Abstract
Congenital structural anomalies of the lower airways of the respiratory tract are uncommon in cats. We describe here a case of cystic pulmonary lesions in a 6-wk-old domestic shorthair cat consistent with congenital pulmonary airway malformation (CPAM; formerly referred to as cystic adenomatoid malformation of the lung, or congenital pulmonary adenomatoid malformation; Stocker type II). CPAM is rarely reported in veterinary species and, to our knowledge, has not been reported in cats. In humans and veterinary species, individuals with CPAM (Stocker types I–IV) can be asymptomatic at birth but are predisposed to developing respiratory abnormalities that typically manifest clinically in the early years of life. We review the pathologic features of CPAM.
Keywords: cats, congenital pulmonary airway malformation
Reports of congenital pulmonary structural anomalies of the lower airways (bronchi, bronchioles, alveoli) are rare in cats. We present here the pathologic findings in a young cat with cystic pulmonary lesions consistent with congenital pulmonary airway malformation (CPAM; formerly known as congenital cystic adenomatoid malformation or congenital pulmonary adenomatoid malformation) in humans. Although one of the more common congenital pulmonary lesions in humans, CPAM is rarely reported in veterinary species.2,8,9,14–16,18,20
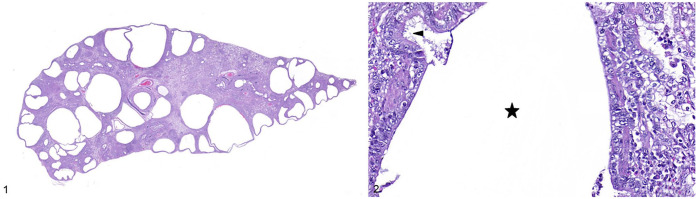
A 6 wk-old female domestic shorthair cat was presented to the Oregon Humane Society because of coughing and dyspnea of unknown duration. Harsh lung sounds were noted on thoracic auscultation. Thoracic radiographs revealed round hyperlucent foci, and suspected cardiomegaly and hepatomegaly. Given the poor prognosis, she was euthanized (Euthansol; Merck). The submitting veterinarian performed an autopsy, noting that cystic structures distorted the lungs grossly, but otherwise no other gross abnormalities were observed in the lungs or other organs. Whole lung, liver, and heart were collected in 10% neutral-buffered formalin and submitted to the Oregon Veterinary Diagnostic Laboratory (OVDL; Corvallis, OR, USA) for histologic examination. Gross examination of the formalin-fixed lung confirmed dozens of randomly distributed, clear, 0.25–20 mm cyst-like structures in the pulmonary parenchyma in all lung lobes (Fig. 1). These cyst-like structures did not appear to communicate with the trachea or bronchi. No gross abnormalities were seen in the liver or heart. The tissues were processed routinely for staining of sections with H&E.
Figures 1, 2.
Congenital pulmonary airway malformation in a cat. Figure 1. A few dozen, randomly distributed, clear, 0.25–20-mm, well-demarcated cyst-like structures in the pulmonary parenchyma. H&E. Figure 2. The cystic spaces in the lung of the kitten were lined by attenuated cuboidal-to-columnar epithelium with fine apical cytoplasmic projections (arrowhead) on the apical surface. The adjacent stroma was composed of fibrous tissue and smooth muscle, with an absence of glandular tissue and cartilage. Star indicates the lumen of the cystic space. H&E.
Histologic examination revealed the cystic spaces to be lined by attenuated cuboidal-to-columnar epithelium with fine cytoplasmic projections resembling cilia on the apical surface, and surrounded by fibrous stroma, with scattered smooth muscle bundles, and an absence of glandular tissue and cartilage (Fig. 2). The alveolar spaces and the larger airways between these cystic structures were filled with variable numbers of macrophages and neutrophils, and eosinophilic-to-pale-eosinophilic amorphous material, interpreted as edema fluid. Interlobular septa were expanded by edema. Multifocally, the pleura was expanded by edema, lymphoplasmacytic infiltrate, and spindle cells interspersed between collagen fibers, with some of these cells lining small vascular spaces (granulation tissue). Bacteria and fungi were not seen on Gram and periodic acid-Schiff–stained sections of lung. Formalin-fixed, paraffin-embedded lung was submitted for a PCR panel for feline herpesvirus, feline calicivirus, and mycoplasma at the Michigan State University–Veterinary Diagnostic Laboratory (Lansing, MI, USA), and was positive for feline calicivirus. No histologic abnormalities were seen in the liver or heart. The final diagnosis was pulmonary cysts with severe, chronic-active, pyogranulomatous, lymphoplasmacytic pleuropneumonia.
Based on the gross appearance of the cystic structures in the lung, differentials considered included congenital pulmonary lobar emphysema, emphysema secondary to pneumonia or pleuropneumonia, and CPAM. The absence of gross and histologic abnormalities in the bronchi made congenital pulmonary lobar emphysema less likely.2,6,7,9,14,15,19,20 Given that the cystic spaces were lined predominantly by epithelium consistent with a bronchiolar morphology and alveolar spaces were relatively spared of cystic distension, emphysema secondary to pneumonia or pleuropneumonia was also considered less likely. Histologically, these cystic structures were lined by bronchiolar-like epithelium, and along with their surrounding stroma, were thus most consistent with a Stocker type II form of CPAM seen in humans.9,12,17
Classification of CPAM is by the Stocker classification system, which is based on the anatomic location of the airway in which the abnormal dilation occurs: Stocker type 0 involves the trachea or proximal bronchi, Stocker type I involves bronchi, Stocker types II and III involve bronchioles, with the latter seen as a solid mass rather than cysts, and Stocker type IV involves alveolar acini.2,9,14,15,20 In veterinary species, descriptions with usage of the term CPAM (or congenital cystic adenomatoid malformation, and congenital pulmonary adeno-matoid malformation, as CPAM was referred to in the past) are limited to single case reports in young calves and a cynomolgus monkey (syn. crab-eating macaque; Macaca fascicularis).8,16,18 We retrieved no cases of described lesions in cats with histologic features consistent with CPAM in a search of Google, PubMed, CABI Direct, Web of Science, Scopus, and archived OVDL pathology reports, suggesting that this condition has not been reported in this species. Stocker types 0 and I CPAM have been diagnosed in calves, and Stocker type I CPAM in a cynomolgus monkey.8,16,18
Newborns with CPAM tend to be asymptomatic at birth.1,2,5,11,13,15,20 The exceptions are individuals with the Stocker type 0 form, which is incompatible with ex utero life given the associated absence of gas exchange in the lung.18,20 CPAM predisposes to the development of infections, asthma, and primary pulmonary neoplasia, particularly mucinous adenocarcinoma in individuals with a Stocker type I form; 25% develop respiratory syndromes such as wheezing, cough, asthma, and lower respiratory tract infections within the first 5 y of life.1–5,10,11,13,15,17,20 This outcome is consistent with that seen in the reports of one of the calves and the cynomolgus monkey with the Stocker type I presentation, which were subclinical at birth but subsequently developed respiratory abnormalities.8,16 A similar sequence of events was presumed to have occurred in our case, given that the kitten had evidence of severe chronic pleuropneumonia that histologically was more likely due to a bacterial infection rather than feline calicivirus. The latter is likely an incidental background finding by PCR.
CPAM is postulated to involve proximal obstruction of the affected airways during the pseudoglandular stage of lung development in utero, as well as increased levels of thyroid transcription factor 1, transforming growth factor β, and fibroblast growth factor 10, which results in atypical hyperplasia, dysplasia, and metaplasia of the fetal epithelium and mesenchyme.2,14,15 In humans, CPAM can also be classified as being composed of macrocystic or microcystic lesions seen during antenatal ultrasound of the fetus.14,15,20
Ultrasonographically, macrocystic lesions are pulmonary cysts of ≥5 mm diameter; microcystic lesions are echogenic pulmonary foci of <5 mm diameter.14,15,20 Microcystic lesions tend to grow faster and thus are associated with a higher risk of developing potentially fatal congenital abnormalities, such as fetal hydrops, polyhydramnios, pulmonary hypoplasia, and deviation of the mediastinum as a result of compression of the adjacent esophagus and pulmonary tissue by the microcystic lesion.2,9,14,15,20 Prognosis is more strongly correlated with antenatal diagnosis of macrocystic or microcystic lesions rather than the histologic subtypes based on the Stocker classification.2,14,15,20
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Deborah L. A. Chong  https://orcid.org/0000-0003-2511-2175
https://orcid.org/0000-0003-2511-2175
References
- 1. Abu Omar M, et al. Congenital pulmonary airway malformation (CPAM) with initial presentation in an adult: a rare presentation of a rare disease. BMJ Case Rep 2016;2016:bcr2016216957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Annunziata F, et al. Congenital lung malformations: unresolved issues and unanswered questions. Front Pediatr 2019;7:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Casagrande A, Pederiva F. Association between congenital lung malformations and lung tumors in children and adults: a systematic review. J Thorac Oncol 2016;11:1837–1845. [DOI] [PubMed] [Google Scholar]
- 4. Chang W-C, et al. Mucinous adenocarcinoma arising in congenital pulmonary airway malformation: clinicopathological analysis of 37 cases. Histopathology 2021;78:434–444. [DOI] [PubMed] [Google Scholar]
- 5. Criss CN, et al. Asymptomatic congenital lung malformations: is nonoperative management a viable alternative? J Pediatr Surg 2018;53:1092–1097. [DOI] [PubMed] [Google Scholar]
- 6. Del Magno, et al. Congenital lobar emphysema in a kitten with concomitant hiatal hernia and nutritional secondary hyperparathyroidism. J Am Anim Hosp Assoc 2022;58:141–145. [DOI] [PubMed] [Google Scholar]
- 7. Demir OF, et al. Congenital lobar emphysema: diagnosis and treatment options. Int J Chron Obstruct Pulmon Dis 2019;14:921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Desrochers A, et al. Type I congenital cystic adenomatoid malformation of the lungs in a Hereford calf. Zentralbl Veterinarmed A 1994;41:709–712. [DOI] [PubMed] [Google Scholar]
- 9. Fowler DJ, Gould SJ. The pathology of congenital lung lesions. Semin Pediatr Surg 2015;24:176–182. [DOI] [PubMed] [Google Scholar]
- 10. Goyal JP, et al. Congenital cyst adenoid malformation masquerading as bronchial asthma. Int J Appl Basic Med Res 2017;7:199–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Indinnimeo L, et al. A surprising finding in an adolescent athlete affected by diffuse congenital cystic adenomatoid malformation (CCAM). Clin Respir J 2013;7:420–422. [DOI] [PubMed] [Google Scholar]
- 12. Jiang Y, et al. Alteration of cystic airway mesenchyme in congenital pulmonary airway malformation. Sci Rep 2019;9:5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kantor N, et al. Symptom development in originally asymptomatic CPAM diagnosed prenatally: a systematic review. Pediatr Surg Int 2018;34:613–620. [DOI] [PubMed] [Google Scholar]
- 14. Kunisaki SM. Narrative review of congenital lung lesions. Transl Pediatr 2021;10:1418–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leblanc C, et al. Congenital pulmonary airway malformations: state-of-the-art review for pediatrician’s use. Eur J Pediatr 2017;176:1559–1571. [DOI] [PubMed] [Google Scholar]
- 16. Okabayashi S, et al. Congenital cystic adenomatoid-like malformation in a cynomolgus monkey (Macaca fascicularis). Vet Pathol 2008;45:232–235. [DOI] [PubMed] [Google Scholar]
- 17. Pogoriler J, et al. Congenital cystic lung lesions: redefining the natural distribution of subtypes and assessing the risk of malignancy. Am J Surg Pathol 2019;43:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van den Ingh TS, van der Gaag I. A congenital adenomatoid malformation of the lungs in a calf. Vet Pathol 1974;11:297–300. [DOI] [PubMed] [Google Scholar]
- 19. Warwick H, et al. Imaging findings in 14 dogs and 3 cats with lobar emphysema. J Vet Intern Med 2021;35:1935–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zobel M, et al. Congenital lung lesions. Semin Pediatr Surg 2019;28:150821. [DOI] [PubMed] [Google Scholar]