Abstract
Background
The benefit of intravenous thrombolysis (IVT) is well established. We aim to study the benefits of IVT in acute ischemic stroke (AIS) patients with large vessel occlusion (LVO) who underwent unsuccessful mechanical thrombectomy (MT).
Methods
We included AIS patients who underwent MT for anterior circulation LVO with failed recanalization (modified treatment in cerebral ischemia [mTICI] score ≤ 2A). Patients who received IVT prior to MT were compared to those who received MT alone. Propensity score matching using demographic, clinical, radiographic and procedural variables was used to match patients with and without IVT. The primary outcome was favorable 90-day good functional outcome (defined as modified Rankin scale of 0–2), and secondary outcomes included intracranial hemorrhage (ICH), symptomatic ICH (sICH), and 90-day mortality.
Results
Totally, 610 AIS patients with unsuccessful MT were included. After propensity matching, 219 patients were identified in each group. Median age was 70 years and 73 years in the IVT + MT and MT alone groups, respectively. In the IVT + MT group, final mTICI scores of 0, 1, and 2A were achieved in 92 (42.0%), 33 (15.1%), and 94 (42.9%) patients, respectively, versus 76 (34.7%), 29 (13.2%), and 114 (52.1%) in the MT alone group. The IVT + MT group had greater odds of 90-day good functional outcome (adjusted odds ratio 2.54, 95% confidence interval 1.53–4.32). There were no significant differences in secondary outcomes.
Conclusions
IVT is associated with improved functional outcomes in AIS patients with LVO despite unsuccessful MT.
Keywords: Stroke, thrombectomy, thrombolysis, LVO
Introduction
Acute ischemic stroke (AIS) is a leading cause of disability and mortality. 1 Since 1995, intravenous thrombolysis (IVT) using tissue plasminogen activator (t-PA) has been the standard treatment for AIS patients presenting within 4.5 hours from the last known normal (LKN). 2 However, in recent years, mechanical thrombectomy (MT) has emerged as a revolutionizing intervention in the treatment of AIS due to large vessel occlusion (LVO).3–5
The current guidelines recommend the administration of IVT before or during MT as a first-line treatment in eligible LVO patients presenting within 4.5 hours of symptom onset. 6 Clinical trials including DEVT, 7 DIRECT-MT, 8 and SKIP, 9 MR CLEAN-NO IV, 10 SWIFT-DIRECT, 11 and DIRECT-SAFE 12 in AIS patients with LVO revealed the non-inferiority of MT alone compared to MT + IVT. While bridging IVT may help in early recanalization, its limited lysis power in large LVO thrombi might soften and fragment the clot, increasing the risks of distal embolization and intracerebral hemorrhage.13,14
Approximately 17% of LVO patients fail to achieve adequate recanalization after MT. 15 Unsuccessful recanalization, defined as modified treatment in cerebral ischemia (mTICI) score ≤ 2A, has been linked to worse clinical outcomes.16–18 However, there are limited data on the impact of bridging IVT on outcomes for LVO-AIS patients who have undergone unsuccessful MT. In this international multicenter cohort study, we aim to investigate the impact of bridging IVT in AIS with LVO who underwent unsuccessful thrombectomy.
Methods
Data collection and patient population
This is a retrospective cohort study from the Stroke Thrombectomy and Aneurysm Registry (STAR), a database including 84 thrombectomy-capable stroke centers in the USA, Europe, and Asia. Ethical approval was obtained from the institutional review board at each participating center, and data analysis was conducted at the core institution.
We included all patients who underwent MT for occlusion of the internal carotid artery (ICA) or proximal middle cerebral artery (M1 and M2 segments) with unsuccessful recanalization (mTICI 0, 1, or 2A) between January 2013 and September 2023. Patients were stratified into “MT only” and “MT + IVT” groups based on whether IVT was administered. This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology statement criteria. 19
Outcomes
The primary outcome was good clinical outcome at 90 days, defined as a modified Rankin score (mRS) of 0–2. The mRS was assessed during follow-up visits or telephone encounters at 90 ± 30 days. Additional outcomes included mRS of 0–3, mortality at 90 days, any intracranial hemorrhage (ICH), and symptomatic ICH (sICH). sICH was defined as ICH with an associated ≥4 points worsening in National Institutes of Health Stroke Scale (NIHSS) score at 24 hours. 20
Statistical analysis
R (version 4.2.2; RStudio Inc, Boston, MA) was used for statistical analysis. Continuous variables were presented as median (interquartile range [IQR]). Wilcoxon rank-sum (Mann–Whitney) test and χ2 tests were used to perform between-group comparisons of characteristics, as appropriate.
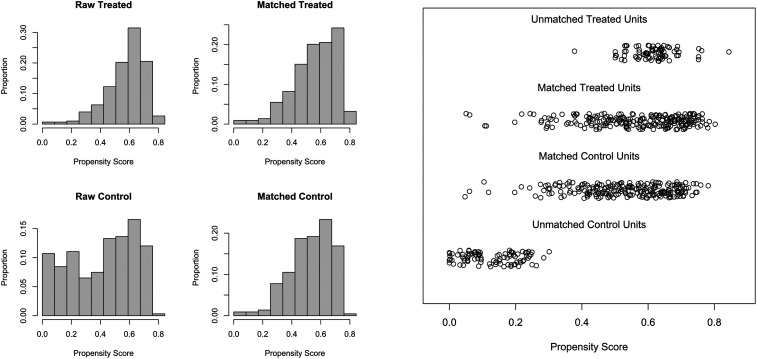
Propensity score (PS) matching was utilized to account for differences in baseline characteristics between IVT + MT and MT only groups. PS matching was performed using age, sex, premorbid mRS, admission NIHSS, occluded vessel location, Alberta Stroke Program Early CT Score (ASPECTS), and time from symptoms onset to arterial puncture. The matching algorithm employed in this study was a 1:1 nearest neighbor greedy matching without replacement, where MT + IVT patients were matched to unique MT only patients. The optimal caliper width was determined as 0.1 times the standard deviation of the logit of the PS of all patients. The model adequacy was validated by comparing the distribution of PS between pre- and post-matched datasets (Figure 1). After PS matching, all variables were reevaluated using univariate hypothesis testing to assess the balance achieved by the PS matching algorithm.21,22 All P-values were derived from two-sided tests, and statistical significance was set at P < 0.05.
Figure 1.
Distribution of propensity scores before and after matching.
Results
Patient characteristics
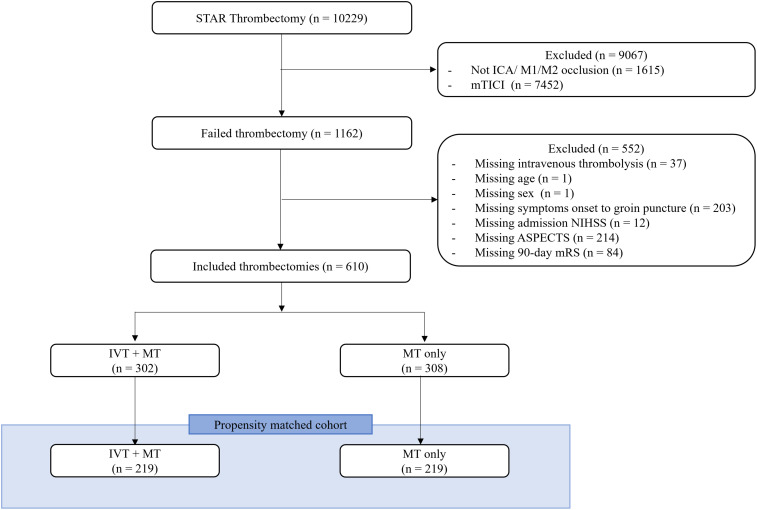
Out of the 10,299 patients in STAR who underwent MT, 610 met the inclusion criteria and 308 (50.5%) were treated with MT + IVT. Propensity matching yielded well-matched cohorts with 219 patients each in IVT + MT and MT only groups (Figure 2). There were no significant differences in demographic, clinical, radiographic or procedural variables between the two matched cohorts (Table 1).
Figure 2.
Patient selection flow chart of anterior circulation large vessel occlusion patients with unsuccessful thrombectomy. ICA, internal carotid artery; M1/M2, M1/M2 segment of the middle cerebral artery; LKN, last known normal; mRS, modified Rankin scale; mTICI, modified thrombolysis in cerebral infarction; ASPECTS, Alberta Stroke Program Early Computed Tomography Score, MT, mechanical thrombectomy; IVT, intravenous thrombolysis.
Table 1.
Baseline demographic, clinical, procedural and imaging characteristics.
| Characteristics | Full cohort | Matched cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| IVT + MT (n = 302) |
MT only (n = 308) |
P-Value | SMD | IVT + MT (n = 219) |
MT only (n = 219) |
P-Value | SD | |
| Female sex | 141 (46.7) | 159 (51.6) | 0.25 | 0.1 | 99 (45.2) | 114 (52.1) | 0.18 | 0.14 |
| Age, (median [IQR]) | 72 [61–81] | 73 [61–81] | 0.65 | 0.007 | 70 [61–80] | 73 [62–81] | 0.24 | 0.1 |
| Race | 0.008 | 0.23 | 0.15 | 0.25 | ||||
| White | 180 (77.9) | 179 (68.8) | 129 (75.4) | 126 (68.9) | ||||
| Black | 14 (6.1) | 35 (13.5) | 10 (5.8) | 21 (11.5) | ||||
| Hispanic | 1 (0.4) | 7 (2.7) | 1 (0.6) | 4 (2.2) | ||||
| Other | 36 (15.6) | 39 (15.0) | 35 (18.3) | 28 (16.0) | ||||
| Diabetes | 72 (24.0) | 93 (30.3) | 0.1 | 0.15 | 53 (24.3) | 63 (28.9) | 0.33 | 0.1 |
| Hypertension | 227 (75.4) | 215 (70.0) | 0.16 | 0.11 | 165 (75.7) | 149 (68.3) | 0.11 | 0.16 |
| Atrial fibrillation | 112 (37.3) | 109 (35.6) | 0.72 | 0.03 | 84 (38.5) | 85 (39.2) | 0.97 | 0.01 |
| Hyperlipidemia | 121 (40.3) | 123 (40.1) | >0.90 | 0.02 | 87 (39.9) | 83 (38.1) | 0.77 | 0.04 |
| Prior stroke | 30 (14.8) | 43 (16.7) | 0.67 | 0.05 | 22 (14.6) | 31 (17.6) | 0.55 | 0.08 |
| Premorbid mRS 0–1 | 173 (83.2) | 192 (77.4) | 0.16 | 0.18 | 188 (85.8) | 186 (84.9) | 0.89 | 0.03 |
| Admission NIHSS, (median [IQR]) | 16 [11–20] | 15 [10–20] | 0.43 | 0.06 | 16 [12–20] | 15 [11–20] | 0.46 | 0.06 |
| Imaging | 0.28 | 0.34 | 0.1 | 0.52 | ||||
| CTA | 24 (40.7) | 61 (53.0) | 21 (44.7) | 51 (63.0) | ||||
| CTA + CTP | 1 (1.7) | 2 (1.7) | 0 (0.0) | 2 (2.5) | ||||
| CTP | 29 (49.2) | 46 (40.0) | 21 (44.7) | 23 (28.4) | ||||
| DWI | 2 (3.4) | 5 (4.3) | 2 (4.3) | 4 (4.9) | ||||
| Others | 3 (5.1) | 1 (0.9) | 3 (6.4) | 1 (1.2) | ||||
| ASPECTS 0–5 | 32 (10.6) | 46 (14.9) | 0.14 | 0.13 | 29 (12.7) | 21 (9.2) | 0.29 | 0.11 |
| Occluded vessel location | 0.69 | 0.16 | 0.54 | 0.2 | ||||
| ICA | 70 (23.2) | 65 (21.1) | 47 (21.5) | 50 (22.8) | ||||
| ICA + M1 | 24 (7.9) | 28 (9.1) | 19 (8.7) | 16 (7.3) | ||||
| ICA + M1 + M2 | 4 (1.3) | 3 (1.0) | 2 (0.9) | 3 (1.4) | ||||
| ICA + M2 | 4 (1.3) | 5 (1.6) | 3 (1.4) | 1 (0.5) | ||||
| M1 | 142 (47.0) | 130 (42.2) | 108 (49.3) | 94 (42.9) | ||||
| M1 + M2 | 11 (3.6) | 15 (4.9) | 10 (4.6) | 12 (5.5) | ||||
| M2 | 47 (15.6) | 62 (20.1) | 30 (13.7) | 43 (19.6) | ||||
| Time from symptoms onset to arterial puncture, minutes, (median [IQR]) | 230 [150–308] | 357 [186–808] | <0.001 | 0.5 | 242 [150–341] | 237 [160–405] | 0.45 | 0.09 |
| Technique | 0.29 | 0.20 | 0.56 | 0.15 | ||||
| Contact aspiration | 98 (40.3) | 132 (48.2) | 77 (42.3) | 85 (45.2) | ||||
| Stent retriever | 47 (19.3) | 45 (16.4) | 32 (17.6) | 37 (19.7) | ||||
| Combined | 89 (36.6) | 82 (29.9) | 67 (36.8) | 57 (30.3) | ||||
| Others | 9 (3.8) | 15 (5.5) | 6 (3.3) | 9 (4.8) | ||||
| Balloon guide catheter | 28 (11.0) | 41 (15.0) | 0.22 | 0.12 | 22 (11.6) | 26 (13.8) | 0.63 | 0.07 |
| Angioplasty | 9 (4.5) | 23 (11.1) | 0.40 | 0.10 | 7 (5.6) | 20 (10.4) | 0.16 | 0.02 |
| Intracranial stent placed | 9 (4.4) | 14 (6.7) | 0.91 | 0.09 | 9 (4.5) | 11 (5.02) | 1.00 | 0.07 |
| Procedure duration, minutes (median [IQR]) | 62 [41–97] | 65 [41–92] | 0.93 | 0.07 | 64 [41–95] | 70 [45–95] | 0.47 | 0.06 |
| Final mTICI | 0.447 | 0.103 | 0.16 | 0.19 | ||||
| 0 | 116 (37.7) | 120 (39.7) | 92 (42.0) | 76 (34.7) | ||||
| 1 | 36 (11.7) | 43 (14.2) | 33 (15.1) | 29 (13.2) | ||||
| 2A | 156 (50.6) | 139 (46.0) | 94 (42.9) | 114 (52.1) | ||||
MT, mechanical thrombectomy; IVT, intravenous thrombolysis; ICA, internal carotid artery; M1/M2, M1/M2 segment of the middle cerebral artery; NIHSS, National Institutes of Health Stroke Scale; ASPECTS, Alberta Stroke Program Early CT Score; mTICI, modified thrombolysis in cerebral infarction; IQR, interquartile range; SD, standard deviation.
Data were expressed as number of cases (%), unless otherwise indicated.
Outcomes
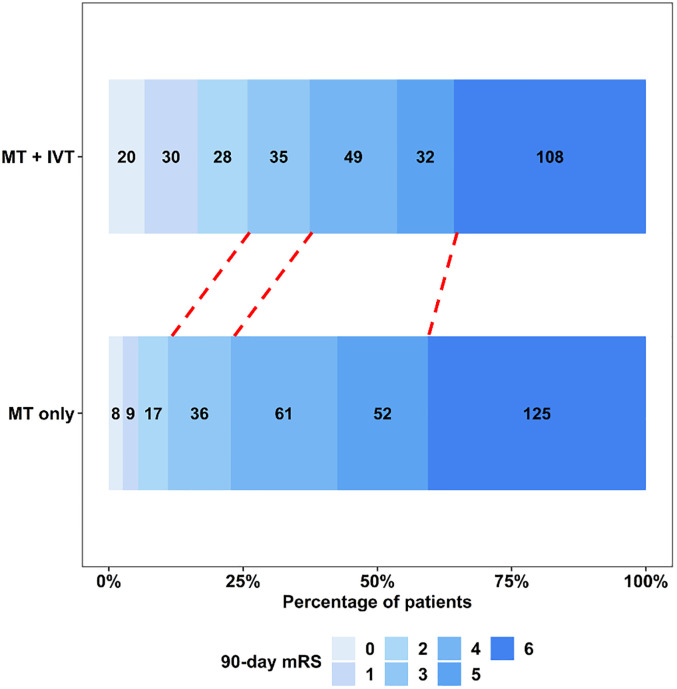
In the full cohort, 78 (25.8%) of 302 IVT + MT patients achieved the primary outcome of good functional outcome at 90 days, compared to 34 (11.0%) of 308 patients in the MT only group (adjusted odds ratio [aOR]: 3.58, 95% confidence interval [CI]: 2.20–5.96; P < 0.001) (Figure 3). The rates of ICH and sICH did not differ significantly between the MT + IVT and MT only groups (aOR: 0.89, 95% CI: 0.61–1.28; P = 0.50 and aOR: 1.01, 95% CI: 0.58–1.76; P > 0.90, respectively) (Table 2).
Figure 3.
90-Day mRS distribution in full cohort of anterior circulation large vessel occlusion patients with unsuccessful thrombectomy. MT, mechanical thrombectomy; IVT, intravenous thrombolysis; mRS, modified Rankin scale.
Table 2.
Outcomes in full cohort analysis.
| Outcome | IVT + MT (n = 302) |
MT only (n = 308) |
Crude OR (95% CI) | P-Value | Adjusted a OR (95% CI) | P-Value |
|---|---|---|---|---|---|---|
| 90-Day mRS 0–2 | 78 (25.8) | 34 (11.0) | 2.81 (1.82–4.40) | <0.001 | 3.58 (2.20–5.96) | <0.001 |
| 90-Day mRS 0–3 | 113 (37.4) | 70 (22.7) | 2.03 (1.43–2.91) | <0.001 | 2.49 (1.65–3.79) | <0.001 |
| ICH | 30 (10.6) | 31 (10.8) | 0.83 (0.59–1.18) | 0.30 | 0.89 (0.61–1.28) | 0.50 |
| sICH | 86 (30.3) | 100 (34.2) | 0.99 (0.58–1.68) | >0.90 | 1.01 (0.58–1.76) | >0.9 |
| 90-Day mortality | 108 (35.8) | 125 (40.6) | 0.82 (0.59–1.13) | 0.20 | 0.79 (0.54–1.14) | 0.20 |
mRS, modified Rankin scale; ICH, intracranial hemorrhage; sICH, symptomatic intracranial hemorrhage.
Data were expressed as number of cases (%), unless otherwise indicated.
Adjusted Factors: age, admission NIHSS, ASPECTS, location of occlusion, and time from symptoms onset to arterial puncture.
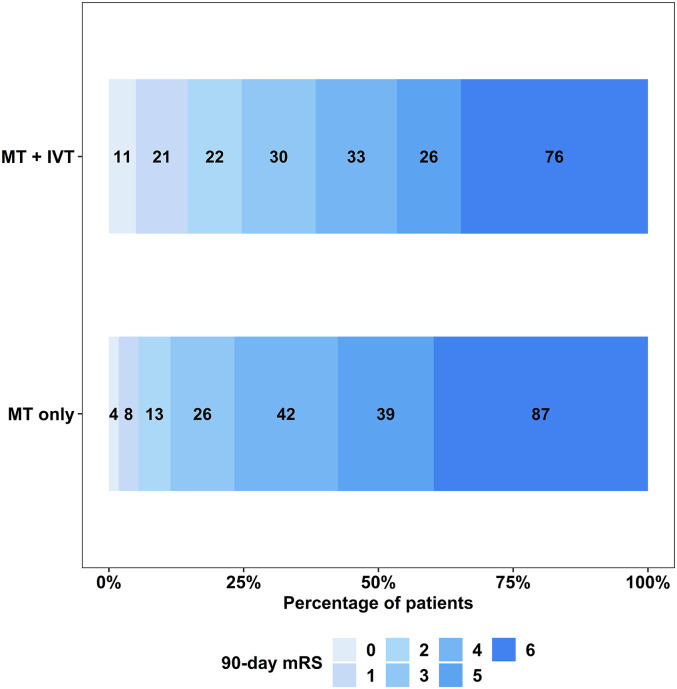
In the matched cohort, 54 (24.7%) of patients in the MT + IVT group achieved the primary outcome, compared to 25 (11.4%) in the MT only group (OR: 2.54, 95% CI: 1.53–4.32; P < 0.001). The distribution of mRS scores for both MT + IVT and MT only groups is presented in Figure 4. There was no significant difference in the incidence of ICH and sICH between the two groups (OR: 0.89, 95% CI: 0.61–1.28; P = 0.50 and OR: 1.01, 95% CI: 0.58–1.76; P > 0.90, respectively). The 90-day mortality rate was 76 (34.7%) in the MT + IVT group compared to 87 (39.7%) in the MT only group (OR: 0.81, 95% CI: 0.55–1.19; P = 0.30) (Table 3).
Figure 4.
90-Day mRS distribution in matched cohort analysis. A shift toward good outcomes was seen in the IVT + MT group compared to the MT only group. MT, mechanical thrombectomy; IVT, intravenous thrombolysis; mRS, modified Rankin scale.
Table 3.
Outcomes in matched cohort analysis.
| Outcome | IVT + MT (n = 219) |
MT only (n = 219) |
OR (95% CI) | P-Value |
|---|---|---|---|---|
| 90-Day mRS 0–2 | 54 (24.7) | 25 (11.4) | 2.54 (1.53–4.32) | <0.001 |
| 90-Day mRS 0–3 | 84 (38.4) | 51 (23.3) | 2.05 (1.36–3.12) | <0.001 |
| ICH | 62 (30.0) | 65 (31.7) | 0.92 (0.61–1.40) | 0.70 |
| sICH | 21 (10.2) | 21 (10.4) | 0.98 (0.51–1.86) | >0.9 |
| 90-Day mortality | 76 (34.7) | 87 (39.7) | 0.81 (0.55–1.19) | 0.30 |
mRS, modified Rankin scale; ICH, intracranial hemorrhage; sICH, symptomatic intracranial hemorrhage.
Data were expressed as number of cases (%), unless otherwise indicated.
A binary logistic regression analysis was conducted to identify the predictors of the 90-day mRS 0–3. The results revealed that younger age (aOR, 0.96; 95% CI, 0.95–0.98; P < 0.001), lower admission NIHSS score (aOR, 0.90; 95% CI, 0.87–0.94; P < 0.001), occlusion location other than ICA (aOR, 0.59; 95% CI, 0.34–0.98; P = 0.05), and IVT (aOR, 3.49; 95% CI, 2.16–5.79; P < 0.001) were significantly associated with the 90-day mRS 0–3 (Table 4).
Table 4.
Predictors of good outcome (mRS 0–2) in full cohort of anterior circulation large vessel occlusion patients with unsuccessful thrombectomy.
| Characteristics | Odds ratio | 95% confidence interval | P-Value |
|---|---|---|---|
| Age | 0.96 | 0.95–0.98 | <0.001 |
| Admission NIHSS | 0.90 | 0.87–0.94 | <0.001 |
| ICA occlusion | 0.59 | 0.34–0.98 | 0.049 |
| ASPECTS | 1.05 | 0.92–1.20 | 0.50 |
| Intravenous thrombolysis | 3.49 | 2.16–5.79 | <0.001 |
| mTICI 2A | 0.92 | 0.58–1.47 | 0.70 |
| Time from symptoms onset to groin puncture, minutes | 1.00 | 1.00–1.00 | 0.40 |
mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale; ASPECTS, Alberta Stroke Program Early CT Score; ICA, internal carotid artery; M1/M2, M1/M2 segment of the middle cerebral artery; mTICI, modified thrombolysis in cerebral infarction.
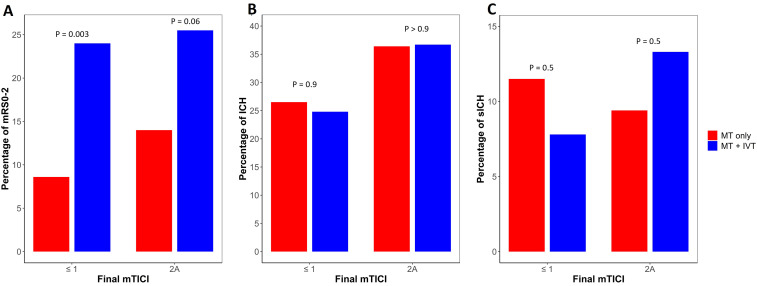
In the subgroup analysis comparing patients with no or minimal recanalization (mTICI 0 or 1) with partial recanalization (mTICI 2A), there was an increase in the percentage of the primary outcome in both the MT only and MT + IVT groups in the setting of no or minimal recanalization (Figure 5(a)). There was no significant difference in ICH or sICH incidence (Figure 5(b) and (c)) between the groups in the setting of no/minimal versus partial recanalization.
Figure 5.
Outcomes in matched cohort analysis among non-recanalized and partially-recanalized groups. Relationship between extent of recanalization and (A) 90-day mRS 0–2; (B) any ICH and (C) sICH. ICH, intracranial hemorrhage; sICH, symptomatic intracranial hemorrhage, mRS, modified Rankin scale.
Discussion
Existing data on bridging thrombolysis before unsuccessful MT are limited, as unsuccessful MT represented only about 20% of patients who underwent MT.23,24 This number is even lower in the non-inferiority bridging thrombolysis clinical trials where the percentage was less than 10% in most trials.7,9,11,12,25 In our large, multicenter cohort study using PS matching, we found that MT + IVT was associated with a higher likelihood of 90-day good functional outcome without increasing the incidence of ICH, sICH, or 90-day mortality.
IVT has been shown to soften thrombus, which may contribute to a higher likelihood of recanalization during MT and a reduction in the number of thrombectomy attempts. 26 Nonetheless, in the non-inferiority trials comparing MT + IVT with MT alone, there minor numerical and no statistically significant differences in recanalization rates.7–9,11,12,25 The effectiveness of IVT in recanalization depends on various factors including the location of occlusion, 27 timing of IVT administration,28,29 clot burden,30,31 and collateral status. 31 A meta-analysis showed that early recanalization after IVT was seen in 4% of ICA, 21% of M1, and 38% of M2 occlusions. 32 In the DIRECT-MT trial, spontaneous recanalization before MT occurred in 7% of patients with IVT versus 2.4% in the MT alone group, 8 while MR-CLEAN NO IV reported 3.7% and 2.8%, respectively. 25 Our study including only patients who underwent MT without adequate recanalization, thereby eliminating the influence of spontaneous recanalization as a potential contributor to improved functional outcomes.
The observed improvement in functional outcomes in the MT + IVT group might be attributed to improved cerebral micro-perfusion enhanced by IVT. 33 Evidence from preclinical studies indicates that early IVT administration induces hypofibrinogenemia which may inhibit platelet aggregation and break down newly formed unstable platelet aggregates, thus preventing microthrombi downstream of arterial occlusion and lysis of newly formed microthrombi. 34 Molecular imaging, using a special FXIIIa-targeted probe, combined with histopathological analysis, showed that fibrin deposition contributes to the microcirculation no-reflow phenomenon in an experimental AIS model, which used C57BL/6 mice and occluded middle cerebral artery via the photothrombotic method. 33 These preclinical studies align with our results on functional independence and are consistent with two recently published cohort studies.35,36
Another potential mechanism for the beneficial effect of the bridging therapy is complete spontaneous and delayed recanalization after failed or partial recanalization of the LVO. Spontaneous recanalization time varies from few hours up to several weeks.37–41 In one study which included 139 AIS patients with LVO or medium vessel occlusions who did not receive IVT or MT, 23 patients (16.6%) achieved spontaneous recanalization on follow-up imaging, 14 of which occurred after 2 weeks. 42 Subgroup analysis of the ESCAPE trial of MT for LVO showed that patients who received IVT in either treatment arm had a significantly higher chance of spontaneous recanalization, up to 8 hours, and that early spontaneous recanalization was associated with better outcomes. 43
Hemorrhagic transformation in AIS is associated with poor outcomes, 44 and IVT has been shown to increase the risk of sICH, especially in patients with underlying intracranial atherosclerosis who may require intracranial stenting and intensive antiplatelet therapy.45,46 ICH mainly occurs due to reperfusion injury that disrupts the blood-brain barrier; such disruption is potentiated by IVT, which increases matrix metalloproteinase secretion, free radical formation, and direct ischemic injury to endothelial cells.47–50
Among the published non-inferiority bridging thrombolysis trials, only two demonstrated that MT + IVT is associated with an increased risk of any ICH, and neither found significant differences in the incidence of sICH.7,9 A cohort study focusing solely on unsuccessful thrombectomy found higher rates of sICH in the IVT + MT groups. 36 In contrast, another study with a similar design aligns with our findings, reporting no difference between the groups in both ICH and sICH. 35 Furthermore, while this study reported a lower 90-day mortality rate in the IVT + MT group, our study found no mortality difference. The discrepancies in the results between these studies may be explained by the differences in stroke etiologies and baseline characteristics of our population. For instance, the median age in our study was 72 years, compared to 78 years in Faizy et al. and 69 years in Rozes et al., respectively.35,36
In a subgroup analysis of partially-recanalized and non-recanalized cohorts, a higher incidence of ICH in cases with partial recanalization was observed, irrespective of whether MT alone or MT + IVT was administered. These findings are consistent with a previous study that found ICH rates were lower with either no recanalization (TICI 0 and 1) or complete recanalization (TICI 3) while partial recanalization (TICI 2a) was associated with highest hemorrhage rates. 51 Interestingly, the incidence of sICH increased with partial recanalization for the MT + IVT group, while it decreased for the MT only group. This observed increase in ICH might arise because the partial opening of the vessel which permits greater distribution of IVT in the infarcted brain tissue, potentially leading to more extensive reperfusion injury. Despite the elevated rates of ICH and sICH with partial recanalization, better outcomes were still seen in this setting in both MT alone and MT + IVT groups, similar to prior study. 35 These findings suggested that even partial recanalization is beneficial.
Limitations
Our study has several limitations, including its retrospective nature, which introduces selection biases. However, this is mitigated to some extent by the use of PS matching. The multicenter nature of the study raises the possibility for variations in management and procedural protocols across different institutions. Additionally, mTICI scores were self-adjudicated by investigators at the participating STAR sites and were not adjudicated by a core laboratory. Our registry did not capture data on AIS etiology, particularly intracranial atherosclerotic disease (ICAD), which is known to impact outcomes in patients who underwent bridging thrombolysis.52,53 Despite this limitation, our data revealed no significant difference in the percentage of patients needing angioplasty or intra-arterial stent placement between the study groups. This suggests that the proportions of ICAD patients were likely comparable. However, the influence of ICAD should be considered when interpreting these findings, including the 90-day outcomes. We also did not collect data regarding the dose and speed of IVT, patient transfer model, and cost. Some studies have shown that IVT might be associated with a delay in the start of MT and an increase in cost.54,55 The absence of detailed data on the specific reasons for non-administration of IVT, including potential contraindications such as anticoagulation, ICH, and hypertension, is a notable limitation of our study. This lack of information could impact the validity of our findings. Future studies that control for potential unmeasured confounding variables, analyze outcomes according to stroke etiology, and conduct cost analysis of IVT among AIS patients with unsuccessful thrombectomy are warranted.
Conclusion
Among AIS patients with anterior circulation LVO who underwent unsuccessful MT, bridging IVT is associated with improved 90-day functional outcomes without increasing the risk of ICH, sICH, or mortality. Prioritizing IVT before MT in all eligible LVO patients is recommended.
Acknowledgements
The authors would like to thank all investigators for their efforts in conducting the Stroke Thrombectomy and Aneurysm Registry (STAR) Collaborators.
Footnotes
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: STAR: Research support from Penumbra, Microvention, Medtronic, Stryker, RapidAI, and Brain Aneurysm Foundation. Dr H. Matsukawa received a lecture fee from Daiichi-Sankyo and Stryker and consulting services fee from B. Braun. Dr Ilko L Maier: speakers’ honoraria from Pfizer and Bristol-Myers Squibb. Dr Robert M Starke: RMS research is supported by the NREF, Joe Niekro Foundation, Brain Aneurysm Foundation, Bee Foundation, Department of Health Biomedical Research Grant (21K02AWD-007000) and by National Institute of Health (R01NS111119-01A1) and (UL1TR002736, KL2TR002737) through the Miami Clinical and Translational Science Institute, from the National Center for Advancing Translational Sciences and the National Institute on Minority Health and Health Disparities. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. RMS has an unrestricted research grant from Medtronic and Balt and has consulting and teaching agreements with Penumbra, Abbott, Medtronic, Balt, InNeuroCo, Cerenovus, Naglreiter, Tonbridge, Von Medical, and Optimize Vascular. Dr Marios-Nikos Psychogios: Grants from the Swiss National Science Foundation (SNF) for the DISTAL trial (33IC30_198783) and TECNO trial (32003B_204977), Grant from Bangerter-Rhyner Stiftung for the DISTAL trial. Unrestricted Grants for the DISTAL trial from Stryker Neurovascular Inc., Phenox GmbH, Penumbra Inc. and Rapid Medical Inc., Sponsor-PI SPINNERS trial (Funded by a Siemens Healthineers AG Grant), Research agreement with Siemens Healthineers AG, Local PI for the ASSIST, EXCELLENT, TENSION, COATING, SURF and ESCAPE-NEXT trials. Speaker fees: Stryker Neurovascular Inc., Medtronic Inc., Penumbra Inc., Acandis GmbH, Phenox GmbH, Siemens Healthineers AG. Dr Edgar Samaniego: consults for Medtronic, microvention, Rapid Medical. Dr S. Yoshimura: received lecture fee from Stryker, Medtronic, Johnson & Johnson, Kaneka Medics. Dr Hugo Cuellar: Consultant for Medtronic and Microvention. Dr Jonathan A Grossberg: Georgia Research Alliance, Emory Medical Care Foundation, Neurosurgery Catalyst, Consultant: Cognition, Imperative Care. Dr Daniele G. Romano: Consultant for Penumbra, Balt, Microvention, Phenox. Dr Omar Tanweer: Consulting Agreements: Viz.AI, Inc., Penumbra, Inc, Balt, Inc, Stryker Inc, Imperative Inc. Proctor: Microvention Inc, Medtronic Inc. Educational/Research Grants: Q’apel Inc, Steinberg Foundation. Dr Charles Matouk: Consultant for Stryker, Medtronic, Microvention, Penumbra, and Silk Road Medical. Speaker for Penumbra and Silk Road Medical. Contact PI for NIH Grant R21NS128641. Dr Min S. Park: Consultant for Medtronic. Dr Michael R Levitt: Unrestricted educational grants from Medtronic and Stryker; consulting agreement with Medtronic, Aeaean Advisers and Metis Innovative; equity interest in Proprio, Stroke Diagnostics, Apertur, Stereotaxis, Fluid Biomed, and Hyperion Surgical; editorial board of Journal of NeuroInterventional Surgery; Data safety monitoring board of Arsenal Medical. Dr Waleed Brinjikji: Holds equity in Nested Knowledge, Superior Medical Editors, Piraeus Medical, Sonoris Medical, and MIVI Neurovascular. He receives royalties from Medtronic and Balloon Guide Catheter Technology. He receives consulting fees from Medtronic, Stryker, Imperative Care, Microvention, MIVI Neurovascular, Cerenovus, Asahi, and Balt. He serves in a leadership or fiduciary role for MIVI Neurovascular, Marblehead Medical LLC, Interventional Neuroradiology (Editor in Chief), Piraeus Medical, and WFITN. Dr Richard Williamson: Consultant for Medtronic, Stryker, and Synaptive Medical. Dr Pedro Navia: Consultant for Penumbra, Medtronic, Stryker, Cerenovus and Balt. Dr Peter Kan: Grants from the NIH (1U18EB029353-01) and unrestricted educational grants from Medtronic and Siemens. Consultant for Imperative Care and Stryker Neurovascular. Stock ownership in Vena Medical. Dr Reade De Leacy: PI for Imperative Trial; Research grants from Siemens Healthineers and Kaneka medical. Consultant for Cerenovus, Stryker Neurovascular and Sim & Cure. Minor equity interest Vastrax, Borvo medical, Synchron, Endostream, Von Vascular, Radical catheters and Precision Recovery Inc. Dr Shakeel A Chowdhry: Consultant and proctor for Medtronic and Microvention Dr Mohammad Ezzeldin: Consultant for Viz.ai and Imperative care. Investments in Galaxy Therapeutics. Dr David J Altschul: Consultant for MicroVention, Stryker, and Cerenovus. Dr Ramesh Grandhi: Consultant for Balt Neurovascular, Cerenovus, Medtronic Neurovascular, Rapid Medical, and Stryker Neurovascular. Dr Alejandro M Spiotta: Consultant for Penumbra, Terumo, RapidAI, Cerenovus. Dr Ali Alaraj: Consultant for Cerenovus. Dr Sameh Samir Elawady, Dr Rahim Abo Kasem, Dr Bhageeradh Mulpur, Dr Conor M. Cunningham, Dr Mohamed Mahdi Sowlat, Dr Atakan Orscelik, Noah L.A. Nawabi, Dr Julio Isidor, Dr Pascal Jabbour, Dr Joon-Tae Kim, Dr Stacey Quintero Wolfe, Dr Ansaar Rai, Dr Ali Alawieh, Dr Justin Mascitelli, Dr Isabel Fragata, Dr Adam Polifka, Dr Fazeel Siddiqui, Dr Joshua Osbun, Dr Roberto Crosa, Dr Mark Moss, Dr Ergun Daglioglu, Dr Nitin Goyal: none.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research support from Penumbra, Microvention, Medtronic, Stryker, RapidAI, and Brain Aneurysm Foundation.
ORCID iDs: Rahim Abo Kasem https://orcid.org/0000-0002-9635-7471
Joshua M Venegas https://orcid.org/0009-0007-6926-1339
Stacey Quintero Wolfe https://orcid.org/0000-0001-7603-2728
Shinichi Yoshimura https://orcid.org/0000-0001-5633-9132
Hugo Cuellar https://orcid.org/0000-0002-8348-4535
Ali Alaraj https://orcid.org/0000-0002-1491-4634
Mohamad Ezzeldin https://orcid.org/0000-0001-7740-8774
Isabel Fragata https://orcid.org/0000-0002-7037-7458
Ramesh Grandhi https://orcid.org/0000-0001-9000-6083
Charles Matouk https://orcid.org/0000-0003-3234-9541
David J Altschul https://orcid.org/0000-0002-5130-1378
References
- 1.Saini V, Guada L, Yavagal DR. Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology 2021; 97: S6–S16. [DOI] [PubMed] [Google Scholar]
- 2.Adams HP, Brott TG, Furlan AJ, et al. Guidelines for thrombolytic therapy for acute stroke: a supplement to the guidelines for the management of patients with acute ischemic stroke. Circulation 1996; 94: 1167–1174. [DOI] [PubMed] [Google Scholar]
- 3.Powers WJ, Derdeyn CP, Biller J, et al. American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015; 2015: 3020–3035. [DOI] [PubMed] [Google Scholar]
- 4.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. [DOI] [PubMed] [Google Scholar]
- 5.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018; 378: 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 7.Zi W, Qiu Z, Li F, et al. Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: the DEVT randomized clinical trial. JAMA 2021; 325: 234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang P, Zhang Y, Zhang L, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med 2020; 382: 1981–1993. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki K, Matsumaru Y, Takeuchi M, et al. Effect of mechanical thrombectomy without vs with intravenous thrombolysis on functional outcome among patients with acute ischemic stroke: the SKIP randomized clinical trial. JAMA 2021; 325: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LeCouffe NE, Kappelhof M, Treurniet KM, et al. A randomized trial of intravenous alteplase before endovascular treatment for stroke. N Engl J Med 2021; 385: 1833–1844. [DOI] [PubMed] [Google Scholar]
- 11.Fischer U, Kaesmacher J, Strbian D, et al. Thrombectomy alone versus intravenous alteplase plus thrombectomy in patients with stroke: an open-label, blinded-outcome, randomised non-inferiority trial. Lancet 2022; 400: 104–115. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell PJ, Yan B, Churilov L, et al. Endovascular thrombectomy versus standard bridging thrombolytic with endovascular thrombectomy within 4·5 h of stroke onset: an open-label, blinded-endpoint, randomised non-inferiority trial. Lancet 2022; 400: 116–125. [DOI] [PubMed] [Google Scholar]
- 13.Kaesmacher J, Boeckh-Behrens T, Simon S, et al. Risk of thrombus fragmentation during endovascular stroke treatment. AJNR Am J Neuroradiol 2017; 38: 991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer U, Kaesmacher J, Pereira VM, et al. Direct mechanical thrombectomy versus combined intravenous and mechanical thrombectomy in large-artery anterior circulation stroke. Stroke 2017; 48: 2912–2918. [DOI] [PubMed] [Google Scholar]
- 15.Tsang COA, Cheung IHW, Lau KK, et al. Outcomes of stent retriever versus aspiration-first thrombectomy in ischemic stroke: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2018; 39: 2070–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rha J-H, Saver JL. The impact of recanalization on ischemic stroke outcome. Stroke 2007; 38: 967–973. [DOI] [PubMed] [Google Scholar]
- 17.Almallouhi E, Al Kasab S, Hubbard Z, et al. Outcomes of mechanical thrombectomy for patients with stroke presenting with low Alberta stroke program early computed tomography score in the early and extended window. JAMA Netw Open 2021; 4: e2137708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manning NW, Chapot R, Meyers PM. Endovascular stroke management: key elements of success. Cerebrovasc Dis 2016; 42: 170–177. [DOI] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344–349. [DOI] [PubMed] [Google Scholar]
- 20.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995; 274: 1017–1025. [PubMed] [Google Scholar]
- 21.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28: 3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin PC. An Introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46: 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeo L, Bhogal P, Gopinathan A, et al. Why does mechanical thrombectomy in large vessel occlusion sometimes fail? A review of the literature. Clin Neuroradiol 2019; 29: 401–414. [DOI] [PubMed] [Google Scholar]
- 24.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 25.Rinkel LA, Treurniet KM, Kappelhof M, et al. Influence of time metrics on the treatment effect of intravenous alteplase prior to endovascular treatment in MR CLEAN-NO IV. J Neurointerv Surg 2023; 15: e54–e59. [DOI] [PubMed] [Google Scholar]
- 26.Gariel F, Lapergue B, Bourcier R, et al. Mechanical thrombectomy outcomes with or without intravenous thrombolysis. Stroke 2018; 49: 2383–2390. [DOI] [PubMed] [Google Scholar]
- 27.Murphy A, Symons SP, Hopyan Jet al. et al. Factors influencing clinically meaningful recanalization after IV-rtPA in acute ischemic stroke. AJNR Am J Neuroradiol 2013; 34: 146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Lorenzo R, Saqqur M, Buletko AB, et al. IV tPA given in the golden hour for emergent large vessel occlusion stroke improves recanalization rates and clinical outcomes. J Neurol Sci 2021; 428: 117580. [DOI] [PubMed] [Google Scholar]
- 29.Czap AL, Parker S, Yamal JM, et al. Immediate recanalization of large-vessel occlusions by tissue plasminogen activator occurs in 28% of patients treated in a mobile stroke unit. Stroke Vasc Interv Neurol 2022; 2: e000101. [Google Scholar]
- 30.Rohan V, Baxa J, Tupy R, et al. Length of occlusion predicts recanalization and outcome after intravenous thrombolysis in middle cerebral artery stroke. Stroke 2014; 45: 2010–2017. [DOI] [PubMed] [Google Scholar]
- 31.Kim YD, Nam HS, Yoo J, et al. Prediction of early recanalization after intravenous thrombolysis in patients with large-vessel occlusion. J Stroke 2021; 23: 244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seners P, Turc G, Maïer B, et al. Incidence and predictors of early recanalization after intravenous thrombolysis. Stroke 2016; 47: 2409–2412. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Wang J, Ge L, et al. A fibrin targeted molecular imaging evaluation of microvascular no-reflow in acute ischemic stroke. Brain Behav 2022; 12: e2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desilles J-P, Loyau S, Syvannarath V, et al. Alteplase reduces downstream microvascular thrombosis and improves the benefit of large artery recanalization in stroke. Stroke 2015; 46: 3241–3248. [DOI] [PubMed] [Google Scholar]
- 35.Faizy TD, Broocks G, Heit JJ, et al. Association between intravenous thrombolysis and clinical outcomes among patients with ischemic stroke and unsuccessful mechanical reperfusion. JAMA Netw Open 2023; 6: e2310213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rozes C, Maier B, Gory B, et al. Influence of prior intravenous thrombolysis on outcome after failed mechanical thrombectomy: ETIS registry analysis. J Neurointerv Surg 2022; 14: 688–692. [DOI] [PubMed] [Google Scholar]
- 37.Wunderlich MT, Goertler M, Postert Tet al. et al. Recanalization after intravenous thrombolysis. Does a recanalization time window exist? Neurology 2007;68:1364–1368. doi: 10.1212/01.wnl.0000260604.26469.8e [DOI] [PubMed] [Google Scholar]
- 38.Klonaris C, Alexandrou A, Katsargyris A, et al. Late spontaneous recanalization of acute internal carotid artery occlusion. J Vasc Surg 2006; 43: 844–847. [DOI] [PubMed] [Google Scholar]
- 39.Kassem-Moussa H, Graffagnino C. Nonocclusion and spontaneous recanalization rates in acute ischemic stroke: a review of cerebral angiography studies. Arch Neurol 2002; 59: 1870–1873. [DOI] [PubMed] [Google Scholar]
- 40.Shah PS, Hingorani A, Ascher E, et al. Spontaneous recanalization of an occluded internal carotid artery. Ann Vasc Surg 2010; 24: e951–e954. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen-Huynh MN, Lev MH, Rordorf G. Spontaneous recanalization of internal carotid artery occlusion. Stroke 2003; 34: 1032–1034. [DOI] [PubMed] [Google Scholar]
- 42.Xu Y, Qian G, Wei L, et al. Predictive factors for the spontaneous recanalization of large and middle cerebral arteries after acute occlusion. J Stroke Cerebrovasc Dis 2016; 25: 1896–1900. [DOI] [PubMed] [Google Scholar]
- 43.Ospel JM, Singh N, Almekhlafi MA, et al. Early recanalization with alteplase in stroke because of large vessel occlusion in the ESCAPE trial. Stroke 2021; 52: 304–307. [DOI] [PubMed] [Google Scholar]
- 44.Kranendonk KRV, Treurniet KM, Boers AMM, et al. Hemorrhagic transformation is associated with poor functional outcome in patients with acute ischemic stroke due to a large vessel occlusion. J Neurointerv Surg 2019; 11: 464–468. [DOI] [PubMed] [Google Scholar]
- 45.Vellimana AK, Washington CW, Yarbrough CK, et al. Thrombolysis is an independent risk factor for poor outcome after carotid revascularization. Neurosurgery 2018; 83: 922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whiteley WN, Emberson J, Lees KR, et al. Risk of intracerebral haemorrhage with alteplase after acute ischaemic stroke: a secondary analysis of an individual patient data meta-analysis. Lancet Neurol 2016; 15: 925–933. [DOI] [PubMed] [Google Scholar]
- 47.Kanazawa M, Takahashi T, Nishizawa Met al. et al. Therapeutic strategies to attenuate hemorrhagic transformation after tissue plasminogen activator treatment for acute ischemic stroke. J Atheroscler Thromb 2017; 24: 240–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Tsuji K, Lee S-R, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke 2004; 35: 2726–2730. [DOI] [PubMed] [Google Scholar]
- 49.Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke 2002; 33: 831–836. [DOI] [PubMed] [Google Scholar]
- 50.Hong JM, Kim DS, Kim M. Hemorrhagic transformation after ischemic stroke: mechanisms and management. Front Neurol 2021; 12: 703258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Desai SM, Tonetti DA, Morrison AA, et al. Relationship between reperfusion and intracranial hemorrhage after thrombectomy. J Neurointerv Surg 2020; 12: 448–453. [DOI] [PubMed] [Google Scholar]
- 52.Mustanoja S, Meretoja A, Putaala J, et al. Outcome by stroke etiology in patients receiving thrombolytic treatment. Stroke 2011; 42: 102–106. [DOI] [PubMed] [Google Scholar]
- 53.Akbik F, Alawieh A, Dimisko L, et al. Bridging thrombolysis in atrial fibrillation stroke is associated with increased hemorrhagic complications without improved outcomes. J Neurointerv Surg 2022; 14: 979–984. [DOI] [PubMed] [Google Scholar]
- 54.Hassan AE, Kotta H, Garza L, et al. Pre-thrombectomy intravenous thrombolytics are associated with increased hospital bills without improved outcomes compared with mechanical thrombectomy alone. J Neurointerv Surg 2019; 11: 1187–1190. [DOI] [PubMed] [Google Scholar]
- 55.Atchaneeyasakul K, Desai S, Malhotra K, et al. Intravenous tPA delays door-to-puncture time in acute ischemic stroke with large vessel occlusion. J Stroke Cerebrovasc Dis 2021; 30: 105732. doi: 10.1016/j.jstrokecerebrovasdis.2021.105732 [DOI] [PubMed] [Google Scholar]