus standard dose;
rabbit antithymocyte globulin (RATG) (Thymoglobulin) versus RATG manufactured by Fresenius (RATG‐Fresenius).
Types of outcome measures
Primary outcomes
The primary outcomes were:
mortality;
severe acute rejection ‐ proven by biopsy ‐ of level 3A or worse according to the classification of the ISHLT, which is equivalent to grade H2R in the recently revised classification (Stewart 2005). Grade H2R is defined according to the ISHLT classification as moderate rejection (that is, two or more foci with infiltrate and associated myocyte damage).
Secondary outcomes
The secondary outcome measures were:
quality of life;
infections as defined in the trials (not including Cytomegalovirus (CMV) infections);
CMV infection;
post‐transplantation lymphoproliferative disorder;
cancer;
steroid‐free immunosuppression;
calcineurin inhibitor reduction/free immunosuppression;
adverse events (not mentioned in any of the other outcomes). Serious adverse events were defined as any untoward medical occurrence that was life threatening, or resulted in death, or persistent or significant disability, or any medical event that might have jeopardised the patient, or required intervention to prevent it. All other adverse events (that is, any medical occurrence not necessarily having a causal relationship with the treatment, that did, however, cause a dose reduction or discontinuation of the treatment) were considered as non‐serious (ICH‐GCP 1996);
chronic allograft vasculopathy;
renal failure requiring chronic dialysis;
serum creatinine;
hypertension.
Search methods for identification of studies
Electronic searches
We searched the following databases:
The Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 11, 2012) (Master List 2009);
MEDLINE (Ovid) (1946 to November Week 1 2012) using the Cochrane highly sensitive strategy for the identification of RCTs (sensitivity‐maximizing version) (Lefebvre 2011);
EMBASE (Ovid) (1946 to 2012 Week 45) using search terms currently used by the UK Cochrane Centre to identify RCTs (Lefebvre 2011);
ISI Web of Science ‐ with Conference Proceedings (14 November 2012) (Royle 2003).
The search strategies were developed with input from the Cochrane Heart Group's Trial Search Co‐ordinator (Appendix 2).
No language restrictions were applied.
Searching other resources
We also searched the following resources:
the World Health Organization International Clinical Trials Registry Platform on www.who.int/trialsearch;
Current Controlled Trials metaRegister of Controlled trials (mRCT) on www.controlled‐trials.com/mrct;
reference lists of cardiology and transplant textbooks, review articles, and relevant studies;
bibliographies of relevant articles.
Data collection and analysis
Selection of studies
The search strategies described were used to obtain titles and abstracts of studies that might be relevant to the review. Two authors (LP and CM) independently assessed trial eligibility. We listed excluded trials with the reasons for their exclusion. We solved disagreements by discussion or consultation with a third author (CG). We contacted authors of the trials when information about methodology or data were unclear or missing.
Data extraction and management
Data extraction was carried out independently by three authors (LP, CM, FG) using standard data extraction forms (Moher 2009; Higgins 2011). Trials reported in non‐English language journals were translated before assessment. Trials were named according to the family name of the first author of the earliest full report of the trial to appear in a peer‐reviewed journal, together with the year of publication. Where no peer‐reviewed journal article was identified, we named the study using the family name of the first author of the earliest report, and the calender year of that report. Where more than one publication of one study existed, reports were grouped together and we included the publication with the most complete data. We used the data when data for relevant outcomes were published in earlier or later versions. We highlighted any discrepancies between published versions. We wrote to request any further information required from the original author and included any relevant information obtained in this manner in the review. Disagreements were resolved by consultation with all authors (Thompson 2002).
We extracted the following information from each trial:
first author;
country of origin;
trial design;
inclusion and exclusion criteria;
number of participants;
patients characteristics;
trial drugs: dose, administration, additional immunosuppressive therapy;
follow‐up period;
primary and secondary outcomes;
adverse events; and
patients lost to follow‐up.
Assessment of risk of bias in included studies
The following points were assessed using the Cochrane 'Risk of bias' assessment tool (Higgins 2011) (see Appendix 1).
Was there adequate sequence generation?
Was allocation adequately concealed?
Was knowledge of the allocated interventions adequately prevented during the trial?
Were incomplete outcome data adequately addressed?
Were reports of the trial free of suggestion of selective outcome reporting?
Was there any risk of bias due to vested interest?
Trials with adequate generation of the allocation sequence, adequate allocation concealment, adequate blinding, adequate outcome data reporting, no selective outcome reporting, and without vested interests were considered to be trials with a low risk of bias, while trials with one or more unclear or inadequate‐quality components were considered to be trials with a high risk of bias (Schulz 1995; Moher 1998; Kjaergard 2001; Savovic 2012). High inter‐rater agreement between blinded and unblinded assessments, as well as between two independent assessors, has been found previously (Kjaergard 2001; Gluud 2006).
Measures of treatment effect
For dichotomous outcomes, results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment, we used the mean difference (MD), and, when different scales were used, we used the standardised mean difference (SMD).
Unit of analysis issues
We planned to take into account unit of analysis issues (e.g. individuals undergoing more than one intervention, or multiple observations for one outcome). In these cases we planned to choose the first intervention, or the observation with longest follow‐up.
Dealing with missing data
We dealt with missing data in the following ways:
we contacted the original investigators to request missing data;
we performed sensitivity analyses to assess how sensitive our results were to reasonable changes in the assumptions that were made;
we addressed the potential impact of missing data on the findings of the review in the 'Discussion'
Assessment of heterogeneity
We analysed heterogeneity using the Chi² test with N‐1 degrees of freedom, and also with the I² test (Higgins 2002), where I² values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity, respectively.
Assessment of reporting biases
Had we identified more than 10 trials that investigated one comparison (for example antibody induction versus no induction), we planned to use a funnel plot to explore the presence of reporting bias (Egger 1997; Macaskill 2001), as asymmetry in funnel plots can be used to assess this bias. We planned to perform linear regression analysis to determine funnel plot asymmetry (Egger 1997).
Data synthesis
We performed the analyses using Review Manager 5 (RevMan 2012). We analysed data with a random‐effects model and a fixed‐effect model. When there was discrepancy between the two models, we reported both results, but otherwise we only reported results from the random‐effects model. Data were analysed according to the intention‐to‐treat principle and presented as risk ratios with 95% confidence intervals.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses for:
an individual antibody preparation compared to other classes of antibody preparation (ATG compared to IL‐2 RAs, etc);
antibody preparation compared to a different formulation of the same class of antibody preparation (basiliximab compared to daclizumab, etc);
trials at low risk of bias compared to trials at high risk of bias.
Sensitivity analysis
Zero‐event trials
RevMan 5 software is unable to handle trials with zero events in both intervention groups when meta‐analyses are performed to calculate risk ratios (RR) or odds ratios. It seems unjustified and unreasonable to exclude zero‐event trials (Keus 2009), and, potentially, to create the risk of inflating the magnitude of the pooled treatment effects. We, therefore, performed a random‐effects meta‐analysis with an empirical continuity correction of 0.01 in trials with zero events (Sweeting 2004).
Trial sequential analysis
Trial sequential analysis was applied, as cumulative meta‐analyses are at risk of producing random errors because of sparse data and repetitive testing on accumulating data (Wetterslev 2008; Thorlund 2011; TSA programme 2012). To minimise random errors we calculated the required information size (i.e. the number of participants needed in a meta‐analysis to detect or reject a certain intervention effect) (Wetterslev 2008; Wetterslev 2009). Information size calculations should also account for heterogeneity present in the meta‐analysis. In our meta‐analysis, the information size calculation was based on the assumption of a plausible RR reduction of 20%, or on the RR reduction observed in the included trials with low risk of bias (Wetterslev 2008). The underlying assumption of trial sequential analysis is that significance testing may be performed each time a new trial is added to the meta‐analysis. We planned to add the trials according to their year of publication, and, if more than one trial was published in a year, trials were to be added alphabetically according to the family name of the first author. On the basis of the risk for type I (5%) and type II (20%) errors, the chosen RR, the proportion with the outcome in the control group, and the observed heterogeneity, the required information size was calculated and the trial sequential alpha‐spending and beta‐spending monitoring boundaries were constructed (Wetterslev 2008; TSA manual 2011). These boundaries determined the statistical inference one may draw regarding a cumulative meta‐analysis that has not reached the required information size; if a trial sequential alpha‐spending or beta‐spending monitoring boundary is crossed before the required information size is reached in a cumulative meta‐analysis, firm evidence may have been established and further trials may be superfluous. On the other hand, if the alpha‐ and beta‐spending boundaries are not surpassed, it is most probably necessary to continue running trials in order to detect or reject a certain intervention effect. We used as default a type I error of 5%, a type II error of 20%, and adjusted information size for diversity unless otherwise stated (Wetterslev 2008; Thorlund 2011).
Results
Description of studies
Results of the search
Figure 1 depicts the results of our search strategy. Our predefined search identified 1495 references, with 53 references identified via additional sources. Exclusion of duplicates and irrelevant references left 22 RCTs published in a total of 31 publications (25 peer‐reviewed journal articles, and six conference abstracts) (Characteristics of included studies; Characteristics of excluded studies). Three of the trials were published both in peer‐reviewed journals, and as conference abstracts (Ippoliti 1991; Mattei 2007; Faggian 2010). Seventeen of the trials were only published in peer‐reviewed journals (Balk 1992; Beniaminovits 2000; Bolling 1989; Bonaros 2006; Carrier 2007; Costanzo‐Nordin 1990; De Santo 2004; Hershberger 2005; Kobashigawa 1993; Kormos 1990; Macdonald 1993; Mehra 2005; Menkis 1992; Schnetzler 2002; Segovia 2006; van Gelder 1996; Wollenek 1989). Two of the trials were published only as conference abstracts (van Gelder 2004; Mullen 2005).
1.

Study flow diagram
Included studies
We included 22 RCTs with a total of 1427 patients: nine trials studied interleukin‐2 receptor antagonists (IL‐2 RA) (four trials studied basiliximab (Mehra 2005; Segovia 2006; Mattei 2007; Carrier 2007); four trials studied daclizumab (Beniaminovits 2000; van Gelder 2004; Hershberger 2005; Mullen 2005); and two trials studied BT563 (van Gelder 1996; Bonaros 2006)). Ten trials studied monoclonal antibodies (other than IL‐2 RA) ‐ muromonab‐CD3 (OKT3) was the monoclonal antibody used in all these trials ‐ (Wollenek 1989; Costanzo‐Nordin 1990; Kormos 1990; Ippoliti 1991; Balk 1992; Menkis 1992; Kobashigawa 1993; Macdonald 1993; van Gelder 1996; Segovia 2006). Fifteen trials studied polyclonal antibodies (Wollenek 1989; Bolling 1989; Costanzo‐Nordin 1990; Kormos 1990; Ippoliti 1991; Balk 1992; Menkis 1992; Macdonald 1993; Schnetzler 2002; De Santo 2004; Mullen 2005; Bonaros 2006; Mattei 2007; Carrier 2007; Faggian 2010). Nine of the 15 trials that studied polyclonal antibodies used rabbit anti‐thymocyte globulin (RATG) (Wollenek 1989; Kormos 1990; Ippoliti 1991; Schnetzler 2002; De Santo 2004; Bonaros 2006; Mattei 2007; Carrier 2007; Faggian 2010), four trials used horse anti‐thymocyte globulin (ATG) (Bolling 1989; Costanzo‐Nordin 1990; Macdonald 1993; Mullen 2005), and three trials used anti‐lymphocyte globulin (ALG) (Bolling 1989; Balk 1992; Menkis 1992).
In our analyses we compared T‐cell antibody induction versus no antibody induction in five trials, with a total of 606 participants (Kobashigawa 1993; Beniaminovits 2000; van Gelder 2004; Hershberger 2005; Mehra 2005); IL‐2 RA versus no induction in four trials, with a total of 576 participants (Beniaminovits 2000; van Gelder 2004; Hershberger 2005; Mehra 2005); monoclonal antibody (other than IL‐2 RA) versus no antibody induction in one trial with 30 participants (Kobashigawa 1993); IL‐2 RA versus monoclonal antibody (other than IL‐2 RA) induction in two trials, with a total of 159 participants (van Gelder 1996; Segovia 2006); IL‐2 RA versus polyclonal antibody induction in four trials with a total of 185 participants (Mullen 2005; Bonaros 2006; Mattei 2007; Carrier 2007); monoclonal antibody (other than IL‐2 RA) versus polyclonal antibody induction in seven trials, with a total of 315 participants (Wollenek 1989; Costanzo‐Nordin 1990; Kormos 1990; Ippoliti 1991; Balk 1992; Menkis 1992; Macdonald 1993); and polyclonal antibody induction versus another kind or dose of polyclonal antibody in four trials, with a total of 162 participants (Bolling 1989; Schnetzler 2002; De Santo 2004; Faggian 2010).
Five of the 22 included trials were multicentre trials (Hershberger 2005; Mehra 2005; Segovia 2006; Mattei 2007; Carrier 2007), and the remaining 17 trials were single‐centre trials.
The population of 15 trials consisted exclusively of adult participants (Wollenek 1989; Bolling 1989; Kormos 1990; Menkis 1992; Kobashigawa 1993; Macdonald 1993; Beniaminovits 2000; Schnetzler 2002; Mehra 2005; Mullen 2005; Bonaros 2006; Segovia 2006; Mattei 2007; Carrier 2007; Faggian 2010); two trials included both adolescent and adult participants (Hershberger 2005 included participants over 13 years of age; van Gelder 1996 included one 14‐year old participant). Five trials did not report whether paediatric participants were included in the trial (Costanzo‐Nordin 1990; Ippoliti 1991; Balk 1992; van Gelder 2004; De Santo 2004).
The mean age of the treatment groups was reported in 21 of the trials, and ranged from 38 to 58 years between the trials; only van Gelder 2004 did not report this. In general, mean age of the treatment groups within the individual trials was comparable, and the largest age difference between treatment groups within a trial was six years.
Participants in the included trials were followed up for between six months and 10 years: follow‐up was six months in two trials (Ippoliti 1991; Menkis 1992); one year in 16 trials (Wollenek 1989; Bolling 1989; Costanzo‐Nordin 1990; Kormos 1990; Kobashigawa 1993; Macdonald 1993; Beniaminovits 2000; Schnetzler 2002; van Gelder 2004; Hershberger 2005; Mehra 2005; Mullen 2005; Segovia 2006; Mattei 2007; Carrier 2007); three years in one trial (De Santo 2004); five years in another (Faggian 2010); and 10 years in two trials (van Gelder 1996; Bonaros 2006).
Baseline immunosuppression
As described in our protocol, we required that maintenance immunosuppressive treatment was the same within all trials. All trials reported on maintenance immunosuppressive treatment, and this varied between trials.
All participants were treated with a calcineurin inhibitor, which in almost all trials was cyclosporine (Wollenek 1989; Bolling 1989; Costanzo‐Nordin 1990; Kormos 1990; Ippoliti 1991; Balk 1992; Menkis 1992; Kobashigawa 1993; Macdonald 1993; van Gelder 1996; Beniaminovits 2000; Schnetzler 2002; van Gelder 2004; De Santo 2004; Hershberger 2005; Mehra 2005; Bonaros 2006; Segovia 2006; Mattei 2007; Carrier 2007; Faggian 2010). Five trials delayed the start of cyclosporine treatment, starting at: between days two and four postoperatively (Kobashigawa 1993); day three (van Gelder 1996); between days three to five (Menkis 1992); and day five (Bonaros 2006; Faggian 2010).
Triple drug maintenance immunosuppression was given in all trials; this included cyclosporine, corticosteroids and an antiproliferative agent. The antiproliferative agents used differed between trials: 14 trials used azathioprine (Wollenek 1989; Bolling 1989; Costanzo‐Nordin 1990; Kormos 1990; Ippoliti 1991; Balk 1992; Menkis 1992; Kobashigawa 1993; Macdonald 1993; van Gelder 1996; Beniaminovits 2000; Schnetzler 2002; Bonaros 2006; Faggian 2010), and the remaining eight trials used mycophenolate mofetil (van Gelder 2004; De Santo 2004; Hershberger 2005; Mehra 2005; Mullen 2005; Segovia 2006; Mattei 2007; Carrier 2007).
Excluded studies
Twenty‐three studies were excluded after we read the full‐text of the articles. These studies were either not randomised, or did not assess T‐cell antibody induction, or used different maintenance immunosuppressive drugs in the treatment arms of individual trials. The reasons for exclusion are described in 'Characteristics of excluded studies'.
Risk of bias in included studies
Trial methodology was inadequately reported in all the included trials (Figure 2; Figure 3). All 22 trials were considered to be at high risk of bias, as one or more of the bias components assessed were either unclear, due to incomplete reporting, or had a high risk of bias.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Generation of the allocation sequence was adequate in seven trials (Kormos 1990; Macdonald 1993; De Santo 2004; Mehra 2005; Mullen 2005; Segovia 2006; Carrier 2007), and unclearly reported in the remaining 15 trials.
The method of allocation concealment was adequate in five trials (Costanzo‐Nordin 1990; Macdonald 1993; Beniaminovits 2000; De Santo 2004; Mehra 2005), and not adequately reported in the remaining 17 trials.
Blinding
Three trials reported that they were 'double‐blind', but provided no other information (van Gelder 2004; Hershberger 2005; Mehra 2005). Two trials were partially blinded (Kormos 1990; Mullen 2005); in one trial only participants were blinded for treatment group (Mullen 2005); and in another the pathologist who examined the endomyocardial biopsy specimens was blinded to the study drug assigned (Kormos 1990). One trial did not report on blinding (Costanzo‐Nordin 1990), and 16 trials were not blinded (Wollenek 1989; Bolling 1989; Ippoliti 1991; Balk 1992; Menkis 1992; Kobashigawa 1993; Macdonald 1993; van Gelder 1996; Beniaminovits 2000; Schnetzler 2002; De Santo 2004; Mullen 2005; Bonaros 2006; Segovia 2006; Mattei 2007; Carrier 2007; Faggian 2010).
Incomplete outcome data
In 18 trials either no data were missing, or missing data were adequately addressed, and it was unlikely that this influenced outcome results. In the remaining four trials, missing data were inadequately addressed, and it was unclear whether this influenced outcome results (Bonaros 2006; Faggian 2010; Mattei 2007; Menkis 1992).
Selective reporting
We only had access to a very limited number of trial protocols, however, all trials reported on the expected clinical outcome measures, including the outcome measures specified in the methods section of each article.
Other potential sources of bias
Five trials reported that they were industry‐sponsored or industry‐affiliated (Menkis 1992; Hershberger 2005; Mehra 2005; Segovia 2006; Mattei 2007); it was unclear whether two further trials were industry‐sponsored (Bolling 1989; Costanzo‐Nordin 1990). Fourteen trials had no other components detected, or described, that could put them at risk of other types of bias (Balk 1992; Beniaminovits 2000; Bonaros 2006; Carrier 2007; Costanzo‐Nordin 1990; De Santo 2004; Faggian 2010; Ippoliti 1991; Kobashigawa 1993; Kormos 1990; Macdonald 1993; Mullen 2005; Schnetzler 2002; van Gelder 1996; van Gelder 2004; Wollenek 1989).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Any kind of T‐cell antibody induction versus no induction
Five trials with 606 participants compared any kind of T‐cell antibody induction versus no antibody induction (Kobashigawa 1993; Beniaminovits 2000; van Gelder 2004; Hershberger 2005; Mehra 2005).
Mortality
All five trials reported adequately on mortality (606 participants; Analysis 1.1) (Kobashigawa 1993; Beniaminovits 2000; van Gelder 2004; Hershberger 2005; Mehra 2005), and no significant difference was found when any kind of antibody induction was compared with no antibody induction (27/299 (9%) versus 18/307 (6%); RR 1.53; 95% CI 0.85 to 2.76). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 11138 patients was not obtained.
1.1. Analysis.

Comparison 1 Antibody induction versus no induction, Outcome 1 Mortality.
Acute rejection
Acute rejection, defined as the number of participants who experienced at least one episode of biopsy‐proven severe acute rejection (equivalent to or worse than 3A), was reported in four of the five trials (576 participants; Analysis 1.2). No significant difference was found when any kind of antibody induction was compared with no induction (93/284 (33%) versus 132/292 (45%); RR 0.73; 95% CI 0.46 to 1.17) when the random‐effects model was applied. However, acute rejection was significantly less frequent when any kind of antibody induction was compared with no induction when the fixed‐effect model was applied (RR 0.73; 95% CI 0.59 to 0.90). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 4707 participants was not obtained.
1.2. Analysis.

Comparison 1 Antibody induction versus no induction, Outcome 2 Acute rejection.
Quality of life
None of the five trials in this category reported on quality of life (Kobashigawa 1993; Beniaminovits 2000; van Gelder 2004; Hershberger 2005; Mehra 2005).
Infection
Infection, defined as the number of participants who experienced at least one episode of infection, was reported in three trials (545 participants; Analysis 1.3). No significant difference was found when any kind of antibody induction was compared with no induction (96/269 (36%) versus 109/276 (39%); RR 0.99; 95% CI 0.79 to 1.24). Trial sequential analysis showed that the required information size of 1255 participants was not obtained, but the beta‐spending monitoring boundary (area of futility) was crossed, which means that we can reject a 20% intervention effect regarding this outcome.
1.3. Analysis.

Comparison 1 Antibody induction versus no induction, Outcome 3 Infection.
Cytomegalovirus (CMV) infection
Cytomegalovirus (CMV) infection was reported in all five trials (606 participants; Analysis 1.4) (Kobashigawa 1993; Beniaminovits 2000; van Gelder 2004; Hershberger 2005; Mehra 2005). No significant difference was found when any kind of antibody induction was compared with no induction (56/299 (19%) versus 68/307 (22%); RR 0.86; 95% CI 0.63 to 1.19). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 2576 participants was not obtained.
1.4. Analysis.

Comparison 1 Antibody induction versus no induction, Outcome 4 Cytomegalovirus infection.
Post‐transplantation lymphoproliferative disorder
Post‐transplantation lymphoproliferative disorder was reported in all five trials (606 participants; Analysis 1.5) (Kobashigawa 1993; Beniaminovits 2000; van Gelder 2004; Hershberger 2005; Mehra 2005). No significant difference was seen when any kind of antibody induction was compared with no induction (2/299 (1%) versus 3/307 (1%); RR 0.74; 95% CI 0.14 to 3.82). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 75005 participants was not obtained.
1.5. Analysis.

Comparison 1 Antibody induction versus no induction, Outcome 5 Post‐transplantation lymphoproliferative disorder.
Cancer
Cancer was reported in four of the five trials (576 participants; Analysis 1.6). No significant difference was seen when any kind of antibody induction was compared with no induction (11/284 (4%) versus 11/292 (4%); RR 1.01; 95% CI 0.45 to 2.28). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 17025 participants was not obtained.
1.6. Analysis.
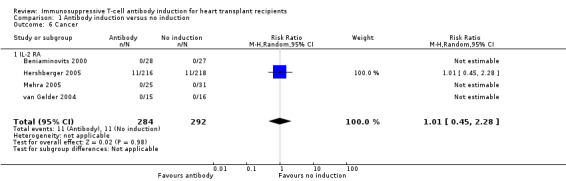
Comparison 1 Antibody induction versus no induction, Outcome 6 Cancer.
Adverse events
Drug‐associated adverse events were reported in two of the five trials (111 participants; Analysis 1.7). No significant difference in reported drug‐associated adverse events was found between any kind of antibody induction compared with no induction (21/53 (40%) versus 19/58 (33%); RR 1.37; 95% CI 0.99 to 1.90). However definitions of drug‐associated adverse events varied widely between trials. Hence in some trials, almost none of the participants were reported to have adverse events, while in other trials adverse events were reported for almost every participant. Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 1505 participants was not obtained.
1.7. Analysis.

Comparison 1 Antibody induction versus no induction, Outcome 7 Adverse events.
Chronic allograft vasculopathy
Chronic allograft vasculopathy was reported in one of the five trials (30 participants; Analysis 1.8). This trial showed no significant difference when antibody induction was compared with no induction (1/15 (7%) versus 1/15 (7%); RR 1.00; 95% CI 0.07 to 14.55). This was confirmed when Fischer's exact test was applied (P value 1.00).
1.8. Analysis.

Comparison 1 Antibody induction versus no induction, Outcome 8 Chronic allograft vasculopathy.
Corticosteroid and calcineurin inhibitor reduction/free immunosuppression
None of the five trials in this category reported on corticosteroid, or calcineurin inhibitor‐free or reduced immunosuppression (Kobashigawa 1993; Beniaminovits 2000; van Gelder 2004; Hershberger 2005; Mehra 2005).
Kidney function
One of the five trials reported on kidney failure requiring chronic dialysis (30 participants; Analysis 1.9). None of the participants in the trial needed chronic dialysis (0/15 (0%) versus 0/15 (0%)).
1.9. Analysis.

Comparison 1 Antibody induction versus no induction, Outcome 9 Renal failure requiring chronic dialysis.
This trial also reported on serum creatinine levels (μmol/L) (30 participants; Analysis 1.10). Serum creatinine was not statistically significantly different when any kind of antibody induction was compared with no induction (MD 26.50 μmol/L; 95% CI ‐2.16 to 55.16).
1.10. Analysis.

Comparison 1 Antibody induction versus no induction, Outcome 10 Serum creatinine.
None of the five trials in this category reported on glomerular filtration rate (GFR) (Kobashigawa 1993; Beniaminovits 2000; van Gelder 2004; Hershberger 2005; Mehra 2005).
Hyperlipidaemia and diabetes mellitus
None of the five trials in this category reported on hyperlipidaemia and diabetes mellitus (Kobashigawa 1993; Beniaminovits 2000; van Gelder 2004; Hershberger 2005; Mehra 2005).
Hypertension
Hypertension was reported in one trial with 56 participants (Analysis 1.11). No significant difference was seen in the number of participants treated for hypertension when any kind of antibody induction was compared with no induction (19/25 (76%) versus 25/31 (81%); RR 0.94; 95% CI 0.71 to 1.25). This was confirmed when Fischer's exact test was applied (P value 0.75).
1.11. Analysis.

Comparison 1 Antibody induction versus no induction, Outcome 11 Hypertension.
Interleukin‐2 receptor antagonist (IL‐2 RA) induction versus no induction
Four trials with 576 participants compared IL‐2 RA induction versus no antibody induction (Beniaminovits 2000; van Gelder 2004; Hershberger 2005; Mehra 2005).
Mortality
All four trials reported adequately on mortality (576 participants; Analysis 2.1) (Beniaminovits 2000; van Gelder 2004; Hershberger 2005; Mehra 2005). No significant difference was found when IL‐2 RA induction was compared with no induction (27/284 (10%) versus 18/292 (6%); RR 1.53; 95% CI 0.85 to 2.76). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 11,138 participants was not obtained (Figure 4). It is important to note that this analysis, Analysis 2.1, is identical to the analysis comparing any kind of T‐cell antibody induction versus no induction, Analysis 1.1. This is due to the fact that the extra trial in Analysis 1.1 did not have any events (no mortality) in both the experimental and the control group.
2.1. Analysis.

Comparison 2 Interleukin‐2 receptor antagonist (IL‐2 RA) versus no induction, Outcome 1 Mortality.
4.

Interleukin‐2 receptor antagonist induction versus no induction: mortality: trial sequential analysis of the effect of interleukin‐2 receptor antagonist induction versus no induction on mortality based on four trials with 576 participants. The diversity adjusted required information size (DARIS) of 11138 participants was calculated based on type I error of 5%, type II error of 20%, risk reduction of 20%, and information size was adjusted for diversity (0%). The cumulative Z‐curve does not cross trial sequential alpha and beta spending monitoring boundaries, and required information size was not reached.
Acute rejection
Acute rejection, defined as the number of participants who experienced at least one episode of biopsy‐proven severe acute rejection (equivalent to or worse than 3A) was reported in all four trials (576 participants; Analysis 2.2) (Beniaminovits 2000; van Gelder 2004; Hershberger 2005; Mehra 2005). No significant difference was found when IL‐2 RA induction was compared with no induction (93/284 (33%) versus 132/292 (45%); RR 0.73; 95% CI 0.46 to 1.17) when the random‐effects model was applied. However, acute rejection was significantly less frequent when IL‐2 RA induction was compared with no induction when the fixed‐effect model was applied (RR 0.73; 95% CI 0.59 to 0.90). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 4707 participants was not obtained (Figure 5).
2.2. Analysis.

Comparison 2 Interleukin‐2 receptor antagonist (IL‐2 RA) versus no induction, Outcome 2 Acute rejection.
5.

Interleukin‐2 receptor antagonist induction versus no induction: acute rejection: trial sequential analysis of the effect of interleukin‐2 receptor antagonist induction versus no induction on acute rejection based on four trials with 576 participants. The diversity adjusted required information size (DARIS) of 4707 participants was calculated based on type I error of 5%, type II error of 20%, risk reduction of 20%, and information size was adjusted for diversity (80%). The cumulative Z‐curve does not cross trial sequential alpha and beta spending monitoring boundaries, and required information size was not reached.
Quality of life
None of the trials reported on quality of life (Beniaminovits 2000; van Gelder 2004; Hershberger 2005; Mehra 2005).
Infection
Infection, defined as the number of participants who experienced at least one episode of infection, was reported in three of the four trials (545 participants; Analysis 2.3). No significant difference was found when IL‐2 RA induction was compared with no induction (96/269 (36%) versus 109/276 (39%); RR 0.99; 95% CI 0.79 to 1.24). Trial sequential analysis showed that the required information size of 1255 participants was not obtained, but the beta‐spending monitoring boundary (area of futility) was crossed, which means that we can reject a 20% intervention effect (Figure 6).
2.3. Analysis.

Comparison 2 Interleukin‐2 receptor antagonist (IL‐2 RA) versus no induction, Outcome 3 Infection.
6.

Interleukin‐2 receptor antagonist induction versus no induction: infection: trial sequential analysis of the effect of interleukin‐2 receptor antagonist induction versus no induction on infection based on three trials with 545 participants. The diversity adjusted required information size (DARIS) of 1255 participants was calculated based on type I error of 5%, type II error of 20%, risk reduction of 20%, and information size was adjusted for diversity (6%). The cumulative Z‐curve does not cross trial sequential alpha spending monitoring boundary, and required information size was not reached. However, the cumulative Z‐curve reaches the area of futility (trial sequential beta spending monitoring boundary), hence we can reject a difference of 20% of more between the groups regarding infection.
Cytomegalovirus (CMV) infection
CMV infection was reported in all four trials (576 participants; Analysis 2.4) (Beniaminovits 2000; van Gelder 2004; Hershberger 2005; Mehra 2005). No significant difference was found when IL‐2 RA induction was compared with no induction (55/284 (19%) versus 67/292 (23%); RR 0.86; 95% CI 0.62 to 1.19). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 2436 participants was not obtained.
2.4. Analysis.

Comparison 2 Interleukin‐2 receptor antagonist (IL‐2 RA) versus no induction, Outcome 4 Cytomegalovirus infection.
Post‐transplantation lymphoproliferative disorder
Post‐transplantation lymphoproliferative disorder was reported in all four trials (576 participants; Analysis 2.5) (Beniaminovits 2000; van Gelder 2004; Hershberger 2005; Mehra 2005). No significant difference was seen when IL‐2 RA induction was compared with no induction (1/284 (0%) versus 2/292 (1%); RR 0.61; 95% CI 0.08 to 4.92). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 70,005 participants was not obtained.
2.5. Analysis.

Comparison 2 Interleukin‐2 receptor antagonist (IL‐2 RA) versus no induction, Outcome 5 Post‐transplantation lymphoproliferative disorder.
Cancer
Cancer was reported in all four trials (576 participants; Analysis 2.6) (Beniaminovits 2000; van Gelder 2004; Hershberger 2005; Mehra 2005). No significant difference was seen when IL‐2 RA induction was compared with no induction (11/284 (4%) versus 11/292 (4%); RR 1.01; 95% CI 0.45 to 2.28). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 17,025 participants was not obtained.
2.6. Analysis.

Comparison 2 Interleukin‐2 receptor antagonist (IL‐2 RA) versus no induction, Outcome 6 Cancer.
Adverse events
Drug‐associated adverse events were reported in two of the four trials (111 participants; Analysis 2.7). No significant difference in reported drug‐associated adverse events was found between IL‐2 RA compared with no induction (21/53 (40%) versus 19/58 (33%); RR 1.37; 95% CI 0.99 to 1.90). However definitions of drug‐associated adverse events varied widely between trials. Hence, in some trials, almost none of the participants were reported to have adverse events, while in other trials adverse events were reported for almost every participant. Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 1505 participants was not obtained.
2.7. Analysis.

Comparison 2 Interleukin‐2 receptor antagonist (IL‐2 RA) versus no induction, Outcome 7 Adverse events.
Chronic allograft vasculopathy
None of the four trials in this category reported on chronic allograft vasculopathy (Beniaminovits 2000; van Gelder 2004; Hershberger 2005; Mehra 2005).
Corticosteroid and calcineurin inhibitor reduction/free immunosuppression
None of the four trials in this category reported on corticosteroid, or calcineurin inhibitor‐free or reduced immunosuppression (Beniaminovits 2000; van Gelder 2004; Hershberger 2005; Mehra 2005).
Kidney function
None of the four trials in this category reported on kidney failure requiring chronic dialysis, serum creatinine levels or GFR (Beniaminovits 2000; van Gelder 2004; Hershberger 2005; Mehra 2005).
Hyperlipidaemia and diabetes mellitus
None of the four trials in this category reported on hyperlipidaemia or diabetes mellitus (Beniaminovits 2000; van Gelder 2004; Hershberger 2005; Mehra 2005).
Hypertension
Hypertension was reported in one trial (56 participants; Analysis 2.8). No significant difference was seen in the number of participants treated for hypertension when IL‐2 RA induction was compared with no induction (19/25 (76%) versus 25/31 (81%); RR 0.94; 95% CI 0.71 to 1.25). This was confirmed when Fischer's exact test was applied (P value 0.74).
2.8. Analysis.

Comparison 2 Interleukin‐2 receptor antagonist (IL‐2 RA) versus no induction, Outcome 8 Hypertension.
Monoclonal antibody (other than IL‐2 RA) induction versus no induction
One trial with 30 participants compared monoclonal antibody (other than IL‐2 RA) induction versus no T‐cell antibody induction (Kobashigawa 1993).
Mortality
The one trial in this category, that compared monoclonal antibody induction with no induction, reported adequately on mortality (Analysis 1.1), but none of the 30 trial participants died (0/15 (0%) versus 0/15 (0%)).
Acute rejection
Acute rejection, using our definition of the number of participants who experienced at least one episode of biopsy‐proven severe acute rejection (equivalent to or worse than 3A), was not reported in this trial (30 participants) (Kobashigawa 1993), although the trial did report on the outcome, which it defined as the number of participants who experienced at least one episode of any kind of acute rejection. The trialists found no significant difference when they compared monoclonal antibody induction with no induction (10/15 (67%) versus 11/15 (73%); RR 0.91; 95% CI 0.57 to 1.45). This was confirmed when Fischer's exact test was applied (P value 1.00).
Quality of life
Quality of life was not reported in the included trial (Kobashigawa 1993).
Infection
Infection, defined as the number of participants who experienced at least one episode of infection, was not reported in the included trial (Kobashigawa 1993).
Cytomegalovirus (CMV) infection
CMV infection was reported in the Kobashigawa 1993 trial with 30 participants (Analysis 1.4). It found no significant difference when monoclonal antibody induction was compared with no induction (1/15 (7%) versus 1/15 (7%); RR 1.00; 95% CI 0.07 to 14.55). This was confirmed when Fischer's exact test was applied (P value 1.00).
Post‐transplantation lymphoproliferative disorder
Post‐transplantation lymphoproliferative disorder was reported in the Kobashigawa 1993 trial with 30 participants (Analysis 1.5). It found no significant difference when monoclonal antibody induction was compared no induction (1/15 (7%) versus 1/15 (7%); RR 1.00; 95% CI 0.07 to 14.55). This was confirmed when Fischer's exact test was applied (P value 1.00).
Cancer
Cancer was not reported in the included trial (Kobashigawa 1993).
Adverse events
Drug‐associated adverse events were not reported in the included trial (Kobashigawa 1993).
Chronic allograft vasculopathy
Chronic allograft vasculopathy was reported in the Kobashigawa 1993 trial with 30 participants (Analysis 1.8). It found no significant difference when monoclonal antibody induction was compared with no induction (1/15 (7%) versus 1/15 (7%); RR 1.00; 95% CI 0.07 to 14.55). This was confirmed when Fischer's exact test was applied (P value 1.00).
Corticosteroid and calcineurin inhibitor reduction/free immunosuppression
The Kobashigawa 1993 trial did not report on corticosteroid, or calcineurin inhibitor‐free or reduced immunosuppression.
Kidney function
The Kobashigawa 1993 trial reported on kidney failure requiring chronic dialysis (Analysis 1.9), but none of the 30 participants required chronic dialysis (0/15 (0%) versus 0/15 (0%)).
The Kobashigawa 1993 trial also reported on serum creatinine levels (μmol/L) (Analysis 1.10). The serum creatinine levels were not statistically significantly different between the intervention groups that compared induction with a monoclonal antibody (other than IL‐2 RA) with no induction (MD 26.50 μmol/L; 95% CI ‐2.16 to 55.16).
The Kobashigawa 1993 trial did not report on GFR.
Hyperlipidaemia, diabetes mellitus, or hypertension
Hyperlipidaemia, diabetes mellitus, or hypertension were not reported in the included trial (Kobashigawa 1993).
Interleukin‐2 receptor antagonist (IL‐2 RA) induction versus monoclonal antibody (other than IL‐2 RA) induction
Two trials, with 159 participants, compared IL‐2 RA induction versus monoclonal antibody induction (van Gelder 1996; Segovia 2006).
Mortality
Both trials in this category (van Gelder 1996; Segovia 2006), with a total of 159 participants, reported adequately on mortality (Analysis 3.1). No significant difference was found when IL‐2 RA induction was compared with monoclonal antibody induction (16/79 (20%) versus 17/80 (21%); RR 0.80; 95% CI 0.19 to 3.41). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 14,065 participants was not obtained.
3.1. Analysis.

Comparison 3 Interleukin‐2 receptor antagonist (IL‐2 RA) versus monoclonal antibody, Outcome 1 Mortality.
Acute rejection
Acute rejection, defined as the number of participants who experienced at least one episode of biopsy‐proven severe acute rejection (equivalent to or worse than 3A), was reported in both trials (159 participants; Analysis 3.2) (van Gelder 1996; Segovia 2006). No significant difference was found when IL‐2 RA induction was compared with monoclonal antibody (other than IL‐2 RA) induction (43/79 (54%) versus 44/80 (55%); RR 0.97; 95% CI 0.77 to 1.23). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 649 participants was not obtained.
3.2. Analysis.

Comparison 3 Interleukin‐2 receptor antagonist (IL‐2 RA) versus monoclonal antibody, Outcome 2 Acute rejection.
Quality of life
Neither of the trials reported on quality of life (van Gelder 1996; Segovia 2006).
Infection
Infection, defined as the number of participants who experienced at least one episode of infection, was reported in both trials (159 participants; Analysis 3.3) (van Gelder 1996; Segovia 2006). No significant difference was found when IL‐2 RA induction was compared with monoclonal antibody (other than IL‐2 RA) induction (43/79 (54%) versus 51/80 (64%); RR 0.91; 95% CI 0.54 to 1.53). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 1801 participants was not obtained.
3.3. Analysis.

Comparison 3 Interleukin‐2 receptor antagonist (IL‐2 RA) versus monoclonal antibody, Outcome 3 Infection.
Cytomegalovirus (CMV) infection
CMV infection was reported in both trials (159 participants; Analysis 3.4) (van Gelder 1996; Segovia 2006). No significant difference was found when IL‐2 RA induction was compared with monoclonal antibody (other than IL‐2 RA) induction (20/79 (25%) versus 23/80 (29%); RR 0.94; 95% CI 0.43 to 2.06). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 3857 participants was not obtained.
3.4. Analysis.

Comparison 3 Interleukin‐2 receptor antagonist (IL‐2 RA) versus monoclonal antibody, Outcome 4 Cytomegalovirus infection.
Post‐transplantation lymphoproliferative disorder
Post‐transplantation lymphoproliferative disorder was reported in both trials (159 participants) (Analysis 3.5). No significant difference was seen when IL‐2 RA induction was compared with monoclonal antibody (other than IL‐2 RA) induction (0/79 (0%) versus 1/80 (1%); RR 0.31; 95% CI 0.01 to 7.38). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 70,005 participants was not obtained.
3.5. Analysis.

Comparison 3 Interleukin‐2 receptor antagonist (IL‐2 RA) versus monoclonal antibody, Outcome 5 Post‐transplantation lymphoproliferative disorder.
Cancer
Cancer was reported in both trials (159 participants; Analysis 3.6) (van Gelder 1996; Segovia 2006). No significant difference was seen when IL‐2 RA induction was compared with monoclonal antibody (other than IL‐2 RA) induction (9/79 (11%) versus 10/80 (13%); RR 0.84; 95% CI 0.40 to 1.77). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 4799 participants was not obtained.
3.6. Analysis.

Comparison 3 Interleukin‐2 receptor antagonist (IL‐2 RA) versus monoclonal antibody, Outcome 6 Cancer.
Adverse events
Drug‐associated adverse events were reported in one trial with participants (Analysis 3.7). This trial reported a significant difference in drug‐associated adverse events when IL‐2 RA induction was compared with monoclonal antibody (other than IL‐2 RA) induction (0/48 (0%) versus 46/51 (90%); (RR 0.01; 95% CI 0.00 to 0.18) applying both the random‐effects and the fixed‐effect model. This was confirmed when Fisher's exact test was applied (P value < 0.0001). In this trial the major difference in drug‐related adverse events could be attributed to the fact that the majority of participants in the monoclonal antibody group experienced acute adverse events, after the infusion of muromonab‐CD3, that were related to the massive release of cytokines. These included headache, fever, hypotension (low blood pressure), neurological events, vomiting and diarrhoea. Definitions of drug‐associated adverse events varied widely between the trials included in this category. This meant that in some trials almost none of the participants were reported to have adverse events, while in other trials adverse events were reported for almost every participant.
3.7. Analysis.

Comparison 3 Interleukin‐2 receptor antagonist (IL‐2 RA) versus monoclonal antibody, Outcome 7 Adverse events.
Chronic allograft vasculopathy
Neither of the trials in this category reported on chronic allograft vasculopathy (van Gelder 1996; Segovia 2006).
Corticosteroid and calcineurin inhibitor reduction/free immunosuppression
Neither of the trials in this category reported on corticosteroid, or calcineurin inhibitor‐free or reduced immunosuppression (van Gelder 1996; Segovia 2006).
Kidney function
Neither of the trials in this category reported on kidney failure requiring chronic dialysis, serum creatinine or GFR (van Gelder 1996; Segovia 2006).
Hyperlipidaemia, diabetes mellitus and hypertension
Neither of the trials in this category reported on hyperlipidaemia, diabetes mellitus or hypertension (van Gelder 1996; Segovia 2006).
Interleukin‐2 receptor antagonist (IL‐2 RA) induction versus polyclonal antibody induction
Four trials with 185 participants compared IL‐2 RA induction versus polyclonal antibody induction (Mullen 2005; Bonaros 2006; Mattei 2007; Carrier 2007).
Mortality
All four trials (185 participants) reported adequately on mortality (Analysis 4.1). No significant difference was found when IL‐2 RA induction was compared with polyclonal antibody induction (21/90 (23%) versus 21/95 (22%); RR 1.10; 95% CI 0.65 to 1.88). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 2765 participants was not obtained.
4.1. Analysis.

Comparison 4 Interleukin‐2 receptor antagonist (IL‐2 RA) versus polyclonal antibody, Outcome 1 Mortality.
Acute rejection
Acute rejection, defined as the number of participants who experienced at least one episode of biopsy‐proven severe acute rejection (equivalent to or worse than 3A), was reported by all four trials (185 participants) (Analysis 4.2). These showed a statistically significant difference when IL‐2 RA induction was compared with polyclonal antibody induction when the fixed‐effect model was used (24/90 (27%) versus 10/95 (11%); RR 2.44; 95% CI 1.25 to 4.74). This was confirmed when the random‐effects model was applied (RR 2.43; 95% CI 1.01 to 5.86). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 5787 participants was not obtained.
4.2. Analysis.

Comparison 4 Interleukin‐2 receptor antagonist (IL‐2 RA) versus polyclonal antibody, Outcome 2 Acute rejection.
Quality of life
None of the four trials reported on quality of life (Mullen 2005; Bonaros 2006; Mattei 2007; Carrier 2007).
Infection
Infection, defined as the number of participants who experienced at least one episode of infection, was reported in three of the four trials (155 participants; Analysis 4.3). These showed no significant difference when IL‐2 RA induction was compared with polyclonal antibody induction (51/75 (68%) versus 64/80 (80%); RR 0.85; 95% CI 0.71 to 1.03). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 248 participants was not obtained.
4.3. Analysis.

Comparison 4 Interleukin‐2 receptor antagonist (IL‐2 RA) versus polyclonal antibody, Outcome 3 Infection.
Cytomegalovirus (CMV) infection
CMV infection was reported in all four trials (185 participants; Analysis 4.4) (Mullen 2005; Bonaros 2006; Mattei 2007; Carrier 2007). These showed no significant difference when IL‐2 RA induction was compared with polyclonal antibody induction (17/90 (19%) versus 19/95 (20%); RR 0.97; 95% CI 0.53 to 1.75). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 2729 participants was not obtained.
4.4. Analysis.

Comparison 4 Interleukin‐2 receptor antagonist (IL‐2 RA) versus polyclonal antibody, Outcome 4 Cytomegalovirus infection.
Post‐transplantation lymphoproliferative disorder
Post‐transplantation lymphoproliferative disorder was reported in all four trials (185 participants; Analysis 4.5) (Mullen 2005; Bonaros 2006; Mattei 2007; Carrier 2007). None of the participants in these four trials had post‐transplantation lymphoproliferative disorder (0/90 (0%) versus 0/95 (0%)). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 2729 participants was not obtained.
4.5. Analysis.

Comparison 4 Interleukin‐2 receptor antagonist (IL‐2 RA) versus polyclonal antibody, Outcome 5 Post‐transplantation lymphoproliferative disorder.
Cancer
Cancer was reported in all four trials (185 participants; Analysis 4.6) (Mullen 2005; Bonaros 2006; Mattei 2007; Carrier 2007). These showed no significant difference when IL‐2 RA induction was compared with polyclonal antibody induction (4/90 (4%) versus 0/95 (0%); RR 9.00; 95% CI 0.52 to 156.91). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 83,875 participants was not obtained.
4.6. Analysis.

Comparison 4 Interleukin‐2 receptor antagonist (IL‐2 RA) versus polyclonal antibody, Outcome 6 Cancer.
Adverse events
Drug‐associated adverse events were reported in three of the four trials (150 participants) (Analysis 4.7). These showed a significant difference in reported drug‐associated adverse events when IL‐2 RA was compared with polyclonal antibody induction (9/73 (12%) versus 27/62 (35%); RR 0.38; 95% CI 0.21 to 0.70) applying both the random‐effects and the fixed‐effect model. However definitions of drug‐associated adverse events varied widely between trials. Hence, in some trials, almost none of the participants were reported to have adverse events, while in other trials adverse events were reported for almost every participant. Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 970 participants was not obtained.
4.7. Analysis.

Comparison 4 Interleukin‐2 receptor antagonist (IL‐2 RA) versus polyclonal antibody, Outcome 7 Adverse events.
Chronic allograft vasculopathy
Chronic allograft vasculopathy was reported in two trials with 70 participants (Analysis 4.8). These showed no significant difference when IL‐2 RA induction was compared with polyclonal antibody induction (8/35 (23%) versus 4/35 (11%); RR 2.00; 95% CI 0.72 to 5.59).
4.8. Analysis.

Comparison 4 Interleukin‐2 receptor antagonist (IL‐2 RA) versus polyclonal antibody, Outcome 8 Chronic allograft vasculopathy.
Corticosteroid and calcineurin inhibitor reduction/free immunosuppression
None of the four trials in this category reported on corticosteroid, or calcineurin inhibitor‐free or reduced immunosuppression (Mullen 2005; Bonaros 2006; Mattei 2007; Carrier 2007).
Kidney function
None of the four trials in this category reported on kidney failure requiring chronic dialysis, serum creatinine, or GFR (Mullen 2005; Bonaros 2006; Mattei 2007; Carrier 2007).
Hyperlipidaemia, diabetes mellitus, or hypertension
None of the four trials in this category reported on hyperlipidaemia, diabetes mellitus, or hypertension (Mullen 2005; Bonaros 2006; Mattei 2007; Carrier 2007).
Monoclonal antibody (other than IL‐2 RA) induction versus polyclonal antibody induction
Seven trials, with 315 participants, compared monoclonal antibody induction versus polyclonal antibody induction (Wollenek 1989; Costanzo‐Nordin 1990; Kormos 1990; Ippoliti 1991; Menkis 1992; Balk 1992; Macdonald 1993). Three trials, with 157 participants, compared rabbit anti‐thymocyte globulin (RATG) with muromonab‐CD3 (Wollenek 1989; Kormos 1990; Ippoliti 1991); two trials, with 64 participants, compared horse anti‐thymocyte globulin (ATG) with muromonab‐CD3 (Costanzo‐Nordin 1990; Macdonald 1993); and two trials with 94 participants compared anti‐lymphocyte globulin (ALG) with muromonab‐CD3 (Balk 1992; Menkis 1992)
Mortality
All seven trials reported adequately on mortality (315 participants; Analysis 5.1). These found no significant difference when monoclonal antibody induction was compared with polyclonal antibody induction (42/160 (26%) versus 43/155 (28%); RR 1.00; 95% CI 0.90 to 1.10). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 1888 participants was not obtained.
5.1. Analysis.

Comparison 5 Monoclonal antibody versus polyclonal antibody, Outcome 1 Mortality.
Acute rejection
Acute rejection, defined as the number of participants who experienced at least one episode of biopsy‐proven acute rejection, was reported in three of the seven trials (146 participants; Analysis 5.2). These found no statistically significant difference when monoclonal antibody induction was compared with polyclonal antibody induction (50/75 (67%) versus 46/71 (65%); RR 0.96; 95% CI 0.67 to 1.37). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 1214 participants was not obtained.
5.2. Analysis.

Comparison 5 Monoclonal antibody versus polyclonal antibody, Outcome 2 Acute rejection.
Quality of life
None of the seven trials in this category reported on quality of life (Wollenek 1989; Costanzo‐Nordin 1990; Kormos 1990; Ippoliti 1991; Menkis 1992; Balk 1992; Macdonald 1993).
Infection
Infection, defined as the number of participants who experienced at least one episode of infection, was reported in five of the seven trials (214 participants; Analysis 5.3). These showed no significant difference when monoclonal antibody induction was compared with polyclonal antibody induction (53/110 (48%) versus 48/104 (46%); RR 1.12; 95% CI 0.91 to 1.39). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 900 participants was not obtained.
5.3. Analysis.

Comparison 5 Monoclonal antibody versus polyclonal antibody, Outcome 3 Infection.
Cytomegalovirus (CMV) infection
CMV infection was reported in four of the seven trials (201 participants; Analysis 5.4). These showed no significant difference when monoclonal antibody induction was compared with polyclonal antibody induction (20/102 (2%) versus 16/99 (16%); RR 1.32; 95% CI 0.77 to 2.28). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 3780 participants was not obtained.
5.4. Analysis.

Comparison 5 Monoclonal antibody versus polyclonal antibody, Outcome 4 Cytomegalovirus infection.
Post‐transplantation lymphoproliferative disorder
Post‐transplantation lymphoproliferative disorder was reported in four of the seven trials with (157 participants; Analysis 5.5). These showed no significant difference when monoclonal antibody induction was compared with polyclonal antibody induction (3/79 (4%) versus 0/78 (0%); RR 3.84; 95% CI 0.45 to 32.96). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 70,005 participants was not obtained.
5.5. Analysis.

Comparison 5 Monoclonal antibody versus polyclonal antibody, Outcome 5 Post‐transplantation lymphoproliferative disorder.
Cancer
Cancer was reported in four of the seven trials (157 participants; Analysis 5.6). These showed no significant difference when monoclonal antibody induction was compared with polyclonal antibody induction (5/79 (6%) versus 2/78 (3%); RR 2.19; 95% CI 0.51 to 9.43). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 22,911 participants was not obtained.
5.6. Analysis.

Comparison 5 Monoclonal antibody versus polyclonal antibody, Outcome 6 Cancer.
Adverse events
Drug‐associated adverse events were reported in five of the seven trials (215 participants; Analysis 5.7). These showed no significant difference in reported drug‐associated adverse events when monoclonal antibody induction was compared with polyclonal antibody induction (40/110 (36%) versus 3/105 (3%); RR 11.62; 95% CI 0.37 to 362.95) when the random‐effects model was applied. However, adverse events were significantly more frequent when monoclonal antibody induction was compared with polyclonal antibody induction applying the fixed‐effect model (RR 11.39; 95% CI 3.91 to 33.14). Definitions of drug‐associated adverse events varied widely between trials. Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 22,911 participants was not obtained.
5.7. Analysis.

Comparison 5 Monoclonal antibody versus polyclonal antibody, Outcome 7 Adverse events.
Chronic allograft vasculopathy
None of the seven trials in this category reported on chronic allograft vasculopathy (Wollenek 1989; Costanzo‐Nordin 1990; Kormos 1990; Ippoliti 1991; Menkis 1992; Balk 1992; Macdonald 1993).
Corticosteroid and calcineurin inhibitor reduction/free immunosuppression
None of the seven trials in this category reported on corticosteroid, or calcineurin inhibitor‐free or reduced immunosuppression (Wollenek 1989; Costanzo‐Nordin 1990; Kormos 1990; Ippoliti 1991; Menkis 1992; Balk 1992; Macdonald 1993).
Kidney function
Two trials reported on serum creatinine levels (μmol/L) (105 participants; Analysis 5.8). These showed that serum creatinine levels were not statistically significantly different when monoclonal antibody induction was compared with polyclonal antibody induction (MD 9.95 μmol/L; 95% CI ‐33.88 to 53.78).
5.8. Analysis.

Comparison 5 Monoclonal antibody versus polyclonal antibody, Outcome 8 Serum creatinine.
Neither of these trials reported on kidney failure that required chronic dialysis, or on GFR.
Hyperlipidaemia, diabetes mellitus, or hypertension
None of the seven trials in this category reported on hyperlipidaemia, diabetes mellitus, or hypertension (Wollenek 1989; Costanzo‐Nordin 1990; Kormos 1990; Ippoliti 1991; Menkis 1992; Balk 1992; Macdonald 1993).
Polyclonal anti‐thymocyte globulin (ATG) induction versus anti‐lymphocyte globulin (ALG) antibody induction
One trial, with 32 participants, compared ATG induction versus ALG induction (Bolling 1989).
Mortality
The one trial in this category reported on mortality (32 participants; Analysis 6.1) (Bolling 1989). It showed no significant difference when ATG induction was compared with ALG induction (1/19 (5%) versus 1/23 (4%); RR 1.21; 95% CI 0.08 to 18.09). This was confirmed when Fisher's exact test was applied (P value 1.00).
6.1. Analysis.

Comparison 6 Anti‐thymocyte globulin (ATG) versus anti‐lymphocyte globulin (ALG), Outcome 1 Mortality.
Acute rejection
Acute rejection, defined by the review authors as the number of participants who experienced at least one episode of biopsy‐proven severe acute rejection (equivalent to or worse than 3A), was not reported in this trial. However, the trial authors defined acute rejection as the number of participants who experienced at least one episode of any kind of biopsy‐proven acute rejection, and reported this outcome. There was no significant difference when ATG induction was compared with ALG induction (18/19 (95%) versus 19/23 (83%); RR 1.15; 95% CI 0.92 to 1.42). This was confirmed when Fisher's exact test was applied (P value 1.00).
Quality of life
The one trial in this category did not report on quality of life (Bolling 1989).
Infection
Infection, defined as the number of participants who experienced at least one episode of infection, was reported in this one trial (32 participants; Analysis 6.2) (Bolling 1989). There was a significant difference when ATG induction was compared with ALG induction (11/19 (58%) versus 6/23 (26%); RR 2.22; 95% CI 1.01 to 4.88) when applying both the random‐effects model and fixed‐effect model. However, this was not confirmed when Fisher's exact test was applied (P value 0.06).
6.2. Analysis.

Comparison 6 Anti‐thymocyte globulin (ATG) versus anti‐lymphocyte globulin (ALG), Outcome 2 Infection.
Cytomegalovirus (CMV) infection, post‐transplantation lymphoproliferative disorder, and cancer
The one trial in this category did not report on CMV infection, post‐transplantation lymphoproliferative disorder, or cancer (Bolling 1989).
Adverse events
Drug‐associated adverse events were reported in this trial (32 participants; Analysis 6.3) (Bolling 1989). There was no significant difference in reported drug‐associated adverse events when ATG induction was compared with ALG induction (0/19 (0%) versus 1/23 (4%); (RR 0.40; 95% CI 0.02 to 9.29).
6.3. Analysis.

Comparison 6 Anti‐thymocyte globulin (ATG) versus anti‐lymphocyte globulin (ALG), Outcome 3 Adverse events.
Chronic allograft vasculopathy, kidney function, hyperlipidaemia, diabetes mellitus, and hypertension
The one trial in this category did not report on chronic allograft vasculopathy, kidney failure requiring chronic dialysis, serum creatinine levels, GFR, hyperlipidaemia, diabetes mellitus, or hypertension (Bolling 1989).
Corticosteroid and calcineurin inhibitor reduction/free immunosuppression
The one trial in this category did not report on corticosteroid, or calcineurin inhibitor‐free or reduced immunosuppression (Bolling 1989).
High‐dose anti‐thymocyte globulin (ATG) induction versus standard dose ATG antibody induction
One trial, with 30 participants, compared high‐dose ATG induction versus standard dose ATG induction (Faggian 2010).
Mortality
The one trial in this category reported on mortality (30 participants; Analysis 7.1). There was no significant difference when high‐dose ATG induction was compared with standard dose ATG induction (1/14 (7%) versus 2/16 (13%); RR 0.57; 95% CI 0.06 to 5.65). This was confirmed when Fisher's exact test was applied (P value 1.00).
Acute rejection
Acute rejection, defined as the number of participants who experienced at least one episode of biopsy‐proven severe acute rejection (equivalent to or worse than 3A), was reported in one trial (30 participants; Analysis 7.2) (Faggian 2010). There was no significant difference when high‐dose ATG induction was compared with standard‐dose ATG induction (5/14 (36%) versus 4/16 (25%); RR 1.43; 95% CI 0.47 to 4.30). This was confirmed when Fisher's exact test was applied (P value 0.46).
7.2. Analysis.

Comparison 7 High‐dose anti‐thymocyte globulin (ATG) versus standard‐dose, Outcome 2 Acute rejection.
Quality of life
The one trial in this category did not report on quality of life (Faggian 2010).
Infection
Infection, defined as the number of participants who experienced at least one episode of infection, was reported in one trial (30 participants; Analysis 7.3) (Faggian 2010).There was no significant difference when high‐dose ATG induction was compared with standard‐dose ATG induction (6/14 (43%) versus 8/16 (50%); RR 0.86; 95% CI 0.39 to 1.87). This was confirmed when Fisher's exact test was applied (P value 0.73).
7.3. Analysis.

Comparison 7 High‐dose anti‐thymocyte globulin (ATG) versus standard‐dose, Outcome 3 Infection.
Cytomegalovirus (CMV) infection
CMV infection was reported in one trial (30 participants; Analysis 7.4) (Faggian 2010). There was no significant difference when high‐dose ATG induction was compared with standard‐dose ATG induction (3/14 (21%) versus 3/16 (19%); RR 1.14; 95% CI 0.27 to 4.78). This was confirmed when Fisher's exact test was applied (P value 1.00).
7.4. Analysis.
Comparison 7 High‐dose anti‐thymocyte globulin (ATG) versus standard‐dose, Outcome 4 Cytomegalovirus infection.
Post‐transplantation lymphoproliferative disorder
Post‐transplantation lymphoproliferative disorder was reported in one trial (30 participants; Analysis 7.5) (Faggian 2010). There was no significant difference when high‐dose ATG induction was compared with standard‐dose ATG induction (0/14 (0%) versus 1/16 (6%); RR 0.38; 95% CI 0.02 to 8.59). This was confirmed when Fisher's exact test was applied (P value 1.00).
7.5. Analysis.

Comparison 7 High‐dose anti‐thymocyte globulin (ATG) versus standard‐dose, Outcome 5 Post‐transplantation lymphoproliferative disorder.
Cancer
Cancer was reported in one trial (30 participants; Analysis 7.6) (Faggian 2010). There was no significant difference when high‐dose ATG induction was compared with standard‐dose ATG induction (1/14 (7%) versus 2/16 (13%); (RR 0.57; 95% CI 0.06 to 5.65). This was confirmed when Fisher's exact test was applied (P value 1.00).
7.6. Analysis.

Comparison 7 High‐dose anti‐thymocyte globulin (ATG) versus standard‐dose, Outcome 6 Cancer.
Adverse events
One trial reported drug‐associated adverse events (30 participants; Analysis 7.7) (Faggian 2010). There was no significant difference in reported drug‐associated adverse events when high‐dose ATG induction was compared with standard‐dose ATG induction (6/14 (43%) versus 4/16 (25%); RR 1.71; 95% CI 0.60 to 4.86). This was confirmed when Fisher's exact test was applied (P value 0.44).
7.7. Analysis.
Comparison 7 High‐dose anti‐thymocyte globulin (ATG) versus standard‐dose, Outcome 7 Adverse events.
Chronic allograft vasculopathy
One trial reported chronic allograft vasculopathy (30 participants; Analysis 7.8) (Faggian 2010). There was no significant difference when high‐dose ATG induction was compared with standard‐dose ATG induction (4/14 (29%) versus 8/16 (50%); RR 0.57; 95% CI 0.22 to 1.50). This was confirmed when Fischer's exact test was applied (P value 0.28).
7.8. Analysis.

Comparison 7 High‐dose anti‐thymocyte globulin (ATG) versus standard‐dose, Outcome 8 Chronic allograft vasculopathy.
Corticosteroid and calcineurin inhibitor reduction/free immunosuppression
The one trial included in this category did not report on corticosteroid, or calcineurin inhibitor‐free or reduced immunosuppression (Faggian 2010).
Kidney function
One trial reported on kidney failure that required chronic dialysis (30 participants; Analysis 7.9) (Faggian 2010). There was no significant difference when high‐dose ATG induction was compared with standard‐dose ATG induction (0/14 (0%) versus 2/16 (13%); RR 0.23; 95% CI 0.01 to 4.36). This was confirmed when Fischer's exact test was applied (P value 0.48).
7.9. Analysis.

Comparison 7 High‐dose anti‐thymocyte globulin (ATG) versus standard‐dose, Outcome 9 Renal failure requiring chronic dialysis.
This trial did not report on serum creatinine levels or GFR.
Hyperlipidaemia, diabetes mellitus, and hypertension
The one trial included in this category did not report on hyperlipidaemia, diabetes mellitus, or hypertension (Faggian 2010).
Rabbit anti‐thymocyte globulin (RATG‐Thymoglobulin) induction versus rabbit anti‐thymocyte globulin (RATG‐Fresenius) induction
Two trials, with a total of 90 participants, compared RATG‐Thymoglobulin versus RATG‐Fresenius induction (Schnetzler 2002; De Santo 2004). Although both are rabbit‐anti‐thymocyte globulins, RATG‐Thymoglobulin and RATG‐Fresenius are produced in different ways (De Santo 2004). Thymoglobulin consists of purified immunoglobulin G, and is obtained by hyper‐immunisation of rabbits with human thymocytes. RATG‐Fresenius is obtained by hyper‐immunisation of rabbits by an immortalized cellular line (Jurkat) (De Santo 2004).
Mortality
Both trials in this category reported on mortality (90 participants; Analysis 8.1) (Schnetzler 2002; De Santo 2004). There was no significant difference when RATG‐Thymoglobulin induction was compared with RATG‐Fresenius induction (5/46 (11%) versus 4/44 (9%); RR 1.18; 95% CI 0.34 to 4.06). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 7214 participants was not obtained.
8.1. Analysis.

Comparison 8 RATG‐Thymoglobulin versus RATG‐Fresenius, Outcome 1 Mortality.
Acute rejection
Acute rejection, defined as the number of participants who experienced at least one episode of biopsy‐proven severe acute rejection (equivalent to or worse than 3A), was reported in both trials (90 participants; Analysis 8.2) (Schnetzler 2002; De Santo 2004). There was no significant difference when RATG‐Thymoglobulin induction was compared with RATG‐Fresenius induction (10/46 (22%) versus 9/44 (20%); RR 1.05; 95% CI 0.48 to 2.26). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 2897 participants was not obtained.
8.2. Analysis.

Comparison 8 RATG‐Thymoglobulin versus RATG‐Fresenius, Outcome 2 Acute rejection.
Quality of life
Neither of the included trials reported on quality of life (Schnetzler 2002; De Santo 2004).
Infection
Infection, defined as the number of participants who experienced at least one episode of infection, was reported in both trials (90 participants; Analysis 8.3) (Schnetzler 2002; De Santo 2004). There was no significant difference when RATG‐Thymoglobulin induction was compared with RATG‐Fresenius induction (19/46 (41%) versus 21/44 (47%); (RR 0.80; 95% CI 0.55 to 1.18). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 868 participants was not obtained.
8.3. Analysis.

Comparison 8 RATG‐Thymoglobulin versus RATG‐Fresenius, Outcome 3 Infection.
Cytomegalovirus (CMV) infection
CMV infection was reported in both trials (90 participants; Analysis 8.4) (Schnetzler 2002; De Santo 2004). There was no significant difference when RATG‐Thymoglobulin induction was compared with RATG‐Fresenius induction (9/46 (20%) versus 8/44 (18%); RR 1.69; 95% CI 0.10 to 28.16). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 26,503 participants was not obtained.
8.4. Analysis.

Comparison 8 RATG‐Thymoglobulin versus RATG‐Fresenius, Outcome 4 Cytomegalovirus infection.
Post‐transplantation lymphoproliferative disorder
Post‐transplantation lymphoproliferative disorder was reported in one trial (40 participants; Analysis 8.5) (De Santo 2004), but none of the participants suffered from it.
8.5. Analysis.

Comparison 8 RATG‐Thymoglobulin versus RATG‐Fresenius, Outcome 5 Post‐transplantation lymphoproliferative disorder.
Cancer
Cancer was reported in one trial (40 participants; Analysis 8.6) (De Santo 2004), but none of the participants developed cancer.
8.6. Analysis.

Comparison 8 RATG‐Thymoglobulin versus RATG‐Fresenius, Outcome 6 Cancer.
Adverse events
Both trials in this category reported drug‐associated adverse events (90 participants; Analysis 8.7) (Schnetzler 2002; De Santo 2004). There was no significant difference in reported drug‐associated adverse events when RATG‐Thymoglobulin induction was compared with RATG‐Fresenius induction (3/46 (7%) versus 2/44 (5%); RR 1.50; 95% CI 0.28 to 8.04). Trial sequential analysis showed that trial sequential monitoring boundaries were not broken by the cumulative Z‐curve, and the required information size of 13,493 participants was not obtained.
8.7. Analysis.

Comparison 8 RATG‐Thymoglobulin versus RATG‐Fresenius, Outcome 7 Adverse events.
Chronic allograft vasculopathy
Only one trial reported chronic allograft vasculopathy (16 participants; Analysis 8.8) (De Santo 2004). There was no significant difference when RATG‐Thymoglobulin induction was compared with RATG‐Fresenius induction (2/9 (22%) versus 2/7 (29%); RR 0.78; 95% CI 0.14 to 4.23). This was confirmed when Fischer's exact test was applied (P value 1.00).
8.8. Analysis.

Comparison 8 RATG‐Thymoglobulin versus RATG‐Fresenius, Outcome 8 Chronic allograft vasculopathy.
Corticosteroid and calcineurin inhibitor reduction/free immunosuppression
Neither of the trials in this category reported on corticosteroid, or calcineurin inhibitor‐free or reduced immunosuppression (Schnetzler 2002; De Santo 2004).
Kidney function
Neither of the trials in this category reported on kidney failure that required chronic dialysis, serum creatinine levels, or GFR (Schnetzler 2002; De Santo 2004).
Hyperlipidaemia, diabetes mellitus, and hypertension
Neither of the trials in this category reported on hyperlipidaemia, diabetes mellitus, or hypertension (Schnetzler 2002; De Santo 2004).
Subgroup analyses
We performed our predefined subgroup analyses on:
the type of antibody applied (i.e. IL‐2 RA compared with monoclonal antibody (other than IL‐2 RA)) when any kind of antibody induction was compared with no antibody induction;
the type of IL‐2 RA applied (i.e. basiliximab compared with daclizumab) when IL‐2 RA induction was compared with no antibody induction;
the type of IL‐2 RA applied (i.e. basiliximab compared with BT563) when IL‐2 RA induction was compared with monoclonal antibody (other than IL‐2 RA) induction;
the type of IL‐2 RA applied (i.e. basiliximab compared with BT563) when IL‐2 RA induction was compared with polyclonal antibody induction;
the type of polyclonal antibody applied (i.e. RATG, horse ATG, and ALG) when polyclonal antibody induction was compared with monoclonal antibody induction.
For the outcome of acute rejection when comparing the type of polyclonal antibody applied (i.e. RATG, horse ATG, and ALG) when polyclonal antibody induction was compared with monoclonal antibody induction, RATG appeared to be better than horse ATG or ALG. We did not find any other significant differences in tests for subgroup differences for any outcome.
We were not able to perform our predefined analyses on trials assessed as being at low risk of bias compared to trials assessed as being at high risk of bias, as all of the included trials in the review were judged to be at high risk of bias.
Assessment of reporting bias
The number of randomised trials was less than 10 for all comparisons, so we did not perform funnel plots to assess reporting bias.
Discussion
Summary of main results
We identified 22 trials with a total of 1427 participants assessing the effects of different types of T‐cell antibody induction in heart transplant recipients. All trials were at a high risk of bias. Our meta‐analysis assessed mortality, acute rejection, infection, Cytomegalovirus (CMV) infection, post‐transplantation lymphoproliferative disorder, cancer, adverse events, chronic allograft vasculopathy, renal function, diabetes mellitus and hypertension. We compared any kind of antibody induction versus no antibody induction, interleukin‐2 receptor antagonists (IL‐2 RA) versus no antibody induction, monoclonal antibody versus no induction, IL2‐RA versus monoclonal antibody (other than IL‐2 RA) induction, monoclonal antibody versus polyclonal antibody induction, anti‐thymocyte globulin (ATG) versus anti‐lymphocyte globulin (ALG) induction, high‐dose ATG versus standard‐dose ATG, and RATG‐Thymoglobulin versus RATG‐Fresenius. Results of the most important outcomes of the main comparisons are summarised in Table 1 to Table 5.
Overall, no significant differences were found for any of the comparison for the outcome measures mortality, infection, CMV infection, post‐transplantation lymphoproliferative disorder, cancer, adverse events, chronic allograft vasculopathy, or renal function. No significant difference was found when IL2‐RA induction was compared with no induction when a random‐effects model was applied, but acute rejection was significantly less frequent when the same analysis was done using a fixed‐effect model. In addition, acute rejection was significantly statistically more frequent ‐ when both fixed‐effect and random‐effects models were applied ‐ in participants given IL2‐RA induction compared with those given polyclonal antibody induction. However, when trial sequential analyses was applied the trial sequential boundaries were not crossed, and the required information sizes were not reached, indicating that we cannot exclude random errors for this result.
We observed some occasional significant differences in adverse events in some of the comparisons, however the definitions of adverse events varied between trials, and numbers of participants and events in these comparisons were too small to allow definitive conclusions to be drawn.
We found no statistically significant differences regarding other outcome measures in any of the analyses. This means we have no evidence for an increase in: infection, CMV infection, cancer, and post‐transplantation lymphoproliferative disorder associated with the use of antibody induction. Though, trial sequential analysis showed that we can reject a 20% risk ratio reduction in infection when any kind of antibody induction was compared with no antibody induction.
Overall completeness and applicability of evidence
This systematic review examined the evidence from 22 included RCTs for the use of T‐cell antibody induction in heart transplant recipients. We could not obtain data regarding all our pre‐defined outcome measures, as the trials identified did not report on all of them.
Almost all trials reported on our primary outcome measures of mortality, and acute rejection. It is important to note that the analysis comparing any kind of T‐cell antibody induction with no induction (Analysis 1.1), and IL‐2 RA with no induction (Analysis 2.1), are identical . This is due to the fact that the extra trial in Analysis 1.1 did not have any events (no mortality) in either the experimental or the control group.
The majority of trials reported on infections, CMV infections, cancer and post‐transplantation lymphoproliferative disorder, though definition of outcomes (e.g. infection) varied between trials limiting the drawing of robust conclusions. None of the trials reported on quality of life, and few trials reported on chronic allograft vasculopathy, or renal function. Furthermore, the included trials reported sparsely on drug‐related adverse effects, and definition of these varied between trials with early trials reporting a very low risk of adverse events in participants, and later trials reporting adverse events in almost every participant. Furthermore, limited data were available to allow for meta‐analysis on drug‐specific adverse events, such as cytokine release syndrome for muromonab‐CD3, and haematological adverse events for anti‐thymocyte globulin. It is important to note that the largest trial performed on antibody induction in heart transplant recipients compared the IL‐2 RA, daclizumab, with placebo, and contributed 72% (434/606) of all participants to the comparison of 'any kind of antibody versus no induction' (Hershberger 2005). In this trial more participants died in the daclizumab group, and it has been suggested that, due to the double‐blinded nature of the trial, many of the participants also received antibodies or other cytolytic agents for treatment of rejection, causing over‐immunosuppression in those participants who had also received daclizumab induction (Hershberger 2005). When the majority of information comes from one trial there can be problems, however, exclusion of this trial from the analysis did not change the results for mortality and acute rejection significantly.
Not all types of T‐cell antibody induction currently available have been studied thoroughly in RCTs. Polyclonal antibodies are the most common type of antibody used in Europe for heart transplant recipients (Stehlik 2012), but we identified no RCTs comparing polyclonal antibody induction against no induction in heart transplantation. Furthermore, alemtuzumab (a monoclonal antibody) has been introduced during the last decade for induction after heart transplantation; although it is now used in approximately 2% of all heart transplant recipients (Stehlik 2012), we only identified one trial that studied alemtuzumab for induction in heart transplant recipients. This trial was excluded from the analysis according to our protocol, as there were differences in concomitant immunosuppressive treatment between the groups (Pham 2010).
In clinical practice the calcineurin inhibitor, tacrolímus, is currently used more often than cyclosporine (Penninga 2010; Stehlik 2012), though cyclosporine was administered in almost all of the RCTs included in our review. It is unclear whether choice of calcineurin inhibitor (cyclosporin or tacrolimus) influences results for antibody induction.
Quality of the evidence
We conducted the review according to the methods outlined in The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and also conducted trial sequential analysis (Wetterslev 2008; TSA programme 2012). The quality and quantity of available evidence limit our findings and interpretation of our findings. A total of 1443 participants were included in the meta‐analysis, but a limited number of participants, events, and trials were included in some of the comparisons. Hence, the occasional positive findings in individual trials and comparisons may be due to random errors. In addition, participants included in RCTs may not be representative of the general patient population. Only few trials included participants bridged to transplantation with mechanical assist devices, which are now used on 30% of all heart transplant recipients. Data from paediatric participants were also very limited. Some of the included trials were from the late 1980s and early 1990s and might not reflect current practice properly. While the age of recipients has not changed during the last decade, the reasons for requiring transplant have changed; there has been a decrease in patients transplanted for ischaemic cardiomyopathy, and the leading indication for transplantation is now non‐ischaemic cardiomyopathy.
Heart transplant recipients are a heterogeneous population where subsets of patients with specific risk factors for rejection (e.g. high antibody titres, difficult cross‐match, etc) might benefit more from T‐cell antibody induction than other groups. The trials did not report sufficiently on this aspect, and we were not able to assess whether males and females benefit differently from antibody induction.
Follow‐up in the included trials was for between six months and 10 years. Consequently, we have no evidence from all included RCTs on long‐term effects of T‐cell antibody induction on our outcome measures. Long‐term effects would be particularly relevant for outcome measures such as mortality, infection, chronic allograft vasculopathy, and cancer.
We explored the presence of statistical heterogeneity by the Chi2 test and measured the quantity of heterogeneity by the I² test (Higgins 2002). The Chi2 test has limited power in a meta‐analysis where trials have small sample sizes or are few in number, as in this review. This means that while a statistically significant result may indicate a problem with heterogeneity, a non‐significant result must not be taken as evidence of homogeneity. To reflect our concern with heterogeneity, we looked at both fixed‐effect and random‐effects models in our analyses, in order to provide more robust estimates of effect, and reported both models when there were differences between them. The random‐effects model takes intertrial variation into account and downgrades information from large trials, while the fixed‐effect model gives more weight to large trials and is less affected by intertrial variation. Indeed, our review showed some significant results when the fixed‐effect model was applied that were not statistically significant when the random‐effects model was applied. Many trials were at a high risk of bias, and overall, according to the 'Summary of findings' tables, the studies were of low quality.
With regard to the precision of our results, many outcomes in the included trials in our meta‐analysis included few participants and few events, and so there are wide confidence intervals around estimates of effect.
Potential biases in the review process
A comprehensive literature search was performed for this systematic review; we prespecified inclusion and exclusion criteria, and conducted data analysis.
It is known that risk of bias impacts on the estimated intervention effect, with trials with a high risk of bias tending to overestimate the beneficial effects of an intervention (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Savovic 2012). Of the 22 trials included in this review: seven (32%) reported adequate generation of the allocation sequence; five (23%) reported adequate allocation concealment; three (14%) were double‐ blinded; one was only blinded for participants (5%); one (5%) blinded the pathologist examining the myocardial biopsies for rejection; 18 (82%) adequately addressed incomplete outcome data; all 22 trials (100%) reported on outcome measures they would reasonably be expected to report; and 14 (64%) appeared to be free of other components that might put the trial at risk of bias. Accordingly, all trials were considered to have a high risk of bias. Therefore, the estimated intervention effect may be due to systematic errors.
The risk of random error is higher when data come from sources of limited information (e.g. individual trials with small sample sizes), so sources of information need to be sufficiently large to reduce the risk of random error and increase the chance of observing a true intervention effect (Wetterslev 2008). Therefore, we also analysed the data using trial sequential analysis. Trial sequential analysis is a statistical method that assesses the risk of random error caused by sparse data and formal or informal repetitive testing of accumulating data. Trial sequential analysis of the different outcomes in this review showed that the trial sequential monitoring boundaries, or the required 'information size', were not reached for any of the outcomes, except for infection. When assessing infection the area of futility was reached when any kind of antibody was compared with no induction, and when IL‐2 RAs were compared with no induction, indicating that we can reject a 20% intervention effect, although as these trials had a high risk of bias, these finding might still be subject to systematic errors.
The majority of trials included in this review recruited only adult participants (15/22 trials), and none of them recruited exclusively paediatric participants. Furthermore it is unlikely that results from the very few included paediatric participants would influence outcomes substantially. Hence, the results of this review are applicable for adult patients. Differences in immunology between adult and paediatric patients exist, and therefore results from adult trials cannot be applied freely in children.
Agreements and disagreements with other studies or reviews
Data from the registry of the International Society for Heart and Lung Transplantation (ISHLT), based on 100,000 heart transplants performed between January 2000 and June 2009, showed that IL‐2RA induction appeared to have a negative effect on survival (Stehlik 2012). We could not confirm this result with a statistically significant difference in our meta‐analysis (RR 1.53; 95% CI 0.85 to 2.76). Furthermore, ISHLT registry data showed that acute rejection during the first year after transplantation occurred less frequently in patients treated with IL‐2RAs than in those who received no induction, and that acute rejection occurred more frequently in patients treated with polyclonal antibody induction and muromonab‐CD3 induction than with no induction (Stehlik 2012). In accordance with the ISHLT registry (Stehlik 2012), we found a possible reduction in acute rejection when IL‐2RAs were compared with no induction, though we lack firm evidence on this point (Stehlik 2012). In addition, the ISHLT registry found that acute rejection was less frequent in patients treated with IL‐2RAs than in those treated with polyclonal antibody induction (Stehlik 2012). In contrast to this ISHLT registry result, we found an increase in acute rejection when IL‐2RAs were compared with polyclonal antibodies (RR 2.43; 95% CI 1.01 to 5.86).
Using data from the Scientific Registry of Transplant Recipients from the USA, which includes 3209 patients, Kobashigawa and colleagues found that induction with the IL‐2RA, daclizumab, reduced acute rejection, and did not increase mortality or risk of infection (Kobashigawa 2005). Our meta‐analysis showed similar results with a possible reduction in acute rejection and no effect on mortality and infection associated with the use of IL‐2RAs. However, as our data were not robust to choice of meta‐analytic model, and assessment with trial sequential analysis, we do not think that our data based on evidence from RCTs can validate the observational data from trial registries. Such observational data are at risk of systematic errors (Deeks 2003)
Higgins and colleagues tried to test the hypothesis that antibody induction improves survival in patients at greatest risk of fatal rejection, and worsens survival in patients at low risk of rejection (Higgins 2005). They analysed data from the Cardiac Transplant Research Database on 5187 patients from North American heart‐transplant institutions who either had received induction (muromonab‐CD3 or polyclonal antibodies) or no induction (Higgins 2005). Survival was slightly better in patients who did not receive antibody induction. Multivariate analysis showed that the patients who benefit most from antibody induction have a combination of young age, black ethnicity, more than four human leukocyte antigen mismatches, and longer‐term (more than six months) mechanical assist device support (Higgins 2005). Our meta‐analysis did not allow identification of patients with different risk factors.
Due to the observational nature of the data, these findings from the different registries should be interpreted with caution because they are not adjusted for category of diagnosis, centre, or other potentially confounding factors, and the risk exists that transplant recipients at high risk of rejection were more likely to receive antibody induction (Higgins 2005; Kobashigawa 2005; Stehlik 2012).
Non‐randomised studies
Our search strategy revealed 32 studies that we excluded from the review after reading the full‐text of the article. These studies were either not randomised, or did not assess T‐cell antibody induction, or used different maintenance immunosuppressive drugs in the treatment arms of the trial (Characteristics of excluded studies). We assessed these studies for harmful effects associated with the use of antibody induction, as adverse effects might be under‐reported in RCTs. In these studies we did not find evidence for any apparent adverse events that differed in incidence or nature from those reported in the RCTs, except from some studies suggesting differences in post‐transplantation lymphoproliferative disorder and cancer. One non‐randomised study found a nine‐fold increase in post‐transplantation lymphoproliferative disorder in patients treated with muromonab‐CD3 compared with no induction (9/79 (11%) versus 1/75 (1%)) (Swinnen 1990). Many of the affected patients in this study had received muromonab‐CD3 both for induction and for treatment of rejection, and a sharp increase in post‐transplantation lymphoproliferative disorder was seen with cumulative doses over 75 mg (Swinnen 1990). This dramatic increase in post‐transplantation lymphoproliferative disorder associated with antibody induction has not been found in other studies (Mallinger 1999; Peraira 2003). We found no increased risk of post‐transplantation lymphoproliferative disorder associated with antibody induction in our review, however, the incidence of post‐transplantation lymphoproliferative disorder is low, and we cannot exclude a potential effect.
A non‐randomised study found an increase in cancer in patients treated with muromonab‐CD3 (4/19 (21%)) and anti‐lymphocyte globulin (13/67 (19%)) compared with RATG (38/388 (10%)) (Rinaldi 2001). However, follow‐up in the patients receiving muromonab‐CD3 and ALG was longer (Rinaldi 2001). Another study found no increased incidence in cancer when RATG was compared with no induction, but suggested that people treated with RATG developed cancer earlier after transplantation (El‐Hamamsy 2005). We did not find an increased cancer risk in any of the comparisons in this review.
Antibody induction in other solid organ transplant recipients
Traditionally, knowledge about immunosuppressive treatment for heart transplantation has been gained from experience from other types of organ transplantation, especially renal transplantation. A Cochrane review investigating the use of IL‐2RAs in kidney transplant recipients patients, which includes 71 studies and a total of 10,537 participants (Webster 2010), compared IL‐2RAs with placebo and showed that treatment with IL‐2RAs reduced graft loss, including death with a functioning graft, by 25% at six months and one year, but not beyond this (Webster 2010). Furthermore, when compared with placebo, IL‐2RAs reduced biopsy‐proven acute rejection (RR 0.75, 95% CI 0.58 to 0.98) and CMV disease (RR 0.81, 95% CI 0.68 to 0.97) (Webster 2010). Where IL‐2RAs were compared with ATG in kidney transplant recipients, biopsy‐proven acute rejection at one year was increased in the IL‐2RA group by 30%, but malignancies (RR 0.25, 95% CI 0.07 to 0.87), and CMV disease (RR 0.68, 95% CI 0.50 to 0.97) were reduced (Webster 2010).
In another Cochrane review, we studied the use of antibody induction in liver transplant recipients; this review included 17 trials with a total of 1951 participants. We found no statistically significant difference when any kind of antibody induction was compared with no antibody induction regarding mortality, acute rejection, infections, or cancer (Penninga 2012).
In a different Cochrane review, we also studied the use of antibody induction in lung transplant recipients; this review included six trials with a total of 278 participants (Penninga 2011). The review found no clear benefits or harms in lung transplant recipients associated with the use of T‐cell antibody induction compared with no induction, or when one type of T‐cell antibody was compared with another type of antibody (Penninga 2011).
Similarly, in this current review, we found no clear evidence for an increase in infections or cancer associated with antibody induction in heart transplant recipients, nor did we find any clear benefits, other than a possible reduction in acute rejection.
Authors' conclusions
Implications for practice.
There are limitations to this review due to the size and nature of the included trials (all trials had a high risk of bias). This systematic review did not show any clear beneficial or harmful effects associated with the use of antibody induction regarding mortality, infection, Cytomegalovirus infection, post‐transplantation lymphoproliferative disorder or cancer. When interleukin‐2 receptor antagonists (IL‐2 RAs)were compared with no induction using a fixed‐effect model a reduction in acute rejection was found, but this could not be replicated using the random‐effects model, and trial sequential analysis could not exclude random errors. Furthermore all trials had a high risk of bias. Accordingly, we cannot support or reject T‐cell antibody induction for heart transplant recipients. When IL‐2 RA induction was compared with polyclonal antibody induction an increase in acute rejection may exist, but random errors could not be excluded by trial sequential analysis.
Implications for research.
Our results suggest that appropriately‐sized randomised trials comparing T‐cell antibodies versus placebo in heart transplant patients receiving contemporarily adjunctive immunosuppression are warranted. These trials should study intervention with basiliximab (currently the only commercially available IL‐2 RA), anti‐thymocyte globulin, or alemtuzumab versus matching placebo. These trials should be conducted with low risks of systematic error (bias) and low risk of random error (play of chance), and should follow the 'CONSORT' guidelines.
Acknowledgements
We would like to thank
the Cochrane Heart Group for their support in preparing this review,
the referees for their comments and feedback during the preparation of the protocol and review.
the investigators and participants of the included RCTs ‐ without their initiative we would have nothing to review.
Appendices
Appendix 1. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
| Was there adequate sequence generation? | Yes (low risk of bias): random‐number table; computer random‐number generator; coin‐tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation (minimisation may be implemented without a random element, and this is considered to be equivalent to being random) |
| No (high risk of bias): sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention | |
| Unclear: insufficient information about the sequence generation process to permit judgement | |
| Was allocation adequately concealed? | Yes (low risk of bias): randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes). |
| No (high risk of bias): using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially‐numbered); alternation or rotation; date of birth; case‐record number; any other explicitly unconcealed procedure | |
| Unclear: randomisation stated but no information on method used is available | |
| Was knowledge of the allocated interventions adequately prevented during the study? | Yes (low risk of bias): no blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken; either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias |
| No (high risk of bias): no blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken; either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias | |
| Unclear: insufficient information to permit judgement of ‘Yes’ or ‘No' | |
| Was there baseline imbalance in important characteristics? | Adequate, if there was no baseline imbalance in important characteristics |
| Unclear, if the baseline characteristics were not reported | |
| Inadequate, if there was a baseline imbalance due to chance or due to imbalanced exclusion after randomisation | |
| Were incomplete outcome data adequately addressed? | Yes (low risk of bias): no missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods |
| No (high risk of bias): reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation | |
| Unclear: insufficient information to permit judgement of ‘Yes’ or ‘No' | |
| Are reports of the study free of suggestion of selective outcome reporting? | Yes (low risk of bias): the study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon) |
| No (high risk of bias): not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: insufficient information to permit judgement of ‘Yes’ or ‘No' | |
| Was 'early stopping' addressed adequately? | Yes, adequate, if sample size calculation was reported and the trial was not stopped, or the trial was stopped early by formal stopping rules at a point where the likelihood of observing an extreme intervention effect due to chance was low |
| Unclear, if sample size calculation was not reported and it is not clear whether the trial was stopped early or not | |
| No, inadequate, if the trial was stopped early due to informal stopping rules | |
| Was there 'vested interest bias'? | No risk of vested interest bias, if the trial's source of funding did not come from parties that might have a conflicting interest or if the authors had not previously published trials on similar interventions |
| Unclear, if the source of funding was not clear | |
| Risk of vested interest bias, if the trial was funded by a drug manufacturer or the authors had previously published trials on similar interventions |
Appendix 2. Detailed search strategies
CENTRAL
#1 MeSH descriptor Heart Transplantation explode all trees #2 (heart or cardiac) near3 transplant* #3 (#1 OR #2) #4 MeSH descriptor Antibodies, Monoclonal explode all trees #5 monoclonal next antibody* #6 polyclonal next antibody* #7 (muromonab* or OKT3 or "OKT 3" or ortho clone) #8 (Zenapax or abciximab or abciximab or daclizumab or daclizumab or LO‐Tact‐1 or BT563) #9 (alemtuzumab or campath* or mabcampath*) #10 (basiliximab or simulect or "chi 621") #11 MeSH descriptor Interleukin‐2 explode all trees #12 interleukin*2 receptor antagonist* or IL*2*RA* #13 MeSH descriptor Antilymphocyte Serum, this term only #14 (antithymocyte or "anti thymocyte" or antithymus or Atgam or ATG* or thymoglobulin* or thymocyte or antithymoglobulin* or anti‐thymoglobulin* or "anti next thymoglobulin*") #15 (ahlbulin or antilymphocyt* or anti‐lymphocyte* or "anti next lymphocyte*" or lymphoglobulin* or ALG) #16 "induction therapy" #17 MeSH descriptor Immunosuppression explode all trees #18 MeSH descriptor Immunosuppressive Agents, this term only #19 (#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18) #20 (#3 AND #19)
MEDLINE (Ovid)
1. exp Heart Transplantation/ 2. ((heart or cardiac) adj3 transplant*).ti,ab. 3. 1 or 2 4. exp antibodies, monoclonal/ 5. monoclonal antibod*.tw. 6. polyclonal antibod*.tw. 7. (muromonab* or OKT3 or OKT 3 or orthoclone).tw. 8. (zenapax or daclizumab or dacliximab or daclizumab or LO‐Tact‐1 or BT563).tw. 9. (alemtuzumab or campath* or mabcampath*).tw. 10. (basiliximab or simulect or chi 621).tw. 11. Interleukin‐2/ 12. (interleukin*2 receptor antagonist* or IL*2*RA*).tw. 13. Antilymphocyte Serum/ 14. (antithymocyte or anti‐thymocyte or antithymus or Atgam or ATG or thymoglobulin* or thymocyte or (thymus adj (antiserum or antibod*)) or antithymoglobulin* or anti‐thymoglobulin*).tw. 15. (ahlbulin or antilymphocyt* or anti‐lymphocyte* or lymphoglobulin* or ALG).tw. 16. induction therapy.tw. 17 or/4‐16 18. 3 and 17 19. randomised controlled trial.pt. 20. controlled clinical trial.pt. 21. randomized.ab. 22. placebo.ab. 23. drug therapy.fs. 24. randomly.ab. 25. trial.ab. 26. groups.ab. 27. 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 28. exp animals/ not humans.sh. 29. 27 not 28 30. 18 and 29
EMBASE (Ovid)
1. exp heart transplantation/ 2. ((heart or cardiac) adj3 transplant*).ti,ab. 3. 1 or 2 4. exp monoclonal antibody/ 5. monoclonal antibod*.tw. 6. polyclonal antibody/ 7. polyclonal antibod*.tw. 8. OKT 3/ 9. (muromonab* or OKT3 or OKT 3 or orthoclone).tw. 10. daclizumab/ 11. (zenapax or daclizumab or dacliximab or daclizumab or LO‐Tact‐1 or BT563).tw. 12. alemtuzumab/ 13. (alemtuzumab or campath* or mabcampath*).tw. 14. basiliximab/ 15. (basiliximab or simulect or chi 621).tw. 16. Interleukin‐2/ 17. (interleukin*2 receptor antagonist* or IL*2*RA*).tw. 18. thymocyte antibody/ 19. (antithymocyte or anti‐thymocyte or antithymus or Atgam or ATG or thymoglobulin* or thymocyte or (thymus adj (antiserum or antibod*)) or antithymoglobulin* or anti‐thymoglobulin*).tw. 20. lymphocyte antibody/ 21. (ahlbulin or antilymphocyt* or anti‐lymphocyte* or lymphoglobulin* or ALG).tw. 22. induction therapy.tw. 23. or/4‐22 24. 3 and 23 25. random$.tw. 26. factorial$.tw. 27. crossover$.tw. 28. cross over$.tw. 29. cross‐over$.tw. 30. placebo$.tw. 31. (doubl$ adj blind$).tw. 32. (singl$ adj blind$).tw. 33. assign$.tw. 34. allocat$.tw. 35. volunteer$.tw. 36. crossover procedure/ 37. double blind procedure/ 38. randomised controlled trial/ 39. single blind procedure/ 40. 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 41. (animal/ or nonhuman/) not human/ 42. 40 not 41 43. 24 and 42
ISI Web of Science with Conference Proceedings
#1 TS="heart transplant*" #2 TS="cardiac transplant*" #3 #2 OR #1 #4 TS=("monoclonal antibod*" or "polyclonal antibod*") #5 TS=(muromonab* or OKT3 or "OKT 3" or orthoclone) #6 TS=(zenapax or daclizumab or dacliximab or daclizumab or LO‐Tact‐1 or BT563) #7 TS=(alemtuzumab or campath* or mabcampath*) #8 TS=(basiliximab or simulect or "chi 621") #9 TS=(interleukin*2 receptor antagonist* or IL*2*RA*).tw. #10 TS=(antithymocyte or anti‐thymocyte or "anti thymocyte" or antithymus or Atgam or ATG or thymoglobulin* or thymocyte or "thymus antiserum" or "thymus antibod*" or antithymoglobulin* or anti‐thymoglobulin* or "anit thymoglobulin*") #11 TS=(ahlbulin or antilymphocyt* or anti‐lymphocyte* or "anti lymphocyt*" or lymphoglobulin* or ALG) #12 TS="induction therapy" #13 #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 #14 #13 AND #3 #15 TS=(random* or blind* or placebo* or "clinical trial") #16 #15 AND #14
World Health Organization International Clinical Trials Registry Platform & Current Controlled Trials metaRegister of Controlled trials (mRCT)
1. heart transplant OR heart transplantation OR cardiac transplant OR cardiac transplantation.
Data and analyses
Comparison 1. Antibody induction versus no induction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 5 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.85, 2.76] |
| 1.1 Interleukin‐2 receptor antagonists (IL‐2 RAs) | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.85, 2.76] |
| 1.2 Monoclonal antibody | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Acute rejection | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.46, 1.17] |
| 2.1 IL‐2 RA | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.46, 1.17] |
| 3 Infection | 3 | 545 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.79, 1.24] |
| 3.1 IL‐2 RA | 3 | 545 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.79, 1.24] |
| 4 Cytomegalovirus infection | 5 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.63, 1.19] |
| 4.1 IL‐2 RA | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.62, 1.19] |
| 4.2 Monoclonal antibody | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.55] |
| 5 Post‐transplantation lymphoproliferative disorder | 5 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.14, 3.82] |
| 5.1 IL‐2 RA | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.08, 4.92] |
| 5.2 Monoclonal antibody | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.55] |
| 6 Cancer | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.45, 2.28] |
| 6.1 IL‐2 RA | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.45, 2.28] |
| 7 Adverse events | 2 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.99, 1.90] |
| 7.1 IL‐2 RA | 2 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.99, 1.90] |
| 8 Chronic allograft vasculopathy | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.55] |
| 8.1 Monoclonal antibody | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.55] |
| 9 Renal failure requiring chronic dialysis | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 Monoclonal antibody | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Serum creatinine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 26.50 [‐2.16, 55.16] |
| 10.1 Monoclonal antibody | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 26.50 [‐2.16, 55.16] |
| 11 Hypertension | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.71, 1.25] |
Comparison 2. Interleukin‐2 receptor antagonist (IL‐2 RA) versus no induction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.85, 2.76] |
| 1.1 Basiliximab | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 3.72 [0.41, 33.61] |
| 1.2 Daclizumab | 3 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.67, 2.74] |
| 2 Acute rejection | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.46, 1.17] |
| 2.1 Basiliximab | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.72, 2.53] |
| 2.2 Daclizumab | 3 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.40, 0.96] |
| 3 Infection | 3 | 545 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.79, 1.24] |
| 3.1 Basiliximab | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.87, 1.48] |
| 3.2 Daclizumab | 2 | 489 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.69, 1.13] |
| 4 Cytomegalovirus infection | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.62, 1.19] |
| 4.1 Basiliximab | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.23, 2.15] |
| 4.2 Daclizumab | 3 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.51, 1.49] |
| 5 Post‐transplantation lymphoproliferative disorder | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.08, 4.92] |
| 5.1 Basiliximab | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Daclizumab | 3 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.08, 4.92] |
| 6 Cancer | 4 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.45, 2.28] |
| 6.1 Basiliximab | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Daclizumab | 3 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.45, 2.28] |
| 7 Adverse events | 2 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.99, 1.90] |
| 7.1 Basiliximab | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.99, 1.90] |
| 7.2 Daclizumab | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Hypertension | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.71, 1.25] |
| 8.1 Basiliximab | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.71, 1.25] |
Comparison 3. Interleukin‐2 receptor antagonist (IL‐2 RA) versus monoclonal antibody.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.19, 3.41] |
| 1.1 Basiliximab | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.10, 1.23] |
| 1.2 BT563 | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.74, 3.13] |
| 2 Acute rejection | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.23] |
| 2.1 Basiliximab | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.60, 1.55] |
| 2.2 BT563 | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.75, 1.27] |
| 3 Infection | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.54, 1.53] |
| 3.1 Basiliximab | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.55, 0.99] |
| 3.2 BT563 | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.72, 2.16] |
| 4 Cytomegalovirus infection | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.43, 2.06] |
| 4.1 Basiliximab | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.50, 1.35] |
| 4.2 BT563 | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.31, 25.48] |
| 5 Post‐transplantation lymphoproliferative disorder | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.38] |
| 5.1 Basiliximab | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 BT563 | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 7.38] |
| 6 Cancer | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.40, 1.77] |
| 6.1 Basiliximab | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 BT563 | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.40, 1.77] |
| 7 Adverse events | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.01 [0.00, 0.18] |
| 7.1 Basiliximab | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.01 [0.00, 0.18] |
Comparison 4. Interleukin‐2 receptor antagonist (IL‐2 RA) versus polyclonal antibody.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 4 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.65, 1.88] |
| 1.1 Basiliximab | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.35, 1.66] |
| 1.2 Daclizumab | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.16, 6.20] |
| 1.3 BT563 | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.75, 3.71] |
| 2 Acute rejection | 4 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 2.43 [1.01, 5.86] |
| 2.1 Basiliximab | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [0.96, 5.62] |
| 2.2 Daclizumab | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 3.85] |
| 2.3 BT563 | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 5.5 [1.39, 21.71] |
| 3 Infection | 3 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.71, 1.03] |
| 3.1 Basiliximab | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.71, 1.07] |
| 3.2 BT563 | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.52, 1.24] |
| 4 Cytomegalovirus infection | 4 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.53, 1.75] |
| 4.1 Basiliximab | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.36, 1.81] |
| 4.2 Daclizumab | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 4.94] |
| 4.3 BT563 | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.4 [0.53, 3.68] |
| 5 Post‐transplantation lymphoproliferative disorder | 4 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Basiliximab | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Daclizumab | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 BT563 | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cancer | 4 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [0.52, 156.91] |
| 6.1 Basiliximab | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Daclizumab | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 BT563 | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [0.52, 156.91] |
| 7 Adverse events | 3 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.21, 0.70] |
| 7.1 Basiliximab | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.21, 0.71] |
| 7.2 Daclizumab | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 BT563 | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.72] |
| 8 Chronic allograft vasculopathy | 2 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.72, 5.59] |
| 8.1 Daclizumab | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 BT563 | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.72, 5.59] |
Comparison 5. Monoclonal antibody versus polyclonal antibody.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 7 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.90, 1.10] |
| 1.1 Rabbit anti‐thymocyte globulin (RATG) | 3 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.39, 2.82] |
| 1.2 Horse anti‐thymocyte globulin (ATG) | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.35, 2.81] |
| 1.3 Anti‐lymphocyte globulin (ALG) | 2 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.90, 1.10] |
| 2 Acute rejection | 3 | 146 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.67, 1.37] |
| 2.1 Rabbit anti‐thymocyte globulin (RATG) | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.87, 1.91] |
| 2.2 Horse anti‐thymocyte globulin (ATG) | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.58, 1.19] |
| 3 Infection | 5 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.91, 1.39] |
| 3.1 Rabbit anti‐thymocyte globulin (RATG) | 3 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.69, 1.39] |
| 3.2 Horse anti‐thymocyte globulin (ATG) | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.89, 1.66] |
| 3.3 Anti‐lymphocyte globulin (ALG) | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.72, 2.11] |
| 4 Cytomegalovirus infection | 4 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.77, 2.28] |
| 4.1 Rabbit anti‐thymocyte globulin (RATG) | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.13, 6.13] |
| 4.2 Horse anti‐thymocyte globulin (ATG) | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.85, 2.89] |
| 4.3 Anti‐lymphocyte globulin (ALG) | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.10, 2.60] |
| 5 Post‐transplantation lymphoproliferative disorder | 4 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 3.84 [0.45, 32.96] |
| 5.1 Horse anti‐thymocyte globulin (ATG) | 2 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 4.62 [0.25, 86.72] |
| 5.2 Anti‐lymphocyte globulin (ALG) | 2 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 3.11 [0.13, 73.11] |
| 6 Cancer | 4 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [0.51, 9.43] |
| 6.1 Horse anti‐thymocyte globulin (ATG) | 2 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.20, 20.33] |
| 6.2 Anti‐lymphocyte globulin (ALG) | 2 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [0.36, 15.20] |
| 7 Adverse events | 5 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 11.62 [0.37, 362.95] |
| 7.1 Rabbit anti‐thymocyte globulin (RATG) | 2 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 55.45 [3.50, 877.43] |
| 7.2 Horse anti‐thymocyte globulin (ATG) | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 3.5 [1.12, 10.90] |
| 7.3 Anti‐lymphocyte globulin (ALG) | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Serum creatinine | 2 | 105 | Mean Difference (IV, Random, 95% CI) | 9.95 [‐33.88, 53.78] |
| 8.1 Rabbit anti‐thymocyte globulin (RATG) | 1 | 82 | Mean Difference (IV, Random, 95% CI) | ‐9.70 [‐11.80, ‐7.60] |
| 8.2 Horse anti‐thymocyte globulin (ATG) | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 35.40 [3.64, 67.16] |
Comparison 6. Anti‐thymocyte globulin (ATG) versus anti‐lymphocyte globulin (ALG).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.08, 18.09] |
| 2 Infection | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.01, 4.88] |
| 3 Adverse events | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.02, 9.29] |
Comparison 7. High‐dose anti‐thymocyte globulin (ATG) versus standard‐dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 Acute rejection | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.47, 4.30] |
| 3 Infection | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.39, 1.87] |
| 4 Cytomegalovirus infection | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.27, 4.78] |
| 5 Post‐transplantation lymphoproliferative disorder | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.02, 8.59] |
| 6 Cancer | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.06, 5.65] |
| 7 Adverse events | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.60, 4.86] |
| 8 Chronic allograft vasculopathy | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.22, 1.50] |
| 9 Renal failure requiring chronic dialysis | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.01, 4.36] |
| 10 Mortality | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.06, 5.65] |
7.10. Analysis.

Comparison 7 High‐dose anti‐thymocyte globulin (ATG) versus standard‐dose, Outcome 10 Mortality.
Comparison 8. RATG‐Thymoglobulin versus RATG‐Fresenius.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.34, 4.06] |
| 2 Acute rejection | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.48, 2.26] |
| 3 Infection | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.55, 1.18] |
| 4 Cytomegalovirus infection | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.10, 28.16] |
| 5 Post‐transplantation lymphoproliferative disorder | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cancer | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Adverse events | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.28, 8.04] |
| 8 Chronic allograft vasculopathy | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.14, 4.23] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Balk 1992.
| Methods | Comparison: polyclonal antibody vs monoclonal antibody Trial design: single‐centre randomised trial Language: English Type of publication: journal article Judgement of trial quality: high risk of bias Cross‐over between treatment groups: no Sample size calculation: not reported Intention to treat analysis: yes |
|
| Participants | Setting: University Hospital Rotterdam Dijkzigt, The Netherlands Inclusion period: 1 August 1989‐1 August 1991 Allocation: 55 participants: ALG n = 28; muromonab‐CD3 n = 27 Sex ratio: ALG 23 (82%) males:5 (18%) females; muromonab‐CD3 22 (81%) males:5 (19%) females Mean age (range): ALG 45 (18‐61) years; muromonab‐CD3 48 (15‐62) years Indication (no (%)): Cardiomyopathy: ALG 16 (57%); muromonab‐CD3 14 (52%) Ishaemic heart disease: ALG 12 (43%); muromonab‐CD3 10 (37%) Vascular cardiac disease: ALG 0 (0%); muromonab‐CD3 3 (11%) Inclusion criteria: all consecutive heart transplant recipients Exclusion criteria: not specified |
|
| Interventions | Intervention therapy: ALG (horse lymphocyte‐specific IgG2, Lymphoglobulin, Institut Merieux) started postoperatively 1‐2 h after arrival at ICU, 425 lymphocytotoxic units daily (0.5 ml//kg body weight) for 7 days Control therapy: muromonab‐CD3 (OKT3, Ortho Pharmaceutical, Raritan NJ), started postoperatively 1‐2 h after arrival at ICU, 5 mg daily for 7 days Concomittant immunosuppressive treatment: cyclosporine, started at day 5 azathioprine corticosteroids |
|
| Outcomes | Survival, rejection, adverse events, infections, cancer Follow‐up period: 15 months (range 3‐25 months) |
|
| Notes | Endomyocardial biopsies in all participants Acute rejection: histological examination of endomyocardial biopsies and graded according to Billingham's criteria of none, mild, moderate and severe rejection Study designer: not reported Sources of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed, but the trial reported on mortality, rejection, infection, cancer, PTLD and adverse events |
| Other bias | Low risk | The trial appeared to be free of other components of bias |
Beniaminovits 2000.
| Methods | Comparison: IL‐2 RA (daclizumab) vs no antibody Trial design: single‐centre RCT, 2 parallel groups Language: English Type of publication: journal article Judgement of trial quality: high risk of bias Cross‐over between treatment groups: no, but 4 participants in each group were switched from cyclosporine to tacrolimus due to adverse events Sample size calculation: not reported Intention to treat analysis: yes |
|
| Participants | Setting: Columbia‐Presbyterian Medical Center, New York USA Inclusion period: January‐December 1998 Allocation: 55 participants: daclizumab n = 28; control n = 27 Sex ratio: daclizumab 22 (79%) men:6 (21%) women; control 21 (78%) men:6 (22%) women Mean age: daclizumab 53 ± 14 years; control 50 ± 13 years Adult:paediatric participants: 100% adults Indication (no (%)): not reported Inclusion criteria: adult, consecutive non‐sensitized recipients of first cardiac transplants Exclusion criteria: recipients of previous allografts or had a positive cross‐match for T‐cell lymphocytes |
|
| Interventions | Intervention therapy: daclizumab 1 mg/kg iv within 12 h of transplantation, and every 2 weeks thereafter for a total of 5 doses Control therapy: no induction Concomittant immunosuppressive treatment: cyclosporine azathioprine steroids |
|
| Outcomes | Primary: biopsy‐confirmed rejection, severity of rejection and length of time to first treatment episode of rejection Secondary: need for anti‐lymphocytic therapy with OKT3 or ATG, indicators of allo‐reactivity, duration of hospitalisation, readmission, incidence of infections and cancers, 12‐month survival Follow‐up period: 12 months |
|
| Notes | Endomyocardial biopsies: weekly for the first month, every 2 weeks for the second month, thereafter monthly until the sixth month and then every second month until 12 months postoperatively OKT3 or ATG antibody induction was used instead of cyclosporine in participants with prolonged renal dysfunction Acute rejection was defined as histologic grade 2 (ISHLT) or higher Sources of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned, not otherwise reported |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed, but the trial reported on mortality, rejection, infection, cancer, PTLD and adverse events |
| Other bias | Low risk | The trial appeared to be free of other components of bias |
Bolling 1989.
| Methods | Comparison: polyclonal antibody vs other type of polyclonal antibody Trial design: single‐centre RCT Language: English Type of publication: journal article Judgement of trial quality: high risk of bias Cross‐over between treatment groups: no Sample size calculation: not reported Intention to treat analysis: yes |
|
| Participants | Setting: University of Michigan, Michigan, USA Inclusion period: December 1986‐August 1988 Allocation: 42 participants: horse ATG (Atgam) n = 19; Minnesota ALG (MALG) n = 23 Sex ratio: Atgam 16 (84%) men:3 (16%) women; MALG 17 (74) men:6 (26%) women Mean age: Atgam 51 ± 1 years; MALG 46 ± 3 years Adult:paediatric participants: 100% adults Indication (no (%)): Not reported, however reported that transplant indication (ischaemic versus idiopathic cardiomyopathy) was not different between Atgam and MALG groups Inclusion criteria: participants who met the standard criteria for end‐stage heart failure, requiring orthotopic heart transplantation Exclusion criteria: age < 30 years, or > 60 years |
|
| Interventions | Intervention A: Atgam 15 mg/kg/day in 500 ml of normal saline given immediately postoperatively in ICU, then daily for a total of 7 doses, or until the serum cyclosporine level reached 250 ng/ml Intervention B: Atgam, 10 mg/kg/day iv in 500 ml of normal saline given immediately postoperatively in ICU, and then daily for a total of 7 doses, or until the serum cyclosporine level reached 250 ng/ml Concomittant immunosuppressive treatment: cyclosporine azathioprine corticosteroids |
|
| Outcomes | Survival, rejection, adverse events, infections Follow‐up period: 10‐13 months |
|
| Notes | Endomyocardial biopsies and right heart catheterisation were performed at 10‐14 days postoperative. Following this, biopsies and catheterisation were obtained as dictated by the results of the first biopsy, and the clinical postoperative course Acute rejection; definition by the criteria of Billingham Study designer: not reported Sources of funding: not reported, but thanked Upjohn Pharmaceuticals (manufacturer of ATGAM) and University of Minnesota for assistance |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated at time of transplant, (however optimal randomisation was not obtained due to unavailability of a specific agent) |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed, but the trial reported on mortality, rejection, infection, and adverse events |
| Other bias | Unclear risk | It was unclear whether the trial was industry‐sponsored; the acknowledgement states, "Upjohn Pharmaceuticals (manufacturer of ATGAM) and University of Minnesota were thanked for assistance." |
Bonaros 2006.
| Methods | Comparison: IL‐2 RA (BT563/BB10) vs polyclonal antibody Trial design: single‐centre, RCT, 2 parallel groups. stratified for recipient/donor CMV serological status Language: English Type of publication: journal article Judgement of trial quality: high risk of bias Cross‐over between treatment groups: no Sample size calculation: not reported Intention to treat analysis: no, 8 participants excluded post‐randomisation |
|
| Participants | Setting: Vienna Medical University, Vienna, Austria Inclusion period: Apr 1991–Apr 1992 Allocation: 40 participants: BT563/BB10 n = 20; RATG = 20 Sex ratio: BT563/BB10 19 (95%) men:1 (5%) woman; RATG 17 (85%) men:3 (15%) women Mean age: BT563/BB10 44.6 ± 9.4 years; RATG 49.7 ± 10.8 years Adult:paediatric participants: 100% adults Indication (no (%)): Congestive cardiomyopathy: BT563/BB10 13 (65%); RATG 11 (55%) Coronary artery disease: BT563/BB10 5 (25%); RATG 9 (45%) Inclusion criteria: orthotopic cardiac transplantation Exclusion criteria: participants < 16 years of age, requiring pre‐operative mechanical support or immunosuppression after cardiac transplantation. Secondary exclusion transfusion > 8 units of blood within the first week postoperatively and non‐adherence to protocol |
|
| Interventions | Intervention therapy: BT563/BB10 10 mg iv on 8 consecutive days Control therapy: RATG (Institute Merieux, Lyon, France) 25 mg/kg iv on 8 consecutive days Concomittant immunosuppressive treatment: cyclosporine, started on day 5 azathioprine steroids |
|
| Outcomes | Primary: survival, overall infection, bacterial infection, CMV infection, CMV disease, other viral infections, side effects, cancer and cardiac allograft vasculopathy Follow‐up period: 10 years |
|
| Notes | Acute rejection defined as ≥ 2 (ISHLT definition) Endomyocardial biopsies: weeks 1, 2, 3, 4, 6, 12 and 24 Routine CMV prophylaxis cytotec 1 mg/kg pre‐operatively, plus day 1, 7, 14, 21, and 28 Prophylactic broad‐spectrum antibiotic for 5 days Excellent tolerance to medication stated Sources of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No participants were lost to follow‐up, but 8 participants were excluded post‐randomisation |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed, but the trial reported on mortality, rejection, cardiac allograft vasculopathy, infection, cancer, PTLD and adverse events |
| Other bias | Low risk | The trial appeared to be free of other components of bias |
Carrier 2007.
| Methods | Comparison: IL‐2 RA (basiliximab) vs polyclonal antibody Trial design: multi‐centre RCT, 2 parallel groups, non‐inferiority trial Language: English Type of publication: journal article Judgement of trial quality: high risk of bias Cross‐over between treatment groups: no Sample size calculation: yes Intention to treat analysis: yes |
|
| Participants | Setting: 2 transplant centres, Montréal and Québec, Canada Inclusion period: Jul 2002–Apr 2004 Allocation: 35 participants: basiliximab n = 17; RATG n = 18 Sex ratio: basiliximab 12 (71%) men:5 (29%) women; RATG 15 (83%) men:3 (17%) women Mean age: basiliximab 54 ± 9 years; RATG 54 ± 12 years Adult:paediatric participants: 100% adults Indication (no (%)): Coronary artery disease: basiliximab 8 (47%); RATG 12 (67%) Idiopathic cardiomyopathy: basiliximab 4 (24%); RATG 1 (6%) Congenital heart disease: basiliximab 1 (6%); RATG 0 (0%) Acute myocarditis: basiliximab 1 (6%); RATG 0 (0%) Valvular heart disease: basiliximab 0 (0%); RATG 2 (11%) Other: basiliximab 3 (18%); RATG 3 (17%) Ventricular assist device: basiliximab 1 (6%); RATG 2 (11%) Inclusion criteria: participant on the waiting list for heart transplantation Exclusion criteria: unclear |
|
| Interventions | Intervention therapy: basiliximab 20 mg iv days 0 and 4 Control therapy: RATG 125 mg/kg iv days 0‐2 Concomittant immunosuppressive treatment: cyclosporine, started at day 5 mycophenolate mofetil steroids |
|
| Outcomes | Primary: incidence of biopsy proven acute rejection at 6 months (freedom from rejection (ISHLT ≥ 3a)) Secondary: mortality, lymphocyte subpopulations, CMV viral load, incidence of infection and impaired renal function at 12 month follow‐up Follow‐up period: 6 and 12‐month follow‐ups |
|
| Notes | Acute rejection defined as ≥ 3a (ISHLT definition) Endomyocardial biopsies: weeks 2, 4, 8, 12 and 24 Participants at high risk of CMV were given prophylactic treatment Study designed by the investigators Sources of funding: supported by Novartis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate, computer‐generated code |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed, but the trial reported on mortality, rejection, cardiac allograft vasculopathy, infection, cancer, PTLD and adverse events |
| Other bias | High risk | The trial was sponsored by industry |
Costanzo‐Nordin 1990.
| Methods | Comparison: monoclonal antibody (muromonab‐CD3) vs polyclonal antibody Trial design: single‐centre RCT Language: English Type of publication: journal article Judgement of trial quality: high risk of bias Cross‐over between treatment groups: no Sample size calculation: not reported Intention to treat analysis: yes |
|
| Participants | Setting: Loyola University Medical Center, Maywood, USA Inclusion period: August 1987‐December 1988 Allocation: 23 participants: muromonab‐CD3 n =12; horse ATG n = 11 Sex ratio: muromonab‐CD3 9 (75%) males:3 (25%) females; horse ATG 9 (82%) males:2 (18%) females Mean age: muromonab‐CD3 51.2 ± 10.4 years; horse ATG 45.1 ± 16.0 years Adult:paediatric participants: not reported Indication (no (%)): not reported Inclusion criteria: participants undergoing heart transplantation Exclusion criteria: leucopenia (white blood cell count < 3000/mm³) or participants who previously had received muromonab‐CD3 |
|
| Interventions | Intervention A: muromonab‐CD3, started 6‐12 h postoperatively 5 mg iv daily Intervention B: horse ATG, preoperatively 5 mg/kg iv, postoperatively 5 mg/kg iv for 9 days Concomittant immunosuppressive treatment: cyclosporine azathioprine corticosteroids |
|
| Outcomes | Survival, rejection, adverse events, infection, cancer, lymphocyte subpopulations, muromonab‐CD3 antibody Follow‐up period: 12 months |
|
| Notes | Endomyocardial biopsies from right ventricular septum, weekly for the first 6 weeks after transplantation and then every 2 weeks for 2 months. Biopsies then performed at progressively longer intervals until a quarterly interval (i.e. 3‐monthly) was reached after 1 year in all participants according to the Stanford criteria. Acute rejection: based on the histologic changes observed on endomyocardial biopsies Study designer: not reported Sources of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Prospective RCT |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes containing the treatment arm were sent from Ortho Pharmaceutical Corporation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed, but the trial reported on mortality, rejection, infection, cancer, and adverse events |
| Other bias | Unclear risk | It is unclear whether the trial was industry‐sponsored, the text states, "Sealed envelopes containing the treatment arm were sent from Ortho Pharmaceutical Corporation." |
De Santo 2004.
| Methods | Comparison: polyclonal antibody vs polyclonal antibody Trial design: single‐centre RCT Language: English Type of publication: journal article Judgement of trial quality: high risk of bias Cross‐over between treatment groups: no Sample size calculation: not reported Intention to treat analysis: yes |
|
| Participants | Setting: V Monaldi Hospital, Naples Hospital, Naples, Italy Inclusion period: January 1998–December 1999 Allocation: 40 participants: RATG‐Thymoglobulin n = 20; RATG‐Fresenius = 20 Sex ratio: RATG‐Thymoglobulin 12 (60%) men:8 (40%) women; RATG‐Fresenius 17 (85%) men:3 (15%) women Mean age: RATG‐Thymoglobulin 38.9 ± 14.6 years; RATG‐Fresenius 42.5 ± 13.5 years Adult:paediatric participants: not reported Indication (no (%)): Idiopathic cardiomyopathy: RATG‐Thymoglobulin 10 (50%); RATG‐Fresenius 10 (50%) Ischemic cardiomyopathy: RATG‐Thymoglobulin 4 (20%); RATG‐Fresenius 6 (30%) Other: RATG‐Thymoglobulin 6 (30%); RATG‐Fresenius 4 (20%) Ventricular assist device: not reported Inclusion criteria: primary heart transplant recipients Exclusion criteria: recipient of a second transplant; previous cancer; pregnancy or female participant of childbearing potential not using a medically approved, effective form of contraception during the study; recent history of systemic infection, severe diarrhoea, active peptic ulcer or gastro‐intestinal diseases interfering with the absorption of oral immunosuppressive agents; previous treatment with ALG or monoclonal antibodies; history of drug addiction, psychiatric disorder, or behavioural attitude interfering with compliance to therapy; recent enrolment in other trials of immunosuppressive drugs |
|
| Interventions | Intervention A: RATG‐Thymoglobulin 2.5 mg/kg daily, days 0‐5 Intervention B: RATG‐Fresenius 2.5 mg/kg daily, days 0‐7 Concomittant immunosuppressive treatment: cyclosporine (Neoral) mycophenolate mofetil corticosteroids |
|
| Outcomes | Primary: incidence of acute rejection episodes at 6 months (ISHLT ≥1b), bouts of steroid‐resistant rejection, and time to first rejection Secondary: survival, graft atherosclerosis, infections, cancer Follow‐up period: mean follow‐up 32.8 ± 8.9 months |
|
| Notes | Acute rejection defined as ≥ 1b (ISHLT definition). Rejection episodes of grade 3a or greater were treated with 1 g/d methyl prednisolone iv for 3 consecutive days. Steroid‐resistant rejections were treated by switching from cyclosporine to tacrolimus and/or by a 9‐day course of OKT3 (5 mg/d) Endomyocardial biopsies: weekly for the first 2 months, then every second week during the third month. Further biopsies were performed as dictated by the participant's clinical condition Antiviral prophylactic treatment: 400 mg acyclovir twice daily for 2 months Sources of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment assignment was recorded on cards that were placed in serially‐numbered sealed envelopes. The ordering of the cards within the envelopes was determined from a table of random numbers |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. Envelopes were opened on the day of transplantation after recipient selection |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed, but the trial reported on acute rejection, mortality, graft atherosclerosis, infections, and cancer |
| Other bias | Low risk | The trial appeared to be free of other components of bias |
Faggian 2010.
| Methods | Comparison: high‐dose polyclonal antibody vs standard‐dose polyclonal antibody Trial design: single‐centre RCT, 2 parallel groups Language: English Type of publication: journal article Judgement of trial quality: high risk of bias Cross‐over between treatment groups: no Sample size calculation: not reported Intention to treat analysis: yes |
|
| Participants | Setting: University of Verona, Verona, Italy Inclusion period: July 1997–March 1999 Allocation: 30 participants: high‐dose RATG n = 14; standard‐dose RATG n= 16 Sex ratio: high‐dose RATG 13 (93%) men:1 (7%) women; standard‐dose RATG 14 (88%) men:2 (12%) women Mean age: high‐dose RATG 52 ± 4 years; standard‐dose RATG 49 ± 7 years Adult:paediatric participants: 100% adults Indication (no (%)): Chronic ischaemic heart disease: high‐dose RATG 8 (57%); standard‐dose RATG 8 (50%) Aortic valve disorder: high‐dose RATG 0 (0%); standard‐dose RATG 1 (6%) Primary cardiomyopathy: high‐dose RATG 5 (36%); standard‐dose RATG 7 (44%) Cardiomegaly: high‐dose RATG 1 (7%); standard‐dose RATG 0 (0%) Inclusion criteria: first heart transplantation Exclusion criteria: participants with thrombocytopenia, leukopenia, or active bacterial, fungal or viral infection |
|
| Interventions | Intervention therapy: high‐dose RATG intraoperative infusion 9 mg/kg in 250 ml saline, postoperatively 3 mg/kg daily for 3 days Control therapy: standard‐dose RATG 3 mg/kg iv, days 0 to 6 Concomittant immunosuppressive treatment: cyclosporine, started at day 5 azathioprine corticosteroids |
|
| Outcomes | Primary: participant and graft survival at 5 years Secondary: vital signs, need for postoperative transfusions, adverse events Follow‐up period: mean 58 ± 16 months (range 43‐83 months) |
|
| Notes | Acute rejection defined according to criteria of ISHLT Endomyocardial biopsies: weekly in postoperative month 1, biweekly in month 2‐3, monthly in months 4‐9 Participants at high risk of CMV (donor positive/recipient negative) were given acyclovir 5 mg/kg/d for 15 days Study designer: not reported Sources of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised, no further details specified |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomised, no further details specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed, but the trial reported on all expected outcome measures |
| Other bias | Low risk | The trial appeared to be free of other components of bias |
Hershberger 2005.
| Methods | Comparison: IL‐2 RA vs no antibody induction Trial design: multi‐centre RCT, placebo‐controlled, two parallel groups Language: English Type of publication: journal article Judgement of trial quality: high risk of bias Cross‐over between treatment groups: no Sample size calculation: yes Intention to treat analysis: yes, but analyses of safety included only participants who had at least received 1 dose of placebo or daclizumab; if a participant received daclizumab any time after randomisation, he or she was included in the daclizumab group |
|
| Participants | Setting: 31 transplant centres, the USA, Canada, Germany and Sweden Inclusion period: Aug 1999‐Apr 2001 Allocation: 434 participants: daclizumab n = 216; placebo n = 218 Sex ratio: daclizumab 171 (79%) men:45 (21%) women; placebo 177 (81%) men:41 (19%) women Mean age: daclizumab 52.4 ± 11.0 years; placebo 53.1 ± 11.9 years Adult:paediatric participants: 100% > 13 years Indication (no (%)): Coronary artery disease: daclizumab 64 (30%); placebo 68 (31%) Dilated cardiomyopathy: daclizumab 138 (64%); placebo 138 (63%) Other: daclizumab 14 (6%); placebo 12 (6%) Ventricular assist device: daclizumab 18 (8%); placebo 16 (7%) Inclusion criteria: primary heart transplantation, age > 13 years, first cardiac allograft Exclusion criteria: known need of cytolytic therapy or requiring ventricular assist device after surgery |
|
| Interventions | Intervention therapy: daclizumab 1 mg/kg iv on days 0, 8, 22, 36 and 50 Control therapy: placebo iv on days 0, 8, 22, 36 and 50 Concomittant immunosuppressive treatment: cyclosporine, initiated within 72 h after transplantation mycophenolate mofetil corticosteroids |
|
| Outcomes | Primary: efficacy endpoint, composite of biopsy showing cellular rejection of grade 3a or higher, haemodynamic compromised, treated with inotropic agents and pulsed doses of immunosuppressants whether or not a biopsy was taken, death, a second transplantation or lost to follow‐up at 6 months Secondary: efficacy endpoints at 12 months, participants at graft survival at 6 and 12 months Follow‐up period: 12 months |
|
| Notes | Endomyocardial biopsies: days 8, 22, 36 and 50, and then every 2rd week up to 3 months, monthly up to 6 months, and every 2rd month up to 12 months Antibiotics to prevent Pneumocystis carinii for 12 months High risk participants for CMV (R‐/D+) were given prophylactic ganciclovir Study designed by Roche with the assistance of a subgroup of the principal investigators. The data were collected and held by Roche, data analyses were performed by Roche statistician Sources of funding: the study was supported by Roche Laboratories, Nutley NJ, USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned, no further details specified |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly assigned, no further details specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blinded, no further details specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blinded, no further details specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 participants were lost to follow‐up, but missing data was unlikely to affect outcome results |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed, but the trial reported on all expected outcome measures |
| Other bias | High risk | The trial was industry‐sponsored |
Ippoliti 1991.
| Methods | Comparison: polyclonal antibody (RATG) vs monoclonal antibody Trial design: single‐centre RCT, 2 parallel groups Language: English Type of publication: journal article Judgement of trial quality: high risk of bias Cross‐over between treatment groups: no Sample size calculation: not reported Intention to treat analysis: yes |
|
| Participants | Setting: University of Pavia, Pavia, Italy Inclusion period: not reported Allocation: 30 consecutive participants: 15 participants, RATG n = 15; OKT3 n = 15 Sex ratio: RATG 12 (80%) men:3 (20%) women; OKT3 14 (93) men:1 (7%) women Mean age: RATG 45.2 ± 12.3 years; OKT3 45.6 ± 11.8 years Adult:paediatric participants: not reported Indication (no (%)): Ischaemic cardiac disease: RATG 7 (47%); OKT3 5 (33%) Dilated cardiomyopathy: RATG 6 (40%); OKT3 9 (60%) Vascular cardiac disease: RATG 2 (13%); OKT3 1 (7%) Inclusion criteria: participants undergoing heart transplantation Exclusion criteria: not specified |
|
| Interventions | Intervention therapy: RATG, preoperatively 5 mg/kg iv; postoperatively 2.5 mg/kg iv for 7 days Control therapy: OKT3, preoperatively 10 mg iv; postoperatively 5 mg iv for 14 days Concomittant immunosuppressive treatment: cyclosporin, started preoperatively azathioprine, started preoperatively corticosteroids, started preoperatively |
|
| Outcomes | Survival, rejection, adverse reactions, OKT3 antibodies, lymphocyte subsets, infections Follow‐up period: 6 months |
|
| Notes | Endomyocardial biopsies in all participants according to the Stanford criteria Acute rejection; definition not specified Study designer: not reported Sources of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, no further details specified |
| Allocation concealment (selection bias) | Unclear risk | Randomised, no further details specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed, but the trial reported on mortality, rejection, adverse reactions, and infections |
| Other bias | Low risk | The trial appeared to be free of other components of bias |
Kobashigawa 1993.
| Methods | Comparison: monoclonal antibody vs no antibody Trial design: single‐centre RCT Language: English Type of publication: journal article Judgement of trial quality: high risk of bias Cross‐over between treatment groups: no Sample size calculation: yes Intention to treat analysis: yes |
|
| Participants | Setting: UCLA School of Medicine, Los Angeles, CA, USA Inclusion period: June 1989–June 1990 Allocation: 30 consecutive participants: muromonab‐CD3 n = 15; control n = 15 Sex ratio: muromonab‐CD3 11 (73%) men:4 (27%) women; Control 12 (80%) men:3 (20%) women Mean age: muromonab‐CD3 48 ± 15 years; control 54 ± 13 years Adult:paediatric participants: 100% adult Indication (no (%)): Coronary artery disease: muromonab‐CD3 7 (47%); control 8 (53%) Other: muromonab‐CD3 8 (53%); control 7 (47%) Ventricular assist device: not reported Inclusion criteria: adult participants undergoing heart transplantation, preoperative serum creatinine level of 1.4 mg/dl or less Exclusion criteria: iv inotrope support in the immediate preoperative period |
|
| Interventions | Intervention therapy: muromonab‐CD3 5 mg daily by iv push for 4‐6 postoperative days, with 3 mg/kg oral cyclosporine started between postoperative days 2‐4 when renal function had stabilised. Muromonab‐CD3 discontinued (usually 4‐6 day course) when cyclosporine reached therapeutic levels (300‐600 ng/ml). During muromonab‐CD3 treatment CD3 levels were monitored daily, and if CD3 levels were not 1% or < 10 mg muromonab‐CD3 was administered Control therapy: no antibody induction; oral cyclosporine 4 mg/kg started before operation, then titrated to serum levels between 300‐600 ng/ml Concomittant immunosuppressive treatment: cyclosporine azathioprine corticosteroids |
|
| Outcomes | Rejection incidence, renal function, infectious complications, CMV infection, coronary allograft vasculopathy Follow‐up period: 6 months and 12 months |
|
| Notes | Endomyocardial biopsies: once weekly for 4 weeks, then every 2 weeks for 4 weeks, then every 3 weeks for 6 weeks, and then every month for 2 months Sources of funding. not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, but no further details specified |
| Allocation concealment (selection bias) | Unclear risk | Randomised, but no further details specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed, but the trial reported on rejection, infections, renal function |
| Other bias | Low risk | The trial appeared to be free of other components of bias |
Kormos 1990.
| Methods | Comparison: polyclonal antibody (RATG) vs monoclonal antibody Trial design:single‐centre RCT, 2 parallel groups Language: English Type of publication: journal article Judgement of trial quality: high risk of bias Cross‐over between treatment groups: yes, 1 OKT3 participant developed severe aseptic meningitis and was switched to RATG Sample size calculation: not reported Intention to treat analysis: no, 5 participants excluded. Reasons for exclusions: RATG: 1 no steroid taper due to delayed wound healing, 1 no steroid taper due to mediastinitis; OKT3: 2 no steroid taper due to mediastinitis, 1 switched to RATG after development of aseptic meningitis |
|
| Participants | Setting: University of Pittsburgh, Pittsburgh, USA Inclusion period: June 1987‐March 1989 Allocation: 87 participants: RATG n = 41; OKT3 n = 46. 5 excluded from analysis: 3 from OKT3 group and 2 from RATG group Sex ratio: RATG 26 (67%) men:13 (33%) women; OKT3 31 (72%) men:12 (7%) women Mean age: RATG 51.8 ± 9.2 years; OKT3 50.3 ± 10.3 years Adult:paediatric participants: 100% adults Indication (no (%)): Ischaemic: RATG 20 (51%); OKT3 23 (54%) Idiopathic: RATG 19 (49%); OKT3 20 (46%) Inclusion criteria: participants undergoing heart transplantation (NYHA class IV heart failure) Exclusion criteria: paediatric participants, participants bridged to transplantation with the Novacor LVAS, and retransplantation Furthermore, excluded those participants who, within 48 h of transplantation, suffered from excessive volume overload, need for > 10 μg/kg/min dobutamine, failure to extubate, and the need for pharmacological control of pulmonary hypertension |
|
| Interventions | Intervention therapy: RATG, 1.5 mg/kg/day IM; for 5 days, beginning on day 2 or 3 Control therapy: OKT3 5 mg/day iv for 14 days, beginning on day 2 or 3 Concomittant immunosuppressive treatment: cyclosporin, started within 24 hours of surgery azathioprine, started preoperatively corticosteroids, started preoperatively |
|
| Outcomes | Survival, rejection, infection, morbidity of prophylactic agents Follow‐up period: 1 year |
|
| Notes | Transvenous endomyocardial biopsies were performed weekly for the first 4 weeks, then biweekly for two months, then monthly for 6 months Acute rejection was defined by the presence of myocyte necrosis in association with an interstitial lymphocytic infiltrate (grade 3 or moderate rejection) or with haemorrhage (grade 4 or severe rejection) Study designer: not reported Sources of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Only the pathologist examining myocardial biopsies was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to affect outcome results |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed, but the trial reported on mortality, rejection, adverse reactions, and infections |
| Other bias | Low risk | The trial appeared to be free of other components of bias |
Macdonald 1993.
| Methods | Comparison: polyclonal antibody (horse ATG) vs monoclonal antibody Trial design:single‐centre RCT, 2 parallel groups Language: English Type of publication: journal article Judgement of trial quality: high risk of bias Cross‐over between treatment groups: no Sample size calculation: not reported Intention to treat analysis: yes |
|
| Participants | Setting: Saint Vincent Hospital, Sydney, Australia Inclusion period: August 1989‐June 1990 Allocation: 41 consecutive participants: horse ATG n = 21; OKT3 n = 20 Sex ratio: horse ATG 18 (86%) men:3 (14%) women; OKT3 17 (85%) men:3 (15%) women Mean age: horse ATG 44.1 years (range 18‐59); OKT3 48.7 years (18‐59) Adult:paediatric participants: 100% adults Indication (no (%)): Cardiomyopathy: horse ATG 13 (62%); OKT3 9 (45%) Ischaemic heart disease: horse ATG 8 (38%); OKT3 9 (45%) Vascular heart disease: horse ATG 0 (0%); OKT3 2 (10%) Inclusion criteria: participants undergoing orthotopic heart transplantation Exclusion criteria: not specified |
|
| Interventions | Intervention therapy: horse ATG, 500 mg iv at time of anaesthesia induction; postoperatively 2.5‐15 mg/kg iv daily for 7 days, with each dose titrated to achieve a daily total CD2 (T11) lymphocyte count of approximately 100 cells/mm³ Control therapy: OKT3, postoperatively 5 mg iv for 10 days (started when the participant was haemodynamically stable) Concomittant immunosuppressive treatment: cyclosporin, started on 1st or 2nd postoperative day, provided renal function stable and adequate urine output azathioprine, started preoperatively corticosteroids, started preoperatively |
|
| Outcomes | Survival, rejection, infection, adverse reactions, hospital stay Follow‐up period: 12 months |
|
| Notes | Endomyocardial biopsies were performed weekly for 4 weeks, every second week for 2 months, monthly from 3‐6 months, at months 9 and 12, and whenever there were clinical signs of rejection Acute rejection based on biopsy results. Biopsies were graded according to Billingham criteria Study designer: not reported Sources of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Serially‐numbered sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to affect outcome results |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed, but the trial reported on mortality, rejection, adverse reactions, and infections |
| Other bias | Unclear risk | The trial appeared to be free of other components of bias |
Mattei 2007.
| Methods | Comparison: IL‐2 RA (basiliximab) vs polyclonal antibody Trial design: multi‐centre RCT, 2 parallel groups, non‐inferiority trial Language: English Type of publication: journal article Judgement of trial quality: high risk of bias Cross‐over between treatment groups: no Sample size calculation: yes Intention to treat analysis: yes, regarding safety endpoints |
|
| Participants | Setting: 12 transplant centres in France Inclusion period: January 2002–November 2003 Allocation: 80 participants: basiliximab n = 38; RATG n = 42 Sex ratio: basiliximab 36 (95%) men:2 (5%) women; RATG 37 (88%) men:5 (12%) women Mean age: basiliximab 52.7 ± 8.1 years; RATG 49.6 ± 11.6 years Adult:paediatric participants: 100% adults Indication (no (%)): Idiopathic cardiomyopathy: basiliximab 15 (39%); RATG 16 (38%) Coronary artery disease: basiliximab 13 (34%); RATG 19 (45%) Congenital heart disease: basiliximab 1 (3%); RATG 2 (5%) Valvular heart disease: basiliximab 6 (16%); RATG 1 (2%) Other: basiliximab 3 (8%); RATG 4 (10%) Ventricular assist device: not reported Inclusion criteria: participants between 18‐65 years of age undergoing a primary heart transplant with a cold ischaemia time < 6 h Exclusion criteria: receipt of a graft from a donor > 60 years of age, or with documented coronary disease or other heart disease; a previous or multiple‐organ transplant; unstable haemodynamic status at the time of randomisation; creatinine level more than 250 μmol/L |
|
| Interventions | Intervention therapy: basiliximab 20 mg iv on days 0 and 4 Control therapy: RATG (Thymoglubin) 2.5 mg/kg iv on days 0 to 3 or 5 Concomittant immunosuppressive treatment: cyclosporine mycophenolate mofetil corticosteroids |
|
| Outcomes | Primary: safety; composite at 6 months of adverse events related to study drug, serum sickness, cutaneous rash, anaphylaxis, treated infections, post‐transplantation lymphoproliferative disorder or episodes of fever, thrombocytopenia and leucopenia Secondary: composite failure criteria at 6 months of death, graft loss, acute rejection of grade > 1 ISHLT, acute rejection associated with haemodynamic compromise, acute rejection treated with antibody therapy, or loss to follow‐up Follow‐up period: 12 months follow‐up |
|
| Notes | Acute rejection as defined by ISHLT Endomyocardial biopsies: days 7, 14, 21, and months 1, 2, 3, 4, 5 and 6 CMV (D+/R‐) received 3‐month prophylactic treatment with ganciclovir or valaciclovir Study designer: not reported; one of the authors was affiliated to Novartis Sources of funding: supported by a grant from Novartis Pharma AG |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised in a 1:1 ratio, no further details specified |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomised, no further details specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14 participants did not complete the 12‐month visit, and missing data might have affected outcome results |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed, but the trial reported on all expected outcome measures |
| Other bias | High risk | The trial was industry‐sponsored |
Mehra 2005.
| Methods | Comparison: IL‐2 RA vs no antibody induction Trial design: multi‐centre RCT, placebo controlled, 2 parallel groups Language: English Type of publication: journal article Judgement of trial quality: high risk of bias Cross‐over between treatment groups: no Sample size calculation: not reported Intention to treat analysis: yes |
|
| Participants | Setting: 5 transplant centres in the United States Inclusion period: not specified Allocation: 56 participants: basiliximab n = 25; placebo n = 31 Sex ratio: basiliximab 18 (72%) men:7 (28%) women; placebo 24 (77%) men:7 (23%) women Mean age: basiliximab 56.3 ± 9.7 years; placebo 53.4 ± 10.2 years Adult:paediatric participants: 100% adults Indication (no (%)): not reported Inclusion criteria: participants between 18‐70 years of age undergoing primary heart transplantation with a donor heart with cold ischaemia time < 6 h Exclusion criteria: donor heart from donor > 60 years of age; previously multiorgan transplantation; received immunosuppressive or investigational therapy within 4 weeks before transplantation; serum creatinine > 3.0 mg/dl; antibodies to HIV or hepatitis C; pregnancy; presence of a severe systemic infection or history of cancer within 5 years of transplantation |
|
| Interventions | Intervention therapy: basiliximab (Simulect) 20 mg iv on days 0 and 4 Control therapy: placebo (lyophilised suspending solution without basiliximab) iv on days 0 and 4 Concomittant immunosuppressive treatment: cyclosporine, starting day 0 for 12 months mycophenolate mofetil, starting day 0 for 12 months corticosteroids, starting day 0 for 12 months |
|
| Outcomes | Primary: safety and tolerability, recording all adverse events and serious adverse events Secondary: efficacy endpoints (time to first biopsy proven acute rejection (ISHLT ≥ 3a) or haemodynamic compromise; severity of biopsy proven acute rejection; numbers of biopsy proven acute rejection/participant; mortality; hospital stay (readmission and rejection required hospitalisation) Follow‐up period: 12 months |
|
| Notes | Acute rejection defined as ≥ 3a by ISHLT Serious adverse events: basiliximab 21/25, placebo 19/31 Study designer: not reported; one of the authors was affiliated to Novartis Sources of funding: supported by a grant from Novartis Pharmaceuticals |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Each centre received drug supplies with sequential randomisation numbers, and a validated system automated the random assignment of treatment groups to randomisation numbers |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blinded for the first 6 months, no further details specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blinded for the first 6 months, no further details specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No protocol assessed, but the trial reported on all expected outcome measures |
| Other bias | High risk | The trial was industry‐sponsored |
Menkis 1992.
| Methods | Comparison: polyclonal antibody (ALG) vs monoclonal antibody induction Trial design: single‐centre RCT, 2 parallel groups Language: English Type of publication: journal article Judgement of trial quality: high risk of bias Cross‐over between treatment groups: no Sample size calculation: not reported Intention to treat analysis: 2 participants who died were excluded for the incidence of acute rejection analysis |
|
| Participants | Setting: University Hospital, London, Ontario, Canada Inclusion period: July 1989–September 1990 Allocation: 39 participants: ALG n = 19; OKT3 n = 20 Sex ratio: ALG 16 (84%) men:3 (16%) women; OKT3 17 (85%) men:3 (15%) women Mean age: ALG 48.7 years; OKT3 50.3 years Adult:paediatric participants: 100% adults Indication (no (%)): Ischaemic heart disease: ALG 11 (58%); OKT3 16 (80%) Valvular heart disease: ALG 2 (11%); OKT3 0 (0%) Idiopathic cardiomyopathy: ALG 6 (32%); OKT3 4 (20%) Inclusion criteria: heart transplant recipients Exclusion criteria: < 18 years of age; no informed consent obtained; prior exposure to OKT3 or ALG; known allergy to OKT3 or ALG; participants of the most urgent pretransplant status requiring intravenous inotropic therapy; mechanical ventilation or mechanical circulatory assistance |
|
| Interventions | Intervention therapy: OKT3 3.5 mg iv on days 0‐7 (discontinued when cyclosporine level was therapeutic) Control therapy: ALG iv at a starting dose of 15 mg/kg over 18 h, thereafter adjusted to maintain total lymphocyte count < 300, total leukocyte count > 4.0, and platelet count > 50,000 on days 0‐7 (discontinued when cyclosporine level was therapeutic) Concomittant immunosuppressive treatment: cyclosporine, started day 3‐5 after heart transplantation when renal function had stabilised and oral fluid intake had started corticosteroids azathioprine: used selectively for following recipients: 1) positive donor‐specific lymphocyte cross match, 2) steroid resistant rejection, 3) complications of cyclosporine therapy requiring lower cyclosporine dosage, 4) steroid‐related complications requiring lower prednisone dosage |
|
| Outcomes | Survival, rejection, infections, cancer, adverse drug reactions, cost of therapy Follow‐up period: minimum of 6 months |
|
| Notes | Endomyocardial biopsies weekly for the first 4 weeks, every 2nd week for the next 8 weeks. If the biopsies were abnormal, additional biopsies were performed at weekly intervals An abnormal biopsy result was considered as an episode of acute rejection only if specific anti‐rejection therapy was instituted. Treatment for acute rejection was initiated for biopsies showing lymphocyte infiltration associated with myocyte loss (ISHLT grade 1B old classification) No dosage modification or adverse event necessitating drug withdrawal occurred in either group Study designer: not reported Sources of funding: in part by grants from Ortho Pharmaceuticals Canada |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised, no further details specified |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomised, no Futcher details specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to affect outcome results |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed, but all expected outcomes were reported |
| Other bias | High risk | The trial was industry‐sponsored |
Mullen 2005.
| Methods | Comparison: IL‐2 RA (daclizumab) vs polyclonal antibody Trial design: single‐centre RCT, 2 parallel groups Language: English Type of publication: abstract Judgement of trial quality: high risk of bias Cross‐over between treatment groups: no Sample size calculation: not reported Intention to treat analysis: not reported |
|
| Participants | Setting: University of Alberta, Edmonton, Canada Inclusion period: June 2001‐April 2005 Allocation: 30 participants: daclizumab n = 15; horse ATG n = 15 Sex ratio: daclizumab 12 (80%) men:3 (20%) women; ATG 11 (73%) men:4 (27%) women Mean age: basiliximab 58 ± 3 years; ATG 58 ± 3 years Adult:paediatric participants: 100% adult participants Indication (no (%)): Ischaemic cardiomyopathy: daclizumab 8 (53%); horse ATG 10 (67%) Dilated cardiomyopathy: daclizumab 3 (20%); horse ATG 1 (7%) Other: daclizumab 4 (27%); horse ATG 4 (26%) Ventricular assist device: not reported Inclusion criteria: all adult participants listed for heart transplantation between June 2001 and April 2005 Exclusion criteria: emergency surgery; previous transplant; multiple‐organ transplant including heart‐lung transplant; active infection; hepatitis C; high positive panel reactive antibodies (> 15%); known sensitivity to daclizumab, ATG or mouse antigens; expected inability to be followed at the study centre for a full year; and inability to give informed consent |
|
| Interventions | Intervention therapy: daclizumab 2 mg/kg iv on day 0, and 1 mg/kg iv on day 4 Control therapy: horse ATG: 10 mg/kg by continuous infusion starting immediately postoperatively and continuing for 5‐7 days followed by a pulse of methylprednisolone 2 mg/kg iv q12h x3 to prevent T‐cell rebound Concomittant immunosuppressive treatment: cyclosporine/tacrolimus mycophenolate mofetil corticosteroids |
|
| Outcomes | Mortality, acute rejection, cancer, infection, adverse reactions, absolute platelet count, absolute lymphocyte count Follow‐up period: 12‐month follow‐up |
|
| Notes | Acute rejection definition: defined as ≥ 3a (ISHLT) Endomyocardial biopsies: not reported Study designer: not reported Sources of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | The study was randomised, no further details specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Only the participants were blinded for treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Only the participants were blinded for treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data, and no participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No protocol assessed, but the trial reported on all expected outcome measures |
| Other bias | Unclear risk | The trial appeared to be free of other components of bias |
Schnetzler 2002.
| Methods | Comparison: polyclonal antibody vs polyclonal antibody Trial design: single‐centre randomised clinical phase‐III trial, 2 parallel groups Language: English Type of publication: journal article Judgement of trial quality: high risk of bias Cross‐over between treatment groups: yes, 1 participant randomised to RATG‐Fresenius received RATG‐Thymoglobulin (Mérieux) by mistake Sample size calculation: not reported Intention to treat analysis: no, one participant randomised to RATG‐Fresenius received RATG‐Thymoglobulin (Mérieux) by mistake, and was analysed as treated |
|
| Participants | Setting: La Pitié Salpétrière, Paris, France Inclusion period: not reported Allocation: 50 consecutive participants: RATG‐Fresenius n = 24, RATG‐Thymoglobulin n = 26 Sex ratio: RATG‐Fresenius 20 (83%) men:4 (17%) women; RATG‐Thymoglobulin 20 (77%) men:6 (23%) women Mean age: RATG‐Fresenius 48 ± 10 years; RATG‐Thymoglobulin 45 ± 11 years Adult:paediatric participants: 100% adults Indication (no (%)): Ischaemic heart disease: RATG‐Fresenius 8 (33%); RATG‐Thymoglobulin 6 (23%) Cardiomyopathy: RATG‐Fresenius 16 (67%); RATG‐Thymoglobulin 17 (65%) Valvular heart disease: RATG‐Fresenius 0 (0%); RATG‐Thymoglobulin 1 (4%) Other: RATG‐Fresenius 0 (0%); RATG‐Thymoglobulin 2 (8%) Ventricular assist device: not reported Inclusion criteria: first heart transplantation, participants between 16‐60 years of age, written informed consent Exclusion criteria: second graft transplantation; serious concomitant diseases or severe thrombocytopenia; viral, fungal, or bacterial infections upon inclusion; pregnancy or lactation; previous treatment with rabbit polyclonal anti‐lymphocytic preparations |
|
| Interventions | Intervention therapy A: RATG‐Fresenius 3.0 mg/kg iv on days 1‐5, dosage increased by 1 mg/kg/day if total lymphocyte count exceeded 300/mm³ Intervention therapy B: RATG Thymoglubin (Mérieux) 2.5 mg/kg iv on days 1‐5, dosage increased by 1 mg/kg/day if total lymphocyte count exceeded 300/mm³ Concomittant immunosuppressive treatment: cyclosporine, started on day 1‐3 azathioprine, started preoperatively corticosteroids, started preoperatively |
|
| Outcomes | Primary: participant survival, incidence and duration of rejection Secondary: infection, pathological laboratory data, adverse events Follow‐up period: 12‐month follow‐up |
|
| Notes | Classified acute rejection episodes as mild, moderate and severe according to the Billingham classification Endomyocardial biopsies conducted weekly during the 1st month, every 2nd week from months 2‐6, every 2nd month from months 6‐12 All recipients who received a heart from a CMV‐positive donor received CMV prophylaxis with ganciclovir for 2 weeks Study designer: not reported Sources of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was randomised, no further details specified |
| Allocation concealment (selection bias) | Unclear risk | The study was randomised, no further details specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data, and per‐protocol analysis instead of intention‐to‐treat analysis of one participant were unlikely to influence outcome results |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed, but the trial reported on all expected outcome measures |
| Other bias | Low risk | The trial appeared to be free of other components of bias |
Segovia 2006.
| Methods | Comparison: IL‐2 RA (basiliximab) vs monoclonal antibody Trial design: multi‐centre RCT, open label, 2 parallel groups Language: English Type of publication: journal article and abstract Judgement of trial quality: high risk of bias Cross‐over between treatment groups: no Sample size calculation: not reported Intention to treat analysis: no, 3 participants were excluded due to protocol violation (2 received at least 1 dose of both study medications, 1 due to hepatitis C) |
|
| Participants | Setting: 8 transplant centres in Spain Inclusion period: not reported Allocation: 102 participants, but 3 withdrawn due to protocol violation making 99 participants: basiliximab n = 48; OKT3 n = 51 Sex ratio: basiliximab 38 (79%) men:10 (21%) women; OKT3 45 (88%) men:6 (12%) women Mean age: basiliximab 56.5 ± 9.5 years; OKT3 55.8 ± 11.2 years Adult:paediatric participants: 100% adults Indication (no (%)): Ischaemic heart disease: basiliximab 25 (52%); OKT3 23 (45%) Idiopathic dilated cardiomyopathy: basiliximab 19 (40%); OKT3 16 (31%) Other: basiliximab 4 (8%); OKT3 12 (24%) Ventricular assist device: not reported Inclusion criteria: participants between 18‐70 years of age undergoing orthotopic primary heart transplantation Exclusion criteria: donor age > 60 years; previous use of basiliximab, OKT3, or any other immunomodulator within 4 weeks before transplant; history of cancer within 5 years before transplantation; ABO incompatibility between participant and donor; severe systemic infection; pregnancy, lactation or non‐use of contraceptives for women of child‐bearing age; seropositivity for HIV or hepatitis B or C; unresolved history of drug abuse; leukocyte count below 3000/mm3 or platelet count below 7000/mm3; serum creatinine higher than 3 mg/dL; multiple organ transplant procedure or previous transplantation |
|
| Interventions | Intervention therapy: basiliximab, 20 mg iv bolus on days 0 and 4 Control therapy: OKT3, 5 mg/kg single daily dose days 0‐7 Concomittant immunosuppressive treatment: cyclosporin, started up to 48 h after transplantation mycophenolate mofetil, started 72 h after transplantation corticosteroids |
|
| Outcomes | Primary: incidence of prespecified adverse events: pulmonary oedema; fever; neurologic disorders; hypotension; headache; diarrhoea; lymphocytic meningitis and any other adverse event thought to be related to study medication; cutaneous, neurological and gastrointestinal complications Secondary: acute rejection incidence and severity; time to first rejection episode ≥ 3A; death; graft loss (defined as being listed for re‐transplant); steroid resistant acute rejection Follow‐up period: 12 months |
|
| Notes | Endomyocardial biopsies on days 10, 20, 30, 45 and in months 2, 3, 4, 5, 6, 9, 12 Acute rejection defined as ≥ 3a (ISHLT) High‐risk participants for CMV (CMV seropositive donor/seronegative recipient) received CMV‐prophylaxis consisting of 2 weeks ganciclovir iv, followed by oral valganciclovir until 3 months post‐transplantation Study designer: investigator‐driven trial in which study design, trial conduct and monitoring, collection and analysis of the data, and manuscript edition entirely performed by clinical investigators Sources of funding: supported by an unrestricted grant from Novartis Pharma |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in blocks of 4 digits, 2 of which corresponded to basiliximab and 2 to muromonab‐CD3 treatment |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants excluded: 1 withdrawn and 1 lost to follow‐up, but missing data were unlikely to influence outcome results |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed, but the trial reported on all expected outcome measures |
| Other bias | High risk | The trial was industry‐sponsored |
van Gelder 1996.
| Methods | Comparison: IL‐2 RA (BT563) vs monoclonal antibody Trial design: single‐centre RCT, open‐label, 2 parallel groups Language: English Type of publication: journal article Judgement of trial quality: high risk of bias Cross‐over between treatment groups: no Sample size calculation: not reported Intention to treat analysis: 2 participants who died were excluded from the analysis of incidence of acute rejection |
|
| Participants | Setting: Erasmus Medical Centre, Rotterdam, The Netherlands Inclusion period: November 1991–February 1994 Allocation: 60 participants: BT563 n = 31; OKT3 n = 29 Sex ratio: BT563 25 (81%) males:6 (19%) females; OKT3 24 (83%) men: 5 17(%) women Mean age: BT563 53 (14‐65) years; OKT3 51 (24‐65) years Adult:paediatric participants: not reported Indication (no (%)): Ischaemic heart disease: BT563 20 (65%); OKT3 19 (66%) Cardiomyopathy: BT563 7 (23%); OKT3 9 (31%) Valvular heart disease: BT563 4 (13%); OKT3 1 (3%) Inclusion criteria: consecutive recipients for cardiac allograft Exclusion criteria: not specified |
|
| Interventions | Intervention therapy: BT563 10 mg iv on days 0‐6 Control therapy: OKT3, 5 mg daily on days 0‐6 Concomittant immunosuppressive treatment: cyclosporine, started on day 3 after transplant azathioprine, when thrombocyte count was > 100 x 109/mL, for the first 6 days corticosteroids |
|
| Outcomes | Freedom from rejection, rejection, infections, duration of pacemaker support, and survival Follow‐up period: 12 months, 2nd publication with 10‐year follow‐up (mean 7.7 years) |
|
| Notes | Endomyocardial biopsies weekly for the first 6 weeks, biweekly for the next month and monthly for the next 4 months Acute rejection defined as ≥ 3a (ISHLT) Donor and recipient were not prospectively matched for CMV Cytokine release syndrome in OKT3 participants: fever 23/29; hypotension 6/29; rash 8/29; altered mental status 19/29 BT563: low grade fever 10/31; psycho syndrome 7/31 CMV disease: 3/31 versus 1/29 Study designer: not reported Sources of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to affect outcome results |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed, but all expected outcomes were reported |
| Other bias | Low risk | The trial appeared to be free of other components of bias |
van Gelder 2004.
| Methods | Comparison: IL‐2 RA vs monoclonal antibody Trial design: single‐centre RCT, double‐blind, placebo‐controlled Language: English Type of publication: abstract Judgement of trial quality: high risk of bias Cross‐over between treatment groups: not reported Sample size calculation: not reported Intention to treat analysis: yes |
|
| Participants | Setting: Erasmus Medical Centre, Rotterdam, The Netherlands Inclusion period: not reported Allocation: 31 participants: daclizumab n = 15; placebo n = 16 Sex ratio: not reported Mean age: not reported Adult:paediatric participants: not reported Indication (no (%)): not reported Inclusion criteria: not specified Exclusion criteria: not specified |
|
| Interventions | Intervention therapy: daclizumab 1 mg/ kg, 5 iv gifts Control therapy: placebo 1 mL/kg, 5 iv gifts Concomittant immunosuppressive treatment: cyclosporine mycophenolate mofetil corticosteroids |
|
| Outcomes | Survival, graft survival, rejection, infections, CMV disease, expression of CD25 on peripheral blood lymphocytes Follow‐up period: 1 year |
|
| Notes | Endomyocardial biopsies: not specified Acute rejection defined as ≥ 3a (ISHLT) Study designer: not reported Sources of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised, no further details specified |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomised, no further details specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, no further details specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind, no further details specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were unlikely to affect outcome results |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed, but all expected outcomes were reported |
| Other bias | Low risk | The trial appeared to be free of other components of bias |
Wollenek 1989.
| Methods | Comparison: IL‐2 RA (RATG) vs monoclonal antibody Trial design: single‐centre RCT, 2 parallel groups Language: English Type of publication: journal article Judgement of trial quality: high risk of bias Cross‐over between treatment groups: no Sample size calculation: not reported Intention to treat analysis: yes |
|
| Participants | Setting: University of Vienna, Vienna, Austria Inclusion period: June 1986‐June 1988 Allocation: 40 participants: RATG n = 20; OKT3 n = 20 Sex ratio: 37 male and 3 female participants in the trial, but distribution between treatment groups not specified Median age: RATG 44 (range 17‐56) years; OKT3 45 (range 30‐59) years Adult:paediatric participants: 100% adults Indication (no (%): not reported Inclusion criteria: adult cardiac transplant participants Exclusion criteria: not specified |
|
| Interventions | Intervention therapy: RATG (Fresenius), 10 mg/kg/day by infusion over 4 h, starting at day 0 for 7‐10 days Control therapy: OKT 5 mg/day iv bolus injection, starting at day 0 for 7‐10 day Concomittant immunosuppressive treatment: cyclosporin, started preoperatively azathioprine, started preoperatively corticosteroids, started intraoperatively |
|
| Outcomes | The primary objective was the efficacy of low‐dose protocols in further follow‐up and analysis, incidence and severity of acute cardiac allograft, infectious complication, nephrotoxicity, and participant survival Follow‐up period: mean 11.5 months (range 0.5‐22 months) |
|
| Notes | Endomyocardial biopsies performed on a routine basis, or when clinically indicated Acute rejection was classified as mild, moderate, or severe, as described by Billingham Study designer: not reported Sources of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised, no further details specified |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomised, no further details specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data was not found |
| Selective reporting (reporting bias) | Low risk | No protocol was assessed, but the trial reported on mortality, graft rejection, infections, and renal function, so all expected outcomes were reported |
| Other bias | Low risk | The trial appeared to be free of other components of bias |
Abbreviations
> = more/greater than < = less than ≥ = more than or equal to ± = plus or minus ALG = anti‐lymphocyte globulin ATG = anti‐thymocyte globulin ATGAM = horse anti‐thymocyte globulin CD2 = Cluster of Differentiation 2 CD25 = Cluster of Differentiation 25 CMV = Cytomegalovirus h = hour(s) HIV = human immunodeficiency virus ICU = intensive care unit IM = intra‐muscular ISHLT = International Society for Heart and Lung Transplantation iv = intraveous(ly) IV = four NYHA = PTLD = post‐transplantation lymphoproliferative disorder RATG = rabbit antithymocyte globulin RCT = randomised controlled trial vs = versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adamson 1998 | Retrospective study comparing muromonab‐CD3 with no antibody induction |
| Almenar 2005 | Retrospective study comparing IL‐2 RAs with muromonab‐CD3 |
| Carlsen 2005 | Retrospective study comparing daclizumab with historic RATG induction group |
| Chien 2000 | Study reporting on RATG induction, no control group |
| Chin 2005 | Study comparing daclizumab with a historic control group receiving muromonab‐CD3 |
| Chou 2008 | Non‐randomised study comparing basiliximab, cyclosporine and everolimus with RATG, cyclosporine/tacrolimus |
| Costanzo‐Nordin 1989 | Non‐randomised study comparing muromonab‐CD3 with horse ATG |
| Delgado 2005 | Non‐randomised study comparing basiliximab with RATG |
| Flaman 2006 | Retrospective non‐randomised study comparing basiliximab with RATG |
| Hegewald 1989 | Not properly randomised; randomised to treatment with or without vincristine |
| Kirklin 1990 | Non‐randomised study comparing RATG with muromonab‐CD3 |
| Koch 2005 | Study comparing T‐cell adapted anti‐thymocyte globulin (ATG‐Sangstat) with historic full‐dose ATG (Merieux) group |
| Laufer 1989 | Study comparing ATG/cyclosporine/azathioprine with ATG/corticosteroids/cyclosporine/azathioprine with muromonab‐CD3/corticosteroids/cyclosporine/azathioprine. Not all participants in the treatment groups were randomised, and results were not reported separately for these participants |
| Petrikovits 2005 | Study comparing daclizumab with historic control group |
| Pham 2010 | RCT investigating alemtuzumab induction. Trial excluded due to differences in concomitant immunosuppressive treatment |
| Renlund 1989 | Study comparing muromonab‐CD3 with horse ATG. Study was not properly randomised: ATG could not be supplied by the manufacturer in a consistent fashion. Consecutive participants were assigned to the ATG protocol when ATG was available, and to the muromonab‐CD3 protocol when ATG was not available |
| Rosenberg 2005 | Study comparing basiliximab with a historic control group receiving no induction |
| Starnes 1989 | Study comparing muromonab‐CD3 with a historic control group receiving (rabbit or horse) ATG |
| Teuteberg 2010 | Retrospective study comparing alemtuzumab induction with no induction |
| Yamani 2008 | RCT comparing tacrolimus, mycophenolate mofetil, corticosteroids, and single doses intraoperatively of RATG versus tacrolimus, mycophenolate mofetil, and RATG day 0‐5: differences in concomitant immunosuppression |
| Zakliczynski 2005 | RCT studying myocardial hypertrophy in participants treated with and without ATG; the abstract did not report on any of our outcomes. Authors were contacted, but did not reply |
| Zuckermann 2000 | Retrospective study comparing two different types of ATG (ATG‐Thymoglobulin versus RATG‐Fresenius) |
| Zuckermann 2003 | Retrospective study reporting on antibody induction in participants over 60 years of age |
Abbreviations
ATG = anti‐thymocyte globulin RATG = rabbit anti‐thymocyte globulin
Differences between protocol and review
At the Cochrane Colloquium, October 2010, Keystone, Colorado, USA, agreement was reached that 'baseline imbalance' and 'early stopping' in an individual trial may cause bias in that trial, but not necessarily in the meta‐analysis. We have therefore removed 'baseline imbalance' and 'early stopping' as criteria of bias.
Contributions of authors
Drafting the protocol: LP, CM, FG. DS, CG.
Study selection: LP, CM.
Extraction of data from studies: LP, CM, FG.
Data‐entry in RevMan: LP, CM.
Performing the analysis: LP, CM, CG.
Interpretation of the analysis: LP, CM, FG, CG.
Drafting the review: LP
Resolution of disagreements: CG.
Critical editing of the review: LP, CM, FG, CG, DS.
Sources of support
Internal sources
-
Rigshospitalet Research Council, Copenhagen, Denmark.
Grant to LP
External sources
No sources of support supplied
Declarations of interest
Luit Penninga: none known Christian H Møller: none known Finn Gustafsson: none known Christian Gluud: none known Daniel A Steinbrüchel: none known
New
References
References to studies included in this review
Balk 1992 {published data only}
- Balk AH, Meeter K, Simoons ML, Brouwer RM, Zondervan PE, Mochtar B, et al. Polyclonal versus monoclonal rejection prophylaxis after heart transplantation: a randomised study. Transplantation International 1992;5 Suppl 1:S476‐9. [DOI] [PubMed] [Google Scholar]
Beniaminovits 2000 {published data only}
- Beniaminovitz A, Itescu S, Lietz K, Donovan M, Burke EM, Groff BD, et al. Prevention of rejection in cardiac transplantation by blockade of the interleukin‐2 receptor with a monoclonal antibody. New England Journal of Medicine 2000;342(9):613‐9. [DOI] [PubMed] [Google Scholar]
Bolling 1989 {published data only}
- Bolling SF, Stirling MC, Miska P, Deeb GM. Comparison of polyclonal antibody sera for early prophylaxis following cardiac transplantation. Journal of Surgical Research 1989;47(4):292‐6. [DOI] [PubMed] [Google Scholar]
Bonaros 2006 {published data only}
- Bonaros N, Dunkler D, Kocher A, Imhof M, Grimm M, Zuckermann A, et al. Ten‐year follow‐up of a prospective, randomized trial of BT563/bb10 versus anti‐thymocyte globulin as induction therapy after heart transplantation. Journal of Heart and Lung Transplantation 2006;25(9):1154‐63. [DOI] [PubMed] [Google Scholar]
Carrier 2007 {published data only}
- Carrier M, Leblanc MH, Perrault LP, White M, Doyle D, Beaudoin D, et al. Basiliximab and rabbit anti‐thymocyte globulin for prophylaxis of acute rejection after heart transplantation: a non‐inferiority trial. Journal of Heart and Lung Transplantation 2007;26(3):258‐63. [DOI] [PubMed] [Google Scholar]
Costanzo‐Nordin 1990 {published data only}
- Costanzo‐Nordin MR, O'Sullivan EJ, Johnson MR, Winters GL, Pifarre R, Radvany R, et al. Prospective randomized trial of OKT3‐ versus horse antithymocyte globulin‐based immunosuppressive prophylaxis in heart transplantation. Journal of Heart Transplantation 1990;9(3 Pt 2):306‐15. [PubMed] [Google Scholar]
De Santo 2004 {published data only}
- Santo LS, Della Corte A, Romano G, Amarelli C, Onorati F, Torella M, et al. Midterm results of a prospective randomized comparison of two different rabbit‐antithymocyte globulin induction therapies after heart transplantation. Transplantation Proceedings 2004;36(3):631‐7. [DOI] [PubMed] [Google Scholar]
Faggian 2010 {published data only}
- Faggian G, Forni A, Milano AD, Chiominto B, Walpoth BH, Scarabelli T, et al. Antithymocyte globulin induction therapy in heart transplantation: prospective randomized study of high vs standard dosage. Transplantation Proceedings 2010;42(9):3679‐87. [DOI] [PubMed] [Google Scholar]
- Forni A, Faggian G, Chiominto B, Mazzucco A. Anti rejection prophylaxis using ATG‐Fresenius S after heart transplantation ‐ long‐term clinical results of a prospective, randomised trial. Transplant International 2007;20:116. [Google Scholar]
Hershberger 2005 {published data only}
- Hershberger RE, Starling RC, Eisen HJ, Bergh CH, Kormos RL, Love RB, et al. Daclizumab to prevent rejection after cardiac transplantation. New England Journal of Medicine 2005;352(26):2705‐13. [DOI] [PubMed] [Google Scholar]
Ippoliti 1991 {published data only}
- Ippoliti G, Negri M, Abelli P, Rovati B, Franco L, Grossi P, et al. Preoperative prophylactic OKT3 vs RATG. A randomized clinical study in heart transplant patients. Transplantation Proceedings 1991;23(4):2272‐4. [PubMed] [Google Scholar]
- Origlia C, Ippoliti G, Martinelli L, Goggi C, Abelli P, Negri M, et al. A randomized clinical‐study of preoperative administration of Okt3 vs Ratg in heart transplanted patients treated with Sandimmun. Transplantation and Clinical Immunology. 1991; Vol. 16:250.
Kobashigawa 1993 {published data only}
- Kobashigawa JA, Stevenson LW, Brownfield E, Moriguchi JD, Kawata N, Hamilton M, et al. Does short‐course induction with OKT3 improve outcome after heart transplantation? A randomized trial. Journal of Heart and Lung Transplantation 1993;12(2):205‐8. [PubMed] [Google Scholar]
Kormos 1990 {published data only}
- Griffith BP, Kormos RL, Armitage JM, Dummer JS, Hardesty RL. Comparative trial of immunoprophylaxis with RATG versus OKT3. Journal of Heart Transplantation 1990;9(3 Pt 2):301‐5. [PubMed] [Google Scholar]
- Kormos RL, Armitage JM, Dummer JS, Miyamoto Y, Griffith BP, Hardesty RL. Optimal perioperative immunosuppression in cardiac transplantation using rabbit antithymocyte globulin. Transplantation 1990;49(2):306‐11. [DOI] [PubMed] [Google Scholar]
- Kormos RL, Herlan DB, Armitage JM, Stein K, Kaufman C, Zeevi A, et al. Monoclonal versus polyclonal antibody therapy for prophylaxis against rejection after heart transplantation. Journal of Heart Transplantation 1990;9(1):1‐9. [PubMed] [Google Scholar]
Macdonald 1993 {published data only}
- Macdonald PS, Mundy J, Keogh AM, Chang VP, Spratt PM. A prospective randomized study of prophylactic OKT3 versus equine antithymocyte globulin after heart transplantation‐‐increased morbidity with OKT3. Transplantation 1993;55(1):110‐6. [DOI] [PubMed] [Google Scholar]
Mattei 2007 {published data only}
- Boissonnat P, Sebbag L, French Heart Nausicaa Study Grp. Less infectious deaths with basiliximab (Simulect (R)) over antihymocyte globulin (Thymoglobuline (R)) induction therapy in heart transplantation: Results of nausicaa, a national multicentre prospective randomized study. 2005; Vol. 5:386.
- Mattei MF, Boissonnat P, Redonnet M, Gandjbakhch I, Bandini AM, Dorent R, et al. Improved safety of basiliximab (Simulect (R)) over antithymocyte globulin (Thymoglobulin (R)) induction therapy in heart transplantation. Journal of Heart and Lung Transplantation 2005;24(2):63. [Google Scholar]
- Mattei MF, Redonnet M, Gandjbakhch I, Bandini AM, Billes A, Epailly E, et al. Lower risk of infectious deaths in cardiac transplant patients receiving basiliximab versus anti‐thymocyte globulin as induction therapy. Journal of Heart and Lung Transplantation 2007;26(7):693‐9. [DOI] [PubMed] [Google Scholar]
Mehra 2005 {published data only}
- Mehra MR, Zucker MJ, Wagoner L, Michler R, Boehmer J, Kovarik J, et al. A multicenter, prospective, randomized, double‐blind trial of basiliximab in heart transplantation. Journal of Heart and Lung Transplantation 2005;24(9):1297‐304. [DOI] [PubMed] [Google Scholar]
Menkis 1992 {published data only}
- Menkis AH, Powell AM, Novick RJ, McKenzie FN, Kostuk WJ, Pflugfelder PW, et al. A prospective randomized controlled trial of initial immunosuppression with ALG versus OKT3 in recipients of cardiac allografts. Journal of Heart and Lung Transplantation 1992;11(3 Pt 1):569‐76. [PubMed] [Google Scholar]
Mullen 2005 {published data only}
- Mullen JC, Dueck A, Bentely MJ, Modry DL, Wang SH, Burton JR, et al. A randomized control trial of daclizumab versus anti‐thymocyte globulin induction for heart transplantation. Journal of Heart and Lung Transplantation 2005;24(2):143. [Google Scholar]
Schnetzler 2002 {published data only}
- Schnetzler B, Leger P, Volp A, Dorent R, Pavie A, Gandjbakhch I. A prospective randomized controlled study on the efficacy and tolerance of two antilymphocytic globulins in the prevention of rejection in first‐heart transplant recipients. Transplant International 2002;15(6):317‐25. [DOI] [PubMed] [Google Scholar]
Segovia 2006 {published data only}
- Segovia J, Rodriguez‐Lambert JL, Crespo‐Leiro MG, Almenar L, Roig E, Gomez‐Sanchez MA, et al. A randomized multicenter comparison of basiliximab and muromonab (OKT3) in heart transplantation: SIMCOR study. Transplantation 2006;81(11):1542‐8. [DOI] [PubMed] [Google Scholar]
van Gelder 1996 {published data only}
- Wabbijn M, Balk AH, Domburg RT, Vantrimpont PJ, Riemsdijk I, Baan CC, et al. Ten‐year follow‐up of recipients of a kidney or heart transplant who received induction therapy with a monoclonal antibody against the interleukin‐2 receptor. Experimental and Clinical Transplantation 2004;2(1):201‐7. [PubMed] [Google Scholar]
- Gelder T, Baan CC, Balk AH, Knoop CJ, Holweg CT, Meer P, et al. Blockade of the interleukin (IL)‐2/IL‐2 receptor pathway with a monoclonal anti‐IL‐2 receptor antibody (BT563) does not prevent the development of acute heart allograft rejection in humans. Transplantation 1998;65(3):405‐10. [DOI] [PubMed] [Google Scholar]
- Gelder T, Balk AH, Jonkman FA, Zietse R, Zondervan P, Hesse CJ, et al. A randomized trial comparing safety and efficacy of OKT3 and a monoclonal anti‐interleukin‐2 receptor antibody (BT563) in the prevention of acute rejection after heart transplantation. Transplantation 1996;62(1):51‐5. [DOI] [PubMed] [Google Scholar]
- Gelder T, Mulder AH, Balk AH, Mochtar B, Hesse CJ, Baan CC, et al. Intragraft monitoring of rejection after prophylactic treatment with monoclonal anti‐interleukin‐2 receptor antibody (BT563) in heart transplant recipients. Journal of Heart and Lung Transplantation 1995;14(2):346‐50. [PubMed] [Google Scholar]
van Gelder 2004 {published data only}
- Gelder T, Wabbijn M, Domburg R, Vantrimpont P, Maat L, Baan C, et al. A randomised, double‐blind, placebo controlled trial of anti interleukin‐2 receptor monoclonal antibody (daclizumab) induction therapy after heart transplantation. American Journal of Transplantation 2004;4:450. [Google Scholar]
Wollenek 1989 {published data only}
- Wollenek G, Laufer G, Laczkovics A, Buxbaum P, Kober I. Comparison of a monoclonal anti‐T cell antibody vs ATG as prophylaxis after heart transplantation. Transplantation Proceedings 1989;21(1 Pt 3):2499‐501. [PubMed] [Google Scholar]
References to studies excluded from this review
Adamson 1998 {published data only}
- Adamson R, Obispo E, Dychter S, Dembitsky W, Moreno‐Cabral R, Jaski B, et al. Long‐term outcome with the use of OKT3 induction therapy in heart transplant patients: a single‐center experience. Transplantation Proceedings 1998;30(4):1107‐9. [DOI] [PubMed] [Google Scholar]
Almenar 2005 {published data only}
- Almenar L, Garcia‐Palomar C, Martinez‐Dolz L, Chamorro C, Moro J, Zorio E, et al. Influence of induction therapy on rejection and survival in heart transplantation. Transplantation Proceedings 2005;37(9):4024‐7. [DOI] [PubMed] [Google Scholar]
Carlsen 2005 {published data only}
- Carlsen J, Johansen M, Boesgaard S, Andersen CB, Arendrup H, Aldershvile J, et al. Induction therapy after cardiac transplantation: a comparison of anti‐thymocyte globulin and daclizumab in the prevention of acute rejection. Journal of Heart and Lung Transplantation 2005;24(3):296‐302. [DOI] [PubMed] [Google Scholar]
Chien 2000 {published data only}
- Chien NC, Lin FL, Chou NK, Hsu RB, Wang SS, Chu SH, et al. Rabbit antithymocyte globulin induction immunosuppression in heart transplantation. Transplantation Proceedings 2000;32(7):2380‐2. [DOI] [PubMed] [Google Scholar]
Chin 2005 {published data only}
- Chin C, Pittson S, Luikart H, Bernstein D, Robbins R, Reitz B, et al. Induction therapy for pediatric and adult heart transplantation: comparison between OKT3 and daclizumab. Transplantation 2005;80(4):477‐81. [DOI] [PubMed] [Google Scholar]
Chou 2008 {published data only}
- Chou NK, Wang SS, Chen YS, Yu HY, Chi NH, Wang CH, et al. Induction immunosuppression with basiliximab in heart transplantation. Transplantation Proceedings 2008;40(8):2623‐5. [DOI] [PubMed] [Google Scholar]
Costanzo‐Nordin 1989 {published data only}
- Costanzo‐Nordin MR, O'Sullivan EJ, Hubbell EA, Zucker MJ, Pifarre R, McManus BM, et al. Long‐term follow‐up of heart transplant recipients treated with murine antihuman mature T cell monoclonal antibody (OKT3): the Loyola experience. Journal of Heart Transplantation 1989;8(4):288‐95. [PubMed] [Google Scholar]
Delgado 2005 {published data only}
- Delgado DH, Miriuka SG, Cusimano RJ, Feindel C, Rao V, Ross HJ. Use of basiliximab and cyclosporine in heart transplant patients with pre‐operative renal dysfunction. Journal of Heart and Lung Transplantation 2005;24(2):166‐9. [DOI] [PubMed] [Google Scholar]
Flaman 2006 {published data only}
- Flaman F, Zieroth S, Rao V, Ross H, Delgado DH. Basiliximab versus rabbit anti‐thymocyte globulin for induction therapy in patients after heart transplantation. Journal of Heart and Lung Transplantation 2006;25(11):1358‐62. [DOI] [PubMed] [Google Scholar]
Hegewald 1989 {published data only}
- Hegewald MG, O'Connell JB, Renlund DG, Lee HR, Burton NA, Karwande SV, et al. OKT3 monoclonal antibody given for ten versus fourteen days as immunosuppressive prophylaxis in heart transplantation. Journal of Heart Transplantation. UNITED STATES, 1989; Vol. 8, issue 4:303‐9. [PubMed]
Kirklin 1990 {published data only}
- Kirklin JK, Bourge RC, White‐Williams C, Naftel DC, Thomas FT, Thomas JM, et al. Prophylactic therapy for rejection after cardiac transplantation. A comparison of rabbit antithymocyte globulin and OKT3. Journal of Thorac and Cardiovascular Surgery 1990;99(4):716‐24. [PubMed] [Google Scholar]
Koch 2005 {published data only}
- Koch A, Daniel V, Dengler TJ, Schnabel PA, Hagl S, Sack FU. Effectivity of a T‐cell‐adapted induction therapy with anti‐thymocyte globulin (Sangstat). Journal of Heart and Lung Transplantation 2005;24(6):708‐13. [DOI] [PubMed] [Google Scholar]
Laufer 1989 {published data only}
- Laufer G, Laczkovics A, Wollenek G, Schreiner W, Sochor H, Holzinger C, et al. Impacts of low‐dose steroids and prophylactic monoclonal versus polyclonal antibodies on acute rejection in cyclosporine‐ and azathioprine‐immunosuppressed cardiac allografts. Journal of Heart Transplantation 1989;8(3):253‐61. [PubMed] [Google Scholar]
Petrikovits 2005 {published data only}
- Petrikovits E, Bedanova H, Necas J, Studenik P, Cerny J. Daclizumab in the induction phase of immunosuppression in heart transplant recipients. Annals of Transplantation 2005;10(3):5‐10. [PubMed] [Google Scholar]
Pham 2010 {published data only}
- Pham SM, Bednar BM, Panos A, Bauerlein EJ, Jimenez F, Rosenkrantz E, et al. A randomized study using humanized monoclonal antibody against Cd52 (Campath‐1h) and tacrolimus in heart transplant recipients. Journal of Heart and Lung Transplantation 2010;29(2):134. [Google Scholar]
Renlund 1989 {published data only}
- Renlund DG, O'Connell JB, Gilbert EM, Hammond ME, Burton NA, Jones KW, et al. A prospective comparison of murine monoclonal CD‐3 (OKT3) antibody‐based and equine antithymocyte globulin‐based rejection prophylaxis in cardiac transplantation. Decreased rejection and less corticosteroid use with OKT3. Transplantation 1989;47(4):599‐605. [PubMed] [Google Scholar]
Rosenberg 2005 {published data only}
- Rosenberg PB, Vriesendorp AE, Drazner MH, Dries DL, Kaiser PA, Hynan LS, et al. Induction therapy with basiliximab allows delayed initiation of cyclosporine and preserves renal function after cardiac transplantation. Journal of Heart and Lung Transplantation 2005;24:1327‐31. [DOI] [PubMed] [Google Scholar]
Starnes 1989 {published data only}
- Starnes VA, Oyer PE, Stinson EB, Dein JR, Shumway NE. Prophylactic OKT3 used as induction therapy for heart transplantation. Circulation 1989;80(5 Pt 2):III79‐83. [PubMed] [Google Scholar]
Teuteberg 2010 {published data only}
- Teuteberg JJ, Shullo MA, Zomak R, Toyoda Y, McNamara DM, Bermudez C, et al. Alemtuzumab induction prior to cardiac transplantation with lower intensity maintenance immunosuppression: one‐year outcomes. American Journal of Transplantation 2010;10(2):382‐8. [DOI] [PubMed] [Google Scholar]
Yamani 2008 {published data only}
- Yamani MH, Taylor DO, Czerr J, Haire C, Kring R, Zhou L, et al. Thymoglobulin induction and steroid avoidance in cardiac transplantation: results of a prospective, randomized, controlled study. Clinical Transplantation 2008;22(1):76‐81. [PubMed] [Google Scholar]
Zakliczynski 2005 {published data only}
- Zakliczynski MW, Nozynski J, Maruszewski M, Zembala M. Thymoglobuline administered electively early after heart transplantation may protect myocardial hypertrophy (Abstract). American Journal of Transplantation. American Transplant Congress, Seattle [United States] 21‐25 May 2005. 2005; Vol. 5, issue Suppl 11:171.
Zuckermann 2000 {published data only}
- Zuckermann AO, Grimm M, Czerny M, Ofner P, Ullrich R, Ploner M, et al. Improved long‐term results with thymoglobuline induction therapy after cardiac transplantation: a comparison of two different rabbit‐antithymocyte globulines. Transplantation 2000;69(9):1890‐8. [DOI] [PubMed] [Google Scholar]
Zuckermann 2003 {published data only}
- Zuckermann A, Dunkler D, Deviatko E, Bodhjalian A, Czerny M, Ankersmit J, et al. Long‐term survival (>10 years) of patients >60 years with induction therapy after cardiac transplantation. European Journal of Cardiothoracic Surgery 2003;24(2):283‐91. [DOI] [PubMed] [Google Scholar]
Additional references
Cantarovich 2007
- Cantarovich M. Renal protective strategies in heart transplant patients. Current Opinion in Cardiology 2007;22(2):133‐8. [DOI] [PubMed] [Google Scholar]
Chatenoud 2008
- Chatenoud L. The long and winding road towards induction of allograft tolerance in the clinic. Transplant International 2008;21:725‐7. [DOI] [PubMed] [Google Scholar]
Chen 2006
- Chen W, Zhang L. Regulatory T‐cell subsets and their roles in transplantation tolerance. Current Opinion in Organ Transplantation 2006;11(4):373‐8. [Google Scholar]
Deeks 2003
- Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non‐randomised intervention studies. Health Technology Assessment 2003;7(27):1‐173. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Hamamsy 2005
- El‐Hamamsy I, Stevens LM, Carrier M, Pelletier G, White M, Tremblay F, et al. Incidence and prognosis of cancer following heart transplantation using RATG induction therapy. Transplant International 2005;18(11):1280‐5. [DOI] [PubMed] [Google Scholar]
Flechner 2008
- Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitor‐sparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity. Clinical Transplantation 2008;22(1):1‐15. [DOI] [PubMed] [Google Scholar]
Gluud 2006
- Gluud LL. Bias in clinical intervention research. American Journal of Epidemiology 2006;163:493‐501. [DOI] [PubMed] [Google Scholar]
Groetzner 2005
- Groetzner J, Wahlers T. Calcineurin inhibitor‐free immunosuppression after heart transplantation: can chronic side effects be avoided?. Current Opinion in Organ Transplantation 2005;10(4):360‐3. [Google Scholar]
Hauptman 2005
- Hauptman PJ, Mehra MR. It is time to stop ignoring malignancy in heart transplantation: a call to arms. Journal of Heart and Lung Transplantation 2005;24:1111‐3. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2005
- Higgins R, Kirklin JK, Brown RN, Rayburn BK, Wagoner L, Oren R, et al. To induce or not to induce: do patients at greatest risk for fatal rejection benefit from cytolytic induction therapy?. Journal of Heart and Lung Transplantation 2005;24(4):392‐400. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICH‐GCP 1996
- ICH‐GCP 1996 International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. Guideline for Good Clinical Practice. E6 (R1). ICH Harmonised Tripartite Guideline 1996. http://ichgcp.net (accessed 2 September 2010).
Keus 2009
- Keus F, Wetterslev J, Gluud C, Gooszen HG, Laarhoven CJ. Robustness assessments are needed to reduce bias in meta‐analyses that include zero‐event randomized trials. American Journal of Gastroenterology 2009;104(3):546‐51. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135:982‐9. [DOI] [PubMed] [Google Scholar]
Kobashigawa 2005
- Kobashigawa J, David K, Morris J, Chu AH, Steffen BJ, Gotz VP, et al. Daclizumab is associated with decreased rejection and no increased mortality in cardiac transplant patients receiving MMF, cyclosporine, and corticosteroids. Transplantation Proceedings 2005;37(2):1333‐9. [DOI] [PubMed] [Google Scholar]
Kobashigawa 2006
- Kobashigawa JA, Patel JK. Immunosuppression for heart transplantation: where are we now?. Nature Clinical Practice Cardiovascular Medicine 2006;3(4):203‐12. [DOI] [PubMed] [Google Scholar]
Lechler 2005
- Lechler RI, Sykes M, Thomson AW, Turka LA. Organ transplantation‐‐how much of the promise has been realized?. Nature Medicine 2005;11:605‐13. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. The Cochrane Collaboration, Available from www.cochrane‐handbook.org.
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20:641‐54. [DOI] [PubMed] [Google Scholar]
Mallinger 1999
- Mallinger R, Chevtchik O, Zuckermann A, Jucewicz M, Moidl R, Kocher A, et al. Development and spectrum of PTLD in 691 cardiac transplant recipients (Htx) receiving induction therapy. Journal of Heart and Lung Transplantation 1999;19(1):77 (abstract 174). [Google Scholar]
Master List 2009
- United States Cochrane Center. Master list of journals being searched. http://apps1.jhsph.edu/cochrane/masterlist.asp (accessed May 2009).
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62:1006‐12. [DOI] [PubMed] [Google Scholar]
Mueller 2004
- Mueller XM. Drug immunosuppression therapy for adult heart transplantation. Part 2: clinical applications and results. Annals of Thoracic Surgery 2004;77:363‐71. [DOI] [PubMed] [Google Scholar]
O'Neill 2004
- O'Neill JOT. Immunosuppression for cardiac transplantation ‐ the past, present and future. Transplantation Proceedings 2004;36(2 SUPPL):309S‐13S. [DOI] [PubMed] [Google Scholar]
Oaks 2001
- Oaks TE, Wannenberg T, Close SA, Tuttle LE, Kon ND. Steroid‐free maintenance immunosuppression after heart transplantation. Annals of Thoracic Surgery 2001;72:102‐6. [DOI] [PubMed] [Google Scholar]
Penninga 2010
- Penninga L, Moller CH, Gustafsson F, Steinbruchel DA, Gluud C. Tacrolimus versus cyclosporine as primary immunosuppression after heart transplantation: systematic review with meta‐analyses and trial sequential analyses of randomised trials. European Journal of Clinical Pharmacology 2010;66(12):1177‐87. [DOI] [PubMed] [Google Scholar]
Penninga 2011
- Penninga L, Møller CH, Penninga EI, Iversen M, Gluud C, Steinbrüchel DA. Antibody induction for lung transplant recipients. Cochrane Database of Systematic Reviews 2011, (1):Art. No.: CD008927. DOI: 10.1002/14651858.CD008927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Penninga 2012
- Penninga L, Wettergren A, Wilson CH, Chan AW, Steinbrüchel DA, Gluud C. Antibody induction versus placebo, no induction, or another type of antibody induction for liver transplant recipients. Cochrane Database of Systematic Reviews 2012. [DOI: 10.1002/14651858.CD007341.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peraira 2003
- Peraira JR, Segovia J, Fuertes B, Fernandez JA, Escudier JM, Salas C, et al. Current induction immunosuppression and post‐heart transplant lymphoproliferative disorders. Transplantation Proceedings 2003;35(5):2009‐10. [DOI] [PubMed] [Google Scholar]
Potter 2005
- Potter B, Giannetti N, Cecere R, Cantarovich M. Long‐term calcineurin inhibitor "holiday" using daclizumab in a heart transplant patient with acute renal dysfunction. Journal of Heart and Lung Transplantation 2005;24:1126‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rinaldi 2001
- Rinaldi M, Pellegrini C, D'Armini AM, Aiello M, Negri M, Arbustini E, et al. Neoplastic disease after heart transplantation: single center experience. European.Journal of Cardiothoracic Surgery 2001;19(5):696‐701. [DOI] [PubMed] [Google Scholar]
Royle 2003
- Royle P, Waugh N. Literature searching for clinical and cost‐effectiveness studies used in health technology assessment reports carried out for the National Institute for Clinical Excellence appraisal system. Health Technology Assessment 2003;7:1‐51. [DOI] [PubMed] [Google Scholar]
Savovic 2012
- Savovic J, Jones HE, Altman DG, Harris RJ, Juni P, Pildal J, et al. Influence of reported study design characteristics on Intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Stehlik 2012
- Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report‐2012. Journal of Heart and Lung Transplantation. 2012;31(10):1052‐64. [DOI] [PubMed] [Google Scholar]
Stevens 2004
- Stevens LM, El‐Hamamsy I, Leblanc M, Perrault LP, Pellerin M, Bouchard D, et al. Continuous renal replacement therapy after heart transplantation. Canadian Journal of Cardiology 2004;20(6):619‐23. [PubMed] [Google Scholar]
Stewart 2005
- Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. Journal of Heart and Lung Transplantation 2005;24:1710‐20. [DOI] [PubMed] [Google Scholar]
Sweeting 2004
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23:1351‐75. [DOI] [PubMed] [Google Scholar]
Swinnen 1990
- Swinnen LJ, Costanzo‐Nordin MR, Fisher SG, O'Sullivan EJ, Johnson MR, Heroux AL, et al. Increased incidence of lymphoproliferative disorder after immunosuppression with the monoclonal antibody OKT3 in cardiac‐transplant recipients. New England Journal of Medicine 1990;323(25):1723‐8. [DOI] [PubMed] [Google Scholar]
Taylor 2009
- Taylor DO, Stehlik J, Edwards LB, Aurora P, Christie JD, Dobbels F, et al. Registry of the International Society for Heart and Lung Transplantation: twenty‐sixth official adult heart transplant report‐2009. Journal of Heart and Lung Transplantation 2009;28:1007‐22. [DOI] [PubMed] [Google Scholar]
Thompson 2002
- Thompson SG, Higgins JP. How should meta‐regression analyses be undertaken and interpreted?. Statistics in Medicine 2002;21:1559‐73. [DOI] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Imberger G, Walsh M, Chu R, Gluud C, Wetterslev J, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta‐analysis‐‐a simulation study. PLoS One 2011;6(10):e25491. [DOI] [PMC free article] [PubMed] [Google Scholar]
TSA manual 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for trial sequential analysis (TSA). Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen, Denmark 2011:1‐115.
TSA programme 2012 [Computer program]
- Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C, Thorlund K. Trial Sequential Analysis Programme. Version 0.9 beta 2012.
Uber 2007
- Uber PA, Mehra MR. Induction therapy in heart transplantation: is there a role?. Journal of Heart and Lung Transplantation 2007;26:205‐9. [DOI] [PubMed] [Google Scholar]
Webster 2010
- Webster AC, Ruster LP, McGee R, Matheson SL, Higgins GY, Willis NS, et al. Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD003897.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61:64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Edition) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zuppan 2009
- Zuppan CW, Wells LM, Kerstetter JC, Johnston JK, Bailey LL, Chinnock RE. Cause of death in pediatric and infant heart transplant recipients: review of a 20‐year, single‐institution cohort. Journal of Heart and Lung Transplantation 2009;28:579‐84. [DOI] [PubMed] [Google Scholar]


