Abstract
Background
The Surpass Evolve (SE) has emerged as a promising alternative treatment from the flow diverter series. The utilization of the SE has gradually increased, however, there is a scarcity of comprehensive data on the solidity of this technology in the endovascular treatment of intracranial aneurysms (IAs). This meta-analysis aimed to evaluate the safety and effectiveness of the SE flow diverter.
Methods
A systematic literature search from inception to April 2024 was conducted across five databases for studies involving IAs treated with the SE. The primary effectiveness outcome was the proportion of complete aneurysm occlusion at the final follow-up, and the primary safety outcome comprised a composite of early and delayed complications. Subgroup analyses based on aneurysm size, anatomical location, and rupture status were also conducted.
Results
Our analysis included nine studies with 645 patients and 722 IAs. Effectiveness outcomes revealed an overall complete aneurysm occlusion rate of 69% (95% confidence interval (CI) = 58%–78%; I2 = 72%) and a favorable aneurysm occlusion rate of 91% (95% CI = 82%–96%; I2 = 49%). Safety outcomes demonstrated an overall complications rate of 6% (95% CI = 3%–12%; I2 = 66%), with an early complications rate of 6% (95% CI = 4%–11%; I2 = 0%), and a delayed complications rate of 0% (95% CI = 0%–7%; I2 = 0%).
Conclusions
Our findings suggest a favorable outcome with a high rate of complete aneurysm occlusion at the last follow-up, with acceptable rates of neurological complications. Future research efforts should focus on larger, prospective studies with standardized outcome measures to further elucidate the clinical utility of the SE flow diverter in the management of IAs.
Keywords: Surpass Evolve, flow diverter, intracranial aneurysm, endovascular treatment
Introduction
The field of interventional neurosurgery has been revolutionized by the introduction of flow diverters for the endovascular treatment of intracranial aneurysms (IAs).1–3 Since their market debut in 2007, flow-diverting stent technology has significantly evolved, yielding advanced models that enhance operational flexibility, patient safety, and treatment efficacy.4,5 Among these advancements, the Surpass Evolve (SE) by Stryker Neurovascular is a treatment alternative that has lower profile features while preserving flow redirection dynamics.6–8 Distinguished by its 64-wires, the SE can be delivered through a 0.027 microcatheter and has enhanced flexibility and navigability, favoring complete aneurysm neck coverage after deployment.6,7,9 Furthermore, it has a fine mesh design, characterized by a rhomboid cell shape which enables a more consistent flow-diverting effect through the aneurysm's neck to promote intra-aneurysmal flow stasis. 7 These features mark an improvement over the surpass streamline, its predecessor, thereby broadening therapeutic avenues for aneurysms that were previously challenging to treat,7,9,10 such as distally located lesions with tortuous anatomy.9,11,12
Since the SE Food and Drug Administration's approval in 2018, the utilization of this device has gradually increased, as noted by the current literature.7,12 Several retrospective studies have reported varying results regarding its safety and effectiveness, although many of these studies are limited by sample size.9,10,13,14 It is crucial to conduct a comprehensive, high-quality meta-analysis that integrates the diverse evidence gathered to date for a thorough and unbiased assessment of this technology's reliability in the endovascular treatment of IAs. Therefore, this systematic review and meta-analysis aimed to evaluate the safety and effectiveness of the SE flow diverter in treating IAs. Furthermore, we conducted comparisons of these outcomes across subgroups stratified by aneurysm size, anatomical location, and rupture status at presentation.
Materials and methods
Protocol and guidance
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guidelines. 15 The study protocol and methods were registered with PROSPERO a priori (CRD42024506429).
Search strategy and eligibility criteria
A systematic literature search was conducted by a medical librarian (CMM) across multiple databases including the Cochrane Library, Ovid Embase, PubMed, Scopus, and Web of Science. The search aimed to identify relevant articles published from the earliest records available in each database up to April 2024. Search algorithms were tailored to the specifications of each database, incorporating keywords such as “aneurysm” or “flow diverter.” Further details of the complete search strategy are provided in Supplemental Table 1.
Included studies utilized a randomized controlled trial or non-randomized design (including open-label trials, real-world or cohort observational studies, and case series) that investigated the safety and effectiveness of the SE and were conducted in adult patients (≥18 years) with ruptured or unruptured IAs in any location. Studies were excluded if they were review articles, abstracts, editorials, letters, animal studies, or case reports.
Study selection and data collection process
The search strategy was systematically applied to each database. All retrieved records were managed using EndNote X9 software, where duplicate entries were identified and removed. Subsequently, the records were exported in .XML format from EndNote X9 to Mendeley for further organization. Mendeley facilitated the conversion of data into RIS format, enabling utilization of the Rayyan tool (https://www.rayyan.ai/) for expert revision. Duplicate articles were meticulously eliminated, and two independent reviewers (NMCH and DEM) conducted an initial screening of titles and abstracts, adhering strictly to the predefined inclusion criteria to identify potentially relevant articles. Any discrepancies between reviewers were resolved through initial discussion, and in cases of persistent disagreement, a third reviewer (NMCH) acted as an arbitrator to reach a consensus.
The extracted data included the following information: year of publication, study design, patient characteristics (age, sex, and comorbidities), procedural specifics (access and number of flow diverters implanted), adjunctive treatments (coiling, balloon angioplasty, and stent), follow-up time, and the prioritized outcomes.
Outcomes and prioritization
The primary effectiveness outcome was determined as the proportion of complete aneurysm occlusion at the final follow-up. Complete occlusion was defined either as a Raymond-Roy class I 16 or O’Kelly Marotta scale Class D. 17 Secondary effectiveness outcomes included technical success, favorable clinical outcome (modified Rankin Scale score 0–2 at the last follow-up), retreatment, and favorable aneurysm occlusion, defined as achieving either a Raymond-Roy class I or II or O’Kelly Marotta scale Class C or D.
The primary safety outcome comprised a composite of both early (<30 days after the procedure) and delayed (>30 days after the procedure) neurological complications (ischemic or hemorrhagic). Secondary safety outcomes encompassed intraprocedural neurologic complications (thromboembolic/ischemic and hemorrhagic), all-cause mortality during follow-up, the occurrence of intraprocedural or during follow-up in-stent stenosis, and the assessment of early and delayed neurological complications independently.
Risk of bias in individual studies
The quality of the studies included in this analysis was evaluated based on the guidelines outlined in the Cochrane Reviewers’ Handbook. 18 For the methodological assessment of non-randomized studies, two independent reviewers (ARC and DEM) utilized the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool. 19
Data synthesis
A meta-analysis was conducted when there were at least three studies reporting the same effect estimate for a specific outcome. To estimate the pooled rates and the 95% confidence intervals (CIs) of the selected outcomes, we used a random-effects meta-analysis of proportions using a generalized linear mixed model. We utilized the Cochran Q and I2 tests to evaluate statistical heterogeneity. An I2 statistic with values of >25%, >50%, and >75% indicated low, considerable, and substantial levels of heterogeneity, respectively. 20 To assess publication bias, we visually inspected funnel plots for any signs of asymmetry. 21 To further assess complete aneurysm occlusion rates, we performed pre-specified subgroup analyses by aneurysm rupture status (acutely ruptured vs. unruptured), size (≥10 mm vs. <10 mm), and by location (anterior vs. posterior circulation). To determine subgroup differences, statistical significance was considered at a P-value ≤ 0.05. All analyses and plots were generated using R statistical software (version 4.1.3) and R Studio.
Results
Study selection and characteristics
The search yielded a total of 214 documents, after which 121 duplicates were removed (Supplemental Figure 1). Following the removal of duplicates, the titles and abstracts of 93 articles were screened for relevance, after which 37 potentially eligible documents remained. In the full-text evaluation, 30 articles were excluded (Supplemental Table 2). Finally, nine studies with 645 patients harboring 722 IAs treated with the SE flow diverter were included in the analysis.
Out of nine studies, five were single-center13,22–25 and four studies were multicenter.9,10,14,26 Six studies were retrospective and two studies were prospective (Table 1). The mean age ranged from 28 to 86 and 80% of the population were females (514 out of 634). Overall, 7% (50 out of 683) of aneurysms were ruptured at presentation. The vast majority of IAs were saccular (626 out of 647, 96.8%). The mean aneurysm size ranged from 3.3 to 18.4 mm. Aneurysms were located more commonly in the anterior circulation (651 out of 722, 90%), being larger the number of aneurysms located in the internal carotid artery (466 out of 722, 64.5%), followed by the middle cerebral artery (40 out of 722, 5.5%). In 40% (221 out of 556) of all IAs, adjunctive treatment was performed (Table 2), balloon angioplasty in 66% (145 out of 221), followed by coiling in 24% (51 out of 221). Dual antiplatelet therapy with aspirin and clopidogrel starting 1 to 14 days before the procedure was the predominant pre-procedural antiplatelet regimen (Supplemental Table 3).
Table 1.
Characteristics of the selected articles.
| Study | Study design | Study scale | Country | Study period | No. of patients | No. of aneurysms |
|---|---|---|---|---|---|---|
| Orru et al. 28 | Prospective | Multicenter study | United States, Canada | 2019 | 25 | 26 |
| Maus et al. 30 | Retrospective | Multicenter study | Germany | 2019–2020 | 42 | 46 |
| Jee et al. 27 | Retrospective | Single-center study | Republic of Korea | 2019–2020 | 31 | 31 |
| Lee et al. 23 | Retrospective | Single-center study | Republic of Korea | 2013–2020 | 5 | 5 |
| Rautio et al. 37 | Retrospective | Multicenter study | Finland | 2019–2020 | 29 | 30 |
| Bibi et al. 22 | Prospective | Single-center study | France | 2019–2022 | 116 | 120 |
| Sayin et al. 24 | Retrospective | Single-center study | Turkey | 2019–2022 | 41 | 52 |
| Vivanco-Suarez et al. 26 | Retrospective | Multicenter registry | United States, Europe | 2020–2022 | 305 | 332 |
| Gupta et al. 25 | Retrospective | Single-center study | United States | 2020–2022 | 51 | 80 |
Table 2.
Participant demographic data, aneurysm, and procedural characteristics.
| Study | Demographics | Aneurysm | Procedure | Follow-up time, months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | Female (%) | Ruptured (%) | Saccular (%) | Size (mm) | Anterior circ. (%) | Posterior circ. (%) | FD per patient | Adj. coils (%) | Adj. balloon angioplasty (%) | Adj. stenting (%) | ||
| Orru et al. 28 | 58 (36–86) | 20 (80) | 0 (0) | 22 (84.7) | 11 (3–30) | 25 (96.1) | 1 (3.8) | 1.12 | 2 (7.7) | 7 (26.9) | 2 (7.7) | 4 [0.1–6] |
| Maus et al. 30 | 58 (28–84) | 32 (76.2) | 6 (13) | 30 (65.2) | 6.6 [4–12.2] | 41 (89.1) | 5 (10.9) | — | 17 (36.9) | 12 (26) | 1 (2.2) | 4 [3.2–4.8] |
| Jee et al. 27 | 56.3 ± 12.2 | 15 (48.4) | — | — | 18.4 ± 7.6 | 20 (64.5) | 11 (35.5) | 1.03 | — | 8 (25.8) | 2 (6.5) | 6 |
| Lee et al. 23 | — | 0 (0) | — | — | 12.8 ± 3.8 | 0 (0) | 5 (100) | — | — | — | — | 6 |
| Rautio et al. 37 | 55.5 (32–72) | 21 (72.4) | 5 (17.2) | 28 (93.3) | 10 ± 7.7 | 24 (80) | 6 (20) | 1.07 | 2 (6.9) | — | — | 6 |
| Bibi et al. 22 | 55 (47–63) | 93 (80.2) | 7 (5.8) | 116 (96.7) | 6.6 ± 4.2 | 102 (85) | 18 (15) | 1.03 | 11 (9.2) | — | 12 (10) | 6 |
| Sayin et al. 24 | 56.3 (37–78) | 29 (70.7) | 4 (7.7) | 45 (86.5) | 3.3 (1.5–15.8) | 47 (90.3) | 5 (9.6) | 1.00 | — | — | — | 16.2 ± 6.6 (6–28) |
| Vivanco-Suarez et al. 26 | 59 [50–67] | 256 (83.9) | 20 (6) | 309 (93) | 5.1 [3.4–9] | 316 (95.1) | 16 (4.8) | 1.00 | 20 (6.6) | 118 (38.7) | 7 (2.3) | 10.2 [6.4–12.9] |
| Gupta et al. 25 | 57.3 ± 12.2 | 48 (86.3) | 8 (10) | 76 (95) | 5.6 | 76 (95) | 4 (5) | 1.07 | — | — | — | 11.4 |
Values within parentheses indicate the range, while values within brackets indicate the interquartile range.
Adj: adjunctive; circ: circulation; FD: flow diverter; y: years.
Risk of bias
The assessment outcomes utilizing the risk of bias in non-randomized studies of interventions framework for non-randomized investigations9,10,14,22–27 indicated a critical level of bias (Supplemental Figure 2). The majority of these studies (77%) were conducted retrospectively, introducing selection bias and a failure to adjust for potential confounders. None of the research included reported any deviation from the planned intervention protocols. However, seven studies9,10,14,23–25 were identified with incomplete data in several sections, leading to a critical assessment due to the absence of essential data pertinent to the outcomes analyzed in the meta-analysis. A considerable proportion (44.4%) of the studies were conducted across multiple centers without any adherence to a predefined patient selection protocol or algorithm, contributing to substantial heterogeneity within the study populations and a significant potential for measurement bias. Additionally, in two of the studies,13,22 there was evident bias in the reporting of outcomes, attributed to the analysis of subgroups without detailed evaluation of each participant to mitigate bias.
Main results
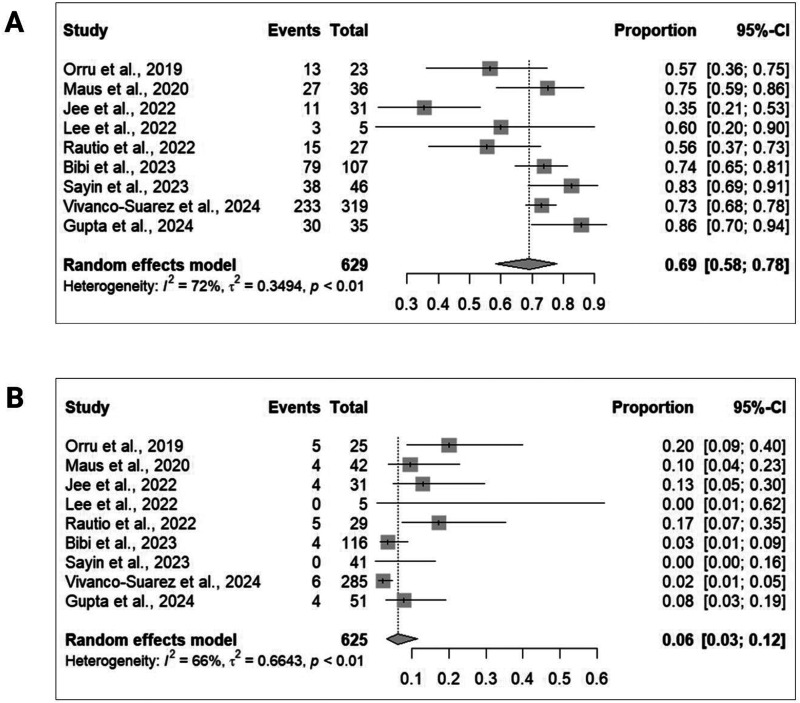
Nine studies9,10,14,22–27 encompassing a total of 629 participants provided data on complete aneurysm occlusion at the last follow-up (Figure 1A). The angiographic follow-up duration varied between 6 and 16.2 months across studies. The pooled analysis revealed an overall complete aneurysm occlusion rate of 69% (95% CI = 58%–78%) with moderate between-study heterogeneity (I2 = 72%). Additionally, the favorable aneurysm occlusion rate was 91% (95% CI = 82%–96%; I2 = 49%; seven studies9,10,14,22–25; 279 participants), and the technical success rate was 100% (95% CI = 90%–100%; I2 = 0%; eight studies9,10,14,22–25,27; 340 participants). Moreover, the retreatment rate was 2% (95% CI = 1%–6%; I2 = 0%; four studies9,22,23,26; 440 participants). In terms of functional outcomes, the favorable functional outcome rate was 95% (95% CI = 46%–100%; I2 = 0%; seven studies10,14,22–27; 532 participants).
Figure 1.
Forest plots illustrating the pooled rates of (A) complete aneurysm occlusion at the last follow-up and (B) the composite of neurological complications.
Concerning safety, nine studies9,10,14,22–27 comprising a total of 625 participants provided data on the composite of early and delayed neurological complications (Figure 1B). The pooled analysis revealed an overall complication rate of 6% (95% CI = 3%–12%) with moderate between-study heterogeneity (I2 = 66%). The individual pooled rate of early and delayed neurological complications was 6% (95% CI = 4%–11%; I2 = 0%; seven studies9,10,14,22–25; 309 participants) and 0% (95% CI = 0%–7%; I2 = 0%; seven studies9,10,14,22–25; 301 participants), respectively. Additionally, the pooled rate of intraprocedural neurological complications was 2% (95% CI = 1%–5%; I2 = 0%; six studies9,10,14,22–24; 258 participants). Specifically, the pooled rate of ischemic intraprocedural events was 1% (95% CI = 0%–3%; I2 = 0%; six studies9,10,14,22–24; 258 participants). The mortality rate was 1% (95% CI = 1%–3%; I2 = 0%; eight studies9,10,14,22,23,25–27; 565 participants). The pooled rate of in-stent stenosis during the procedure was 1% (95% CI = 0%–9%; I2 = 0%; three studies9,10,23; 72 participants) and 8% (95% CI = 3%–17%; I2 = 63%; nine studies9,10,14,22–27; 532 participants) during follow-up.
Subgroup analysis
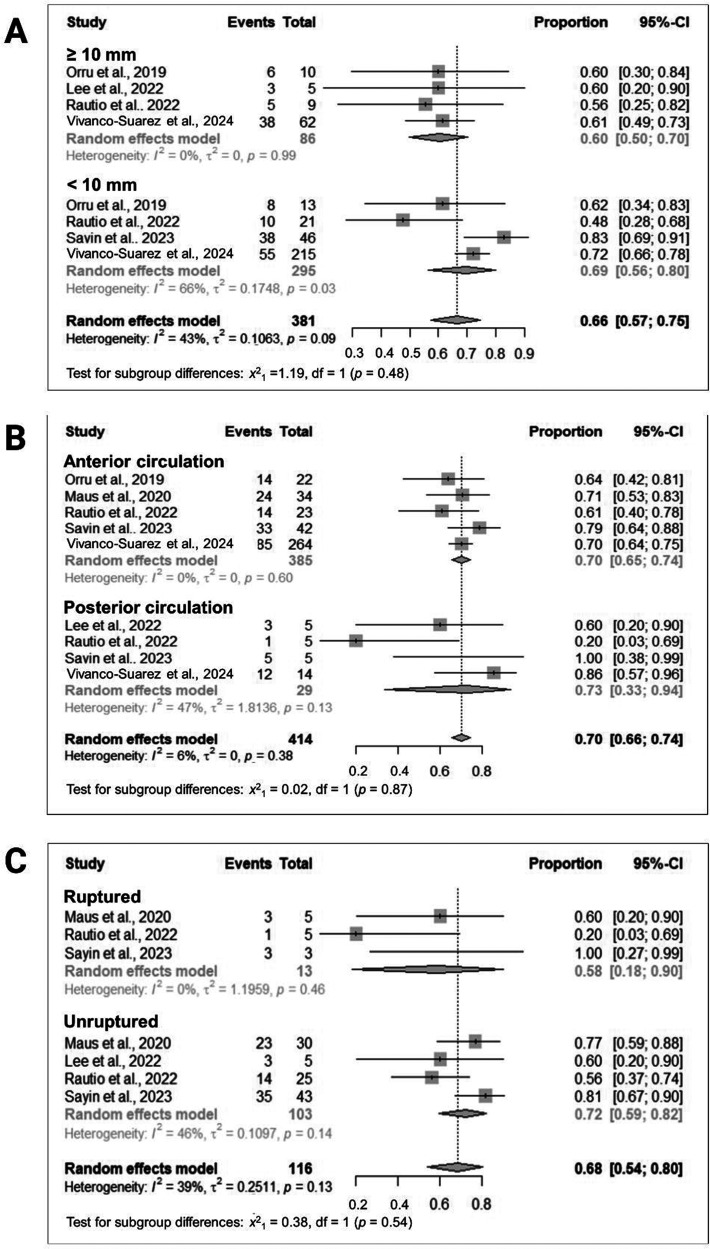
Subgroup analysis of studies with IAs measuring ≥10 mm revealed a pooled rate of complete occlusion at the last follow-up of 60% (95% CI = 50%–70%; I2 = 0%; four studies10,14,23,26; 86 participants), contrasting with 69% (95% CI = 57%–75%; I2 = 66%; four studies10,14,24,26; 295 participants) observed in studies with IAs measuring <10 mm (Figure 2A).
Figure 2.
Subgroup analyses stratified by (A) aneurysm size (≥10 mm vs. <10 mm), (B) location (anterior vs. posterior circulation), and (C) rupture status (acutely ruptured vs. unruptured).
Subgroup analyses based on anatomical location revealed a pooled rate of complete occlusion of 70% (95% CI = 65%–74%; I2 = 0; five studies9,10,14,24,26; 385 participants) for IAs situated in the anterior circulation, while studies focusing on IAs within the posterior circulation reported a similar rate of 70% (95% CI = 66%–74%; I2 = 47%; four studies14,23,24,26; 29 participants) (Figure 2B).
Subgroup analyses based on rupture status at presentation revealed a pooled rate of complete occlusion of 58% (95% CI = 18%–90%; I2 = 0%; three studies9,14,24; 13 participants) in acutely ruptured IAs, contrasted with a rate of 72% (95% CI = 59%–82%; I2 = 46; four studies9,14,23,24; 103 participants) observed in unruptured IAs (Figure 2C).
Discussion
To the best of our knowledge, this was the first meta-analysis of 645 participants from nine studies9,10,14,22–27 to assess the safety and effectiveness of the SE flow diverter for treating IAs. The complete and favorable aneurysm occlusion at an angiographic follow-up ranging between 6 and 16.2 months was 69% and 91%, respectively. The rate of neurological complications was 6%, with a 2% rate of intraprocedural neurologic complications. Of note, a favorable functional outcome was evidenced in 95% of the patients. Finally, the retreatment rate was 2%, and the overall mortality was 1%.
As new devices and technologies are continually developed and incorporated for the endovascular treatment of IAs, worldwide access to these new devices is limited, and the clinical evidence is mainly derived from early reports that lack robustness in their sample size and generalizability. This study offers valuable cumulative evidence about the safety and effectiveness of the SE to aid the neurovascular operator in the tool selection from the current endovascular armamentarium.
Safety
Compared to a previously reported meta-analysis that evaluated the ischemic and hemorrhagic events after implantation of a nitinol 64-wire p64 flow modulation device (p64, Phenox, GmbH, Germany), in this study, we found a higher rate (6% vs. 2%). 29 Aspects to consider for the higher rate we found in this study are the definitions of the safety outcomes, the clinical effects of the events, and the heterogeneity of patients and aneurysms across the different studies. In this study, we considered all the safety events reported regardless of the clinical effect. Asymptomatic ischemic lesions found on follow-up magnetic resonance imaging, such as the ones reported by Maus et al. 30 could explain the higher rate we evidenced. Furthermore, the imaging protocols used for follow-up varied between studies so a direct comparison in terms of safety events is limited. Nevertheless, a meta-analysis on safety outcomes after implantation of the early generation Pipeline Embolization Device (PED, Medtronic Neurovascular, CA, USA) reported a 6.6% rate of ischemic events and 3% of hemorrhagic events. 31 Safety events might decrease with a rate of 1.8% major complications after implantation of the PED Flex (Medtronic Neurovascular) in unruptured aneurysms, as reported by Bhatia et al. 32 in a meta-analysis. Of note, in our study, we included ruptured aneurysms in the pooled results, and this should be taken into consideration due to the increased risk of safety events when endovascular treatment with flow diversion is performed in patients with aneurysmal subarachnoid hemorrhage.
A meta-analysis of the flow redirection endoluminal device (MicroVention, Aliso Viejo, CA, USA) reported a morbidity rate of 3.9%. Additionally, a meta-analysis of the Derivo embolization device (Acandis GmbH & Co, KG, Pforzheim, Germany) reported a 4.9% rate of ischemic and hemorrhagic complications.33,34 After implantation of the Silk (Balt, Montmorency, France), a meta-analysis reported 6.1% of thromboembolic events and 1.6% of hemorrhagic events. 34 Finally, the standardization of safety measures across different studies will allow a better understanding of the most common severe complications encountered after implantation.
Efficacy
The implantation of the SE resulted in a 69% complete occlusion and 91% favorable occlusion at a follow-up ranging between 6 and 16.2 months. This rate is lower than the 75% to 87% range reported in previously published meta-analyses and single-arm studies.33–36 It should be noted that in six of the included studies, the maximum time to follow-up was 6 months, and in several of the reported cases, the last follow-up was <6 months.13,22,23,28,30,37 Considering that aneurysm occlusion and vascular remodeling after flow diverter implantation is a gradual and progressive process that improves with longer follow-up times, a lower rate of complete occlusion is expected due to the short follow-up times. 38 However, other factors, such as aneurysm size and location, did not significantly change the rate of complete occlusion at follow-up, as shown by the subgroup analyses. The range of aneurysm sizes treated in the included studies was wide, and, therefore, one would expect that the occlusion rates are different (lower and higher) when compared to studies that evaluated occlusion in a subset of aneurysms with similar sizes, such as the meta-analysis reported by Fiorella et al. 35 Although complete occlusion was higher in the unruptured aneurysm subgroup, the number of ruptured cases evaluated in this study was small. Furthermore, considering morphology, most aneurysms were saccular, which favors the adequate performance of flow diversion. Of note, most of the included studies presented self-adjudicated outcomes, which could influence the occlusion outcomes, in general, favoring complete occlusion.
In terms of technical considerations, we found a 66% rate of balloon angioplasty and a 24% rate of adjunctive coiling. Of note, in all the studies, the mean number of flow diverters implanted per patient was 1. Theoretically, the number of wires can influence the mechanical properties of the device during deployment, but this is not completely intuitive because a meta-analysis reporting outcomes after implantation of the p64 found a significantly lower rate of balloon-angioplasty after deployment (2% vs. 66%). To our knowledge, there is no evidence that demonstrates that balloon angioplasty influences the effect of flow diversion, but it should be considered that performing balloon angioplasty could change the mechanical properties of the device by increasing the pore density in the non-neck areas and decreasing it in the neck area. 39
Limitations
The primary limitation of this meta-analysis is the inclusion of non-randomized cohort studies with small sample sizes, along with the absence of a central adjudication center. Additionally, inconsistency in defining safety outcomes across studies poses another significant limitation. Generally, our composite safety outcome had to be based on the definitions provided in the individual studies. Furthermore, all studies exhibited a serious risk of bias, largely due to their small sample sizes, uncontrolled confounders, and retrospective designs. To mitigate confounding factors and biases, we conducted subgroup meta-analyses and utilized qualitative methods such as the ROBINS-I tool (Supplemental Figure 2) to address methodological issues that could affect the validity of our findings. These steps are recommended for meta-analyzing non-randomized studies. 40 Additional data to stratify our cohort into saccular versus fusiform subgroups was not available, potentially explaining the high rates of occlusion observed in the posterior circulation subgroup.
Conclusion
In conclusion, this systematic review and meta-analysis provide a comprehensive evaluation of the safety and effectiveness of the SE flow diverter in treating IAs. Our findings suggest favorable outcomes with a high rate of complete aneurysm occlusion at the last follow-up, with acceptable rates of neurological complications. Future research efforts should focus on larger, prospective studies with standardized outcome measures to further elucidate the clinical utility of the SE flow diverter in the management of IAs.
Supplemental Material
Supplemental material, sj-docx-1-ine-10.1177_15910199241284412 for Performance assessment of the Surpass Evolve flow diverter for the treatment of intracranial aneurysms: A systematic review and meta-analysis by Aaron Rodriguez-Calienes, Juan Vivanco-Suarez, Nicole M. Castillo-Huerta, David Espinoza-Martinez, Cristian Morán-Mariños, Ximena Espiritu-Vilcapoma, Valeria Rivera-Angles and Santiago Ortega-Gutierrez in Interventional Neuroradiology
Footnotes
Declaration of conflicting interest: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Ortega-Gutierrez has received grants from NIH-NINDS (R01NS127114–01 and RO3NS126804–01), Stryker, Medtronic, Microvention, Methinks, Viz.ai, and consulting fees from Medtronic and Stryker Neurovascular.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Juan Vivanco-Suarez https://orcid.org/0000-0001-5326-1907
Nicole M. Castillo-Huerta https://orcid.org/0000-0002-7787-3745
David Espinoza-Martinez https://orcid.org/0000-0002-1166-2531
Ximena Espiritu-Vilcapoma https://orcid.org/0009-0006-2547-8977
Valeria Rivera-Angles https://orcid.org/0009-0004-8940-5246
Santiago Ortega-Gutierrez https://orcid.org/0000-0002-3408-1297
Supplemental material: Supplemental material for this article is available online.
References
- 1.Kallmes DF, Ding YH, Dai D, et al. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke 2007; 38: 2346–2352. [DOI] [PubMed] [Google Scholar]
- 2.Kallmes DF, Brinjikji W, Boccardi E, et al. Aneurysm study of pipeline in an observational registry (ASPIRe). Interv Neurol 2016; 5: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hilditch CA, Brinjikji W, Schaafsma J, et al. Flow-diverter stents for internal carotid artery reconstruction following spontaneous dissection: A technical report. Clin Neuroradiol 2019; 29: 707–715. [DOI] [PubMed] [Google Scholar]
- 4.Brinjikji W, Murad MH, Lanzino G, et al. Endovascular treatment of intracranial aneurysms with flow diverters: A meta-analysis. Stroke 2013; 44: 442–447. [DOI] [PubMed] [Google Scholar]
- 5.Cancelliere NM, Nicholson P, Radovanovic I, et al. Comparison of intra-aneurysmal flow modification using optical flow imaging to evaluate the performance of evolve and pipeline flow diverting stents. J Neurointerv Surg 2020; 12: 814–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyers PM, Coon AL, Kan PT, et al. SCENT Trial: One-year outcomes. Stroke 2019; 50: 1473–1479. [DOI] [PubMed] [Google Scholar]
- 7.Sadasivan C, Fiorella D. Preliminary in vitro angiographic comparison of the flow diversion behavior of Evolve and Pipeline devices. J Neurointerv Surg 2020; 12: 616–620. [DOI] [PubMed] [Google Scholar]
- 8.Hanel RA, Kallmes DF, Lopes DK, et al. Prospective study on embolization of intracranial aneurysms with the pipeline device: The PREMIER study 1 year results. J Neurointerv Surg 2020; 12: 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maus V, Weber W, Berlis A, et al. Initial experience with surpass evolve flow diverter in the treatment of intracranial aneurysms. Clin Neuroradiol 2021; 31: 681–689. [DOI] [PubMed] [Google Scholar]
- 10.Orru E, Rice H, De Villiers L, et al. First clinical experience with the new surpass evolve flow diverter: Technical and clinical considerations. J Neurointerv Surg 2020; 12: 974–980. [DOI] [PubMed] [Google Scholar]
- 11.De Vries J, Boogaarts J, Van Norden A, et al. New generation of flow diverter (surpass) for unruptured intracranial aneurysms: A prospective single-center study in 37 patients. Stroke 2013; 44: 1567–1577. [DOI] [PubMed] [Google Scholar]
- 12.Issa R, Al-Homedi Z, Syed DH, et al. Surpass evolve flow diverter for the treatment of intracranial aneurysm: A systematic review. Brain Sci 2022; 12: 810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jee TK, Yeon JY, Kim KH, et al. Early clinical experience of using the surpass evolve flow diverter in the treatment of intracranial aneurysms. Neuroradiology 2022; 64: 343–351. [DOI] [PubMed] [Google Scholar]
- 14.Rautio R, Alpay K, Sinisalo M, et al. Treatment of intracranial aneurysms using the new surpass evolve flow diverter: Safety outcomes and six-month imaging follow-up. J Neuroradiol 2022; 49: 80–86. [DOI] [PubMed] [Google Scholar]
- 15.Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. Br Med J 2020; 368: l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003; 34: 1398–1403. [DOI] [PubMed] [Google Scholar]
- 17.O'Kelly CJ, Krings T, Fiorella D, et al. A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol 2010; 16: 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JPTTJ, Chandler J, Cumpston M, et al. Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester, UK: John Wiley & Sons; 2019. [Google Scholar]
- 19.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. Br Med J 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 21.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Br Med J 2011; 343: d4002. [DOI] [PubMed] [Google Scholar]
- 22.Bibi R, Bankole NDA, Donnard B, et al. Safety and efficacy of surpass evolve flow diverter for intracranial aneurysms: A study of 116 patients. Neuroradiol J 2024; 37: 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee W, Han HJ, Kim J, et al. Flow diverter for the treatment of large (>10 mm) vertebral artery dissecting aneurysms. Acta Neurochir (Wien) 2022; 164: 1247–1254. [DOI] [PubMed] [Google Scholar]
- 24.Sayin B, Dereli B, Senol YC, et al. The evaluation of low-profile surpass evolve( < TM) flow diverter for endovascular treatment of distal cerebral artery aneurysms: A single-center experience. Turk Neurosurg 2023; 33: 477–487. [DOI] [PubMed] [Google Scholar]
- 25.Gupta G, Sreenivasan S, Baek SW, et al. Does small aneurysm size (10 mm) predict complete occlusion after flow diversion? A surpass evolve single-center study. J Endovasc Ther 2024: 15266028241240943 Epub ahead of print 2024. [DOI] [PubMed] [Google Scholar]
- 26.Vivanco-Suarez J, Dibas M, Lopes DK, et al. Safety and effectiveness assessment of the surpass evolve (SEASE): A post-market international multicenter study. J Neurointerv Surg Epub ahead of print 2024. [DOI] [PubMed] [Google Scholar]
- 27.Jee TK, Yeon JY, Kim KH, et al. Early clinical experience of using the surpass evolve flow diverter in the treatment of intracranial aneurysms. Neuroradiology 2022; 64: 343–351. [DOI] [PubMed] [Google Scholar]
- 28.Orru E, Rice H, De Villiers L, et al. First clinical experience with the new surpass evolve flow diverter: Technical and clinical considerations. J Neurointerv Surg 2020; 12: 974–980. [DOI] [PubMed] [Google Scholar]
- 29.Vivanco-Suarez J, Salem MM, Sioutas GS, et al. Safety and efficacy of the p48 MW and p64 flow modulation devices: A systematic review and meta-analysis. Neurosurg Focus 2023; 54: E7. [DOI] [PubMed] [Google Scholar]
- 30.Maus V, Weber W, Berlis A, et al. Initial experience with surpass evolve flow diverter in the treatment of intracranial aneurysms. Clin Neuroradiol 2021; 31: 681–689. [DOI] [PubMed] [Google Scholar]
- 31.Texakalidis P, Bekelis K, Atallah E, et al. Flow diversion with the pipeline embolization device for patients with intracranial aneurysms and antiplatelet therapy: A systematic literature review. Clin Neurol Neurosurg 2017; 161: 78–87. [DOI] [PubMed] [Google Scholar]
- 32.Bhatia KD, Kortman H, Orru E, et al. Periprocedural complications of second-generation flow diverter treatment using pipeline flex for unruptured intracranial aneurysms: A systematic review and meta-analysis. J Neurointerv Surg 2019; 11: 817–824. [DOI] [PubMed] [Google Scholar]
- 33.Waqas M, Dossani RH, Alkhaldi M, et al. Flow redirection endoluminal device (FRED) for treatment of intracranial aneurysms: A systematic review. Interv Neuroradiol 2022; 28: 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monteiro A, Burke SM, Baig AA, et al. A systematic review and meta-analysis of the derivo embolization device: A novel surface-modified flow diverter for intracranial aneurysm treatment. J Neurointerv Surg 2022; 14: 1125–1129. [DOI] [PubMed] [Google Scholar]
- 35.Fiorella D, Gache L, Frame D, et al. How safe and effective are flow diverters for the treatment of unruptured small/medium intracranial aneurysms of the internal carotid artery? Meta-analysis for evidence-based performance goals. J Neurointerv Surg 2020; 12: 869–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vivanco-Suarez J, Salem MM, Sioutas GS, et al. Safety and efficacy of the p48 MW and p64 flow modulation devices: A systematic review and meta-analysis. Neurosurg Focus 2023; 54: E7. [DOI] [PubMed] [Google Scholar]
- 37.Rautio R, Alpay K, Sinisalo M, et al. Treatment of intracranial aneurysms using the new surpass evolve flow diverter: Safety outcomes and six-month imaging follow-up. J Neuroradiol 2022; 49: 80–86. [DOI] [PubMed] [Google Scholar]
- 38.Becske T, Potts MB, Shapiro M, et al. Pipeline for uncoilable or failed aneurysms: 3-year follow-up results. J Neurosurg 2017; 127: 81–88. [DOI] [PubMed] [Google Scholar]
- 39.Bae HJ, Park YK, Cho DY, et al. Predictors of the effects of flow diversion in very large and giant aneurysms. AJNR Am J Neuroradiol 2021; 42: 1099–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathur MB, VanderWeele TJ. Methods to address confounding and other biases in meta-analyses: Review and recommendations. Annu Rev Public Health 2022; 43: 19–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ine-10.1177_15910199241284412 for Performance assessment of the Surpass Evolve flow diverter for the treatment of intracranial aneurysms: A systematic review and meta-analysis by Aaron Rodriguez-Calienes, Juan Vivanco-Suarez, Nicole M. Castillo-Huerta, David Espinoza-Martinez, Cristian Morán-Mariños, Ximena Espiritu-Vilcapoma, Valeria Rivera-Angles and Santiago Ortega-Gutierrez in Interventional Neuroradiology