Abstract
The olfactory mucosa is a component of the nasal airway that mediates the sense of smell. Recent studies point to an important role for the olfactory mucosa as a barrier to both respiratory pathogens and to neuroinvasive pathogens that hijack the olfactory nerve and invade the CNS. In particular, the COVID-19 pandemic has demonstrated that the olfactory mucosa is an integral part of a heterogeneous nasal mucosal barrier critical to upper airway immunity. However, our insufficient knowledge of olfactory mucosal immunity hinders attempts to protect this tissue from infection and other diseases. This Review summarizes the state of olfactory immunology by highlighting the unique immunologically relevant anatomy of the olfactory mucosa, describing what is known of olfactory immune cells, and considering the impact of common infectious diseases and inflammatory disorders at this site. We will offer our perspective on the future of the field and the many unresolved questions pertaining to olfactory immunity.
Introduction
The ongoing coronavirus disease 2019 (COVID-19) pandemic has highlighted the importance of vaccines that prevent severe illness, hospitalization and death1,2. At the same time, the widespread prevalence of breakthrough infections and reinfections3–5, even in previously vaccinated individuals, illustrates the shortcomings of vaccines. Breakthrough infections typically present with milder symptoms that are contained to the upper respiratory tract, but these still come with serious consequences. Upper respiratory severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can lead to smell loss6–8, long COVID8,9 and potential systemic viral dissemination. Perhaps most critically, upper airway infection allows for continued pathogen transmission10,11. This not only presents a danger to immunocompromised individuals and the unvaccinated but also provides an opportunity for viral evolution leading to immune evasion. Data from a plethora of SARS-CoV-2 animal studies consistently indicate that nasal tissue is less well protected than the lung from reinfection following prior immunization or infection11–23. Indeed, across airborne infection models, the nasal mucosa is generally unprotected even in the presence of systemic immunity24–28.
Moving forward, the most pressing challenge for vaccinology is to design vaccines that generate sterilizing immunity at all portals of infection. Defending the entire upper airway from infection should be a key correlate for vaccine-induced protective responses, both against SARS-CoV-2 and future respiratory pandemics. The nasal airway is the entry point for many pathogens, and establishing protective immunity in this tissue is essential to break the chain of transmission. Many approaches have been suggested to orchestrate locally protective immunity at the nasal surface, including mucosal immunization routes, specific antigen formulations and distinct adjuvant signalling29–35. The efficacy of these mucosal vaccination strategies is currently limited by two considerations: what immune parameters are required to protect the nasal passages? And how can this tissue-specific immunity be generated? Common wisdom has held that a tissue-tailored mucosal immune response is required for upper airway protection more so than in the lower respiratory tract36–38. Numerous hypotheses have been offered insofar as to what constitutes this mucosal response: secretory IgA antibodies, tissue-resident T cells and mucosal cytokines are frequently mentioned22,23,38–41. Moreover, how to best elicit these protective responses is unclear14,18,32–35,42 (Box 1). On top of that, how do we determine efficacy? Although peripheral blood is easily sampled, studies of mucosal tissue, particularly the nasal mucosa, are impeded by difficulty in tissue acquisition. Most prior work relies on nasal washes39,43–47 that disproportionately sample the lower nasal turbinates and cannot capture the complete mucosal antibody response, particularly in the superior nasal turbinates. Therefore, determining how vaccination or infection impacts local nasal mucosal immunity and viral control is a technical challenge we have yet to overcome. But one consideration looms over the above questions: the nasal mucosa contains at least two distinct tissue types that require protection, namely the olfactory mucosa and the respiratory mucosa, and each possesses unique immune considerations (Fig. 1a).
Box 1. Vaccine approaches to protect the upper airway.
What immunization approaches most effectively protect the olfactory mucosa? Vaccines variably protect the olfactory mucosa and respiratory mucosa, and the tropism of many infectious diseases also differs between these tissues, but recent data measuring general infection of the nasal passages may provide clues. Prior infection has been shown to generally providew more complete protection of the URT than immunizations, and accordingly, live attenuated vaccines show improved nasal passage protection in animal models compared with inactivated antigen100,310, although the olfactory mucosa (OM) can still be left exposed14. Mucosal immunization, particularly intranasal dosing, is one putative approach to induce local immunity. Intranasal vaccines often struggle to induce strong antibody titres311, probably owing to poor antigen and adjuvant retention, but can reduce nasal replication better than parenteral immunizations in some cases14,18,312. ‘Prime and pull’ strategies try to synergize the efficacy of parenteral and mucosal immunization313 and can improve nasal protection314,315. Regardless of approach, immunization should engender lymphocytes that home to the olfactory mucosa to confer local protection. This homing is imprinted in the lymph node by the inflammatory signals induced either by infection or by a vaccine adjuvant. If adjuvants can successfully mimic the lymphocyte activation induced by infection, olfactory homing and protection would occur regardless of the route of immunization. Indeed, several studies show that parenteral immunization with non-conventional adjuvants can induce superior protection of the nasal passages17,48,316, consistent with data from other mucosal tissues317. More analysis of the adaptive response in the lymph node, and dissection of olfactory-specific protection, is needed to produce the best immunization formula.
Fig. 1 |. Heterogeneity of the upper respiratory tract: the olfaction fraction.
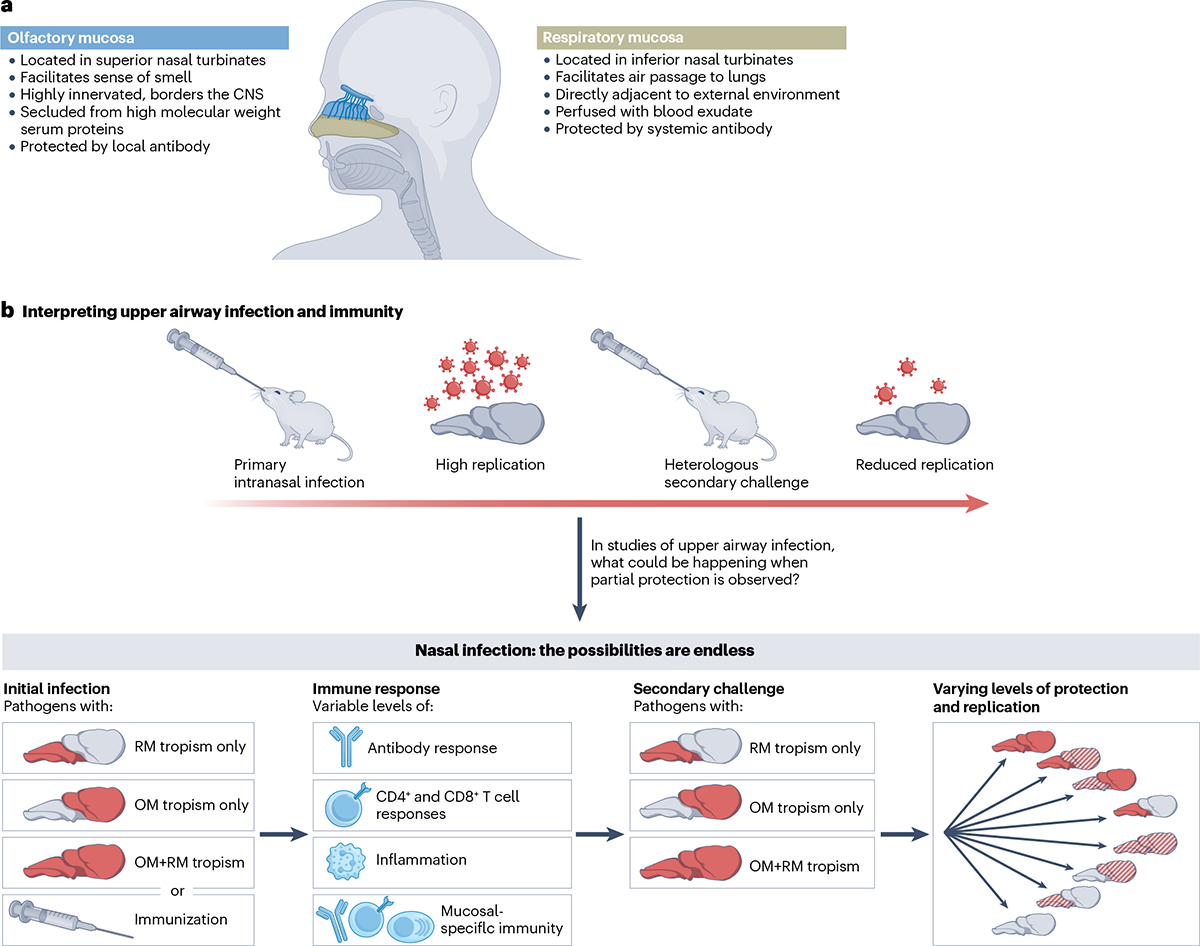
a, The upper respiratory tract consists of two distinct tissues with important implications for immunity, namely the olfactory mucosa (blue) and the respiratory mucosa (beige). The olfactory mucosa must balance olfaction with immune defence and acts as a barrier to the CNS. The olfactory and respiratory mucosae have different requirements for immune protection, including local humoral protection from resident plasma cells in the olfactory mucosa. b, Prior studies of intranasal infection and immunity often indicate that upon rechallenge with a pathogen, pathogen replication is reduced. However, these studies treat the nasal passages as a homogeneous tissue. In actual fact, the overall reduced pathogen replication that is observed could represent many different scenarios depending on the tropism of the pathogens used and the quality of the immune response they induce. The lower panel indicates some hypothetical examples, considering whether a pathogen shows tropism for the olfactory mucosa (OM) alone, the respiratory mucosa (RM) alone or for both the olfactory mucosa and respiratory mucosa. Differences between the olfactory mucosa and respiratory mucosa, both in pathogen tropism and protective immune parameters, must be carefully considered and analysed to yield interpretable data regarding consequences of infection or immunization.
Our recent work has provided an essential new insight into these conundrums48. By focusing on infection in the olfactory mucosa, we identified a novel endothelial barrier, termed the blood–olfactory barrier (BOB), that prevents circulating serum antibodies from accessing the olfactory mucosa. Therefore, even in situations in which highly neutralizing blood-borne antibody is present, the olfactory mucosa is still vulnerable to infection. Interestingly, circulating antibody accesses and protects the respiratory mucosal surface in the nasal passages, and only the olfactory mucosa is strictly segregated from serum antibodies. This gap in immunity at the olfactory barrier can be overcome by a population of extravascular plasma cells that are driven to reside in the olfactory mucosa following infection, secreting antibodies that directly reach the mucosal surface. Although many of these plasma cells were IgA+, protection did not depend on IgA, consistent with other studies of nasal IgG49. Plasma cell-mediated olfactory protection is dependent on signals given to B cells in the lymph node, but intriguingly, these mucosal plasma cells are not always generated following immunization. Using multiple mouse models of viral infection, we demonstrated that these olfactory plasma cells are absolutely required to protect the olfactory mucosa, and furthermore, to protect the CNS from neuroinvasive viruses. These results answer several outstanding questions about upper airway protection from infection. Blood-derived antibodies provide a critical layer of protection for the respiratory epithelium, but the BOB prevents circulating antibody from accessing and protecting the olfactory mucosa48. This difference in antibody transudation may resolve conflicting studies on upper airway antibodies and infection11–23,39,43–47, in which conclusions were confounded by nasal washes failing to distinguish olfactory and respiratory antibody and by pathogens infecting olfactory mucosa and respiratory mucosa to differing levels (Fig. 1b). Olfactory protection can, however, be achieved by locally protective mucosal plasma cells, and the generation of these plasma cells is dependent on the signals engendered by infection or immunization. Differences in vaccine formulation and delivery may at least partially explain why different vaccination strategies could protect the upper airway from infection to variable degrees (Box 1). These findings have illuminated the significance of a previously neglected field: olfactory immunology.
Perhaps most importantly for immunologists and vaccinologists, these discoveries emphasize the heterogeneity of the upper respiratory tract; with distinct respiratory and olfactory mucosae that exhibit different correlates for immune protection, the nasal tissue cannot be treated as a monolithic tissue (Fig. 1). Although protecting the olfactory mucosa is critical to breaking the transmission chain for respiratory pathogens, it is perhaps even more essential in the context of neuroinvasive pathogens. The olfactory nerve acts as a direct portal to the brain, forming a single-cell connection from the airway to the CNS that is essential for the sense of smell but can be subverted by pathogens. The olfactory mucosa is, therefore, a CNS mucosal barrier, the sole line of defence between the external environment and catastrophic neurological disease50,51. Neuroimmunologists have subjected CNS barrier tissues such as the blood–brain barrier (BBB) and meninges to detailed examinations across neuroimmune diseases. These studies have yielded rich and comprehensive characterizations of their anatomical minutiae52, and yet, the olfactory barrier has been largely overlooked. This can be likened to a castle guard preparing for siege defence: the walls have been fortified, cracks in the battlements repaired, secret entrances have been sealed — but the front gate has been left open! Breaching other CNS barrier tissues, such as the tissues of the eye or meninges, requires penetrating several cell layers and structures to reach the CNS;52,53 only the olfactory mucosa contains neurons that directly interface with the outside world and the CNS. Fortunately, despite our relative neglect, the olfactory mucosa has evolved several structural and immune barriers that safeguard this entryway (Fig. 2).
Fig. 2 |. Cell types and effector mechanisms in the olfactory mucosa.
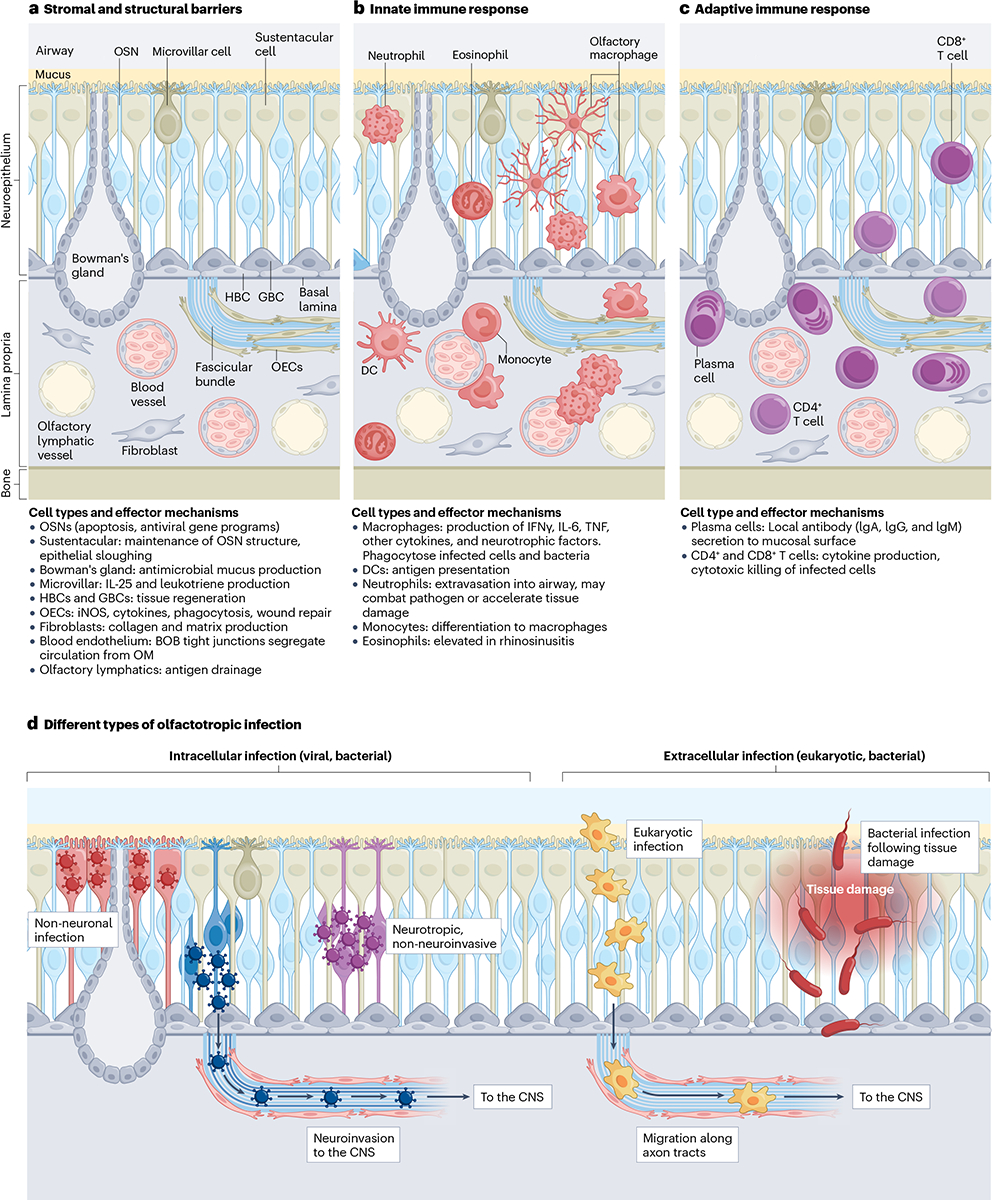
a, The figure indicates the parenchymal cell types that compose the olfactory mucosa. The luminal side is coated in mucus and directly exposed to the airway. The neuroepithelium contains olfactory sensory neurons (OSN), sustentacular cells, microvillar cells and the Bowman’s glands. Lining the basal lamina are horizontal basal stem cells (HBCs) and globose basal stem cells (GBCs). Within the lamina propria, OSN axon tracts run directly towards the olfactory bulb of the brain. Olfactory ensheathing cells (OECs) are interwoven within these axon bundles. Also, within the lamina propria are lymphatic and blood endothelial cells. The blood–olfactory barrier (BOB) prevents antibodies and other large circulating molecules from entering the olfactory mucosa. The exact composition of the BOB is unknown; beyond endothelial cells, pericytes, macrophages or olfactory ensheathing cells could contribute to barrier integrity. b, Innate immune cells of the olfactory mucosa are indicated in dark red. At homoeostasis, macrophages can be observed within the neuroepithelium and lamina propria with several distinct morphologies. During inflammation, dendritic cells (DCs), neutrophils, monocytes and eosinophils can infiltrate the tissue and contribute to the immune response. c, Following infection or immunization, T cells and B lineage cells migrate to the olfactory mucosa and take up long-term residence. These lymphocytes (shown in purple) can provide protective local immunity against future challenge. d, Intracellular olfactotropic pathogens can be neuroinvasive or non-neuroinvasive. Viruses and bacteria may infect non-neuronal epithelial cells or OSNs. Neurotropic pathogens that infect OSNs may either be cleared before reaching the CNS or migrate through OSN axons into the olfactory bulb. Extracellular pathogens, such as bacteria and eukaryotes, can migrate along axon bundles to reach the brain. These pathogens are better able to infect the olfactory mucosa when the tissue has been previously damaged, compromising existing structural impediments. IFN, interferon; iNOS, inducible nitric oxide synthase; OM, olfactory mucosa; TNF, tumour necrosis factor.
And yet, to this point, little work has been done to characterize the olfactory mucosa as an immune tissue (Box 2), and several outstanding questions remain. Which haematopoietic populations reside within this tissue? How does infectious disease uniquely impact the olfactory mucosa compared with the rest of the upper respiratory tract (URT)? What mechanisms does the olfactory mucosa use to protect the brain from invasive threats? How can we augment olfactory mucosal tissue defence to lessen disease burden imposed by airborne pathogens? In this Review, we highlight the existing literature on immunology of the olfactory mucosa. This is an emerging field, and we will offer our perspective on the many unexplored questions in olfactory mucosal, URT and CNS immunity.
Box 2. Sniffing out the historical link between olfaction and immunity.
Emerging data have stirred memories of a long-suspected link between olfaction and immunity. Fundamentally, these two systems attempt to perform similar functions, namely, to recognize a foreign substance and coordinate a rapid physiological response. Olfaction can lead to changes in behavioural immunity318, such as the ‘disgust’ response which is known to trigger a ‘prepared’ immune state in response to noxious and potentially threatening stimuli319,320. Pleasant odours can also impact the immune system: exposure to soothing fragrances following stress leads to a decrease in inflammation321. Removal of the olfactory bulbs leads to depression and a dysfunctional immune system in a manner not fully understood312,322, but the neuroimmune signalling pathways that coordinate olfactory–immune crosstalk have been profiled in drosophila323. An additional structural parallel between olfactory chemosensation and immune recognition has been hypothesized to explain the tendency for mammals to choose mates with dissimilar major histocompatibility complex (MHC) polymorphisms. Peptides that bind to particular MHC molecules have been shown to bind receptors in the olfactory and vomeronasal organs324–326, demonstrating a mechanistic link between olfactory and immune non-self-discrimination that could serve to increase MHC repertoire diversity and, thereby, disease resistance at the population level. Another structural analogue between olfactory and immune cells has recently been demonstrated, as olfactory receptors expressed by leukocytes have important roles in immunity327. Olfactory–immune communication is not unidirectional; infection and subsequent immune activation in drosophila led to altered olfaction328 and there is even growing evidence that mammals, such as dogs, can smell when a person is ill329–333. This olfactory discernment is responsive to common immune or inflammatory substrates, especially volatile organic compounds, but can identify molecules specific to particular diseases334. Although poorly understood, a diverse body of literature indicates that the immune and olfactory systems can dynamically interact.
Immune barriers in the olfactory mucosa
Immunologic analyses of the URT have primarily addressed the respiratory regions of the nasal mucosa54, especially in humans, neglecting olfactory regions almost entirely. The reasons for this are probably twofold. The first reason is a technical hurdle: inferior nasal turbinates or nasal polyps can be sampled with relative ease compared with more superior olfactory regions. The second reason is that there persists a false assumption that URT mucosae are homogeneous — that any sample is representative — ignoring that olfaction exists and requires special equipment. Thus, the human respiratory mucosa has been extensively characterized in contexts including rhinosinusitis, allergy and viral diseases such as COVID-19. Far less work has been done on immune cell populations and functions within the olfactory mucosa. In animal models, analysing the tissue-resident cells of the olfactory mucosa is further complicated by cell isolation from the nasal turbinates. When generating single-cell suspensions, tissue mincing causes turbinate bone marrow to intermix with mucosal cells. We have overcome this complication by developing an intranasal antibody labelling technique that distinguishes true olfactory mucosa CD45+ cells48. In the following sections, we will review the constituent olfactory mucosal cells and structures and discuss how they contribute to immunity.
Anatomical barriers
Situated within the upper turbinates of the superior nasal cavity, the anatomical structure of the olfactory mucosa provides intrinsic obstacles to an incoming pathogen. In some species, the olfactory mucosa dominates the nasal space, but in humans, olfactory tissue is present only on the upper of three nasal turbinate pairs and is proportionally limited in size, covering approximately 5 cm2. Air is needed to sample environmental odourants, but only 15% of air passing across the lower respiratory turbinates reaches the upper ‘olfactory recess’ in which air slows to increase odourant detection55. Limiting air volume exposure may restrict pathogen exposure, but the olfactory system has several physiological barriers that support olfaction and counteract environmental threats.
Olfactory mucus.
The first structural barrier encountered by an olfactory pathogen is the surface mucus layer loaded with antimicrobial peptides56–58. Although the entire nasal mucosa is lined by mucus, olfactory mucus is characterized by a tissue-specific combination of mucin 1, mucin 5AC and mucin 5B proteins that distinguish it from the neighbouring respiratory mucosa59. Mucin specialization suggests that antimicrobial molecule production within the URT may also have spatially regulated expression patterns between respiratory mucosa and olfactory mucosa. Indeed, other proteins secreted into the olfactory mucus have unique immunomodulatory properties, such as olfactory binding proteins, which are essential for receptor-mediated olfactory chemosensation but also possess antimicrobial functions60,61. Moreover, mucus collected from the human olfactory cleft has increased enzymatic activity compared with respiratory mucus, which may contribute to interactions with olfactory microfauna and their metabolites62. Future studies on olfactory mucus may yield further insight into tissue-specific properties that facilitate both odourant detection and immune defence.
Tissue structure.
Beneath the mucus layer lies the olfactory neuroepithelium63,64, which has been extensively characterized by neurobiologists63. This avascular pseudostratified columnar epithelium is packed full of olfactory sensory neuron (OSN) cell bodies that project dendrites into the mucus layer, wherein their specialized cilia access airway odourants. The neuroepithelium itself (Fig. 2) contains large numbers of structurally supportive sustentacular cells, as well as at least two distinct microvillar cell types65 and Bowman’s gland ducts. Beneath these cells lie basal stem cell populations, globose basal cells and horizontal basal cells, that repopulate the neuroepithelial layer66. To speedily convey sensory information, OSN axons extend basally from the cell body, pass through the basal lamina67, converge in fascicular bundles and tunnel through olfactory lamina propria en route to the olfactory bulb of the brain. These fascicular bundles contain immune cells and olfactory ensheathing cells (OECs, refer to the following sections)68. Between axon tracts, secretory Bowman’s glands and endothelial vessels, the olfactory lamina propria (which is proportionally larger in humans than in mice) contains numerous immune cell types and fibroblasts (Fig. 2a).
Endothelial barriers.
Endothelial cells within the nasal mucosa have distinct and unusual phenotypes, as demonstrated by a recent study that has identified atypical venous sinusoids and lymphatic vessels in respiratory and olfactory nasal tissue69. Olfactory antigen delivery to draining lymph nodes has not been studied directly; however, unconventional LYVE1−VEGFR3+ collecting vessels in the olfactory regions probably have significant roles in tissue surveillance70 (Fig. 2a). Several animal studies have demonstrated cerebrospinal fluid drainage through cribriform plate lymphatics before connecting to nasal lymphatics71–77. Although the CNS is primarily drained by a cranial lymphatic network52,78–80, the olfactory route may sometimes have an important physiological role as well. Supporting this hypothesis, a recent study has demonstrated that lymphangiogenesis induced by vascular endothelial growth factor C during autoimmune disease promotes CNS drainage through the olfactory lymphatics, emphasizing that olfactory lymphatics can also contribute to battling neuroinflammation in certain contexts73. In humans, it is unclear how the olfactory lymphatic route may complement the CNS drainage known to occur through meningeal lymphatics70, but post-mortem tissues81,82 and in vivo nasal cerebrospinal fluid studies82–84 suggest that material from the CNS may egress through the cribriform plate into the olfactory mucosa under certain conditions.
Blood–olfactory barrier.
Our group recently reported a novel endothelial barrier, the BOB, that has important implications for olfactory mucosa and CNS protection from airborne infections48. This barrier restricts the movement of large circulating molecules, including antibodies, into the olfactory mucosa. Prior studies have sought to detect a blood–nerve barrier within the olfactory mucosa85,86, and although these studies have concluded that some lower molecular weight compounds can access the olfactory mucosa from the bloodstream, these studies lacked the granularity and ability to distinguish between the endothelial and nerve fascia barriers. What might be the teleological purpose of the BOB? We propose that the BOB is a functional BBB extension, preventing circulating factors and pathogens from entering the olfactory tissues and travelling along the ‘nose-to-brain’ axis. Indeed, exploiting the olfactory nerve tracts to circumvent canonical CNS barriers has been used to intranasally deliver therapeutics into the brain87,88, but also suggests a CNS vulnerability that the BOB may protect. The BOB probably also protects olfactory neurogenic potential and function from harmful circulating substances, much like the blood–retina barrier in the eye.
Like the BBB, the BOB dramatically restricts local tissue availability of larger serum proteins, requiring a fundamental reconsideration of what can and cannot support URT (olfactory) immune protection. We propose that upper airway breakthrough SARS-CoV-2 infections in vaccinated individuals could in large part be owing to the inability of circulating antibodies to protect olfactory tissues. Large molecular weight serum proteins such as antibodies and complement are probably excluded from the olfactory mucosa, but what are the exact molecular size restrictions imposed by the BOB? Can some molecules — especially drugs targeting the olfactory mucosa — be actively transported across? Do BOB formation and persistence depend upon the presence of neurons, and would it be retained in cases of tissue metaplasia in which neuroepithelium is replaced with respiratory cells? Are multiple cell types involved in BOB integrity, as with the neurovascular unit in the brain? These and other fundamental questions remain to be answered.
Adaptive immunity
B cells.
MHC class I expression within the olfactory mucosa is variable across cell types89, yet OSNs are effectively devoid of MHC class I, making them especially reliant on humoral immunity. As previously noted, the BOB prevents serum antibody from accessing the olfactory mucosa, but local antibody secretion in the olfactory mucosa is highly protective. Without this pre-existing antibody protection directly at the mucosal surface, intracellular pathogens can infect OSN dendrites and translocate through axons into the brain without ever encountering other immune cells48. Humoral immunity is, therefore, vital for protection against neuroinvasive microbes, but because the BOB prevents serum antibody from protecting all the cells of the olfactory mucosa, local antibodies would be essential to defending against even non-neurotropic airborne pathogens with olfactory tropism, such as SARS-CoV-2 (refs. 48,90–92). Local antibody production may also prevent early replication and continued pathogen transmission, while also preventing the olfactory mucosa from serving as a foothold for further pathogen selection. Influenza infection studies in ferrets indicate that nasal passage viral replication, but not lung replication, leads to transmission between individuals93. Although this work has detected virus in both respiratory and olfactory mucosa, no study has yet to directly test whether olfactory infection alone permits transmission. Nevertheless, passive antibody transfer experiments suggest that olfactory viral replication may be sufficient for transmission. The BOB means that antibody transfer should protect the respiratory mucosa, but not the olfactory mucosa, and studies of murine influenza infection indicate that passive antibody transfer does not block viral transmission94,95. Formally testing this possibility will require researchers to carefully design experiments to analyse transmission using viruses with known respiratory and olfactory tropism (Fig. 1b). But these studies are critical because selective replication in olfactory and not respiratory tissues could drive evolution of variants with enhanced olfactotropic and neuroinvasive qualities.
Olfactory B cells are detected in the lamina propria of human biopsies, often localized near secretory Bowman’s glands that stain positive for Ig molecules, perhaps indicating that these glands may assist in luminal antibody secretion96 (Fig. 2c). However, intranasal antibody administration in mice demonstrates that IgG antibodies can freely diffuse throughout the tissue without being impeded by structural barriers such as the basal lamina48. These B lineage cells appeared to produce all antibody isotypes, consistent with concurrent studies in salamanders and rats that demonstrated multiple antibody isotypes differentially distributed across the tissue97. In response to olfactory infection, B cells from fish respond to challenge by producing IgT, a mucosal antibody that protects the olfactory surface98,99. In addition to recruitment following infection, local live attenuated immunization increases the frequency of IgA+ plasma cells in the olfactory mucosa48,100. Single-cell RNA sequencing studies have found B lineage populations, particularly class-switched plasma cells, in samples from mice and humans89,101,102.
Our recent work suggests critical non-redundant roles for these olfactory plasma cells in olfactory mucosal defence against viral infection48. These protective plasma cells appear to secrete several antibody isotypes, provide long-term protection, and intriguingly, can be driven to olfactory mucosa residence following non-local priming in distal lymph nodes, suggesting that parenteral immunization can imprint an olfactory mucosa-homing phenotype48 (Fig. 2c). In addition to further elucidating the signals that dictate olfactory mucosa migration, numerous outstanding questions about olfactory plasma cells remain. Which local cells provide tissue retention signals and what is their relationship to long-lived plasma cells in the bone marrow or other mucosal sites? Our efforts have focused on neutralizing antibodies, but studies of olfactory mucosa murid herpesvirus infection indicate that antibodies can limit local viral replication in an Fc-dependent manner26,103. Plasma cell-derived pre-existing antibody is critical for preventing olfactory mucosa infection, but memory B cell populations may also help maintain the plasma cell pool or respond to re-infection, as has been found in the lower respiratory tract104. Fully understanding these local B lineage populations is paramount for vaccination against respiratory and neurotropic pathogens.
T cells.
Adaptive immunity in the olfactory mucosa also includes contributions from T cells. T cell populations (CD4+, CD8+ and γδ T cells) have been described in mouse nasal passages, but whether these were truly olfactory or contained respiratory or bone marrow contaminant is unclear105,106. However, following influenza virus infection, nasal CD8+ resident memory T (TRM) cells accumulated within the olfactory mucosa41. These antigen-specific T cells were extravascular long-term resident cells that, upon rechallenge, provided superior and more durable viral control than lung TRM cells. Furthermore, the nasal passage TRM cells alone were able to reduce viral dissemination to the lung, emphasizing the role olfactory CD8+ T cells can perform in respiratory virus defence41 (Fig. 2c). CD8+ olfactory T cells have also been observed during an olfactotropic viral infection in fish107. Post-COVID-19, patients with smell loss were found to have olfactory infiltration of interferon-γ (IFNγ)-producing T cells, indicating that T cell-mediated inflammation can persist long after olfactory viral clearance108. Similarly, another study has found that patients with long COVID had elevated olfactory mucosal interferon signatures and T cell-associated genes109. Similar long-term changes in the olfactory chemokine expression, interferon-stimulated genes and T cell markers were observed in SARS-CoV-2-infected hamsters109 (Fig. 2c). These studies and similar transcriptional analyses from humans suggest that T cell pathogenesis may have a role in dysosmia (altered sense of smell)110. In agreement with this, CD8+ T cell and natural killer T cell signatures are associated with human age-related olfactory loss and have been proposed to directly signal to the olfactory stem cell niche to disrupt neurogenesis110, and T cell cytokine production is observed in mouse models of chronic nasal inflammation111,112. Together, these data suggest that olfactory T cells can control infections but may also instigate chronic olfactory changes. More work to characterize olfactory T cell subsets, including CD4+ T cells and γδ T cells, is needed.
Innate immunity
Macrophages.
Macrophages represent the most abundant immune cell type in mouse and human olfactory mucosa89,101,102 (Fig. 2b). Tissue-resident macrophages are known to mediate various functions — including neuronal maintenance, wound repair and infection responses — and macrophages have been shown to perform each of these roles in the olfactory mucosa. Macrophages can be found in close association with OSNs, both within the neuroepithelium and within olfactory nerve fascicles113. Olfactory macrophages express Cx3cr1, and deficiency in this receptor leads to a reduction in dendritic morphology for intraepithelial macrophages114, a phenotype that mirrors morphological changes seen in CX3CR1-deficient microglia115. Following OSN death, clodronate-mediated macrophage depletion reduced neurogenesis116 and expression of immune response genes such as Cxcr4 (ref. 117). Similar analyses implicate macrophage Lif and Msr1 expression in tissue regeneration118, perhaps directed by IL-1β signalling from dying OSNs and subsequent expression of Ccl2 and Ccl3 (refs. 119–121). Macrophages have also been shown to be important for defence against olfactotropic pathogens. Macrophage numbers are increased in mice following porcine hemagglutinating encephalomyelitis virus (PHEV) infection, correlating with upregulation of inflammatory mediators such as IFNs, IL-6 and tumour necrosis factor (TNF)122. Influenza A virus-infected OSNs became apoptotic and were engulfed by macrophages, preventing viral spread to the brain123. Similarly, when Staphylococcus aureus was delivered intranasally after OSN damage, macrophages within the nerve fascicle were able to phagocytose the bacteria124. Macrophages are further implicated in chronic olfactory mucosal inflammation, as patients with chronic rhinosinusitis (CRS) who present with smell loss have elevated macrophage numbers compared with controls125,126. Mouse inflammation models recapitulate the elevated macrophage numbers127,128 and macrophage skewing to an immune defence (IL-6 expressing) phenotype111. How macrophages balance these conflicting roles in neuronal support and immune defence remains to be thoroughly examined, but these data suggest that there are functionally or ontologically distinct olfactory mucosal macrophage subsets.
Other innate immune cells.
Although less well studied than macrophages, other innate leukocytes can be detected within the olfactory mucosa, including dendritic cells89,101,102 (Fig. 2b). Circulating myeloid cells, although probably not residing in the olfactory mucosa long-term, can impact the olfactory mucosa during inflammation. Monocytes may differentiate into macrophages in the olfactory mucosa following recruitment from blood, and monocytic inflammation has been observed in an olfactory listeriosis model129. Neutrophilic olfactory mucosal inflammation occurs in numerous contexts and is often severe. Mice given intranasal poly(I:C) treatments see a rapid neutrophil influx112 that subsequently launch themselves into the nasal airway127, a phenotype also witnessed in amoeba infection130. Intranasal lipopolysaccharide administration similarly led to neutrophil influx131, but the role of neutrophils in human CRS is less clear. Although elevated neutrophil levels have been observed125, neutrophilia is not associated with smell loss in CRS132. Neutrophils may also have either beneficial or pathogenic roles in acute infection. Olfactory neutrophils are elevated in mouse and hamster SARS-CoV-2 infections90,133. Upon neutrophil depletion or blockade, SARS-CoV-2 titres were actually decreased and olfactory mucosal damage was mitigated, suggesting that neutrophils contribute to olfactory mucosa destruction and permit increased viral replication133. Among the other granulocytes (mast cells, basophils and eosinophils), only eosinophils have been definitively reported within the olfactory mucosa, typically in the context of CRS or amoebic infection134 (Fig. 2b). Although eosinophil numbers in the olfactory mucosa seem to be elevated in human rhinosinusitis126,135, they are not associated with impaired olfaction. Natural killer cells have been observed in human olfactory biopsies and may express inflammatory genes that signal to basal progenitors and OSNs, inhibiting their ability to properly regenerate110. To our knowledge, no studies have directly searched for other innate-like lymphocytes in the olfactory mucosa.
Stromal immune barriers
In addition to haematopoietic immune cells, parenchymal cells in the olfactory mucosa may contribute to immunity through cell-autonomous pathogen clearance, the production of antimicrobial and inflammatory compounds, and communication with the haematopoietic compartment (Fig. 2a).
Olfactory sensory neurons
OSNs represent a curious case in intrinsic immunity. Compared with CNS neurons, OSNs are accustomed to much higher rates of death and regeneration, suggesting that they may respond differently to inflammation. As OSNs are a single-cell gateway to the CNS, evidence suggests that they use unique antiviral signalling pathways to stifle intracellular infections that attempt to invade the brain parenchyma (Fig. 2d). Type III IFNs are a critical component of early mucosal responses to infection, and indeed, IFNλ reduces murid herpesvirus infection at the olfactory mucosa136. Similarly, IFNλ signalling prevents influenza virus spreading from the olfactory mucosa to the lung137. Conversely, vesicular stomatitis virus, a virus highly sensitive to type I IFN signalling, replicates aggressively in OSNs138, suggesting inherent vulnerabilities in OSN type I IFN responses. Yet, other studies indicate that type I IFN has a critical role in combating URT viral infection prophylactically and after disease onset139–142, although differences in olfactory and respiratory infection have not been quantified in these studies. Our work has demonstrated that OSNs can non-lytically clear influenza B virus infection more quickly than neighbouring respiratory epithelial cells by using a rapidly induced antiviral response143. OSNs can also prevent virus from reaching the brain by quickly inducing apoptosis107,123. In summary, OSNs certainly exhibit vulnerabilities to infection but can respond swiftly to some pathogens, and their antimicrobial capabilities remain to be carefully characterized.
Other epithelial cells.
Sustentacular cells provide structural support within the olfactory neuroepithelium and make up the largest portion of non-OSN cells. These cells are the target of SARS-CoV-2 infection in humans owing to their ACE2 expression90–92,144. Following sustentacular cell infection, chemosensory function is impaired and the entire neuroepithelial layer appears to slough off, as the olfactory mucosa structure is compromised. Also within the neuroepithelium, olfactory microvillar tuft-like cells express Il25 and genes for cysteinyl leukotriene production, which they produce upon airway allergen exposure resulting in eosinophilia145. An ensuing study demonstrated that microvillar Ltc4 expression induced by allergens stimulated olfactory stem cell proliferation65, suggesting these cells may coordinate the immune response and neurogenesis (Fig. 2a).
Olfactory ensheathing cells.
OECs surround OSN axon bundles as they pass through the olfactory lamina propria into the CNS. OECs are related to astrocytes and Schwann cells, acting as an important glial component of the olfactory nerve68,86,146. OECs are promising cellular therapies for treating brain and spinal cord injuries, probably owing to their neuroprotective and neurogenic functions, but studies also suggest that they have potent immune-modifying abilities147. OEC phenotypes within the olfactory bulb are geared towards axon regeneration, whereas olfactory mucosa OECs express genes associated with the defence response, inflammation and immunomodulation148. Advantageously poised to patrol olfactory nerve tracts (Fig. 2a), olfactory mucosa OECs can produce inducible nitric oxide synthase in response to bacterial invasion of the damaged olfactory nerve149 and phagocytose infected or dying olfactory axons150,151. In the context of OSN death, OECs recognize phosphatidylserine produced by dying axons, phagocytosing a greater OSN number than olfactory mucosa macrophages150 in a process that may be enhanced by MIF and HTRA1151.
Together, these adaptive, innate and stromal cells coordinate to maintain olfactory function and combat disease. But what are the specific threats, infectious or otherwise, that impact the olfactory system? Next, we will review disease pathogenesis within the olfactory mucosa, with an emphasis on how the local immune response ameliorates or exacerbates disease.
Disease in the olfactory mucosa
Olfactotropic pathogens
Airborne pathogens initiate infection in the upper airway, in which they first encounter host defences. However, the specific impact pathogens have on the olfactory mucosa is poorly described, in large part owing to the technical difficulty in measuring microbial replication and corresponding inflammation of the human superior nasal turbinates (nasal swabs sample the lower respiratory turbinates of the nose). Consequently, the olfactory tropism of many common airborne pathogens is unknown, and we probably drastically underestimate the number of airway infections that impact the olfactory mucosa. Pathogens currently known to infect the olfactory mucosa, which we refer to as olfactotropic infections, are reviewed in Table 1. Here, we will highlight infections that have special implications for olfactory immunity. We can think of olfactotropic pathogens in two broad categories: neuroinvasive and non-neuroinvasive (Fig. 2d). The olfactory mucosa is heavily innervated by OSNs and pathogens can hijack OSNs for direct CNS invasion, resulting in potentially lethal meningitis or encephalitis. Many pathogens are known to exploit this entryway to the CNS50,152, but ascertaining the proportion of meningitis and encephalitis cases that originate from olfactory infection is difficult. At the same time, many non-neuroinvasive respiratory pathogens infect both the olfactory mucosa and the respiratory mucosa, and olfactory immune defence must limit viral dissemination, break the chain of community transmission, and prevent olfactory mucosal damage and smell loss.
Table 1 |.
Infection stinks: pathogens in the olfactory mucosa
| Pathogen | Notes | Refs. |
|---|---|---|
| Naegleria fowleri | Free-living amoeba that causes catastrophic lethal disease known as primary amoebic meningoencephalitis through the olfactory nerve in humans134,165; similar pathogenesis seen in animal models168 | 134,165,168 |
| Balamuthia madrillaris | Free-living amoeba, opportunistic agent of lethal granulomatous amoebic encephalitis in humans and animals228; olfactory neuroinvasion seen in immunocompromised mice229 but not in immunocompetent organisms230 | 228–230 |
| Ichthyophthirius multifiliis | Trout parasite that infects the olfactory epithelium but with overt CNS invasion | 98 |
| Mucorales spp. | Fungal pathogens have not been formally shown to invade the olfactory nerve, but Mucorales order fungi commonly cause rhino-orbital-cerebral mucormycosis, suggesting fungal invasion along the olfactory pathway | 231 |
| Streptococcus pneumoniae | Common human respiratory bacterial infection displays olfactory neuroinvasion in immunocompromised mice232,233; in immunocompetent mice, it traverses along the olfactory nerve to the meninges but does not infect the brain parenchyma234 | 232–234 |
| Chlamydia spp. | Chlamydia muridarium and Chlamydia pneumoniae invade the olfactory nerve during intranasal infection in mice227,235, with C. pneumoniae upregulating brain inflammatory genes, suggesting olfactory contribution to neurodegenerative disorders associated with human C. pneumoniae infection227,236 | 227,235,236 |
| Neisseria meningitidis | Bacterial human pathogen shown in transgenic mice to cause olfactory mucosa damage and neuroinvasion without blood dissemination | 237 |
| Listeria monocytogenes | Bacterial pathogen thought to access the human brain primarily through the trigeminal nerve but can enter via the olfactory epithelium in neonatal mice | 129 |
| Burkholderia pseudomallei | Intracellular bacterium that infects the olfactory mucosa of mice238, causing OSN death, exposing the axon tracts and allowing for stark neuroinvasion189; B. pseudomallei can shelter within the nerve fascicles and neuroinvasion is enhanced following prior olfactory mucosa damage190 | 189,190,238 |
| Staphylococcus aureus | Common human bacterial infection that in mice can opportunistically invade the CNS through the olfactory nerve following olfactory mucosa damage | 124 |
| Flavobacterium columnare | Bacterial pathogen that invades the trout olfactory organ and provokes an immune response | 99 |
| SARS-CoV-2 | Coronavirus that infects sustentacular cells of human olfactory mucosa, frequently causing inflammatory damage and loss of smell90–92,144,153; olfactory neuroinvasion does not seem to occur in humans, but animal models, such as K18-hACE2 mice, display CNS invasion via the olfactory nerve158,159,161; in Syrian golden hamsters, some later-emerging variants infect the brain through the olfactory nerve162,164 | 90–92,144,153,158,159,161,162,164 |
| SARS-CoV-1 | Does not penetrate CNS in wild-type mice but shows olfactory neuroinvasion in the K18-hACE2 mouse model239; autopsy study in humans detected CNS virus but did not sample nasal mucosa240 | 239,240 |
| Human coronavirus OC43 (HCoV-OC43) | Intranasal inoculation in mice leads to CNS infection that focally radiates from the olfactory bulb241,242; frequently detected in human post-mortem brain samples243 | 241–243 |
| Middle Eastern respiratory syndrome coronavirus (MERS-CoV) | Neurologic symptoms in humans;244,245 transgenic mouse studies indicated possible olfactory involvement in CNS infection246 | 244–246 |
| Porcine hemagglutinating encephalomyelitis virus (PHEV) | Following mouse intranasal infection, invades the brain via the olfactory and trigeminal nerves | 122 |
| Influenza virus | In mice, several strains infect the nasal passages but not the brain41,137,143,247–250; ferret studies indicated olfactory mucosa infection in highly pathogenic strains all without reaching the brain179,181; olfactotropic CNS neuroinvasion occurs for some strains in either mice or ferrets178–182 | 41,137,143,178–182,247–250 |
| Vesicular stomatitis virus (VSV) | A cytopathic rhabdovirus that infects livestock but rarely humans251; in mice, VSV rapidly infects OSNs and transmits to the brain252 before being cleared by T cells253; excellent olfactory immunity model owing to its strict olfactory tropism during intranasal infection and its ability to generate robust adaptive immunity254 | 251–254 |
| Rabies | A case of fatal rabies encephalitis reported via airborne exposure, and with rabies virions detected in the olfactory bulb255; intranasal rabies challenge in mice demonstrated OSN infection and transsynaptic transmission into the brain256 | 255,256 |
| Vaccinia | Vaccinia virus has been shown to infect animals intranasally and reach the brain, possibly through olfactory transmucosal invasion | 257–259 |
| Cytomegalovirus (CMV) | Human CMV causes olfactory bulb lesions in infants185 and childhood loss of olfaction186; murine-adapted CMV (MCMV) spreads between animals nasally, and inactive MCMV binds to OSNs, suggesting potential intranasal transmission260 | 185,186,260 |
| Herpes simplex virus 1 (HSV-1) | Invades the brain through olfactory nerve in rats261; infects olfactory mucosa of mice but is not neuroinvasive262; HSV1 may infect human OSNs and reach the brain263, given the large number of associated neurological sequelae226, post-mortem HSV1 detection264,265 and long-term loss of olfaction in convalescent herpetic meningoencephalitis patients266; HSV1 accesses the brain through the trigeminal nerve and may enter these cells through neuron termini at the olfactory surface | 226,261–266 |
| Human herpesvirus 6 (HHV-6) | May infect human olfactory mucosa and invade the brain based on post-mortem antigen detection in the brain and in the nasal cavity | 267 |
| Murid herpesvirus 4 (MuHV-4) | Infects mouse OSNs before reaching B cells in the draining lymph node but does not undergo CNS neuroinvasion | 26,268 |
| Aujeszky’s disease virus | Known as pseudorabies, this herpesvirus infects pigs and invades along the olfactory tract following intranasal inoculation | 269,270 |
| Poliovirus | Intranasal instillation in primates leads to olfactory CNS neuroinvasion271–274, although biopsy analysis indicates this is unlikely to occur in humans275; mice expressing a poliovirus receptor transgene are susceptible to olfactory neuroinvasion following intranasal challenge276 | 271–276 |
| Eastern equine encephalitis virus (EEEV) | Highly fatal but poorly transmitted virus in humans; brain invasion appears to occur via the olfactory nerve in guinea pigs, mice and rhesus macaques | 277–279 |
| Venezuelan equine encephalitis virus (VEEV) | Invades the brain via the olfactory route in both mice and macaques | 28,280–282 |
| Chikungunya virus | Infects olfactory neurons in immunodeficient mice | 283 |
| Zika virus | Zika virus infects olfactory ensheathing cells in neonatal mice, causing transient olfactory deficits; broad olfactory tropism seen in immunodeficient mice, and human olfactory organoids are also susceptible to infection | 284 |
| West Nile virus (WNV) | The leading global cause of arboviral encephalitis, but the CNS entry route is unknown; experimental aerosol administration leads to mouse olfactory neuroinvasion | 285,286 |
| Other flaviviruses | Japanese encephalitis virus, Murray Valley encephalitis virus and St. Louis encephalitis virus infect the olfactory epithelium and olfactory bulb | 287–293 |
| Parainfluenza | A large portion of patients with olfactory dysfunction tested positive for human parainfluenza virus 3 (ref. 294); mice intranasally infected with parainfluenza virus 1 (Sendai virus) had persistent viral RNA in the olfactory epithelium and bulb long after infection, suggesting viral immune evasion in the olfactory system295,296 | 294–296 |
| Paramyxoviruses | Nipah and Hendra virus cause acute, severe respiratory disease and encephalitis in humans, and although haematogenous spread to the brain is probably involved, animal models demonstrate olfactory neuroinvasion for both viruses297–300; measles virus can be highly neuroinvasive and invades the olfactory nerve in hamsters301 | 297–301 |
| Borna disease virus | Devastating CNS disease of livestock following faecal matter inhalation; when administered intranasally to rats, OSN neuroinvasion drives fatal encephalitis | 302–304 |
| Hepatitis virus | Murine hepatitis virus enters the CNS via the olfactory pathway305–308, causing significant OSN death and rapidly inducing brain chemokines and cytokines309 | 305–309 |
CoV, coronavirus; MERS, Middle East respiratory syndrome; OSN, olfactory sensory neuron; SARS, severe acute respiratory syndrome.
SARS-CoV-2.
The COVID-19 pandemic brought the impact of olfactory mucosal viral infection to the forefront of much scientific and public discourse. SARS-CoV-2 directly infects both the olfactory and respiratory epithelia in humans91,92,144 (Fig. 2d). Fortunately, evidence suggests that OSN infection and subsequent CNS neuroinvasion do not occur91,92,144. Instead, SARS-CoV-2 mediates olfactory pathology by infecting sustentacular cells leading to transient damage, inflammation and subsequent tissue structure loss90,91,108,144. Without the structural support provided by sustentacular cells, olfactory neurons die or become dysfunctional, and smell is compromised to varying degrees8,153. This partial or complete smell loss (clinically, hyposmia or anosmia) is typically short-term, as the olfactory epithelium is regenerated by the underlying stem cell populations66, although persistent inflammation can lead to long-term hyposmia or anosmia108. Interestingly, chemosensory deficits also strongly predict the humoral response in SARS-CoV-2 infection154, suggesting a functional link between olfactory infection and immunity induction. The olfactory pathogenesis of SARS-CoV-2 is mirrored in rhesus macaques, as the typical URT viral replication is observed in the absence of frank CNS neuroinvasion15,23. Replicating virus has not been detected in long-term hyposmic or anosmic olfactory biopsies, indicating that innate and adaptive immune responses can clear virus from the olfactory system108. However, prolonged viral shedding has been observed from nasal swabs155,156, suggesting that the olfactory mucosa could harbour virus in some individuals157. Patients with long COVID often present with neurological symptoms, including olfactory deficits8, but whether this occurs because of viral persistence, cell-intrinsic OSN alterations or continued olfactory mucosal inflammation is unknown.
In contrast to human infections, animal models of SARS-CoV-2 and other coronavirus infections are characterized by olfactory neuroinvasion, raising concerns that future variants could gain neurovirulent capabilities as they repeatedly passage through olfactory tissues. In the commonly used K18-hACE2 mouse SARS-CoV-2 model, nearly ubiquitous epithelial hACE2 expression directs OSN infection and consequent CNS pathology is observed158–160, resulting in lethal neuroinvasion across multiple SARS-CoV-2 variants161. Much like in humans, SARS-CoV-2 was initially only believed to infect olfactory mucosal sustentacular cells in hamsters162, but more recent variants have been shown to infect OSNs and invade the CNS163,164. Hamsters also have lasting olfactory perturbations following SARS-CoV-2 infection, indicating that they may be useful for post-COVID olfaction studies109. Given the frequency of coronavirus epidemic outbreaks this century (Table 1), more research on their olfactotropism is needed.
Naegleria fowleri.
Naegleria fowleri, the ‘brain-eating amoeba’, is perhaps the most notorious pathogen capable of olfactory transmucosal infection. This free-living amoeba is ubiquitously present in warm bodies of freshwater but only drives disease when it contacts olfactory tissue in the nasal turbinates165. N. fowleri crosses the olfactory epithelium and quickly rampages through the olfactory nerve into the CNS (Fig. 2d) in which it causes an almost universally fatal inflammatory condition known as primary amoebic meningoencephalitis. Olfactory immunity against N. fowleri is complex: immunization against N. fowleri in animal models offers limited protection166, but neutrophils and other myeloid cells slow disease progression but also contribute to disease pathogenesis134,167. Because N. fowleri is only pathogenic across the olfactory mucosa, and olfactory neuroinvasion is conserved across mammals including mice, this infection serves as a powerful model system to highlight the unique immune properties and vulnerabilities of the olfactory mucosa168. Seropositivity studies suggest that many humans may have some protection owing to subclinical exposure169–171; but does the BOB prevent antibodies and/or complement from slowing pathogenesis? Are neutrophils or other cells able to respond to the amoeba more quickly in non-olfactory tissues?
Influenza virus.
Analysis of patients with influenza has shown that subjective olfactory dysfunction increases as vaccination rate decreases172, suggesting not only that frequent olfactory influenza virus infection occurs, but also a preventative immune capacity. Olfactotropism seems to depend on strain, but influenza virus infection is associated with neurologic symptoms and sequelae173,174, and in some cases, influenza infection coincides with meningitis or encephalitis175–177. Direct CNS neuroinvasion has been reported in several mammalian infection models. Influenza A/WSN/33 (H1N1) infects OSNs and translocates to the CNS in mice178, and highly pathogenic and pandemic strains are predisposed to olfactory neuroinvasion in ferrets179–182. In addition, influenza virus-derived antigen has been identified in human post-mortem olfactory nerves, lending credibility to an olfactory route of CNS infection183. The recent highly pathogenic avian H5N1 strain of influenza virus has been shown to infect the brains of some animals184, raising concerns that olfactory neuroinvasion may contribute to future emergent pandemics. Whereas such highly pathogenic cases are rare, the olfactotropism of more common, lowly pathogenic strains is understudied, and much remains to be learned about the viral and host factors that determine the neuroinvasive potential of various influenza virus strains. More common influenza virus strains may infect the olfactory mucosa, and potentially even reach the brain, but olfactory tropism is generally not explored unless a patient develops severe CNS disease. Supporting this, some influenza viruses infect the olfactory mucosa but innate mechanisms allow OSNs to quench viral replication to prevent neuroinvasion123,143. The olfactory mucosa can also have a role in limiting influenza virus dissemination to the lung, as type I IFNs and type III IFNs were shown to be crucial for containing two different strains of influenza virus to the nasal passages of mice137. Interestingly, comparing nasal and lung infection across multiple influenza virus strains in ferrets indicates that only nasal infection supports airborne transmission between organisms, whereas lung infections are not spread93. These data reinforce the importance of preventing influenza virus infection in the upper airways to limit propagation.
Opportunistic infections.
Some pathogens may act opportunistically to infect the olfactory mucosa and reach the CNS. For example, cytomegalovirus (CMV) is a congenital disease in humans that frequently leads to neurological disorders such as hearing and smell loss, but whether CMV uses OSNs to infect the brain is unknown. Olfactory bulb lesions have been observed in infants with CMV185, and olfactory defects are reported throughout childhood186 in a manner that is decoupled from hearing loss187. Furthermore, a human olfactory receptor was identified as a CMV entry receptor188, opening the possibility that olfactory invasion may explain some CNS pathologies. Damage of the olfactory mucosa, whether acute or chronic, can expose it to opportunistic pathogens that may infect the tissue and penetrate the CNS. Olfactory mucosa damage in mice allows for bacteria such as Burkholderia pseudomallei124,189,190, Streptococcus agalactiae191 and S. aureus124 to subsequently colonize the olfactory mucosa and invade the brain, either intracellularly through OSNs or extracellularly along the axon tract50 (Fig. 2d). The olfactory mucosa is damaged throughout life, from pathogenic and other insults, and maintaining structural and cellular olfactory mucosal barriers is critical for preventing opportunistic infections.
Post-viral olfactory dysfunction.
Post-viral olfactory dysfunction is one of the most frequently reported acute and chronic side effects of upper respiratory illness153,192–195, including well-documented short-term and long-term olfactory loss following COVID-19196–201. Potential mechanisms driving post-viral olfactory dysfunction include direct OSN infection and death, infection of other olfactory mucosal cells leading to inflammation and/or neuroepithelial damage, CNS consequences resulting in olfactory bulb dysfunction, or respiratory infection leading to nasal inflammation and airflow blockage. The immune response is heavily involved in all these pathologies, ranging from the rapid antiviral responses of epithelial cells to haematopoietic cell recruitment during sustained inflammation. Indeed, a recent study of olfactory biopsies following clearance of SARS-CoV-2 has identified that long-term smell loss was associated with immune cell infiltration and inflammatory gene expression108. Persistent T cell infiltration was accompanied by a shift in myeloid cell populations away from an anti-inflammatory, wound-healing phenotype, reflecting a disruption in the balance between productive and deleterious immune responses to infection. This immune dysfunction extended to an inflammatory gene signature in sustentacular cells of the olfactory epithelium, as well as lower OSN numbers in dysosmic patients108. Exactly how other pathogens mediate the loss of chemosensation warrants further study.
Damage of the olfactory mucosa
Olfactory injury, inflammation and regeneration.
Respiration brings a constant stream of airborne environmental pollutants, microbial toxins or inorganic compounds that can damage the olfactory mucosa and drive inflammation202. CRS is a persistent inflammation of the upper airways that frequently coincides with dysosmia. Biopsies from patients with CRS indicate that inflammation in the olfactory mucosa is associated with hyposmia126, and comparison of healthy control and CRS samples indicates increased olfactory metaplasia that can be characterized histologically by the type of epithelial deformation125,135. Interestingly, one study of olfactory function found that type II cytokines were associated with worse olfaction prior to corrective CRS surgery, but improved olfaction postoperatively203. By contrast, type III cytokines correlated with better olfactory scores preoperatively, but corresponded with worse scores after surgery, suggesting the immune response may shape olfactory potentiation. Indeed, immune and glial cell activation supports phagocytosis of apoptotic debris and tissue regeneration after OSN ablation204, and infiltrating immune cells facilitate OSN regeneration128. Furthermore, deficiencies in TNFR1 or basal progenitor cell NF-κB signalling led to defective olfactory regeneration, emphasizing the importance of immune crosstalk in neurogenesis128. Overall, resident stem cells in the olfactory mucosa display remarkable regenerative capacity and therapies targeting these cells are currently being explored to combat dysosmia205,206.
To understand the olfactory implications of chronic inflammatory conditions such as CRS and neurodegeneration, mouse lines that inducibly express inflammatory mediators such as TNF111 and IL-13 (ref. 207) in the olfactory mucosa have been developed131,208. The inducible olfactory inflammation mouse drives TNF expression from CYP2G1+ sustentacular cells209 to recapitulate CRS-induced olfactory loss and progressive olfactory neurodegeneration. Studies in inducible olfactory inflammation mice have indicated cognitive functional defects, epithelial reorganization, macrophage-mediated and T cell-mediated cytokine production, and reprogramming of basal progenitor cells from a proliferative to an inflammatory phenotype111,209,210. Likewise, olfactory damage and inflammation can drive acute cytokine expression in the olfactory mucosa, neutrophil infiltration and tissue deformation112,125,211, but critically, this peripheral inflammation can be communicated into the CNS131. In zebrafish, olfactory epithelial damage leads to rapid neutrophil recruitment into the olfactory organ of the brain212. Similarly, in hamsters, SARS-CoV-2-mediated olfactory mucosa inflammation is sufficient to induce changes in the olfactory bulb90. The connection between mucosal and brain inflammation has important consequences, as frequent olfactory inflammation may contribute to neurodegenerative pressure213 over time. Further work is needed to understand how olfactory mucosa inflammation not only drives immune-mediated olfactory disorders but also supports inflammatory communication between the olfactory mucosa and the CNS.
Presbyosmia and neurodegeneration.
Age-associated olfactory loss, or presbyosmia, is extremely common in elderly patients (occurring in >50% of adults over 65 years and in 60–80% of those aged over 80 years214–217). Presbyosmia is associated with olfactory metaplasia, the replacement of olfactory epithelium with respiratory epithelium218,219. This tissue conversion mirrors that observed in models of chronic olfactory mucosal inflammation209 and is probably owing to inflammation-related changes in olfactory stem cell progenitors110. Transcriptional evidence of strong cytokine stimuli and elevated immune cells in a presbyosmic cohort compared with controls suggest a direct role for sustained inflammation in age-associated olfactory loss110. Concordant with age-associated neuroinflammation, impaired olfaction is associated with (and is one of the strongest predictors of) cognitive decline220, Alzheimer disease221, dementia222 and Parkinson disease223. Myriad factors probably contribute to this association224, but prior olfactory infections may accelerate cognitive decline through repeated inflammatory stimuli. To this end, individuals with familial Alzheimer disease were found to express signatures of antiviral inflammation in the olfactory bulb and olfactory tract225. Corroborating this, HSV1, which can infect sensory fibres within the olfactory neuroepithelium, has been implicated in Alzheimer disease226. More directly supporting the olfactory infection hypothesis, in a mouse model, Chlamydia was shown to invade the CNS through the olfactory nerve and upregulate Alzheimer disease-associated gene signatures227. Much remains to be investigated about the bidirectional link between olfaction and neurodegeneration and the role infection might have in both.
Conclusion
The olfactory mucosa must be considered as a critical component of pathogen defence, both as part of the respiratory tract and as a mucosal barrier for the brain. Immune protection of the olfactory mucosa is vital for protection against continued respiratory pathogen transmission and neurotropic microbial invasion. Previously, it was difficult to reconcile our assumptions about peripheral immunity with many studies revealing incomplete URT immunity. We believe that appreciating the unique immunological considerations of the olfactory mucosa is not only critical for vaccine-induced URT immunity but also provides clarity into prior data and informs better experimental design. For instance, nasal washes and swabs insufficiently capture the olfactory mucosal immune response, and increased upper nasal turbinate sampling may reveal that many URT pathogens have distinct olfactotropism. An improved understanding of pathogen-induced olfactory dysfunction and neurodegeneration could lead to better and more targeted therapeutics for diseases such as COVID-19. A key consideration in drugs that target the olfactory system will be the BOB. Additional characterization of the BOB, and the role it has in immune defence, is also of interest for intranasal drug delivery and CNS anatomy. The BOB confers a degree of immune isolation and privilege to the olfactory mucosa, but very little is known about the tissue-resident immune cells in this tissue. Analysing these cells, and how they interact with stromal cells in both health and disease, will shed new light on URT and CNS defence. The olfactory mucosa deserves more attention as a mucosal immune barrier, and we smell the dawning of a new age in its study (pun intended).
Acknowledgements
E.A.M. is supported by R01NS121067, R21DC021260 and R21AG074324, and by the Duke School of Medicine Whitehead Family Scholarship.
Glossary
- Anosmia
The complete loss of smell, typically defined clinically by the University of Pennsylvania Smell Identification Test scores <19
- Blood–olfactory barrier
(BOB). A blood–endothelial barrier that prevents the movement of large molecules from circulation into the olfactory mucosa
- Dysosmia
A general term for an altered sense of smell
- Hyposmia
A reduced sense of smell, typically defined clinically by the University of Pennsylvania Smell Identification Test scores in the 19–33 range, although scoring can be adjusted by age and sex
- Olfactory binding proteins
Soluble proteins in the nasal mucus that bind to odourants to facilitate recognition by olfactory receptors. They have also been shown to have antimicrobial effects
- Olfactotropic
A pathogen that is capable of infecting cells within the olfactory mucosa
- Presbyosmia
An age-associated loss of smell
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Baden LR et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med 383, 2603–2615 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergwerk M et al. Covid-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med 385, 1629–1630 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Terreri S et al. Persistent B cell memory after SARS-CoV-2 vaccination is functional during breakthrough infections. Cell Host Microbe 30, 400–408.e404 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall V et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N. Engl. J. Med 386, 1207–1220 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giacomelli A et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin. Infect. Dis 71, 889–890 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee Y, Min P, Lee S & Kim S-W Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J. Korean Med. Sci 10.3346/jkms.2020.35.e174 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Premraj L et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J. Neurol. Sci 434, 120162 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crook H, Raza S, Nowell J, Young M & Edison P Long covid — mechanisms, risk factors, and management. BMJ 374, n1648 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Eyre DW et al. Effect of Covid-19 vaccination on transmission of alpha and delta variants. N. Engl. J. Med 386, 744–756 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horiuchi S et al. Immune memory from SARS-CoV-2 infection in hamsters provides variant-independent protection but still allows virus transmission. Sci. Immunol 10.1126/sciimmunol.abm3131 (2021). [DOI] [PubMed] [Google Scholar]; Demonstrated that SARS-CoV-2 could be transmitted between hamsters even in the presence of systemic immune memory.
- 12.Brouwer PJM et al. Two-component spike nanoparticle vaccine protects macaques from SARS-CoV-2 infection. Cell 184, 1188–1200.e19 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Routhu NK et al. A modified vaccinia Ankara vector-based vaccine protects macaques from SARS-CoV-2 infection, immune pathology, and dysfunction in the lungs. Immunity 54, 542–556.e9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bricker TL et al. A single intranasal or intramuscular immunization with chimpanzee adenovirus-vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters. Cell Rep 36, 109400 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Doremalen N et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 586, 578–582 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Case JB et al. Replication-competent vesicular stomatitis virus vaccine vector protects against SARS-CoV-2-mediated pathogenesis in mice. Cell Host Microbe 28, 465–474.e4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pino M et al. A yeast expressed RBD-based SARS-CoV-2 vaccine formulated with 3M-052-alum adjuvant promotes protective efficacy in non-human primates. Sci. Immunol 10.1126/sciimmunol.abh3634 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Doremalen N et al. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces viral shedding after SARS-CoV-2 D614G challenge in preclinical models. Sci. Transl. Med 13, eabh0755 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou D et al. Robust SARS-CoV-2 infection in nasal turbinates after treatment with systemic neutralizing antibodies. Cell Host Microbe 29, 551–563.e5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; Shows that the nasal turbinates could still be infected with SARS-CoV-2 despite prior neutralizing antibody administration.
- 20.Gagne M et al. Protection from SARS-CoV-2 delta one year after mRNA-1273 vaccination in rhesus macaques coincides with anamnestic antibody response in the lung. Cell 185, 113–130.e5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen F et al. SARS-CoV-2 reinfection prevents acute respiratory disease in Syrian hamsters but not replication in the upper respiratory tract. Cell Rep 10.1016/j.celrep.2022.110515 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang J et al. Respiratory mucosal immunity against SARS-CoV-2 following mRNA vaccination. Sci. Immunol 0, eadd4853 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J et al. CD8 T cells contribute to vaccine protection against SARS-CoV-2 in macaques. Sci. Immunol 7, eabq7647 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramphal R, Cogliano RC, Shands JW & Small PA Serum antibody prevents lethal murine influenza pneumonitis but not tracheitis. Infect. Immun 25 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subbarao K et al. Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J. Virol 78, 3572–3577 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glauser DL, Milho R, Lawler C & Stevenson PG Antibody arrests γ-herpesvirus olfactory super-infection independently of neutralization. J. Gen. Virol 100, 246–258 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Williamson LE et al. Human antibodies protect against aerosolized Eastern equine encephalitis virus infection. Cell 183, 1884–1900.e23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kafai NM et al. Neutralizing antibodies protect mice against Venezuelan equine encephalitis virus aerosol challenge. J. Exp. Med 219, e20212532 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukuiwa T et al. A combination of Flt3 ligand cDNA and CpG ODN as nasal adjuvant elicits NALT dendritic cells for prolonged mucosal immunity. Vaccine 26, 4849–4859 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sealy R, Webby RJ, Crumpton JC & Hurwitz JL Differential localization and function of antibody forming cells responsive to inactivated or live attenuated influenza virus vaccines. Int. Immunol 25, 183–195 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martini V et al. Simultaneous aerosol and intramuscular immunization with influenza vaccine induces powerful protective local T cell and systemic antibody immune responses in pigs. J. Immunol 206, ji2001086 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arunachalam PS et al. Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature 594, 253–258 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Ochsner SP et al. FcRn-targeted mucosal vaccination against influenza virus infection. J. Immunol 207, 1310–1321 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lavelle EC & Ward RW Mucosal vaccines — fortifying the frontiers. Nat. Rev. Immunol, 1–15, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lund FE & Randall TD Scent of a vaccine. Science (2021). [DOI] [PubMed] [Google Scholar]
- 36.Smith N et al. Distinct systemic and mucosal immune responses during acute SARS-CoV-2 infection. Nat. Immunol 22, 1428–1439 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fröberg J et al. SARS-CoV-2 mucosal antibody development and persistence and their relation to viral load and COVID-19 symptoms. Nat. Commun 12, 5621 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mettelman RC, Allen EK & Thomas PG Mucosal immune responses to infection and vaccination in the respiratory tract. Immunity 55, 749–780 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renegar KB, Small PA, Boykins LG & Wright PF Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J. Immunol 173, 1978–1986 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Horton RE & Vidarsson G Antibodies and their receptors: different potential roles in mucosal defense. Front. Immunol 10.3389/fimmu.2013.00200 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pizzolla A et al. Resident memory CD8+ T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci. Immunol 2, eaam6970 (2017). [DOI] [PubMed] [Google Scholar]; Describes resident memory T cells in the olfactory mucosa and shows their importance in combating influenza viral spread to the lung.
- 42.Sheikh-Mohamed S et al. A mucosal antibody response is induced by intra-muscular SARS-CoV-2 mRNA vaccination 2021.2008.2001.21261297 (2021).
- 43.Wagner DK et al. Analysis of immunoglobulin G antibody responses after administration of live and inactivated influenza A vaccine indicates that nasal wash immunoglobulin G is a transudate from serum. J. Clin. Microbiol 25, 559–562 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazanec MB, Nedrud JG, Liang XP & Lamm ME Transport of serum IgA into murine respiratory secretions and its implications for immunization strategies. J. Immunol 142, 4275–4281 (1989). [PubMed] [Google Scholar]
- 45.Pakkanen SH et al. Expression of homing receptors on IgA1 and IgA2 plasmablasts in blood reflects differential distribution of IgA1 and IgA2 in various body fluids. Clin. Vaccin. Immunol 17, 393–401 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mades A et al. Detection of persistent SARS-CoV-2 IgG antibodies in oral mucosal fluid and upper respiratory tract specimens following COVID-19 mRNA vaccination. Sci. Rep 11, 24448 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ladel S et al. Impact of glycosylation and species origin on the uptake and permeation of IgGs through the nasal airway mucosa. Pharmaceutics 12, 1014 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wellford SA et al. Mucosal plasma cells are required to protect the upper airway and brain from infection. Immunity 10.1016/j.immuni.2022.08.017 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; Has discovered the blood–olfactory barrier and demonstrates the requirement for local antibody production by mucosal plasma cells in olfactory mucosa protection.
- 49.Rajini B, Zeng J, Suvas PK, Dech HM & Onami TM Both systemic and mucosal LCMV immunization generate robust viral-specific IgG in mucosal secretions, but elicit poor LCMV-specific IgA. Viral Immunol 23, 377–384 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dando SJ et al. Pathogens penetrating the central nervous system: infection pathways and the cellular and molecular mechanisms of invasion. Clin. Microbiol. Rev 27, 691–726 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ampie L & McGavern DB Immunological defense of CNS barriers against infections. Immunity 55, 781–799 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mastorakos P & McGavern D The anatomy and immunology of vasculature in the central nervous system. Sci. Immunol 4, eaav0492 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Paiva CS, Leger AJS & Caspi RR Mucosal immunology of the ocular surface. Mucosal Immunol 15, 1143–1157 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hewitt RJ & Lloyd CM Regulation of immune responses by the airway epithelial cell landscape. Nat. Rev. Immunol 21, 347–362 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eiting TP, Smith TD, Perot JB & Dumont ER The role of the olfactory recess in olfactory airflow. J. Exp. Biol 217, 1799–1803 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Lee SH, Kim JE, Lee HM, Lim HH & Choi JO Antimicrobial defensin peptides of the human nasal mucosa. Ann. Otol. Rhinol. Laryngol 111, 135–141 (2002). [DOI] [PubMed] [Google Scholar]
- 57.Thienhaus ML et al. Antimicrobial peptides in nasal secretion and mucosa with respect to Staphylococcus aureus colonization in chronic rhinosinusitis with nasal polyps. Rhinology 49, 554–561 (2011). [DOI] [PubMed] [Google Scholar]
- 58.Podlesnaja M, Pilmane M & Sumeraga G Cytokines, proliferation markers, antimicrobial factors and neuropeptide-containing innervation in human nasal mucosa after rhinoseptoplasty procedure. Med. Sci 9, 25 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kennel C et al. Differential expression of mucins in murine olfactory versus respiratory epithelium. Chem. Senses 44, 511–521 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bryche B, Baly C & Meunier N Modulation of olfactory signal detection in the olfactory epithelium: focus on the internal and external environment, and the emerging role of the immune system. Cell Tissue Res 384, 589–605 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bianchi F et al. Vertebrate odorant binding proteins as antimicrobial humoral components of innate immunity for pathogenic microorganisms. PLoS One 14, e0213545 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shirai T et al. Functions of human olfactory mucus and age-dependent changes. Sci. Rep 13, 971 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen CR, Kachramanoglou C, Li D, Andrews P & Choi D Anatomy and cellular constituents of the human olfactory mucosa: a review. J. Neurol. Surg. B Skull Base 75, 293–300 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barrios AW, Nunez G, Sanchez Quinteiro P & Salazar I Anatomy, histochemistry, and immunohistochemistry of the olfactory subsystems in mice. Front. Neuroanat 8, 63 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ualiyeva S et al. Olfactory microvillar tuft cells direct neurogenesis during allergic inflammation. Preprint at bioRxiv 10.1101/2022.09.26.509561 (2022). [DOI] [Google Scholar]
- 66.Schwob JE et al. Stem and progenitor cells of the mammalian olfactory epithelium: taking poietic license. J. Comp. Neurol 525, 1034–1054 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morrison EE & Costanzo RM Scanning electron microscopic study of degeneration and regeneration in the olfactory epithelium after axotomy. J. Neurocytol 18, 393–405 (1989). [DOI] [PubMed] [Google Scholar]
- 68.Perera SN et al. Insights into olfactory ensheathing cell development from a laser-microdissection and transcriptome-profiling approach. GLIA 10.1002/glia.23870 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hong SP et al. Three-dimensional morphologic and molecular atlases of nasal vasculature. Nat. Cardiovasc. Res, 1–18 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jacob L et al. Conserved meningeal lymphatic drainage circuits in mice and humans. J. Exp. Med 219 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Norwood JN et al. Anatomical basis and physiological role of cerebrospinal fluid transport through the murine cribriform plate. eLife 10.7554/eLife.44278 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; Illustrates lymphatic drainage from the CNS through the olfactory portal.
- 72.Goldmann J et al. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. J. Leukoc. Biol 80, 797–801 (2006). [DOI] [PubMed] [Google Scholar]
- 73.Hsu M et al. Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat. Commun 10.1038/s41467-018-08163-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; Shows the role of olfactory-specific lymphangiogenesis in CNS drainage.
- 74.Walter BA, Valera VA, Takahashi S & Ushiki T The olfactory route for cerebrospinal fluid drainage into the peripheral lymphatic system. Neuropathol. Appl. Neurobiol 32, 388–396 (2006). [DOI] [PubMed] [Google Scholar]
- 75.Zakharov A, Papaiconomou C & Johnston M Lymphatic vessels gain access to cerebrospinal fluid through unique association with olfactory nerves. Lymphat. Res. Biol 2, 139–146 (2004). [DOI] [PubMed] [Google Scholar]
- 76.Jackson RT, Tigges J & Arnold W Subarachnoid space of the CNS, nasal mucosa, and lymphatic system. Arch. Otolaryngol 105, 180–184 (1979). [DOI] [PubMed] [Google Scholar]
- 77.Spera I et al. Open pathways for cerebrospinal fluid outflow at the cribriform plate along the olfactory nerves. eBioMedicine 91 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Louveau A et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aspelund A et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med 212, 991–999 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Papadopoulos Z, Herz J & Kipnis J Meningeal lymphatics: from anatomy to central nervous system immune surveillance. J. Immunol 204, 286–293 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johnston M, Zakharov A, Papaiconomou C, Salmasi G & Armstrong D Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res 1, 1–13 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mehta NH et al. The brain-nose interface: a potential cerebrospinal fluid clearance site in humans. Front. Physiol 12, 769948 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou Y et al. Impaired peri-olfactory cerebrospinal fluid clearance is associated with ageing, cognitive decline and dyssomnia. eBioMedicine 86, 104381 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Melin E, Eide PK & Ringstad G In vivo assessment of cerebrospinal fluid efflux to nasal mucosa in humans. Sci. Rep 10, 14974–14974 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mori I Highlighting the ‘blood-nerve barrier’ in virology research. Acta Virol 62, 28–32 (2018). [DOI] [PubMed] [Google Scholar]
- 86.Wolburg H et al. Epithelial and endothelial barriers in the olfactory region of the nasal cavity of the rat. Histochem. Cell Biol 130, 127–140 (2008). [DOI] [PubMed] [Google Scholar]
- 87.Crowe TP & Hsu WH Evaluation of recent intranasal drug delivery systems to the central nervous system. Pharmaceutics 14, 629 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lochhead JJ & Thorne RG Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev 64, 614–628 (2012). [DOI] [PubMed] [Google Scholar]
- 89.Ziegler CGK et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is Detected in specific cell subsets across tissues. Cell 181, 1016–1035.e19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Verma AK, Zheng J, Meyerholz DK & Perlman S SARS-CoV-2 infection of sustentacular cells disrupts olfactory signaling pathways. JCI Insight e160277 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; Shows that SARS-CoV-2 infection of sustentacular cells contributes to loss of smell.
- 91.Khan M et al. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell 184, 5932–5949. e15 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brann DH et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv 6, 5801–5832 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Richard M et al. Influenza A viruses are transmitted via the air from the nasal respiratory epithelium of ferrets. Nat. Commun 11, 766 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kolpe A, Schepens B, Ye L, Staeheli P & Saelens X Passively transferred M2e-specific monoclonal antibody reduces influenza A virus transmission in mice. Antivir. Res 158, 244–254 (2018). [DOI] [PubMed] [Google Scholar]
- 95.Price GE, Lo C-Y, Misplon JA & Epstein SL Reduction of influenza A virus transmission in mice by a universal intranasal vaccine candidate is long-lasting and does not require antibodies. J. Virol 96, e00320–e00322 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mellert TK, Getchell ML, Sparks L & Getchell TV Characterization of the immune barrier in human olfactory mucosa (1991). [PubMed] [Google Scholar]; Shows characterization of olfactory immune cells in human samples.
- 97.Getchell ML & Getchell TV Immunohistochemical localization of components of the immune barrier in the olfactory mucosae of salamanders and rats. Anat. Rec 231, 358–374 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu Y-Y et al. Mucosal immunoglobulins protect the olfactory organ of teleost fish against parasitic infection. PLoS Pathog 14, e1007251 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; Has determined the importance of mucosal immunoglobulins in protecting the olfactory organ of fish.
- 99.Dong F et al. IgT plays a predominant role in the antibacterial immunity of rainbow trout olfactory organs. Front. Immunol 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nouailles G et al. Live-attenuated vaccine sCPD9 elicits superior mucosal and systemic immunity to SARS-CoV-2 variants in hamsters. Nat. Microbiol 8, 860–874 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Durante MA et al. Single-cell analysis of olfactory neurogenesis and differentiation in adult humans. Nat. Neurosci 23, 323–326 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kazer SW et al. Primary nasal viral infection rewires the tissue-scale memory response. Preprint at bioRxiv 10.1101/2023.05.11.539887 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tan CSE & Stevenson PG B cell response to herpesvirus infection of the olfactory neuroepithelium. J. Virol 88, 14030–14039 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; Describes a role for B cells in olfactory herpesvirus infection.
- 104.Tan H-X et al. Lung-resident memory B cells established after pulmonary influenza infection display distinct transcriptional and phenotypic profiles. Sci. Immunol 7, eabf5314 (2022). [DOI] [PubMed] [Google Scholar]
- 105.Hiroi T et al. Nasal immune system: distinctive Th0 and Th1/Th2 type environments in murine nasal-associated lymphoid tissues and nasal passage, respectively. Eur. J. Immunol 28, 3346–3353 (1998). [DOI] [PubMed] [Google Scholar]
- 106.Lim JME et al. SARS-CoV-2 breakthrough infection in vaccinees induces virus-specific nasal-resident CD8+ and CD4+ T cells of broad specificity. J. Exp. Med 10.1084/jem.20220780 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sepahi A et al. Olfactory sensory neurons mediate ultrarapid antiviral immune responses in a TrkA-dependent manner. Proc. Natl Acad. Sci. USA 201900083 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; Persistent inflammation following SARS-CoV-2 infection contributes to long-term olfactory deficits.
- 108.Finlay JB et al. Persistent post-COVID-19 smell loss is associated with immune cell infiltration and altered gene expression in olfactory epithelium. Sci. Transl. Med 14, eadd0484 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Frere JJ et al. SARS-CoV-2 infection in hamsters and humans results in lasting and unique systemic perturbations post recovery. Sci. Transl. Med 0, eabq3059 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; Shows that in age-related olfactory loss, lymphocytic inflammation altered olfactory stem cell regenerative capacity.
- 110.Oliva AD et al. Aging-related olfactory loss is associated with olfactory stem cell transcriptional alterations in humans. J. Clin. Investig 10.1172/JCI155506 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; Shows that inflammation drives an olfactory stem cell switch from regeneration to immune defense and that NF-κB signalling drives alterations in olfactory function.
- 111.Chen M & Reed R Chronic inflammation directs an olfactory stem cell functional switch from neuroregeneration to immune defense. Cell Stem Cell 1–13, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kagoya R et al. Immunological status of the olfactory bulb in a murine model of Toll-like receptor 3-mediated upper respiratory tract inflammation. J. Neuroinflamm 19, 13 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Smithson LJ & Kawaja MD Microglial/macrophage cells in mammalian olfactory nerve fascicles. J. Neurosci. Res 88, 858–865 (2010). [DOI] [PubMed] [Google Scholar]
- 114.Ruitenberg MJ et al. CX3CL1/fractalkine regulates branching and migration of monocyte-derived cells in the mouse olfactory epithelium. J. Neuroimmunol 205, 80–85 (2008). [DOI] [PubMed] [Google Scholar]
- 115.Savage JC, Carrier M & Tremblay M-È Morphology of microglia across contexts of health and disease. Microglia Meth. Protoc 13–26 (2019). [DOI] [PubMed] [Google Scholar]
- 116.Borders AS et al. Macrophage depletion in the murine olfactory epithelium leads to increased neuronal death and decreased neurogenesis. J. Comp. Neurol 501, 206–218 (2007). [DOI] [PubMed] [Google Scholar]
- 117.Borders AS et al. Macrophage-mediated neuroprotection and neurogenesis in the olfactory epithelium. Physiol. Genom 31, 531–543 (2007). [DOI] [PubMed] [Google Scholar]
- 118.Nan B, Getchell ML, Partin JV & Getchell TV Leukemia inhibitory factor, interleukin-6, and their receptors are expressed transiently in the olfactory mucosa after target ablation. J. Comp. Neurol 435, 60–77 (2001). [DOI] [PubMed] [Google Scholar]
- 119.Getchell ML et al. Temporal gene expression profiles of target-ablated olfactory epithelium in mice with disrupted expression of scavenger receptor A: impact on macrophages. Physiol. Genom 27, 245–263 (2006). [DOI] [PubMed] [Google Scholar]
- 120.Getchell TV, Shah DS, Partin JV, Subhedar NK & Getchell ML Leukemia inhibitory factor mRNA expression is upregulated in macrophages and olfactory receptor neurons after target ablation. J. Neurosci. Res 67, 246–254 (2002). [DOI] [PubMed] [Google Scholar]
- 121.Getchell TV et al. Chemokine regulation of macrophage recruitment into the olfactory epithelium following target ablation: involvement of macrophage inflammatory protein-1α and monocyte chemoattractant protein-1. J. Neurosci. Res 70, 784–793 (2002). [DOI] [PubMed] [Google Scholar]
- 122.Shi J et al. PHEV infection: a promising model of betacoronavirus-associated neurological and olfactory dysfunction. PLoS Pathog 18, e1010667 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yoshida T et al. Olfactory receptor neurons prevent dissemination of neurovirulent influenza A virus into the brain by undergoing virus-induced apoptosis. J. Gen. Virol 83, 2109–2116 (2002). [DOI] [PubMed] [Google Scholar]
- 124.Herbert RP et al. Cytokines and olfactory bulb microglia in response to bacterial challenge in the compromised primary olfactory pathway. J. Neuroinflamm 9, 109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yee KK et al. Analysis of the olfactory mucosa in chronic rhinosinusitis. Ann. N. Y. Acad. Sci 1170, 590–595 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kern RC Chronic sinusitis and anosmia: pathologic changes in the olfactory mucosa. Laryngoscope 110, 1071–1077 (2000). [DOI] [PubMed] [Google Scholar]
- 127.Kanaya K et al. Innate immune responses and neuroepithelial degeneration and regeneration in the mouse olfactory mucosa induced by intranasal administration of poly(I:C). Cell Tissue Res 357, 279–299 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen M, Reed RR & Lane AP Acute inflammation regulates neuroregeneration through the NF-κB pathway in olfactory epithelium. Proc. Natl Acad. Sci. USA 114, 8089–8094 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pägelow D et al. The olfactory epithelium as a port of entry in neonatal neurolisteriosis. Nat. Commun 9, 4269 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rojas-Hernández S, Jarillo-Luna A, Rodríguez-Monroy M, Moreno-Fierros L & Campos-Rodríguez R Immunohistochemical characterization of the initial stages of Naegleria fowleri meningoencephalitis in mice. Parasitol. Res 94, 31–36 (2004). [DOI] [PubMed] [Google Scholar]
- 131.Yeh C-F, Huang W-H, Lan M-Y & Hung W Lipopolysaccharide-initiated rhinosinusitis causes neuroinflammation and olfactory dysfunction in mice. Am. J. Rhinol. Allergy 10.1177/19458924221140965 (2022). [DOI] [PubMed] [Google Scholar]
- 132.Farrell NF et al. Mucosal eosinophilia and neutrophilia are not associated with QOL or olfactory function in chronic rhinosinusitis. Am. J. Rhinol. Allergy 1945892420987439 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bourgon C et al. Neutrophils play a major role in the destruction of the olfactory epithelium during SARS-CoV-2 infection in hamsters. Cell. Mol. Life Sci 79, 616 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cervantes-Sandoval I, Serrano-Luna Jd. J., García-Latorre E, Tsutsumi V & Shibayama M Characterization of brain inflammation during primary amoebic meningoencephalitis. Parasitol. Int 57, 307–313 (2008). [DOI] [PubMed] [Google Scholar]
- 135.Yee KK et al. Neuropathology of the olfactory mucosa in chronic rhinosinusitis. Am. J. Rhinol. Allergy 24, 110–120 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jacobs S, Zeippen C, Wavreil F, Gillet L & Michiels T IFN-λ decreases murid herpesvirus-4 infection of the olfactory epithelium but fails to prevent virus reactivation in the vaginal mucosa. Viruses 11, 757 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Klinkhammer J et al. IFN-λ prevents influenza virus spread from the upper airways to the lungs and limits virus transmission. eLife 7, e33354 (). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Trottier M, Lyles D & Reiss CS Peripheral, but not central nervous system, type I interferon expression in mice in response to intranasal vesicular stomatitis virus infection. J. Neurovirol 13, 433–445 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lawler C & Stevenson PG Type I interferon signaling to dendritic cells limits murid herpesvirus 4 spread from the olfactory epithelium. J. Virol 91, e00951–00917 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu G et al. Prevention of lethal respiratory vaccinia infections in mice with interferon-α and interferon-γ. FEMS Immunol. Med. Microbiol 40, 201–206 (2004). [DOI] [PubMed] [Google Scholar]
- 141.Bessière P et al. Intranasal type I interferon treatment is beneficial only when administered before clinical signs onset in the SARS-CoV-2 hamster model. PLoS Pathog 17, e1009427 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chong Z et al. Nasally delivered interferon-λ protects mice against infection by SARS-CoV-2 variants including Omicron. Cell Rep 39, 110799 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; Shows that OSN-mediated clearance of influenza prevents viral trafficking to the CNS.
- 143.Dumm RE, Wellford SA, Moseman EA & Heaton NS Heterogeneity of antiviral responses in the upper respiratory tract mediates differential non-lytic clearance of influenza viruses. Cell Rep 32, 108103 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Khan M et al. Anatomical barriers against SARS-CoV-2 neuroinvasion at vulnerable interfaces visualized in deceased COVID-19 patients. Neuron S0896–S6273 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ualiyeva S et al. Airway brush cells generate cysteinyl leukotrienes through the ATP sensor P2Y2. Sci. Immunol 10.1126/sciimmunol.aax7224 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Beiersdorfer A et al. Sublamina-specific organization of the blood brain barrier in the mouse olfactory nerve layer. Glia 68, 631–645 (2020). [DOI] [PubMed] [Google Scholar]
- 147.Denaro S et al. Neurotrophic and immunomodulatory effects of olfactory ensheathing cells as a strategy for neuroprotection and regeneration. Front. Immunol 13 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lan Y-X et al. Gene and protein expression profiles of olfactory ensheathing cells from olfactory bulb versus olfactory mucosa. Neural Regen. Res 17, 440 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Harris JA, West AK & Chuah MI Olfactory ensheathing cells: nitric oxide production and innate immunity. Glia 57, 1848–1857 (2009). [DOI] [PubMed] [Google Scholar]; Shows that olfactory ensheathing cells are able to monitor and phagocytose OSN axons.
- 150.Su Z et al. Olfactory ensheathing cells: the primary innate immunocytes in the olfactory pathway to engulf apoptotic olfactory nerve debris. Glia 61, 490–503 (2013). [DOI] [PubMed] [Google Scholar]
- 151.Wright AA, Todorovic M, Murtaza M, St John JA & Ekberg JA Macrophage migration inhibitory factor and its binding partner HTRA1 are expressed by olfactory ensheathing cells. Mol. Cell. Neurosci 102, 103450 (2020). [DOI] [PubMed] [Google Scholar]
- 152.van Riel D, Verdijk R & Kuiken T The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J. Pathol 235, 277–287 (2015). [DOI] [PubMed] [Google Scholar]
- 153.Meng X, Deng Y, Dai Z & Meng Z COVID-19 and anosmia: a review based on up-to-date knowledge. Am. J. Otolaryngol. Head Neck Med. Surg 41, 102581–102581 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Overdevest JB et al. Chemosensory deficits are best predictor of serologic response among individuals infected with SARS-CoV-2. PLoS One 17, e0274611 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hossain ME et al. Prolonged viral shedding in patients with mild to moderate COVID-19 disease: a regional perspective. Infect. Dis 14, 11786337211010428 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Long H et al. Prolonged viral shedding of SARS-CoV-2 and related factors in symptomatic COVID-19 patients: a prospective study. BMC Infect. Dis 21, 1282 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.de Melo GD et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci. Transl. Med 13, eabf8396 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zheng J et al. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature 10.1038/s41586-020-2943-z (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kumari P et al. Neuroinvasion and encephalitis following intranasal inoculation of SARS-CoV-2 in K18-hACE2 mice. Viruses 13, 132 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fumagalli V et al. Administration of aerosolized SARS-CoV-2 to K18-hACE2 mice uncouples respiratory infection from fatal neuroinvasion. Sci. Immunol 10.1126/sciimmunol.abl9929 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Seehusen F et al. Neuroinvasion and neurotropism by SARS-CoV-2 variants in the K18-hACE2 mouse. Viruses 14, 1020 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bryche B et al. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav. Immun 89, 579–586 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen M et al. Evolution of nasal and olfactory infection characteristics of SARS-CoV-2 variants. Preprint at bioRxiv 10.1101/2022.04.12.487379 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Melo GDD et al. Neuroinvasion and anosmia are independent phenomena upon infection with SARS-CoV-2 and its variants. Preprint at bioRxiv 10.1101/2022.08.31.505985 (2022). [DOI] [Google Scholar]
- 165.Siddiqui R, Ali IKM, Cope JR & Khan NA Biology and pathogenesis of Naegleria fowleri. Acta Tropica 164, 375–394 (2016). [DOI] [PubMed] [Google Scholar]
- 166.Weik RR & Adams AC Immunization of mice against Naegleria fowleri. Infection 16, 817–820 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ferrante A et al. Depression of immunity to Naegleria fowleri in mice by selective depletion of neutrophils with a monoclonal antibody. Infect. Immun 56, 2286–2291 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jarolim KL, McCosh JK, Howard MJ & John DT A light microscopy study of the migration of Naegleria fowleri from the nasal submucosa to the central nervous system during the early stage of primary amebic meningoencephalitis in mice. J. Parasitol 86, 50–55 (2000). [DOI] [PubMed] [Google Scholar]
- 169.Dubray BL, Wilhelm WE & Jennings BR Serology of Naegleria fowleri and Naegleria lovaniensis in a hospital survey. J. Protozool 34, 322–327 (1987). [DOI] [PubMed] [Google Scholar]
- 170.Marciano-Cabral F, Cline ML & Bradley SG Specificity of antibodies from human sera for Naegleria species. J. Clin. Microbiol 25, 692–697 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rivera V et al. IgA and IgM anti-Naegleria fowleri antibodies in human serum and saliva. Can. J. Microbiol 47, 464–466 (2001). [PubMed] [Google Scholar]
- 172.Flanagan CE, Wise SK, DelGaudio JM & Patel ZM Association of decreased rate of influenza vaccination with increased subjective olfactory dysfunction. JAMA Otolaryngol. Head Neck Surg 141, 225–228 (2015). [DOI] [PubMed] [Google Scholar]
- 173.Mizuguchi M Influenza encephalopathy and related neuropsychiatric syndromes. Influenza Other Respir. Viruses 7, 67–71 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Studahl M Influenza virus and CNS manifestations. J. Clin. Virol 28, 225–232 (2003). [DOI] [PubMed] [Google Scholar]
- 175.Lee N et al. Acute encephalopathy associated with influenza A infection in adults. Emerg. Infect. Dis 16, 139–142 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Newland JG et al. Encephalitis associated with influenza B virus infection in 2 children and a review of the literature. Clin. Infect. Dis 36, e87–e95 (2003). [DOI] [PubMed] [Google Scholar]
- 177.Britton PN et al. The spectrum and burden of influenza-associated neurological disease in children: combined encephalitis and influenza sentinel site surveillance from Australia, 2013–2015. Clin. Infect. Dis 65, 653–660 (2017). [DOI] [PubMed] [Google Scholar]
- 178.Aronsson F, Robertson B, Ljunggren H-G & Kristensson K Invasion and persistence of the neuroadapted influenza virus A/WSN/33 in the mouse olfactory system. Viral Immunol 16, 415–423 (2003). [DOI] [PubMed] [Google Scholar]
- 179.Plourde JR et al. Neurovirulence of H5N1 infection in ferrets is mediated by multifocal replication in distinct permissive neuronal cell regions. PLoS One 7, e46605 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jang H et al. Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc. Natl Acad. Sci. USA 106, 14063–14068 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.van den Brand JMA et al. Comparison of temporal and spatial dynamics of seasonal H3N2, pandemic H1N1 and highly pathogenic avian influenza H5N1 virus infections in ferrets. PLoS One 7, e42343 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Schrauwen EJA et al. The multibasic cleavage site in H5N1 virus is critical for systemic spread along the olfactory and hematogenous routes in ferrets. J. Virol 86, 3975–3984 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.van Riel D et al. Evidence for influenza virus CNS invasion along the olfactory route in an immunocompromised infant. J. Infect. Dis 210, 419–423 (2014). [DOI] [PubMed] [Google Scholar]
- 184.Kobasa D et al. Transmission of lethal H5N1 clade 2.3. 4.4 b avian influenza in ferrets (2023).
- 185.Bianchi A et al. In vivo magnetic resonance imaging evidence of olfactory bulbs changes in a newborn with congenital citomegalovirus: a case report. Ital. J. Pediatr 47, 227 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lazarini F et al. Olfactory function in congenital cytomegalovirus infection: a prospective study. Eur. J. Pediatr 181, 1859–1869 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lazarini F et al. Congenital cytomegalovirus infection alters olfaction before hearing deterioration in mice. J. Neurosci 38, 10424–10437 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xiaofei E et al. OR14I1 is a receptor for the human cytomegalovirus pentameric complex and defines viral epithelial cell tropism. Proc. Natl Acad. Sci. USA 116, 7043–7052 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.St. John JA et al. Burkholderia pseudomallei penetrates the brain via destruction of the olfactory and trigeminal nerves: implications for the pathogenesis of neurological melioidosis. mBio 5, e00025–14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Walkden H et al. Burkholderia pseudomallei invades the olfactory nerve and bulb after epithelial injury in mice and causes the formation of multinucleated giant glial cells in vitro. PLoS Negl. Tropical Dis 14, e0008017 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chacko A et al. Streptococcus agalactiae infects glial cells and invades the central nervous system via the olfactory and trigeminal nerves. Front. Cell. Infect. Microbiol 12, 793416 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sugiura M, Aiba T, Mori J & Nakai Y An epidemiological study of postviral olfactory disorder. Acta Oto-Laryngol 118, 191–196 (1998). [DOI] [PubMed] [Google Scholar]
- 193.Welge-Lüssen A & Wolfensberger M Olfactory disorders following upper respiratory tract infections. Adv. Oto-Rhino-Laryngol 63, 125–132 (2006). [DOI] [PubMed] [Google Scholar]
- 194.Seiden AM Postviral olfactory loss. Otolaryngol. Clin. North Am 37, 1159–1166 (2004). [DOI] [PubMed] [Google Scholar]
- 195.Hura N et al. in International Forum of Allergy & Rhinology 1065–1086 (Wiley; ). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Othman BA et al. Olfactory dysfunction as a post-infectious symptom of SARS-CoV-2 infection. Ann. Med. Surg 75, 103352 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lechien JR, Vaira LA & Saussez S Prevalence and 24-month recovery of olfactory dysfunction in COVID-19 patients: a multicentre prospective study. J. Intern. Med 293, 82–90 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tan HQM, Pendolino AL, Andrews PJ & Choi D Prevalence of olfactory dysfunction and quality of life in hospitalised patients 1 year after SARS-CoV-2 infection: a cohort study. BMJ Open 12, e054598 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cardoso CC et al. Olfactory dysfunction in patients with mild COVID-19 during gamma, delta, and omicron waves in Rio de Janeiro, Brazil. JAMA 328, 582–583 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Narayanan SN et al. The prevalence and pathophysiology of chemical sense disorder caused by the novel coronavirus. Front. Public Health 10 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kapoor D, Verma N, Gupta N & Goyal A Post viral olfactory dysfunction after SARS-CoV-2 infection: anticipated post-pandemic clinical challenge. Indian J. Otolaryngol. Head Neck Surg 1–8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Imamura F & Hasegawa-Ishii S Environmental toxicants-induced immune responses in the olfactory mucosa. Front. Immunol 7, 475 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gomes SC et al. Olfaction in nasal polyp patients after Reboot surgery: an endotype-based prospective study. Eur. Arch. Oto-Rhino-Laryngol 1–10, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kim J et al. Microglial and astroglial reaction in the olfactory bulb of mice after Triton X-100 application. Acta Histochem 121, 546–552 (2019). [DOI] [PubMed] [Google Scholar]
- 205.Lakshmanan HG, Miller E, White-Canale A & McCluskey LP Immune responses in the injured olfactory and gustatory systems: a role in olfactory receptor neuron and taste bud regeneration? Chem. Senses 47 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Choi R & Goldstein BJ Olfactory epithelium: cells, clinical disorders, and insights from an adult stem cell niche. Laryngosc. Investig. Otolaryngol 3, 35–42 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Saraswathula A, Liu MM, Kulaga H & Lane AP Chronic interleukin-13 expression in mouse olfactory mucosa results in regional aneuronal epithelium. Int. Forum Allergy Rhinol 10.1002/alr.23073 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ozaki S et al. Impaired olfactory function in mice with allergic rhinitis. Auris Nasus Larynx 37, 575–583 (2010). [DOI] [PubMed] [Google Scholar]
- 209.Lane AP, Turner J, May L & Reed R A genetic model of chronic rhinosinusitis-associated olfactory inflammation reveals reversible functional impairment and dramatic neuroepithelial reorganization. J. Neurosci 30, 2324–2329 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hasegawa Y et al. Causal impact of local inflammation in the nasal cavity on higher brain function and cognition. Neurosci. Res 10.1016/j.neures.2021.04.009 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; Shows that olfactory mucosa inflammation can impact cognitive function.
- 211.Bryche B et al. IL-17c is involved in olfactory mucosa responses to poly(I:C) mimicking virus presence. Brain Behav. Immun 79, 274–283 (2019). [DOI] [PubMed] [Google Scholar]
- 212.Palominos MF et al. The olfactory organ is a unique site for neutrophils in the brain. Front. Immunol 2110 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.LaFever BJ & Imamura F Effects of nasal inflammation on the olfactory bulb. J. Neuroinflamm 19, 1–11 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]; Shows that OM inflammation can directly affect inflammation in the brain.
- 214.Cain WS, Goodspeed RB, Gent JF & Leonard G Evaluation of olfactory dysfunction in the Connecticut Chemosensory Clinical Research Center. Laryngoscope 98, 83–88 (1988). [DOI] [PubMed] [Google Scholar]
- 215.Murphy C et al. Prevalence of olfactory impairment in older adults. JAMA 288, 2307–2312 (2002). [DOI] [PubMed] [Google Scholar]
- 216.Mackay-Sim A, Johnston AN, Owen C & Burne TH Olfactory ability in the healthy population: reassessing presbyosmia. Chem. Senses 31, 763–771 (2006). [DOI] [PubMed] [Google Scholar]
- 217.Lafreniere D & Mann N Anosmia: loss of smell in the elderly. Otolaryngol. Clin. North Am 42, 123–131 (2009). [DOI] [PubMed] [Google Scholar]
- 218.Paik SI, Lehman MN, Seiden AM, Duncan HJ & Smith DV Human olfactory biopsy: the influence of age and receptor distribution. Arch. Otolaryngol. Head Neck Surg 118, 731–738 (1992). [DOI] [PubMed] [Google Scholar]
- 219.Fitzek M et al. Integrated age-related immunohistological changes occur in human olfactory epithelium and olfactory bulb. J. Comp. Neurol 530, 2154–2175 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Dintica CS et al. Impaired olfaction is associated with cognitive decline and neurodegeneration in the brain. Neurology 92, e700–e709 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Murphy C Olfactory and other sensory impairments in Alzheimer disease. Nat. Rev. Neurol 15, 11–24 (2019). [DOI] [PubMed] [Google Scholar]
- 222.Alves J, Petrosyan A & Magalhães R Olfactory dysfunction in dementia. World J. Clin. Cases WJCC 2, 661 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Doty RL Olfactory dysfunction in Parkinson disease. Nat. Rev. Neurol 8, 329–339 (2012). [DOI] [PubMed] [Google Scholar]
- 224.Strous RD & Shoenfeld Y To smell the immune system: olfaction, autoimmunity and brain involvement. Autoimmun. Rev 6, 54–60 (2006). [DOI] [PubMed] [Google Scholar]
- 225.Bubak AN et al. Signatures for viral infection and inflammation in the proximal olfactory system in familial Alzheimer’s disease. Neurobiol. Aging 123, 75–82 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Itzhaki RF Overwhelming evidence for a major role for herpes simplex virus type 1 (HSV1) in Alzheimer’s disease (AD); underwhelming evidence against. Vaccines 9, 679 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chacko A et al. Chlamydia pneumoniae can infect the central nervous system via the olfactory and trigeminal nerves and contributes to Alzheimer’s disease risk. Sci. Rep 12, 2759 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cope JR et al. The epidemiology and clinical features of Balamuthia mandrillaris disease in the United States, 1974–2016. Clin. Infect. Dis 68, 1815 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kiderlen AF & Laube U Balamuthia mandrillaris, an opportunistic agent of granulomatous amebic encephalitis, infects the brain via the olfactory nerve pathway. Parasitol. Res 94, 49–52 (2004). [DOI] [PubMed] [Google Scholar]
- 230.Hu J et al. Encephalomyelitis caused by Balamuthia mandrillaris in a woman with breast cancer: a case report and review of the literature. Front. Immunol 12 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Góralska K, Blaszkowska J & Dzikowiec M Neuroinfections caused by fungi. Infection 46, 443–459 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Rake G The rapid invasion of the body through the olfactory mucosa. J. Exp. Med 65, 303–315 (1937). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.van Ginkel FW et al. Pneumococcal carriage results in ganglioside-mediated olfactory tissue infection. Proc. Natl Acad. Sci. USA 100, 14363–14367 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Audshasai T et al. Streptococcus pneumoniae rapidly translocate from the nasopharynx through the cribriform plate to invade the outer meninges. mBio 13, e0102422 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Nazareth L et al. Chlamydia muridarum can invade the central nervous system via the olfactory and trigeminal nerves and infect peripheral nerve glial cells. Front. Cell. Infect. Microbiol 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Stratton CW & Sriram S Association of Chlamydia pneumoniae with central nervous system disease. Microbes Infect 5, 1249–1253 (2003). [DOI] [PubMed] [Google Scholar]
- 237.Sjölinder H & Jonsson A-B Olfactory nerve — a novel invasion route of Neisseria meningitidis to reach the meninges. PLoS One 5, e14034 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Owen SJ et al. Nasal‐associated lymphoid tissue and olfactory epithelium as portals of entry for Burkholderia pseudomallei in murine melioidosis. J. Infect. Dis 199, 1761–1770 (2009). [DOI] [PubMed] [Google Scholar]
- 239.Netland J, Meyerholz DK, Moore S, Cassell M & Perlman S Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol 82, 7264–7275 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Gu J et al. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med 202, 415–424 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Dubé M et al. Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J. Virol 92, e00404–e00418 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Jacomy H & Talbot PJ Vacuolating encephalitis in mice infected by human coronavirus OC43. Virology 315, 20–33 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Arbour N, Day R, Newcombe J & Talbot PJ Neuroinvasion by human respiratory coronaviruses. J. Virol 74, 8913–8921 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Arabi YM et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV). Infection 43, 495–501 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Saad M et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int. J. Infect. Dis 29, 301–306 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Li K et al. Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J. Infect. Dis 213, 712–722 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zielinski MR, Souza G, Taishi P, Bohnet SG & Krueger JM Olfactory bulb and hypothalamic acute-phase responses to influenza virus: effects of immunization. Neuroimmunomodulation 20, 323–333 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Leyva-Grado VH et al. Influenza virus- and cytokine-immunoreactive cells in the murine olfactory and central autonomic nervous systems before and after illness onset. J. Neuroimmunol 211, 73–83 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Schlesinger RW Incomplete growth cycle of influenza virus in mouse brain. Proc. Soc. Exp. Biol. Med 74, 541–548 (1950). [DOI] [PubMed] [Google Scholar]
- 250.Bouvier NM & Lowen AC Vol. 2 1530–1563 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Whelan SPJ in Encyclopedia of Virology 3rd edn (eds Mahy BWJ & Van Regenmortel MHV) 291–299 (Academic, 2008). [Google Scholar]
- 252.Reiss CS, Plakhov IV & Komatsu T Viral replication in olfactory receptor neurons and entry into the olfactory bulb and brain. Ann. N. Y. Acad. Sci 855, 751–761 (1998). [DOI] [PubMed] [Google Scholar]
- 253.Moseman EA, Blanchard AC, Nayak D & McGavern DB T cell engagement of cross-presenting microglia protects the brain from a nasal virus infection. Sci. Immunol 5, eabb1817 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Kalinke U et al. The role of somatic mutation in the generation of the protective humoral immune response against vesicular stomatitis virus. Immunity 5, 639–652 (1996). [DOI] [PubMed] [Google Scholar]
- 255.Conomy JP, Leibovitz A, McCombs W & Stinson J Airborne rabies encephalitis: demonstration of rabies virus in the human central nervous system. Neurology 27, 67–67 (1977). [DOI] [PubMed] [Google Scholar]
- 256.Lafay F et al. Spread of the CVS strain of rabies virus and of the avirulent mutant AvO1 along the olfactory pathways of the mouse after intranasal inoculation. Virology 183, 320–330 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Turner G Respiratory infection of mice with vaccinia virus. J. Gen. Virol 1, 399–402 (1967). [DOI] [PubMed] [Google Scholar]
- 258.Martinez MJ, Bray MP & Huggins JW A mouse model of aerosol-transmitted orthopoxviral disease: morphology of experimental aerosol-transmitted orthopoxviral disease in a cowpox virus-BALB/c mouse system. Arch. Pathol. Lab. Med 124, 362–377 (2000). [DOI] [PubMed] [Google Scholar]
- 259.Luker KE, Hutchens M, Schultz T, Pekosz A & Luker GD Bioluminescence imaging of vaccinia virus: effects of interferon on viral replication and spread. Virology 341, 284–300 (2005). [DOI] [PubMed] [Google Scholar]
- 260.Farrell HE et al. Murine cytomegalovirus exploits olfaction to enter new hosts. mBio 7, e00251–16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Jennische E, Eriksson CE, Lange S, Trybala E & Bergström T The anterior commissure is a pathway for contralateral spread of herpes simplex virus type 1 after olfactory tract infection. J. Neurovirol 21, 129–147 (2015). [DOI] [PubMed] [Google Scholar]
- 262.Shivkumar M et al. Herpes simplex virus 1 targets the murine olfactory neuroepithelium for host entry. J. Virol 87, 10477–10488 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Mori I Transolfactory neuroinvasion by viruses threatens the human brain. Acta Virol 59, 338–349 (2015). [DOI] [PubMed] [Google Scholar]
- 264.Twomey JA, Barker CM, Robinson G & Howell DA Olfactory mucosa in herpes simplex encephalitis. J. Neurol. Neurosurg. Psychiatry 42, 983–987 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Esiri MM Herpes simplex encephalitis. An immunohistological study of the distribution of viral antigen within the brain. J. Neurol. Sci 54, 209–226 (1982). [DOI] [PubMed] [Google Scholar]
- 266.Landis BN, Vodicka J & Hummel T Olfactory dysfunction following herpetic meningoencephalitis. J. Neurol 257, 439–443 (2010). [DOI] [PubMed] [Google Scholar]
- 267.Harberts E et al. Human herpesvirus-6 entry into the central nervous system through the olfactory pathway. Proc. Natl Acad. Sci. USA 108, 13734–13739 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Milho R, Frederico B, Efstathiou S & Stevenson PG A heparan-dependent herpesvirus targets the olfactory neuroepithelium for host entry. PLoS Pathog 8, e1002986 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Narita M, Imada T & Haritani M Immunohistological demonstration of spread of Aujeszky’s disease virus via the olfactory pathway in HPCD pigs. J. Comp. Pathol 105, 141–145 (1991). [DOI] [PubMed] [Google Scholar]
- 270.Kritas SK, Pensaert MB & Mettenleiter TC Role of envelope glycoproteins gI, gp63 and gIII in the invasion and spread of Aujeszky’s disease virus in the olfactory nervous pathway of the pig. J. Gen. Virol 75, 2319–2327 (1994). [DOI] [PubMed] [Google Scholar]
- 271.Faber HK & Gebhardt LP Localizations of the virus of poliomyelitis in the central nervous system during the preparalytic period, after intranasal instillation. J. Exp. Med 57, 933–954 (1933). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Schultz EW & Gebhardt LP Olfactory tract and poliomyelitis. Proc. Soc. Exp. Biol. Med 31, 728–730 (1934). [Google Scholar]
- 273.Sabin AB & Olitsky PK The olfactory bulbs in experimental poliomyelitis: their pathologic condition as an indicator of the portal of entry of the virus. J. Am. Med. Assoc 108, 21–24 (1937). [Google Scholar]
- 274.Faber HK, Silverberg RJ & Dong L Poliomyelitis in the cynomolgus monkey: III. Infection by inhalation of droplet nuclei and the nasopharyngeal portal of entry, with a note on this mode of infection in rhesus. J. Exp. Med 80, 39–57 (1944). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sabin AB The olfactory bulbs in human poliomyelitis. Am. J. Dis. Child 60, 1313–1318 (1940). [Google Scholar]
- 276.Crotty S, Hix L, Sigal LJ & Andino R Poliovirus pathogenesis in a new poliovirus receptor transgenic mouse model: age-dependent paralysis and a mucosal route of infection. J. Gen. Virol 83, 1707–1720 (2002). [DOI] [PubMed] [Google Scholar]
- 277.Roy CJ et al. Pathogenesis of aerosolized Eastern equine encephalitis virus infection in guinea pigs. Virol. J 6, 170 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Honnold SP et al. Eastern equine encephalitis virus in mice II: pathogenesis is dependent on route of exposure. Virol. J 12, 154 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Williams JA et al. Eastern equine encephalitis virus rapidly infects and disseminates in the brain and spinal cord of cynomolgus macaques following aerosol challenge. PLoS Negl. Tropical Dis 16, e0010081 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Charles PC, Walters E, Margolis F & Johnston RE Mechanism of neuroinvasion of Venezuelan equine encephalitis virus in the mouse. Virology 208, 662–671 (1995). [DOI] [PubMed] [Google Scholar]
- 281.Ryzhikov AB, Ryabchikova EI, Sergeev AN & Tkacheva NV Spread of Venezuelan equine encephalitis virus in mice olfactory tract. Arch. Virol 140, 2243–2254 (1995). [DOI] [PubMed] [Google Scholar]
- 282.Danes L, Kufner J, Hrusková J & Rychterová V The role of the olfactory route on infection of the respiratory tract with Venezuelan equine encephalomyelitis virus in normal and operated Macaca rhesus monkeys. I. Results of virological examination. Acta Virol 17, 50–56 (1973). [PubMed] [Google Scholar]
- 283.Gardner J et al. Infectious Chikungunya virus in the saliva of mice, monkeys and humans. PLoS One 10, e0139481 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Zhou J et al. Zika virus leads to olfactory disorders in mice by targeting olfactory ensheathing cells. eBioMedicine 89 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Nir Y, Beemer A & Goldwasser RA West Nile virus infection in mice following exposure to a viral aerosol. Br. J. Exp. Pathol 46, 443–449 (1965). [PMC free article] [PubMed] [Google Scholar]
- 286.Brown AN, Kent KA, Bennett CJ & Bernard KA Tissue tropism and neuroinvasion of West Nile virus do not differ for two mouse strains with different survival rates. Virology 368, 422–430 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Yamada M, Nakamura K, Yoshii M, Kaku Y & Narita M Brain lesions induced by experimental intranasal infection of Japanese encephalitis virus in piglets. J. Comp. Pathol 141, 156–162 (2009). [DOI] [PubMed] [Google Scholar]
- 288.Han W et al. Precise localization and dynamic distribution of Japanese encephalitis virus in the rain nuclei of infected mice. PLoS Negl. Tropical Dis 15, e0008442 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.McMinn PC, Dalgarno L & Weir RC A comparison of the spread of Murray Valley encephalitis viruses of high or low neuroinvasiveness in the tissues of Swiss mice after peripheral inoculation. Virology 220, 414–423 (1996). [DOI] [PubMed] [Google Scholar]
- 290.Andrews DM, Matthews VB, Sammels LM, Carrello AC & McMinn PC The severity of Murray Valley encephalitis in mice is linked to neutrophil infiltration and inducible nitric oxide synthase activity in the central nervous system. J. Virol 73, 8781–8790 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Matthews V et al. Morphological features of Murray Valley encephalitis virus infection in the central nervous system of Swiss mice. Int. J. Exp. Pathol 81, 31–40 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Webster LT & Clow AD The limited neurotropic character of the encephalitis virus (St. Louis type) in susceptible mice. J. Exp. Med 63, 433–448 (1936). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Monath TP, Cropp CB & Harrison AK Mode of entry of a neurotropic arbovirus into the central nervous system. Reinvestigation of an old controversy. Lab. Investig 48, 399–410 (1983). [PubMed] [Google Scholar]
- 294.Wang JH, Kwon HJ & Jang YJ Detection of parainfluenza virus 3 in turbinate epithelial cells of postviral olfactory dysfunction patients. Laryngoscope 117, 1445–1449 (2007). [DOI] [PubMed] [Google Scholar]
- 295.Mori I et al. Parainfluenza virus type 1 infects olfactory neurons and establishes long-term persistence in the nerve tissue. J. Gen. Virol 76, 1251–1254 (1995). [DOI] [PubMed] [Google Scholar]
- 296.Tian J et al. Sendai virus induces persistent olfactory dysfunction in a murine model of PVOD via effects on apoptosis, cell proliferation, and response to odorants. PLoS One 11, e0159033 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Weingartl H et al. Invasion of the central nervous system in a porcine host by Nipah Virus. J. Virol 79, 7528–7534 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Dups J et al. A new model for Hendra virus encephalitis in the mouse. PLoS One 7, e40308 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Munster VJ et al. Rapid Nipah virus entry into the central nervous system of hamsters via the olfactory route. Sci. Rep 2, 736 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Baseler L et al. Identifying early target cells of Nipah virus infection in Syrian hamsters. PLoS Negl. Tropical Dis 10, e0005120 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Zlotnik I & Grant DP Further observations on subacute sclerosing encephalitis in adult hamsters: the effects of intranasal infections with Langat virus, measles virus and SSPE-measles virus. Br. J. Exp. Pathol 57, 49–66 (1976). [PMC free article] [PubMed] [Google Scholar]
- 302.Carbone KM, Duchala CS, Griffin JW, Kincaid AL & Narayan O Pathogenesis of Borna disease in rats: evidence that intra-axonal spread is the major route for virus dissemination and the determinant for disease incubation. J. Virol 61, 3431–3440 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Morales JA, Herzog S, Kompter C, Frese K & Rott R Axonal transport of Borna disease virus along olfactory pathways in spontaneously and experimentally infected rats. Med. Microbiol. Immunol 177, 51–68 (1988). [DOI] [PubMed] [Google Scholar]
- 304.Sauder C & Staeheli P Rat model of Borna disease virus transmission: epidemiological implications. J. Virol 77, 12886–12890 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Barnett EM & Perlman S The olfactory nerve and not the trigeminal nerve is the major site of CNS entry for mouse hepatitis virus, strain JHM. Virology 194, 185–191 (1993). [DOI] [PubMed] [Google Scholar]
- 306.Barthold S Olfactory neural pathway in mouse hepatitis virus nasoencephalitis. Acta Neuropathol 76, 502–506 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Barnett E, Cassell M & Perlman S Two neurotropic viruses, herpes simplex virus type 1 and mouse hepatitis virus, spread along different neural pathways from the main olfactory bulb. Neuroscience 57, 1007–1025 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Schwob JE, Saha S, Youngentob SL & Jubelt B Intranasal inoculation with the olfactory bulb line variant of mouse hepatitis virus causes extensive destruction of the olfactory bulb and accelerated turnover of neurons in the olfactory epithelium of mice. Chem. Senses 26, 937–952 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Cupovic J et al. Central nervous system stromal cells control local CD8+ T cell responses during virus-induced neuroinflammation. Immunity 44, 622–633 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Ogra PL, Karzon DT, Righthand F & MacGillivray M Immunoglobulin response in serum and secretions after immunization with live and inactivated poliovaccine and natural infection. N. Engl. J. Med 279, 893–900 (1968). [DOI] [PubMed] [Google Scholar]
- 311.Furuyama W et al. Rapid protection from COVID-19 in nonhuman primates vaccinated intramuscularly but not intranasally with a single dose of a vesicular stomatitis virus-based vaccine. mBio, e0337921 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Diallo BK et al. Intranasal COVID-19 vaccine induces respiratory memory T cells and protects K18-hACE mice against SARS-CoV-2 infection. npj Vaccines 8, 68 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Shin H & Iwasaki A A vaccine strategy protects against genital herpes by establishing local memory T cells. Nature 491, 463–467 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Zhou R et al. Nasal prevention of SARS-CoV-2 infection by intranasal influenza-based boost vaccination in mouse models. eBioMedicine 75, 103762 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Mao T et al. Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses. Science 378, eabo2523 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Saunders KO et al. Neutralizing antibody vaccine for pandemic and pre-emergent coronaviruses. Nature, 1–9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Clements JD & Norton EB The mucosal vaccine adjuvant LT(R192G/L211A) or dmLT. mSphere 3, e00215–e00218 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Tizard I & Skow L The olfactory system: the remote-sensing arm of the immune system. Anim. Health Res. Rev 22, 14–25 (2021). [DOI] [PubMed] [Google Scholar]
- 319.Anja Juran S et al. Disgusting odors trigger the oral immune system. Evol. Med. Public Health 11, 8–17 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Angelucci F et al. Physiological effect of olfactory stimuli inhalation in humans: an overview. Int. J. Cosmet. Sci 36, 117–123 (2014). [DOI] [PubMed] [Google Scholar]
- 321.Shibata H, Fujiwara R, Iwamoto M, Matsuoka H & Yokoyama MM Immunological and behavioral effects of fragrance in mice. Int. J. Neurosci 57, 151–159 (1991). [DOI] [PubMed] [Google Scholar]
- 322.Song C & Leonard B The effect of olfactory bulbectomy in the rat, alone or in combination with antidepressants and endogenous factors, on immune function. Hum. Psychopharmacol. Clin. Exp 10, 7–18 (1995). [Google Scholar]
- 323.Madhwal S et al. Metabolic control of cellular immune-competency by odors in Drosophila. eLife 9, e60376 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Leinders-Zufall T et al. MHC class I peptides as chemosensory signals in the vomeronasal organ. Science 306, 1033–1037 (2004). [DOI] [PubMed] [Google Scholar]
- 325.Thompson RN, McMillon R, Napier A & Wekesa KS Pregnancy block by MHC class I peptides is mediated via the production of inositol 1,4,5-trisphosphate in the mouse vomeronasal organ. J. Exp. Biol 210, 1406–1412 (2007). [DOI] [PubMed] [Google Scholar]
- 326.Milinski M A review of suggested mechanisms of MHC odor signaling. Biology 11, 1187 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Orecchioni M, Matsunami H & Ley K Olfactory receptors in macrophages and inflammation. Front. Immunol 13 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Cai XT et al. Gut cytokines modulate olfaction through metabolic reprogramming of glia. Nature 596, 97–102 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Jendrny P et al. Scent dog identification of samples from COVID-19 patients — a pilot study. BMC Infect. Dis 20, 1–7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Pirrone F et al. Sniffer dogs performance is stable over time in detecting COVID-19 positive samples and agrees with the rapid antigen test in the field. Sci. Rep 13, 3679 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Grandjean D et al. Diagnostic accuracy of non-invasive detection of SARS-CoV-2 infection by canine olfaction. PLoS One 17, e0268382 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Kantele A et al. Scent dogs in detection of COVID-19: triple-blinded randomised trial and operational real-life screening in airport setting. BMJ Glob. Health 7, e008024 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Juge AE, Foster MF & Daigle CL Canine olfaction as a disease detection technology: a systematic review. Appl. Anim. Behav. Sci 105664 (2022). [Google Scholar]
- 334.Buljubasic F & Buchbauer G Scent of human diseases: a review on specific volatile organic compounds as diagnostic biomarkers. Flavour Fragr. J (2015). [Google Scholar]


