Abstract
Background
The retina is affected by Parkinson’s disease (PD).
Purpose
We aimed to assess the anatomical and vascular deterioration of the retina in PD.
Methods
Sixty-six patients with PD and 66 healthy volunteers were evaluated in this study. Choriocapillaris vessel density (CCVD), superficial vascular density (SVD), deep vascular densities (DVD), central macular thickness (CMT), retinal nerve fibre layer (RNFL), ganglionic cell layer (GCL), and choroidal thickness (CT) were assessed.
Results
RNFL, GCL, CMT, and CT were thinner than in HC, and also the differences between the groups were statistically significant (P < .05). SVD and DVD were not statistically different between the groups (P > .05). There was a decrease in vascular density in all quadrants of the choriocapillary layer. The decrease in vascular density was statistically significant in the nasal, inferior and central quadrants (P < .05).
Conclusion
These results supported vascular thinning in the choroidal layer. Also showed that vascular and neural layers were affected together. It could help clinicians in the follow-up of Parkinson’s patients.
Keywords: Neurology, clinical neuroscience, Parkinson’s disease, vascular density, eye
Introduction
Different regions of the brain are affected by Parkinson’s disease (PD). Many different clinical conditions such as stiffness, cognitive impairment, instability, bradykinesia, and impaired contrast sensitivity are seen.1, 2
The relationship between PD and the eye has been investigated for many years. It is accepted that neurodegeneration and vascular components, which are key factors for occurring disease, may be effective in retinal and vascular involvement in the eye.3, 4
Thinning of the central macular thickness (CMT) and ganglion cell thickness (GCL) was demonstrated in many studies.5–8 However, different results have been reported about the change in choroidal thickness (CT). It has been reported that CT is increased.9, 10 Conversely, Eraslan et al. announced that CT decreases as disease severity increases. 11
In previous years, we used to measure CT mostly manually. However, with the developments in optical coherence tomography (OCT) technology, precise results with automatic measurements made by the device are possible.
On the other hand, advances in OCT technology allow for more detailed measurements of retinal layers. In particular, using OCT angiography (OCT-A), vascular density measurement in the retina and choroid layer can be done non-invasively.
Studies evaluating vascular density generally report a decrease.1, 12, 13 However, Rascuna et al. and Li et al. reported increased retinal vascular density values.5, 14 Although there are many studies evaluating retinal vascular density, only a few studies evaluating choriocapillary vascular density attracted our attention.
In those two studies, Zhang et al. and Robbins et al. reported decreased vascular density in the choriocapillary area.15, 16 So, we wanted to reevaluate the choriocapillary vascular density to increase the literature on this subject. Also, choriocapillary vascular density changes have not been evaluated together with retinal vascular density changes, CT, and retinal structural changes. Therefore, we aimed to comprehensively evaluate all parameters.
Methods
Patients with confirmed idiopathic PD diagnosis and healthy subjects from the outpatient clinic of the Izmir Tepecik Research Hospital and the University of Economics Medical Point Hospital were enrolled in this observational cross-sectional study.
Sixty-six patients with PD and 66 healthy individuals were enrolled in the study. The groups were age and gender-matched. Only the data from the right eyes were analysed in both groups. The Ethics Committee of Bakircay University (Decision No: 817 - Search No: 797) approved the study, and written informed consent from all individuals was taken. Neurologists (ŞB and HAU) recorded disease onset, Unified Parkinson’s Disease Rating Scale Part 3 (UPDRS-3), and HoehnYahr scale (H-Y scale). The Montreal Cognitive Assessment (MoCA) test was used to assess the cognitive status of groups.
The anterior and posterior length of the eye (axial length), blood pressure, and eye pressure were measured. Cornea, lens, and retina examinations were performed. Patients with controlled hypertension without any systemic problems were enrolled in the study.
Patients with any systemic disease affecting the eye tissues, with diseases such as cataracts and corneal clouding that affect the scan quality, who have previously undergone eye surgery for any reason, patients with an anterior-posterior eye length greater than 22 mm, and patients with any neurological disorder other than PD were excluded from the study. The DRI OCT Triton device (Topcon, NJ, USA) was used to scan the retinal structure and vessel network. Images with a signal strength of 60 or higher were carefully analysed. All images were taken by the same individual (SB). A fovea-centred 6×6 mm area was used for analyses. In the macular area, vessel density was calculated as a percentage (%) in the outer circle with a diameter of 3 mm and the inner circle with a diameter of 1 mm (Figure 1A–C).
Figure 1. (A) Angiography image of superficial vascular plexus at 6 mm × 6 mm area of macula. (B) The superficial vascular plexus is highlighted with red dots on structural OCT. (C) The vessel density of the fovea, temporal, inferior, nasal, and superior quadrants.
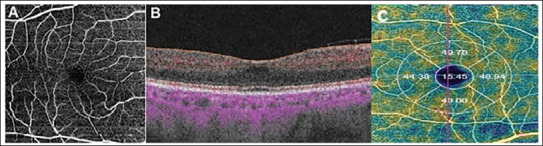
Superficial vascular density (SVD) was measured at a distance of 2.6 µm–15.6 µm, and deep vascular density (DVD) was measured at a distance of 15.6–70.2 µm under ILM. Choriocapillaris vessel density (CCVD) was measured at a distance of 30 µm – 60 µm under the RPE layer. 17 Measurement distances may vary depending on the light and wavelength used by different devices. 18
The following layer complexes were calculated: CMT, retinal nerve fibre layer (RNFL), ganglionic cell layer (GCL), and CT (BM-SCI).
We used IBM SPSS Statistics 21.0 for statistical analyses. Numerical variables were presented as mean ± SD, and categorical variables were presented as percentages and numbers. Kolmogorov-Smirnov test and Levene test were used to analyse the normality of the distribution of continuous variables and homogeneity of variance. Independent samples t-test and Mann Whitney U test were used for normally distributed data and non-normally distributed data, respectively. P values of .05 and smaller were considered statistically significant.
Results
UPDRS-3, H-Y scale, and MoCA test results for PD patients were 16 ± 3.8; 1.8 ± 0.8, and 23.5 ± 4.8, respectively. According to these scores, our patient group included in the study were early to mid-stage patients. Mean axial length, age, gender, and intraocular pressure (IOP) were similar for both groups (Table 1).
Table 1. Clinical Characteristics of the Groups.
| PD Group | Control Group | P | |
| Age (years) | 69 ± 10 | 67 ± 9 | .467 |
| Sex (F/M) | 29/37 | 22/44 | .211 |
| IOP (mmHg) | 14.8 ± 2.2 | 15.7 ± 3.0 | .051 |
| AL (mm) | 23.3 ± 1.0 | 23.5 ± 0.9 | .54 |
| Hypertension (n,%) | 18 (27%) | 12 (18%) | .213 |
| MoCA | 23.5 ± 4.8 | 24.42 ± 3.44 | .099 |
| H-Y scale | 1.8 ± 0.8 | ||
| UPDRS | 16 ± 3.8 |
Notes: Values are mean ± SD (*P ≤ .05), AL, axial eye length; H-Y scale, Hoehn -Yahr scale; IOP, Intraocular pressure; MoCA, Montreal Cognitive Assessment; PD, Parkinson’s disease; UPDRS, Unified Parkinson’s Disease Rating Scale.
The RNFL, GCL, CMT, and CT were thinner ‘in PD’ than in controls, and also the differences between the groups were statistically significant (Table 2). SVD and DVD were not statistically different between the groups (Table 3). There was a decrease in vascular density in all quadrants of the choriocapillary layer. The decrease in vascular density was statistically significant in the nasal, inferior, and central quadrants (Table 3).
Table 2. Retinal and Choroidal Values.
| PD Group | Control Group | P | |
| Retinal nerve fibre layer (µm) | 37.56 ± 4.71 | 39.18 ± 3.69 | .03 |
| Ganglionic cell layer (µm) | 62.75 ± 6.39 | 65.09 ± 5.01 | .021 |
| Central macular thickness (µm) | 231.39 ± 20.28 | 240.46 ± 17.42 | .007 |
| Choroidal thickness (µm) | 216.69 ± 53.66 | 256.37 ± 74.80 | .001 |
Note: Values are mean ± SD (*P ≤ .05).
Table 3. Vascular Density Values.
| Parkinson’s Disease | Controls | P | ||
| Superficial vascular density (%) | Temporal | 46.69 ± 2.73 | 46.42 ± 2.36 | .538 |
| Nasal | 44.73 ± 3.69 | 45.25 ± 2.02 | .315 | |
| Superior | 48.40 ± 8.57 | 48.90 ± 1.98 | .645 | |
| Inferior | 48.48 ± 5.26 | 48.77 ± 2.91 | .692 | |
| Central | 18.62 ± 3.47 | 18.75 ± 3.34 | .825 | |
| Deep vascular density (%) | Temporal | 46.19 ± 3.43 | 45.99 ± 1.86 | .671 |
| Nasal | 46.81 ± 3.95 | 47.16 ± 2.52 | .550 | |
| Superior | 48 ± 4.39 | 48.22 ± 8.92 | .854 | |
| Inferior | 49.83 ± 3.57 | 50.6 ± 4.26 | .262 | |
| Central | 17.05 ± 3.97 | 17.66 ± 3.94 | .374 | |
| Choriocapillaris vascular density (%) | Temporal | 53.54 ± 2.33 | 54.10 ± 2.31 | .171 |
| Nasal | 53.22 ± 2.06 | 53.97 ± 1.94 | .033 | |
| Superior | 52.74 ± 2.33 | 53.31 ± 1.69 | .113 | |
| Inferior | 52.91 ± 1.96 | 53.87 ± 1.95 | .006 | |
| Central | 52.83 ± 3.16 | 53.83 ± 2.57 | .049 |
Note: Values are mean ± SD (*P ≤ .05).
Discussion
Our main goal in the study is to assess the choriocapillary vascular change. We also wanted to show the anatomical changes of the retina and choroid in mild to moderate PD. Abnormal alpha-synuclein (α-SYN) accumulation is important in PD. This protein has been shown to similarly accumulate in the retina,19, 20 Katie et al. reported the increased immunoreactivity of α-SYN protein in the RNFL and ganglion cell inner plexiform layer. It is thought that neurodegeneration due to increased immune reactivity and deterioration can cause thinning in retinal nerve fibre and ganglion cell layers. 21 We found a decrease in the RNFL, retinal thickness, and ganglion cell thickness. Similarly, when we looked at other studies in the literature, we saw that there was harmony in this regard. Generally, a decrease in these layers is reported in mild, moderate, and severe patient groups.6, 7, 12, 22
BM-SCI representing CT was another parameter we evaluated. Kamata et al. and Zhang et al. reported a reduction in CT.6, 15 Similarly, we found a thinning of the CT. This thinning of the CT could be explained by the decrease in blood flow due to autonomic dysfunction in PD patients. Furthermore, Brown et al. reported that higher CT was associated with better clinical performance. 9
It is argued that deterioration in vascular structures is a factor responsible for the development of PD. But this issue continues to be discussed.23, 24 First, Kwapong et al. found a decrease in SVD and DVD in the early stages of PD, and the decrease in superior vascular density was more pronounced. 1
Rascuna et al. showed non-significant thickening in SVD and almost no change in DVD values in the early stages of PD compared to the healthy group. 5 However, Li et al. reported lower macular microvascular density and microvascular impairments in the superficial and deep retinal capillary layer in PD patients with higher UPDRS scores. They commented that as the severity of the disease increased, there was a decrease in vascular density. 14
In a recently published review, Katsimpris et al. searched MEDLINE and EMBASE and reported an inverse relationship between SVD and PD. 25 Similarly, we found a slight decrease in SVD and DVD in mild to moderate PD patients. These results show that vascular density measurements can be used in routine examination and screening at all stages of the disease. When studies on CT are examined, it is generally accepted that there is choroidal thinning. In this case, it is also a matter of curiosity how the choriocapillary vascular density is affected. Therefore, the choriocapillary vascular system has been a subject of interest and research lately. Although there are many studies on CT and retinal vascular density changes in PD, there are only two studies on choriocapillary vascular changes.
Zhang et al. reported that there was a decrease in CC flow density in all quadrants, especially in the superior and inferior quadrants, which was statistically significant. 15 Furthermore, Robbins et al. evaluated the choroidal vascularity index (CVI), which is used as an indicator of choroidal vascular status, and they found CVI to be significantly lower in Parkinson’s patients with MoCA score of 23 and above. 16
In our study, CCVD was decreased in all quadrants of PD. Moreover, the reduction in the nasal and central quadrants in addition to the inferior quadrant was statistically significant. When we compared these studies, the severity of PD was almost the same. The choroid is responsible for feeding the outer retinal layers. Due to the high metabolic rate and rapid turnover rate in the photoreceptors, it can be seriously affected by the deterioration of the choroidal system. 26 As a result, the decreased choroidal flow may cause an increase in free radicals and metabolic toxins, which may affect PD more negatively in addition to neurodegenerative deterioration.
We have some limitations. First is a small sample size that reduces the statistical power of the data. Second, we could not evaluate the subgroups according to the severity of the disease due to the small sample size.
Conclusion
Since there is limited information about choriocapillary vascular density in the literature, we aimed to contribute to this field. These results indicated that microvascular deterioration and neurodegenerative processes are involved together in PD, and that retinal microvascular abnormality may contribute to neurodegenerative progression. Evaluation of RNLF, GCL, CT, retina, and choriocapillaris vascular changes together displayed the effect of PD on eye tissues in a comprehensive way. Considering the disruption of the choriocapillary layer in PD may help clinicians in the follow-up of the disease.
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Şule Bilgin  https://orcid.org/0000-0001-5901-1933
https://orcid.org/0000-0001-5901-1933
Acknowledgement
Dr. Bilgin contributed to the design and writing of the method section. Reference to the previous study of Bilgin et al was made while writing the method section (Reference 17).
Authors’ Contribution
ŞB, HAU, OYK, SB, designed the research protocol, and ŞB, HAU, OYK, SB, and ECY collected and analysed data. ŞB, HAU wrote the manuscript.
Declaration of Patient Consent
Informed consent was obtained from all patients included in the study.
Statement of Ethics
It was approved by the Ethics Committee of Bakircay University. Decision No: 817 - Search No: 797.
References
- 1.Kwapong WR, Ye H, Peng C, et al. Retinal microvascular impairment in the early stages of Parkinson’s disease. Invest Ophthalmol Vis Sci 2018; 59(10): 4115–4122. [DOI] [PubMed] [Google Scholar]
- 2.Mohana DS, Mahalaxmi I, Aswathy NP, et al. Does retina play a role in Parkinson’s disease. Acta Neurol Belg 2020; 120(2): 257–265. [DOI] [PubMed] [Google Scholar]
- 3.Murueta-Goyena A, Del Pino R, Galdós M, et al. Retinal thickness predicts the risk of cognitive decline in Parkinson disease. Ann Neurol 2012; 89(1): 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang P, Pavlovic D, Waldvogel H, et al. String vessel formation is increased in the brain of Parkinson disease. J Parkinsons Dis 2015; 5(4): 821–836. [DOI] [PubMed] [Google Scholar]
- 5.Rascunà C, Cicero CE, Chisari CG, et al. Retinal thickness and microvascular pathway in Idiopathic Rapid eye movement sleep behaviour disorder and Parkinson’s disease. Parkinsonism Relat Disord 2021; 88: 40–45. [DOI] [PubMed] [Google Scholar]
- 6.Kamata Y, Hara N, Satou T, et al. Investigation of the pathophysiology of the retina and choroid in Parkinson’s disease by optical coherence tomography. Int Ophthalmol 2022; 42(5): 1437–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins CB, Thompson AC, Bhullar PK, et al. Characterization of retinal microvascular and choroidal structural changes in Parkinson disease. JAMA Ophthalmol 2021; 139(2): 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou M, Wu L, Hu Q, et al. Visual impairments are associated with retinal microvascular density in patients with Parkinson’s disease. Front Neurosci 2021; 15: 718820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown GL, Camacci ML, Kim SD, et al. Choroidal thickness correlates with clinical and imaging metrics of Parkinson’s disease: A pilot study. J Parkinsons Dis 2021; 11(4): 1857–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satue M, Obis J, Alarcia R, et al. Retinal and choroidal changes in patients with Parkinson’s disease detected by swept-source optical coherence tomography. Curr Eye Res 2018; 43(1): 109–115. [DOI] [PubMed] [Google Scholar]
- 11.Eraslan M, Cerman E, Yildiz BS.. The choroid and lamina cribrosa is affected in patients with Parkinson’s disease: Enhanced depth imaging optical coherence tomography study. Acta Ophthalmol 2016. (1); 94: 68–75. [DOI] [PubMed] [Google Scholar]
- 12.Murueta GA, Barrenechea M, Erramuzpe A, et al. Foveal remodeling of retinal microvasculature in Parkinson’s disease. Front Neurosci . 2021; 15: 708700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu B, Wang X, Guo J, et al. Retinal microvascular density was associated with the clinical progression of Parkinson’s disease. Front Aging Neurosci 2022; 14: 818597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Wang X, Zhang Y, et al. Retinal microvascular impairment in Parkinson’s disease with cognitive dysfunction. Parkinsonism Relat Disord 2022; 98: 27–31. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Yang L, Gao Y, et al. Choroid and choriocapillaris changes in early-stage Parkinson’s disease: A swept-source optical coherence tomography angiography-based cross-sectional study. Alzheimers Res Ther 2022; 14(1): 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robbins CB, Thompson AC, Bhullar PK, et al. Characterization of retinal microvascular and choroidal structural changes in Parkinson disease. JAMA Ophthalmol 2021; 139(2): 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilgin S and Tüzün AY. Evaluation of vascular density and structural changes in inflammatory bowel diseases. Eur J Inflamm 2023; 21. DOI: 10.1177/1721727X231190246 [Google Scholar]
- 18.Miller AR, Roisman L, Zhang Q, et al. Comparison between spectral-domain and swept-source optical coherence tomography angiographic ımaging of choroidal neovascularization. Invest Ophthalmol Vis Sci 2017; 58(4): 1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veys L, Devroye J, Lefevere E. et al. Characterizing the retinal phenotype of the Thy1-h[A30P]α-syn mouse model of Parkinson’s disease. Front Neurosci 2021; 15: 726476. DOI: 10.3389/fnins.2021.726476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beach TG, Carew J, Serrano G. et al. Phosphorylated α-synuclein-immunoreactive retinal neuronal elements in Parkinson’s disease subjects. Neurosci Lett 2014; 571: 34–38. DOI: 10.1016/j.neulet.2014.04.027. Epub 2014 Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tran KKN, Wong VHY, Hoang A. et al. Retinal alpha-synuclein accumulation correlates with retinal dysfunction and structural thinning in the A53T mouse model of Parkinson’s disease. Front Neurosci 2023; 17: 1146979. DOI: 10.3389/fnins.2023.1146979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rascunà C, Russo A, Terravecchia C. et al. Retinal thickness and microvascular pattern in early Parkinson’ s disease. Front Neurol 2020; 11 : 533375. DOI: 10.3389/fneur.2020.533375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz RS, Halliday GM, Cordato DJ. et al. Small-vessel disease in patients with Parkinson’s disease: A clinicopathological study. Mov Disord 2012; 27(12): 1506–1512. DOI: 10.1002/mds.25112 [DOI] [PubMed] [Google Scholar]
- 24.Guan J, Pavlovic D, Dalkie N. et al. Vascular degeneration in Parkinson’s disease. Brain Pathol 2013; 23(2): 154–164. DOI: 10.1111/j.1750-3639.2012. 00628.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katsimpris A, Papadopoulos I, Voulgari N. et al. Optical coherence tomography angiography in Parkinson’s disease: a systematic review and meta-analysis. Eye (Lond) 2023. DOI: 10.1038/s41433-023-02438-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forrester JV, Dick AD, McMenamin PG, et al. Anatomy of the eye and orbit. Eye Basic Sci Pract 2002; 2: 66–90. [Google Scholar]


