Abstract
Microscopic vascular invasion (MVI) has been demonstrated as a strong risk factor associated with tumor recurrence and poor overall survival among hepatocellular carcinoma (HCC) patients after resection, but the preoperative prediction of MVI is still challenging. We aimed to build and validate a novel model to predict MVI in the preoperative setting. We retrospectively collected 857 patients with Barcelona Clinic Liver Cancer (BCLC) stage 0 or A HCC who underwent primary resection at Kaohsiung Chang Gung Hospital between January 2001 and June 2016. The patients were randomized into derivation (n = 648) and validation groups (n = 209). Logistic regression analysis was used to screen out independent risk factors for MVI and further constructed a predictive model for MVI. Prediction performance was compared by the area under the receiver operating characteristic curve (AUC). The multivariable logistic regression analysis of the training cohort found that alpha-fetoprotein (AFP) ≥ 20 ng/mL (OR = 1.96, 95% CI: 1.41-2.73, P < 0.001), albumin < 3.5 g/dL (OR = 1.48, 95% CI: 1.06-2.05, P = 0.019) and tumor burden score (TBS) ≥ 8.6 (OR = 2.54, 95% CI: 1.49-4.35, P = 0.001) to be independent risk factors for MVI. The three factors were chosen to build a model for prediction of MVI. The AUC for the training and validation group was 0.619 (95% CI: 0.575-0.663) and 0.642 (95% CI: 0.562-0.722), respectively, and the calibration plot showed good performance of the prediction model, with a low mean absolute error at 0.01. In conclusion, the new model comprised AFP, albumin, and TBS that can predict risk of MVI for early-stage HCC.
Keywords: Hepatocellular carcinoma, recurrence, microvascular invasion, tumor burden score, AFP
Introduction
Liver cancer is highly prevalent globally, with over 900,000 estimated new cases annually. Hepatocellular carcinoma (HCC) constitutes more than 90% of these instances [1]. Hepatocellular carcinoma (HCC) is the predominant form of liver malignancy, estimated to account for over 80% of primary cases [2]. Presently, hepatectomy stands as a prevalent and safe treatment for early-stage HCC patients. Despite successful surgical resections, recurrence rates for HCC exceed 50% [1,3]. Identified risk factors for HCC recurrence post-hepatectomy include tumor size, serum α-fetoprotein (AFP) levels, tumor differentiation, cirrhosis, surgical margins, HBV viral load, and microvascular invasion (MVI) [3-7].
MVI represents a histologic diagnosis that can only be determined post-hepatectomy. Numerous models aimed at predicting MVI have been developed, facilitating the stratification of high-risk groups preoperatively and enhancing treatment decision-making for HCC [8-11]. While biomarkers such as alpha-fetoprotein (AFP) and des-carboxy prothrombin are utilized in clinical practice, their accuracy in detecting vascular invasion remains constrained [12]. Some investigations have identified specific radiographic and radiomic features as independent risk factors for MVI, leading to the development of various risk models [13-16]. Nevertheless, many radiomic and radiographic features are obtained by delineating volumes of interest and extracting features, leading to models that are vulnerable to operator-dependent variability and reduced robustness. Lee et al. employed total tumor volume (TTV), measured manually via length, width, and height in preoperative computed tomography, to characterize tumor burden [8]. However, this approach is labor-intensive, and interobserver variability poses a challenge.
Sasaki K et al. initially introduced the concept of tumor burden score (TBS), which is derived through tailored computations incorporating tumor size and number as continuous variables. They elucidated that TBS holds promise as a precise tool for prognostic stratification among patients with colorectal liver metastases undergoing resection [17]. Subsequent investigations have corroborated the utility of TBS in prognosticating outcomes among patients with hepatocellular carcinoma (HCC) undergoing hepatectomy [18]. Nonetheless, it is important to note that TBS lacks reflection of the biological intricacies of tumors as it solely encompasses morphological characteristics.
As a pivotal prognostic determinant, the preoperative anticipation of MVI enhances the optimization of treatment decisions for HCC. Numerous investigations have endeavored to forecast MVI employing preoperative parameters. Risk scoring models and nomogram frameworks have been constructed utilizing preoperative clinical, laboratory, and imaging variables to enhance the accuracy of MVI prediction [19,20]. Evidently, the efficacy of these models in predicting MVI in HCC has been extensively validated. Although scant studies exist on this subject, increasing attention is being directed towards it. Therefore, the aim of the present study was to develop and validate a novel model for predicting MVI in HCC within the preoperative context.
Patients and methods
Ethics statement
This study adhered to the principles outlined in the Declaration of Helsinki and current ethical standards. Approval was obtained from the Ethics Committee of Chang Gung Memorial Hospital (Institutional Review Board No. 201901103B0). The need for informed consent for this study was waived by the Institutional Review Board (IRB), and all data were analyzed anonymously.
Patients
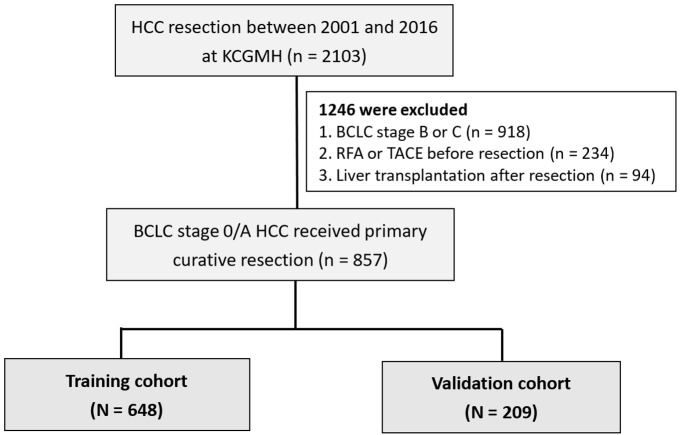
A total of 2,103 patients who underwent primary resection for HCC at Kaohsiung Chang Gung Memorial Hospital (KCGMH) between January 2001 and June 2016 were consecutively included. Exclusion criteria comprised patients aged less than 20 years, those classified as BCLC stage B or C, individuals who had received radiofrequency ablation (RFA) or transcatheter arterial chemoembolization (TACE) prior to resection, and those who underwent liver transplantation following resection. Ultimately, 857 patients with BCLC stage 0 or A HCC who underwent primary resection were enrolled in the study. Patients were randomized into training (n = 648) and validation groups (n = 209) at a ratio of 3:1 for internal validation (Figure 1). Baseline characteristics, including laboratory examinations (hematology, biochemistry, tumor marker AFP, and hepatitis serology tests), and pathological features were comprehensively documented. The number of tumors and their sizes (length and width) were measured based on post-operative pathological reports.
Figure 1.
Flowchart of the study population.
Definition
The diagnosis of cirrhosis was documented in the pathological reports for resected non-tumor tissues. The diagnosis of HCC was made in accordance with the guidelines set forth by the European Association for the Study of Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) [21,22]. Histopathological confirmation of MVI was conducted by experienced pathologists at our hospital, defined as tumor invasion into the portal vein, capsule vein, or both [23].
TBS is defined as the distance from the origin of a Cartesian plane composed of two variables: maximum tumor size and the number of tumors. TBS is calculated using the following formula: TBS^2 = (maximum tumor size)^2 + (number of tumors)^2 [17,18]. In our study, cut-offs for TBS were determined by calculating the maximal Youden index in the receiver operating characteristic curve. Subsequently, we categorized TBS into two groups: low (≤ 8.6) and high (> 8.6).
Statistical analysis
Continuous data were expressed as mean ± standard deviation, while categorical data were presented as numbers (percentages). Demographic data were compared between groups using the χ2 test or Fisher’s exact test, as appropriate. Logistic regression analysis was employed to identify independent risk factors for MVI and to construct a predictive model for MVI. Prediction performance was assessed by calculating the area under the receiver operating characteristic curve (AUC). Diagnostic performance of the model was evaluated using calibration curves in both the training and validation groups. Additionally, sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the model were computed. Statistical significance was set at a two-sided P-value less than 0.05. All statistical analyses were performed using SPSS version 24.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics
A total of 857 patients were enrolled in the study based on our inclusion and exclusion criteria. Their baseline characteristics are summarized in Table 1. Within this cohort, the mean age of patients was 58.6 ± 11.5 years, with 670 (78.2%) being male. The majority of patients were classified as Child-Pugh grade A (91.7%) and BCLC stage A (85.5%). The mean tumor size was 2.9 ± 1.0 cm, with 780 patients (91.0%) presenting with a single tumor. Diabetes mellitus (DM) was present in 222 (25.9%) patients, while liver cirrhosis was observed in 400 patients (46.7%). The etiologies of hepatitis were hepatitis B (485/857; 56.6%), hepatitis C (300/857; 35.0%), hepatitis B+C (42/857; 4.9%), and non-B non-C (NBNC) (114/857; 13.3%). Elevated AFP (≥ 20 ng/mL) was found in 331 (38.6%) patients. 306, 514, and 35 patients were categorized as ALBI (albumin-bilirubin) grade I, II, and III, respectively. The mean FIB-4 (Fibrosis-4) level was 0.92 ± 0.75. Pathologically, microvascular invasion was present in 327 patients (38.2%). There were no significant differences between the training and validation datasets regarding all preoperative characteristics and the incidence of MVI (39.0% and 35.4%, respectively; Table 1).
Table 1.
Patient characteristics of HCC patients in the training and validation cohorts
| Total (n = 857) | Training cohort (n = 648) | Validation cohort (n = 209) | P value | |
|---|---|---|---|---|
| Age (years), mean ± SD | 58.6 ± 11.5 | 58.6 ± 11.5 | 58.7 ± 11.7 | 0.906 |
| Male, n (%) | 670 (78.2) | 513 (79.2) | 157 (75.1) | 0.218 |
| Diabetes mellitus, n (%) | 222 (25.9) | 176 (27.2) | 46 (22) | 0.139 |
| Viral hepatitis status, n (%) | 0.845 | |||
| HBV | 443 (51.7) | 338 (52.2) | 105 (50.2) | |
| HCV | 258 (30.1) | 190 (29.3) | 68 (32.5) | |
| HBV + HCV | 42 (4.9) | 32 (4.9) | 10 (4.8) | |
| NBNC | 114 (13.3) | 88 (13.6) | 26 (12.4) | |
| Platelets < 150 103/µL, n (%) | 411 (48.0) | 304 (46.9) | 107 (51.2) | 0.281 |
| Albumin (g/dL); mean ± SD | 3.6 ± 0.6 | 3.63 ± 0.61 | 3.67 ± 0.63 | 0.384 |
| Total bilirubin(mg/dL), mean ± SD | 0.80 ± 0.34 | 0.83 ± 0.34 | 0.80 ± 0.32 | 0.300 |
| ALBI score, mean ± SD | -2.36 ± 0.53 | -2.35 ± 0.53 | -2.39 ± 0.54 | 0.339 |
| FIB-4 index, mean ± SD | 0.92 ± 0.75 | 0.93 ± 0.82 | 0.88 ± 0.51 | 0.385 |
| Liver cirrhosis, n (%) | 400 (46.7) | 296 (45.7) | 104 (49.8) | 0.304 |
| Child-Pugh grade, n (%) | 0.504 | |||
| A | 786 (91.7) | 592 (91.4) | 194 (92.8) | |
| B | 71 (8.3) | 56 (8.6) | 15 (7.2) | |
| BCLC stage, n (%) | 0.510 | |||
| 0 | 124 (14.5) | 106 (16.4) | 29 (13.9) | |
| A | 733 (85.5) | 542 (83.6) | 180 (86.1) | |
| AFP (ng/mL), n (%) | 0.052 | |||
| < 20 | 526 (61.4) | 395 (61.0) | 131 (62.7) | |
| 20-200 | 167 (19.5) | 137 (21.1) | 30 (14.4) | |
| > 200 | 164 (19.1) | 116 (17.9) | 48 (23.0) | |
| Tumor size (cm)a; mean ± SD | 2.9 ± 1.0 | 2.86 ± 1.02 | 2.90 ± 0.99 | 0.680 |
| Tumor number, n (%) | 2.9 ± 1.0 | 0.512 | ||
| Single | 780 (91.0) | 588 (90.7) | 188 (90.0) | |
| Multiple | 77 (9.0) | 60 (9.3) | 21 (10.0) | |
| Tumor burden score; mean ± SD | 10.6 ± 6.2 | 10.6 ± 6.3 | 10.5 ± 6.0 | 0.837 |
| Microvascular invasion | 0.347 | |||
| Yes | 327 (38.2) | 263 (39.0) | 74 (35.4) | |
| No | 530 (61.8) | 395 (61.0) | 135 (64.6) | |
| Recurrence, n (%) | 486 (56.7) | 371 (57.3) | 115 (55.0) | 0.572 |
| Death, n (%) | 324 (37.8) | 247 (38.1) | 77 (36.8) | 0.741 |
Data are expressed as mean ± standard deviation (SD) or n (%).Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; NBNC, non HBV non HCV; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, alpha fetoprotein; ALBI, albumin-bilirubin; FIB-4, fibrosis-4; BCLC, Barcelona clinical liver cancer.
Diameter of the largest tumor nodule.
Analysis of MVI-related predictors
Univariate logistic regression analysis of the training dataset identified HCV infection, elevated AFP (≥ 20 ng/mL), low albumin (< 3.5 g/dL), thrombocytopenia (< 150 103/µL), and high TBS (≥ 8.6) as potential risk factors for the presence of MVI. Subsequently, stepwise multivariable analysis of the training cohort revealed that AFP ≥ 20 ng/mL (Odds ratio [OR] = 1.951; 95% CI = 1.404-2.711; P < 0.001), albumin < 3.5 g/dL (OR = 1.498; 95% CI = 1.081-2.075; P = 0.015), and TBS ≥ 8.6 (OR = 1.586; 95% CI = 1.143-2.200; P = 0.006) were independent risk factors for MVI (Table 2).
Table 2.
Preoperative predictors of MVI in patients with HCC in a training cohort
| Variable | Comparison | Univariable | Multivariable | ||
|---|---|---|---|---|---|
|
|
|
||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| Age (years) | > 60 vs. ≤ 60 | 1.066 (0.777-1.463) | 0.690 | ||
| Sex | Male vs. Female | 1.159 (0.782-1.716) | 0.462 | ||
| Hypertension | Yes vs. No | 1.078 (0.777-1.494) | 0.654 | ||
| Diabetes mellitus | Yes vs. No | 1.077 (0.757-1.535) | 0.679 | ||
| CHB | Yes vs. No | 1.400 (0.922-2.127) | 0.114 | ||
| CHC | Yes vs. No | 1.628 (1.057-2.056) | 0.027 | ||
| AFP (ng/mL) | ≥ 20 vs.< 20 | 1.877 (1.358-2.594) | 0.000 | 1.951 (1.404-2.711) | < 0.001 |
| Albumin (g/dL) | < 3.5 vs.≥ 3.5 | 1.545 (1.123-2.125) | 0.008 | 1.498 (1.081-2.075) | 0.015 |
| Platelet (103/µL) | ≥ 150 vs.< 150 | 1.378 (1.004-1.891) | 0.047 | ||
| Liver cirrhosis | Yes vs. No | 1.183 (0.862-1.624) | 0.299 | ||
| Child-Pugh grade | B vs. A | 1.189 (0.683-2.070) | 0.541 | ||
| FIB-4 index | Per 1 score increase | 1.155 (0.952-1.401) | 0.143 | ||
| ALBI grade¶ | Grade 2 vs. grade 1 | 1.423 (1.012-2.001) | 0.043 | ||
| Grade 3 vs. grade 1 | 1.703 (0.751-3.862) | 0.203 | |||
| BCLC stage | A vs. 0 | 1.177 (0.763-1.814) | 0.461 | ||
| Tumor number | Multiple vs. Single | 0.701 (0.397-1.238) | 0.221 | ||
| Tumor burden score* | ≥ 8.6 vs. < 8.6 | 1.585 (1.151-2.183) | 0.005 | 1.586 (1.143-2.200) | 0.006 |
Tumor burden score divided into 2 groups: Low: < 8.6 and High: ≥ 8.6.
ALBI grade divided into 3 grades: Grade1: ≤ -2.60; Grade 2: -2.60 to ≤ -1.39, Grade 3: ≥ -1.39.
Development and validation of a risk score model for predicting MVI in HCC
The three factors were selected to construct a model for predicting MVI, as detailed in Table 3. Score points were assigned to each independent risk factor based on their regression coefficients, as outlined in Table 3. AFP values exceeding 20 ng/mL were assigned 2 points, albumin values below 3.5 g/dL were assigned 1 point, and TBS values of 8.6 cm^2 and above were assigned 1 point. With a total of five possible points, incidences of MVI ranged from 23.5% to 62.1% across scores of 0 to 4, yielding a significant p-value of less than 0.001 (Table 3). The area under the curve (AUC) of the receiver operating characteristic (ROC) curve was 0.619 (Figure 2A), and the calibration plot demonstrated favorable performance of the prediction model, with a low mean absolute error of 0.01 (Figure 3). Subsequently, a validation dataset was utilized to validate the risk score model, yielding an AUC of the ROC curve of 0.642 (Figure 2B). Nagelkerke’s R^2 values and the results of the Hosmer-Lemeshow test indicated good overall model fit, with a significant effect size (Table 4).
Table 3.
Risk score model for the prediction of microvascular invasion
| Predictor variables | Regression coefficients (β) | Categories | Points | ||
|---|---|---|---|---|---|
| AFP | < 20* | 0 | |||
| 1.951 | ≥ 20 | 2 | |||
| Albumin | ≥ 3.5* | 0 | |||
| 1.498 | < 3.5 | 1 | |||
| Tumor burden score | < 8.6* | 0 | |||
| 1.586 | ≥ 8.6 | 1 | |||
| Total score | 0 | 1 | 2 | 3 | 4 |
| Probability of MVI | 23.5% | 33.3% | 41.8% | 45.7% | 62.1% |
Reference category.
Figure 2.
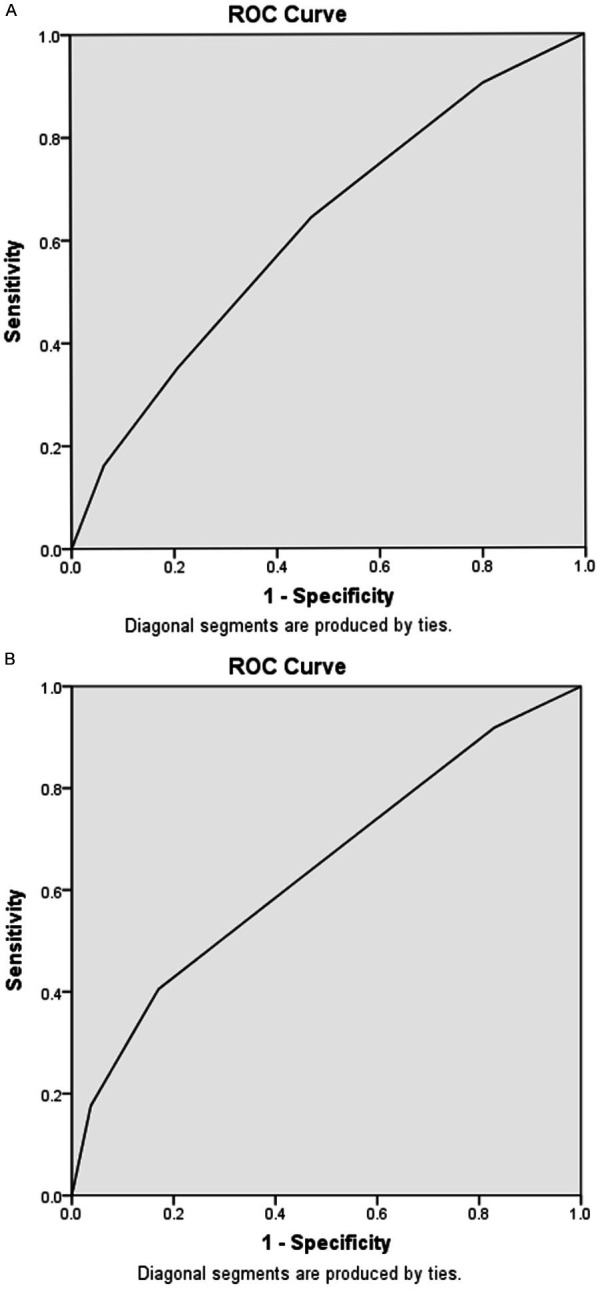
Receiver operating characteristic (ROC) curve of the risk score model for predicting MVI: (A) training dataset with an area under the curve (AUC) of 0.619 and (B) validation dataset with an AUC of 0.642.
Figure 3.
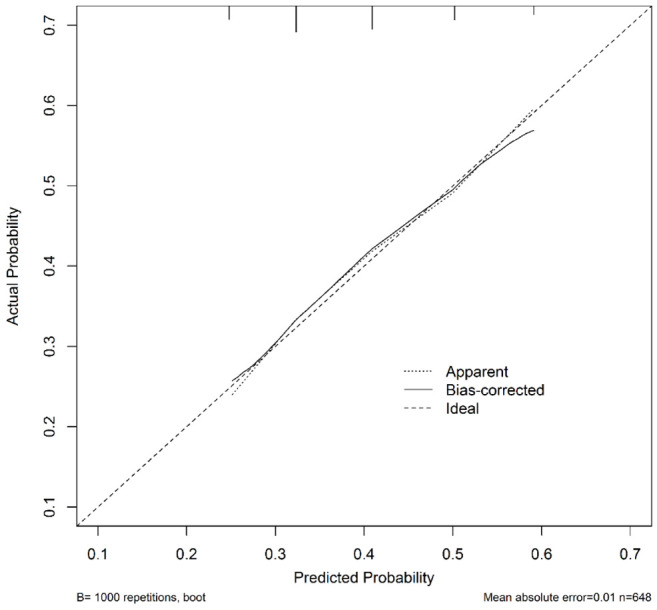
Calibration plot of actual versus predicted probability of MVI in the training dataset, demonstrating a mean absolute error of 0.01.
Table 4.
The comparison of performance measures by using logistic regression mode in MVI probability
| Model | Overall Performance Measure | Discrimination | Calibration |
|---|---|---|---|
|
| |||
| Nagelkerke R2 | C Statistic | Hosmer-Lemeshow Test | |
| Training cohort | 0.060 | 0.619 | 0.738 |
| Validation cohort | 0.094 | 0.642 | 0.473 |
Discussion
MVI has been proven by many studies to be the most consistent risk factor for HCC recurrence and survival [24-26]. Several novel systems based on MVI were proposed and proven to have better prognostic value than conventional staging systems [17,27]. However, MVI can only be recognized by histopathologic examination of postoperative specimen currently. The results of the present study indicated that pre-operative serum AFP and albumin levels and TBS to be independent predictors for MVI for early-stage HCC, which can thus be used to build an effective risk score model. A novel model was then developed based on albumin, AFP, and TBS for patients with early-stage HCC to identify MVI before surgery.
In our study, multivariate analysis revealed that preoperative albumin, AFP, and TBS were independent risk factors for MVI. A risk score model for predicting MVI was constructed using these three factors, which respectively represent tumor burden and biology. These factors, along with cut-points selected via a classification approach, are straightforward to calculate and exhibit objectivity, minimizing user bias. These characteristics render the prediction model more applicable across various subspecialties, without being constrained by a patient’s viral hepatitis status, which was not significant in predicting MVI in univariate analysis. This predictive model was convenient to use for its easily available preoperative clinical data.
In recent years, numerous studies have explored the use of radiomic features to develop prediction models for MVI with promising levels of accuracy. Other prediction models for MVI integrate radiographic, tumor characteristics, serum biochemistry, tumor markers, and inflammatory indicators. For instance, Li et al. constructed a nomogram based on inflammatory scores, AFP levels, and tumor size [28]. Lee et al. devised a model utilizing AFP levels and total tumor volume for patients with early-stage HCC undergoing liver resection [8]. However, many imaging features rely on subjective assessments by radiologists and lack standardization. Zhao et al. demonstrated that variations in regions of interest significantly impact the performance of CT-based radiomics models for predicting MVI in HCC patients [29]. Total tumor volume serves as a surrogate for both tumor size and number and is a robust predictor of MVI. Nonetheless, the manual acquisition of total tumor volume, involving measurements of length, width, and height, is time-consuming.
Our study revealed that preoperative TBS and AFP were independent risk factors for MVI, consistent with previous findings [8,30,31]. However, a noteworthy difference is the identification of albumin as an independent risk factor for predicting MVI in our study, a factor not previously reported in the literature. We hypothesize that this discrepancy may be attributed to the specific characteristics of the HCC cohort examined in our study. Specifically, we focused on patients with early-stage HCC, characterized by relatively small tumor sizes and numbers. Under such circumstances, the impact of TBS on MVI may be diminished. Conversely, albumin, being produced in the liver and serving as an indicator of hepatic synthetic function and nutritional status, emerges as a relevant and readily available parameter for preoperative assessment.
According to guidelines, various treatment options are available for early-stage HCC, including resection, RFA, radiotherapy, and liver transplantation. Among these, only surgery provides sufficient tissue for MVI confirmation. In cases of early-stage HCC treated with RFA or radiotherapy, MVI status remains unknown. However, our model, utilizing preoperative albumin, AFP, and TBS, offers the ability to predict MVI in such cases. This preoperative prediction of MVI enables optimization of HCC treatment planning. Notably, liver transplantation should be prioritized post-tumor treatment in patients at high risk of MVI to address potential intrahepatic micro-metastases. Recent advancements in systemic therapies, including immune checkpoint inhibitors, tyrosine kinase inhibitors, and monoclonal antibodies, have shown promising outcomes in advanced HCC treatment. The combination of atezolizumab (anti-PDL1 antibody) and bevacizumab (anti-VEGF antibody) has emerged as a superior option to sorafenib in terms of overall survival [32,33]. Consequently, this combination has become the standard first-line therapy for advanced HCC. With the rapid progress of immunotherapy in HCC, MVI may serve as a potential indicator for adjuvant or neoadjuvant immunotherapy in the future. The findings of our study contribute valuable insights into MVI, particularly in the context of early-stage HCC undergoing non-surgical treatments.
Protein induced by vitamin K absence II (PIVKA-II) was detected in 80-90% of HCC patients, suggesting its potential use as an HCC biomarker [34]. Although multiple studies have explored its utility in surveillance, treatment monitoring, and predicting recurrence of HCC, it is not yet recommended as a routine test. In Taiwan, PIVKA-II has been covered by health insurance since June 2020 for patients with cirrhosis or those who have undergone curative treatment for HCC. In the present study, the HCC cohort was enrolled from 2001 to 2016; thus, PIVKA-II was not available in clinical practice at that time. We believe that incorporating PIVKA-II into our model could enhance the prediction rate. In the future, we plan to extend our cohort and use PIVKA-II to predict MVI.
The study has some limitations. Firstly, we utilized pathologic TBS, which cannot be obtained preoperatively. While pathologic TBS provides theoretically accurate data, we extrapolate that imaging TBS effectively represents its pathological counterpart in our study. However, further investigation is warranted to validate this assumption. Secondly, patient enrollment was conducted retrospectively, resulting in potential selection bias. Nevertheless, we contend that the risk of bias is minimal given that patients were consistently monitored by the same physicians throughout their disease trajectory. Additionally, they underwent standardized clinical and laboratory assessments, as well as HCC screening via ultrasonography every 3 to 6 months. Finally, the study is limited by its single-center design and lacks external validation. Therefore, a multi-center study is needed to perform external validation.
Conclusion
In conclusion, we have developed a model incorporating AFP, albumin, and TBS, which effectively predicts the risk of MVI in early-stage HCC. This model holds potential for routine clinical application, aiding in treatment decision-making for early-stage HCC patients undergoing non-surgical interventions.
Acknowledgements
We thank the service provided by the Cancer Registration of Cancer Center Department, Kaohsiung Chang Gung Memorial Hospital. We also appreciated the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital for statistics work.
Disclosure of conflict of interest
None.
References
- 1.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 3.Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947–955. doi: 10.1097/SLA.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 4.McHugh PP, Gilbert J, Vera S, Koch A, Ranjan D, Gedaly R. Alpha-fetoprotein and tumour size are associated with microvascular invasion in explanted livers of patients undergoing transplantation with hepatocellular carcinoma. HPB (Oxford) 2010;12:56–61. doi: 10.1111/j.1477-2574.2009.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu JC, Huang YH, Chau GY, Su CW, Lai CR, Lee PC, Huo TI, Sheen IJ, Lee SD, Lui WY. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009;51:890–897. doi: 10.1016/j.jhep.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Hirokawa F, Hayashi M, Asakuma M, Shimizu T, Inoue Y, Uchiyama K. Risk factors and patterns of early recurrence after curative hepatectomy for hepatocellular carcinoma. Surg Oncol. 2016;25:24–29. doi: 10.1016/j.suronc.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Shinkawa H, Tanaka S, Kabata D, Takemura S, Amano R, Kimura K, Kinoshita M, Kubo S. The prognostic impact of tumor differentiation on recurrence and survival after resection of hepatocellular carcinoma is dependent on tumor size. Liver Cancer. 2021;10:461–472. doi: 10.1159/000517992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JC, Hung HC, Wang YC, Cheng CH, Wu TH, Lee CF, Wu TJ, Chou HS, Chan KM, Lee WC. Risk score model for microvascular invasion in hepatocellular carcinoma: the role of tumor burden and alpha-fetoprotein. Cancers (Basel) 2021;13:4403. doi: 10.3390/cancers13174403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Li T, Hu J, Liu J. A nomogram to predict microvascular invasion in early hepatocellular carcinoma. J Cancer Res Ther. 2021;17:652–657. doi: 10.4103/jcrt.JCRT_1714_20. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Zeng F, Jiang S, Tang H, Zhang J. Preoperative prediction model of microvascular invasion in patients with hepatocellular carcinoma. HPB (Oxford) 2023;25:45–53. doi: 10.1016/j.hpb.2022.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W, Liu Z, Chen J, Dong S, Cen B, Zheng S, Xu X. A preoperative model for predicting microvascular invasion and assisting in prognostic stratification in liver transplantation for HCC regarding empirical criteria. Transl Oncol. 2021;14:101200. doi: 10.1016/j.tranon.2021.101200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamazaki S, Takayama T, Kurokawa T, Shimamoto N, Mitsuka Y, Yoshida N, Higaki T, Sugitani M. Next-generation des-r-carboxy prothrombin for immunohistochemical assessment of vascular invasion by hepatocellular carcinoma. BMC Surg. 2020;20:201. doi: 10.1186/s12893-020-00862-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong XQ, Tao YY, Wu YK, Liu N, Yu X, Wang R, Zheng J, Liu N, Huang XH, Li JD, Yang G, Wei XQ, Yang L, Zhang XM. Progress of MRI radiomics in hepatocellular carcinoma. Front Oncol. 2021;11:698373. doi: 10.3389/fonc.2021.698373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q, Li C, Zhang J, Hu X, Fan Y, Ma K, Sparrelid E, Brismar TB. Radiomics models for predicting microvascular invasion in hepatocellular carcinoma: a systematic review and radiomics quality score assessment. Cancers (Basel) 2021;13:5864. doi: 10.3390/cancers13225864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei J, Jiang H, Zeng M, Wang M, Niu M, Gu D, Chong H, Zhang Y, Fu F, Zhou M, Chen J, Lyv F, Wei H, Bashir MR, Song B, Li H, Tian J. Prediction of microvascular invasion in hepatocellular carcinoma via deep learning: a multi-center and prospective validation study. Cancers (Basel) 2021;13:2368. doi: 10.3390/cancers13102368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang K, Xie SS, Li WC, Ye ZX, Shen ZW, Shen W. Prediction of microvascular invasion in HCC by a scoring model combining Gd-EOB-DTPA MRI and biochemical indicators. Eur Radiol. 2022;32:4186–4197. doi: 10.1007/s00330-021-08502-8. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki K, Morioka D, Conci S, Margonis GA, Sawada Y, Ruzzenente A, Kumamoto T, Iacono C, Andreatos N, Guglielmi A, Endo I, Pawlik TM. The tumor burden score: a new “metro-ticket” prognostic tool for colorectal liver metastases based on tumor size and number of tumors. Ann Surg. 2018;267:132–141. doi: 10.1097/SLA.0000000000002064. [DOI] [PubMed] [Google Scholar]
- 18.Tsilimigras DI, Moris D, Hyer JM, Bagante F, Sahara K, Moro A, Paredes AZ, Mehta R, Ratti F, Marques HP, Silva S, Soubrane O, Lam V, Poultsides GA, Popescu I, Alexandrescu S, Martel G, Workneh A, Guglielmi A, Hugh T, Aldrighetti L, Endo I, Sasaki K, Rodarte AI, Aucejo FN, Pawlik TM. Hepatocellular carcinoma tumour burden score to stratify prognosis after resection. Br J Surg. 2020;107:854–864. doi: 10.1002/bjs.11464. [DOI] [PubMed] [Google Scholar]
- 19.Zhao WC, Fan LF, Yang N, Zhang HB, Chen BD, Yang GS. Preoperative predictors of microvascular invasion in multinodular hepatocellular carcinoma. Eur J Surg Oncol. 2013;39:858–864. doi: 10.1016/j.ejso.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Lei Z, Li J, Wu D, Xia Y, Wang Q, Si A, Wang K, Wan X, Lau WY, Wu M, Shen F. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the Milan criteria. JAMA Surg. 2016;151:356–363. doi: 10.1001/jamasurg.2015.4257. [DOI] [PubMed] [Google Scholar]
- 21.Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul JL, Schirmacher P, Vilgrain V. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 23.Huang DS, Liu TT, Lu WT, Wang CC, Lin CC, Yong CC, Chen KD, Liu YW, Kuo YH, Yen YH, Hu TH, Tsai MC. Comparison of portal and capsular microscopic vascular invasion in the outcomes of early HCC after curative resection. Am J Cancer Res. 2022;12:2659–2672. [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Liu FC, Li L, Zhou WP, Jiang BG, Pan ZY. Nomograms to predict the long-time prognosis in patients with alpha-fetoprotein negative hepatocellular carcinoma following radical resection. Cancer Med. 2020;9:2791–2802. doi: 10.1002/cam4.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodríguez-Perálvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20:325–339. doi: 10.1245/s10434-012-2513-1. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Li J, Shen F, Lau WY. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2018;33:347–354. doi: 10.1111/jgh.13843. [DOI] [PubMed] [Google Scholar]
- 27.Ho SY, Liu PH, Hsu CY, Huang YH, Liao JI, Su CW, Hou MC, Huo TI. A new tumor burden score and albumin-bilirubin grade-based prognostic model for hepatocellular carcinoma. Cancers (Basel) 2022;14:649. doi: 10.3390/cancers14030649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S, Zeng Q, Liang R, Long J, Liu Y, Xiao H, Sun K. Using systemic inflammatory markers to predict microvascular invasion before surgery in patients with hepatocellular carcinoma. Front Surg. 2022;9:833779. doi: 10.3389/fsurg.2022.833779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao H, Feng Z, Li H, Yao S, Zheng W, Rong P. Influence of different region of interest sizes on CT-based radiomics model for microvascular invasion prediction in hepatocellular carcinoma. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022;47:1049–1057. doi: 10.11817/j.issn.1672-7347.2022.220027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Endo Y, Alaimo L, Lima HA, Moazzam Z, Ratti F, Marques HP, Soubrane O, Lam V, Kitago M, Poultsides GA, Popescu I, Alexandrescu S, Martel G, Workneh A, Guglielmi A, Hugh T, Aldrighetti L, Endo I, Pawlik TM. A novel online calculator to predict risk of microvascular invasion in the preoperative setting for hepatocellular carcinoma patients undergoing curative-intent surgery. Ann Surg Oncol. 2023;30:725–733. doi: 10.1245/s10434-022-12494-0. [DOI] [PubMed] [Google Scholar]
- 31.Schlichtemeier SM, Pang TC, Williams NE, Gill AJ, Smith RC, Samra JS, Lam VW, Hollands M, Richardson AJ, Pleass HC, Nozawa S, Albania M, Hugh TJ. A pre-operative clinical model to predict microvascular invasion and long-term outcome after resection of hepatocellular cancer: the Australian experience. Eur J Surg Oncol. 2016;42:1576–1583. doi: 10.1016/j.ejso.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 32.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL IMbrave150 Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 33.Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Ma N, Nicholas A, Wang Y, Li L, Zhu AX, Finn RS. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862–873. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 34.Weitz IC, Liebman HA. Des-gamma-carboxy (abnormal) prothrombin and hepatocellular carcinoma: a critical review. Hepatology. 1993;18:990–997. doi: 10.1002/hep.1840180434. [DOI] [PubMed] [Google Scholar]