Abstract
Lung adenocarcinoma (LUAD) carries a poor prognosis at advanced stages underscoring the need to elucidate the underlying molecular mechanisms driving its pathogenesis. This study aimed to investigate the roles of eukaryotic translation initiation factor 3 subunit M (EIF3M) and its associated effector, serum amyloid A-like 1 (SAAL1), in LUAD development and progression. Bioinformatic analyses such as TNMplot, The Cancer Genome Atlas (TCGA), Gene Expression Omnibus (GEO), and other public databases were used to evaluate EIF3M and SAAL1 expression levels, methylation status, clinical associations, and potential transcriptional regulators across LUAD datasets. Patient samples were analyzed for EIF3M/SAAL1 expression by qRT-PCR, immunohistochemistry, and ELISA. EIF3M and SAAL1 were overexpressed in LUAD tumor tissues compared with normal lung tissues, correlated with advanced stage, nodal metastasis, and poor survival outcomes. High EIF3M/SAAL1 levels associated with increased cell proliferation, epithelial-mesenchymal transition, metastasis, and regulatory T cell dysfunction based on gene set enrichment analysis (GSEA). Mechanistically, EIF3M/SAAL1 upregulation was linked to promoter hypomethylation, and transcriptionally regulated by JMJD1C, via hTFtarget prediction. The EIF3M/SAAL1 promote oncogenic cellular programs and immunosuppressive microenvironments that conferred unfavorable prognosis. These findings nominate EIF3M/SAAL1 as potential therapeutic targets and biomarkers in LUAD.
Keywords: EIF3M, JMJD1C, lung adenocarcinoma, SAAL1
Introduction
Lung cancer remains the most common cancer-related death worldwide [1]. Basically, lung cancer is divided into small cell and non-small cell lung cancer (NSCLC), which then could be further divided into adenocarcinoma (LUAD), squamous cell carcinoma (LUSC), large cell carcinoma, etc [2]. In the past decades, scientists devoted lots of efforts to delineate the possible mechanisms mediating carcinogenesis and metastasis [3]. Drug development such as targeted therapy, immune checkpoint inhibitors, chemotherapy, etc has improved the clinical outcomes [4]. However, the prognosis of advanced stage LUAD is still not satisfactory. For these critical situatoin, more efforts are emergent need to solve the cancer related events.
The occurrence of cancer starts from genetic mutation as initiation [5]. Later, the complexity among promotion and progression in lung cancer remains unsolved despite the elucidated molecular events by advanced techniques [6]. Dysregulated oncogene expression, tumor suppressor inhibition, and eukaryotic translation initiation factors (EIFs) are necessary for tumorigenesis [7]. EIFs are frequently involved in tumorigenic transformation, cancer development, and progression, and is the target of for cancer therapy development [8]. Translation into protein and its regulation are recognized as a major determinant in inducing adaptive stress responses to overcome various stress conditions imposed cancer cells by their surrounding microenvironment, immunological cells, and cytotoxicity of antitumor drugs [9]. Tumor-induced immunological changes affect the progression to metastatic disease, even before disseminated cancer cells have reached a secondary organ [10].
Eukaryotic translation initiation requires ribosome subunits and EIFs [8]. For decades, studies have revealed that a group of initiation factors such as EIF2, EIF3, EIF4, and EIF5 are implicated in different types of cancer [8]. Also, they contribute to the hallmark of cancer, such as sustained proliferation, uncontrolled growth, angiogenesis, aggressive behaviors including invasion, metastasis, etc [9,11-14]. Epigenetic regulations on the genome result either in gene activation or silencing [5]. These modifications have been contributed to the development and progression of different lung cancer subtypes [15]. As we know that the major epigenetic signals include DNA methylation and demethylation, modifications of the histone proteins, such as acetylation and methylation, and gene regulation by non-coding RNAs [15,16]. Among these processes, the methyltransferases and demethylases are responsible for maintaining the hemostasis between levels of methylation and gene expression [16]. The methylation associated with addition of methyl group at lysine residues of core histones plays a critical role in the epigenetic modification of transcription and chromatin structures [16]. These modifications might enhance mRNA expression to execute its specific physiologic functions such as ageing process, wound healing or pathophysiologic ones such as cancer initiation, progression, and metastasis [16,17].
Therefore, to meet the unsatisfactory parts in treating lung cancer, more efforts are required. To depict the molecular pathways regulating initiation, promotion, and progression would be critical one to solve the long-lasting issue concerning lung cancer. This study devoted efforts to elucidate the role of a member of eIF family, eIF3m, cooperating with SAAL1 in regulating LUAD pathogenesis and its impact on clinical outcomes. We also found the possible epigenetic regulation. The further detailed mechanism and clinical study might be worthy of further investigation.
Materials and methods
Bioinformatic analysis
The expressions of EIF3M from different cancer types were acquired from TNMplot (https://tnmplot.com/analysis/; accessed on 12th March, 2023) [18]. The differential expressions of EIF3M and SAAL1 between different types of lung cancer and normal lung tissue were extracted from The Cancer Genome Atlas (TCGA) using UALCAN website (http://ualcan.path.uab.edu/; accessed on 12th March, 2023) [19]. The protein expression level was extracted from the Clinical Proteomic Tumor Analysis Consortium (CPTAC) LUAD cohort via the UALCAN website. GSVA (Gene Set Variation Analysis) score of gene sets correlated with EIF3M or SAAL1 was calculated by the GSCAL website (http://bioinfo.life.hust.edu.cn/GSCA; accessed on 12th March 2023) [20]. The immune cell infiltration by either eIF3m or SAAL1 was analyzed from Gene Set Cancer Analysis (GSCA; https://guolab.wchscu.cn/GSCA/#/; accessed on 25 Aug. 2024) [21]. The pathways of gene sets correlated with EIF3M or SAAL1 were assessed by DAVID website (https://david.ncifcrf.gov/; accessed on 20 April 2023) [22]. Prognostic analysis Kaplan-Meier (K-M) survival analysis of various genes for overall survival (OS) and relapse-free survival (RFS) was conducted using the KM plotter website (https://kmplot.com/analysis/; accessed on 20 April 2023) [23]. The hazard ratios (HR) with 95% confidence intervals (CI) and p-values were extracted from the KM plotter webpage and considered significant with p-values < 0.05. The existence of the expression of eIF3M was acquired from the Tumor Immune Single-cell Hub 2 (TISCH2) (http://tisch.comp-genomics.org/home/; accessed on 2 Sep. 2024) [24]. Potential transcription factors of EIF3M and SAAL1 were curated from the website hTFtarget (http://bioinfo.life.hust.edu.cn/hTFtarget#!/; accessed on 21 April 2023) [25]. The expression of EIF3M and SAAL1 also extracted from Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE31210; accessed on 13 March 2023) [26].
Bulk RNA-sequencing of in-house LUAD dataset
The fifteen pairs of adjacent non-tumor lungs and tumors were harvested from the Division of Thoracic Surgery and Division of Pulmonary and Critical Care Medicine, Kaohsiung Medical University Hospital (Kaohsiung, Taiwan, KMUH-IRB-20130054; KMUH-IRB-20180023). The RNA sequencing for the pairs of LUAD and lung normal tissue was performed by the Welgene biotechnology company (Taipei, Taiwan).
Gene set enrichment analysis (GSEA)
To investigate the role of eIF3m and SAAL1 in LUAD, the patients of the TCGA were divided into EIF3M and SAAL1 high expression and low expression groups, and GSEA was conducted to analyze the enrichment of datasets between the high and low expressing selected genes groups. False discovery rate (FDR) < 0.005 and nominal p-value < 0.001 were set as the cut-off criteria.
Immunohistochemistry and serum SAAL1 assay
The 7 pairs of normal lung tissues and malignant tissues were collected from the Division of Thoracic Surgery and Division of Pulmonary and Critical Care Medicine, Kaohsiung Medical University Hospital (Kaohsiung, Taiwan, KMUH-IRB-20180023, KMU-IRB-20200038; KMU-IRB-E(II)-20220175). EIF3M antibody (Abcam, UK, cat no.: ab24688) was used for the immunohistochemistry stain.
The levels of SAAL1 in the healthy donors and lung cancer patients were assessed by SAAL1 ELISA kit (Biorbyt, USA).
Statistical analysis
Results are presented as mean ± standard deviation (SD). Differences between the two tested groups were compared using the Student’s t-test with GraphPad Prism software (9.0.2 version, Graphpad Software, San Diego, CA, USA). Statistical significance was defined as p-value < 0.05. Pearson correlation and multiple linear regression by R package (estimatr) were used for correlation.
Results
Increased EIF3M expression in non-small cell lung cancer (NSCLC)
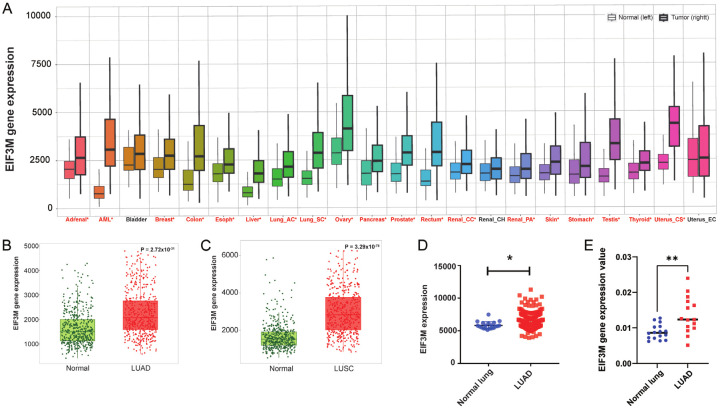
The mechanism of EIF3M in mediating lung cancer was not clear, therefore, we took advantage a cohort from the TNMplot [18] to elucidate the expression levels of it. From TNMplot, the pan-cancer analysis page displays the expression levels for EIF3M gene across common tumor types based on RNAseq data from normal and cancer tissues (Figure 1A). The expression levels of the most popular types of lung cancer, LUAD and LUSC, were higher in tumor parts compared with normal parts. Therefore, EIF3M expression was increased in primary cancer tissues compared with normal lung tissues in these two major cancer types (Figure 1B, 1C). In addition, the result of LUAD was also consistent with Caucasian population (TCGA) in Asian population (GSE31210) (Figure 1D) and our in-house lung cancer specimens (Figure 1E). Taken together, EIF3M expression increased in NSCLC.
Figure 1.
Tumor cells expressed higher level of EIF3M in lung cancer. Expression levels of EIF3M in different types of cancer (A) from TNMplot. The levels of EIF3M expressed in LUAD (B) and LUSC (C) compared with adjancent normal parts. The gene expression of EIF3M in GSE31210 dataset (D) and in the KMUH cohort (E). LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma. *, P < 0.05; **, P < 0.01.
Higher levels of EIF3M conferred worse clinical outcomes in in LUAD
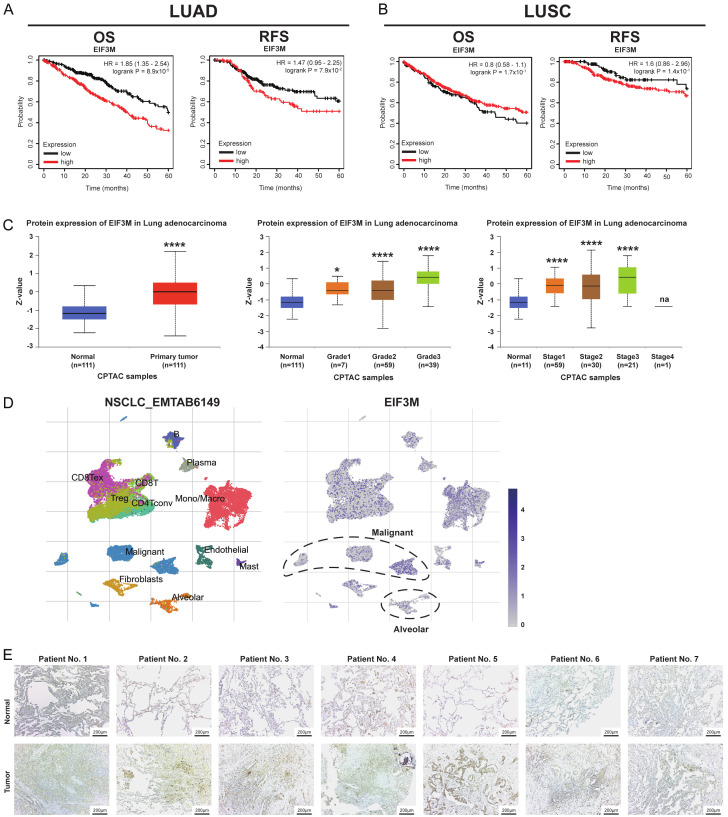
Next step, the clinical implications as survival analyses of EIF3M were dissected. First, In terms of overall survival (OS) and relapse free survival (RFS), high expression group revealed survival disadvantage compared with low group in LUAD only but not LUSC (Figure 2A, 2B). The OS analysis was not significant regarding treatment strategy, either chemotherapy or radiotherapy (Figure S1A, S1B). Furthermore, protein expression of eIF3m was also increased primary tumor, higher tumor grades, and cancer stages in LUAD (Figure 2C). The cohort as EMTAB6149 from the TISCH2 revealed higher levels of EIF3M in malignant cells compared with alveolar cells (Figure 2D). In addition, we evaluated EIF3M expression from our 15 paired clinical specimens from LUAD patients. The expression levels using immunohistochemical staining were higher in tumor parts compared with normal parts (Figure 2E) and secondary antibody staining (Figure S2). These results meant that increased EIF3M expression in both mRNA and protein resulted in poor clinical prognosis.
Figure 2.
Higher expression of EIF3M conferred poor prognosis in LUAD. Survival analysis as overall survival (OS) and relapse-free survival (RFS) in LUAD (A) and LUSC (B) using Kaplan-Meier survival analysis. The protein expression of eIF3m in primary tumor tissue, tumor grades, and cancer stages in LUAD from CPTAC dataset (C). The expression of EIF3M in the tumor part of NSCLC_EMTAB6149 from the TISCH2 (D). Immunohistochemical staining of eIF3m from in-house LUAD patients (E). *, P < 0.05; ****, P < 0.001; na = not applicable.
Higher expression of EIF3M possed more aggressive biologic functions
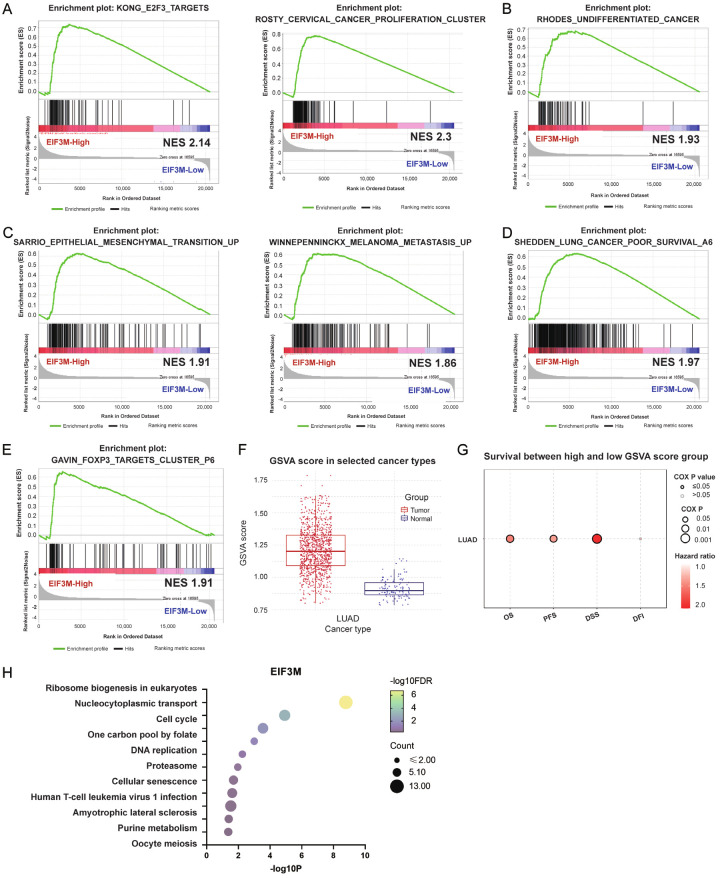
For functions of EIF3M, GSEA and GSVA were used to delineate its cellular behaviors in LUAD. GSEA revealed that high expression of EIF3M compared with low expression was positively associated with cell proliferation (Figure 3A), undifferentiation (Figure 3B), and epithelial mesenchymal transition & cancer cell metastasis (Figure 3C). In terms of clinical outcome, poor survival was associated with high expression of EIF3M than low group (Figure 3D). Moreover, GSEA results also showed that high EIF3M expression was involved in tumor immune microenvironment remodeling by increasing regulatory T cell (Treg) (Figure 3E). The immune infiltration affected by EIF3M using GSCA showed positive correlation with Effector_memory, Th1, Exhausted, nTreg, etc (Figure S3A). Furthermore, a positively correlated gene set of EIF3M presented as GSVA score (top 200) in TCGA cohort was high in the tumor part compared with the normal part (Figure 3F). The high GSVA score compared with low one was associated with poor clinical outcomes including overall survival, progression free survival, and disease-specific survival (Figure 3G). KEGG pathway analysis using EIF3M associated gene set showed its association with cell cycle (Figure 3H). These data suggesting EIF3M and its co-regulatory factors acted as an oncogenic network in LUAD.
Figure 3.
Higher EIF3M expression held aggressive biologic functions in LUAD. The functions of EIF3M of LUAD disclosed by the gene set enrichment analysis (GSEA) as cell proliferation (A), cell undifferentiation (B), epithelial mesenchymal transition, and metastasis (C), poor survival (D), and regulatory T cell (E). Gene set variation analysis (GSVA) score of EIF3M between the tumor tissue and normal tissue (F) showed higher hazard ratio as OS, PFS, DSS, but DFI was not correlated with GSVA score. (G) KEGG pathway analysis using a gene set of EIF3M associated 200 genes (H). PFS, progression free survival; DSS, disease free survival; DFI, disease free interval.
SAAL1 correlated with EIF3M signified the survival disadvantage
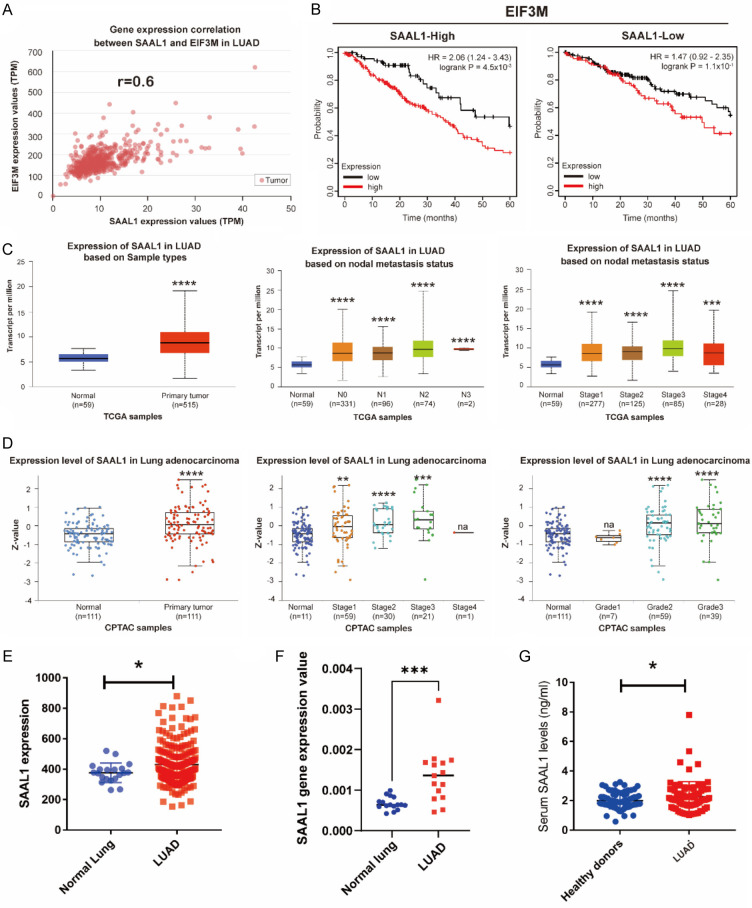
Next, we investigated the potential candidate genes which co-operated the oncogenic effect of EIF3M in LUAD. Base on the bioinformatics analysis in LUAD cohort from TCGA, SAAL1 was the highest expression gene and positively correlated with EIF3M in LUAD (r = 0.6) (Figure 4A). Cross analysis of overall survival revealed the impact of EIF3M only in SAAL1-high expression group, but not in SAAL1-low expression one, suggesting that the co-operation of SAAL1 and EIF3M had impact on the clinical outcome of LUAD (Figure 4B). Additionally, the expression levels of SAAL1 mRNA were higher in tumor parts compared with normal ones, advanced nodal metastasis status, and stages from TCGA cohort (Figure 4C). Moreover, the protein expression levels of SAAL1 were higher in tumor parts compared with normal ones, advanced stages, and higher grades but not stage nor grade dependent from CPTAC cohort (Figure 4D). The expression levels of SAAL1 were higher in GSE31210, an Asian cohort (Figure 4E). SAAL1 expression was higher in tumor parts compared with normal ones in our in-house cohort (Figure 4F). Meanwhile, collected serum SAAL1 levels were higher in LUAD patients compared with healthy donors (Figure 4G). These results showed the evidence that SAAL1 was correlated with EIF3M and exerted its functions.
Figure 4.
SAAL1 synergized with EIF3M exerted poor survival outcome. The correlation between EIF3M and SAAL1 using TCGA cohort (A). The effects of SAAL1 on EIF3M was elucdated using cross-analysis as OS (B). The gene expression of SAAL1 expression in primary tumor parts, nodal metastasis status, and cancer stage from TCGA in LUAD (C). Protein expression of SAAL1 in LUAD in primary tumor, tumor stage, and grade from CPTAC dataset for LUAD (D). SAAL1 expression in tumor parts of LUAD from GSE31210 dataset (E) and in-house cohort (F). Serum SAAL1 levels from healthy donors and LUAD patients (G). *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****P < 0.001; na = not-applicable.
Higher levels of SAAL1 expression conferred poor prognosis
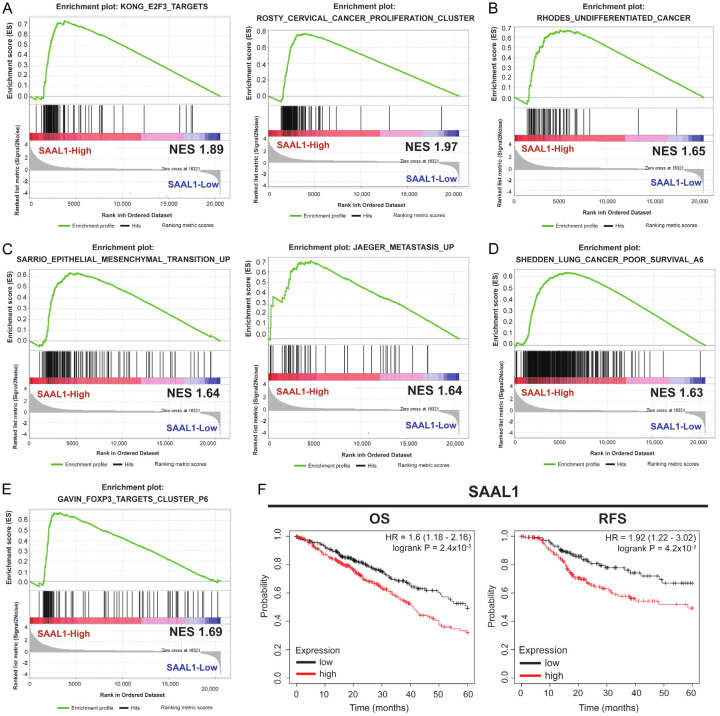
Functional investigation for SAAL1 by GSEA analysis in LUAD revealed that higher expression of SAAL1 was correlated with cancer cell proliferation (Figure 5A), cell undifferentiation (Figure 5B), epithelial mesenchymal transition and cancer metastasis (Figure 5C), and poor survival (Figure 5D). The immune infiltration affected by SAAL1 using GSCA showed positive correlation with nTreg, Effector_memory, Th1, Exhausted, etc (Figure S3B). The role of Treg involving SAAL1 was consistent with EIF3M acting as a main function in LUAD (Figure 5E). In terms of survival outcomes, higher expression levels of SAAL1 had poor OS and RFS than lower group in LUAD (Figure 5F). The OS analysis was not significant regarding chemotherapy (Figure S1C). Thererfore, SAAL1 played a critical factor regulating cell proliferation, mesenchymal transition and metastasis, and its higher expression conferred poor clinical prognosis in LUAD.
Figure 5.
Higher SAAL1 expression held poor survival in LUAD. The functions of SAAL1 through GSEA revealed assoication with cell proliferation (A), cell undifferentiation (B), epithelial mesenchymal transition and metastasis (C), poor survival (D), and Treg cells (E) in LUAD. The survival analysis of SAAL1 regarding OS and RFS (F) (HR = 1.6 and 1.92, respectively, P < 0.05).
High expression of a transcription factor, JMJD1C, resulted in high expression of EIF3M and SAAL1
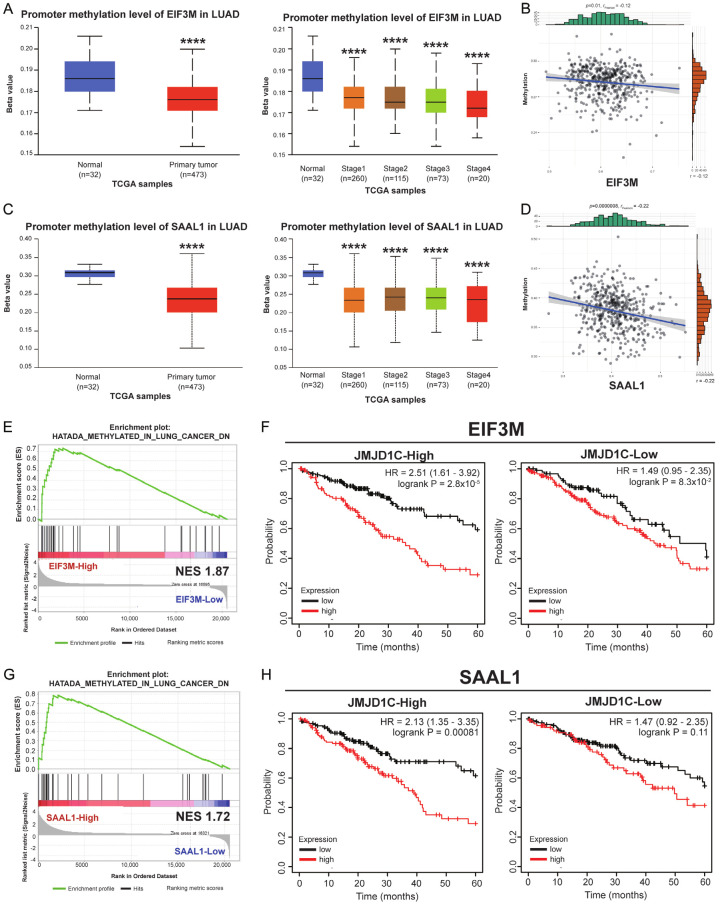
We then investigated the mechanisms involving in the dysregulation of EIF3M and SAAL1. In TCGA cohort, promoter methylation levels of EIF3M were decreased which resulted in unrepressed gene expression in primary tumor and each cancer stage in LUAD (Figure 6A). In addition, there was a negative correlation in DNA methylation status with EIF3M expression (Figure 6B). At the same time, DNA methylation levels of SAAL1 was decreased either in tumor or advanced stages (Figure 6C). DNA methylation levels and the gene expression of SAAL1 was in a negative correlation pattenr in LUAD (Figure 6D). Therefore, the regualory mechanism might lie on transcription facors, and a list of possible factors displayed in Table 1. The transcription factors or chromosome_remodeling_factors regulated EIF3M and SAAL1 were predicted by hTFtarget website, and the result showed 70 factors contributed to the transcriptional modification of both EIF3M and SAAL1. GSEA showed higher expression levels of EIF3M was related to DNA demethylation (Figure 6E). The cross analysis of OS revealed that high-expressed JMJD1C increased the impact of EIF3M on the poor OS in LUAD (Figure 6F). The higher expression levels of SAAL1 was also related to DNA demethylation (Figure 6G). Cross analysis of OS revealed that high-expressed JMJD1C enhanced the impact of SAAL1 on the poor OS in LUAD (Figure 6H). These results suggested that the epigenetic modification by JMJDC1 involved in dysregulation of EIF3M and SAAL1 in LUAD.
Figure 6.
JMJD1C regulated EIF3M and SAAL1 in LUAD. The gene expression is under the regulation of different mechanisms. Promoter methylation levels of EIF3M presented as either in normal/tumor or tumor stages (A) and the correlation between levels of methylation and EIF3M expression (B) in LUAD. The expression of SAAL1 regarding promoter methylation levels either in normal/tumor or tumor stages (C) and the correlation between levels of methylation and SAAL1 expression (D). GSEA showed the association of EIF3M promoter methylation and gene expression (E). The cross analysis revealed the impact of JMJD1C on the OS by levels of EIF3M in LUAD (F). GSEA showed the association of SAAL1 promoter methylation and gene expression (G). The cross analysis revealed the impact of JMJD1C on the OS by levels of SAAL1 in LUAD (H). ****, P < 0.001.
Table 1.
The transcription factors co-regulated the expression of EIF3M and SAAL1
| Transcription factors | EIF3M | SAAL1 | ||
|---|---|---|---|---|
|
|
|
|||
| High | Low | High | Low | |
| BPTF | 2.28* | 1.76* | 2* | 1.45 |
| TBX21 | 1.85* | 1.97* | 1.81* | 1.72* |
| SUPT5H | 1.87* | 1.81* | 2.21* | 1.65* |
| TAZ | 1.91* | 2.08* | 1.55 | 2.07* |
| ETS1 | 1.82* | 2.1* | 1.47 | 1.76* |
| ZNF574 | 2.25* | 1.64* | 2.23* | 1.73* |
| RELB | 1.85* | 2.4* | 1.86* | 1.7* |
| MYC | 1.92* | 1.91* | 2.13* | 1.52 |
| MYB | 1.98* | 1.75* | 2.19* | 1.55* |
| ZSCAN22 | 1.91* | 1.84* | 2.11* | 1.57* |
| LARP7 | 2.34* | 1.76* | 1.89* | 1.57* |
| FLI1 | 1.76* | 1.95* | 1.9* | 1.35 |
| CREB1 | 2.13* | 1.72* | 2.07* | 1.83* |
| BRD4 | 1.98* | 1.94* | 2.07* | 1.83* |
| PCGF1 | 1.86* | 2.01* | 1.4 | 2.22* |
| MAZ | 2.08* | 1.75* | 1.85* | 1.83* |
| RUNX1 | 1.46 | 2.53* | 1.51 | 1.88* |
| MAF | 1.75* | 2.14* | 1.8* | 1.62* |
| APC | 2.22* | 1.68* | 1.97* | 1.61* |
| SKI | 2.17* | 1.7* | 2.24* | 1.56* |
| MYCN | 1.75* | 2.11* | 1.56 | 1.92 |
| GTF2B | 1.93* | 2.39* | 1.72* | 1.79* |
| NFE2 | 2.14* | 1.73* | 1.84* | 1.5 |
| JMJD1C | 2.51* | 1.49 | 2.13* | 1.26 |
| SMC1A | 1.92* | 1.77* | 1.53* | 1.71* |
| PPARG | 2.41* | 1.52 | 2.33* | 1.32 |
| TP53 | 2.01* | 1.97* | 1.55 | 1.98* |
| KDM2B | 2.43* | 1.55* | 2.18* | 2.26* |
| HDAC1 | 1.83* | 2.01* | 1.55* | 1.86* |
| ZNF335 | 2.14* | 1.58* | 1.81* | 1.73* |
| USF1 | 2.5* | 1.57* | 1.84* | 1.67* |
| NFKB1 | 1.81* | 2.14* | 1.36 | 2.23* |
| NFKB2 | 2.3* | 2.16* | 1.81* | 2.02* |
| EP300 | 2.19* | 1.74* | 2.22* | 1.62* |
| BCOR | 2.26* | 1.67* | 1.67* | 1.59* |
| TAP1 | 2.13* | 1.68* | 1.88* | 1.47 |
| ZNF639 | 1.8* | 2.07* | 1.87* | 1.4 |
| NEUROD1 | 1.99* | 1.79* | ||
| GABPA | 1.67* | 2.24* | 1.64* | 1.62* |
| FOXO3 | 2.53* | 1.76* | 1.86* | 1.55* |
| FOXO1 | 1.91* | 1.93* | 1.72* | 1.84* |
| ERG | 1.32 | 2.64* | 1.63* | 1.58* |
| KLF10 | 1.91* | 2.1* | 1.47 | 1.93* |
| KLF5 | 2.52* | 1.53 | 1.88* | 1.55 |
| NR4A1 | 1.64* | 2.19* | 1.6* | 1.71* |
| HOXB13 | 2.3* | 1.71* | 2.2* | 1.58 |
| E2F1 | 1.97* | 1.77* | 1.79* | 1.7* |
| ILF3 | 1.97* | 1.77* | 1.92* | 1.56* |
| ATF2 | 2.04* | 1.83* | 2.05* | 1.67* |
| ATF3 | 1.65* | 2.45* | 1.63* | 1.73* |
P < 0.05.
Discussion
Till now, lung cancer is still a deadly disease worldwide with around 1.8 million death in 2018 [27]. The treatment relies on surgical resection and/or radiation therapy in early stage lung cancer. However, once it goes beyond resectable ranges, outcomes of lung cancer patients would be ominous. Our study demonstrated that an increase in either EIF3M or SAAL1 expression was associated with poor clinical prognosis in LUAD, suggesting its potential involvement in tumor proliferation, aggressiveness, and immunity reprograming compared with lower ones. In addition, EIF3M, correlated with SAAL1, has impacts on survival by cross analysis. Further investigation would be required to meet the high expectation for modern medicine.
Cancer development require oncogenic signaling leading to enhanced cell survival, proliferation and resistance to apoptosis, protein synthesis and translation control, and cell cycle regulation [8,13,28]. Dysregulated translations of genes for cancer formation including oncogenes, tumor suppressors and eukaryotic translation initiation factors (EIFs) are essential for tumorigenesis [7]. eIF3, the largest and most complex eIF (800 kDa) comprises 13 subunits (eIF3a-m) to form the eIF3 complex to execute their physiologic functions [8] as various cellular functions, including cell growth and regulation of the cell cycle, and the control of gene expression [14]. It has also been linked to cancer formation and progression, where its dysregulation can lead to tumor cell proliferation, [13,28] including triple negative breast cancer [29], colon cancer [12], and lung adenocarcinoma [11]. Also ablation of EIF3M would retard the translation and cancer cell proliferation [12]. This study used TCGA, in-house cohort, and bioinformatics algorithms to confirms higher expression of EIF3M (both in mRNA and protein) in lung cancer, which carried to wore clinical outcomes as overall survival (OS) and relapse free survival (RFS). Furthermore, higher EIF3M levels meant worse pathophysiologic functions as proliferation, undifferentiation, EMT, metastasis, presence of immunosuppressive Treg cells, and clinical outcomes (OS, PFS, etc) in LUAD.
SAAL1 belongs to the serum amyloid A superfamily of proteins, among which C-reactive protein serves as a clinically crucial marker for inflammation. The functions of SAAL1 have been scarcely explored, with few studies indicating its overexpression in synovial fibroblasts within rheumatoid joints. Recently, SAAL1 has also been found to participate in the progression of hepatocellular carcinoma by modulating the HGF/Met-regulated Akt/mTOR pathway [30]. Additionally, SAAL1 has been demonstrated to regulate PD-1 expression, thus influencing the efficacy of immunotherapy [19]. EIF3M has been reported to bind the 5’UTR of CAPRIN1, thereby promoting tumor growth and migration in LUAD [31]. Our findings provide the evidence that, both in TCGA and our in-house cohort, mRNA and protein levels of SAAL1 increase in the tumor parts of patients with LUAD compared with adjacent normal ones. Moreover, there is a high correlation between the expressions of SAAL1 and EIF3M. Cross-analysis further demonstrates their shared involvement as prognostic factors for OS in patients. Furthermore, functional analysis reveals that, similar to EIF3M, SAAL1 is associated with cancer undifferentiation, cell proliferation, metastasis, and even the increase of Treg cells. Our findings indicate its association with the expression of EIF3M and SAAL1. Therefore, the EIF3M-SAAL1 axis plays a crucial role in the progression of LUAD and shaping the microenvironment.
Gene expression is under tight control through epigenetic methods including DNA methylation, histone modification, non-coding RNAs, et al [17]. Epigenetic regulation have been involving in physiologic functions and cancer development [32]. The GSEA analysis suggested that high levels of EIF3M and SAAL1 were of low methylation status indicating a negative correlation between the expression levels of both EIF3M and SAAL1 and DNA methylation levels. Therefore, we found that histone lysine demethylases, JMJD1C, strongly associated with the impact of both EIF3M and SAAL1, supporting by the decrease influence of EIF3M and SAAL1 on OS in the lung cancer patients with lower JMJD1C. JMJD1C belongs to the JMJD family of proteins, which are epigenetic regulators and histone lysine demethylases [31,33]. JMJD1C has negative impacts on disease severity in rheumatoid arthritis [30]. The expression and activity of JMJD1C have been shown to regulate spermatogonia [34]. The involvement of JMJD1C in the pathogenesis of acute myeloid leukemia (AML) has been reported. The pharmacological inhibition of JMJD1C has shown the ability to trigger apoptosis in AML cells, thereby offering therapeutic direction [35]. Recent studies have also indicated that JMJD1C deletion remodels the tumor microenvironment, including increasing interferon-γ production and enhancing tumor Treg cell fragility, ultimately leading to tumor eradication [36]. So far, the impact of JMJD1C on lung cancer remains unexplored.
There are some limitations that need to be addressed. First, the interaction between EIF3M and SAAL1 was predicted using TCGA cohort. Second, the epigenetic regulator, JMJD1C, was also analyzed from hTFtarget. For the uncertainty such as whether EIF3M regulates SAAL1 translation or other mechanisms, further confirmation, eg. co-IP would be required. In addition, further investigating the effect of JMJD1C inhibitor in lung cancer treatment, and its relationship with the two oncogenes, EIF3M and SAAL1, could serve as a direction for future research.
In conclusion, our findings underscore the pivotal involvement of the eIF3m-SAAL1 axis in both the genesis of lung cancer and the sculpting of the tumor microenvironment. The observed overexpression of EIF3M/SAAL1 correlates with DNA and histone hypomethylation, implicating epigenetic dysregulation in lung carcinogenesis. Consequently, compounds targeting these pathways, such as DNA demethylation suppressors and JMJD1C inhibitors, emerge as promising candidates for the development of novel therapeutic interventions against lung cancer.
Acknowledgements
The authors would thank the Center for Research Resources and Development at Kaohsiung Medical University for the technical support. The results of gene expression profile analyzed here are in whole or part based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga. This study was supported by grants from the Ministry of Science and Technology (MOST 110-2314-B-037-124-MY3), National Science and Technology Council (NSTC 112-2314-B-037-126-MY3), and the Kaohsiung Medical University Hospital Research Funding (KMUH-104-4M23; KMUH-110-0R14; KMUHR112-2R18).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Kratzer TB, Bandi P, Freedman ND, Smith RA, Travis WD, Jemal A, Siegel RL. Lung cancer statistics, 2023. Cancer. 2024;130:1330–1348. doi: 10.1002/cncr.35128. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA, Dacic S, Jain D, Kerr KM, Lantuejoul S, Noguchi M, Papotti M, Rekhtman N, Scagliotti G, van Schil P, Sholl L, Yatabe Y, Yoshida A, Travis WD. The 2021 WHO classification of lung tumors: impact of advances since 2015. J Thorac Oncol. 2022;17:362–387. doi: 10.1016/j.jtho.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Xie S, Wu Z, Qi Y, Wu B, Zhu X. The metastasizing mechanisms of lung cancer: recent advances and therapeutic challenges. Biomed Pharmacother. 2021;138:111450. doi: 10.1016/j.biopha.2021.111450. [DOI] [PubMed] [Google Scholar]
- 4.Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med. 2021;27:1345–1356. doi: 10.1038/s41591-021-01450-2. [DOI] [PubMed] [Google Scholar]
- 5.Teimouri H, Kochugaeva MP, Kolomeisky AB. Elucidating the correlations between cancer initiation times and lifetime cancer risks. Sci Rep. 2019;9:18940. doi: 10.1038/s41598-019-55300-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoddart RW. The generation of cancer: initiation, promotion, progression and the multiple influences of the environment. Nutrition and Health. 1983;2:153–162. [Google Scholar]
- 7.Franzmann TM, Alberti S. Protein phase separation as a stress survival strategy. Cold Spring Harb Perspect Biol. 2019;11:a034058. doi: 10.1101/cshperspect.a034058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomes-Duarte A, Lacerda R, Menezes J, Romao L. eIF3: a factor for human health and disease. RNA Biol. 2018;15:26–34. doi: 10.1080/15476286.2017.1391437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu D, Mihoayi M, Ablikim Y, Arikin A. RNA splicing regulator EIF3D regulates the tumor microenvironment through immunogene-related alternative splicing in head and neck squamous cell carcinoma. Aging (Albany NY) 2024;16:5929–5948. doi: 10.18632/aging.205681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, Pittet MJ, Jain RK, Zou W, Howcroft TK, Woodhouse EC, Weinberg RA, Krummel MF. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Xiang D, Xu C, Chai R. EIF3m promotes the malignant phenotype of lung adenocarcinoma by the up-regulation of oncogene CAPRIN1. Am J Cancer Res. 2021;11:979–996. [PMC free article] [PubMed] [Google Scholar]
- 12.Goh SH, Hong SH, Hong SH, Lee BC, Ju MH, Jeong JS, Cho YR, Kim IH, Lee YS. eIF3m expression influences the regulation of tumorigenesis-related genes in human colon cancer. Oncogene. 2011;30:398–409. doi: 10.1038/onc.2010.422. [DOI] [PubMed] [Google Scholar]
- 13.Han W, Zhang C, Shi CT, Gao XJ, Zhou MH, Shao QX, Shen XJ, Wu CJ, Cao F, Hu YW, Yuan JL, Ding HZ, Wang QH, Wang HN. Roles of eIF3m in the tumorigenesis of triple negative breast cancer. Cancer Cell Int. 2020;20:141. doi: 10.1186/s12935-020-01220-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee AS, Kranzusch PJ, Cate JH. eIF3 targets cell-proliferation messenger RNAs for translational activation or repression. Nature. 2015;522:111–114. doi: 10.1038/nature14267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoang PH, Landi MT. DNA methylation in lung cancer: mechanisms and associations with histological subtypes, molecular alterations, and major epidemiological factors. Cancers (Basel) 2022;14:961. doi: 10.3390/cancers14040961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Recillas-Targa F. Cancer epigenetics: an overview. Arch Med Res. 2022;53:732–740. doi: 10.1016/j.arcmed.2022.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Viscarra JA, Wang Y, Nguyen HP, Choi YG, Sul HS. Histone demethylase JMJD1C is phosphorylated by mTOR to activate de novo lipogenesis. Nat Commun. 2020;11:796. doi: 10.1038/s41467-020-14617-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartha A, Gyorffy B. TNMplot.com: a web tool for the comparison of gene expression in normal, tumor and metastatic tissues. Int J Mol Sci. 2021;22:2622. doi: 10.3390/ijms22052622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandrashekar DS, Karthikeyan SK, Korla PK, Patel H, Shovon AR, Athar M, Netto GJ, Qin ZS, Kumar S, Manne U, Creighton CJ, Varambally S. UALCAN: an update to the integrated cancer data analysis platform. Neoplasia. 2022;25:18–27. doi: 10.1016/j.neo.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu CJ, Hu FF, Xia MX, Han L, Zhang Q, Guo AY. GSCALite: a web server for gene set cancer analysis. Bioinformatics. 2018;34:3771–3772. doi: 10.1093/bioinformatics/bty411. [DOI] [PubMed] [Google Scholar]
- 21.Liu CJ, Hu FF, Xie GY, Miao YR, Li XW, Zeng Y, Guo AY. GSCA: an integrated platform for gene set cancer analysis at genomic, pharmacogenomic and immunogenomic levels. Brief Bioinform. 2023;24:bbac558. doi: 10.1093/bib/bbac558. [DOI] [PubMed] [Google Scholar]
- 22.Dennis G Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 23.Gyorffy B. Transcriptome-level discovery of survival-associated biomarkers and therapy targets in non-small-cell lung cancer. Br J Pharmacol. 2024;181:362–374. doi: 10.1111/bph.16257. [DOI] [PubMed] [Google Scholar]
- 24.Han Y, Wang Y, Dong X, Sun D, Liu Z, Yue J, Wang H, Li T, Wang C. TISCH2: expanded datasets and new tools for single-cell transcriptome analyses of the tumor microenvironment. Nucleic Acids Res. 2022;51:D1425–D1431. doi: 10.1093/nar/gkac959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Q, Liu W, Zhang HM, Xie GY, Miao YR, Xia M, Guo AY. hTFtarget: a comprehensive database for regulations of human transcription factors and their targets. Genomics Proteomics Bioinformatics. 2020;18:120–128. doi: 10.1016/j.gpb.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edgar R, Domrachev M, Lash AE. Gene expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thandra KC, Barsouk A, Saginala K, Aluru JS, Barsouk A. Epidemiology of lung cancer. Contemp Oncol (Pozn) 2021;25:45–52. doi: 10.5114/wo.2021.103829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin Y, Long J, Sun Y, Li H, Jiang E, Zeng C, Zhu W. The function and clinical significance of eIF3 in cancer. Gene. 2018;673:130–133. doi: 10.1016/j.gene.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 29.Han W, Zhang C, Shi CT, Gao XJ, Zhou MH, Shao QX, Shen XJ, Wu CJ, Cao F, Hu YW, Yuan JL, Ding HZ, Wang QH, Wang HN. Roles of eIF3m in the tumorigenesis of triple negative breast cancer. Cancer Cell Int. 2020;20:141. doi: 10.1186/s12935-020-01220-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin Y, Yang X, Wu S, Ding X, Zhu H, Long X, Wang Y, Zhai S, Chen Y, Che N, Chen J, Wang X. Jmjd1c demethylates STAT3 to restrain plasma cell differentiation and rheumatoid arthritis. Nat Immunol. 2022;23:1342–1354. doi: 10.1038/s41590-022-01287-y. [DOI] [PubMed] [Google Scholar]
- 31.Manni W, Jianxin X, Weiqi H, Siyuan C, Huashan S. JMJD family proteins in cancer and inflammation. Signal Transduct Target Ther. 2022;7:304. doi: 10.1038/s41392-022-01145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butler JS, Koutelou E, Schibler AC, Dent SY. Histone-modifying enzymes: regulators of developmental decisions and drivers of human disease. Epigenomics. 2012;4:163–177. doi: 10.2217/epi.12.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sui Y, Gu R, Janknecht R. Crucial functions of the JMJD1/KDM3 epigenetic regulators in cancer. Mol Cancer Res. 2021;19:3–13. doi: 10.1158/1541-7786.MCR-20-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakajima R, Okano H, Noce T. JMJD1C exhibits multiple functions in epigenetic regulation during spermatogenesis. PLoS One. 2016;11:e0163466. doi: 10.1371/journal.pone.0163466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qi D, Wang J, Zhao Y, Yang Y, Wang Y, Wang H, Wang L, Wang Z, Xu X, Hu Z. JMJD1C-regulated lipid synthesis contributes to the maintenance of MLL-rearranged acute myeloid leukemia. Leuk Lymphoma. 2022;63:2149–2160. doi: 10.1080/10428194.2022.2068004. [DOI] [PubMed] [Google Scholar]
- 36.Long X, Zhang S, Wang Y, Chen J, Lu Y, Hou H, Lin B, Li X, Shen C, Yang R, Zhu H, Cui R, Cao D, Chen G, Wang D, Chen Y, Zhai S, Zeng Z, Wu S, Lou M, Chen J, Zou J, Zheng M, Qin J, Wang X. Targeting JMJD1C to selectively disrupt tumor T(reg) cell fitness enhances antitumor immunity. Nat Immunol. 2024;25:525–536. doi: 10.1038/s41590-024-01746-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.