Abstract
Background:
Uncertainty exists concerning the optimal utilization and effectiveness of pectoralis minor tenotomy (PMT) in neurogenic thoracic outlet syndrome (NTOS).
Methods:
Between January 2020 and July 2023, 355 patients with NTOS underwent primary surgical treatment. Prospectively collected data were analyzed retrospectively.
Results:
Overall mean patient age was 35.9 ± 1.9 years, 76% were female, and the Quick Disabilities of the Arm, Shoulder, and Hand (QuickDASH) score at presentation was 60.3 ± 3.2, reflecting substantial disability. Surgical treatment was based on localized tenderness/symptoms to palpation, with 322 (91%) undergoing combined supraclavicular decompression and PMT (SCD + PMT) and 33 (9%) selected for isolated PMT when findings were solely confined to the subcoracoid space. Mean operative time (29 ± 5 vs 164 ± 9 min, P < .01) and hospital stay (0.3 ± 0.1 vs 4.0 ± 0.2 days, P < .01) were both lower after isolated PMT, with no significant differences in postoperative complications or rehospitalization. During follow-up of 26.7 ± 1.5 months, QuickDASH scores declined by 41.2% ± 2.3% (P < .0001) and patient-rated outcomes were excellent in 34%, good in 41%, fair in 22%, and poor in 4%. Fewer patients had poor-rated outcomes after SCD + PMT (2%) than after isolated PMT (19%) (P < .01). Recurrent symptoms requiring supraclavicular reoperation occurred in 16 patients after SCD + PMT (5%) and in 5 patients after isolated PMT (15%) (P < .05).
Conclusions:
Pectoralis minor tenotomy (PMT) has an important role in surgical treatment of NTOS, mainly as an adjunct in combination with SCD. While highly selected patients can do well after isolated PMT as a short outpatient procedure with rapid recovery, there is a greater potential for poor outcomes and supraclavicular reoperation than after SCD + PMT.
Keywords: thoracic outlet syndrome, pectoralis minor muscle, brachial plexus, compression neuropathy, surgical treatment, patient-reported outcomes measures, reoperation
Introduction
There has been substantial progress in the past several decades toward understanding the pathophysiology, diagnostic approaches, and optimal treatment strategies for neurogenic thoracic outlet syndrome (NTOS).1 -3 Owing largely to complex developmental anatomy, predisposing structural factors for NTOS include cervical ribs, anomalous first ribs, muscle variations within the scalene triangle, and anomalous fibro-fascial bands.4,5 External acquired factors also contribute, including neck or upper extremity trauma, repetitive strain injury, postural changes, and altered shoulder girdle mechanics, along with development of fibrosis and sustained spasm within the scalene and pectoralis minor muscles.6 -8 Symptoms associated with NTOS include pain, numbness, paresthesia, and weakness of the neck, shoulder, upper chest, back, arm, and/or hand, which can be promptly exacerbated by position-related dynamic neural compression, which can occur within the scalene triangle, costoclavicular space, and/or subcoracoid (pectoralis minor) space. The clinical diagnosis of NTOS requires the exclusion of other conditions as a principal source of symptoms and the presence of specific clinical criteria.9,10
Conservative treatment of NTOS with physical therapy and multimodal pain management strategies can result in symptomatic relief for many patients, but surgical decompression is recommended for those with persistent and disabling symptoms that affect daily function and quality of life.11 -13 Surgical treatment usually involves either supraclavicular or transaxillary approaches, including resection of any cervical rib and/or the first rib, anterior and middle scalenectomy, removal of anomalous fibro-fascial bands, and external brachial plexus neurolysis.3,14 -16 Each approach has its specific advantages and disadvantages, but both have demonstrated excellent results in the hands of experienced surgeons at high volume centers.12,17 -21
One of the unresolved issues regarding surgical treatment for NTOS is the best management of brachial plexus nerve compression within the subcoracoid space underneath the pectoralis minor muscle. The pectoralis minor muscle was previously identified as a source of persistent or recurrent symptoms during follow-up after surgery for NTOS, with pectoralis minor tenotomy (PMT) emerging as a potentially effective approach to reoperative treatment.22 -24 It was subsequently shown that subcoracoid compression is frequently associated with nerve compression at the scalene triangle at the time of initial clinical evaluation, and that for some patients isolated PMT might suffice to prevent the need for scalene triangle decompression.25,26 Investigations built on this work found that for selected patients, the inclusion of PMT in combination with either supraclavicular decompression (SCD) or transaxillary first rib resection was an effective approach in treatment of NTOS with excellent early results, suggesting that PMT is a valuable adjunct to achieve sustained, long-term symptom relief.27,28
The purpose of the present study was to elucidate current utilization and long-term effectiveness of PMT in patients with NTOS, in a case series comparison of patients that underwent primary surgical treatment by either combined SCD + PMT or by isolated PMT.
Materials and Methods
Derivation of the Study Population
The study population was derived from patients referred to our institution for evaluation and management of NTOS, for whom surgical treatment was performed between January 1, 2020, and July 1, 2023. Those with arterial or venous forms of TOS or those undergoing treatment for recurrent NTOS were excluded. Patient information was obtained from a prospectively maintained database and summarized from office notes, hospital charts, and other medical records. This study was approved by the institutional review board at our center, with all patients providing written informed consent to study participation.
Clinical Diagnosis
Each patient met predefined clinical diagnostic criteria for NTOS as developed by the Consortium for Outcomes Research and Education on Thoracic Outlet Syndrome and those described in the 2016 Society for Vascular Surgery reporting standards publication.9 -11 Imaging studies and electrophysiological tests were not considered necessary to establish a clinical diagnosis of NTOS, but were routinely used to identify bony abnormalities (eg, cervical ribs) and to help assess or exclude other conditions. In some patients, anesthetic injections into the anterior scalene muscle and/or pectoralis minor muscle were used as an adjunct to clinical diagnosis and to help predict responsiveness to treatment. In each patient the level of functional disability was assessed using the 11-item version of the Quick Disabilities of the Arm, Shoulder, and Hand (QuickDASH) survey instrument. 29
Treatment Algorithm
All patients underwent initial physical therapy management guided by a therapist aligned with our TOS Center. Patients who experienced an improvement in symptoms were encouraged to continue with conservative management. Surgical treatment was offered for patients who had a sound clinical diagnosis of NTOS, a significant level of functional disability, and insufficient improvement with conservative management. Patients with concomitant brachial plexus compression at the level of the scalene triangle and the subcoracoid (pectoralis minor) space were offered combined SCD + PMT, whereas those with brachial plexus compression confined solely to the subcoracoid space were offered isolated PMT.
Surgical Treatment
Surgical treatment in the SCD + PMT group consisted of standardized supraclavicular exposure with complete anterior and middle scalenectomy, first rib resection (including a cervical rib when present), external brachial plexus neurolysis to remove perineural scar tissue (including full mobilization of all 5 nerve roots and 3 trunks), and adjunctive PMT without brachial plexus neurolysis performed through a separate deltopectoral groove incision. 15 In other patients, surgical treatment consisted of isolated PMT alone without brachial plexus neurolysis, when examination findings were confined to the subcoracoid space. 30 Postoperative complications, hospital stay, and readmissions were all recorded in the prospective database.
Follow-up and Outcome Measures
Patients resumed physical therapy 3-4 weeks after the operation and were seen for office visits every 3-4 months after the initial recovery from surgery, or more frequently when necessary. At each visit, patients were asked to complete the QuickDASH survey instrument and to rate their outcome of treatment on a Derkash scale using the following descriptors: “excellent” (no or minimal pain, easy return to professional and leisure activities, relief of almost all major symptoms with only some mild residual symptoms that do not significantly limit enjoyment of life); “good” (intermittent pain well-tolerated, possible to return to professional and leisure activities, relief of most major symptoms with some mild residual symptoms that do not significantly limit enjoyment of life); “fair” (intermittent or permanent pain not consistently well-tolerated, difficult or no return to professional and leisure activities, partial relief of some symptoms while other major symptoms persist); and “poor” (symptoms not improved or aggravated after surgery, difficult or no return to professional and leisure activities, not enough relief in symptoms to have made the operation worthwhile).31,32
Statistical Analysis
Descriptive data are presented as mean ± standard error (SE), median and range, or the frequency (percent incidence). For two-group comparisons, Fisher’s exact test (for categorical variables) or the unpaired student t-test with two-tailed distribution (for continuous variables) was used to determine statistical significance. All statistical tests were performed using Prism version 10.0.2 (GraphPad Software Inc., San Diego, CA), with P-values < .05 considered significant.
Results
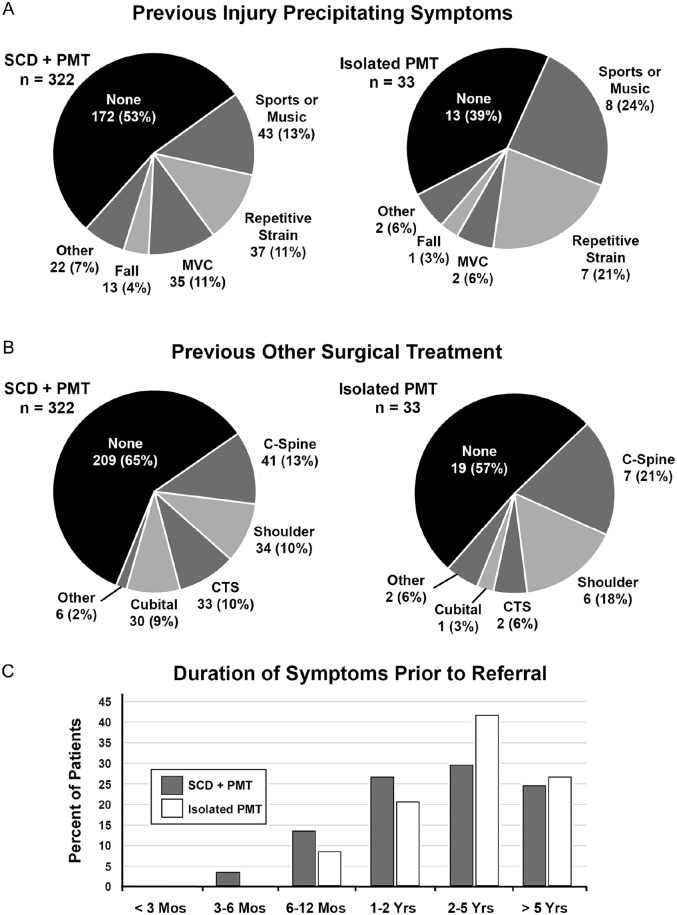
During the study interval, there were 355 patients who underwent primary surgical treatment for NTOS, with 322 (91%) undergoing SCD + PMT and 33 (9%) undergoing isolated PMT. The presenting characteristics of the patient population are shown in Table 1, with the overall study population consisting of 270 women (76%) and 85 men (24%) with a mean age of 35.8 ± 1.9 years (median 37, range 13-70) at the time of presentation. The proportion of women was slightly greater for the combined SCD + PMT group compared to the isolated PMT group (P < .05), but there were no significant differences between groups in the side affected (right side in 54%), body mass index (mean 28.2 + 1.5 kg/m2), history of injury that precipitated symptoms (48%), duration of symptoms > 2 years prior to referral (57%), presence of a cervical rib (8%), previous surgery for the presenting symptoms (36%), overall abnormalities on upper extremity electrodiagnostic testing (34%), mild lower trunk brachial plexopathy on electrodiagnostic testing (6%), current use of opioid pain medications (15%), or >3 patient-reported allergies (17%) (Table 1). The types of previous injury precipitating symptoms included competitive sports or performance music (14%), repetitive strain activity (12%), motor vehicle collision (10%), and a fall on the arm (4%) (Figure 1A). Other surgical operations previously performed for the presenting symptoms included cervical spine procedures (14%), shoulder procedures (11%), carpal tunnel decompression (10%), cubital canal decompression (9%), and others (2%) (Figure 1B). Nearly 60% of patients had had symptoms for more than 2 years prior to referral (Figure 1C).
Table 1.
Presenting Characteristics of the Patient Population.
| SCD + PMT | Isolated PMT | P-value e | |
|---|---|---|---|
| Number of operations | 322 (91%) | 33 (9%) | |
| Patient age (years) | 35.6 ± 2.0 | 37.7 ± 6.6 | NS |
| Female gender | 251 (78%) | 19 (57%) | P < .05 |
| Right side affected | 176 (55%) | 16 (48%) | NS |
| Body mass index (kg/m2) | 28.1 ± 1.6 | 29.3 ± 5.1 | NS |
| History of injury precipitating symptoms | 150 (46%) | 20 (61%) | NS |
| Duration of symptoms > 2 years | 178 (55%) | 23 (70%) | NS |
| Bilateral NTOS symptoms | 27 (8%) | 6 (18%) | NS |
| Cervical rib or other bone anomaly | 28 (9%) | 0 (0%) | NS |
| Abnormal electrodiagnostic testing | 112 (35%) a | 10 (30%) b | NS |
| Previous other surgery for same symptoms | 113 (35%) | 14 (42%) | NS |
| Current opioid pain medications | 47 (15%) | 5 (15%) | NS |
| Patient-reported medication allergies > 3 | 58 (18%) | 4 (12%) | NS |
| Supraclavicular palpation | |||
| Tenderness (rated 0-3) | 1.4 ± 0.1 | 0.4 ± 0.1 | P < .05 |
| Reproduction of arm/hand symptoms | 314 (98%) | 7 (21%) | P < .05 |
| Subcoracoid palpation | |||
| Tenderness (rated 0-3) | 1.3 ± 0.1 | 1.6 ± 0.3 | P < .05 |
| Reproduction of arm/hand symptoms | 302 (94%) | 33 (100%) | NS |
| Provocative maneuvers | |||
| Upper limb tension test positive | 306 (95%) | 29 (88%) | NS |
| Duration of 3-min EAST (s) | 120 ± 7 | 143 ± 25 | P < .05 |
| Diagnosis and disability | |||
| Total number of clinical diagnostic criteria c | 10.2 ± 0.6 | 9.5 ± 1.7 | P < .05 |
| QuickDASH score (0-100) | 60.6 ± 3.4 | 57.7 ± 10.5 | NS |
| Able to work or attend school | 230 (71%) | 22 (67%) | NS |
| Preoperative muscle block d | |||
| Muscle block performed | 111 (34%) | 14 (42%) | NS |
| Proportion of muscle blocks positive | 104 (94%) | 10 (71%) | P < .05 |
EAST, 3-min elevated arm stress test; QuickDASH, 11-item version of the Disabilities of the Arm, Shoulder, and Hand survey; PMT, pectoralis minor tenotomy; SCD, supraclavicular decompression; CORE-TOS, Consortium for Outcomes Research and Education on Thoracic Outlet Syndrome; NTOS, neurogenic thoracic outlet syndrome.
Patients undergoing primary surgical treatment for NTOS from January 1, 2020 to July 1, 2023. Data shown indicate the mean ± SE or the number and proportion (%) of patients in the study population with various presenting characteristics.
Mild brachial plexopathy (n = 19, 6%), carpal tunnel syndrome (n = 59, 18%), cubital canal syndrome (n = 14, 4%), cervical radiculopathy (n = 17, 5%), or other (n = 3, 1%).
Mild brachial plexopathy (n = 4, 12%), carpal tunnel syndrome (n = 3, 9%), or cubital canal syndrome (n = 3, 9%).
Total number of 14 criteria met in accord with the CORE-TOS Clinical Diagnostic Criteria for NTOS.10,11
Muscle blocks were performed by imaging-guided local anesthetic injection into the anterior scalene and/or pectoralis minor muscles. 33
Comparison between SCD + PMT versus isolated PMT groups, unpaired student t-test (continuous variables) or Fisher’s exact test (categorical variables); NS, P > .05.
Figure 1.
Presenting characteristics of the study population. (A) Pie chart showing the proportion of patients with various types of previous injury prior to development of the presenting symptoms. (B) Pie chart showing the proportion of patients having had various previous surgical procedures for the presenting symptoms, prior to referral. (C) Bar graph depicting the duration of symptoms prior to referral for NTOS.
C-spine, cervical spine; CTS, carpal tunnel syndrome; MVC, motor vehicle collision; PMT, pectoralis minor tenotomy; SCD, supraclavicular decompression; NTOS, neurogenic thoracic outlet syndrome.
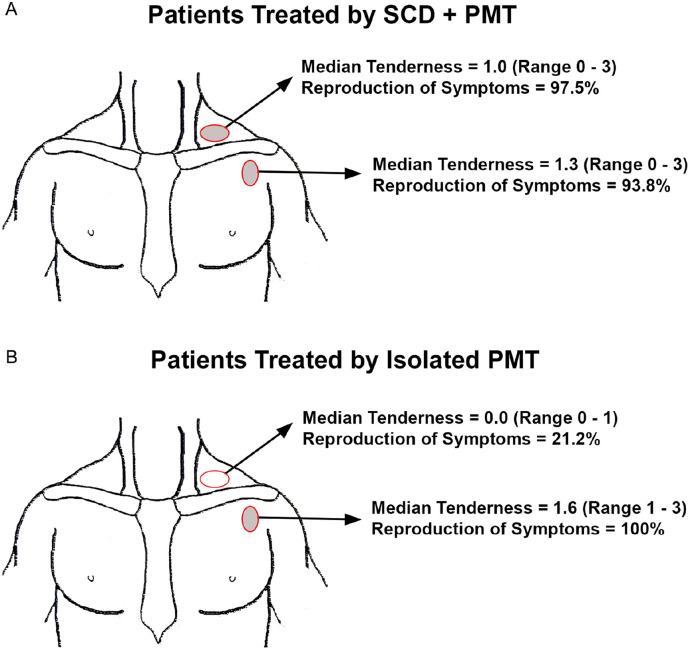
Each patient presented with symptoms consisting of pain, numbness, and paresthesia affecting the neck and upper extremity. Characteristic physical examination findings of localized tenderness in the supraclavicular and/or subcoracoid spaces, with reproduction of upper extremity symptoms upon palpation, were crucial in diagnosis and in determining the principal site(s) of brachial plexus compression. Using a 0-3 point scale on examination, patients in the SCD + PMT group had significant tenderness and reproduction of symptoms upon palpation at the supraclavicular space (mean rating 1.4 ± 0.1, 98% reproduction) as well as the subcoracoid space (mean rating 1.3 ± 0.1, 94% reproduction), whereas those in the isolated PMT group had minimal tenderness or reproduction of symptoms upon palpation at the supraclavicular space (mean rating 0.4 ± 0.1, 21% reproduction) but significant findings at the subcoracoid space (mean rating 1.6 ± 0.3, 100% reproduction) (Table 1 and Figure 2). The mean duration of the 3-min Elevated Arm Stress Test was somewhat lower in the SCD + PMT group compared to the isolated PMT group (120 ± 7 vs 143 ± 25 s, P < .05) and the total number of positive diagnostic criteria was slightly greater for the SCD + PMT group (10.2 ± 0.6 vs 9.5 ± 1.7, P < .05) (Table 1). The overall mean QuickDASH score upon referral was 60.3 ± 3.2, reflecting a substantial level of disability, but there was no difference In QuickDASH scores between the SCD + PMT and isolated PMT groups (Table 1). Overall there were 125 patients (35%) that had an anterior scalene and/or pectoralis minor muscle anesthetic block following initial clinical assessment. There was no difference in the utilization of muscle blocks between groups (SCD + PMT, 34%; isolated PMT, 42%; P > .05), but there was a higher proportion of positive muscle blocks in the SCD + PMT group compared to the isolated PMT group (104 of 111, 94% vs 10 of 14, 71%; P < .05) (Table 1).
Figure 2.
Tenderness to palpation. Illustrations demonstrating the sites of localized tenderness to palpation and reproduction of symptoms on initial physical examination, in patients that underwent SCD + PMT (A) and those underwent isolated PMT (B).
PMT, pectoralis minor tenotomy; SCD, supraclavicular decompression.
All patients underwent successful SCD and/or PMT with no intraoperative complications. As expected, the operation time was longer in the SCD + PMT group compared to isolated PMT (164.5 ± 9.2 vs 29.3 ± 5.1 min, P < .05), as was the hospital length of stay (4.0 ± 0.2 vs 0.3 ± 0.1 days, P < .05) (Table 2). There were no significant differences between the SCD + PMT and isolated PMT groups with respect to the proportion of those having prolonged hospital stays more than 6 days, postoperative complications, early reoperations, or 30-day readmissions, largely due to the low incidence of these events.
Table 2.
Operative Treatment.
| SCD + PMT | Isolated PMT | P-value a | |
|---|---|---|---|
| Number of operations | 322 (91%) | 33 (9%) | |
| Operative time (min) | 164.5 ± 9.2 | 29.3 ± 5.1 | P < .05 |
| Postoperative hospital stay (days) | 4.0 ± 0.2 | 0.3 ± 0.1 | P < .05 |
| Postoperative hospital stay > 6 days | 7 (2.2%) | 0 (0%) | NS |
| Postoperative complication | 2 (0.6%) | 0 (0%) | NS |
| 30-day hospital readmission | 12 (3.7%) | 0 (0%) | NS |
PMT, pectoralis minor tenotomy; SCD, supraclavicular decompression; NTOS, neurogenic thoracic outlet syndrome.
Patients undergoing primary surgical treatment for NTOS from January 1, 2020 to July 1, 2023. Data shown indicate the mean ± SE or the number and proportion (%) of patients in the study population.
Comparison between SCD + PMT versus isolated PMT groups, unpaired student t-test (continuous variables) or Fisher’s exact test (categorical variables); NS, P > .05.
The overall mean duration of follow-up after surgery was 26.7 ± 1.5 months (median 27.8, range 5-48 months) (Table 3). For the patients with complete follow-up data available (n = 325, 91%), the mean lowest follow-up QuickDASH score was 36.3 ± 2.0, reflecting a significant difference compared to preoperative QuickDASH scores, for both SCD + PMT and isolated PMT patients (P < .0001) (Figure 3A). For individual patients the mean decline in QuickDASH scores was 24.4 ± 1.4 and the mean percent improvement was 41.2% ± 2.3%. There were no differences between the SCD + PMT and isolated PMT groups with regard to the extent of decline or percent improvement in QuickDASH scores during follow-up.
Table 3.
Postoperative Follow-Up.
| SCD + PMT | Isolated PMT | P-value c | |
|---|---|---|---|
| Number of operations | 322 (91%) | 33 (9%) | |
| Mean duration of follow-up (months) | 27.9 ± 1.6 | 24.7 ± 4.3 | NS |
| Median, range of follow-up (months) | 28.9, 5-48 | 24.9, 7-46 | NS |
| Insufficient follow-up data | 25 (8%) | 5 (15%) | NS |
| Functional outcome measures | n = 297 | n = 28 | |
| Mean duration of follow-up (months) | 27.1 ± 1.6 | 23.0 ± 4.3 | NS |
| Median, range of follow-up (months) | 27.5, 5-47 | 24.7, 7-39 | NS |
| Preoperative QuickDASH score (0-100) | 61.1 ± 3.5 | 57.7 ± 10.5 | NS |
| Follow-up QuickDASH score (0-100) | 36.1 ± 2.1 b | 39.3 ± 7.1 b | NS |
| Decline in QuickDASH score | 25.0 ± 1.4 | 18.8 ± 3.5 | NS |
| Percent improvement in QuickDASH score | 41.8 ± 2.4% | 35.3 ± 6.7% | NS |
| Patient-rated outcomes a | n = 284 | n = 21 | |
| Follow-up Derkash score = excellent | 100 (35%) | 4 (19%) | NS |
| Follow-up Derkash score = good | 118 (42%) | 6 (28%) | NS |
| Follow-up Derkash score = fair | 59 (21%) | 7 (33%) | NS |
| Follow-up Derkash score = poor | 7 (2%) | 4 (19%) | P < .01 |
| Late reoperation | |||
| SC reoperation during follow-up | 16 (5.0%) | 5 (15.1%) | P < .05 |
| Interval to SC reoperation (months) | 19.1 ± 4.8 | 7.6 ± 3.4 | P < .05 |
PMT, pectoralis minor tenotomy; SCD, supraclavicular decompression; QuickDASH, 11-item version of the Disabilities of the Arm, Shoulder, and Hand survey; SC, supraclavicular; NTOS, neurogenic thoracic outlet syndrome.
Patients undergoing primary surgical treatment for NTOS from January 1, 2020 to July 1, 2023. Data shown indicate the mean ± SE or the number and proportion (%) of patients in the study population.
P < .0001, preoperative versus follow-up, paired student t-test.
Comparison between SCD + PMT versus isolated PMT groups, unpaired student t-test (continuous variables) or Fisher’s exact test (categorical variables); NS, P > .05.
Figure 3.
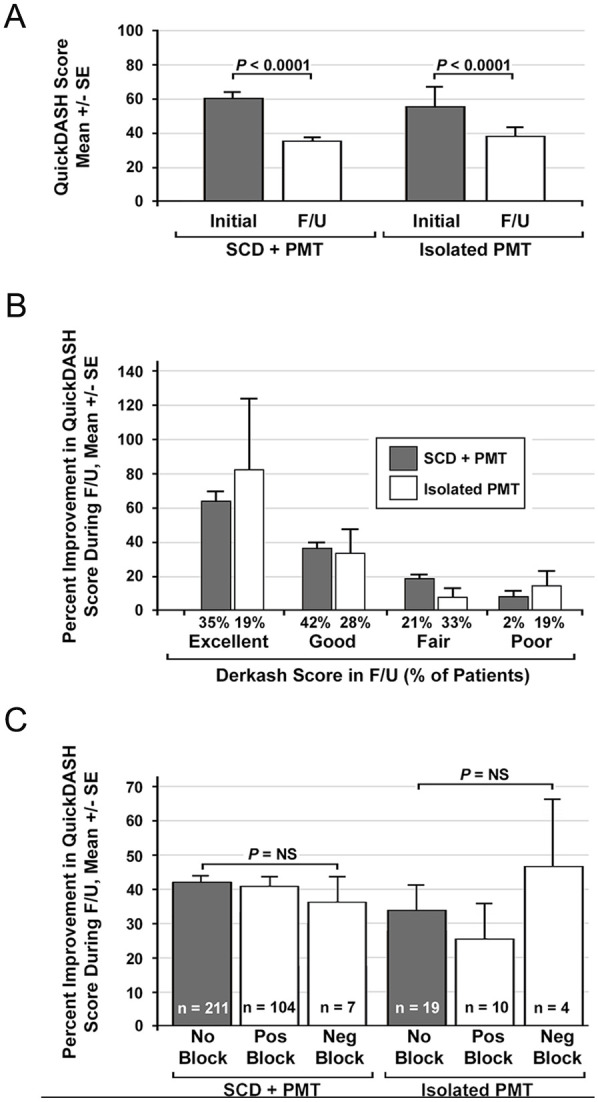
Outcome measures. (A) Bar graph comparing mean QuickDASH scores at initial presentation and in follow-up after surgical treatment. Comparisons were made using the paired student t-test. (B) Bar graph comparing the percent improvement in QuickDASH scores for different patient-reported outcome (Derkash score) groups for patients undergoing SCD + PMT and isolated PMT. (C) Bar graph comparing the percent improvement in QuickDASH scores for patients who did or did not have a previous ASM and/or PMM block with local anesthetic prior to undergoing SCD + PMT or isolated PMT.
ASM, anterior scalene muscle; F/U, follow-up; Neg, negative; NS, not significant (P > .05, unpaired student t-test); PMM, pectoralis minor muscle; PMT, pectoralis minor tenotomy; Pos, positive; QuickDASH, 11-item version of the Disabilities of the Arm, Shoulder, and Hand survey; SCD, supraclavicular decompression.
The assessment of patient-rated outcomes during follow-up corresponded with outcomes measured by changes in QuickDASH score, with 34% of patients rating their outcome as excellent, 41% as good, 22% as fair, and only 4% as poor (Table 3 and Figure 3B). The proportion of patients with outcomes rated as poor was significantly lower after SCD + PMT (2%) than after isolated PMT (19%) (P < .01). The percent improvement in follow-up QuickDASH scores was no different between patients that had undergone an anesthetic muscle block compared to those who had no muscle block, and for those who had a muscle block there was no difference in outcome measures between those who had positive versus negative blocks (Figure 3C).
During long-term follow-up, 16 patients (5.0%) in the SCD + PMT group and 5 patients (15.1%) in the isolated PMT group underwent later supraclavicular reoperation for recurrent symptoms (P < .05), at mean intervals of 19.1 ± 4.8 versus 7.6 ± 3.4 months after the initial operation, respectively (P < .05) (Table 3). No patient required reoperation solely for recurrent symptoms localized to the subcoracoid space.
Discussion
There is ample evidence that surgical treatment can have substantial benefit for patients with a sound clinical diagnosis of NTOS, disabling symptoms, and an unsatisfactory response to targeted physical therapy.11 -13 Successful outcomes can be achieved by either supraclavicular or transaxillary approaches, with 85% to 90% of patients having a substantial improvement in symptoms and function.12,17 -21 In the present study, we sought to elucidate the current utilization and effectiveness of PMT in patients undergoing primary surgical treatment for NTOS. One of the most important findings is to reinforce previous observations that the large majority of patients presenting with NTOS have physical examination evidence for symptomatic brachial plexus compression at both supraclavicular and subcoracoid sites, with fewer than 10% having compression solely at the subcoracoid space. This indicates that in most patients subcoracoid compression is not a primary cause of NTOS or a precursor to scalene triangle compression, but likely arises as a later secondary feature due to prolonged postural changes, chronic muscle spasm, and imbalances in shoulder girdle mechanics. How to best assess and manage brachial plexus compression at the pectoralis minor level is therefore an unresolved consideration in surgical treatment of NTOS.
Careful physical examination is crucial in determining the location of brachial plexus nerve compression in patients with NTOS, particularly for surgical planning. In expanding the use of telemedicine during the COVID-19 pandemic, we found it possible to establish a reliable clinical diagnosis of NTOS in part by physician-guided patient self-examination. 34 However, as our use of telemedicine progressed it became clearer that defining the site of brachial plexus compression by this approach was less reliable than desired, and in making recommendations for surgical treatment it was still necessary to have an in-person examination to distinguish those patients that were candidates for SCD + PMT versus isolated PMT. To avoid recommending isolated PMT for patients subsequently found to have significant tenderness at the scalene triangle, it has remained our recommendation to conduct an in-person preoperative physical examination before reaching a final determination of the surgical procedure to be performed.
Some specialists advocate routine use of scalene and/or pectoralis minor muscle injections with local anesthetic (or botulinum toxin) prior to considering surgical treatment.33,35 We have used muscle blocks much more selectively, both as an adjunct to clinical diagnosis and as a potential predictor of symptom responsiveness to treatment. During this study muscle blocks were performed in 35% of patients and were considered most helpful in patients with equivocal physical examination findings or concomitant conditions associated with overlapping symptoms, such as shoulder pathology or degenerative cervical spine disease, but not in patients with electrodiagnostic findings of brachial plexopathy, osseus abnormalities (eg, cervical ribs), or strong physical examination findings. It is of interest that we observed no difference in long-term outcome measures in patients with or without a previous muscle block, or between patients with a positive or negative block, in either the SCD + PMT or isolated PMT groups. These findings indicate that scalene and/or pectoralis minor muscle injections are not necessarily predictive of clinical outcomes and that clinical decisions should be primarily based on other factors.
With respect to surgical treatment, brachial plexus compression at the pectoralis minor level can be addressed either as part of a primary scalene triangle decompression or later as a secondary consideration, if symptoms are not sufficiently improved by the initial procedure. Indeed, unresolved/recurrent symptoms attributable to the pectoralis minor led to the reintroduction of PMT as a potential treatment for recurrent NTOS.22 -24 Isolated PMT can also be considered as the initial procedure, with scalene triangle decompression performed only if there is insufficient improvement. Sanders et al found that this approach is not optimal, as a substantial number of patients went on to require SCD after failing to improve after isolated PMT.23,25 Vemuri et al championed use of PMT as an adjunctive procedure to be conducted in combination with primary scalene triangle decompression, demonstrating excellent early outcomes. 27 The timing and sequence of scalene triangle decompression and PMT is therefore an unresolved consideration in surgical treatment of NTOS.
Isolated PMT is an attractive surgical option for patients with NTOS who present solely with subcoracoid space tenderness. Open operation with an incision in the deltopectoral groove allows for easy exposure and complete division of the tendinous attachment of the pectoralis minor to the coracoid process. Additional maneuvers require seeking the “costocoracoid ligament” and/or fascia of the subclavius muscle laterally, as well as addressing additional anatomic variations, such as Langer’s axillary arch, but extensive neurolysis of the brachial plexus is not necessary. Isolated PMT is typically an outpatient operation, requiring less operative time and with fewer potential complications than combined SCD + PMT, and it can also be conducted under monitored local anesthesia if desired. 30 These factors may bias providers toward performing isolated PMT as a more attractive intervention for patients who are older or have more medical comorbidity, or perhaps in younger, athletic patients who wish to attempt an earlier return to full activity. We did not find any measurable selection bias toward recommending isolated PMT in this study and there were generally no differences in demographic or preoperative characteristics between the two groups.
Recurrence requiring supraclavicular reoperation can occur after any primary operation for NTOS, most typically related to a retained first rib or long first rib remnant(s), reattachment of partially resected scalene muscle, or fibrous perineural scar tissue around the brachial plexus.36,37 It has been previously reported that the recurrence rate after anatomically complete SCD (combined with PMT) is approximately 5%, with recurrences following transaxillary first rib resection as high as 20% to 30%.21,38,39 The recurrence rate (5%) observed for SCD combined with PMT in the present study is thereby consistent with previous reports. In contrast, the 3-fold higher recurrence rate requiring SCD after isolated PMT (15%), even in this highly selected patient group, raises concern that this “minimally invasive” procedure may be more frequently incomplete than previously recognized. This finding, along with the higher frequency of poor-rated outcomes, suggests that with less rigorous patient selection for isolated PMT one can expect an even greater level of dissatisfying outcomes and the need for reoperation. This should serve as a caution against expanding the use of isolated PMT in patients with NTOS. While recent reports have described other minimally invasive approaches for treating NTOS centered around arthroscopic PMT and/or endoscopic neurolysis of the brachial plexus, we recommend these procedures be conducted in the context of well-designed clinical trials with appropriate quantitative endpoints. 40
One limitation of this and other studies is the reliance on QuickDASH scores and Derkash ratings as outcome measures to assess symptomatic improvement after surgical treatment. Such measurements taken at different times after surgery may vary with fluctuating symptoms that might be present at any given follow-up visit. Patient-specific factors, such as some individuals being more aggressive with physical therapy or having greater adherence to postoperative restrictions, may also influence these outcome scores. Finally, QuickDASH and Derkash scores do not easily distinguish laterality, such that bilateral symptoms can complicate accurate measurements of surgical outcomes. We expect to develop more robust quantitative measures in the future to address some of these limitations.
Conclusions
Pectoralis minor tenotomy (PMT) has an important role in the surgical treatment of NTOS, mainly as an adjunct combined with SCD. While highly selected patients can do well after isolated PMT as a short outpatient procedure with rapid recovery, there is a greater potential for poor outcomes and the need for supraclavicular reoperation than after SCD + PMT. These observations emphasize that isolated PMT should be considered with caution in highly selected circumstances, in which physicians and patients understand the limitations of this approach and the significant potential need for further treatment should outcomes not meet expectations.
Footnotes
Ethical Approval: This study was approved by the Institutional Review Board at our center.
Statement of Human and Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Written informed consent was obtained from all patients for being included in the study.
Statement of Informed Consent: Written informed consent was obtained from all individual participants included in the study.
Data Availability Statement: Anonymized data that support the findings of this study are available from the corresponding author upon reasonable request and after local institutional review board approval for data sharing.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported in part by the Thoracic Outlet Syndrome Research and Education Fund of the Foundation for Barnes Jewish Hospital, BJC Healthcare, St. Louis, Missouri.
ORCID iD: Robert W. Thompson  https://orcid.org/0000-0002-2609-1284
https://orcid.org/0000-0002-2609-1284
References
- 1. Povlsen B, Hansson T, Povlsen SD. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev. 2014;11:CD007218. doi: 10.1002/14651858.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lim C, Kavousi Y, Lum YW, Christo PJ. Evaluation and management of neurogenic thoracic outlet syndrome with an overview of surgical approaches: a comprehensive review. J Pain Res. 2021;14:3085-3095. doi: 10.2147/JPR.S282578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Panda N, Hurd J, Madsen J, et al. Efficacy and safety of supraclavicular thoracic outlet decompression. Ann Surg. 2023;278(3):417-425. [DOI] [PubMed] [Google Scholar]
- 4. Roos DB. Congenital anomalies associated with thoracic outlet syndrome: anatomy, symptoms, diagnosis, and treatment. Am J Surg. 1976;132(6):771-778. doi: 10.1016/0002-9610(76)90456-6 [DOI] [PubMed] [Google Scholar]
- 5. Connolly MR, Auchincloss HG. Anatomy and embryology of the thoracic outlet. Thorac Surg Clin. 2021;31(1):1-10. doi: 10.1016/j.thorsurg.2020.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Machleder HI, Moll F, Verity MA. The anterior scalene muscle in thoracic outlet compression syndrome: histochemical and morphometric studies. Arch Surg. 1986;121(10):1141-1144. doi: 10.1001/archsurg.1986.01400100047009 [DOI] [PubMed] [Google Scholar]
- 7. Sanders RJ, Jackson CG, Banchero N, Pearce WH. Scalene muscle abnormalities in traumatic thoracic outlet syndrome. Am J Surg. 1990;159(2):231-236. doi: 10.1016/S0002-9610(05)80269-7 [DOI] [PubMed] [Google Scholar]
- 8. Dubuisson A, Lamotte C, Foidart-Dessalle M, et al. Post-traumatic thoracic outlet syndrome. Acta Neurochir (Wien). 2012;154(3):517-526. doi: 10.1007/s00701-011-1269-x [DOI] [PubMed] [Google Scholar]
- 9. Illig KA, Donahue DM, Duncan A, et al. Reporting standards of the Society for Vascular Surgery for thoracic outlet syndrome. J Vasc Surg. 2016;64(3):e23-e35. doi: 10.1016/j.jvs.2016.04.039 [DOI] [PubMed] [Google Scholar]
- 10. Thompson RW. Diagnosis of neurogenic thoracic outlet syndrome: 2016 consensus guidelines and other strategies. In: Illig KA, Thompson RW, Freischlag JA, et al. , eds. Thoracic Outlet Syndrome (TOS). 2nd ed. Springer Nature; 2021:67-97. [Google Scholar]
- 11. Balderman J, Holzem K, Field BJ, et al. Associations between clinical diagnostic criteria and pre-treatment patient-reported outcomes measures in a prospective observational cohort of patients with neurogenic thoracic outlet syndrome. J Vasc Surg. 2017;66(2):533-544.e2. doi: 10.1016/j.jvs.2017.03.419 [DOI] [PubMed] [Google Scholar]
- 12. Balderman J, Abuirqeba AA, Eichaker L, et al. Physical therapy management, surgical treatment, and patient-reported outcomes measures in a prospective observational cohort of patients with neurogenic thoracic outlet syndrome. J Vasc Surg. 2019;70(3):832-841. doi: 10.1016/j.jvs.2018.12.027 [DOI] [PubMed] [Google Scholar]
- 13. Goeteyn J, Pesser N, Houterman S, van Sambeek MRHM, van Nuenen BFL, Teijink JAW. Surgery versus continued conservative treatment for neurogenic thoracic outlet syndrome: the first randomised clinical trial (STOPNTOS Trial). Eur J Vasc Endovasc Surg. 2022;64(1):119-127. doi: 10.1016/j.ejvs.2022.05.003 [DOI] [PubMed] [Google Scholar]
- 14. Jayaraj A, Duncan AA, Kalra M, Bower TC, Gloviczki P, Outcomes of transaxillary approach to cervical and first-rib resection for neurogenic thoracic outlet syndrome. Ann Vasc Surg. 2018;51:147-149. doi: 10.1016/j.avsg.2018.02.029 [DOI] [PubMed] [Google Scholar]
- 15. Thompson RW, Ohman JW. Surgical techniques: operative decompression using the supraclavicular approach for neurogenic thoracic outlet syndrome. In: Illig KA, Thompson RW, Freischlag JA, et al. , eds. Thoracic Outlet Syndrome (TOS). 2nd ed. Springer Nature; 2021:265-285. [Google Scholar]
- 16. Teijink SBJ, Goeteyn J, Pesser N, van Nuenen BFL, Thompson RW, Teijink JAW. Surgical approaches for thoracic outlet decompression in the treatment of thoracic outlet syndrome. J Thorac Dis. 2023;15(12):7088-7099. doi: 10.21037/jtd-23-546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caputo FJ, Wittenberg AM, Vemuri C, et al. Supraclavicular decompression for neurogenic thoracic outlet syndrome in adolescent and adult populations. J Vasc Surg. 2013;57(1):149-157. doi: 10.1016/j.jvs.2012.07.025 [DOI] [PubMed] [Google Scholar]
- 18. Rochlin DH, Gilson MM, Likes KC, et al. Quality-of-life scores in neurogenic thoracic outlet syndrome patients undergoing first rib resection and scalenectomy. J Vasc Surg. 2013;57(2):436-443. doi: 10.1016/j.jvs.2012.08.112 [DOI] [PubMed] [Google Scholar]
- 19. Nejim B, Alshaikh HN, Arhuidese I, et al. Perioperative outcomes of thoracic outlet syndrome surgical repair in a nationally validated database. Angiology. 2017;68(6):502-507. doi: 10.1177/0003319716677666 [DOI] [PubMed] [Google Scholar]
- 20. Ransom EF, Minton HL, Young BL, et al. Intermediate and long-term outcomes following surgical decompression of neurogenic thoracic outlet syndrome in an adolescent patient population. Hand (N Y). 2022;17(1):43-49. doi: 10.1177/1558944719901319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Talutis SD, Ulloa JG, Gelabert HA. Adolescent athletes can get back in the game after surgery for thoracic outlet syndrome. J Vasc Surg. 2023;77(2):599-605. doi: 10.1016/j.jvs.2022.10.002 [DOI] [PubMed] [Google Scholar]
- 22. Ambrad-Chalela E, Thomas GI, Johansen KH. Recurrent neurogenic thoracic outlet syndrome. Am J Surg. 2004;187(4):505-510. doi: 10.1016/j.amjsurg.2003.12.050 [DOI] [PubMed] [Google Scholar]
- 23. Sanders RJ, Rao NM. The forgotten pectoralis minor syndrome: 100 operations for pectoralis minor syndrome alone or accompanied by neurogenic thoracic outlet syndrome. Ann Vasc Surg. 2010;24(6):701-708. doi: 10.1016/j.avsg.2010.02.022 [DOI] [PubMed] [Google Scholar]
- 24. Sanders RJ. Recurrent neurogenic thoracic outlet syndrome stressing the importance of pectoralis minor syndrome. Vasc Endovascular Surg. 2011;45(1):33-38. doi: 10.1177/1538574410388311 [DOI] [PubMed] [Google Scholar]
- 25. Sanders RJ, Annest SJ, Goldson E. Neurogenic thoracic outlet and pectoralis minor syndromes in children. Vasc Endovasc Surg. 2013;47(5):335-341. doi: 10.1177/1538574413481858 [DOI] [PubMed] [Google Scholar]
- 26. Sanders RJ, Annest SJ. Pectoralis minor syndrome: subclavicular brachial plexus compression. Diagnostics (Basel). 2017;7(3):46. doi: 10.3390/diagnostics7030046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vemuri C, Wittenberg AM, Caputo FJ, et al. Early effectiveness of isolated pectoralis minor tenotomy in selected patients with neurogenic thoracic outlet syndrome. J Vasc Surg. 2013;57(5):1345-1352. doi: 10.1016/j.jvs.2012.11.045 [DOI] [PubMed] [Google Scholar]
- 28. Ammi M, Péret M, Henni S, et al. Frequency of the pectoralis minor compression syndrome in patients treated for thoracic outlet syndrome. Ann Vasc Surg. 2018;47:253-259. doi: 10.1016/j.avsg.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 29. Hudak PL, Amadio PC, Bombardier C, et al. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder, and hand). The Upper Extremity Collaborative Group (UECG). Am J Ind Med. 1996;29(6):602-608.<602::AID-AJIM4>3.0.CO;2-L [DOI] [PubMed] [Google Scholar]
- 30. Vemuri C, Thompson RW. Surgical techniques: pectoralis minor tenotomy for neurogenic thoracic outlet syndrome. In: Illig KA, Thompson RW, Freischlag JA, et al. , eds. Thoracic Outlet Syndrome (TOS). 2nd ed. Springer Nature; 2021:295-301. [Google Scholar]
- 31. Derkash RS, Goldberg VM, Mendelson H, Mevicker R. The results of first rib resection in thoracic outlet syndrome. Orthopedics. 1981;4(9):1025-1029. doi: 10.3928/0147-7447-19810901-08 [DOI] [PubMed] [Google Scholar]
- 32. Lingyun W, Ke S, Jinmin Z, Yu Q, Jun Q. Derkash's classification and VAS visual analog scale to access the long-term outcome of neurothoracic outlet syndrome: a meta-analysis and systematic review. Front Neurol. 2022;13:899120. doi: 10.3389/fneur.2022.899120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lum YW, Brooke BS, Likes K, et al. Impact of anterior scalene lidocaine blocks on predicting surgical success in older patients with neurogenic thoracic outlet syndrome. J Vasc Surg. 2012;55(5):1370-1375. doi: 10.1016/j.jvs.2011.11.132 [DOI] [PubMed] [Google Scholar]
- 34. Ohman JW, Annest SJ, Azizzadeh A, et al. Evaluation and treatment of thoracic outlet syndrome during the global pandemic due to SARS-CoV-2 and COVID-19. J Vasc Surg. 2020;72(3):790-798. doi: 10.1016/j.jvs.2020.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Donahue DM, Godoy IRB, Gupta R, Donahue JA, Torriani M. Sonographically guided botulinum toxin injections in patients with neurogenic thoracic outlet syndrome: correlation with surgical outcomes. Skeletal Radiol. 2020;49(5):715-722. doi: 10.1007/s00256-019-03331-9 [DOI] [PubMed] [Google Scholar]
- 36. Phillips WW, Donahue DM. Reoperation for persistent or recurrent neurogenic thoracic outlet syndrome. Thorac Surg Clin. 2021;31(1):89-96. doi: 10.1016/j.thorsurg.2020.08.011 [DOI] [PubMed] [Google Scholar]
- 37. Jammeh ML, Ohman JW, Vemuri C, Abuirqeba AA, Thompson RW. Anatomically complete supraclavicular reoperation for recurrent neurogenic thoracic outlet syndrome: clinical characteristics, operative findings, and long-term outcomes. Hand (NY). 2022;17(6):1055-1064. doi: 10.1177/1558944720988079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Altobelli GG, Kudo T, Haas BT, Chandra FA, Moy JL, Ahn SS. Thoracic outlet syndrome: pattern of clinical success after operative decompression. J Vasc Surg. 2005;42(1):122-128. doi: 10.1016/j.jvs.2005.03.029 [DOI] [PubMed] [Google Scholar]
- 39. Jammeh ML, Yang A, Abuirqeba AA, Ohman JW, Thompson RW. Reoperative brachial plexus neurolysis after previous anatomically complete supraclavicular decompression for neurogenic thoracic outlet syndrome: a 10-year single-center case series. Oper Neurosurg (Hagerstown). 2022;23(2):125-132. doi: 10.1227/ons.0000000000000252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haeni D, Martinez-Catalan N, Esper RN, Wagner ER, El Hassan BT, Sanchez-Sotelo J. Arthroscopic release of the pectoralis minor tendon from the coracoid for pectoralis minor syndrome. J Exp Orthop. 2022;9(1):57. doi: 10.1186/s40634-022-00491-x [DOI] [PMC free article] [PubMed] [Google Scholar]