Abstract
Background: Medications requiring refrigeration for stability are commonly used across hospitals. Temperature-sensitive medications may not have their temperature requirements maintained due to breaches in the cold chain, especially refrigerator failure. This is usually caused by malfunction of the refrigerator unit or by power outage. After multiple power outages at our institution involving refrigerators with temperature probes located in different areas of the refrigerator, we hypothesised that the fixed temperature probe may not accurately reflect the temperature of the medications themselves. Methods: We conducted simulations of power outages in a commonly used medication refrigerator, using additional temperature data loggers, placed on the refrigerator shelf and inside a cardboard box to replicate the temperature inside medication containers to determine if there was a difference in the time to breach cold chain conditions (>8°C) and to return to appropriate refrigerated temperatures (<8°C) when power was restored. Results: All data loggers took a longer time to breach cold chain conditions than the refrigerator probe (12.5 minutes vs 23-26 minutes) but took longer to return to acceptable temperature after power was restored (17.5 minutes vs 70.5-89 minutes). Conclusion: This exploratory research suggests that temperatures vary within a refrigerator depending on the type and location of probe and that medications within may take longer to breach cold chain conditions but also take longer to return to cold chain conditions compared with fixed refrigerator temperature probes. Further research is required to determine whether these variations occur across different sizes/brands of refrigerators and the effect on stability on refrigerated medications.
Keywords: temperature, medication stability, refrigerator, electricity outage, temperature sensitive medications
Introduction
Medications have temperature range and exposure to light storage conditions specified by the pharmaceutical manufacturer in order to maintain chemical stability over the shelf life. 1 The number of medications and vaccines requiring refrigerated or low temperature storage to maintain stability has increased in recent years, most notably with the introduction of mRNA vaccines for COVID-19 prevention. Medications requiring refrigerated storage must be maintained within 2°C to 8°C during the cold chain, the period from product manufacture to patient administration. 2 Maintaining the cold chain is crucial as exposure outside these temperatures can result in chemical or physical changes to the medication, including protein aggregation or denaturation (chemical changes that are undetectable on visualisation), or visible changes in viscosity or colour. Temperature breaches during the cold chain may result in the loss of potency or stability of the affected medication and have the potential to cause patient harm through reduced or no efficacy of the medication, harmful degradation products or adverse events.1,3,4 Wasted medications from temperature breaches also have an economic impact.4-6
Temperature breaches can occur at any point during the manufacture, transport, storage and supply of a medication. A cold chain breach is defined as exposure to temperatures outside of 2°C to 8°C for longer than 15 minutes or any period below 2°C, as per the Australian national vaccine storage guidelines. 2 (There is no specific Australian guidance for refrigerated medication storage, and hospitals commonly extrapolate the vaccine guidelines to medications). This was originally applied to vaccines, but has been generally adopted to include all medications requiring refrigeration. To ensure cold chain maintenance, specialized medication refrigerators with inbuilt temperature probe(s) are used that monitor the temperature electronically. 7 However, these refrigerators can experience unexpected power outages, failure due to human error (eg, leaving the door open) or mechanical failure. 1
There is limited literature available investigating how temperatures of medications inside refrigerators vary from the temperature probe reading within the refrigerator and whether this difference is significant.5,8 A site wide power outage across our institution resulted in the need to research the potential differences further. 9 Standard temperature monitoring of the fridges failed due to their electricity requirement and battery backup systems were inconsistent in their ability to capture temperature data due to varying maintenance schedules resulting in failure of the battery to function. Based on this event and multiple power outages since, we hypothesised that a medication in its original packaging would be cooler than the temperature of the refrigerator during a power outage, potentially not reaching temperatures greater than those expected when the fridge is functioning normally.
Methods
This study performed a duplicated simulation of sustained power loss to a specialized medication refrigerator (model ESCO HR1-140T), assessing the time to reach a temperature excursion and resolution post power restoration. This medication refrigerator has a capacity of 128 L with glass doors, wired shelving and a lower drawer compartment. The ESCO refrigerators are calibrated according to ISO standards 9001 and 13 485 prior to shipping. The inpatient pharmacy refrigerator used in this study had been in operation for 12 years and underwent scheduled maintenance to ensure it was fit for purpose. The ESCO HR1-140T refrigerator was chosen as it is the most commonly used medication refrigerator (54%) on the wards at our institution (Fiona Stanley Hospital, Western Australia). ESCO provide guidance on how to load the refrigerator for maximum performance (eg, ensuring space between the walls of the refrigerator and medication, limiting how often the door is opened), however this can be difficult practically on the wards.
Temperature monitoring was performed with the ESCO refrigerators (inbuilt) temperature measuring probe (PT100 resistance temperature detector with platinum sensor), assessing ambient air, and with data loggers (TempTale Ultra®, negative temperature coefficient thermistor probe), which have an accuracy of ±0.5°C within the standard operating range of −10°C to 45°C. 10 The TempTales are calibrated to the ISO standards 9001 and 17 025 and also the National Institute of Standards and Technology (NIST) standard (there is no Australian equivalent). One TempTale (serial number: KAE4N09YH0, not expired at time of experiment) was placed on the bottom shelf inside the refrigerator and a second (serial number: KAE4N09ZQ0, not expired at time of experiment) was placed inside an insulin medication box (Novorapid, Novo Nordisk, Denmark), stored on the middle shelf inside the refrigerator. The bottom shelf was chosen as a site for the TempTale monitor as it was located away from the refrigerator temperature probe, giving insight as to the variation in temperature within the refrigerator. Likewise, the TempTale monitor in the cardboard medication box was placed on the middle shelf to replicate common placement for medication within the fridge and to investigate the temperature variation in the middle of the refrigerator.
Temperature measurements from the refrigerator were documented at 5-minute intervals as suggested in the Australian national vaccine storage guidelines and temperature measurements from the TempTale monitors were documented at 1-minute intervals. The refrigerator was filled with expired medication according to usual practice to simulate a standard medication refrigerator. All temperature monitors inside the refrigerator were confirmed to be at 2°C to 8°C prior to simulation commencement. Power loss was simulated by switching the refrigerator off at the general power outlet. Measurements were recorded? from 25 minutes prior to power loss, during 2.5 hours of power loss and continued until temperatures returned to within 2°C to 8°C consistently for 15 minutes.
This study was approved as a quality improvement project by the Institutional Safety and Quality Committee (GEKO #49353) and was exempt from requiring Human Research and Ethics Committee approval.
Results
The study completed 2 simulations of power loss for a medication refrigerator. The mean time to reach a temperature excursion of greater than 8°C (displayed in Table 1), was 12.5 minutes for the refrigerator monitor compared to 23 and 26 minutes for the TempTale monitors stored on the shelf and in medication box respectively.
Table 1.
Temperature Times by Monitor Type.
| Power loss simulation | Fridge monitor | TempTale shelf monitor | TempTale box monitor |
|---|---|---|---|
| Time to >8°C when turned off—mean (range) | 12.5 mins (10-15 mins) | 23 mins (23 mins) | 26 mins (23-29 mins) |
| Time to <8°C after power restored—mean (range) | 17.5 mins (15-20 mins) | 89 mins (88-90 mins) | 70.5 mins (70-71 mins) |
The mean time for the temperature monitors to return to less than 8°C, was 17.5 minutes for the refrigerator monitor compared to the TempTale monitors; which were 89 minutes (shelf monitor) and 70.5 minutes (box monitor), as shown in Figure 1 and in the Appendices.
Figure 1.
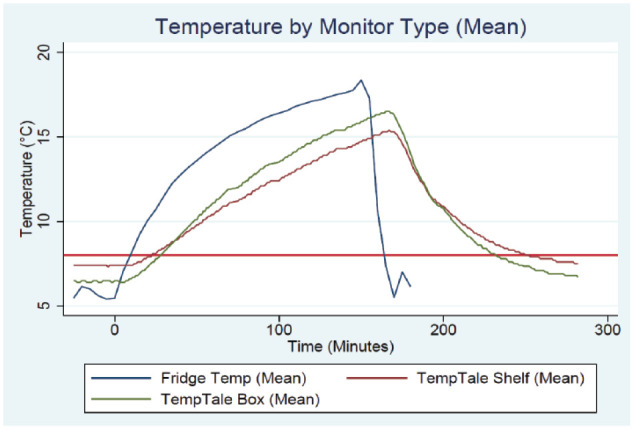
Refrigerator temperatures recorded by monitor types, during and after a simulated power loss.
The temperature variation between the 2 simulations for the refrigerator monitor at the measurement timepoints was minimal, with no difference for 80% of measurements and a maximum variation of 1.8°C. There was no temperature variation between simulations observed with the TempTale shelf monitor at the specified measurement time points. There was very minor temperature variation observed between simulations with the TempTale box, with no difference observed for 98.5% of measurements and a maximum variation of 0.1°C.
Discussion
The TempTale monitors took an additional 12 minutes (range 8-14 minutes) to exceed 8°C once the refrigerator was turned off compared to the standard refrigerator monitor however the TempTale temperature monitors took an additional 63 minutes (range 55-75 minutes) to return to below 8°C once the refrigerator was powered-up. This indicates that the method of measuring the temperature to maintain medication cold chain is different when using a TempTale monitor and a standard refrigerator monitor. These differences in temperature monitoring methods may have a substantial impact on the quality of the medication administered to patients, medication wastage and financial consequences. Both TempTale monitors showed relatively similar times to breach 8°C and then return to below 8°C despite different location placement, indicating further research is needed to determine if the TempTale placement within the refrigerator has a significant impact. More investigation needs to be conducted on different sizes and brands of refrigerators. It is also unknown whether the length of time a refrigerator has been in use or time between maintenance affects the variation in temperature.
A study in German general practices found that 44% of medication refrigerators reached temperatures >8°C, demonstrating a need for research investigating these issues. 11 However, most refrigerators studied were household rather than pharmaceutical grade which is not reflective of the hospital environment but suggests that household refrigerators are not fit for the purpose of storing medications safely. 11
Temperature excursions are also not isolated events, D’Onise et al. 6 reported 597 cold chain breach reports from vaccine providers in South Australia across a 2 years period. In a survey of community pharmacies in the Australian Capital Territory, 50% of pharmacies reported 1 to 4 temperature excursions in a 12-month period. 5 With the advent of COVID-19 vaccines, there is also a need to explore the effects of ultra-low freezer temperature excursions, and for medications requiring freezer storage.
Published data on the stability of temperature sensitive medications when cold chain protocols are not maintained is limited, focusing predominantly on vaccines in resource-limited settings. 12 There is some literature addressing the stability of temperature sensitive products and how this affects the shelf life.1,13 This information is often brand and/or country specific and may not apply to the Australian setting. Determining medication stability is often a lengthy process involving review of the product information and communication with sponsors of the medication. Stability data outside of the recommended temperature may also not be available for some medications due to a lack of available data to clinicians.1,5 Disposing of medications when there is no stability data to support use may be costly depending on the medications involved. One study in community pharmacies described a cost of $13168 AUD (2018 prices) to replace medications due to power outages. 5 In GP practices, the costs to replace vaccines lost in a temperature excursion ranged from $1339.06 to $2321.20 AUD (2011 prices), despite generally robust data available to clinicians on the stability of vaccines outside recommended storage conditions. 6 The impact on hospitals is likely to be much higher due to the wider range of medications used, in addition to the regular use of specialised high-cost medications, including those for parenteral use. In addition, we did not conduct stability data on actual medications, so the effect on the medications themselves is unknown.
This exploratory brief report has several limitations. Our study only utilized 1 refrigerator that had been in service for a considerable period, so further research investigating multiple refrigerator types is needed to fully ascertain the extent and accuracy of temperature monitoring in medication refrigerators. Different types of medication refrigerators may perform differently depending on their design. Zamani et al. 14 found that brands of refrigerators varied in the ability to provide a stable and uniform temperature throughout, possibly due to their position or proximity to air vents which could cause fluctuations in temperature when the door was opened. Mean kinetic temperature was also not assessed in this study due to its investigative nature and that medication stability and potency was not evaluated. We have attempted to provide data from the Australian hospital setting on real-world use of a medication refrigerator that reflects use in the ward setting.
Conclusion
There appears to be a substantial difference in 2 separate temperature monitoring methods within a medication refrigerator at our institution. It is unknown whether this relates to the temperature monitoring method used, the location of the temperature probes within the refrigerator, or a combination of these factors. Using individual monitoring devices within medication containers themselves may provide a more accurate reflection of the temperature of the medication itself rather than the surrounding temperature of the storage device itself. This warrants further investigation, not only for refrigerated medications but those requiring storage in standard and ultra-low freezers due to the potential quality control concerns and economic consequences of medication breaching cold chain conditions.
Supplemental Material
Supplemental material, sj-docx-1-hpx-10.1177_00185787241282245 for Temperature Excursions in Cold Chain Management—Assessing the Accuracy of Refrigerator Temperature Probes by Kayla Ferraz, Melissa Cato, Emma Fox, Matthew Rawlins and Jeanie Misko in Hospital Pharmacy
Acknowledgments
None.
Footnotes
Authorship Statement: Conceptualization: JM, EF. Research question: JM, MR, and EF. Methodology: JM, KF, MC, and EF. Data recording: KF and MC. Data synthesis: KF, MC, and EF. Data analysis: KF, MC, and EF. Writing—original draft: KF, MC, JM, and EF. Writing—reviewing and editing: KF, MC, JM, EF, and MR.
Data Availability Statement: The data that supports the findings of this study is available from the corresponding author upon reasonable request.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Statement: This study was approved as a quality improvement project by the institutional Safety and Quality Committee (GEKO #49353) and was exempt from requiring Human Research and Ethics Committee approval.
ORCID iD: Jeanie Misko  https://orcid.org/0000-0003-1136-2683
https://orcid.org/0000-0003-1136-2683
Open Access Statement: This research received no specific open access funding.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Orth LE, Ellingson AS, Azimi SF, et al. Allowable room temperature excursions for refrigerated medications: a 20-year review. Am J Health Syst Pharm. 2022;79(15):1296-1300. doi: 10.1093/ajhp/zxac118 [DOI] [PubMed] [Google Scholar]
- 2. Department of Health. National vaccine storage guidelines—strive for 5. Updated 2019. Accessed March 15, 2023. https://www.health.gov.au/sites/default/files/documents/2020/04/national-vaccine-storage-guidelines-strive-for-5.pdf
- 3. Cohen V, Jellinek SP, Teperikidis L, Berkovits E, Goldman WM. Room-temperature storage of medications labeled for refrigeration. Am J Health Syst Pharm. 2007;64(16):1711-1715. doi: 10.2146/ajhp060262 [DOI] [PubMed] [Google Scholar]
- 4. Sakly H, Chakroun I, Ben Jeddou K. Application of failure mode, effects, and criticality analysis to the medication-use process for temperature-sensitive drugs in a university hospital. Can J Hosp Pharm. 2022;75(3):159-168. doi: 10.4212/cjhp.3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kosari S, Walker EJ, Anderson C, et al. Power outages and refrigerated medicines: the need for better guidelines, awareness and planning. J Clin Pharm Ther. 2018;43(5):737-739. doi: 10.1111/jcpt.12716 [DOI] [PubMed] [Google Scholar]
- 6. D’Onise K, Almond S, MacDonald B, Watson M, Scrimgeour S. Have purpose-built vaccine refrigerators reduced the cost of vaccine losses in South Australia? Aust N Z J Public Health. 2012;36(6):572-576. doi: 10.1111/j.1753-6405.2012.00932.x [DOI] [PubMed] [Google Scholar]
- 7. Department of Health. National Vaccines Storage Guidelines—Strive for 5. Updated 2019. Accessed April 19, 2023. https://www.health.gov.au/sites/default/files/documents/2020/04/national-vaccine-storage-guidelines-strive-for-5.pdf
- 8. Beatty ME, Phelps S, Rohner C, Weisfuse I. Blackout of 2003: public health effects and emergency response. Public Health Rep. 2006;121(1):36-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ridley B, Bint L, Misko J, Sweeney M, Thorson R. Management of refrigerated medications during and after a hospital-wide power outage. Paper presented at: Medicines Management 2019, the 45th SHPA National Conference; November 14-16, 2019; Gold Coast, Australia. [Google Scholar]
- 10. Sensitech Inc. TempTale Ultra. Sensitech Inc. Updated 2023. Accessed 19 February, 2024. https://www.shareddocs.com/hvac/docs/2004/Public/05/tt-ultra-life-sciences.pdf
- 11. Thielmann A, Puth MT, Kersting C, Porz J, Weltermann B. Vaccine cold chain in general practices: a prospective study in 75 refrigerators (Keep Cool study). PLoS One. 2019;14(11):e0224972. doi: 10.1371/journal.pone.0224972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dadari IK, Zgibor JC. How the use of vaccines outside the cold chain or in controlled temperature chain contributes to improving immunization coverage in low- and middle-income countries (LMICs): A scoping review of the literature. J Glob Health. 2021;11:04004. doi: 10.7189/jogh.11.04004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jenkins D, Cancel A, Layloff T. Mean kinetic temperature evaluations through simulated temperature excursions and risk assessment with oral dosage usage for health programs. BMC Public Health. 2022;22(1):300. doi: 10.1186/s12889-022-12660-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zamani M, Wembridge P. Evaluating an automated temperature-monitoring system in medicine and vaccine storage facilities of a hospital network. Asia Pacific J Health Manag. 2022;17(1):1–10. doi: 10.24083/apjhm.v17i1.899 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-hpx-10.1177_00185787241282245 for Temperature Excursions in Cold Chain Management—Assessing the Accuracy of Refrigerator Temperature Probes by Kayla Ferraz, Melissa Cato, Emma Fox, Matthew Rawlins and Jeanie Misko in Hospital Pharmacy


