Abstract
Background and Aims
Risk stratification of sudden cardiac death after myocardial infarction and prevention by defibrillator rely on left ventricular ejection fraction (LVEF). Improved risk stratification across the whole LVEF range is required for decision-making on defibrillator implantation.
Methods
The analysis pooled 20 data sets with 140 204 post-myocardial infarction patients containing information on demographics, medical history, clinical characteristics, biomarkers, electrocardiography, echocardiography, and cardiac magnetic resonance imaging. Separate analyses were performed in patients (i) carrying a primary prevention cardioverter-defibrillator with LVEF ≤ 35% [implantable cardioverter-defibrillator (ICD) patients], (ii) without cardioverter-defibrillator with LVEF ≤ 35% (non-ICD patients ≤ 35%), and (iii) without cardioverter-defibrillator with LVEF > 35% (non-ICD patients >35%). Primary outcome was sudden cardiac death or, in defibrillator carriers, appropriate defibrillator therapy. Using a competing risk framework and systematic internal–external cross-validation, a model using LVEF only, a multivariable flexible parametric survival model, and a multivariable random forest survival model were developed and externally validated. Predictive performance was assessed by random effect meta-analysis.
Results
There were 1326 primary outcomes in 7543 ICD patients, 1193 in 25 058 non-ICD patients ≤35%, and 1567 in 107 603 non-ICD patients >35% during mean follow-up of 30.0, 46.5, and 57.6 months, respectively. In these three subgroups, LVEF poorly predicted sudden cardiac death (c-statistics between 0.50 and 0.56). Considering additional parameters did not improve calibration and discrimination, and model generalizability was poor.
Conclusions
More accurate risk stratification for sudden cardiac death and identification of low-risk individuals with severely reduced LVEF or of high-risk individuals with preserved LVEF was not feasible, neither using LVEF nor using other predictors.
Keywords: Implantable cardioverter-defibrillator, Myocardial infarction, Primary prevention, Sudden cardiac death
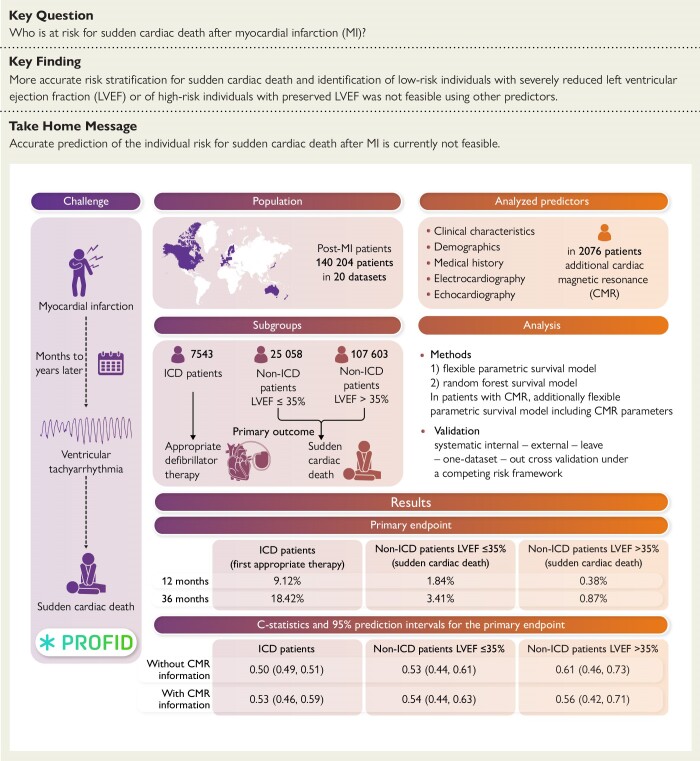
Structured Graphical Abstract
Structured Graphical Abstract.
Brief summary of the background, methodology, and results of the PROFID data analysis. ICD patients, patients with left ventricular ejection fraction ≤ 35% who had received a cardioverter-defibrillator implantation for primary prevention of sudden cardiac death; non-ICD patients ≤35%, patients who did not carry a cardioverter-defibrillator and had a left ventricular ejection fraction ≤ 35%; and non-ICD patients >35%, patients who did not carry a cardioverter-defibrillator and had a left ventricular ejection fraction > 35%. Detailed inclusion and exclusion criteria are provided in the text. CMR, cardiac magnetic resonance; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; MI, myocardial infarction.
See the editorial comment for this article ‘Refining the stratification of sudden cardiac death risk after myocardial infarction—beyond ejection fraction’, by E.C. Ajufo and U.B. Tedrow, https://doi.org10.1093/eurheartj/ehae272.
Introduction
Sudden cardiac death is the leading cause of death, accounting for ≈20% of deaths.1,2 Patients with previous myocardial infarction are at particular risk due to life-threatening ventricular arrhythmias.3 The implantable cardioverter-defibrillator detects and terminates these arrhythmias. However, defibrillator therapy is limited by the profound difficulty to identify patients at elevated sudden cardiac death risk as candidates for implantation.
Historic trials, which is restricted by design inclusion to patients with reduced left ventricular ejection fraction (LVEF), found that cardioverter-defibrillator implantation improves survival in patients with severely impaired LVEF,4,5 a non-specific risk factor for both sudden and non-sudden cardiac death.6 Current guidelines therefore recommend prophylactic cardioverter-defibrillator implantation in these patients.7,8 This strategy has significant shortcomings.9 Treatment advances, in particular guideline-directed medical therapy, have led to substantial reduction of the sudden cardiac death risk in patients with reduced LVEF,10 and most of currently implanted cardioverter-defibrillators are not required during their life cycle.11 The identification of the many low-risk patients not requiring defibrillator protection is crucial to avoid unnecessary implantations. Additionally, many sudden cardiac deaths occur in patients with mildly reduced or preserved left ventricular function.1,2 In this population, risk stratification attempts remain scarce.
The aim of this analysis was to investigate whether use of LVEF and of a broad spectrum of further candidate predictors allows identification of low-risk patients with severely reduced LVEF not needing defibrillator protection and of high-risk patients with mildly reduced or preserved LVEF as candidates for targeted defibrillator implantation. The presented work is part of the PROFID project.
Methods
The structure and reporting follow the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) statement.12
Data sources and study population
Twenty data sets from Europe, the USA, and Israel were analysed including (i) cohort data sets of patients who had coronary artery disease with previous myocardial infarction and/or ischaemic cardiomyopathy with reduced LVEF (<50%) and were entered into the data set at some time after the infarction; (ii) cohort data sets of patients who had acute myocardial infarction and were entered into the data set at the time of the acute event; (iii) cohort data sets of patients who underwent prophylactic cardioverter-defibrillator implantation for primary prevention of sudden cardiac death after previous myocardial infarction and were entered into the data set at the time of device implantation, and (iv) data sets from randomized controlled trials, which compared different cardioverter-defibrillator programming settings or the outcome of patients receiving a cardioverter-defibrillator against medical treatment in patients with prior myocardial infarction and/or ischaemic cardiomyopathy with severely reduced LVEF. Details are given in Supplementary data online, Materials.
Participant inclusion and exclusion criteria
Included in the analysis were patients older than 18 years with either (i) previous ST-elevation and/or non-ST-elevation myocardial infarction regardless of LVEF or (ii) coronary artery disease with ischaemic cardiomyopathy and reduced LVEF (<50%).8 The following patients were excluded: (i) patients carrying at baseline a cardioverter-defibrillator for secondary sudden cardiac death prevention; (ii) patients who had received a cardioverter-defibrillator within 40 days after infarction; (iii) patients with a cardiac resynchronization therapy device at baseline; (iv) patients with coexisting non-ischaemic cardiomyopathy (such as dilated, hypertrophic, or restrictive cardiomyopathy), coexisting primary electrical arrhythmic disease (such as long QT or Brugada syndrome), or coexisting congenital heart disease; and (v) patients who died or experienced the outcome within the first 40 days after the index infarction.
To account for the use of a surrogate endpoint in patients carrying a defibrillator, we analysed separately (i) patients with LVEF ≤ 35% who had received a cardioverter-defibrillator implantation for primary prevention of sudden cardiac death, hitherto called ‘ICD patients’, and (ii) patients who did not carry a cardioverter-defibrillator within 3 months after the index myocardial infarction event, hitherto called ‘non-ICD patients’. In the latter, separate analyses were performed in (iia) patients with LVEF ≤ 35%, called ‘non-ICD patients ≤35%’, and (iib) patients with LVEF > 35% called ‘non-ICD patients >35%’.
Primary outcome
In non-ICD patients, the primary outcome was sudden cardiac death/sudden cardiac arrest. The definition of sudden cardiac death that was applied in each data set is given in the ‘Description of data sets’ in the Supplementary data online, Material. In most cases, the Hinkle–Thaler definition was applied.13 In two data sets with non-ICD patients, the primary outcome included additionally life-threatening ventricular arrhythmias (ventricular fibrillation or ventricular tachycardia). We modelled time-to-the-primary outcome accounting for the competing risk of death from other causes through the Fine and Gray competing risk framework.14 Time zero was defined as 40 days after the index infarction or at the start of study enrolment, whichever occurred latest.
In ICD patients, the primary outcome, as best available surrogate for sudden cardiac death in this population, was defined as appropriate therapy (anti-tachycardia pacing or shock) delivered by the defibrillator or, if anti-tachycardia pacing data were not collected, appropriate shock. Time-to-first-appropriate therapy/shock was modelled accounting for the competing risk of death from other cause (prior to first appropriate shock/therapy) through the Fine and Gray competing risk framework.14 Time zero was defined as the point of defibrillator implantation.
Some non-ICD patients underwent defibrillator implantation during follow-up. If implantation took place for secondary prevention of sudden cardiac death, such events were considered to constitute the primary outcome. If implantation occurred for primary prevention, this was not considered an outcome; patients were continued to be modelled until they either died suddenly, died from other causes, or were censored (intention-to-treat analysis).
Candidate predictors
We used individual participant data pertaining to demographics, medical history, clinical parameters, biomarkers, medication, electrocardiography, and echocardiography (see Supplementary data online, Table S1) and, in a subset of data sets and patients, cardiac magnetic resonance imaging. To address the expected heterogeneity in prevalence of the primary outcome across data sets, a categorical ‘risk geography’ predictor variable based on the cardiovascular disease risk regions of the World Health Organization was added (see Supplementary data online, Figures S1 and S2).
Missing data
Only those predictors were considered that were present in ≥75% of observations and recorded in the majority of data sets (see Supplementary data online, Table S2). Missing data in predictor variables (both systematically missing across entire data sets and sporadically missing within certain data sets) were imputed using fuzzy K-means.15 As sensitivity, an ‘uncapped analysis’ was performed considering all available candidate predictors (all variables listed in Supplementary data online, Table S1; results were quantitatively similar and are available upon request).
Statistical analysis
Continuous variables are reported as mean (standard deviation) or median (interquartile range) as appropriate and categorical variables as frequencies of occurrence (percentage). Time-to-event was visually explored by cumulative incidence plots of the outcome or death from other causes.
To account for the fact that cardiac magnetic resonance imaging was available only in a patient subset, the analysis consisted of two phases. In Phase 1, all candidate predictors were considered except parameters derived from cardiac magnetic resonance imaging. In Phase 2, the analysis was updated with inclusion of candidate predictors from cardiac magnetic resonance imaging.
In Phase 1, we first assessed the predictive performance of LVEF for sudden cardiac death. Using systematic internal–external leave-one-data set-out cross-validation,16,17 a flexible parametric survival model was fit under a Fine and Gray competing risk framework with LVEF as sole, continuous predictor variable. In brief, leave-one-data set-out cross-validation means that each time one data set was left out, a model with LVEF as sole predictor was built in all remaining data sets and the model was then validated in the data set that had been left out. This cycle was repeated for every data set. The resulting estimates of predictive performance of LVEF for sudden cardiac death, one per data set, were then combined by random effects meta-analysis providing the overall estimate of the predictive performance of LVEF across all data sets as well as the associated prediction interval, which gives the expected performance in a new data set that is similar to the analysed ones.18 This was done in each of the three patient subgroups separately.
To assess whether consideration of further candidate predictors apart from LVEF improved the prediction of sudden cardiac death, we developed and externally validated (again using systematic internal–external leave-one-data set-out cross-validation) multivariable prediction models for sudden cardiac death considering two different analytical methods within a competing risk framework: (i) flexible parametric survival model19,20 and (ii) random forest survival model21 applying again the process described above. To select the candidate predictors for the flexible parametric survival models, backwards selection under Bayesian information criteria stopping rule was applied.
In Phase 2, we assessed whether parameters from cardiac magnetic resonance imaging improved prediction of sudden cardiac death over LVEF alone. Seven data sets included information from cardiac magnetic resonance imaging and were included in the Phase 2 analysis: six of the data sets from Phase 1 analysis and an additional data set that was not included in Phase 1 as it contained mainly information related to cardiac magnetic resonance imaging. Within these data sets, two models were fit: (i) a flexible parametric survival model with age, sex, and LVEF as only covariates and (ii) a flexible parametric survival model with age, sex, and LVEF, plus core scar and greyzone. For definition of core scar and greyzone, the application of the full-width-half-maximum, 2 SD, 3 SD, and 5 SD methods was considered as previously described.22 The definition resulting in the best predictive performance across all data sets was selected. As with Phase 1, systematic leave-one-data set-out cross-validation was performed to validate the models. Given the smaller number of data sets and events, results were pooled using fixed effect meta-analysis.
The predictive performance of all models was assessed using discrimination and calibration within the competing risk framework at a prediction horizon of 36 months post time zero. Discrimination was assessed using time-dependent c-statistics with ideal value of 1, whereas 0.5 indicates random performance. Calibration was assessed by comparing the observed cumulative incidence function for the primary endpoint with the cumulative incidence function predicted by the model at 36 months, hereto called ‘observed:expected ratio’, with ideal value of 1. To visualize discrimination performance, within each data set of each study population, patients were split into three equally sized groups based on the 36-month risk for each model separately. A cumulative incidence plot was produced in each of the three study populations, stratified by the three risk groups.
Analyses were undertaken in R version 3.6.1,23 Stata (version. 16.1; StataCorp), and Python.
Results
Phase 1 analysis
In Phase 1, i.e. the analysis excluding magnetic resonance imaging information, 140 204 patients across all data sets fulfilled the inclusion and exclusion criteria, comprised of 7543 ICD patients, 25 058 non-ICD patients ≤35%, and 107 603 non-ICD patients >35%. Mean age was 63.8, 72.6, and 68.3 years, respectively. Baseline characteristics are shown in Table 1. The whole range of LVEF was represented. There were 1326 primary outcomes in ICD patients, 1193 non-ICD patients ≤35% and 1567 non-ICD patients >35% during mean follow-up of 30.0, 46.5, and 57.6 months. The cumulative incidence function of the primary outcome is given in Table 2 and Supplementary data online, Figure S3.
Table 1.
Baseline characteristics across the three subgroups in the Phase 1 analysis, i.e. the analysis excluding cardiac magnetic resonance imaging parameters, and in the Phase 2 analysis, i.e. the analysis including cardiac magnetic resonance imaging parameters
| Phase 1 analysis (excluding cardiac magnetic resonance imaging parameters) | ||||
|---|---|---|---|---|
| Variable | ICD patients (n = 7543) | Non-ICD patients ≤35% (n = 25 058) | Non-ICD patients >35% (n = 107 603) | Missing data n (%) |
| Demographics | ||||
| Age (years) | 63.8 (10.7) | 72.6 (11.7) | 68.3 (11.9) | 2 (0%) |
| Sex (male) | 6410 (85.0%) | 17 145 (68.4%) | 72 813 (67.7%) | 1 (0%) |
| Medical history | ||||
| Prior percutaneous coronary intervention | 1571 (20.8%) | 3576 (14.3%) | 16 048 (14.9%) | 10 140 (7.2%) |
| Prior coronary bypass graft surgery | 1528 (20.3%) | 2870 (11.5%) | 7428 (6.9%) | 8401 (6.0%) |
| Smoking | 2113 (28.0%) | 13 740 (54.8%) | 60 681 (56.4%) | 11 230 (8.0%) |
| Clinical characteristics | ||||
| Body mass index, kg/m2 | 28.2 (5.4) | 26.6 (4.6) | 27.3 (4.6) | 18 583 (13.3%) |
| Hypertension | 2402 (31.8%) | 12 528 (50.0%) | 53 561 (49.8%) | 5586 (4.0%) |
| Diabetes | 2049 (27.2%) | 6847 (27.3%) | 21 848 (20.3%) | 1958 (1.4%) |
| Myocardial infarction type | 11 903 (8.49%) | |||
| ST-segment elevation | Not availablea | 8293 (33.1%) | 28 013 (26.0%) | |
| Non-ST-segment elevation | Not availablea | 14 312 (57.1%) | 70 140 (65.2%) | |
| Atrial fibrillation or atrial flutter | 1273 (16.9%) | 5379 (21.5%) | 11 473 (10.7%) | 9723 (6.9%) |
| Estimated glomerular filtration rate (mL/min/1.73 m2), median (interquartile range) | 69.0 (51.4–85.0) | 68.0 (49.9–85.0) | 79.6 (62.6–91.8) | 9020 (6.4%) |
| Electrocardiography | ||||
| Left bundle branch block | 391 (5.2%) | 3100 (12.4%) | 3177 (3.0%) | 11 654 (8.3%) |
| Medication | ||||
| Angiotensin-converting enzyme inhibitors and/or angiotensin receptor blockers | 5029 (66.7%) | 21 527 (85.9%) | 82 760 (76.9%) | 2259 (1.6%) |
| Beta-blockers | 5249 (69.6%) | 22 381 (89.3%) | 95 197 (88.5%) | 1581 (1.1%) |
| Diuretics | 4276 (56.7%) | 13 086 (52.2%) | 23 988 (22.3%) | 1828 (1.3%) |
| Antiplatelet drugs | 4266 (56.6%) | 22 642 (90.4%) | 99 610 (92.6%) | 6981 (5.0%) |
| Oral anticoagulants | 1582 (21.0%) | 3977 (15.9%) | 7444 (6.9%) | 12 711 (9.0%) |
| Lipid-lowering medication | 3163 (41.9%) | 20 669 (82.5%) | 98 954 (92.0%) | 3440 (2.5%) |
| Echocardiography | ||||
| Left ventricular ejection fraction (%) | 26.5 (5.9) | 29.9 (6.3) | 55.2 (7.1) | 94 (0.1%) |
| Phase 2 analysis (including cardiac magnetic resonance imaging parameters) | ||||
|---|---|---|---|---|
| Variable | ICD patients (n = 514) | Non-ICD patients ≤35% (n = 576) | Non-ICD patients >35% (n = 986) | Missing data n (%) |
| Sex (male) | 381 (74.1%) | 466 (80.9%) | 771 (78.2%) | 0 |
| Age (years) | 63.0 (11.3) | 65.9 (11.5) | 64.1 (11.8) | 0 |
| Left ventricular ejection fraction (%) | 25.2 (6.7) | 25.9 (6.3) | 48.9 (10.8) | 0 |
| Extent of core scar (g) median (interquartile range) | 24.5 (9.3–44.8) | 30.5 (11.6–49.8) | 18.6 (3.2–35.4) | 0 |
| Extent of greyzone (g) median (interquartile range) | 12.7 (4.9–20.7) | 15.0 (9.4–21.1) | 9.8 (4.2–14.8) | 0 |
Unless stated otherwise, values give mean numbers with standard deviation in parentheses or absolute numbers with percentages in parentheses as appropriate. Characteristics shown are for primary data, i.e. prior to imputation of missing values. ICD patients, patients with left ventricular ejection fraction ≤ 35% who had received a cardioverter-defibrillator implantation for primary prevention of sudden cardiac death. Non-ICD patients ≤35%, patients who did not carry a cardioverter-defibrillator and had a left ventricular ejection fraction ≤ 35%. Non-ICD patients >35%, patients who did not carry a cardioverter-defibrillator and had a left ventricular ejection fraction > 35%. The column ‘Missing data’ gives numbers of patients with missing data across all three subgroups.
aICD patients were entered into the data sets at some time after the myocardial infarction event and the information on ST- or non-ST-segment elevation infarction was not available. Core scar was defined by the 5 SD method, and greyzone was defined by the 3 SD minus 5 SD method.
Table 2.
Fine and Gray cumulative incidence of the primary endpoint in the three patient subgroups at 12 and 36 months in the Phase 1 analysis, i.e. the analysis excluding cardiac magnetic resonance imaging parameters, and in the Phase 2 analysis, i.e. the analysis including cardiac magnetic resonance imaging parameters
| Time point (months after time zero) | ICD patients incidence (95% confidence interval) of the endpoint, first appropriate therapy | Non-ICD patients ≤35% incidence (95% confidence interval) of the endpoint, sudden cardiac deatha | Non-ICD patients >35% incidence (95% confidence interval) of the endpoint, sudden cardiac deatha |
|---|---|---|---|
| Phase 1 analysis (excluding cardiac magnetic resonance imaging parameters) | |||
| 12 | 9.12% (8.48%, 9.77%) | 1.84% (1.68%, 2.01%) | 0.38% (0.34%, 0.41%) |
| 36 | 18.42% (17.44%, 19.39%) | 3.41% (3.18%, 3.63%) | 0.87% (0.81%, 0.92%) |
| Phase 2 analysis (including cardiac magnetic resonance imaging parameters) | |||
| 12 | 7.49% (5.19%, 9.78%) | 3.66% (2.12%, 5.19%) | 0.41% (0.01%, 0.81%) |
| 36 | 17.43% (14.05%, 20.82%) | 7.34% (5.14%, 9.54%) | 1.89% (1.02%, 2.75%) |
ICD patients, patients with left ventricular ejection fraction ≤ 35% who had received a cardioverter-defibrillator implantation for primary prevention of sudden cardiac death. Non-ICD patients ≤35%, patients who did not carry a cardioverter-defibrillator and had a left ventricular ejection fraction ≤ 35%. Non-ICD patients >35%, patients who did not carry a cardioverter-defibrillator and had a left ventricular ejection fraction > 35%.
aIn two data sets, the primary outcome included additionally life-threatening ventricular arrhythmias (ventricular fibrillation or ventricular tachycardia).
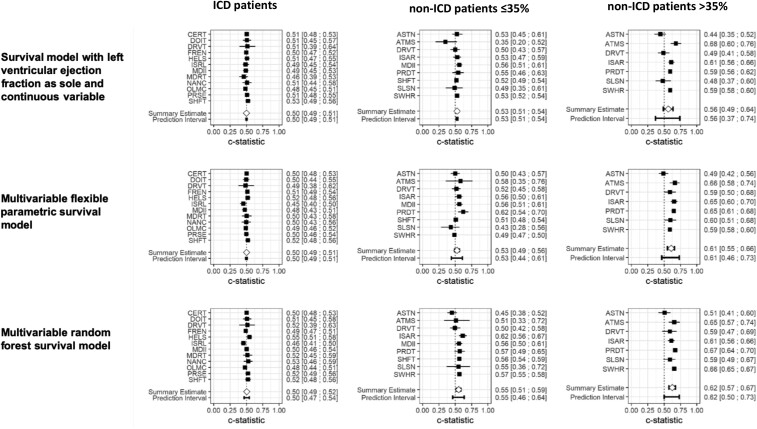
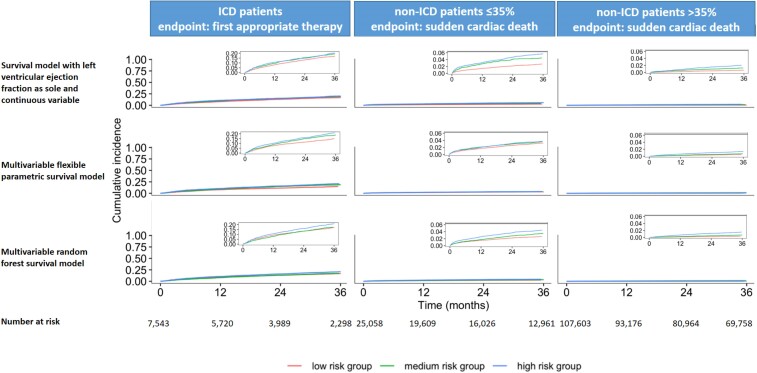
The sudden cardiac death risk was low in patients with severely reduced LVEF and very low in mildly reduced or preserved LVEF (Table 2). The risk for the primary outcome was considerable in the ICD patients (appropriate defibrillator therapy). Left ventricular ejection fraction was a poor predictor of the primary outcome in all three patient subgroups. Upon external validation, LVEF as sole continuous predictor had a c-statistic of 0.50 (95% prediction interval 0.49–0.51) for the risk of first appropriate defibrillator therapy at 36 months in ICD patients, 0.53 (95% prediction interval 0.51–0.54) for the risk of sudden cardiac death in non-ICD patients ≤35%, and 0.56 (95% prediction interval 0.37–0.74) in non-ICD patients >35% (Figure 1). Risk stratifying the three study populations into low, medium, and high risk based on LVEF produced very similar cumulative incidence curves, particularly in ICD patients and in the non-ICD patients ≤35%. Separation of the curves was marginally better in non-ICD patients >35% (Figure 2). Calibration was similarly poor (see Supplementary data online, Table S3).
Figure 1.
Forest plot of the c-statistic for the prediction of risk for the primary endpoint at 36 months across the three subgroups in the Phase 1 analysis, i.e. the analysis excluding cardiac magnetic resonance imaging parameters. ICD patients, patients with left ventricular ejection fraction ≤ 35% who had received a cardioverter-defibrillator implantation for primary prevention of sudden cardiac death; non-ICD patients ≤35%: patients who did not carry a cardioverter-defibrillator and had a left ventricular ejection fraction ≤ 35%; and non-ICD patients >35%, patients who did not carry a cardioverter-defibrillator and had a left ventricular ejection fraction > 35%. In ICD patients, endpoint was first appropriate therapy, and in non-ICD patients ≤35% and non-ICD patients >35%, endpoint was sudden cardiac death. In two data sets with non-ICD patients, the primary endpoint included additionally life-threatening ventricular arrhythmias (ventricular fibrillation or ventricular tachycardia). Please note that the leave-one-data set-out cross-validation was applied meaning that each time one data set was left out, a model was built in all remaining data sets and the model was then applied in the data set that had been left out. This cycle was then repeated for every data set. The resulted estimates of predictive performance for the primary endpoint, one per data set, were then combined by random effects meta-analysis providing the overall estimate of the predictive performance of ejection fraction across all data sets as well as the associated prediction interval, which gives the expected performance in a new data set that is similar to the analysed ones. A wide prediction interval indicates limited generalizability to a new data set. To select the candidate predictors for the multivariable models, only those predictors were considered that were present in ≥75% of observations and recorded in the majority of data sets. For the multivariable flexible parametric survival models, backwards selection under Bayesian information criteria stopping rule was applied. The named data sets on the y-axis denote the data set left-out for model development and then used to validate the subsequent model to produce the corresponding performance estimates shown. For abbreviations of the individual data sets, please see the ‘Description of data sets’ in the Supplementary data online, Material
Figure 2.
Fine and Gray cumulative incidence of the primary endpoint stratified by predicted risk categories obtained from each model for the three subgroups in the Phase 1 analysis. The figure depicts Fine and Gray cumulative incidence plots of the primary endpoint stratified by predicted risk, for the three cohorts in the Phase 1 analysis. In each iteration of the leave-one-data set cross-validation loop, patients in the validation data set were split into three equally sized groups (low, medium, and high) based on 36-month risk, predicted by the model that was developed in the remaining data sets. After completing the leave-one-data set cross-validation process, all patients in the low-risk group were pooled to produce the cumulative incidence plot, and the same happened for the medium-risk and high-risk groups. ICD patients, patients with left ventricular ejection fraction ≤ 35% who had received a cardioverter-defibrillator implantation for primary prevention of sudden cardiac death; non-ICD patients ≤35%, patients who did not carry a cardioverter-defibrillator and had a left ventricular ejection fraction ≤ 35%; and non-ICD patients >35%, patients who did not carry a cardioverter-defibrillator and had a left ventricular ejection fraction > 35%. In ICD patients, endpoint was first appropriate therapy, and in non-ICD patients ≤35% and non-ICD patients >35%, endpoint was sudden cardiac death. In two data sets with non-ICD patients, the primary endpoint included additionally life-threatening ventricular arrhythmias (ventricular fibrillation or ventricular tachycardia). To select the candidate predictors for the multivariable models, only those predictors were considered that were present in ≥75% of observations and recorded in the majority of data sets. For the multivariable flexible parametric survival models, backwards selection under Bayesian information criteria stopping rule was applied
The consideration of further candidate predictors in addition to LVEF did not improve the predictive performance (Figures 1 and 2). In all three study populations, the c-statistic of all multivariable models, upon external validation, was very close to 0.5 with very wide prediction intervals indicating large heterogeneity across data sets and demonstrating inability to risk stratify based on these models (Figures 1 and 2). The predictive performance was moderate in some databases for the non-ICD patients >35% (Figure 1), but with large heterogeneity across data sets. Calibration of the models was reasonable on average (observed:expected ratio close to 1; Supplementary data online, Table S3), but again with very large heterogeneity across data sets indicating low generalizability of the models across data sets.
Phase 2 analysis
Magnetic resonance imaging information was available in 2076 patients for the Phase 2 analysis. Table 1 presents the baseline information of these patients, and Table 2 gives the cumulative incidence estimates of the primary outcome. Across these data sets, the combination of the 5 SD method for defining core scar and 3 SD minus 5 SD method for defining greyzone consistently performed best at predicting sudden cardiac death risk (see Supplementary data online, Tables S4 and S5). Thus, these definitions were used in the multivariable modelling of Phase 2. Upon external validation, the predictive performance for the primary outcome at 36 months achieved by the model consisting only of LVEF, age, and sex was almost identical with the predictive performance achieved by the model consisting of LVEF, age, sex, extent of core scar, and extent of greyzone, across all three study populations (Table 3), indicating a low additive value of the information derived from cardiac magnetic resonance.
Table 3.
Meta-analysis results of the predictive performance results for the primary endpoint at 36 months for the three study groups in the Phase 2 analysis
| Model | C-statistic (95% prediction interval) | Observed:expected ratio (95% prediction interval) |
|---|---|---|
| ICD patients | ||
| Model without CMR information: age, sex, and left ventricular ejection fraction as predictors | 0.51 (0.45, 0.58) | 1.10 (0.94, 1.29) |
| Model with CMR information: age, sex, left ventricular ejection fraction, core scar, and greyzone as predictors | 0.53 (0.46, 0.59) | 1.09 (0.93, 1.27) |
| Non-ICD patients ≤35% | ||
| Model without CMR information: age, sex, and left ventricular ejection fraction as predictors | 0.40 (0.31, 0.49) | 0.94 (0.76, 1.16) |
| Model with CMR information: age, sex, left ventricular ejection fraction, core scar, and greyzone as predictors | 0.54 (0.44, 0.63) | 0.91 (0.74, 1.12) |
| Non-ICD patients >35% | ||
| Model without CMR information: age, sex, and left ventricular ejection fraction as predictors | 0.52 (0.36, 0.67) | 0.37 (0.27, 0.50) |
| Model with CMR information: age, sex, left ventricular ejection fraction, core scar, and greyzone as predictors | 0.56 (0.42, 0.71) | 0.36 (0.27, 0.49) |
All models are flexible parametric survival models. ICD patients, patients with left ventricular ejection fraction ≤ 35% who had received a cardioverter-defibrillator implantation for primary prevention of sudden cardiac death. Non-ICD patients ≤35%, patients who did not carry a cardioverter-defibrillator and had a left ventricular ejection fraction ≤ 35%. Non-ICD patients >35%, patients who did not carry a cardioverter-defibrillator and had a left ventricular ejection fraction > 35%.
CMR, cardiac magnetic resonance.
Discussion
This pooled cohort analysis of 20 international data sets representing 140 204 patients after myocardial infarction provided two main findings: (i) LVEF had poor predictive performance for the risk of sudden cardiac death among patients with severely reduced LVEF and among those with moderately reduced or preserved LVEF, and (ii) the consideration of a large number and wide spectrum of readily available clinical variables did not improve the predictive performance (Structured Graphical Abstract). Thus, more accurate risk stratification of patients among these two subgroups and in particular identification of low-risk individuals with severely reduced LVEF as candidates for omission of defibrillator protection or of high-risk individuals with preserved LVEF as candidates for targeted defibrillator protection was not feasible, neither using LVEF nor using various other candidate predictors.
Limitations of current practice and inherent limitations in risk prediction for sudden cardiac death
Guidelines recommend LVEF ≤ 35% as criterion for primary prevention defibrillator following myocardial infarction7,8 based on historical trial evidence. In the present analysis, LVEF had poor predictive performance for sudden cardiac death with c-statistics between 0.50 and 0.56 in all three studied populations corroborating previous reports on limitations of LVEF as sole risk stratification tool.2,3 Thus, LVEF did not prove useful in further risk stratifying patients with severely reduced LVEF or patients with moderately reduced or preserved LVEF. Notably, the results of this analysis do not prove that LVEF is in general not useful for risk prediction of sudden cardiac death across the whole spectrum of LVEF.
While the limitations of current practice are evident, there is uncertainty about alternative strategies. The current results contrast with previous studies reporting good discrimination of patients at high risk for sudden cardiac death or for appropriate defibrillator intervention achieved by various models.6,24–26 Whether the non-generalizability of results obtained in single or few data sets to a greater number of larger data sets and larger populations provides a plausible explanation is speculative. Previous research efforts were hampered by relatively small patient numbers, neglect of patients with preserved LVEF, and focus on certain predictor categories. To overcome these shortcomings, we analysed a large pooled data set of post-infarction patients with data sets of considerable breadth and depth, including a large patient number with mildly reduced or preserved LVEF, and considered the majority of previously proposed predictors. We applied different analytical methods and consistently used competing risks framework to adjust for risk of death by other causes. Systematic internal–external leave-one-data set-out cross-validation was used to assess generalizable predictive model performance.
Nevertheless, the current analysis did not yield a tool that predicted the individual sudden cardiac death risk with satisfactory accuracy. Whether this result is related to the nature of sudden cardiac death and to inherent limitations of respective analyses rendering prediction not feasible cannot be answered. A key inherent limitation lies on correct adjudication of death cause. Misclassifications of cause of death are indeed frequent,27 reducing the performance of models for sudden cardiac death prediction. Furthermore, sudden cardiac death is the result of a complex, highly dynamic interplay of multiple factors that is difficult to capture, especially with single-time assessments.
Limitations of the present analysis
Some other limitations should be noted. A wide variable spectrum was considered. However, variable availability varied across data sets and may have led to exclusion of variables with potential predictive value. Analysis of electrocardiographic data was limited to data derived from conventional surface electrocardiogram (ECG). Previously reported candidate predictors such as T-wave alternans or baroreflex sensitivity were not analysed due to a paucity of analysable data sets containing information on these parameters. Further, although the analysed subset of patients with available cardiac magnetic resonance imaging constitutes with more than 2100 patients a large pooled data set in this setting, the number of observed events was still relatively small limiting the power of the analysis. Additionally, the higher event rates in patients with magnetic resonance information compared with patients without this information may indicate differences among these populations due to the indication for performing magnetic resonance imaging or the design of the respective cohorts. Notwithstanding these limitations, the results of the Phase 2 analysis, which did not demonstrate a predictive value of cardiac magnetic resonance imaging, are noteworthy particularly considering the costs associated with the examination.
As the aim was to derive a risk stratification scheme that would be easily applicable in everyday clinical practice, invasive risk stratification tools such as programmed ventricular stimulation were not considered. Previous data have demonstrated usefulness of invasive risk stratification, particularly if preceded by non-invasive risk stratification in a two-step approach.28 Genetic markers were not considered because up to conduction of the analyses, there was not sufficient evidence for an association between specific genetic markers and risk for sudden cardiac death in patients with coronary artery disease in the stable, post-remodelling phase after infarction.29 Nevertheless, a recent report indicates a potential role of genetic information.30
A further significant limitation of the analysis is the large heterogeneity of the analysed data sets. This was dictated by the need to combine different data sets in order to achieve the size that is necessary for the study of the rare outcome of sudden cardiac death. These differences in design, data, outcome ascertainment, and follow-up of the analysed cohorts limit the strength of the conclusions.
Recently introduced drugs for heart failure treatment such as sodium–glucose co-transporter 2 inhibitors or angiotensin receptor–neprilysin inhibitors were not available or not yet standard at the time of most cohorts. Therefore, it is unclear whether the results of the presented analysis are well applicable in patients treated with contemporary optimal therapy including these recently introduced agents and contemporary revascularization strategies.
Rates of defibrillator therapies vs. rates of sudden cardiac death
Overall, the sudden cardiac death risk was low in patients with severely reduced LVEF and very low in moderately reduced or preserved LVEF. Notably, the risk of appropriate defibrillator therapy was considerable in defibrillator carriers. This difference between sudden cardiac death rates in patients with LVEF ≤ 35% not carrying a defibrillator and rates of appropriate defibrillator therapies in defibrillator carriers in our analysis is noteworthy and may have different explanations. It is well known that not all arrhythmias treated by the defibrillator would be fatal if left untreated. An analysis restricted to appropriate defibrillator therapies caused by ventricular fibrillation only could have served as a more accurate surrogate in ICD patients. Such an analysis was not feasible, since information on the arrhythmia triggering the appropriate defibrillator therapy was not consistently available in the analysed data sets. Differences in patient characteristics between these two study populations due to selection bias may have further contributed to the observed differences in endpoint rates. Programming parameters of the devices, which were not controlled for in the analysis, have also an impact on the rates of defibrillator therapy.
Implications of the findings
These findings have substantial implications in the field of sudden cardiac death prevention after myocardial infarction. In patients with severely reduced LVEF, the lack of appropriate risk stratification tools questions the feasibility of approaches for personalized decision-making on defibrillator implantation. Considering the declining risk for sudden death,10 the effect of recently introduced heart failure drugs,31,32 the fact that non-sudden deaths account for the large majority of deaths in this population, and the still considerable complication rate of the devices,33 a re-evaluation of the benefit of routine prophylactic defibrillator implantation in patients with LVEF ≤ 35% appears necessary.
Similarly, in patients with LVEF > 35%, the lack of acceptably accurate risk stratification tools combined with the very low sudden cardiac death risk questions the feasibility of attempts at identification of high-risk candidates for targeted protection by defibrillator.
Conclusions
In patients with previous myocardial infarction, LVEF had poor predictive performance for the risk of sudden cardiac death among patients with severely impaired LVEF and among those with moderately reduced or preserved LVEF. The consideration of a large variety and wide spectrum of further candidate predictors did not improve the predictive performance. Thus, more accurate risk stratification and in particular identification of low-risk individuals with severely reduced LVEF as candidates for omission of defibrillator protection or of high-risk individuals with preserved LVEF as candidates for targeted defibrillator protection was not feasible, neither using LVEF nor using various other candidate predictors.
Supplementary Material
Acknowledgements
We acknowledge the PROFID collaborators, which includes PRE-DETERMINE collaborators, DERIVATE collaborators, and DO-IT investigators.
Contributor Information
Niels Peek, Division of Informatics, Imaging and Data Science, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, UK; The Healthcare Improvement Studies Institute (THIS Institute), Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK.
Gerhard Hindricks, Department of Cardiology, Angiology and Intensive Care Medicine, Deutsches Herzzentrum der Charité, Campus Charité Mitte, Charitéplatz 1, 10117 Berlin, Germany; Department of Electrophysiology, Heart Center Leipzig, Strumpellstr. 39, 04289 Leipzig, Germany.
Artur Akbarov, Division of Informatics, Imaging and Data Science, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, UK.
Jan G P Tijssen, Clinical Epidemiology and Biostatistics, The AMC, Amsterdam, The Netherlands.
David A Jenkins, Division of Informatics, Imaging and Data Science, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, UK.
Zoher Kapacee, Division of Informatics, Imaging and Data Science, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, UK.
Le Mai Parkes, Division of Informatics, Imaging and Data Science, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, UK.
Rob J van der Geest, Department of Radiology, Leiden University Medical Center, Leiden, The Netherlands.
Enrico Longato, Department of Information Engineering, University of Padova, Padova, Italy.
Daniel Sprague, Spectra Analytics, London, UK.
Youssef Taleb, Spectra Analytics, London, UK.
Marcus Ong, Spectra Analytics, London, UK.
Christopher A Miller, Division of Cardiovascular Sciences, School of Medical Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester Academic Health Science Centre, Manchester, UK.
Alireza Sepehri Shamloo, Department of Cardiology, Angiology and Intensive Care Medicine, Deutsches Herzzentrum der Charité, Campus Charité Mitte, Charitéplatz 1, 10117 Berlin, Germany; Department of Electrophysiology, Heart Center Leipzig, Strumpellstr. 39, 04289 Leipzig, Germany.
Christine Albert, Department of Cardiology, Smidt Heart Institute, Cedars Sinai Medical Center, Los Angeles, CA, USA.
Petra Barthel, Klinikum rechts der Isar, Technische Universität München, Munich, Germany.
Serge Boveda, Cardiology—Heart Rhythm Management Department, Clinique Pasteur, Toulouse, France.
Frieder Braunschweig, Department of Cardiology, Karolinska University Hospital, Stockholm, Sweden.
Jens Brock Johansen, Department of Cardiology, Department of Cardiology Odense, Odense University Hospital, Syddanmark, Denmark.
Nancy Cook, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Christian de Chillou, Département de Cardiologie, CHRU de Nancy, Nancy, France.
Petra Elders, Department of General Practice and Elderly Care, Medicine, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Jonas Faxén, Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden.
Tim Friede, Department of Medical Statistics, University Medical Center Göttingen, Göttingen, Germany.
Laura Fusini, Department of Cardiovascular Imaging, Centro Cardiologico Monzino IRCCS, Milan, Italy.
Chris P Gale, Leeds Institute of Cardiovascular and Metabolic Medicine, University of Leeds, Leeds, UK.
Jiri Jarkovsky, Institute of Biostatistics and Analyses, Faculty of Medicine, Masaryk University, Brno, Czech Republic.
Xavier Jouven, Medical and Surgical Department of Cardiology, Georges Pompidou European Hospital, Paris, France.
Juhani Junttila, Research Unit of Internal Medicine, Medical Research Center Oulu, University of Oulu and Oulu University Hospital, Oulu, Finland.
Josef Kautzner, Institute of Clinical and Experimental Medicine, University Hospital Olomouc, Moravia, Czech Republic.
Antti Kiviniemi, Research Unit of Internal Medicine, Medical Research Center Oulu, University of Oulu and Oulu University Hospital, Oulu, Finland.
Valentina Kutyifa, University of Rochester Medical Center, Clinical Cardiovascular Research Center, Rochester, NY, USA.
Christophe Leclercq, Service de Cardiologie et Maladies Vasculaires, CHU Pontchaillou, Rennes, France.
Daniel C Lee, School of Medicine, Northwestern University Feinberg, Chicago, USA.
Jill Leigh, Boston Scientific Corporation, St. Paul, MN, USA.
Radosław Lenarczyk, Division of Medical Sciences in Zabrze, Department of Cardiology, Congenital Heart Diseases and Electrotherapy, Silesian Center of Heart Diseases, The Medical University of Silesia, Katowice, Poland.
Francisco Leyva, Aston Medical School, Aston University, Aston Triangle, Birmingham, UK.
Michael Maeng, Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark.
Andrea Manca, Centre for Health Economics, University of York, York, UK.
Eloi Marijon, Division of Cardiology, European Georges Pompidou Hospital, Paris, France.
Ursula Marschall, Barmer, Germany.
Jose Luis Merino, Arrhythmia and Robotic Electrophysiology Unit, La Paz University Hospital, Madrid, Spain.
Lluis Mont, Hospital Clinic, University of Barcelona, Catalonia, Spain.
Jens Cosedis Nielsen, Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark; Department of Clinical Medicine, Aarhus University, Aarhus, Denmark.
Thomas Olsen, Department of Cardiology, Department of Cardiology Odense, Odense University Hospital, Syddanmark, Denmark.
Julie Pester, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Gianluca Pontone, Department of Cardiovascular Imaging, Centro Cardiologico Monzino IRCCS, Milan, Italy.
Ivo Roca, Hospital Clinic, University of Barcelona, Catalonia, Spain.
Georg Schmidt, Klinikum rechts der Isar, Technische Universität München, Munich, Germany.
Peter J Schwartz, Center for Cardiac Arrhythmias of Genetic Origin, IRCCS Istituto Auxologico Italiano, Milan, Italy.
Christian Sticherling, Department of Cardiology, University Hospital Basel, University Basel, Basel, Switzerland.
Mahmoud Suleiman, Department of Cardiology, Rambam Health Care Campus, Haifa, Israel.
Milos Taborsky, Department of Internal Medicine I—Cardiology, Olomouc University Hospital, Moravia, Czech Republic.
Hanno L Tan, Department of Clinical and Experimental Cardiology, Amsterdam University Medical Center AMC, University of Amsterdam, Amsterdam, Netherlands.
Jacob Tfelt-Hansen, Department of Cardiology, The Heart Centre, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark.
Holger Thiele, Heart Center Leipzig at the University of Leipzig, Leipzig, Germany.
Gordon F Tomaselli, Albert Einstein College of Medicine, Bronx, NY.
Tom Verstraelen, Department of Cardiology, Amsterdam UMC location University of Amsterdam, Amsterdam, The Netherlands.
Manickavasagar Vinayagamoorthy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Kevin Kris Warnakula Olesen, Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark.
Arthur Wilde, Department of Cardiology, Amsterdam UMC location University of Amsterdam, Amsterdam, The Netherlands.
Rik Willems, Department of Cardiovascular Sciences, University of Leuven, Leuven, Belgium; Cardiology, University Hospitals Leuven, Leuven, Belgium.
Katherine C Wu, Division of Cardiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Markus Zabel, Department of Cardiology and Pneumology, Heart Center, University Medical Center Goettingen, Göttingen, Germany.
Glen P Martin, Division of Informatics, Imaging and Data Science, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, UK.
Nikolaos Dagres, Department of Cardiology, Angiology and Intensive Care Medicine, Deutsches Herzzentrum der Charité, Campus Charité Mitte, Charitéplatz 1, 10117 Berlin, Germany; Department of Electrophysiology, Heart Center Leipzig, Strumpellstr. 39, 04289 Leipzig, Germany.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
N.P., G.H., A.A., J.G.P.T., D.A.J., Z.K., L.M.P., R.J.v.d.G., A.S.S., P.B., J.F., L.F., Ji.J., Ju.J., J.K., A.K., V.K., J.L., U.M., J.L., T.O., J.P., I.R., G.S., P.J.S., M.S., M.T., H.L.T., J.T.-H., H.T., T.V., M.V., K.K.W.O., K.C.W., G.P.M., and N.D.—no disclosures.
D.S., Y.T., and M.O.—subcontracted under European Union's Horizon 2020 Grant Agreement No. 847999.
S.B.—consulting fees from Medtronic, Boston Scientific, MicroPort, and Zol; support for meeting attendance from Medtronic, Boston Scientific, and MicroPort; leadership roles on French Society of Cardiology and European Heart Rhythm Association.
F.B.—leadership roles with Medtronic and Biotronik.
X.J.—consulting fees from Schiller Medical.
J.B.J.—consulting fees from Medtronic; honoraria payments from Merit Medical.
N.C.—grant from the National Institute of Health.
C.d.C.—grants from the French Federation of Cardiology; license with Biosense Webster; honoraria payment from Biosense Webster, Abbott, and Boston Scientific; support for meeting attendance from Biosense Webster, Abbott, and Boston Scientific; patents with Biosense Webster.
F.L.—honoraria payments from Medtronic, Boston Scientific, and Abbott Medical; support for meeting attendance from Medtronic, Boston Scientific, and Abbott Medical.
D.C.L.—grants from St. Jude Medical, National Heart, Lung and Blood Institute, and Abbott Laboratories.
C.L.—consulting fees and honoraria payments from Medtronic, Boston Scientific, Biotronik, MicroPort, and Abbott.
R.L.—payments from European Union's Horizon 2020 Grant Agreement No. 847999; honoraria payments from Boston Scientific and Abbott.
C.A.M.—NIHR advanced fellowship (NIHR301338), British Heart Foundation Accelerator Award (AA/18/4/342221), NIHR Manchester Biomedical Research Centre (NIHR203308); grants from Roche, Univar Solutions, Amicus Therapeutics, Guerbet Laboratories, and AstraZeneca; consulting fees from AstraZeneca and PureTech Health; honoraria payments from Novo Nordisk, Boehringer Ingelheim, and AstraZeneca; participation on advisory boards for Boehringer Ingelheim, Lilly Alliance, AstraZeneca, and Haya Therapeutics.
T.F.—grant from European Community's 7th Framework Programme FP7/2007-2013 (602299), German Research Foundation, German Ministry of Education and Research, Federal Joint Committee; consulting fees from Actimed, Bayer, Galapagos, Minoryx, Vifor, Novartis, LivaNova, Relaxera, and Recardio; honoraria payments from Fresenius Kabi; advisory board participation on Novartis, Enanta, Asian, PPD, IQVIA, Vico Therapeutics, Bayer, Biosense Webster, Janssen, Roche, and Galapagos.
J.C.N.—grants from Novo Nordisk Foundation and the Danish Heart Foundation.
M.M.—grants from Novo Nordisk; consulting fees from Novo Nordisk; honoraria payments from Novo Nordisk and Boehringer Ingelheim; advisory board participation on Novo Nordisk and Boehringer Ingelheim; stock options from Novo Nordisk, Eli Lilly, and Verve Therapeutics.
A.M.—grant from NIHR.
R.W.—grant from the fund for Scientific Research Flanders; consulting fees, honoraria payments, and support for meeting attendance from Biotronik, Boston Scientific, Abbott, and Medtronic; leadership roles on Belgian Heart Rhythm Association, Belgian Society of Cardiology, and Belgian Alliance on Cardiovascular Health.
G.F.T.—grant from R01 HL132181 Cardiac MR to Improve Clinical Risk Prediction in Defibrillator Patients.
A.W.—consulting fees from ARMGO, Thryy Therapeutics; advisory board on LEAP trial.
G.P.—grants and consulting fees from GE Healthcare, Bracco, and Boehringer Ingelheim; honoraria payments from GE Healthcare.
C.S.—consulting fees from Medtronic and Boston Scientific; honoraria payments from Biotronik and MicroPort; advisory board on Reset CRT trial.
C.A.—grants from NHLBI, St. Jude Medical Foundation; consulting fees from Medtronic and Novartis; advisory board on Element Science, Boston Scientific, and Illumina; leadership role on Heart Rhythm Society.
L.M.—consulting fees and honoraria payments from Abbott Medical, Medtronic, Boston Scientific, and Biosense Webster; payment for expert testimonies from Medtronic, Boston Scientific, and Abbott Medical; support for meeting attendance from Johnson & Johnson; advisory board on Medtronic and Boston Scientific; stockholder of Galgo Medical.
E.L.—research assistant contract under European Union's Horizon 2020 Grant Agreement No. 847999.
C.P.G.—grants from Horizon 2020, Alan Turing Institute, British Heart Foundation, National Institute for Health Research, Abbott Diabetes, Bristol Myers Squibb, and European Society of Cardiology; consultancy fees from AI Nexus, AstraZeneca, Amgen, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, CardioMatics, Chiesi, Daiichi Sankyo, GPRI Research B.V., Menarini, Novartis, iRhythm, Organon, and The Phoenix Group; honoraria payments from AstraZeneca, Boston Scientific, Menarini, Novartis, Raisio Group, Wondr Medical, and Zydus; support for meeting attendance from AstraZeneca; advisory board on DANBLCOK trial and TARGET CTCA trial; leadership roles on Deputy Editor: EHJ Quality of Care and Clinical Outcomes, NICE Indicator Advisory Committee, Chair ESC Quality Indicator Committee; stock option with Cardiomatics; equipment received from Kosmos device.
P.E.—honoraria payments from Health Investments; advisory board on Dutch Health Council.
E.M.—grants from Medtronic, Boston Scientific, Abbott Medical, Biotronik, MicroPort, and Zoll; consulting fees from Medtronic, Boston Scientific, Abbott Medical, and Zoll; honoraria payments from Medtronic, Boston Scientific, and Zoll; support for meeting attendance from Medtronic, Boston Scientific, and Abbott Medical.
M.Z.—grant from EU FP7.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Raw data, including de-identified participant data, statistical code, and supplementary material, will be provided to researchers who meet the criteria for access, following a formal request to the corresponding author. Data sharing is subject to applicable data protection laws and regulations, and the sharing of certain data may require further permissions or ethical approvals. Researchers interested in obtaining the data should contact N.D. at nikolaos.dagres@dhzc-charite.de to initiate the data request process. Additionally, summary data and relevant findings are provided within the manuscript, tables, and supplementary materials, allowing for a comprehensive understanding of the study’s results. We are committed to promoting transparency and facilitating access to data to foster scientific collaboration and further research in the field of cardiology.
Funding

This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 847999.
Ethical Approval
Ethics committee approvals were obtained wherever this was necessary according to national and local regulations.
Pre-registered Clinical Trial Number
None supplied.
References
- 1. Wellens HJJ, Schwartz PJ, Lindemans FW, Buxton AE, Goldberger JJ, Hohnloser SH, et al. Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J 2014;35:1642–51. 10.1093/eurheartj/ehu176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gorgels APM, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJJ. Out-of-hospital cardiac arrest–the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J 2003;24:1204–9. 10.1016/S0195-668X(03)00191-X [DOI] [PubMed] [Google Scholar]
- 3. Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation 2012;125:1043–52. 10.1161/CIRCULATIONAHA.111.023846 [DOI] [PubMed] [Google Scholar]
- 4. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–83. 10.1056/NEJMoa013474 [DOI] [PubMed] [Google Scholar]
- 5. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–37. 10.1056/NEJMoa043399 [DOI] [PubMed] [Google Scholar]
- 6. Younis A, Goldberger JJ, Kutyifa V, Zareba W, Polonsky B, Klein H, et al. Predicted benefit of an implantable cardioverter-defibrillator: the MADIT-ICD benefit score. Eur Heart J 2021;42:1676–84. 10.1093/eurheartj/ehaa1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2022;43:3997–4126. 10.1093/eurheartj/ehac262 [DOI] [PubMed] [Google Scholar]
- 8. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2021;42:3599–726. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 9. Dagres N, Hindricks G. Risk stratification after myocardial infarction: is left ventricular ejection fraction enough to prevent sudden cardiac death? Eur Heart J 2013;34:1964–71. 10.1093/eurheartj/eht109 [DOI] [PubMed] [Google Scholar]
- 10. Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JGF, et al. Declining risk of sudden death in heart failure. N Engl J Med 2017;377:41–51. 10.1056/NEJMoa1609758 [DOI] [PubMed] [Google Scholar]
- 11. Sabbag A, Suleiman M, Laish-Farkash A, Samania N, Kazatsker M, Goldenberg I, et al. Contemporary rates of appropriate shock therapy in patients who receive implantable device therapy in a real-world setting: from the Israeli ICD registry. Heart Rhythm 2015;12:2426–33. 10.1016/j.hrthm.2015.08.020 [DOI] [PubMed] [Google Scholar]
- 12. Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Eur Urol 2015;67:1142–51. 10.1016/j.eururo.2014.11.025 [DOI] [PubMed] [Google Scholar]
- 13. Hinkle LEJ, Thaler HT. Clinical classification of cardiac deaths. Circulation 1982;65:457–64. 10.1161/01.CIR.65.3.457 [DOI] [PubMed] [Google Scholar]
- 14. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 15. Li D, Deogun J, Spaulding W, Shuart B.. A study of fuzzy K-means clustering method. In: Tsumoto S, Słowiński R, Komorowski J, Grzymała-Busse JW, (eds.), Rough Sets and Current Trends in Computing (RSCTC 2004). Lecture Notes in Computer Science, Vol. 3066. Berlin, Heidelberg: Springer; 2004. 10.1007/978-3-540-25929-9. [DOI] [Google Scholar]
- 16. Takada T, Nijman S, Denaxas S, Snell KIE, Uijl A, Nguyen T-L, et al. Internal-external cross-validation helped to evaluate the generalizability of prediction models in large clustered datasets. J Clin Epidemiol 2021;137:83–91. 10.1016/j.jclinepi.2021.03.025 [DOI] [PubMed] [Google Scholar]
- 17. Steyerberg EW, Harrell FE. Prediction models need appropriate internal, internal-external, and external validation. J Clin Epidemiol 2016;69:245–7. 10.1016/j.jclinepi.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Snell KIE, Ensor J, Debray TPA, Moons KGM, Riley RD. Meta-analysis of prediction model performance across multiple studies: which scale helps ensure between-study normality for the C-statistic and calibration measures? Stat Methods Med Res 2018;27:3505–22. 10.1177/0962280217705678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nelson CP, Lambert PC, Squire IB, Jones DR. Flexible parametric models for relative survival, with application in coronary heart disease. Stat Med 2007;26:5486–98. 10.1002/sim.3064 [DOI] [PubMed] [Google Scholar]
- 20. Mozumder SI, Rutherford MJ, Lambert PC. Stpm2cr: a flexible parametric competing risks model using a direct likelihood approach for the cause-specific cumulative incidence function. Stata J 2017;17:462–89. 10.1177/1536867X1701700212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ishwaran H, Kogalur UB, Blackstone EH, Lauer MS. Random survival forests. Ann Appl Stat 2008;2:841–60. 10.1214/08-AOAS169 [DOI] [Google Scholar]
- 22. Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance—2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. J Cardiovasc Magn Reson 2020;22:19. 10.1186/s12968-020-00610-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, 2022. https://www.R-project.org.
- 24. Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol 2012;59:2075–9. 10.1016/j.jacc.2012.02.036 [DOI] [PubMed] [Google Scholar]
- 25. Docherty KF, Ferreira JP, Sharma A, Girerd N, Gregson J, Duarte K, et al. Predictors of sudden cardiac death in high-risk patients following a myocardial infarction. Eur J Heart Fail 2020;22:848–55. 10.1002/ejhf.1694 [DOI] [PubMed] [Google Scholar]
- 26. Zegard A, Okafor O, de Bono J, Kalla M, Lencioni M, Marshall H, et al. Myocardial fibrosis as a predictor of sudden death in patients with coronary artery disease. J Am Coll Cardiol 2021;77:29–41. 10.1016/j.jacc.2020.10.046 [DOI] [PubMed] [Google Scholar]
- 27. Chernomordik F, Jons C, Klein HU, Kutyifa V, Nof E, Zareba W, et al. Death with an implantable cardioverter-defibrillator: a MADIT-II substudy. Europace 2019;21:1843–50. 10.1093/europace/euz263 [DOI] [PubMed] [Google Scholar]
- 28. Gatzoulis KA, Tsiachris D, Arsenos P, Antoniou C-K, Dilaveris P, Sideris S, et al. Arrhythmic risk stratification in post-myocardial infarction patients with preserved ejection fraction: the PRESERVE EF study. Eur Heart J 2019;40:2940–9. 10.1093/eurheartj/ehz260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ashar FN, Mitchell RN, Albert CM, Newton-Cheh C, Brody JA, Müller-Nurasyid M, et al. A comprehensive evaluation of the genetic architecture of sudden cardiac arrest. Eur Heart J 2018;39:3961–9. 10.1093/eurheartj/ehy474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sandhu RK, Dron JS, Liu Y, Moorthy MV, Chatterjee NA, Ellinor PT, et al. Polygenic risk score predicts sudden death in patients with coronary disease and preserved systolic function. J Am Coll Cardiol 2022;80:873–83. 10.1016/j.jacc.2022.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rohde LE, Chatterjee NA, Vaduganathan M, Claggett B, Packer M, Desai AS, et al. Sacubitril/valsartan and sudden cardiac death according to implantable cardioverter-defibrillator use and heart failure cause: a PARADIGM-HF analysis. JACC Heart Fail 2020;8:844–55. 10.1016/j.jchf.2020.06.015 [DOI] [PubMed] [Google Scholar]
- 32. Curtain JP, Docherty KF, Jhund PS, Petrie MC, Inzucchi SE, Køber L, et al. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Eur Heart J 2021;42:3727–38. 10.1093/eurheartj/ehab560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Knops RE, Pepplinkhuizen S, Delnoy PPHM, Boersma LVA, Kuschyk J, El-Chami MF, et al. Device-related complications in the subcutaneous and transvenous ICD: a secondary analysis of the PRAETORIAN trial. Eur Heart J 2022;43:4872–83. 10.1093/eurheartj/ehac496 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Raw data, including de-identified participant data, statistical code, and supplementary material, will be provided to researchers who meet the criteria for access, following a formal request to the corresponding author. Data sharing is subject to applicable data protection laws and regulations, and the sharing of certain data may require further permissions or ethical approvals. Researchers interested in obtaining the data should contact N.D. at nikolaos.dagres@dhzc-charite.de to initiate the data request process. Additionally, summary data and relevant findings are provided within the manuscript, tables, and supplementary materials, allowing for a comprehensive understanding of the study’s results. We are committed to promoting transparency and facilitating access to data to foster scientific collaboration and further research in the field of cardiology.