Abstract
Endovascular mechanical thrombectomy has significantly improved recovery in acute ischemic stroke (AIS). While traditional patient selection has relied on factors such as last known well and penumbra volume, emerging research highlights the importance of collateral circulation in influencing thrombectomy success. However, current methods to assess collateral circulation are often unreliable and lack standardization, limiting their integration into clinical decision-making and prompting the need for innovative approaches. This study introduces trans-clot manometry as a promising approach for quantitatively assessing collateral blood flow before thrombectomy. Two patients were included in this study: a 64-year-old female with a left M1 near-complete occlusion and an 81-year-old male with a left P1 occlusion. After receiving intravenous tenecteplase, each patient underwent emergent thrombectomy where intraoperative trans-clot manometry revealed significant trans-clot mean arterial pressure (MAP) gradients (66.7% for Patient 1 and 96.9% for Patient 2). Both patients had successful first-pass thrombectomy (Patient 1: TICI 3; Patient 2: TICI 3), with substantial clinical improvement (Patient 1: NIHSS 11 to 1; Patient 2: NIHSS 19 to 8). Intraoperative trans-clot manometry offers a simple yet powerful, objective, and generalizable measure of collateral circulation, applicable to a wide range of AIS cases regardless of clot location or vessel size. In addition, real-time correlations with heart-rate variability and radial artery pressures provide an intrinsic quality control, ensuring proper execution of the technique and accuracy of the resulting MAP gradient. Future research will focus on validating this approach, determining its generalizability, and establishing MAP gradient thresholds to enhance device selection and predict first-pass success.
Keywords: Acute ischemic stroke, thrombectomy, collaterals, manometry, MAP gradient
Introduction
Endovascular mechanical thrombectomy (MT) has revolutionized the management of acute ischemic stroke (AIS), dramatically enhancing recovery rates and significantly reducing disability. 1 Traditionally, patient selection for thrombectomy has relied on criteria from clinical trials, focusing on patient age, last known well time, Alberta Stroke Program Early CT score (ASPECTS), location of occlusion, infarct core volume, size of penumbra, and other factors.2–4 There is ongoing discussion about the role of collateral circulation, with emerging research suggesting that it may significantly influence thrombectomy success and patient outcomes.2,5–16 Despite this recognized importance, a reliable method for quantifying the strength of collateral blood flow has yet to be established, 17 thus limiting the integration of collateral status into clinical treatment decisions and underscoring the need for innovative quantifiable approaches. Here, we demonstrate a simple yet effective intraoperative technique utilizing trans-clot manometry to assess collateral circulation prior to thrombectomy in two patients, and discuss its applicability for guiding device selection and predicting success, as well as its broader implications for clinical practice.
Case report: Patient 1
Patient 2 is a 64-year-old female with a medical history of diabetes mellitus and hyperlipidemia (HLD). The patient was not on any antiplatelet or anticoagulation agents. She presented from home with acute onset of aphasia and right-sided weakness. Upon arrival at the emergency department, he was hypertensive (SBP 160 s) but otherwise vital signs and basic labs were unremarkable. Neurological examination revealed global aphasia, right homonymous hemianopia, and right upper and lower extremity weakness (motor research council scores 3/5 for muscle strength). His initial NIH Stroke Scale score was 11. An emergent CT scan showed no intracranial hemorrhage, no loss of gray-white differentiation, and no hyperdense vessel signs. CTA revealed near-complete occlusion of the distal left M1 segment and decreased opacification of an M2 branch.
The patient was an intravenous TNK candidate and received TNK approximately 50 min after the last known well time. The patient was then taken for endovascular MT. Under fluoroscopic guidance, the right common femoral artery was accessed using a micropuncture technique. An 8 French short sheath was placed, and 2000 units of heparin were administered. A 5F Bernstein select catheter and an 8F EmboGuard balloon guide catheter were introduced. A Rebar18 microcatheter and Synchro2 microwire were advanced through the clot to the left M2 branch.
The Synchro2 microwire was withdrawn, and the Rebar18 microcatheter was connected to an arterial pressure transducer. The transducer was calibrated to account for atmospheric pressure, and it was kept level with the patient's chest for at least 10 s to ensure accurate readings. Arterial pressures distal to the clot were measured, revealing a mean arterial pressure (MAP) of 20 mmHg with a stable waveform. At this point, the arterial line in the patient's left radial artery recorded a pressure of 120/58, corresponding to a MAP of 83. The Rebar18 microcatheter was then repositioned just proximal to the clot and reconnected to the arterial pressure transducer, which was recalibrated in the same manner as before. The pressures proximal to the clot were measured, showing a MAP of 60 with a stable waveform. Concurrently, the arterial line in the left radial artery recorded a pressure of 145/71, translating to a MAP of 101 (see Figure 1).
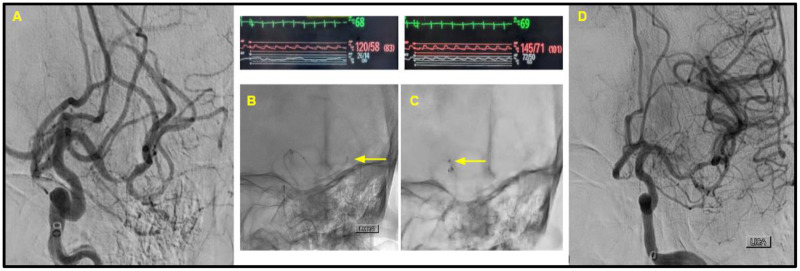
Figure 1.
Patient 1. (A) The initial selective digital subtraction angiogram from the left internal carotid artery, noting the left M1 near-complete occlusion and decreased opacification of the M2 branch. (B and C) The position of the Rebar18 microcatheter (with yellow arrow pointing to the distal tip). Immediately above these images is noted the heart rate (green), blood pressure from radial artery line (red), and manometry waveforms from the Rebar18 microcatheter (white). Trans-clot manometry shows a MAP of 20 mmHg distal to the clot, in comparison to the MAP of 60 mmHg proximal to the clot. (D) The successful first-pass recanalization with TICI score of 3.
The Synchro2 microwire was reinserted, and the Rebar18 microcatheter and Synchro2 microwire were readvanced through the clot. Continuous aspiration was applied from the reperfusion catheter using the Vaclock 20 cc for 3 min as it was advanced towards the left M1 MCA occlusion. The reperfusion catheter and microcatheter were removed from the body with suctioning from the reperfusion catheter (Vaclock) and from the balloon guide catheter (manual suctioning, 60 cc syringe). This achieved successful recanalization with a TICI 3 score. Red thrombus was visualized. Postprocedure, the patient was transferred to the NeuroICU and showed significant improvement. Her ability to follow commands and verbalize basic information improved markedly, though she continued to have some paraphasic errors. Her right-sided weakness in the arm and leg showed steady improvement and returned to baseline (motor research council scores 5/5 for muscle strength). His NIH Stroke Scale score decreased to 1, indicating substantial clinical recovery.
Case report: Patient 2
Patient 2 is an 84-year-old male with a medical history of a transient ischemic attack, coronary artery disease (CAD), hypertension, and HLD. The patient was taking Plavix and Effient for CAD. He presented from home with acute onset of aphasia and right-sided weakness. Upon arrival at the emergency department, he was hypertensive (SBP 180 s) but otherwise vital signs and basic labs were unremarkable. Neurological examination revealed expressive aphasia, slurred speech, right facial droop, and right upper and lower extremity weakness (motor research council scores 4/5 for muscle strength). His initial NIH Stroke Scale score was 19. An emergent CT scan showed no intracranial hemorrhage and no loss of gray-white differentiation but indicated a hyperdense left PCA. CTA revealed a left P1 occlusion.
The patient was an intravenous TNK candidate and received TNK approximately 1 h after the last known well time. The patient was then taken for endovascular MT. Under fluoroscopic guidance, the right common femoral artery was accessed using a micropuncture technique. An 8 French short sheath was placed, and 2000 units of heparin were administered. A 5F Bernstein select catheter and an 8F EmboGuard balloon guide catheter were introduced. A Rebar18 microcatheter and Synchro2 microwire were advanced through the clot to the left PCA P2.
The Synchro2 microwire was withdrawn, and the Rebar18 microcatheter was connected to an arterial pressure transducer. The transducer was calibrated to account for atmospheric pressure, and it was kept level with the patient's chest for at least 10 s to ensure accurate readings. Arterial pressures distal to the clot were measured, revealing a MAP of 3 mmHg with a stable waveform. At this point, the arterial line in the patient's left radial artery recorded a pressure of 173/61, corresponding to a MAP of 98. The Rebar18 microcatheter was then repositioned just proximal to the clot and reconnected to the arterial pressure transducer, which was recalibrated in the same manner as before. The pressures proximal to the clot were measured, showing a MAP of 97 with a stable waveform. Concurrently, the arterial line in the left radial artery recorded a pressure of 168/58, translating to a MAP of 102 (see Figure 2).
Figure 2.
A conceptual representation of the trans-clot MAP gradient.
The Synchro2 microwire was reinserted, and the Rebar18 microcatheter and Synchro2 microwire were readvanced through the clot. Thrombectomy was performed using a 3 × 32 mm Trevo stent retriever, achieving successful recanalization with a TICI 3 score. Red thrombus was visualized. Postprocedure, the patient was transferred to the NeuroICU and showed significant improvement. His ability to follow commands and verbalize basic information improved markedly. His facial droop reduced to mild, and right-sided weakness in the arm and leg showed steady improvement (motor research council scores 4+/5 for muscle strength). His NIH Stroke Scale score decreased to 8, indicating substantial clinical recovery.
Discussion
This case report introduces trans-clot manometry as a novel technique for quantitatively assessing collateral blood flow prior to endovascular MT. Mean arterial pressure measurements taken distal to and proximal to the clot reliably demonstrated a significant gradient in both patients, suggesting limited collateral circulation during an acute possibly embolic event, which would be consistent with the clinical history and first-pass thrombectomy success observed.
Collateral circulation plays a crucial role in the outcomes of AIS, as evidenced by a growing body of literature.2,5–9 The ESCAPE trial, the only randomized clinical study to date that included collateral blood flow as assessed using multiphase CT angiography in the selection of patients undergoing thrombectomy, suggested that rapid endovascular treatment in patients with moderate-to-good collateral circulation enhanced functional outcomes and lowered mortality. 10 Post hoc analyses of prominent endovascular thrombectomy trials including IMS 3, 11 SWIFT, 12 MR CLEAN,13,14 DAWN, 15 and DEFUSE 3 16 have all demonstrated that robust collateral flow can influence infarct growth, improve recanalization success, reduce hemorrhagic transformation, and potentially extend the therapeutic time window for thrombectomy.
Despite this recognized importance, a reliable method for quantifying the strength of collateral blood flow has yet to be established. The ESCAPE trial used a noninvasive radiological measure utilizing multiphase CT angiography, which was further described by Menon et al.10,17 Another approach, popularized by the UCLA group, involves using the baseline digital subtraction angiogram prior to thrombectomy to assign an ASITN/SIR collateral score. This score categorizes collateral circulation into five grades: grades 0 and 1 indicate poor collaterals, grade 2 indicates fair collaterals, and grades 3 and 4 indicate good collateral circulation.11,12,15 While these approaches are commendable, they pose significant limitations given their subjective nature, possibility of interrater bias, and lack of standardization or generalizability given the heterogeneity of ischemic stroke lesions and patient populations. These drawbacks have thus far limited the integration of collateral status in clinical treatment decisions and underscore the need for innovative quantifiable approaches.
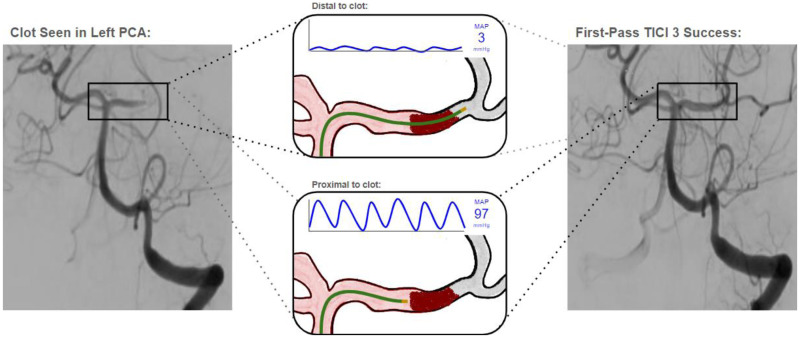
Intraoperative trans-clot manometry is a simple yet effective technique that offers a promising approach for assessing collateral circulation (see Figure 3 for a conceptual representation). The technique as described in this report yields quantifiable measures of MAP proximal to and distal to the ischemic clot, which can be expressed as an adjusted percentage gradient to indicate the strength of the collateral circulation using the formula: (MAPproximal – MAPdistal) / MAPdistal * 100%. This MAP gradient is inherently quantifiable, objective, targeted to the specific ischemic clot, indifferent to anterior or posterior circulation or large or small vessel lesions, and generalizable across patient populations. In addition, analysis of heart-rate variability on the pressure waveform and its correlation with the waveform derived from the radial artery provides an intrinsic real-time quality control, ensuring proper execution of the trans-clot manometry technique and the validity of the resulting MAP gradient.
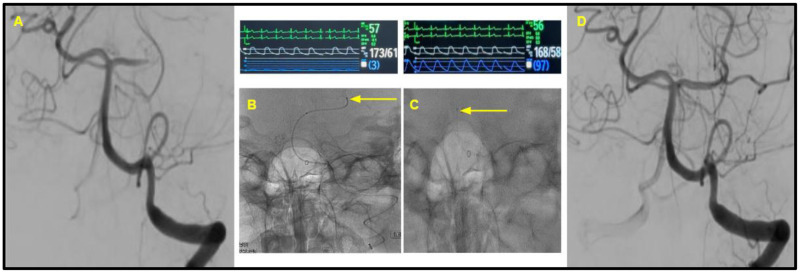
Figure 3.
Patient 2. (A) The initial selective digital subtraction angiogram from the left vertebral artery, noting the left P1 occlusion. (B and C) The position of the Rebar18 microcatheter (with yellow arrow pointing to the distal tip). Immediately above these images is noted the heart rate (green), blood pressure from radial artery line (white), and manometry waveforms from the Rebar18 microcatheter (blue). Trans-clot manometry shows a MAP of 3 mmHg distal to the clot, in comparison to the MAP of 97 mmHg proximal to the clot. (D) The successful first-pass recanalization with TICI score of 3.
Intraoperative trans-clot manometry showed a significant MAP gradient for both patients included in this study, with Patient 1 showing a 66.7% gradient (see Figure 1) and Patient 2 showing a 96.9% gradient (see Figure 2). The reliable waveforms and the consistency with radial artery measurements served to validate the observed gradient. While this was not a prospective or blinded study, we theorize that this intraoperative finding may independently suggest limited or absent collateral circulation, thus favoring an acute possibly embolic event that would be consistent with the patients’ clinical histories and witnessed acuity of symptom onset, rather than a subacute or chronic intracranial atherosclerotic disease. Further research is needed to determine whether the observed trans-clot manometry could have predicted the excellent first-pass success achieved in these two patients.
While the results from these two cases are promising, they represent preliminary data and pose several limitations. First, the limited sample size restricts the generalizability of the findings and underscores the need for further research with larger cohorts to validate the technique and to establish definitive thresholds for MAP gradients. Second, the measurements were neither prospective nor blinded, which may have introduced an inadvertent selection or observer bias. Third, the current technique involves passing the microcatheter through the clot twice, which may not be ideal and suggests that further optimization is needed to enhance procedural efficiency and minimize potential complications. One potential approach may be to obtain manometric measurements simultaneously from the microcatheter distal to the clot and the aspiration catheter proximal to the clot, but this may have its own limitations given the differences in luminal diameter and that the partial obstruction of flow within the aspiration catheter. Lastly, the effectiveness of the trans-clot manometry technique in predicting first-pass outcomes may vary across different patient populations and ischemic stroke locations, necessitating further studies to evaluate its broader applicability and reliability.
In conclusion, trans-clot manometry during thrombectomy offers a valuable, quantifiable, and generalizable method for the assessment of collateral circulation in AIS. This may help discern the potential etiology of the clot, with, acute embolic clots characterized by inadequate collateralization expected to exhibit more pronounced MAP gradients, whereas clots associated with intracranial atherosclerotic disease that develop progressively expected to show lower gradients indicative of more robust collateral flow. These real-time intraoperative insights can empower neurointerventionalists in their selection of thrombectomy devices, thereby enhancing the chances of achieving successful first-pass recanalization. Future research will focus on validating the trans-clot manometry technique across different patient populations and aim to establish MAP gradient thresholds to further enhance device selection and predict first-pass success during thrombectomy.
Acknowledgements
The authors gratefully acknowledge the patients who participated in this study, as well as the dedicated technicians, nurses, and healthcare professionals whose expertise and support were instrumental in making this research possible. Their contributions were invaluable to the success of this study. Special thanks to MeiLani Renger RN for inspiring the artwork.
Authors’ note: Writing assistance: No writing assistance or third-party services were used in the preparation of this manuscript. All work, including writing and editing, was completed solely by the authors themselves.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical considerations and informed consent: All procedures adhered to established clinical guidelines and ethical standards. Patients provided informed consent for the procedures as part of their routine clinical care. No special consents or research approvals were required for this study.
ORCID iD: Amit Chaudhari https://orcid.org/0000-0001-9051-3499
References
- 1.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American stroke association. Stroke 2019; 50: e344–e418. Epub 2019 Oct 30. Erratum in: Stroke. 2019 Dec;50(12):e440-e441. doi: 10.1161/STR.0000000000000215. PMID: 31662037. [DOI] [PubMed] [Google Scholar]
- 2.Nogueira RG, Jadhav AP, Haussen DC, et al. DAWN Trial investigators. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. Epub 2017 Nov 11. PMID: 29129157. [DOI] [PubMed] [Google Scholar]
- 3.Albers GW, Marks MP, Kemp S, et al. DEFUSE 3 Investigators. thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018; 378: 708–718. Epub 2018 Jan 24. PMID: 29364767; PMCID: PMC6590673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JS, Demchuk AM. Choosing a hyperacute stroke imaging protocol for proper patient selection and time efficient endovascular treatment: lessons from recent trials. J Stroke 2015; 17: 221–228. Epub 2015 Sep 30. PMID: 26437989; PMCID: PMC4612767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JS, Bang OY. Collateral status and outcomes after thrombectomy. Transl Stroke Res 2023; 14: 22–37. Epub 2022 Jun 10. PMID: 35687300. [DOI] [PubMed] [Google Scholar]
- 6.Regenhardt RW, González RG, He J, et al. Symmetric CTA collaterals identify patients with slow-progressing stroke likely to benefit from late thrombectomy. Radiology 2022; 302: 400–407. Epub 2021 Nov 2. PMID: 34726532; PMCID: PMC8792270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bang OY, Saver JL, Buck BH, et al. Liebeskind DS; UCLA collateral investigators. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry 2008; 79: 625–629. Epub 2007 Dec 12. PMID: 18077482; PMCID: PMC2702489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nannoni S, Cereda CW, Sirimarco G, et al. Collaterals are a major determinant of the core but not the penumbra volume in acute ischemic stroke. Neuroradiology 2019; 61: 971–978. Epub 2019 May 23. PMID: 31123760. [DOI] [PubMed] [Google Scholar]
- 9.Chung JW, Kim BJ, Jeong HG, et al. Selection of candidates for endovascular treatment: characteristics according to three different selection methods. J Stroke 2019; 21: 332–339. Epub 2019 Sep 30. PMID: 31590477; PMCID: PMC6780015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goyal M, Demchuk AM, Menon BK, et al. Hill MD; ESCAPE trial investigators. randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. Epub 2015 Feb 11. PMID: 25671798. [DOI] [PubMed] [Google Scholar]
- 11.Liebeskind DS, Tomsick TA, Foster LD, et al. Broderick JP; IMS III investigators. Collaterals at angiography and outcomes in the interventional management of stroke (IMS) III trial. Stroke 2014; 45: 759–764. Epub 2014 Jan 28. PMID: 24473178; PMCID: PMC3977615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liebeskind DS, Jahan R, Nogueira RG, et al. SWIFT Investigators. Impact of collaterals on successful revascularization in solitaire FR with the intention for thrombectomy. Stroke 2014; 45: 2036–2040. Epub 2014 May 29. PMID: 24876081; PMCID: PMC4157911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berkhemer OA, Jansen IG, Beumer D, et al. MR CLEAN investigators. Collateral Status on baseline computed tomographic angiography and intra-arterial treatment effect in patients with proximal anterior circulation stroke. Stroke 2016; 47: 768–776. Epub 2016 Jan 28. PMID: 26903582. [DOI] [PubMed] [Google Scholar]
- 14.Boers AM, Jansen IG, Berkhemer OA, et al. MR CLEAN trial investigators †. Collateral status and tissue outcome after intra-arterial therapy for patients with acute ischemic stroke. J Cereb Blood Flow Metab 2017; 37: 3589–3598. Epub 2016 Nov 19. PMID: 27864462; PMCID: PMC5669341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liebeskind DS, Saber H, Xiang B, et al. DAWN Investigators. Collateral circulation in thrombectomy for stroke after 6 to 24 hours in the DAWN trial. Stroke 2022; 53: 742–748. Epub 2021 Nov 3. PMID: 34727737. [DOI] [PubMed] [Google Scholar]
- 16.de Havenon A, Mlynash M, Kim-Tenser MA, et al. DEFUSE 3 Investigators. Results from DEFUSE 3: good collaterals are associated with reduced ischemic core growth but not neurologic outcome. Stroke 2019; 50: 632–638. PMID: 30726184; PMCID: PMC6628906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menon BK, d'Esterre CD, Qazi EM, et al. Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology 2015; 275: 510–520. Epub 2015 Jan 29. PMID: 25633505. [DOI] [PubMed] [Google Scholar]