Abstract
Background
There has been growing data about the association between D-dimer levels and thrombectomy outcomes in acute ischemic stroke patients (AIS) with no cumulative evidence. This systematic review and meta-analysis aim to discuss and analyze the findings of the current studies to provide more robust evidence in this regard.
Methods
A systematic search was conducted through PubMed, Web of Science, Embase, and Scopus to retrieve all relevant investigations. A meta-analysis was conducted, and the results were presented in odds ratio (ORs) for binary variables and ratio of means (ROM) for continuous variables, each accompanied by its respective 95% confidence intervals (CIs).
Results
After searching and screening, 14 studies were included. The analysis showed that the low D-dimer group had significantly higher rates of favorable functional outcome (OR: 4.40; 95%CI: 2.65–7.30; p < 0.001, n = 3) and recanalization (OR: 4.13; 95%CI: 1.57–10.84; p = 0.004, n = 3) than the high one. The association between D-dimer levels and first-pass effect and re-occlusion risk was also demonstrated. Eventually, two studies also demonstrated a significant association between high D-dimer levels and deep venous thrombosis and symptomatic intracranial hemorrhage as post-thrombectomy complications.
Conclusion
Current evidence indicates a significant association between D-dimer levels and post-thrombectomy outcomes in AIS patients. However, current data are remarkably heterogeneous, and additional comparative investigations are needed.
Keywords: D-dimer, thrombectomy, stroke, functional outcome, recanalization, deep venous thrombosis, hemorrhage
Introduction
Endovascular thrombectomy (EVT) is now the gold standard for the management of patients with acute ischemic stroke (AIS) due to large vessel occlusion (LVO) who meet certain eligibility criteria. 1 Multiple factors might affect the outcome of EVT in AIS-LVO patients, and these factors can be periprocedural, such as the use of intravenous thrombolysis (IVT), 2 the access site for the procedure, 3 the revascularization success, 4 the pre-stroke collateral status, 5 and the presence of pre-EVT cerebral microbleeds. 6 Moreover, there has been a growing interest in the literature regarding the effect of different biomarkers, like D-dimer, on the clinical outcomes of AIS.7–24
In thrombogenic diseases, such as pulmonary embolism, deep vein thrombosis (DVT), and aggressive malignancy, D-dimer levels are usually elevated.22,25–27 Elevated D-dimer levels are strongly linked to certain stroke etiologies (e.g. cancer-related stroke) and poor prognoses (e.g. stroke progression, lesion enlargement, and unfavorable functional outcome) for acute ischemic stroke.22,28–31 It is well-known that EVT outcomes could be affected by thrombus characteristics. Different D-dimer levels may affect the circumstances of thrombus formation because plasma D-dimer represents the induction of the coagulation system and associated hyperfibrinolysis. For instance, platelet-rich or white thrombi are typically seen in cancer-related stroke, which is invariably associated with a relatively elevated D-dimer level.32,33 There are a few studies that looked into the potential impact of D-dimer level on EVT outcomes.11,22–24 Plasma D-dimer levels likely influence EVT and subsequent clinical outcomes by influencing different thrombogenic states or thrombus types. However, there is still uncertainty regarding the clinical relevance of D-dimer levels in EVT. 20 Therefore, in this systematic review and meta-analysis, we will discuss and analyze the findings of the available studies that investigated the association between D-dimer levels and EVT outcomes in AIS patients to provide more robust evidence in this regard.
Methods
Selection process
This meta-analysis was conducted based on the Preferred Reporting Items for Systematic Review and Meta-analyses Statement (PRISMA) guidelines. 34 Subsequently, a comprehensive search strategy was developed to obtain relevant articles from PubMed, Web of Science (WoS), Embase, and Scopus. We conducted the search process on 20 December 2023 through all databases, with the following terms: ((Endovascular OR thrombectomy) AND stroke) AND (D-dimer), which were adapted to fit each database's standards for an ideal search. The search was further done on 5 March 2024 on PubMed to find any additional published research. Moreover, we manually searched Google Scholar and the references of the included articles to detect any potentially missed articles during our main selection process.
Articles were included if they: (1) were original investigations; (2) reported data about the association between D-dimer levels and outcome; (3) recruited AIS patients having thrombectomy (regardless of the technique); (4) reported any procedure-related outcome like functional independence, successful reperfusion, first-pass effect (FPE), symptomatic intracranial hemorrhage (sICH), procedure-related DVT, and others; (5) reported human data only; and (6) were published in English. Studies that were not original like reviews, protocols, thesis, conference papers, commentaries, or editorials did not report D-dimer levels or reported it among patients that did not have thrombectomy, did not report any post-procedural outcome and its association with D-dimer levels, reported non-human data, or were not published in English were excluded from this study. Defining each outcome (e.g. defining functional independence) was part of the data to be extracted and reported in this study, which was done for the reported post-thrombectomy events.
All the search results were exported into a single Endnote library for organization and to remove potential duplicates. After removing duplicates, the remaining articles were organized and prepared for the selection process. At least two reviewers participated in the selection process, which was done in two main screening steps, including title/abstract and full-text screening. Decisions on study selection were blindly made following the pre-defined criteria. These decisions were then compared to each other, and discrepancies were resolved via proper discussions between the two authors and a senior third party whenever needed.
Data extraction
An extraction sheet was designed to include all the outcomes of interest. It mainly consisted of two parts: one for extracting each study's baseline characteristics, and another for the outcomes of interest. The baseline characteristics included the reference for each study (the last name of the first author and the year of publication), settings where they were conducted, total population number, National Institutes of Health Stroke Scale (NIHSS) score at baseline, age, gender, D-dimer at baseline, thrombectomy-related data, including the number of patients having thrombectomy, and the definition and numbers of successful perfusion and functional independence for the whole population. The outcome part included D-dimer levels, cutoff values, and other predictive parameters of functional independence, successful perfusion, FPE rates, reocclusion risk sICH, and DVT. Based on the cutoff values for each study, some authors also compared the rates of patients achieving these outcomes based on their D-dimer levels (low or high), which were also extracted and analyzed when applicable.
Quality assessment
We used the National Institutes of Health (NIH) methodology to evaluate the methodological quality of the studies that were included. Each research was assessed by two separate reviewers using a standardized evaluation sheet. Studies were reviewed using a 14-question sheet. The ultimate decision was reached after a discussion among the reviewers. A score was assigned to each investigation based on the fulfillment of the 14 questions. A score ranging from 11 to 14 was deemed to indicate high quality, a score ranging from 6 to 10 was deemed to indicate moderate quality, and scores below 6 were deemed to indicate poor quality.
Statistical analysis
We performed random-effects meta-analysis using R software version 4.3.2 and the ‘meta’ statistical package. We calculated the risk ratio (OR) for binary variables and the ratio of means (ROM) for continuous variables, 35 each accompanied by its respective 95% confidence intervals (CIs). To calculate the confidence interval of the random-effects estimate, we used a restricted maximum likelihood (REML) estimator and assessed the presence of heterogeneity in the data using Cochran's Q and I² tests, considering a significance level of p < 0.05 for the Q statistic and I² values above 50% as indicative of significant heterogeneity. Due to the limited number of studies included in each analysis (fewer than 10), we were unable to conduct Egger's regression test to assess publication bias or perform meta-aggression. 36
Results
Search results
Searching databases resulted in 646 relevant articles, among which 257 duplicates were removed via Endnote. The remaining articles (n = 389) were screened against our criteria of inclusion by title/abstract, which resulted in excluding 336 articles, and only 53 studies have had their full texts screened. On making a search update via PubMed on 5 March 2024, an additional four citations were found and screened, but did not meet the inclusion criteria. Eventually, 14 studies satisfied our criteria and were considered for data extraction and evidence synthesis. Supplemental Figure 1 shows the selection process of the relevant studies on a PRISMA flow chart.
Baseline characteristics
Among the 14 included studies,11–24 13 (92.86%) were retrospective,11–20,22–24 while only one (7.14%) was prospective. 21 Regarding publication setting, most studies were conducted in China (n = 6, 42.86%),13,16–18,22,24 followed by Japan (n = 4, 28.57%),11,14,19,20 the Republic of Korea (n = 3, 21.43%),12,15,23 and South Korea (n = 1, 7.14%). 21 A total of 3268 AIS patients (range: 59 to 614) were recruited in these studies, including 1974 (60.4%) male patients. EVT was done in 3236 patients. A total of 10 (71.43%) studies performed aspiration and/or stent retriever or adopted a combined approach for their population,11,12,14–16,19–22,24 while two (14.29%) studies established stent retriever approaches.18,23 One (7.14%) study adopted aspiration only, 13 while the other study did not specify their EVT approach. 17 The reported successful perfusion and functional outcome with their definitions per investigation, together with other baseline characteristics, such as NIHSS score at baseline, D-dimer at baseline, age, and outcome of interest, are presented in Table 1. Regarding the quality of the included studies, all studies were categorized as having a fair quality (Supplemental Table 1).
Table 1.
Baseline characteristics.
| Author | Year | Data collection | Country | Age (years), median (IQR) OR mean (SD) | Gender, N (%) | NIHSS score at baseline, median (IQR) OR mean (SD) | Population (N) | Preoperative D-dimer, median (IQR) OR mean (SD) | EVT | Outcome of interest | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Technique | Successful reperfusion | Favorable outcome | ||||||||||||
| Definition | N | Definition | N | ||||||||||||
| Aoki et al. 11 | 2020 | R | Japan | sMR: 82 (76–86); nsMR: 77 (71–83) | M = 72 (56.69) | sMR: 18 (13–24); nsMR: 19 (12–24) | 127 | sMR: 2.0 (1.1–3.4); nsMR: 1.6 (0.9–3.3) | 127 | Aspiration & Stent | TICI 3 | 53 | mRS 0–2 | 32 | Functional outcome |
| Baek et al. 12 | 2023 | R | Republic of Korea | 71.3 (±12.9) | M = 113 (52.6) | 14.0 (9.0–19.0) | 215 | 757.0 (392.0–2562.5) ng/mL | 215 | Aspiration & Stent | mTICI ≥ 2b-3 | 203; FPE (mTICI 2c-3): 71 | mRS 0–2 | 87 | Successful reperfusion, FPE, and functional outcome |
| Han et al. 13 | 2023 | R | China | 67.08 ± 11.67 | F = 87 (36) | 14 (11, 19) | 245 | 1.28 (0.7, 2.61) mg/L | 245 | Aspiration | - | - | mRS 0–2 | 122 | Deep venous thrombosis |
| Hisamitsu et al. 14 | 2022 | R | Japan | 74.75 (±11.11) | M = 43 (49.4) | 15.51 (±5.95) | 87 | Favorable outcome: 1.74 (2.02); Unfavorable: 3.33 (3.16) µg/mL | 87 | Aspiration & Stent | mTICI ≥ 2b-3 | 80 | mRS 0–2 | 47 | Functional outcome |
| Lee et al. 15 | 2021 | R | Republic of Korea | 68.46 ± 13.2 | M = 178 (52.2) | CRS: 18 (11–23); Control: 15 (8–19) | 341 | Favorable outcome: 10.7 (6.66–17.71); Unfavorable: 16.95 (14.55–26.60) | 341 | Aspiration & Stent | mTICI ≥ 2b-3 | 295 | mRS 0–2 | CRS: 11/34 | Functional outcome |
| Li W. et al. 18 | 2020 | R | China | Reocclusion: 57.3 (±10.3); Non-reocclusion: 63.6 (±12.4) | M = 464 (75.57) | Occlusion: 15 (12–20); Non-reocclusion: 16 (12–23) | 614 | Reocclusion: 2 (0.8–4.7); Non-reocclusion: 0.8 (0.4–1.9) mmol/L | 614 | Stent | mTICI ≥ 2b-3 | - | mRS 0–2 | 230 | Re-occlusion rate |
| Li J. et al. 16 | 2023 | R | China | 63.6 ± 12.6 | F = 104 (30.3) | 15.2 ± 4.5 | 343 | 1.3 (0.7–3.1) | 343 | Aspiration & Stent | mTICI ≥ 2b-3 | 259 | mRS <2 | 162 | Functional outcome |
| Li L. et al. 17 | 2023 | R | China | Validation = favorable: 67 (59–71); unfavorable: 66 (59–70); Test = favorable: 54.5 (32–71.5); unfavorable: 61 (55.5–76) | M = 64 | Validation = favorable: 12 (9–15); unfavorable: 15 (13–18); Test = favorable: 14 (10.25–15.75); unfavorable: 13 (11–15) | 102 | Validation = favorable: 0.96 (0.59–2.24); unfavorable: 2.60 (0.92–190); Test = favorable: 1.00 (0.75–2.52); unfavorable: 0.59 (0.55–7.54) | 102 | NR | mTICI ≥ 2b-3 | 93 | mRS 0–2 | 46 | Functional outcome |
| Mitsuhashi et al. 19 | 2023 | R | Japan | 85 (83–87) | F = 27 (61.02) | 24 (18–27) | 59 | Favorable: 1.25 (1.2–4.05); Unfavorable: 2 (0.875–1.8) µg/mL | 59 | Aspiration & Stent | mTICI ≥ 2b-3 | 44 | mRS 0–3 | 28 | Functional outcome |
| Ohbuchi et al. 20 | 2022 | R | Japan | 76 ± 12 | F = 49 (40) | 18 ± 8 | 121 | 4.4 ± 6.6 µg/mL | 121 | Aspiration & Stent | mTICI ≥ 2b-3 | 88 | mRS 0–3 | 39 | Functional outcome and successful reperfusion |
| Pan et al. 21 | 2022 | P | South Korea | 65.59 | M = 36 (52.9) | 15 (10–20) | 68 | 16.07 μg/mL | 36 | Aspiration & Stent | mTICI ≥ 2b-3 | 20 | mRS 0–2 | 18 | Functional outcome and successful reperfusion |
| Qiu et al. 22 | 2022 | R | China | 69.7 ± 12.7 | M = 207 (52.4) | 16 (12–21) | 395 | 0.87 (0.42–1.83) mg/L | 395 | Aspiration & Stent | mTICI ≥ 2b-3 | 328 | - | - | sICH |
| Song et al. 23 | 2020 | R | Republic of Korea | FPE: 69.6 (13.5); non-FPE: 68.0 (13.9) | M = 133 (55.88) | FPE: 10 (3–17); non-FPE: 9 (2–18) | 238 | FPE: 4 (3.86); non-FPE: 4.01 (3.75) mg/L | 238 | Stent | mTICI ≥ 2b-3 | 228 | mRS 0–2 | 141 | FPE |
| Xie and Tang 24 | 2023 | R | China | 69.5 ± 12.9 | M = 163 (52.1) | 15 (12–21) | 313 | 0.85 (0.40–1.76) mg/L | 313 | Aspiration & Stent | mTICI ≥ 2b-3 | 254 | mRS 0–2 | 111 | FPE |
R: retrospective; P: prospective; M: male; F: female; sMR and nsMR: significant and non-significant mitral regurge; CRS: cancer-related stroke; FPE: first-pass effect; NR: not reported; mTICI: modified thrombolysis in cerebral infarction; sICH; symptomatic intracranial hemorrhage; mRS: modified Rankin scale; NIHSS: National Institute of Health Stroke Scale; DVT: deep venous thrombosis.
Regarding outcomes of interest, not all studies reported all of the intended outcomes. Functional outcome was reported by nine studies, and successful reperfusion and FPE rates were reported by three studies each, while reocclusion, sICH, and DVT were each reported by one study only. Table 2 shows the outcomes reported by each study and whether their reported data were eligible for a meta-analysis.
Table 2.
Outcomes reported by each study and outcomes analyzed.
| Reference | Year | Successful recanalization | FPE | Reocclusion | Functional outcome | sICH | DVT | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reporting | Def. | Cutoff | Reporting | Def. | Reporting | Def. | Cut-off | |||||
| Aoki et al. | 2020 | - | - | - | - | - | - | ** | 0–2 | - | - | - |
| Baek et al. | 2023 | ** | 2b-3 | 1.09 | ** | 2b-3 | - | ** | 0–2 | 0.67 | - | - |
| Han et al. | 2023 | - | - | - | - | - | - | - | - | - | - | * |
| Hisamitsu et al. | 2022 | - | - | - | - | - | - | ** | 0–2 | - | - | - |
| Lee et al. | 2021 | - | - | - | - | - | - | ** | 0–3 | - | - | - |
| Li W. et al. | 2020 | - | - | - | - | - | * | - | - | - | - | - |
| Li J. et al. | 2023 | - | - | - | - | - | - | * | - | - | - | - |
| Li L. et al. | 2023 | - | - | - | - | - | - | ** | 0–2 | - | - | - |
| Mitsuhashi et al. | 2023 | - | - | - | - | - | - | ** | 0–3 | - | - | - |
| Ohbuchi et al. | 2022 | ** | 2b-3 | 6.7 | - | - | - | ** | 0–3 | 1.9 | - | - |
| Pan et al. | 2022 | ** | 2b-3 | 3.83 | - | - | - | ** | - | 3.83 | - | - |
| Qiu et al. | 2022 | - | - | - | - | - | - | - | - | - | * | - |
| Song et al. | 2020 | - | - | - | ** | 2b-3 | - | - | - | - | - | - |
| Xie and Tang | 2023 | - | - | - | ** | 2c-3 | - | - | - | - | - | - |
*Outcome reported by this study; **Reported outcome included in a meta-analysis; - Outcome was not reported by this study. Presented cutoff values (all in µg/mL) were used by their corresponding studies to define low and high D-dimer levels. Successful recanalization and FPE were defined by mTICI scores. Functional outcome was defined by the mRS score.
Functional outcome
Seven studies compared the D-dimer levels between patients having favorable outcomes and others with unfavorable outcomes. Lee et al. 15 investigated the risk factors associated with having favorable outcomes among cancer-related stroke patients who received EVT. The authors demonstrated that median D-dimer levels were remarkably lower among the group having favorable outcomes than others having unfavorable outcomes (10.7 (6.66–17.71) vs 16.95 (14.55–26.60) µg/mL; p = 0.028). Functional outcome association with preoperative D-dimer levels was also reported in a prospective investigation of 121 patients by Ohbuchi et al. 20 The authors demonstrated that D-dimer levels were significantly lower among patients having favorable than unfavorable outcomes (2.5 ± 2.6 vs 5.3 ± 7.6 µg/mL; p = 0.0309). Furthermore, among 87 patients undergoing EVT for AIS-LVO, Hisamitus et al. 14 investigated the ability of D-dimer to predict patients’ functional outcomes. The mean D-dimer levels were significantly higher among patients with unfavorable than favorable functional outcomes (3.33 (3.16) vs 1.74 (2.02) µg/mL; p = 0.008). According to Baek et al., 12 D-dimer levels were lower among individuals with favorable outcomes than others with non-favorable outcomes (495.0 (290.0–856.0) vs 1189.0 (526.0–3208.0) ng/mL; p < 0.001). Similarly, mean D-dimer levels were remarkably lower in the population with favorable than non-favorable outcomes (1.29 (1.31) vs 6.44 (12.58) µg/mL). 17 Moreover, in a population of 59 patients undergoing EVT, Mitsuhashi et al. 19 reported that the median D-dimer levels were significantly lower in the group with favorable functional outcomes (1.25 (1.2–4.05) vs 2 (0.875–1.8); p = 0.01). Eventually, Aoki et al. 11 demonstrated that the median D-dimer levels were comparable between the two groups (Favorable = 1.1 (0.8–2.5); Unfavorable = 1.6 (0.9–3.1) mg/dL; p = 0.202).
Furthermore, some studies established a multivariate regression analysis to assess the association between different variables and achieving a favorable outcome.12,14,17,19,20 These studies demonstrated that D-dimer levels were significant predictors of achieving favorable outcomes following EVT (Table 3). By contrast, Mitsuhashi et al. 19 showed that D-dimer levels could not predict functional outcomes after EVT based on binomial regression analysis (standard error = 0.99; p = 0.644). Some studies have also reported a cutoff value and estimated the sensitivity and specificity of D-dimer level for predicting favorable outcome after EVT. According to Ohbuchi et al. 20 and Hisamitus et al., 14 the estimated cutoff value of D-dimer in relation to functional outcome was 1.9 µg/mL. Nevertheless, Baek et al. 12 estimated a different cutoff value of 667.0 ng/mL. It should be noted that Pan et al. 21 also reported that the optimal cutoff point in their population was 3.825 µg/mL. Subsequently, the authors demonstrated that a high D-dimer level (>4 µg/mL) was significantly correlated with unfavorable functional outcomes and mortality (OR: 0.098 and 0.297; p = 0.005 and 0.027, respectively). However, these estimates were reported for all patients regardless of whether they had EVT or not without clear discrimination.
Table 3.
D-dimer predictive ability of different post-thrombectomy outcomes.
| Reference | Cutoff (µg/mL) | OR (95%CI) | Specificity | Sensitivity | PPV | NPV | Accuracy | AUC |
|---|---|---|---|---|---|---|---|---|
| Successful recanalization | ||||||||
| Ohbuchi et al. | 6.7 | 5.51 (1.80–16.88) | 36% | 91% | 79% | 60% | 76% | - |
| First-pass effect | ||||||||
| Xie and Tang | 0.97 | 0.81 (0.52–0.96) | 79% | 79.30% | - | 87% | - | 0.761 |
| Baek et al. | 1.09 | 0.92 (0.85–0.98)/500 ng/mL | 47.90% | 76.10% | - | - | - | 0.631 |
| Reocclusion | ||||||||
| Li W. et al. | - | 1.06 (1.01–1.12) | - | - | - | - | - | - |
| Favorable outcome | ||||||||
| Baek et al. | 0.67 | 0.88 (0.81–0.97) per 500 ng/mL | 70.30% | 67% | - | - | - | 0.722 |
| Li J. et al. | - | 1.14 (1.04–1.26) | - | - | - | - | - | - |
| Ohbuchi et al. | 1.9 | 3.86 (1.27–11.75) | 62% | 64% | 45% | 78% | 63% | - |
| Hisamitus et al. | 1.9 | 1.27 (1.0–1.6) | 80% | 60.60% | - | - | - | - |
| Lee et al. | - | 1.524 (1.043–2.226)* | - | - | - | - | - | - |
| Low DVT | ||||||||
| Han et al. | 1.62 | 1.350 (1.150–1.585) | 70.20% | 71.60% | - | - | - | 0.749 |
| sICH | ||||||||
| Qiu et al. | 2.27 | 2.45 (1.75–3.43) | 86.20% | 64.60% | - | 94.60% | - | 0.774 |
OR: odds ratio; CI: confidence interval; PPV and NPV: positive and negative predictive values; AUC: area under the curve.
*Association with unfavorable functional outcome; - Outcome was not reported by this study.
The estimated sensitivity and specificity of D-dimer in predicting favorable outcome were nearly similar among the included studies, ranging from 60.6–67% and 62–80%, respectively. Additionally, NPV, PPV, and accuracy were estimated by Ohbuchi et al. 20 as 78%, 45%, and 63%, respectively. It should be noted that most of the included studies defined having a favorable outcome by an mRS score of 0–2, while an unfavorable outcome was defined as a score of 3–6 at 3 months or 90 days postoperatively.11,12,14,17,21 However, three studies used an mRS score of 0–3 as a definition of having a favorable outcome.15,19,20 Moreover, Ohbuchi et al. 20 reported the outcome at hospital discharge (mean stay = 41 ± 40 days).
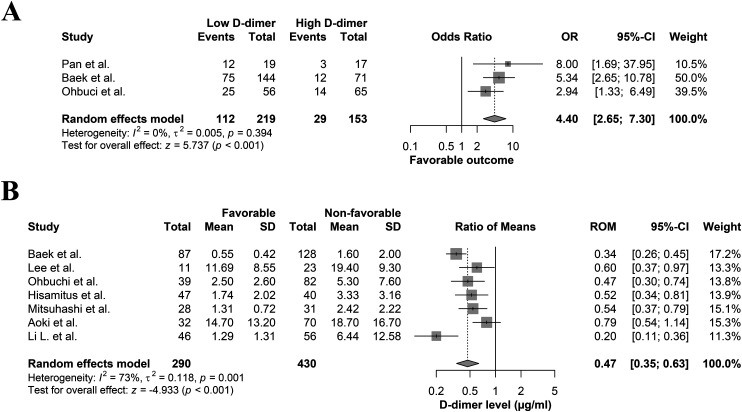
Moreover, three studies compared the rates of patients having favorable outcomes between the low and high D-dimer groups, based on their established cutoff points.12,20,21 All of these studies demonstrated that the rates of patients having favorable outcomes were significantly higher in the low than high D-dimer groups. The meta-analysis of three studies of favorable functional outcome rates showed that the low D-dimer group had significantly higher rates compared to the high D-dimer group (OR: 4.40; 95%CI: 2.65–7.30; p < 0.001) (Figure 1(a)). In addition, analyzing the data from seven studies comparing D-dimer levels based on favorable outcome bases showed significantly lower levels (∼50%) of D-dimer level in those achieving favorable functional outcomes as opposed to those who did not (ROM: 0.47; 95%CI: 0.35–0.63; p < 0.001) (Figure 1(b)).
Figure 1.
Forest plot comparing (a) the rates of patients having favorable functional outcomes following EVT between the low and high D-dimer groups and (b) mean D-dimer levels between patients with and without favorable functional outcome after EVT.
Reperfusion outcomes
Successful recanalization
Three investigations demonstrated the association between D-dimer levels and achieving successful recanalization following EVT. Among these three, Baek et al. 12 and Ohbuchi et al. 20 compared the D-dimer levels between patients who achieved successful reperfusion and others who did not, following EVT. Baek et al. 12 reported that the median D-dimer levels were not different among patients achieving overall successful recanalization compared to others that did not (757.0 (392.0–2520.0) vs 732.0 (315.0–2690.0) ng/mL; p = 0.962). Similarly, Ohbuchi et al. 20 demonstrated that mean D-dimer levels were not different between patients having successful and unsuccessful cerebral reperfusion (3.8 ± 6.4 µg/mL vs 6.1 ± 6.9 µg/mL; p = 0.0778).
All of the included studies reported that successful reperfusion was considered at mTICI 2b-3.12,20,21 Moreover, the cutoff value of D-dimer in relation to achieving successful recanalization was reported by two studies. Ohbuchi et al. 20 estimated a cutoff value of 6.7 µg/mL, while Baek et al. 12 reported a value of 1085.0 ng/mL. Moreover, multivariate regression by Ohbuchi et al. 20 revealed a significant association between high D-dimer levels and not achieving cerebral reperfusion following EVT (OR, 5.51; 95% CI, 1.80–16.88; p = 0.0021). The authors also estimated the accuracy, PPV, NPV, specificity, and sensitivity of low D-dimer levels in predicting cerebral reperfusion following EVT to be 76%, 79%, 60%, 36%, and 91%, respectively.
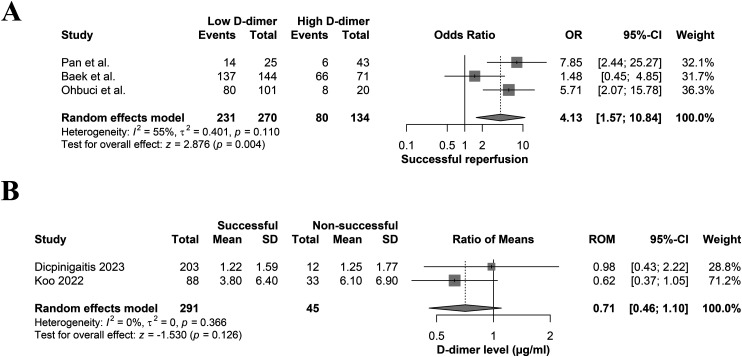
All three studies compared the rates of patients who achieved successful reperfusion based on the D-dimer levels. Ohbuchi et al. 20 and Pan et al. 21 demonstrated that the rates were significantly higher in the low than high D-dimer group. By contrast, Baek et al. 12 mentioned that the rate of patients having successful recanalization was comparable between the low and high D-dimer groups (Low = 95.14 (137/144), High = 93% (66/71)). A meta-analysis of the three studies showed significantly higher successful reperfusion rates in the low D-dimer group compared to the high D-dimer one (OR: 4.13; 95%CI: 1.57–10.84; p = 0.004) (Figure 2(a)). Moreover, pooling two studies that compared D-dimer levels in terms of achieving successful recanalization showed comparable levels in both successful and non-successful reperfusion groups (ROM: 0.71; 95%CI: 0.46–1.10; p = 0.126) (Figure 2(b)).
Figure 2.
Forest plot comparing (a) the rates of patients having successful recanalization following EVT between the low and high D-dimer groups and (b) mean D-dimer levels between patients with and without successful recanalization after EVT.
FPE
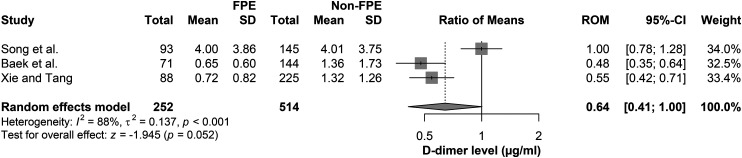
Three studies investigated the association between preprocedural D-dimer levels and achieving the FPE. Baek et al. 12 reported that D-dimer level was the only significant predictor of FPE in their investigation (OR: 0.92; 95%CI: 0.85–0.98/500 ng/mL; p = 0.022), as the median was lower among individuals who achieved it than others who did not (606.0 (268.0–1062.0) vs 879.0 (437.0–2748.0) ng/mL; p = 0.002), with a 1085.0 ng/mL cut-off value. The estimated specificity and sensitivity for D-dimer levels to predict FPE were 47.9% and 76.1%, respectively. Similarly, Xie and Tang 24 reported that D-dimer level was a significant predictor of FPE in their population on multivariate regression (aOR: 0.81; 95%CI: 0.52–0.96; p = 0.015) as the median D-dimer levels were remarkably lower in FPE than non-FPE groups (0.53 (0.27–1.36 vs 1.12 (0.58–2.27); p < 0.001), with a cutoff value of 0.97 mg/L. The estimated specificity, sensitivity, and NPV were 79%, 97.3%, and 87%, respectively. On the contrary, Song et al. 23 estimated that the mean D-dimer levels were comparable between the FPE and non-FPE groups (4 (3.86) vs 4.01 (3.75); p = 0.992). The meta-analysis results of those three studies showed comparable D-dimer levels between those who achieved FPE and those who did not (ROM: 0.64; 95%CI: 0.41–1.00; p = 0.052) (Figure 3).
Figure 3.
Forest plot comparing the mean D-dimer levels between patients with and without FPE after EVT.
Reocclusion risk
A comparative investigation by Li et al. 18 compared two AIS cohorts with prior EVT based on having a subsequent re-occlusion (N = 44) or not (N = 570). The authors demonstrated that a D-dimer level was a significant independent predictor of re-occlusion following EVT based on multivariate regression analysis (median (IQR) = reocclusion: 2 (0.8–4.7) vs non-reocclusion: 0.8 (0.4–1.9); aOR: 1.06; 95%CI: 1.01–1.12; p = 0.029).
Postprocedural complications
Deep vein thrombosis (low extremity)
In a 245 AIS patient population, Han et al. 13 investigated the factors associated with low extremity DVT following EVT. It was demonstrated that the median D-dimer levels were significantly higher among AIS patients with DVT than others without it (2.6 (1.38, 4.49) vs 1.08 (0.6, 1.96) mg/L; p < 0.001). Moreover, it was shown that D-dimer had specificity and sensitivity levels of 70.2% and 71.6%, respectively, with an estimated cutoff value of 1.62 mg/L for predicting DVT after EVT. Multivariate analysis demonstrated a significant association between high D-dimer levels (≥1.62 mg/L) and DVT (OR: 1.350; 95%CI: 1.150–1.585; p < 0.001).
sICH
The association between D-dimer levels and sICH following EVT for AIS-LVO patients was reported by one study only. In a retrospective investigation of 395 patients, Qiu et al. 22 reported that D-dimer levels were significantly higher among patients with sICH than patients without it (2.70 vs 0.74 mg/L, p < 0.001). The estimated NPV and specificity of D-dimer ability to predict sICH in this population were 94.6% and 86.2%, respectively, indicating the significant association between D-dimer and sICH, even after adjustment of confounders (adjusted OR = 2.45, 95% CI 1.75 to 3.43, p < 0.001).
Discussion
Our systematic review and meta-analysis included 14 observational studies with a total of 3268 patients. The results of our meta-analysis showed that the rates of AIS patients having favorable functional outcomes and successful recanalization were remarkably higher in the low-D-dimer group than the high one. Moreover, it was found that the D-dimer level was significantly lower in patients who achieved favorable functional outcomes after EVT as compared with those who did not. Most of the included studies in this systematic review demonstrated the ability of D-dimer levels to significantly predict the favorable functional outcome and recanalization success. By contrast, the D-dimer levels were comparable with no significant differences in the D-dimer levels among patients who achieved FPE and successful recanalization versus those who did not after EVT. It should be noted that these studies did not report adequately homogeneous data. For instance, the definition of successful recanalization and FPE was not consistent among the included studies. Moreover, the cutoff values, which were used to subgroup AIS patients into the high and low D-dimer groups, were also different (Table 2). Besides, some studies reported a significant association between high D-dimer levels and the risk of re-occlusion, deep venous thrombosis, and sICH. However, current evidence is limited to these studies because not much data could be found in the literature to make a meta-analysis.
Thus, there is a need to understand how much the D-dimer level's variation might influence the procedural and clinical outcomes of the EVT procedure, and this need stems from the fact that a high D-dimer level is already known to be an indicator of hypercoagulability, which makes it a potential marker of active thrombus formation.20,37 Moreover, it is speculated that there might be a direct relation between increased D-dimer levels and the thrombus burden in patients with AIS-LVO, which might factor in making the clot retrieval process during the EVT procedure more difficult. 38 Furthermore, there might be variations of the D-dimer levels in AIS-LVO patients according to the underlying etiology of AIS, 39 which might provide insight for clinicians not only regarding what to expect for the EVT outcomes, but also regarding the secondary prevention planning according to the AIS etiology. It is suggested that D-dimer levels might be higher in AIS-caused cardioembolism and cancer than in AIS attributed to other etiologies.15,39 It is to be noted that histological characteristics of the thrombi causing LVO vary according to the etiology of AIS, which might also influence the recanalization process. 32 In the same regard, elevated D-dimer levels in patients with cancer-related AIS-LVO might increase the risk of reperfusion injury, 40 which might lead to an increased risk of post-EVT hemorrhage in those patients. 15
Despite the aforementioned potential implications of D-dimer level variations in both the thrombus burden and the histological composition, as well as the underlying etiology of LVO, our results did not demonstrate a significant impact of the D-dimer level on the possibility of achieving FPE or successful recanalization. However, it should not escape one's notice that the number of studies pooled in the analysis of those two outcomes was limited. Moreover, the data received from the analyzed investigations were remarkably heterogeneous, which might contribute to these potential differences. Nevertheless, our results still showed that lower D-dimer levels were associated with better functional outcomes after EVT. This finding might be related to the underlying etiology of LVO, which by itself, can trigger the changes in the D-dimer levels.
Multiple limitations should be considered when interpreting the findings of this systematic review and meta-analysis. First, almost all studies were retrospective, which could have introduced bias in the reported outcomes within these studies. Second, the reported data were not homogenous in defining their cutoff values and reported outcomes. Third, the sample size of many studies was small. Therefore, future comparative investigations are still needed to verify the current reported outcomes.
Conclusion
The results of this systematic review and meta-analysis show a potential association between low D-dimer levels and favorable functional outcomes and successful recanalization. Some included studies also reported an association between D-dimer levels and FPE, re-occlusion risk, sICH, and DVT. However, further comparative data are required to make a solid conclusion in this regard.
Supplemental Material
Supplemental material, sj-docx-1-ine-10.1177_15910199241289628 for Impact of D-dimer on the outcomes of endovascular thrombectomy for acute ischemic stroke: A systematic review and meta-analysis by Abdullah Reda, Sherief Ghozy, Mohamed Elfil, Eris Spirollari, Aryan Gajjar and Fawaz Al-Mufti in Interventional Neuroradiology
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Abdullah Reda https://orcid.org/0009-0005-1298-2034
Sherief Ghozy https://orcid.org/0000-0001-5629-3023
Fawaz Al-Mufti https://orcid.org/0000-0003-4461-7005
Supplemental material: Supplemental material for this article is available online.
References
- 1.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
- 2.Elfil M, Ghaith HS, Elsayed H, et al. Intravenous thrombolysis plus mechanical thrombectomy versus mechanical thrombectomy alone for acute ischemic stroke: a systematic review and updated meta-analysis of clinical trials. Interv Neuroradiol 2022: 15910199221140276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elfil M, Ghaith HS, Doheim MF, et al. Transradial versus transfemoral access for mechanical thrombectomy: a systematic review and meta-analysis. Stroke 2023; 3: e000758. [Google Scholar]
- 4.Kobeissi H, Ghozy S, Amoukhteh M, et al. 2b Or 2c-3? A meta-analysis of first pass thrombolysis in cerebral infarction 2b vs multiple pass thrombolysis in cerebral infarction 2c-3 following mechanical thrombectomy for stroke. Interv Neuroradiol 2023:15910199231193925. [DOI] [PubMed] [Google Scholar]
- 5.Al-Mufti F, Elfil M, Ghaith HS, et al. Time-to-treatment with endovascular thrombectomy in patients with large core ischemic stroke: the ‘late window paradox’. J Neurointerv Surg 2023; 15: 733–734. [DOI] [PubMed] [Google Scholar]
- 6.Elfil M, Ghaith HS, Bayoumi A, et al. Impact of pre-treatment cerebral microbleeds on the outcomes of endovascular thrombectomy: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis 2023; 32: 107324. [DOI] [PubMed] [Google Scholar]
- 7.Al-Mufti F, Khandelwal P, Sursal T, et al. Neutrophil–lymphocyte ratio is associated with poor clinical outcome after mechanical thrombectomy in stroke in patients with COVID-19. Interv Neuroradiol 2023; 29: 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S, Yi HJ, Lee DHet al. et al. Association of high-sensitivity C-reactive protein with patient prognosis following mechanical thrombectomy for acute ischemic stroke. Curr Neurovasc Res 2020; 17: 402–410. [DOI] [PubMed] [Google Scholar]
- 9.Kisialiou A, Pelone G, Carrizzo A, et al. Blood biomarkers role in acute ischemic stroke patients: higher is worse or better? Immun Ageing 2012; 9: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sayed A, Munir M, Nabet MS, et al. Galectin-3: a novel marker for the prediction of stroke incidence and clinical prognosis. Mediators Inflamm 2022; 2022: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aoki J, Suzuki K, Kanamaru T, et al. Association between mitral regurgitation and clinical outcome after endovascular thrombectomy in stroke patients. Neurol Res 2020; 42: 605–611. [DOI] [PubMed] [Google Scholar]
- 12.Baek JH, Heo JH, Nam HS, et al. Preprocedural D-dimer level as a predictor of first-pass recanalization and functional outcome in endovascular treatment of acute ischemic stroke. J Clin Med 2023; 12(19): 6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han L, Yang JM, Qian WY, et al. Risk factors for lower extremity deep vein thrombosis in acute stroke patients following endovascular thrombectomy: a retrospective cohort study. Front Neurol 2023; 14: 1249365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hisamitsu Y, Kubo T, Fudaba H, et al. High D-dimer concentration is a significant independent prognostic factor in patients with acute large vessel occlusion undergoing endovascular thrombectomy. World Neurosurg 2022; 160: e487–ee93. [DOI] [PubMed] [Google Scholar]
- 15.Lee EJ, Bae J, Jeong HB, et al. Effectiveness of mechanical thrombectomy in cancer-related stroke and associated factors with unfavorable outcome. BMC Neurol 2021; 21: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Duan J, Zhang L, et al. Low (0-5) Alberta Stroke Program Early Computed Tomography Score on admission predictive of worse functional outcome after mechanical thrombectomy for anterior circulation large vessel occlusion. Eur J Med Res 2023; 28: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L, Li M, Chen Z, et al. Prognostic value of radiomics-based hyperdense middle cerebral artery sign for patients with acute ischemic stroke after thrombectomy strategy. Front Neurol 2022; 13: 1037204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W, Ding J, Sui X, et al. Prognosis and risk factors for reocclusion after mechanical thrombectomy. Ann Clin Transl Neurol 2020; 7: 420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitsuhashi T, Teranishi K, Tokugawa J, et al. Prognostic determinants of anterior large vessel occlusion in acute stroke in elderly patients. Geriatrics (Basel) 2024; 9(1): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohbuchi H, Kanazawa R, Hagiwara S, et al. Preoperative plasma D-dimer level may be predictive for success of cerebral reperfusion and outcome after emergency mechanical thrombectomy for intracranial large vessel occlusion. J Clin Neurosci 2022; 97: 75–81. [DOI] [PubMed] [Google Scholar]
- 21.Pan KH, Kim J, Chung JW, et al. Significance of D-dimer in acute ischemic stroke patients with large vessel occlusion accompanied by active cancer. Front Neurol 2022; 13: 843871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu K, Jia ZY, Cao Y, et al. Emergency admission plasma D-dimer: a novel predictor for symptomatic intracranial hemorrhage after thrombectomy in acute ischemic stroke. J Neurointerv Surg 2023; 15: e375–ee80. [DOI] [PubMed] [Google Scholar]
- 23.Song K, Yi HJ, Lee DHet al. et al. Association of blood viscosity with first-pass reperfusion in mechanical thrombectomy for acute ischemic stroke. Clin Hemorheol Microcirc 2021; 77: 233–244. [DOI] [PubMed] [Google Scholar]
- 24.Xie T, Tang WW. Could emergency admission plasma D-dimer level predict first pass effect of stent retriever thrombectomy in acute ischemic stroke? Acta Radiol (Stockholm, Sweden: 1987) 2023; 65: 2841851231218375. [DOI] [PubMed] [Google Scholar]
- 25.Li W, Tang Y, Song Y, et al. Prognostic role of pretreatment plasma D-dimer in patients with solid tumors: a systematic review and meta-analysis. Cell Physiol Biochem 2018; 45: 1663–1676. [DOI] [PubMed] [Google Scholar]
- 26.Keller K, Beule J, Balzer JOet al. et al. D-dimer and thrombus burden in acute pulmonary embolism. Am J Emerg Med 2018; 36: 1613–1618. [DOI] [PubMed] [Google Scholar]
- 27.Sartori M, Migliaccio L, Favaretto E, et al. D-dimer for the diagnosis of upper extremity deep and superficial venous thrombosis. Thromb Res 2015; 135: 673–678. [DOI] [PubMed] [Google Scholar]
- 28.Bang OY, Chung JW, Lee MJ, et al. Cancer-related stroke: an emerging subtype of ischemic stroke with unique pathomechanisms. J Stroke 2020; 22: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Song Y, Shan B, et al. Elevated level of D-dimer increases the risk of stroke. Oncotarget 2018; 9: 2208–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welsh P, Barber M, Langhorne P, et al. Associations of inflammatory and haemostatic biomarkers with poor outcome in acute ischaemic stroke. Cerebrovasc Dis (Basel, Switzerland) 2009; 27: 247–253. [DOI] [PubMed] [Google Scholar]
- 31.Dougu N, Takashima S, Sasahara E, et al. Predictors of poor outcome in patients with acute cerebral infarction. J Clin Neurol (Seoul, Korea) 2011; 7: 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park H, Kim J, Ha J, et al. Histological features of intracranial thrombi in stroke patients with cancer. Ann Neurol 2019; 86: 143–149. [DOI] [PubMed] [Google Scholar]
- 33.Ikeda H, Ishibashi R, Kinosada M, et al. Factors related to white thrombi in acute ischemic stroke in cancer patients. Neuroradiol J 2023; 36: 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedrich JO, Adhikari NK, Beyene J. Ratio of means for analyzing continuous outcomes in meta-analysis performed as well as mean difference methods. J Clin Epidemiol 2011; 64: 556–564. [DOI] [PubMed] [Google Scholar]
- 36.Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. Hoboken, NJ: John Wiley & Sons, 2019. [Google Scholar]
- 37.Koch HJ, Horn M, Bogdahn Uet al. et al. The relationship between plasma D-dimer concentrations and acute ischemic stroke subtypes. J Stroke Cerebrovasc Dis 2005; 14: 75–79. [DOI] [PubMed] [Google Scholar]
- 38.Ramos-Pachon A, Lopez-Cancio E, Bustamante A, et al. D-dimer as predictor of large vessel occlusion in acute ischemic stroke. Stroke 2021; 52: 852–858. [DOI] [PubMed] [Google Scholar]
- 39.Montaner J, Perea-Gainza M, Delgado P, et al. Etiologic diagnosis of ischemic stroke subtypes with plasma biomarkers. Stroke 2008; 39: 2280–2287. [DOI] [PubMed] [Google Scholar]
- 40.Yao T, Tian BL, Li G, et al. Elevated plasma D-dimer levels are associated with short-term poor outcome in patients with acute ischemic stroke: a prospective, observational study. BMC Neurol 2019; 19: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ine-10.1177_15910199241289628 for Impact of D-dimer on the outcomes of endovascular thrombectomy for acute ischemic stroke: A systematic review and meta-analysis by Abdullah Reda, Sherief Ghozy, Mohamed Elfil, Eris Spirollari, Aryan Gajjar and Fawaz Al-Mufti in Interventional Neuroradiology