Abstract
High-grade dural arteriovenous fistulas (DAVFs) are known to demonstrate classical dural supply and can demonstrate pre-existing dural supply and ‘pure’ arterial supply from pial branches. The latter two are examples of congenital versus acquired pial to dural shunting, respectively. We describe the recognition of dural to pial supply during combined transarterial and transvenous embolization of a high-grade DAVF with holocephalic venous reflux, stressing the importance of careful assessment of this condition with micro catheter injections.
Keywords: Dural arteriovenous fistula, pial supply, dural supply, endovascular treatment, VEGF
Introduction
Dural arteriovenous fistulas (DAVFs) are acquired and abnormal connections between meningeal arteries, shunting into dural venous sinus and / or cortical veins. Retrograde flow into cortical veins defines a DAVF as high grade, with an annual risk of adverse events and mortality up to 15.0% and 10.4%, respectively. 1
Various types of arterial supply of DAVFs have been identified: (1) classical dural to fistulous supply from extradural arteries; (2) supply from dilated pre-existing, physiological or congenital dural branches of intradural pial arteries (e.g., the artery of Davidoff and Schechter); (3) de novo ‘pure’ pial supply from dilated tortuous vessels present in locations other than the known physiological or congenital connections between pial arteries and dura (e.g., distal branches of the anterior, middle and posterior cerebral arteries). 2
Dural arteriovenous fistulas with pial arterial supply (either pre-existing or de novo shunting) are more likely: (1) to demonstrate aggressive angioarchitectural characteristics (such as venous dilatation); (2) to present with hemorrhage; (3) located at the tentorium (either torcular or galenic type) and (4) have a male predisposition.2,3 Supply from pre-existing dural branches of pial arteries is present in up to 8.3%–9.0% of patients with a DAVF, whereas supply from ‘pure’ pial arteries is present in up to 3.9%–8.0%. A minority of DAVFs 1.0%–3.5% show coexistence of both types of pial arterial supply.2,3
Large retrospective case series have shown a low-risk profile of endovascular treatment of DAVFs with ‘pure’ arterial supply;2,3 however, case reports have pointed out a risk of severe hemorrhage from this type of arterial supply (including ‘pure’ distal ACA and PCA supply). Suggestions have made to close off ‘pure’ arterial supply at the start of an endovascular embolization procedure, prior to the steps towards curing the DAVF, as closing of the venous outflow route of fragile ‘pure’ arterial vessels might result in rupture of the latter.4,5
Apart from classical dural supply, pre-existing dural supply from pial arteries and ‘pure’ pial arterial supply, we would like to point out a rare fourth type of shunting in high-grade DAVFs, the so-called dural to pial supply, wherein a dural branch supplying the DAVF, demonstrates pial supply to a significant area of brain parenchyma during and after embolization. Here, we present a technical note of a high-grade DAVF, with all four subtypes of dural to pial or pial to dural shunting.
Case description
Clinical presentation and angioarchitecture
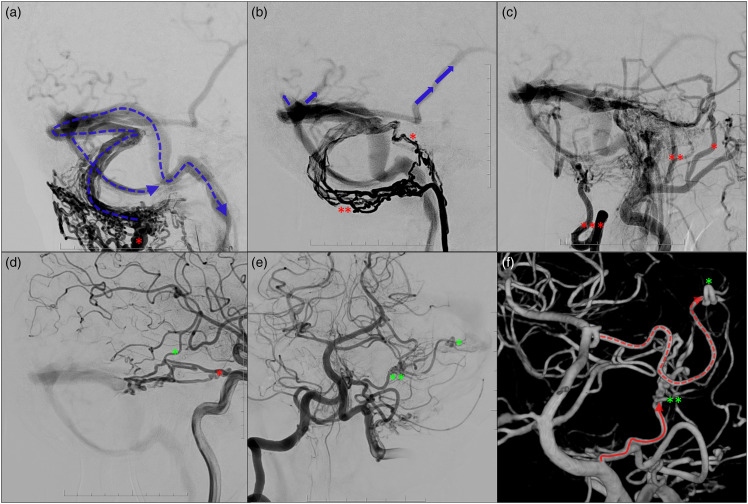
A patient in their 60s presented with cognitive decline, weight loss, lethargy and difficulty swallowing over the last months. He underwent a left subtotal petrosectomy for resection of a schwannoma, limited left neck dissection and reconstruction of a skull base defect with a flap three years ago, in which the patient developed acute thrombosis of the left transverse sinus post-operatively. Cross-sectional imaging and diagnostic cerebral angiogram during the current presentation demonstrated a multi-hole high-grade DAVF located at the left distal transverse sinus, continuing along with an adjacent parasinus in the posterior fossa (Figure 1). There was classical dural supply from left external carotid artery (ECA) branches: the left occipital artery (OA), neuromeningeal trunk of the left ascending pharyngeal artery (ApharA), left superficial temporal artery, left posterior auricular artery, left deep temporal artery and left distal internal maxillary artery branches. There was classic dural supply from the left internal carotid artery (ICA; lateral and medial tentorial branches) and right ECA branches (right OA transosseous branches, neuro-meningeal trunk arising from and the right proximal OA). There was supply from pre-existing dural to pial branches: the posterior meningeal arteries from the right and left postero-inferior cerebrellar artery and the left antero-inferior cerebellar artery (or subarcuate artery). There was ‘pure’ arterial supply from the left P2 posterior cerebral artery and small distal right MCA branches. Lastly, there was extensive transosseous supply from the left deep cervical artery, originating from the left subclavian artery. There was retrograde drainage into the ipsilateral dural venous system, with dural venous reflux into the distal superior sagittal sinus and straight sinus and cortical venous reflux to the left cerebral and cerebellar hemisphere, as well as reflux into the left ophthalmic vein. The left sigmoid sinus was removed during the petrosectomy. Late venous phase angiography demonstrated extensive holocephalic venous congestion above and below the tentorium cerebelli.
Figure 1.
DSA shows a multi-hole high-grade DAVF located at the left distal transverse sinus, continuing along an adjacent parasinus in the posterior fossa. There is extensive transosseous supply from the left deep cervical artery (a; red asterisk), originating from the left subclavian artery, classical dural supply from left external carotid artery branches including the neuromeningeal trunk of the left ascending pharyngeal artery (b; single and double red asterisks), the left occipital artery (c; triple red asterisks) and left superficial temporal artery, left posterior auricular artery, left deep temporal artery and left distal internal maxillary artery branches (c). There was supply from the left internal carotid artery lateral and medial tentorial branches (d; red asterisk). There was supply from pre-existing dural to pial branches: the posterior meningeal arteries from the right and left postero-inferior cerebrellar artery (PICA; e) and left antero-inferior cerebellar artery (or subarcuate artery; e; double green asterisks). There was ‘pure’ arterial supply from the left P2 posterior cerebral artery (e; single green asterisk) and smaller more distal right MCA branches (d; single green asterisk). There was retrograde drainage into the ipsilateral dural venous system (a), with dural venous reflux into the distal superior sagittal sinus and straight sinus and cortical venous reflux to the left cerebral and cerebellar hemisphere, including the contralateral vein of Labbe (b).
Treatment
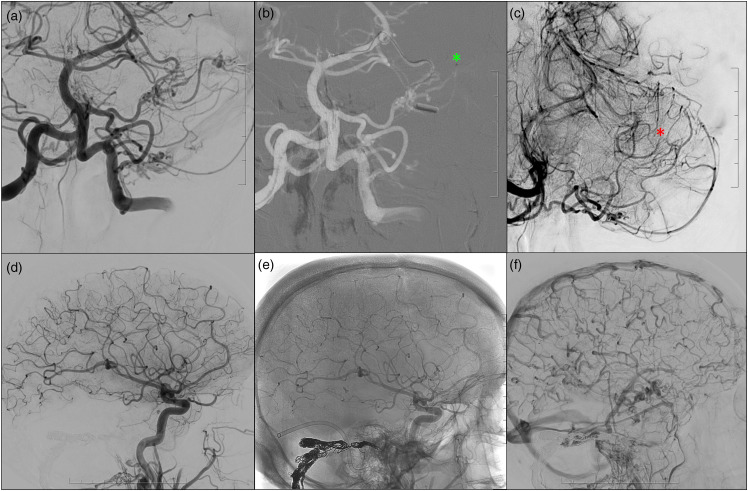
Transvenous coil embolization was the preferred endovascular treatment modality given: (1) the multihole fistulous segment; (2) absence of right cerebral and cerebellar venous drainage in the affected sinus; (3) involvement of multiple dural arteries supplying various cranial nerves (e.g., tentorial arteries – CN III, IV, V, VI; arteries of the facial arcade; neuromeningeal trunk of the APharA – CN IX, X, XI). Prior to commencing transvenous coil embolization via a contralateral transjugular approach, the ‘pure’ arterial supply of the left distal PCA branch was obliterated via superselective embolization with 50% Gluebran: 50% Lipiodol through a 1.2 Magic flow-directed microcatheter (BALT neurovascular). The pre-existing dural supply from the left subarcuate artery was left untouched. Subsequently, the affected parasinus in the posterior fossa and right distal transverse sinus were occluded with a dual-microcatheter technique with coils (Figure 2). Given the global sluggish venous flow after closure of the DAVF (Figure 2), the patient was kept on low-dose heparin overnight to prevent overshooting venous thrombosis formation. The follow-up CT brain the next day was unremarkable. The patient had no new neurological deficits postoperatively, with gradual cognitive improvement. Three-month follow-up DSA demonstrated complete cure of this high-grade DAVF.
Figure 2.
Prior to transvenous coil embolization via a contralateral transjugular approach, the ‘pure’ arterial supply of the left distal PCA branch was obliterated via superselective embolization with 50% Gluebran: 50% Lipiodol through a 1.2 Magic flow-directed microcatheter (BALT neurovascular), pre embolization (b; green asterisk) and post embolization (c; red asterisk). The pre-existing dural supply from the left subarcuate artery was left untouched. Subsequently, the affected parasinus in the posterior fossa and right distal transverse sinus were occluded with a dual-microcatheter technique with coils, with complete occlusion of the DAVF after embolization (d, subtracted image; e, unsubtracted images demonstrating the coil skeleton). There is global slow flow in cortical veins with normalized antegrade flow in the contralateral vein of Labbe on post embolization angiogram (f).
Angioarchitecture related ‘dural to pial supply’
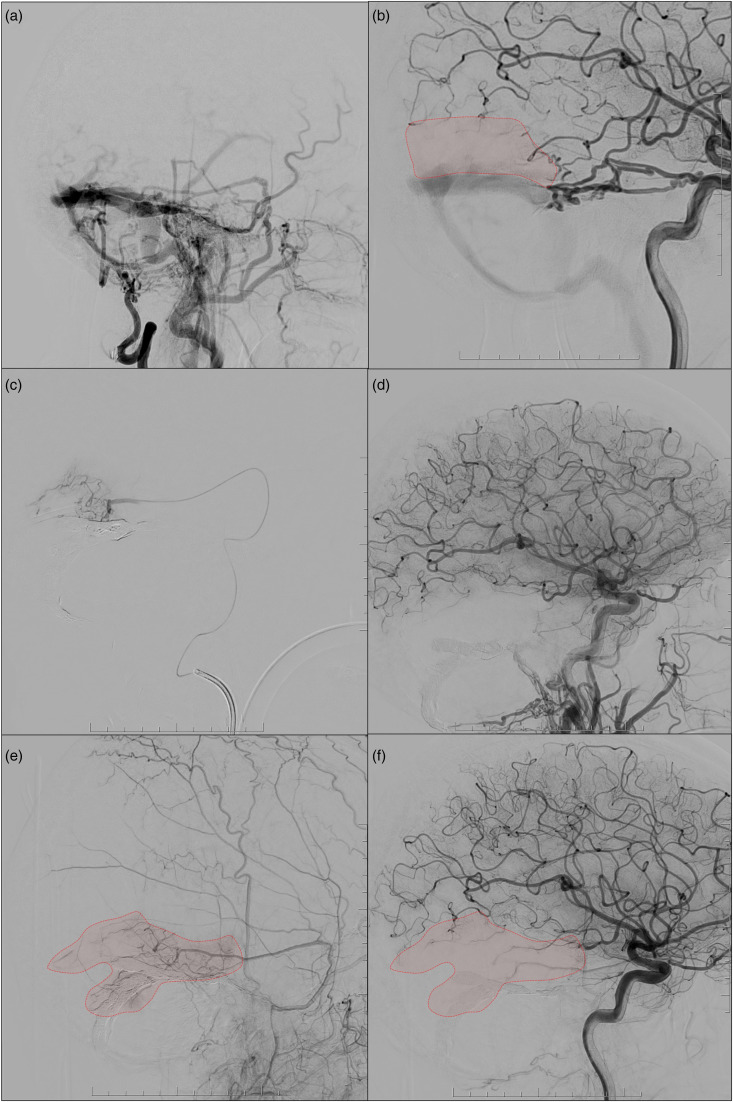
The left ICA injection showed a relatively avascular area (posterior middle temporal gyrus) distal to the pial supply of several MCA branches. This relatively avascular area was supplied by dural to pial arterial supply from the left MMA petrosquamosal branch, discovered on superselective microcatheter injection of this arterial branch to confirm closure of the DAVF post embolization. This dural to pial supply was again demonstrated and confirmed on three-month follow-up cerebral angiography (Figure 3).
Figure 3.
The pre-treatment external carotid injection shows numerous classical feeders, but no cortical supply of the MMA (a). On the other hand, the internal carotid artery (ICA) injection showed an avascular area distal to the pial supply of the MCA branch (b, red territory). A superselective injection of the petrosquamosal branch of the right MMA was performed to confirm closure of the DAVF during the procedure. Microinjection demonstrated dural to pial supply to a large area of the right posterior middle temporal gyrus (c) and possibly a smaller area of the left superior cerebellar artery territory. ICA injection performed after closure of the DAVF showed slow flow of the distal MCA where the pial supply was located, and the distal parenchyma was replaced by blood flow from the MMA. The dural to pial supply was confirmed on three-month follow-up cerebral angiography, with established supply from the MMA to the right temporal lobe (e and f).
Discussion
We present a case of a high-grade DAVF successfully treated with a combined strategy of trans-arterial and trans-venous embolization, involving four subtypes of shunting: classical dural arterial branches, pre-existing dural branches of pial arteries, de novo ‘pure’ pial supply, 2 and dural to pial supply. The presence of all four subtypes of arterial supply to fistulas in this case is an unusual finding, which suggests underlying pathophysiological alterations leading to significant angiogenesis. It is important to recognize these different subtypes of arterial supply as they have significant implications for treatment.
Pial arterial supply of DAVFs has been recognized and clinically characterized,4,5 with cases exhibiting both types of pial supply being rare.2,3 Although the pathogenesis of DAVFs is not fully understood, analysis of human specimens6,7 and animal models8,9 based on venous hypertension has revealed the crucial role of angiogenic factors such as hypoxia-inducible factor 1 (HIF-1) and vascular endothelial growth factor (VEGF). Importantly, these human and animal models have shown high expression levels of angiogenic factors not only in the endothelium but also in the perivascular tissue, dura and cortex.7–9 This phenomenon is believed to promote pial angiogenesis and contribute to the formation of pial supply in DAVFs.
This case demonstrated a fourth type of shunting: dural to pial supply. Generally, dural to pial supply is associated with conditions such as Moya Moya Disease (MMD) or brain arteriovenous malformations (BAVMs). In MMD, dural to pial supply is formed due to decreased cerebral blood flow (CBF), 10 and surgically induced pial synangiosis via indirect revascularization has proven effective in improving CBF. 11 This type of supply to the ischemic brain has been shown to be inducible in pathological conditions other than MMD and has been utilized in treatment. 12 Similarly, pial supply has been reported in BAVM, with studies indicating pial supply in approximately 11% of BAVMs. This suggests that VEGF production by BAVMs may induce angiogenesis. 13 Interestingly, the pial supply of BAVM is predominantly observed in the temporooccipital lobe, in close proximity to the pial supply of tentorial DAVFs, suggesting a homology between dura and pia connections in DAVFs and BAVMs. In high-grade DAVF leading to venous reflux, regional hypoperfusion of the brain has been observed in the quantitative regional CBF studies.14–16 Furthermore, cerebral ischemia is known to occur in pial AVFs due to steal phenomenon. 17 Therefore, it is reasonable that focal ischemia of the brain parenchyma progresses in high-grade DAVF with a pial supply. Dural arteriovenous fistulas are believed to potentially express HIF-1 and VEGF in both the dura and parenchyma, secondary to ischemia in the brain parenchyma, implicating the formation of dural to pial supply via these angiogenic factors.
In this technical note, the ‘pure’ pial supply was first selectively embolized, followed by double-catheter technique transvenous embolization of the fistulous segment to ensure complete fistulous occlusion. Additional selective injection from the left MMA post embolization identified a dural to pial supply. Recognition of this dural to pial supply prevented potential complication of cerebral infarction from overzealous embolization. Pial supply has been identified as an independent risk factor for ischemic complications in the treatment of DAVF. 18 Hetts et al. analyzed ischemic complications in DAVF treatment with pial supply and noted that some of these complications could be attributed to the influx of liquid embolic material into the pial arteries that supply the brain parenchyma rather than the DAVF. 18 Our case shows that identification of this rare dural to pial supply is just as important to prevent similar complications, which was best recognized on microcatheter injections and especially after shunt reduction or obliteration. Therefore, performing selective microcatheter injections prior to embolization and re-assessment of angiographic findings after partial or complete embolization would be the critical steps to identify possible dural and pial anastomosis.
In conclusion, this case report illustrates the four different types of arterial shunting in fistulas and potential development of anastomosis between dural and pial arteries. It is especially important to recognize the ‘pure’ pial arterial supply and the dural – pial supply to minimize potential complications from embolization.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: EH is consultant for Medtronic.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: EH and TK thank UMIT for their kind support.
ORCID iDs: Shigeta Miyake https://orcid.org/0000-0002-1887-3227
Eef Jacobus Hendriks https://orcid.org/0000-0003-2100-7992
References
- 1.van Dijk JMC, terBrugge KG, Willinsky RA, et al. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke 2002; 33: 1233–1236. [DOI] [PubMed] [Google Scholar]
- 2.Osada T, Krings T. Intracranial dural arteriovenous fistulas with pial arterial supply. Neurosurgery 2019; 84: 104–115. [DOI] [PubMed] [Google Scholar]
- 3.Brinjikji W, Cloft HJ, Lanzino G. Clinical, angiographic, and treatment characteristics of cranial dural arteriovenous fistulas with pial arterial supply. J Neurointerv Surg 2021; 13: 331–335. 20200629. [DOI] [PubMed] [Google Scholar]
- 4.Wu Q, Zhang X-S, Wang H-D, et al. Onyx embolization for tentorial dural arteriovenous fistula with pial arterial supply: case series and analysis of complications. World Neurosurg 2016; 92: 58–64. 20160420. [DOI] [PubMed] [Google Scholar]
- 5.Sato K, Matsumoto Y, Endo H, et al. A hemorrhagic complication after onyx embolization of a tentorial dural arteriovenous fistula: a caution about subdural extension with pial arterial supply. Interv Neuroradiol 2017; 23: 307–312. 20170101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uranishi R, Nakase H, Sakaki T. Expression of angiogenic growth factors in dural arteriovenous fistula. J Neurosurg 1999; 91: 781–786. [DOI] [PubMed] [Google Scholar]
- 7.Tirakotai W, Bertalanffy H, Liu-Guan B, et al. Immunohistochemical study in dural arteriovenous fistulas and possible role of local hypoxia for the de novo formation of dural arteriovenous fistulas. Clin Neurol Neurosurg 2005; 107: 455–460. 2005/10/06. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Zhang Q, Huang Q-H, et al. A pivotal role of the vascular endothelial growth factor signaling pathway in the formation of venous hypertension-induced dural arteriovenous fistulas. Mol Med Rep 2014; 9: 1551–1558. 2014/03/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S-S, Li C-H, Zhang X-J, et al. Investigation of the mechanism of dural arteriovenous fistula formation induced by high intracranial venous pressure in a rabbit model. BMC Neurosci 2014; 15: 101. 2014/08/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol 1969; 20: 288–299. 1969/03/01. [DOI] [PubMed] [Google Scholar]
- 11.Lin N, Aronson JP, Manjila S, et al. Treatment of moyamoya disease in the adult population with pial synangiosis. J Neurosurg 2014; 120: 612–617. 2014/01/11. [DOI] [PubMed] [Google Scholar]
- 12.Hong JM, Choi MH, Park GH, et al. Transdural revascularization by multiple burrhole after erythropoietin in stroke patients with cerebral hypoperfusion: a randomized controlled trial. Stroke 2022; 53: 2739–2748. 2022/05/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozaki T, Lee H, Krings T. Characteristics of pial brain arteriovenous malformations with transdural arterial supply. Eur J Radiol 2021; 139: 109670. 2021/04/13. [DOI] [PubMed] [Google Scholar]
- 14.Lagares A, Millán JM, Ramos A, et al. Perfusion computed tomography in a dural arteriovenous fistula presenting with focal signs: vascular congestion as a cause of reversible neurologic dysfunction. Neurosurgery 2010; 66: E226–E227. discussion E227. 2009/12/22. [DOI] [PubMed] [Google Scholar]
- 15.Kanemaru K, Kinouchi H, Yoshioka H, et al. Cerebral hemodynamic disturbance in dural arteriovenous fistula with retrograde leptomeningeal venous drainage: a prospective study using (123)I-iodoamphetamine single photon emission computed tomography. J Neurosurg 2015; 123: 110–117. 2015/04/11. [DOI] [PubMed] [Google Scholar]
- 16.Kanemaru K, Yoshioka H, Hashimoto K, et al. Neuronal dysfunction and hemodynamic disturbance due to venous congestion in dural arteriovenous fistula revealed by 123I-iomazenil SPECT. J Neurosurg 2023; 138: 760–767. 2022/07/31. [DOI] [PubMed] [Google Scholar]
- 17.Yu J, Shi L, Lv X, et al. Intracranial non-galenic pial arteriovenous fistula: a review of the literature. Interv Neuroradiol 2016; 22: 557–568. 2016/07/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hetts SW, Yen A, Cooke DL, et al. Pial artery supply as an anatomic risk factor for ischemic stroke in the treatment of intracranial dural arteriovenous fistulas. AJNR Am J Neuroradiol 2017; 38: 2315–2320. 2017/10/04. [DOI] [PMC free article] [PubMed] [Google Scholar]