Abstract
Background
Some patients with postoperative hip fractures (HF) experience persistent severe pain. In this longitudinal study, we examined the characteristics of patients with persistent pain after HF surgery, and the factors influencing pain intensity.
Methods
We conducted an 8-week prospective study in patients with postsurgical HF. Verbal rating scale (VRS), and multifaceted outcomes, including pressure pain threshold (PPT) (affected site and biceps), were evaluated at 2, 4, and 8 weeks postoperatively. Patients were divided into mild (VRS ≤1) and severe (VRS ≥2) groups according to pain intensity at 8 weeks postoperatively. Statistical analyses were performed using two-way ANOVA and decision-tree analysis.
Results
VRS, PPT at the affected site and biceps, and physical activity (PA) time were significantly lower in the severe group than in the mild group 2 weeks postoperatively. VRS, PPT at the affected site, pain catastrophizing (PCS)-13, and the Tampa Scale for Kineshiophobia (TSK)-11 did not show significant improvements in the severe group. Decision tree analysis revealed that the VRS and PCS-13 at 4 weeks, PA time at 2 weeks, and TSK-11 change between 4 weeks and 2 weeks were factors influencing severe pain intensity at 8 weeks after HF surgery.
Conclusion
Persistent severe pain after HF surgery was characterised by high peripheral and central sensitisation, pain catastrophizing, and reduced PA at 2 weeks after HF surgery. In addition, early pain intensity, pain catastrophizing, and PA may be hierarchically influential factors for persistent pain 8 weeks after HF surgery.
Keywords: Hip fracture, postsurgical pain, pressure pain threshold, multifaceted assessment, decision-tree analysis
Introduction
Hip fracture (HF) is one of the most common fragility fractures, and its incidence is expected to increase. 1 In most cases, surgery is indicated after injury and rehabilitation is initiated immediately in the postoperative period to restore activities of daily living (ADL). Chronic postsurgical pain (CPSP) is an important problem in these patients and is known to adversely affect their long-term postsurgical recovery and physical function. 2 The risk factors for CPSP include age, female sex, and surgery-related factors such as surgical technique, surgery time, and revision surgery. 3 Psychological factors such as pain catastrophizing, fear of movement, and depression are important risk factors that increase the incidence of CPSP.4,5 Conversely, peripheral and central sensitisation are key factors of transition from acute to chronic pain. Thus, it is important to objectively and quantitatively assess abnormal pain sensitivity using the quantitative sensory test (QST), which is a psychophysical assessment method. 6 Previous studies using pressure pain threshold (PPT) have reported that a decrease in PPT at the affected site reflects peripheral sensitisation. In addition, a decrease in PPT at a site distant from the affected area may indicate central sensitisation. 7 Patients with hip osteoarthritis have been shown to exhibit systemic decrease in the PPT. 8
Pain during the post-acute recovery phases after HF surgery is considered to be associated with these patients’ limited mobility, thus it is important to manage pain appropriately after HF surgery. 9 We previously found that patients with persistent moderate or severe pain during the post-acute recovery phases, up to 8 weeks after HF surgery had higher subjective pain intensity and pain catastrophizing at two and four weeks postoperatively. 10 Considering the association between abnormal pain sensitivity and CPSP, 11 the PPT at the affected or at a distal site may decrease in patients with persistent moderate or severe pain after HF surgery. However, no studies have examined these aspects in the acute phase after HF surgery. Therefore, it is necessary to examine the characteristics of patients with persistent moderate or severe pain after HF surgery during 8 weeks based on multifaceted outcomes, including PPT. In addition, factors affecting the intensity of persistent pain after HF surgery remain unknown. Therefore, clarifying these points would be useful for developing rehabilitation strategies to prevent the development of CPSP. This study aimed to longitudinally examine the multifaceted characteristics of patients with persistent severe pain and factors associated with pain intensity at eight weeks after HF surgery.
Methods
Participants
The study was conducted between September 2021 and January 2024. Patients aged ≥65 years who were admitted to the emergency department of Nagasaki Memorial Hospital with a femoral neck fracture or femoral transverse fracture following a fall and who were diagnosed by radiography were included in the study. Exclusion criteria were conservative treatment, inability to understand the questionnaire due to cognitive decline, age <65 years, acute exacerbation, and declined to participate in the study. The study protocol was approved by the Research Ethical Committee of the Graduate School of Biomedical Sciences at Nagasaki University (approval number: 21080501). All the patients provided signed informed consent. Pain intensity, PPT, pain catastrophizing, fear of movement, depression, ADL, and physical activity (PA) were measured by physical therapists at 2, 4, and 8 weeks postoperatively. Physical function was assessed 4 and 8 weeks after surgery.
Rehabilitation protocol
During the preoperative period, the therapist instructed the patient on strength training and range of motion exercises (upper and lower extremities without fractures). No rehabilitation was performed on the day of the surgery. After surgery, depending on the surgical treatment (internal fixation or arthroplasty), patients were initiated on stand up and gait training programs. The physiotherapist spent more than 60 min/day with each patient.
Epidemiological background
Height and weight were measured, and the body mass index (BMI) was calculated. Age, sex, type of fracture (femoral neck or trochanteric fracture), preoperative period, type of surgery (bipolar hip arthroplasty [BHA] or compression hip screw [CHS] or γ-nail or pinning), and pain medications (celecoxib, acetaminophen, loxoprofen, and pregabalin), were also recorded. Pre-admission patient characteristics such as pain (lower back, hip, knee), comorbidities (Charlson Comorbidity Index [CCI]), ADL (Barthel Index [BI]), nutritional status (mini nutritional assessment short-form [MNA-SF]), and clinical frailty scale (CFS) were also recorded. The CFS was classified into subcategories (1–3, 4–5 and 6–9). 12 In addition, C-reactive protein (CRP) was measured as an inflammatory response at 1 week postoperative, and mini-mental state examination (MMSE) as cognitive function at 2 weeks postoperative.
Measures
Pain intensity
Pain intensity, defined as the level of the most intense pain of the day, was measured using a 5 point verbal rating scale (VRS; 0: no pain, 1: mild pain, 2: moderate pain, 3: severe pain, and 4: intolerable pain). 13
Pressure pain threshold
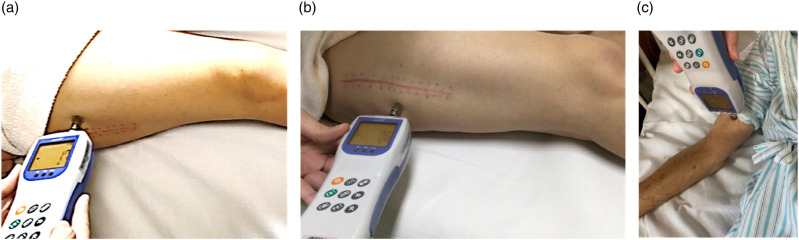
Deep-tissue pain sensitivity was assessed using a digital force gauge (RZE-100; Aikoh Engineering Company, Japan). We assessed the PPT at the affected and remote sites with the participants lying in bed. The affected PPT was measured at six points (2 or 3 cm) around the surgical incision. 14 The PPT at the remote site was measured at the biceps brachii. 15 The circular probe was placed at a 90° angle to the skin and was constantly and gradually pressed. When the pressure turned into a sharp pain, participants were instructed to say ‘stop’. Three measurements were repeated at 60 s intervals at each point, and the average value was used for analysis (Figure 1). 14
Figure 1.
Participants assessed two points of the surgical incision (3 cm ventral and 3 cm dorsal) and biceps brachii using a digital force gauge (RZE-100, Aikoh Engineering Company, Japan) while lying on bed. (a) surgical incision (3 cm ventral). (b) surgical incision (3 cm dorsal). (c) biceps brachii.
Pain catastrophizing, fear of movement, and depression symptoms
The pain catastrophizing scale-13 (PCS-13) consists of 13 items, and is rated on a 5 point scale from 0 (not at all) to 4 (always present), with total scores ranging from 0 to 52 and higher scores indicating greater catastrophizing. 16 The Tampa scale for kinesiophobia-11 (TSK-11) was used to evaluate fear of movement and consisted of 11 items, each rated on a 4-point scale from 1 (not at all agree) to 4 (strongly agree). Therefore, the range of scores is 11 to 44, with higher scores indicating stronger fear. 17 The Japanese version of the 5 item geriatric depression scale (GDS-5) was used to assess depressive symptoms. It consists of five items, with scores ranging from 0 to 5, with higher scores indicating stronger depression. 18
Activities of daily living
We used motor-FIM to measure general ADL. The motor-FIM consists of 13 items and measures the degree of impairment in performing basic ADL. The motor-FIM is a 7-point scale with scores ranging from 13 to 91 points. 19
Physical activity
Life coder GS, which is a uniaxial accelerometer, was used to evaluate the amount of PA. This is a compact device that classifies exercise intensity (1.8-8.3 METs) based on acceleration during movement and can calculate activity time for each exercise intensity; the validity of exercise intensity and accuracy of step count measurement has been verified.20,21 The participants were asked to always wear the Life coder GS near the superior anterior iliac spine on the nonoperative side, except when bathing, and the amount of PA was monitored. The data used for the analysis were the average of the total activity time during 2, 4, and 8 weeks postoperatively, which was defined as the PA time.
Physical function
Physical function was assessed using the 30 s chair stand test (CS-30) and 6 min walk distance (6 MWD). The CS-30 test measures the number of times a person stands up from a chair in 30 s, with higher standing numbers indicating greater lower limb muscle strength. 22 The 6 MWD was measured to assess whether functional exercise capacity correlated with physical fitness. 23 Participants were instructed to walk as far as possible for 6 min and rest while standing during the test; however, the test would be stopped in case they sat down. To prevent the participant from falling, the evaluator accompanied the participant to the rear side.
Statistical analysis
Persistent postoperative pain was defined as pain that persisted for 8 weeks after surgery. Persistent severe pain during movement at 8 weeks after surgery was defined as moderate to severe pain on the VRS (score = 2–4; severe group), and mild pain was defined as no or mild pain on the VRS (score = 0–1; mild group) at 8 weeks after surgery, based on our previous study. 10 Unpaired t-tests or Chi-square tests were used to evaluate significant differences in patient characteristics before and after hospital admission between the mild and severe groups. A repeated-measures two-way ANOVA was applied to each rating item, with group (mild and severe) and rating period as factors, to examine the main effect of group, main effect of rating period, and interaction effects. The Bonferroni method was applied as a subtest to examine simple main effects such as differences in time within each group and differences between groups at each time point.
A decision tree analysis was performed to clarify the factors influence pain intensity at 8 weeks after surgery by using classification and regression tree (CART). The Gini coefficient was used as the branching criterion. The 2 week postoperative decision tree was labelled Model 1, the 4 week postoperative decision tree was labelled Model 2; and the 2 and 4 week postoperative and 4 week-2 week (Δ-score) were labelled Model 3. In Model 1, 8 week postoperative VRS score served as the dependent variable, while the 10 items, including the baseline survey items (age and MMSE) and each assessment item served as the independent variables. In Model 2, the 8 week postoperative VRS was the dependent variable, and the 12 items, including baseline survey items (age, MMSE) and each assessment item, were entered as independent variables. In Model 3, the decision tree analysis with VRS at 8 weeks postoperatively served as the dependent variable while the 28 items, including baseline survey items, each assessment item at 2 and 4 weeks, and their Δ-score, served as independent variables. In the decision tree analysis, the maximum tree depth was set to 5, the minimum number of cases before analysis was set to 20, and the minimum number of cases after analysis was set to 6. Statistical analyses of patient characteristics and two-way ANOVA were performed using IBM SPSS Statistics version 22 software, with a significance level of <5%. The decision tree analysis was performed using JASP 0.18.1.
Results
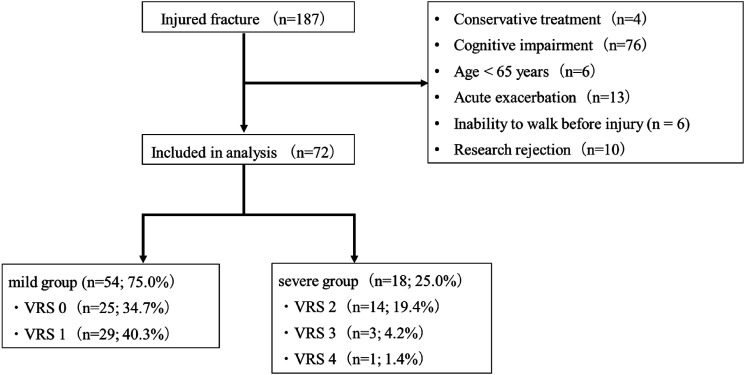
Between September 2021 and January 2024, 187 patients with HF were admitted to our hospital. Patients who received conservative treatment (n = 4), those who could not understand questions due to cognitive dysfunction (n = 76), those under 65 years of age (n = 6), those with acute exacerbations (n = 13), those who were unable to walk before the injury (n = 6), and those who declined to participate, (n = 10), were excluded. Overall, 72 patients were included in the analysis (Figure 1). Regarding pain during movement at 8 weeks postoperatively, 25 (34.7%), 29 (40.3%), 14 (19.4%), 3 (4.2%), and 1 (1.4%) of the 72 patients had a VRS score of 0, 1, 2, and 4, respectively (Figure 2).
Figure 2.
Flow chart of the number of participants in each study arm.
Patient characteristics
No significant differences was observed in age, sex, BMI, type of fracture, pain (lower, hip, knee), CCI, BI, MNA-SF, CFS, preoperative period, type of surgery, pain medications, blood loss, operative time, or CRP levels between the two groups. In contrast, the severe group had a significantly lower MMSE than the mild group at 2 weeks postoperatively (Table 1).
Table 1.
Patient characteristics.
| all participants (n = 72) | Mild group (n = 54) | Severe group (n = 18) | P value | |||
|---|---|---|---|---|---|---|
| Age (years) | 84.0 ± 8.2 | 83.1 ± 8.2 | 86.7 ± 7.7 | 0.107 | ||
| Sex | Male: 11 Female: 61 |
Male: 8 Female: 46 |
Male: 3 Female: 15 |
0.557 | ||
| BMI | 21.5 ± 3.4 | 21.4 ± 3.1 | 21.5 ± 4.2 | 0.914 | ||
| Type of fracture | Femoral neck fracture (n) | 43 | 35 | 8 | 0.168 | |
| Trochanteric fracture (n) | 29 | 19 | 10 | |||
| Pre-admission | Pain | Lower back pain | 0.450 | |||
| No (n) | 61 | 14 | 47 | |||
| Yes (n) | 11 | 4 | 7 | |||
| Hip pain | 0.152 | |||||
| No (n) | 69 | 16 | 53 | |||
| Yes (n) | 3 | 2 | 1 | |||
| Knee pain | 0.479 | |||||
| No (n) | 60 | 14 | 46 | |||
| Yes (n) | 12 | 4 | 8 | |||
| Comorbidities | CCI (point) | 0.8 ± 1.2 | 0.8 ± 1.1 | 0.9 ± 1.2 | 0.625 | |
| ADL | BI (point) | 91.7 ± 12.5 | 93.1 ± 10.5 | 87.2 ± 16.9 | 0.176 | |
| Nutritional status | MNA-SF (point) | 9.3 ± 2.6 | 9.5 ± 2.6 | 8.9 ± 2.6 | 0.425 | |
| Frailty | CFS 1-3 (n) | 18 | 8 | 10 | 0.606 | |
| CFS 4-5 (n) | 54 | 24 | 30 | |||
| Preoperative period (days) | 5.1 ± 3.3 | 4.9 ± 3.6 | 5.4 ± 2.3 | 0.624 | ||
| Type of surgery | BHA (n) | 29 | 22 | 7 | 0.146 | |
| Pinning (n) | 10 | 10 | 0 | |||
| CHS (n) | 9 | 5 | 4 | |||
| γ-nail (n) | 24 | 17 | 7 | |||
| Pain medications | Celecoxib | 11 | 6 | 5 | 0.352 | |
| Acetaminophen | 46 | 36 | 10 | |||
| Loxoprofen | 12 | 10 | 2 | |||
| Pregabalin | 3 | 2 | 1 | |||
| Surgery | Blood loss (ml) | 108.6 ± 90.4 | 94.5 ± 75.3 | 152.0 ± 119.8 | 0.140 | |
| Operative time (min) | 60.8 ± 13.7 | 59.2 ± 14.2 | 65.5 ± 10.9 | 0.108 | ||
| CRP at 1week post surgery (mg/dl) | 2.5 ± 2.1 | 2.3 ± 1.9 | 3.0 ± 2.7 | 0.379 | ||
| MMSE at 2weeks after surgery (point) | 24.0 ± 3.8 | 24.6 ± 3.7 | 22.2 ± 3.9 | 0.028 | ||
Data are presented as mean ± standard deviation or number of patients (%). χ2 Tests: Sex, Type of fracture, Pain, Frailty, Type of surgery, Pain medications. Unpaired t-tests: BMI, Age, Preoperative period, Blood loss, Operative time, CRP. Mann–Whitney’s U test: CCI, BI, MNA-SF, MMSE. Significance level: p < .05. BMI, body mass index; CCI, charlson comorbidity index; ADL, activities of daily living; BI, barthel index; MNA-SF, mini nutritional assessment-short form; CFS, clinical frailty scale; BHA, bipolar hip arthroplasty; CHS, compression hip screw; CRP, C-reactive protein; MMSE, mini mental state examination.
Effects of persistent severe pain on pain intensity, PPT, pain catastrophizing, fear of movement, depression, ADL, PA, and physical function
Table 2 shows the results of the repeated-measures two-way ANOVA with group and evaluation period as the factors. There were significant time-by-group interactions for the VRS (F = 9.952, p < .001), PPT at the affected site (F = 9.450, p < .001), PPT at the biceps (F = 4.424, p = .016), PCS-13 (F = 4.424, p = .016), and GDS-5 scores (F = 5.051, p = .008). The VRS, PPT at the affected site, PCS-13, TSK-11, and GDS-5 in the mild group improved significantly at 8 weeks compared with those at 2 weeks, while the severe group showed no significant improvement. Both groups showed significant improvements in PPT at the biceps, motor-FIM, and PA time points at 8 weeks compared with 2 weeks. Both groups also showed significant improvements in CS-30 and 6 MWD at 8 weeks compared with those at 4 weeks. In contrast, there were significant main effects of group on the VRS, PPT at the affected site, PPT at the biceps, PCS-13, TSK-11, GDS-5, PA times, CS-30, and 6 MWD. There were no significant main effects of group on motor-FIM.
Table 2.
Comparison of each assessment item.
| Items | Mild group (n = 54) | Severe group (n = 18) | Main effect | Time-by-group interaction | p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 weeks | 4 weeks | 8 weeks | 2 weeks | 4 weeks | 8 weeks | Group F-value |
p-value | Times F-value |
p-value | ||||
| VRS | 1.5 ± 1.0 | 0.9 ± 0.9† | 0.5 ± 0.9†,# | 2.3 ± 0.8∗ | 2.1 ± 0.6∗ | 2.3 ± 0.6∗ | 53.343 | <0.001 | 9.279 | <0.001 | 9.952 | <0.001 | |
| PPT | Affected site (N) | 17.5 ± 4.8 | 21.0 ± 6.1† | 24.3 ± 6.9†,# | 14.1 ± 5.0∗ | 15.6 ± 4.0∗ | 16.0 ± 3.7∗ | 16.091 | <0.001 | 27.495 | <0.001 | 9.450 | <0.001 |
| Biceps (N) | 13.5 ± 3.5 | 14.8 ± 3.4† | 16.3 ± 3.9†,# | 10.2 ± 3.0∗ | 11.3 ± 2.9†,∗ | 11.7 ± 2.8†,∗ | 18.110 | <0.001 | 27.853 | <0.001 | 4.424 | 0.016 | |
| PCS-13 (points) | 17.1 ± 10.1 | 12.1 ± 8.6† | 10.0 ± 9.0† | 23.5 ± 10.8∗ | 21.4 ± 9.4∗ | 23.3 ± 11.2†,∗ | 18.122 | <0.001 | 5.707 | 0.005 | 4.424 | 0.016 | |
| TSK-11 (points) | 23.9 ± 5.7 | 22.5 ± 5.7 | 21.0 ± 5.9† | 26.7 ± 4.9 | 27.4 ± 5.8∗ | 27.3 ± 5.7∗ | 12.623 | 0.001 | 1.468 | 0.238 | 3.019 | 0.055 | |
| GDS-5 (points) | 2.3 ± 1.4 | 1.6 ± 1.3† | 1.6 ± 1.4† | 2.6 ± 1.8 | 2.8 ± 1.5∗ | 2.9 ± 1.5∗ | 7.486 | 0.008 | 0.710 | 0.493 | 5.051 | 0.008 | |
| Motor-FIM (points) | 45.4 ± 15.3 | 59.2 ± 16.2† | 74.8 ± 12.8†,# | 41.7 ± 13.6 | 53.2 ± 13.0† | 64.2 ± 10.4†,#,∗ | 3.674 | 0.059 | 146.917 | <0.001 | 2.521 | 0.088 | |
| PA times (seconds) | 311.0 ± 257.4 | 616.4 ± 524.0† | 1191.1 ± 965.4†,# | 158.9 ± 74.9∗ | 278.1 ± 140.7∗ | 679.5 ± 724.8†,#,∗ | 20.782 | <0.001 | 40.590 | <0.001 | 2.262 | 0.106 | |
| CS-30 (times) | 5.4 ± 6.8 | 9.2 ± 7.4# | 2.4 ± 3.8 | 5.3 ± 5.4#,∗ | 4.151 | 0.045 | 30.693 | <0.001 | 0.606 | 0.439 | |||
| 6MWD (m) | 177.3 ± 110.7 | 255.5 ± 120.2# | 109.9 ± 56.5∗ | 179.6 ± 82.1# | 7.077 | 0.010 | 48.332 | <0.001 | 0.160 | 0.690 | |||
Values are expressed as mean ± standard deviation.
VRS, verbal rating scale; PPT, pressure pain threshold; PCS-13, pain catastrophizing scale-13; TSK-11, tampa scale for kinesiophobia-11; GDS-5, geriatric depression scale-5; ADL, activities of daily living; motor-FIM, motor functional independence measure; PA, physical activity; CS-30, 30-second chair stand test; 6 MWD, 6 min walk distance. † Significant intragroup difference from 2week. # Significant intragroup difference from 4 week. * Significant inter-group differences.
Decision tree analysis
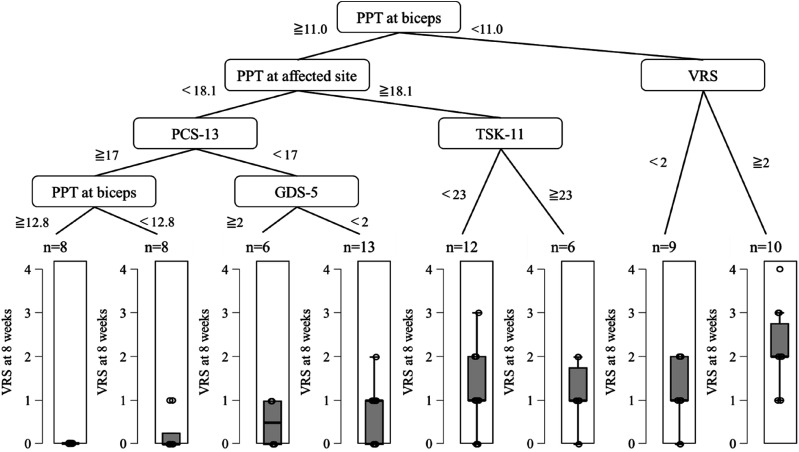
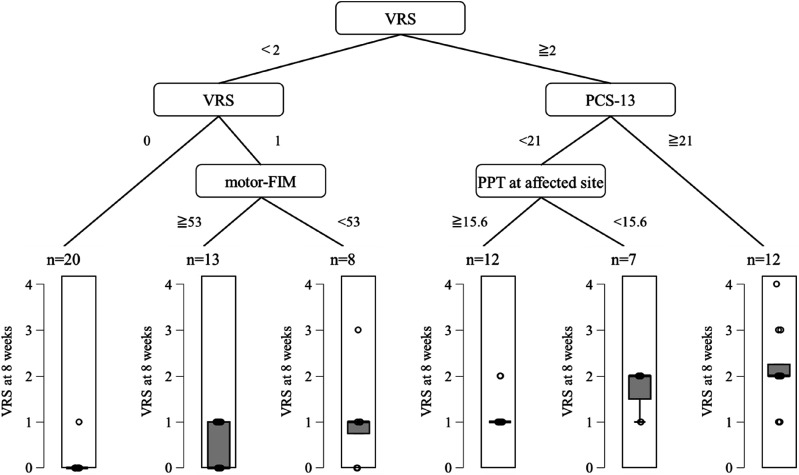
The results of the decision tree analysis are shown in Figures 3, 4, 5. For Model 1, the R2 was 0.279 and the root mean squared error (RMSE) was 1.078. For Model 2, the R2 was 0.731 and the RMSE was 0.690. The variable of the first split was VRS (<2 or ≧2, Figure 4). In the group with VRS of ≧2, the variable of the second split was PCS-13 score (<21 or ≧21 point). In the group with PCS-13 score of ≧21 point, persistent pain intensity was higher. In the group with PCS-13 of <21 point, the variable of the third split was PPT at affected site (≧15.6 or <15.6 N). On the other hand, in the group with VRS of <2, the variable of the second split was the VRS (0 or 1). In the group with a VRS of 1, the variable of the third split was motor-FIM (≧53 or <53 points). In the group with motor-FIM score of <53 points, persistent pain intensity was mild.
Figure 3.
Results of the decision tree analysis with 2 weeks. PPT, pressure pain threshold; VRS, verbal rating scale; PCS-13, pain catastrophizing scale-13; TSK-11, tampa scale for kinesiophobia-11; GDS-5, geriatric depression scale-5.
Figure 4.
Results of the decision tree analysis with 4 weeks. VRS, verbal rating scale; PCS-13, pain catastrophizing scale-13; motor-FIM, motor-functional independence measure; PPT, pressure pain threshold.
Figure 5.
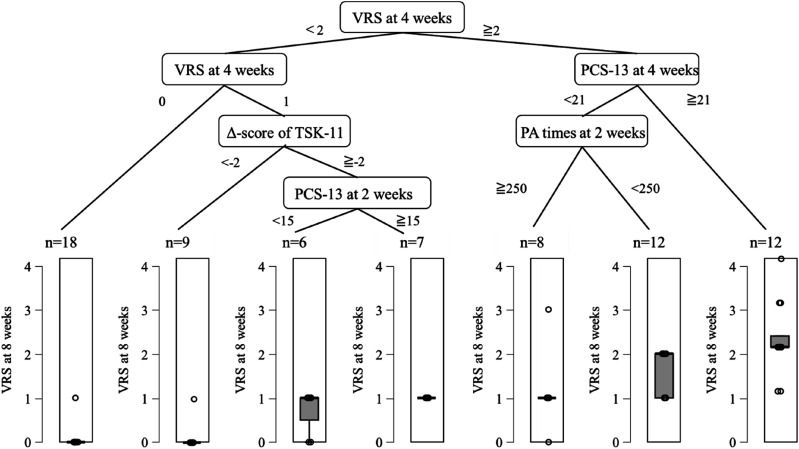
Results of the decision tree analysis with Δ-score. VRS, verbal rating scale; PCS-13, pain catastrophizing scale-13; TSK-11, tampa scale for kinesiophobia-11; PA, physical activity.
For Model 3, the R2 was 0.753 and the RMSE was 0.672. The variable of the first split was VRS at 4 weeks (<2 or ≧2, Figure 5). In the group with VRS at 4 weeks of ≧2, the variable of the second split was PCS-13 at 4 weeks score (<21 or ≧21 points). In the group with PCS-13 at 4 weeks of ≧21 point, persistent pain intensity was higher. In the group with PCS-13 at 4 weeks of <21 point, the variable of the third split was PA times at 2 weeks (≧250 or <250 s). In the group with VRS at 4 weeks of <2, the variable of the second split was VRS at 4 weeks (0 or 1). In the group with VRS of 1, the variable of the third split was Δ-score of TSK-11. In the group of the Δ-score ≧-2, the variable of the fourth split was PCS-13 at 2 weeks (<-15 or ≧15 points). In the group with PCS-13 at 2 weeks ≧15 points, persistent pain intensity was mild.
Discussion
In this longitudinal study, we investigated the multifaceted characteristics of patients with persistent severe pain after HF surgery. We found that 25.0% of patients with HF had persistent severe pain at eight weeks postoperatively, and these patients had multifaceted psychological and physical problems. The results of the decision-tree analysis indicate that factors at 4 weeks postoperatively and those change are more relevant to pain intensity at 8 weeks postoperatively than factors at 2 weeks postoperatively.
Regarding patient characteristics, MMSE was significantly lower in the severe group than in the mild group. A previous study reported that 15.8% of patients with postoperative HF and cognitive impairment continue to experience moderate or severe pain 3 months after surgery. 24 Thus, our result is consistent with previous study 24 and cognitive dysfunction may contribute to persistent severe pain after surgery for HF.
Patients with persistent severe pain 8 weeks after HF surgery had moderate or severe pain, peripheral sensitisation, central sensitisation, pain catastrophizing, and decreased PA from 2 weeks postoperatively. Goto et al. reported that patients with persistent severe pain 8 weeks after HF surgery had higher subjective pain intensity and pain catastrophizing at 2 and 4 weeks postoperatively. 10 In addition, peripheral and central sensitisations were more pronounced in the early postoperative period. Patients with severe pain 4 months after HF experienced peripheral sensitisation during the same period. 14 Although no previous studies have investigated the PPT in the early postoperative period, these findings are consistent with our results.
Furthermore, in patients with persistent severe pain 8 weeks after HF surgery, the amount of PA was low at 2 weeks after HF surgery. Talkowski et al. reported that PA in the early postoperative period of HF surgery affects improvement of disability at 3 and 6 months after surgery. 25 Although, there are no reports examining whether PA in the early postoperative period affects persistent severe pain in HF patients, our results indicate that PA in the early postoperative period may effect to reduce pain after HF surgery.
Improvements in pain catastrophizing, fear of movement, and depression were poorer in the severe than in mild group. In our previous study, patients with persistent severe pain 8 weeks after HF surgery were found to have more pain catastrophizing at 2 and 4 weeks postoperatively. 10 Alsaleem et al. also reported that fear of movement at 4–8 weeks was strongly associated with postoperative pain in patients who undergone total hip arthroplasty. 26 Furthermore, Archer et al. reported that the more depressed patients were 4 weeks after lower extremity trauma surgery, the more likely they were to have persistent severe pain 1 year after surgery. 27 Thus, patients with high levels of pain catastrophizing, fear of movement, and depression are characterised by persistent severe pain.
Although both the mild and severe groups showed improvements in ADL and physical function, these outcomes were poorer in the severe group than in the mild group 8 weeks after HF surgery. Postoperative rehabilitation after HF surgery improves ADL and physical function. 28 However, pain during the acute and early post-acute recovery periods after HF surgery may contribute to functional impairment in these patients.9,10 In addition, considering these previous studies and our results, ADL and physical function may be poorly improved in patients with persistent severe pain at 8 weeks postoperatively for HF.
The results of the decision tree analysis showed that the first bifurcation was the VRS at 4 weeks in Model 2. In previous studies, a VRS of ≥2 is classified as severe pain. 29 Therefore, patients complaining of severe pain at 4 weeks are likely to experience severe pain. Second, the intensity of persistent pain was higher when the VRS was ≥2 and the PCS-13 score was ≥21. Birch S, et al. reported that patients with high levels of preoperative pain catastrophizing (>21 point) have lower physical function and more pain after knee arthroplasty than patients without elevated pain catastrophizing. 30 This report supports our result and, it is consider that pain catastrophizing would be related to a higher intensity of persistent pain. In the group with PCS-13 of <21 point, the variable of the third layer was PPT at affected site (≧15.6 or <15.6 N). Campos et al. reported that the PPT at the affected site was 14.4 ± 10.5 N in patients with persistent severe pain even 4 months following HF surgery. 14 Thus, a 14.0–16.0 N or lower for PPT at the affected site would be associated with severe pain and, we speculate that increased peripheral sensitisation 4 weeks after HF surgery is associated with higher persistent pain intensity. However, when the 4 weeks VRS was <2 in the first branch, the intensity of persistent pain was higher than that in the other branches when the motor-FIM was less than 53 points. Thus, even patients with low pain intensity at week 4 were likely to experience more pain at week 8 if their ADL declined.
The results of Model 3 showed that the first deviation was the 4 weeks VRS. Persistent pain intensity increased when the 4 weeks VRS was >2 and the 4 weeks PCS-13 was >21 points, which is similar to Model 2. In the group with PCS-13 at 4 weeks of <21 points, the variable of the third layer was PA times at 2 weeks (≧250 or <250 s). Thus, lower PA at 2 weeks after HF surgery may be associated with a higher intensity of persistent pain. In contrast, in the group with a VRS of 1, the variable of the third layer was the TSK-11 at Δ-score. Thus, an improvement in the fear of movement after HF surgery may be associated with a higher intensity of persistent pain.
Considering the results of this study, it is necessary to modify peripheral sensitisation, pain catastrophizing and fear of movement, and to increase PA in order to prevent CPSP. Therefore, it is important to combine not only basic rehabilitation but also a tailored approach based on a multifaceted evaluation from an early stage after HF surgery, and future intervention studies are needed.
This study has certain limitations. First, it was not possible to examine cases that developed CPSP. In the present study, we focused on pain in patients 8 weeks after HF surgery during hospitalisation to provide early postoperative pain management. However, long-term studies are required. Second, Dynamic QST, which reflects pain modulation in the central nervous system, was not measured. The Dynamic QST involves the assessment of temporal summation of pain and conditioned pain modulation. 7 However, owing to the characteristics of the measurement method, it is difficult to perform these assessments in older adults and cognitively impaired individuals, and there have been few previous studies. Therefore, the implementation of these evaluations will be a subject for further study. Third, 47 (38.2%) patients were excluded because of cognitive decline. Future studies should examine the characteristics of patients with HF and their cognitive decline. Finally, we were not able to examine the anaesthesia, nerve blocks, and opioids. Collecting these data from the first postoperative day would help us understand the persistence of severe pain.
Conclusions
Patients with persistent severe pain 8 weeks after HF surgery were characterised by early severe peripheral and central sensitisation, pain catastrophizing, and decreased PA. In addition, the postoperative pain-related outcomes were poor. In contrast, early pain intensity, pain catastrophizing, peripheral sensitisation, improvement in fear of movement, and PA may be hierarchical factors influencing persistent pain intensity 8 weeks after HF surgery. The results of this study demonstrate the importance of a longitudinal assessment of nervous system sensitisation, psychological problems, ADL, and PA.
Acknowledgements
We are grateful to the contributions of all the patients who provided data. The work of the hospital staff and laboratory members is gratefully acknowledged.
Footnotes
Author contributions: YN, HK, and KG contributed to the study design, literature review, and manuscript writing. YN, HK, and YN performed statistical analysis and interpretation. YN, HK, and KG designed the database and the protocol for data collection. YN, YK, and KN conducted the experiments. JY, KM, JS, and MO supervised the study.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI [grant number JP 23K16612].
ORCID iD
Hideki Kataoka https://orcid.org/0000-0002-6727-639X
Data availability statement
The data associated with the paper are not publicly available but are available from the corresponding author upon reasonable request.
References
- 1.Omsland TK, Magnus JH. Forecasting the burden of future postmenopausal hip fractures. Osteoporos Int 2014; 25(10): 2493–2496. [DOI] [PubMed] [Google Scholar]
- 2.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006; 367(9522): 1618–1625. [DOI] [PubMed] [Google Scholar]
- 3.Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet 2019; 393(10180): 1537-1546. [DOI] [PubMed] [Google Scholar]
- 4.Edwards RR, Haythornthwaite JA, Smith MT, et al. Catastrophizing and depressive symptoms as prospective predictors of outcomes following total knee replacement. Pain Res Manag 2009; 14(4): 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kocic M, Stankovic A, Lazovic M, et al. Influence of fear of movement on total knee arthroplasty outcome. Ann Ital Chir 2015; 86(2): 148–155. [PubMed] [Google Scholar]
- 6.Graven-Nielsen T, Arendt-Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol 2010; 6(10): 599–606. [DOI] [PubMed] [Google Scholar]
- 7.Arendt-Nielsen L, Nie H, Laursen MB, et al. Sensitization in patients with painful knee osteoarthritis. Pain 2010; 149(3): 573–581. [DOI] [PubMed] [Google Scholar]
- 8.Izumi M, Petersen KK, Laursen MB, et al. Facilitated temporal summation of pain correlates with clinical pain intensity after hip arthroplasty. Pain 2017; 158(2): 323–332. [DOI] [PubMed] [Google Scholar]
- 9.Morrison SR, Magaziner J, McLaughlin MA, et al. The impact of post-operative pain on outcomes following hip fracture. Pain 2003; 103(3): 303–311. [DOI] [PubMed] [Google Scholar]
- 10.Goto K, Kataoka H, Honda A, et al. Factors affecting persistent postoperative pain in patients with hip fractures. Pain Res Manag 2020; 4: 8814290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fregoso G, Wang A, Tseng K, et al. Transition from acute to chronic pain: evaluating risk for chronic postsurgical pain. Pain Physician 2019; 22(5): 479–488. [PubMed] [Google Scholar]
- 12.Kastora S, Kounidas G, Perrott S, et al. Clinical frailty scale as a point of care prognostic indicator of mortality in COVID-19: a systematic review and meta-analysis. EClinicalMedicine 2021; 23(36): 100896. DOI: 10.1016/j.eclinm.2021.100896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caraceni A, Cherny N, Fainsinger R, et al. Pain measurement tools and methods in clinical research in palliative care: recommendations of an Expert Working Group of the European Association of Palliative Care. J Pain Symptom Manage 2002; 23(3): 239–255. [DOI] [PubMed] [Google Scholar]
- 14.Campos HLM, Liebano RE, Lima CA, et al. Multidimensional investigate on of chronic pain experience and physical functioning following hip fracture surgery clinical implications. Br J Pain 2020; 14: 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaegter HB, Handberg G, Graven-Nielsen T. Similarities between exercise-induced hypoalgesia and conditioned pain modulation in humans. Pain 2014; 155(1): 158–167. [DOI] [PubMed] [Google Scholar]
- 16.Iwaki R, Arimura T, Jensen MP, et al. Global catastrophizing vs catastrophizing subdomains: assessment and associations with patient functioning. Pain Med 2012; 13(5): 677–687. [DOI] [PubMed] [Google Scholar]
- 17.Woby SR, Roach NK, Urmston M, et al. Psychometric properties of the TSK-11: a shortened version of the tampa scale for kinesiophobia. Pain 2005; 117(1-2): 137–144. [DOI] [PubMed] [Google Scholar]
- 18.Hoyl MT, Alessi CA, Harker JO, et al. Development and testing of a five-item version of the geriatric depression scale. J Am Geriatr Soc 1999; 47(7): 873–878. [DOI] [PubMed] [Google Scholar]
- 19.Pollak N, Rheault W, Stoecker JL. Reliability and validity of the FIM for persons aged 80 years and above from a multilevel continuing care retirement community. Arch Phys Med Rehabil 1996; 77(10): 1056–1061. [DOI] [PubMed] [Google Scholar]
- 20.Schneider PL, Crouter SE, Bassett DR. Pedometer measures of free-liveng physical activity: comparison of 13 models. Med Sci Sports Exerc 2004; 36(2): 331–335. [DOI] [PubMed] [Google Scholar]
- 21.McClain JJ, Craig CL, Sisson SB, et al. Comparison of Lifecorder EX and ActiGaraph accelerometers under freeliving conditions. Appl Physiol Nutr Metab 2007; 32(4): 753–761. [DOI] [PubMed] [Google Scholar]
- 22.Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport 1999; 70(2): 113–119. [DOI] [PubMed] [Google Scholar]
- 23.ATS committee on proficiency standards for clinical pulmonary function laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166(1), 111–117. [DOI] [PubMed] [Google Scholar]
- 24.Casafont C, Gonzalez-Garcia MJ, Maranon-Echeverria AM, et al. Profile of patients with dementia or cognitive impairment hospitalized with a proximal femur fracture requiring surgery. Int Environ Public Health 2022; 19(5): 2799. DOI: 10.3390/ijerph19052799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talkowski JB, Lenze EJ, Munin MC, et al. Patient participation and physical activity during rehabilitation and future functional outcomes in patients following hip fracture. Arch Phys Med Rehabil 2009; 90(4): 618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alsaleem MK, Alkhars AM, Alalwan HA, et al. Kinesiophobia post total hip arthroplasty: a retrospective study. Cureus 2021; 13(6): e15991. DOI: 10.7759/cureus.15991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Archer KR, Abraham CM, Obremskey WT. Psychosocial factors predict pain and physical health after lower extremity trauma. Clin Orthop Relat Res. 2015; 473(11): 3519–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Auais MA, Eilayyan O, Mayo NE. Extended exercise rehabilitation after hip fracture improves patients' physical function: a systematic review and meta-analysis. Phys Ther 2012; 92(11): 1437–1451. [DOI] [PubMed] [Google Scholar]
- 29.Kristensen MT. Hip fracture−related pain strongly influences functional performance of patients with an intertrochanteric fracture upon discharge from the hospital. PM R. 2013; 5(2): 135–141. [DOI] [PubMed] [Google Scholar]
- 30.Birch S, Stilling M, Mechlenburg I, et al. Association between pain catastrophizing, physical function and pain at first visit in the outpatient knee clinic. Knee 2019; 26(6): 1286–1291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data associated with the paper are not publicly available but are available from the corresponding author upon reasonable request.