Abstract
Objective: To evaluate the potential of leukocyte-specific protein 1 (LCP1) and adenosine diphosphate-dependent glucokinase (ADPGK) as predictive biomarkers for immunotherapy-related adverse events in late-stage non-small cell lung cancer (NSCLC) patients with the KARS G12C mutation undergoing treatment with programmed cell death protein-1 (PD-1) monoclonal antibodies. Methods: A total of 160 late-stage NSCLC patients with the KARS G12C mutation receiving PD-1 monoclonal antibody treatment were retrospectively analyzed. LCP1 and ADPGK expression levels were assessed at both mRNA and protein levels using validated methods. Statistical analyses, including correlation analysis, logistic regression, and receiver operating characteristic (ROC) curve analysis, were conducted to explore the association between LCP1 and ADPGK expression levels and the occurrence of immunotherapy-related adverse events. Results: The mRNA levels of LCP1 (2.43 ± 0.72 vs. 2.14 ± 0.67, t=2.311, P=0.023) and ADPGK (2.31 ± 0.61 vs. 1.98 ± 0.59, t=3.145, P=0.002) were significantly elevated in patients with adverse reactions. Similarly, protein levels of LCP1 (1.22 ± 0.28 vs. 1.07 ± 0.25, t=3.179, P=0.002) and ADPGK (1.01 ± 0.18 vs. 0.93 ± 0.19, t=2.488, P=0.015) were higher in this group. Correlation and logistic regression analyses revealed positive correlations between LCP1 and ADPGK mRNA levels and adverse event occurrence (LCP1: rho=0.186, P=0.019, OR=1.842; ADPGK: rho=0.246, P=0.002, OR=2.549). Protein levels of LCP1 and ADPGK also correlated with immunotherapy-related adverse events (LCP1: rho=0.254, P=0.001, OR=9.554; ADPGK: rho=0.19, P=0.016, OR=10.058). The combined assessment of LCP1 and ADPGK expression showed strong predictive power for identifying patients at increased risk of adverse events during PD-1 treatment (AUC=0.808), with the validation group achieving an AUC of 0.751. Conclusion: LCP1 and ADPGK are potential independent predictive biomarkers for immunotherapy-related adverse events in late-stage NSCLC patients with the KARS G12C mutation. Their combined assessment may offer a valuable tool for risk stratification during PD-1 monoclonal antibody treatment.
Keywords: Immunotherapy-related adverse events, late-stage non-small cell lung cancer, KARS G12C mutation
Introduction
The advent of immune checkpoint inhibitors, particularly programmed cell death protein-1 (PD-1) inhibitors, has transformed cancer treatment, offering promising outcomes for patients with advanced non-small cell lung cancer (NSCLC) [1-3]. These agents are now central to first-line and subsequent therapies, either as monotherapy or in combination with other immunotherapies or chemotherapy, showing superior efficacy and manageable toxicity profiles compared to traditional chemotherapy [4]. The inclusion of PD-1 inhibitors in clinical guidelines reflects their established role in improving overall survival and progression-free survival in NSCLC patients [5]. While immunotherapy has significantly improved survival rates and treatment efficacy, especially in patients with late-stage NSCLC [6,7], it is also associated with immune-related adverse events, which can impact patient well-being and disrupt treatment [8-10]. Biomarker-based risk assessment could play a crucial role in predicting and mitigating these adverse events, enabling personalized treatment approaches and proactive management.
Leukocyte-specific protein 1 (LCP1), also known as L-plastin, is a cytoskeletal protein involved in T-cell activation and migration [11,12]. Elevated LCP1 expression has been linked to effector T-cell function and is associated with autoimmune diseases and inflammatory conditions [13]. Previous studies suggest LCP1’s involvement in regulating immune response dynamics, making it a compelling candidate for use as a predictive biomarker for immunotherapy-related adverse events [14]. Similarly, adenosine diphosphate-dependent glucokinase (ADPGK), an enzyme crucial for energy metabolism, glycolysis, and redox balance, represents a novel target for investigating the relationship between cellular metabolic pathways and immune-related adverse events during PD-1 inhibitor therapy [15]. The role of ADPGK in modulating immune function, particularly in the context of immunotherapy, highlights the growing interest in the interaction between cellular metabolism and immune response regulation.
Despite the therapeutic benefits, the risk of immunotherapy-related adverse events with PD-1 inhibitors underscores the need for reliable predictive biomarkers [16]. Identifying such biomarkers could help clinicians stratify patients based on their risk of developing these adverse events, allowing for personalized treatment planning and proactive monitoring. This study’s focus on LCP1 and ADPGK expression as potential predictors of immunotherapy-related adverse events in patients with the KARS G12C mutation treated with PD-1 inhibitors addresses a critical gap in understanding immunotherapy toxicities. The findings may lead to the development of refined risk assessment tools, contributing to safer and more effective immunotherapy practices for NSCLC patients.
Materials and methods
Study population
This study retrospectively analyzed clinical data from January 2021 to December 2023 for patients with late-stage NSCLC harboring the KRAS G12C mutation admitted to Peking University Shougang Hospital. A total of 160 patients received PD-1 monoclonal antibody treatment, of whom 113 did not experience immune-related adverse events, while 47 did. During PD-1 inhibitor therapy, patients were closely monitored for vital signs, and the occurrence of fever, chills, rash, diarrhea, palpitations, chest tightness, or similar symptoms was classified as immune-related adverse events. This study was approved by the Ethics Committee of Peking University Shougang Hospital, with a waiver of informed consent.
Inclusion and exclusion criteria
Inclusion criteria: Patients over 18 years of age with histologically or cytologically confirmed unresectable advanced or metastatic NSCLC [17], carrying the KRAS G12C mutation, receiving PD-1 monoclonal antibody treatment, having normal cognitive function, and possessing complete medical records.
Exclusion criteria: Patients using immunosuppressive agents other than PD-1 inhibitors, those with primary malignancies at other sites, severe liver or kidney dysfunction, concomitant cardiovascular or cerebrovascular diseases, chronic infections, non-healing wounds, local suppuration, brain metastases, or communication impairments.
General information acquisition
General information from the validation group and study patients was obtained from medical records, including age, gender, BMI, smoking history, alcohol consumption, comorbidities (e.g., hypertension and diabetes), Eastern Cooperative Oncology Group (ECOG) performance status, family cancer history, history of central nervous system disease, liver metastasis status, histological classification, and disease staging.
Baseline imaging examination
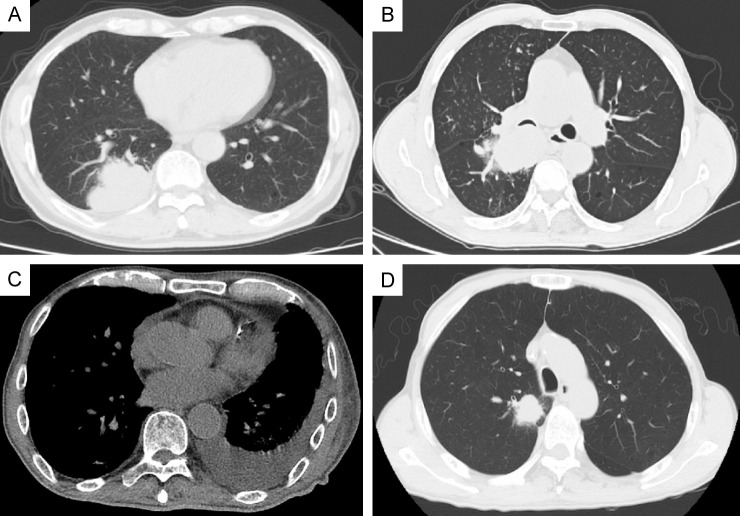
Baseline chest computed tomography (CT) images were collected and analyzed for four morphological features (Figure 1): lobulation, bronchial invasion, pleural effusion, and spiculation. CT scans were performed using GE Discovery CT750, LightSpeed VCT, Optima CT660, NeuViz 128, and Philips Brilliance 64 scanners. The scanning range extended from the thoracic inlet to the bilateral adrenal glands. The tube voltage was set at 120 kV, with automatic tube current modulation, and a scan layer thickness of 5 mm. Lung window settings were 1500 Hounsfield Units (HU) for window level and 500 HU for window width, while mediastinal window settings were 300 HU for window level and 35 HU for window width. All images were transferred to the medical image management system. At least two experienced radiologists reviewed the images, and in cases of disagreement, a joint review by two physicians was conducted, followed by re-evaluation by a senior radiologist.
Figure 1.
Representative morphological computed tomography features of involved patients. A: Lobulation; B: Bronchial invasion; C: Pleural effusion; D: Spiculation.
Baseline hematological examination
Baseline hematological tests were performed by collecting 5 ml of fasting venous blood from patients at 8 am. The blood was analyzed using a fully automated blood analyzer (DxH800 by Beckman Coulter), measuring parameters such as absolute neutrophil count, absolute lymphocyte count, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and absolute eosinophil count.
Baseline inflammatory factor analysis
After routine blood tests, the remaining blood samples were stored in the clinical sample bank at -80°C for future use. These samples were centrifuged at 3000 rpm for 10 minutes under low-temperature, high-speed conditions to isolate the serum. The serum samples were stored at -80°C until analysis. Enzyme-linked immunosorbent assay (ELISA) was used to measure the levels of IFN-γ (ab174443, Abcam, UK), TNF-α (ab181421, Abcam, UK), IL-10 (ab185986, Abcam, UK), and IL-4 (ab215089, Abcam, UK).
Pulmonary function testing
Baseline pulmonary function tests were conducted after patients had rested for 20 minutes. The tests measured vital capacity (VC), forced vital capacity (FVC), forced expiratory volume in one second (FEV1), and maximum voluntary ventilation (MVV). Testing was performed while the patient was seated, with instructions provided to ensure proper cooperation. The tests used the Quark PFT4 pulmonary function testing device (COSMED, Italy) at a room temperature of 22°C.
Detection of ADPGK and LCP1
ADPGK and LCP1 detection was conducted using biopsy tissue samples from both patient groups and the validation group. RNA was extracted from the biopsy tissue using the RNAsimple Total RNA Kit (Tiangen Biotech). The RNA was then reverse-transcribed to cDNA using the FastKing RT kit (Tiangen Biotech) and subjected to real-time quantitative polymerase chain reaction (RT-qPCR) with SuperReal PreMix Plus (SYBR Green). Data analysis followed the 2-ΔΔCt method. Para-cancer tissues were used for normalization. The primer sequences used were: ADPGK: Forward primer: 5’-CGTGGCAGTGGGAGTCAAT-3’; Reverse primer: 5’-TGAATGCAGAATGCTGTGATCT-3’. LCP1: Forward primer: 5’-GATCAGTGTCCGATGAGGAAATG-3’; Reverse primer: 5’-CCAGATCACCTGTAGCCATCA-3’. GAPDH: Forward primer: 5’-ACGGATTTGGTCGTATTGGG-3’; Reverse primer: 5’-CGCTCCTGGAAGATGGTGAT-3’.
Protein levels of ADPGK and LCP1 in tissue samples were assessed using an ELISA kit (KL-EL001380HU and KL-LCP1-Hu, KALANG, China) according to the manufacturer’s instructions.
Statistical analysis
Patients were grouped based on the occurrence of adverse reactions during treatment. Following the principle of EPV (Events per Variable) >10, the sample sizes in both groups met the requirements for model stability and prediction accuracy.
Statistical analysis was performed using SPSS 29.0 (SPSS Inc., Chicago, IL, USA). Categorical data were presented as [n (%)]. For sample sizes ≥40 and theoretical frequency (T) ≥5, the chi-square test was performed using the basic formula. When the sample size was ≥40 but 1≤T<5, the chi-square test was conducted with a correction formula. For sample sizes <40 or T<1, Fisher’s exact test was used. Normality of continuous variables was tested with the Shapiro-Wilk test. Normally distributed continuous variables were expressed as mean ± standard deviation (X±sd), and the t-test with corrected variance was applied. Non-normally distributed data were presented as median (25th percentile, 75th percentile), and the Wilcoxon rank-sum test was used. A two-tailed P<0.05 was considered statistically significant. Spearman correlation analysis was used to examine the relationship between LCP1 mRNA expression, ADPGK mRNA expression, LCP1 protein levels, ADPGK protein levels, and the occurrence of immune-related adverse events. The diagnostic performance of LCP1 mRNA expression, ADPGK mRNA expression, LCP1 protein levels, and ADPGK protein levels, alone or in combination, in predicting immune-related adverse events was evaluated using the area under the receiver operating characteristic (ROC) curve (AUC). A joint prediction model was constructed using the xgbTree method.
Results
Comparison of general information
An analysis of the demographic and general information of 160 late-stage NSCLC patients with the KRAS G12C mutation treated with PD-1 inhibitors identified 47 patients who experienced adverse reactions and 113 who did not (Table 1). There were no statistically significant differences between the two groups in terms of age (58.52 ± 5.24 vs. 59.81 ± 4.75 years, t=1.523, P=0.131), smoking history (29.2% vs. 25.53%, χ2=0.077, P=0.781), alcohol consumption (16.81% vs. 19.15%, χ2=0.016, P=0.900), or pre-existing comorbidities, including hypertension (10.62% vs. 10.64%, χ2=0, P=1) and diabetes (15.93% vs. 19.15%, χ2=0.069, P=0.792). Similarly, there were no differences in ECOG performance status (P=1), family cancer history (22.12% vs. 19.15%, χ2=0.043, P=0.836), history of CNS involvement (30.09% vs. 34.04%, χ2=0.093, P=0.761), liver metastasis (16.81% vs. 19.15%, χ2=0.016, P=0.900), histological classification, or disease stage (P=1). These results indicate no significant statistical differences between the two groups, making them comparable. However, there were significant differences in gender (χ2=3.888, P=0.049) and BMI (22.99 ± 3.06 vs. 24.09 ± 3.15, t=2.020, P=0.047), suggesting that these factors may serve as potential risk predictors for immune-related adverse reactions.
Table 1.
Comparison of general information and demographic characteristics
| Parameter | No adverse reactions (n=113) | Adverse reactions (n=47) | t/χ2 | P |
|---|---|---|---|---|
| Age (years) | 58.52 ± 5.24 | 59.81 ± 4.75 | 1.523 | 0.131 |
| Gender (M/F) | 69 (61.06%)/44 (38.94%) | 20 (42.55%)/27 (57.45%) | 3.888 | 0.049 |
| Body mass index (kg/m2) | 22.99 ± 3.06 | 24.09 ± 3.15 | 2.020 | 0.047 |
| Smoking history | 33 (29.2%) | 12 (25.53%) | 0.077 | 0.781 |
| Drinking history | 19 (16.81%) | 9 (19.15%) | 0.016 | 0.9 |
| Comorbidities (%) | ||||
| Hypertension | 12 (10.62%) | 5 (10.64%) | 0 | 1 |
| Diabetes | 18 (15.93%) | 9 (19.15%) | 0.069 | 0.792 |
| ECOG performance status | 0 | 1 | ||
| 0 | 37 (32.74%) | 15 (31.91%) | ||
| 1 | 76 (67.26%) | 32 (68.09%) | ||
| Family history of cancer | 25 (22.12%) | 9 (19.15%) | 0.043 | 0.836 |
| History of CNS involvement | 34 (30.09%) | 16 (34.04%) | 0.093 | 0.761 |
| Liver metastasis | 19 (16.81%) | 9 (19.15%) | 0.016 | 0.9 |
| Histology | 0 | 1 | ||
| Squamous | 2 (1.77%) | 1 (2.13%) | ||
| Non-squamous | 111 (98.23%) | 46 (97.87%) | ||
| Disease stage | 0 | 1 | ||
| Locally advanced and unresectable | 5 (4.42%) | 2 (4.26%) | ||
| Metastatic | 108 (95.58%) | 45 (95.74%) |
Note: ECOG: Eastern Cooperative Oncology Group.
Comparison of imaging characteristics
Figure 1 illustrates the imaging evaluation of four typical features in both patient groups: tumor lobulation, bronchial invasion, pleural effusion, and spiculation. No statistically significant differences were observed between the groups (P>0.05) (Table 2). These findings suggest that baseline imaging characteristics are not associated with the risk of immune-related adverse events in the treatment of advanced NSCLC with the KRAS G12C mutation using PD-1 inhibitors.
Table 2.
Comparison of imaging characteristics
| Parameter | No adverse reactions (n=113) | Adverse reactions (n=47) | t/χ2 | P |
|---|---|---|---|---|
| Tumor Lobe (Y/N) | 51 (45.13%)/62 (54.87%) | 23 (48.94%)/24 (51.06%) | 0.07 | 0.791 |
| Bronchial Invasion (Y/N) | 35 (30.97%)/78 (69.03%) | 16 (34.04%)/31 (65.96%) | 0.037 | 0.847 |
| Pleural Effusion (Y/N) | 15 (13.27%)/98 (86.73%) | 5 (10.64%)/42 (89.36%) | 0.039 | 0.844 |
| Spiculation (Y/N) | 42 (37.17%)/71 (62.83%) | 16 (34.04%)/31 (65.96%) | 0.038 | 0.846 |
Comparison of routine hematological examination
Baseline routine hematological examinations revealed no significant differences between the two groups in terms of absolute neutrophil count, absolute lymphocyte count, or neutrophil-lymphocyte ratio (all P>0.05) (Table 3). However, significant differences were observed in the platelet-lymphocyte ratio (195.13 ± 66.98 vs. 172.24 ± 65.56, t=1.999, P=0.049) and absolute eosinophil count (1.41 ± 0.81 vs. 1.68 ± 0.73, t=2.040, P=0.044). These results indicate that the platelet-lymphocyte ratio and absolute eosinophil count may be associated with the risk of immune-related adverse events in the treatment of advanced cancer with the KRAS G12C mutation using PD-1 inhibitors.
Table 3.
Comparison of routine hematological examination
| Parameter | No adverse reactions (n=113) | Adverse reactions (n=47) | t/χ2 | P |
|---|---|---|---|---|
| Neutrophil Count | 5.11 ± 1.48 | 5.42 ± 1.56 | 1.146 | 0.255 |
| Lymphocyte Count | 1.53 ± 0.55 | 1.44 ± 0.47 | 1.079 | 0.283 |
| Neutrophil to Lymphocyte Ratio | 4.29 ± 2.51 | 4.57 ± 2.84 | 0.578 | 0.565 |
| Platelet to Lymphocyte Ratio | 195.13 ± 66.98 | 172.24 ± 65.56 | 1.999 | 0.049 |
| Absolute Eosinophil Count | 1.41 ± 0.81 | 1.68 ± 0.73 | 2.040 | 0.044 |
Comparison of inflammatory factors
Baseline inflammatory factor analyses, including tumor necrosis factor-alpha (TNF-α), interleukin-10 (IL-10), and interleukin-4 (IL-4), showed no statistically significant differences between the two groups (all P>0.05) (Table 4). These results indicate that baseline inflammatory levels were consistent across both patient groups.
Table 4.
Comparison of inflammatory factors
| Parameter | No adverse reactions (n=113) | Adverse reactions (n=47) | t/χ2 | P |
|---|---|---|---|---|
| TNF-α/(ng·L-1) | 18.54 ± 5.86 | 17.83 ± 5.51 | 0.723 | 0.471 |
| IL-10 (ng·L-1) | 14.02 ± 4.34 | 14.63 ± 4.24 | 0.833 | 0.407 |
| IL-4 (ng·L-1) | 16.67 ± 4.38 | 15.83 ± 4.01 | 1.162 | 0.248 |
Note: TNF-α: tumor necrosis factor-α; IL-10: Interleukin-10; IL-4: Interleukin-4.
Comparison of lung function examination
Similarly, in the pulmonary function tests, there were no statistically significant differences between the two groups for vital capacity (VC), forced vital capacity (FVC), forced expiratory volume in one second (FEV1), or maximum voluntary ventilation (MVV) (Table 5) (all P>0.05).
Table 5.
Comparison of lung function examination
| Parameter | No adverse reactions (n=113) | Adverse reactions (n=47) | t/χ2 | P |
|---|---|---|---|---|
| Vital Capacity (ml) | 98.71 ± 9.79 | 96.11 ± 8.92 | 1.634 | 0.106 |
| Forced Vital Capacity (%) | 99.36 ± 8.33 | 97.34 ± 8.03 | 1.434 | 0.155 |
| Forced Expiratory Volume in 1 second (%) | 80.63 ± 6.81 | 81.45 ± 5.97 | 0.759 | 0.45 |
| Maximal Voluntary Ventilation (L) | 87.33 ± 4.61 | 86.62 ± 4.08 | 0.955 | 0.342 |
Comparison of expression levels of LCP1 and ADPGK
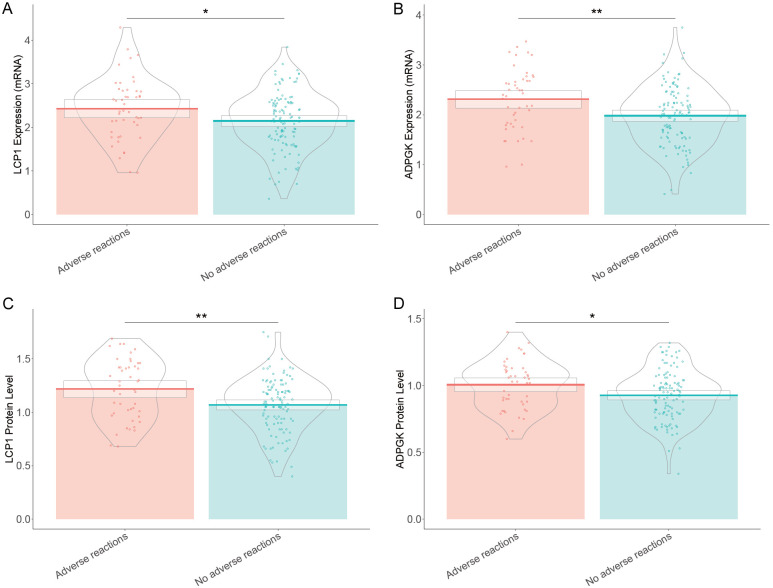
The mRNA expression levels of LCP1 (2.43 ± 0.72 vs. 2.14 ± 0.67, t=2.311, P=0.023) and ADPGK (2.31 ± 0.61 vs. 1.98 ± 0.59, t=3.145, P=0.002) were significantly higher in the group that experienced adverse reactions (Figure 2). Likewise, the protein levels of LCP1 (1.22 ± 0.28 vs. 1.07 ± 0.25, t=3.179, P=0.002) and ADPGK (1.01 ± 0.18 vs. 0.93 ± 0.19, t=2.488, P=0.015) were also significantly elevated in patients with adverse reactions. These findings suggest that higher expression levels of both LCP1 and ADPGK, at both mRNA and protein levels, are associated with an increased risk of immunotherapy-related adverse events in late-stage NSCLC patients with the KRAS G12C mutation treated with PD-1 inhibitors.
Figure 2.
Comparison of expression levels of LCP1 and ADPGK in late-stage non-small cell lung cancer patients with KARS G12C mutation treated with PD-1 inhibitors. Note: LCP1: Leukocyte-specific protein 1; ADPGK: adenosine diphosphate-dependent glucokinase. *: P<0.05; **: P<0.01.
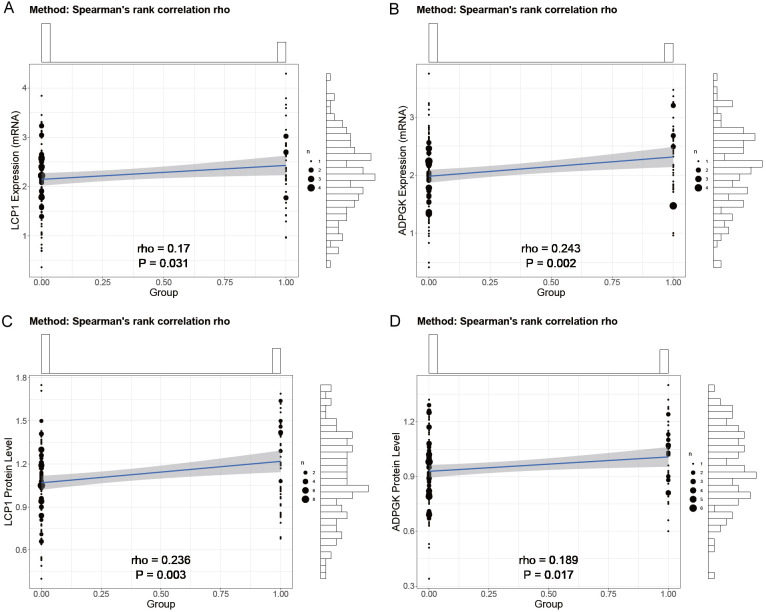
Correlation analysis
In our investigation of the relationship between LCP1 and ADPGK expression levels and immunotherapy-related adverse events in late-stage NSCLC patients with the KRAS G12C mutation treated with PD-1 inhibitors, correlation analyses showed statistically significant positive correlations between the mRNA expression levels of LCP1 (rho=0.186, P=0.019) and ADPGK (rho=0.246, P=0.002) and the occurrence of adverse events (Table 6; Figure 3). Additionally, similar positive correlations were observed between the protein levels of LCP1 (rho=0.254, P=0.001) and ADPGK (rho=0.19, P=0.016) and immunotherapy-related adverse events. These results indicate that higher expression levels of LCP1 and ADPGK at both mRNA and protein levels are significantly correlated with an increased likelihood of experiencing immunotherapy-related adverse events. The other four risk factors showed relatively insignificant correlations with the occurrence of immune-related adverse events, and thus only the four factors with more pronounced effects are discussed in detail.
Table 6.
Correlation analysis of risk factors with immunotherapy-related
| Parameter | rho | P |
|---|---|---|
| Gender (M/F) | -0.17 | 0.032 |
| Body mass index (kg/m2) | 0.161 | 0.043 |
| Platelet to Lymphocyte Ratio | -0.156 | 0.049 |
| Absolute Eosinophil Count | 0.154 | 0.052 |
| LCP1 Expression (mRNA) | 0.186 | 0.019 |
| ADPGK Expression (mRNA) | 0.246 | 0.002 |
| LCP1 Protein Level | 0.254 | 0.001 |
| ADPGK Protein Level | 0.19 | 0.016 |
Note: LCP1: Leukocyte-specific protein 1; ADPGK: adenosine diphosphate-dependent glucokinase; mRNA: Messenger Ribonucleic Acid.
Figure 3.
Correlation analysis of LCP1 and ADPGK expression with immunotherapy-related. Note: LCP1: Leukocyte-specific protein 1; ADPGK: adenosine diphosphate-dependent glucokinase.
Logistic regression analysis
Logistic regression analysis revealed that LCP1 and ADPGK expression levels were significantly associated with an increased risk of adverse reactions in late-stage NSCLC patients with the KRAS G12C mutation treated with PD-1 monoclonal antibodies (Table 7). Specifically, the mRNA expression of LCP1 (coef=0.611, odds ratio =1.842, B=2.305, beta=0.611, P=0.021) and ADPGK (coef=0.936, odds ratio =2.549, B=3.022, beta=0.936, P=0.003), as well as the protein levels of LCP1 (coef=2.257, odds ratio =9.554, B=3.111, beta=2.257, P=0.002) and ADPGK (coef=2.308, odds ratio =10.058, B=2.364, beta=2.308, P=0.018), were statistically significant predictors of the likelihood of experiencing immunotherapy-related adverse events. These results suggest that LCP1 and ADPGK expression levels serve as independent risk factors for adverse reactions in late-stage NSCLC patients with the KRAS G12C mutation undergoing PD-1 monoclonal antibody treatment.
Table 7.
Risk prediction on PD-1 monoclonal antibody treatment-related adverse reactions in late-stage non-small cell lung cancer with KARS G12C mutation
| Parameter | coef | Odds ratio | B | beta | P Value |
|---|---|---|---|---|---|
| LCP1 Expression (mRNA) | 0.611 | 1.842 | 2.305 | 0.611 | 0.021 |
| ADPGK Expression (mRNA) | 0.936 | 2.549 | 3.022 | 0.936 | 0.003 |
| LCP1 Protein Level | 2.257 | 9.554 | 3.111 | 2.257 | 0.002 |
| ADPGK Protein Level | 2.308 | 10.058 | 2.364 | 2.308 | 0.018 |
Note: LCP1: Leukocyte-specific protein 1; ADPGK: adenosine diphosphate-dependent glucokinase; mRNA: Messenger Ribonucleic Acid.
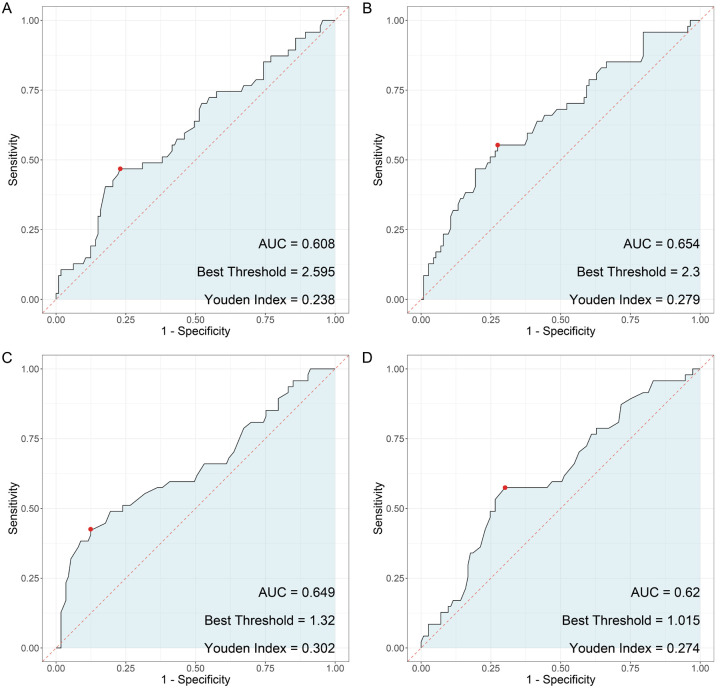
ROC analysis
In our investigation of the predictive value of LCP1 and ADPGK expression levels for PD-1 monoclonal antibody treatment-related adverse reactions in late-stage NSCLC patients with the KRAS G12C mutation, the analysis revealed notable sensitivities and specificities (Figure 4). The mRNA expression levels of LCP1 showed a sensitivity of 0.468 and a specificity of 0.770, with an area under the curve (AUC) of 0.608 and a Youden index of 0.238. Similarly, the mRNA expression levels of ADPGK exhibited a sensitivity of 0.553 and a specificity of 0.726, with an AUC of 0.654 and a Youden index of 0.279. The protein levels of LCP1 demonstrated a sensitivity of 0.426 and a specificity of 0.876, with an AUC of 0.649 and a Youden index of 0.302. Additionally, the protein levels of ADPGK showed a sensitivity of 0.574 and a specificity of 0.699, with an AUC of 0.620 and a Youden index of 0.273.
Figure 4.
Predictive value of combined LCP1 and ADPGK expression levels on PD-1 monoclonal antibody treatment-related adverse reactions in late-stage non-small cell lung cancer with KARS G12C mutation. Note: LCP1: Leukocyte-specific protein 1; ADPGK: adenosine diphosphate-dependent glucokinasel; KARS: lysyl-tRNA synthetase gene.
Combined model
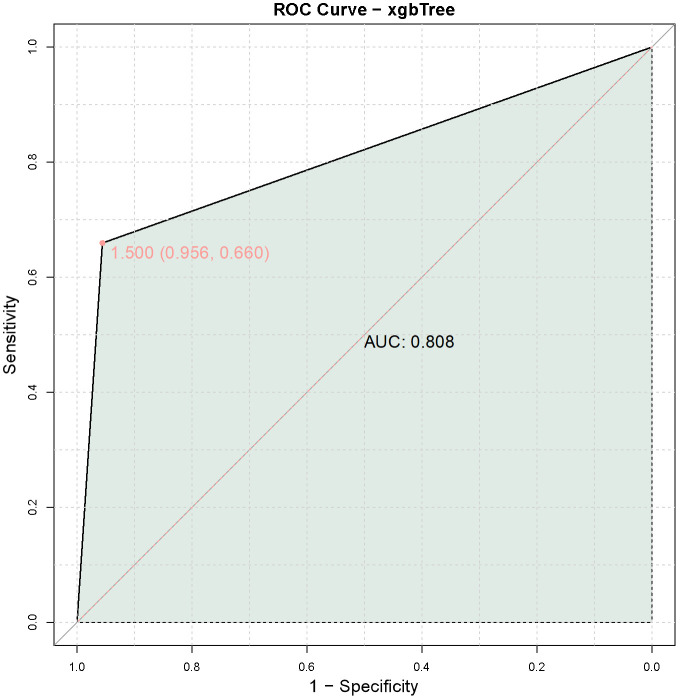
A combined model was constructed using the expression levels of LCP1 and ADPGK to predict immune-related adverse events in late-stage NSCLC patients with the KRAS G12C mutation undergoing PD-1 monoclonal antibody treatment. The results demonstrated an AUC of 0.808, indicating that the combined model of LCP1 and ADPGK expression levels provides high predictive value for immune-related adverse events in this patient population (Figure 5).
Figure 5.
Predictive value of the combined model of LCP1 and ADPGK expression levels for PD-1 monoclonal antibody treatment-related immune-related adverse events in late-stage non-small cell lung cancer with KARS G12C mutation. Note: LCP1: Leukocyte-specific protein 1; ADPGK: adenosine diphosphate-dependent glucokinasel; KARS: lysyl-tRNA synthetase gene; AUC: Area Under the Curve.
Validation group patient data
An additional cohort of 127 late-stage NSCLC patients, all with the KRAS G12C mutation and treated with PD-1 inhibitors, was designated as the validation group. Among them, 94 patients did not experience immune-related adverse events, while 33 patients did. No statistically significant differences were observed between patients with and without adverse reactions in terms of age, gender distribution, BMI, smoking history, alcohol consumption, comorbidities (hypertension and diabetes), ECOG performance status, family cancer history, CNS involvement, liver metastasis, histology, or disease stage (P>0.05) (Table 8). However, significant differences were observed in the expression levels of LCP1 mRNA (2.28 ± 0.36 vs. 2.48 ± 0.43, t=2.416, P=0.020), ADPGK mRNA (1.89 ± 0.69 vs. 2.31 ± 0.78, t=2.733, P=0.009), LCP1 protein (1.16 ± 0.24 vs. 1.29 ± 0.26, t=2.398, P=0.020), and ADPGK protein (0.97 ± 0.21 vs. 1.07 ± 0.22, t=2.338, P=0.023) between patients with and without adverse reactions to PD-1 inhibitors.
Table 8.
Patients data in the validation group
| Parameter | No adverse reactions (n=94) | Adverse reactions (n=33) | t/χ2 | P |
|---|---|---|---|---|
| Age (years) | 57.68 ± 3.18 | 58.91 ± 4.09 | 1.568 | 0.124 |
| Gender (M/F) | 52 (55.32%)/42 (44.68%) | 14 (42.42%)/19 (57.58%) | 1.151 | 0.283 |
| Body mass index (kg/m2) | 22.19 ± 2.83 | 22.67 ± 1.38 | 1.259 | 0.211 |
| Smoking history | 18 (19.15%)/76 (80.85%) | 5 (15.15%)/28 (84.85%) | 0.063 | 0.802 |
| Drinking history | 15 (15.96%)/79 (84.04%) | 6 (18.18%)/27 (81.82%) | 0.001 | 0.981 |
| Comorbidities (%) | ||||
| Hypertension | 11 (11.7%) | 5 (15.15%) | 0.044 | 0.835 |
| Diabetes | 15 (15.96%) | 6 (18.18%) | 0.001 | 0.981 |
| ECOG performance status | 0.009 | 0.923 | ||
| 0 | 37 (39.36%) | 12 (36.36%) | ||
| 1 | 57 (60.64%) | 21 (63.64%) | ||
| Family history of cancer | 24 (25.53%) | 7 (21.21%) | 0.068 | 0.794 |
| History of CNS involvement | 32 (34.04%) | 9 (27.27%) | 0.249 | 0.618 |
| Liver metastasis | 18 (19.15%) | 5 (15.15%) | 0.063 | 0.802 |
| Histology | 0.285 | 0.594 | ||
| Squamous | 2 (2.13%) | 2 (6.06%) | ||
| Non-squamous | 92 (97.87%) | 31 (93.94%) | ||
| Disease stage | 0.003 | 0.955 | ||
| Locally advanced and unresectable | 5 (5.32%) | 1 (3.03%) | ||
| Metastatic | 89 (94.68%) | 32 (96.97%) | ||
| LCP1 Expression (mRNA) | 2.28 ± 0.36 | 2.48 ± 0.43 | 2.416 | 0.020 |
| ADPGK Expression (mRNA) | 1.89 ± 0.69 | 2.31 ± 0.78 | 2.733 | 0.009 |
| LCP1 Protein Level | 1.16 ± 0.24 | 1.29 ± 0.26 | 2.398 | 0.020 |
| ADPGK Protein Level | 0.97 ± 0.21 | 1.07 ± 0.22 | 2.338 | 0.023 |
Note: ECOG: Eastern Cooperative Oncology Group; LCP1: Leukocyte-specific protein 1; ADPGK: adenosine diphosphate-dependent glucokinase; mRNA: Messenger Ribonucleic Acid.
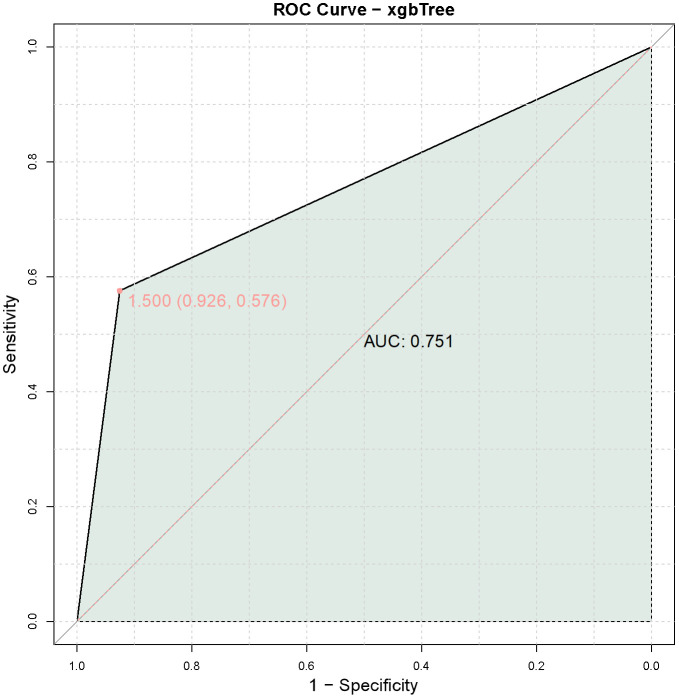
The ROC curve analysis of the validation group showed an AUC of 0.751, further supporting the high predictive value of the combined model of LCP1 and ADPGK expression levels for immune-related adverse events in late-stage NSCLC patients with the KRAS G12C mutation treated with PD-1 monoclonal antibodies (Figure 6).
Figure 6.
Predictive value of the combined model of LCP1 and ADPGK expression levels for PD-1 monoclonal antibody treatment-related immune-related adverse events in the validation group. Note: LCP1: Leukocyte-specific protein 1; ADPGK: adenosine diphosphate-dependent glucokinasel; AUC: Area Under the Curve.
Discussion
The identification of predictive biomarkers for immunotherapy-related adverse events is critical in oncology, particularly in the context of NSCLC, where immunotherapy has become a cornerstone of treatment [18-20]. Immune checkpoint inhibitors, such as PD-1 inhibitors, have transformed the management of advanced NSCLC, improving survival and treatment outcomes for select patients [21-23]. However, the clinical use of these agents is limited by the risk of immune-related adverse events, which can vary in severity and negatively impact patient well-being and treatment adherence [24]. Consequently, biomarker-based risk assessment for predicting and mitigating these adverse events holds significant clinical value, allowing for personalized treatment strategies and proactive management.
In this study, we identified eight potential risk indicators for immunotherapy-related adverse events, but we focus on the four most significant: LCP1 mRNA expression, ADPGK mRNA expression, LCP1 protein levels, and ADPGK protein levels. The remaining four risk factors showed relatively minor associations with adverse events and thus are not discussed in detail.
The roles of LCP1 and ADPGK as potential predictive biomarkers for immunotherapy-related adverse events in NSCLC patients treated with PD-1 inhibitors are supported by previous studies investigating their involvement in immune regulation and cellular metabolism [25]. For instance, Pan et al. [26] demonstrated that elevated LCP1 expression was associated with enhanced T-cell activation and migration. Our findings align with prior research, suggesting that elevated LCP1 expression increases the risk of immunotherapy-related adverse events, reinforcing LCP1’s role in immune response regulation [27,28]. LCP1, also known as plastin-1, interacts with the actin cytoskeleton to regulate T-cell activation and migration [29]. Its elevated expression has been linked to effector T-cell function and has been implicated in the development of autoimmune diseases and inflammatory conditions [30]. The positive correlation between LCP1 expression and immunotherapy-related adverse events observed in this study underscores its potential as a predictive biomarker. Furthermore, the significant associations found at both mRNA and protein levels suggest LCP1’s utility as a predictive marker at various stages of gene expression regulation.
Similarly, our findings on ADPGK, an enzyme involved in energy metabolism, offer new insights into the potential link between cellular metabolic pathways and immune-related adverse events in the context of PD-1 inhibitor treatment. ADPGK has been implicated in key cellular processes, including glycolysis regulation and redox balance [31-33]. Our study identified a significant association between elevated ADPGK expression levels and an increased risk of immunotherapy-related adverse events. This suggests that metabolic reprogramming may influence susceptibility to immune-related adverse events during PD-1 inhibitor therapy. This finding aligns with recent research emphasizing the role of cellular metabolism in shaping immune responses to immunotherapy [34]. The identification of ADPGK as a predictive biomarker underscores the complex interplay between metabolism and immune function and its potential impact on immunotherapy outcomes [35,36].
The strong positive correlations between LCP1 and ADPGK expression levels and the occurrence of immunotherapy-related adverse events reflect the multifaceted nature of the immune response and its regulation by various cellular processes. Furthermore, the logistic regression analysis confirmed the independent predictive value of both LCP1 and ADPGK, reinforcing their potential as standalone biomarkers for identifying patients at higher risk of adverse events during PD-1 inhibitor treatment.
Of particular importance is the predictive value demonstrated by the combined assessment of LCP1 and ADPGK expression levels. The high sensitivities, specificities, and AUC observed in the ROC analysis highlight the potential of this combined model as a robust tool for predicting immunotherapy-related adverse events in late-stage NSCLC patients with the KRAS G12C mutation treated with PD-1 inhibitors. The impressive AUC value further indicates the model’s strong predictive performance. These findings suggest that a multi-marker approach, incorporating LCP1 and ADPGK expression levels, may provide greater predictive accuracy compared to individual biomarkers alone.
Our study has several clinical and research implications. First, the identification of LCP1 and ADPGK as predictive biomarkers for immunotherapy-related adverse events lays the groundwork for future research into the mechanisms linking these biomarkers to treatment outcomes. Further studies could investigate the biological pathways through which LCP1 and ADPGK influence immune response dynamics and contribute to the development of adverse events during PD-1 inhibitor therapy. Additionally, the development of validated biomarker panels, such as the combined assessment of LCP1 and ADPGK, could support personalized risk stratification strategies in clinical decision-making. By identifying patients at higher risk of adverse events, such panels could enable proactive monitoring and early intervention, ultimately improving treatment safety and patient care.
It is important to acknowledge the limitations of our study, including its retrospective design and the single-center nature of the patient cohort. Additionally, while the sample size was sufficient to detect significant associations, it may limit the generalizability of our findings. Therefore, multi-center studies with larger and more diverse patient populations are needed to validate the predictive utility of LCP1 and ADPGK expression levels in the context of PD-1 inhibitor treatment for late-stage NSCLC with the KRAS G12C mutation. Furthermore, prospective investigations should explore the functional roles of LCP1 and ADPGK in immune response modulation and the development of immune-related adverse events, ultimately clarifying the biological underpinnings of these predictive biomarkers.
In conclusion, our study contributes to the growing body of evidence supporting the potential of LCP1 and ADPGK expression levels as predictive biomarkers for immunotherapy-related adverse events in late-stage NSCLC patients with the KRAS G12C mutation undergoing PD-1 inhibitor treatment. The findings emphasize the importance of understanding the interplay between immune function, cellular metabolism, and gene expression regulation in relation to immunotherapy outcomes. Future research should focus on further elucidating the biological mechanisms underlying these associations and translating these insights into clinically actionable strategies for personalized risk assessment and patient management during PD-1 inhibitor therapy.
Disclosure of conflict of interest
None.
References
- 1.Brueckl WM, Ficker JH, Zeitler G. Clinically relevant prognostic and predictive markers for immune-checkpoint-inhibitor (ICI) therapy in non-small cell lung cancer (NSCLC) BMC Cancer. 2020;20:1185. doi: 10.1186/s12885-020-07690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desai AP, Adashek JJ, Reuss JE, West HJ, Mansfield AS. Perioperative immune checkpoint inhibition in early-stage non-small cell lung cancer: a review. JAMA Oncol. 2023;9:135–142. doi: 10.1001/jamaoncol.2022.5389. [DOI] [PubMed] [Google Scholar]
- 3.Mountzios G, Remon J, Hendriks LEL, García-Campelo R, Rolfo C, Van Schil P, Forde PM, Besse B, Subbiah V, Reck M, Soria JC, Peters S. Immune-checkpoint inhibition for resectable non-small-cell lung cancer - opportunities and challenges. Nat Rev Clin Oncol. 2023;20:664–677. doi: 10.1038/s41571-023-00794-7. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Alduais Y, Chen B. Therapeutic and systemic adverse events of immune checkpoint inhibitors targeting the PD-1/PD-L1 axis for clinical management of NSCLC. Cell Transplant. 2021;30:9636897211041587. doi: 10.1177/09636897211041587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdayem P, Planchard D. Safety of current immune checkpoint inhibitors in non-small cell lung cancer. Expert Opin Drug Saf. 2021;20:651–667. doi: 10.1080/14740338.2021.1867100. [DOI] [PubMed] [Google Scholar]
- 6.Shankar B, Zhang J, Naqash AR, Forde PM, Feliciano JL, Marrone KA, Ettinger DS, Hann CL, Brahmer JR, Ricciuti B, Owen D, Toi Y, Walker P, Otterson GA, Patel SH, Sugawara S, Naidoo J. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol. 2020;6:1952–1956. doi: 10.1001/jamaoncol.2020.5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang S, Qin C, Hu H, Liu T, He Y, Guo H, Yan H, Zhang J, Tang S, Zhou H. Immune checkpoint inhibitors in non-small cell lung cancer: progress, challenges, and prospects. Cells. 2022;11:320. doi: 10.3390/cells11030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geisler AN, Phillips GS, Barrios DM, Wu J, Leung DYM, Moy AP, Kern JA, Lacouture ME. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255–1268. doi: 10.1016/j.jaad.2020.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Zhang X, Zhou S, Zhou Y, Liu X. Association between PD-1 inhibitor-related adverse events and frailty assessed by frailty index in lung cancer patients. Cancer Med. 2023;12:9272–9281. doi: 10.1002/cam4.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okiyama N, Tanaka R. Immune-related adverse events in various organs caused by immune checkpoint inhibitors. Allergol Int. 2022;71:169–178. doi: 10.1016/j.alit.2022.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Joshi H, Morley SC. Efficient T cell migration and activation require L-plastin. Front Immunol. 2022;13:916137. doi: 10.3389/fimmu.2022.916137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wabnitz GH, Honus S, Habicht J, Orlik C, Kirchgessner H, Samstag Y. LFA-1 cluster formation in T-cells depends on L-plastin phosphorylation regulated by P90(RSK) and PP2A. Cell Mol Life Sci. 2021;78:3543–3564. doi: 10.1007/s00018-020-03744-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahat U, Garg B, Yang CY, Mehta H, Hanna R, Rogers HJ, Flagg A, Ivanov AI, Corey SJ. Lymphocyte cytosolic protein 1 (L-plastin) I232F mutation impairs granulocytic proliferation and causes neutropenia. Blood Adv. 2022;6:2581–2594. doi: 10.1182/bloodadvances.2021006398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Yin Q, Yang D, Jin H, Yao Y, Song J, Liu C, Nie Y, Yin H, Wang W, Xu B, Xue L, Ji X, Chen X, Zhao H. LCP1 knockdown in monocyte-derived macrophages: mitigating ischemic brain injury and shaping immune cell signaling and metabolism. Theranostics. 2024;14:159–175. doi: 10.7150/thno.88678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang Z, Cui X, Yang L, Hu Q, Li D, Zhang X, Han L, Shi S, Shen Y, Zhao W, Ju Q, Deng X, Wu Y, Sheng W. Co-assembled nanocomplexes of peptide neoantigen Adpgk and Toll-like receptor 9 agonist CpG ODN for efficient colorectal cancer immunotherapy. Int J Pharm. 2021;608:121091. doi: 10.1016/j.ijpharm.2021.121091. [DOI] [PubMed] [Google Scholar]
- 16.Yin Q, Wu L, Han L, Zheng X, Tong R, Li L, Bai L, Bian Y. Immune-related adverse events of immune checkpoint inhibitors: a review. Front Immunol. 2023;14:1167975. doi: 10.3389/fimmu.2023.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Remon J, Soria JC, Peters S ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Early and locally advanced non-small-cell lung cancer: an update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol. 2021;32:1637–1642. doi: 10.1016/j.annonc.2021.08.1994. [DOI] [PubMed] [Google Scholar]
- 18.Reck M, Remon J, Hellmann MD. First-line immunotherapy for non-small-cell lung cancer. J. Clin. Oncol. 2022;40:586–597. doi: 10.1200/JCO.21.01497. [DOI] [PubMed] [Google Scholar]
- 19.Lazzari C, Spagnolo CC, Ciappina G, Di Pietro M, Squeri A, Passalacqua MI, Marchesi S, Gregorc V, Santarpia M. Immunotherapy in early-stage non-small cell lung cancer (NSCLC): current evidence and perspectives. Curr Oncol. 2023;30:3684–3696. doi: 10.3390/curroncol30040280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai A, Peters S. Immunotherapy-based combinations in metastatic NSCLC. Cancer Treat Rev. 2023;116:102545. doi: 10.1016/j.ctrv.2023.102545. [DOI] [PubMed] [Google Scholar]
- 21.Fang Q, Yu J, Li W, Luo J, Deng Q, Chen B, He Y, Zhang J, Zhou C. Prognostic value of inflammatory and nutritional indexes among advanced NSCLC patients receiving PD-1 inhibitor therapy. Clin Exp Pharmacol Physiol. 2023;50:178–190. doi: 10.1111/1440-1681.13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inomata M, Hirai T, Seto Z, Tokui K, Taka C, Okazawa S, Kambara K, Ichikawa T, Imanishi S, Yamada T, Miwa T, Hayashi R, Tobe K. Clinical parameters for predicting the survival in patients with squamous and non-squamous-cell NSCLC receiving PD-1 inhibitor therapy. Pathol Oncol Res. 2020;26:327–333. doi: 10.1007/s12253-018-0473-x. [DOI] [PubMed] [Google Scholar]
- 23.Huang Y, Chau YF, Bai H, Wu X, Duan J. Biomarkers for immunotherapy in driver-gene-negative advanced NSCLC. Int J Mol Sci. 2023;24:14521. doi: 10.3390/ijms241914521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poto R, Troiani T, Criscuolo G, Marone G, Ciardiello F, Tocchetti CG, Varricchi G. Holistic approach to immune checkpoint inhibitor-related adverse events. Front Immunol. 2022;13:804597. doi: 10.3389/fimmu.2022.804597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karger A, Mansouri S, Leisegang MS, Weigert A, Günther S, Kuenne C, Wittig I, Zukunft S, Klatt S, Aliraj B, Klotz LV, Winter H, Mahavadi P, Fleming I, Ruppert C, Witte B, Alkoudmani I, Gattenlöhner S, Grimminger F, Seeger W, Pullamsetti SS, Savai R. ADPGK-AS1 long noncoding RNA switches macrophage metabolic and phenotypic state to promote lung cancer growth. EMBO J. 2023;42:e111620. doi: 10.15252/embj.2022111620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan S, Wan M, Jin H, Ning R, Zhang J, Han X. LCP1 correlates with immune infiltration: a prognostic marker for triple-negative breast cancer. BMC Immunol. 2024;25:42. doi: 10.1186/s12865-024-00635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaffner-Reckinger E, Machado RAC. The actin-bundling protein L-plastin-A double-edged sword: beneficial for the immune response, maleficent in cancer. Int Rev Cell Mol Biol. 2020;355:109–154. doi: 10.1016/bs.ircmb.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Zeng Q, Li L, Feng Z, Luo L, Xiong J, Jie Z, Cao Y, Li Z. LCP1 is a prognostic biomarker correlated with immune infiltrates in gastric cancer. Cancer Biomark. 2021;30:105–125. doi: 10.3233/CBM-200006. [DOI] [PubMed] [Google Scholar]
- 29.Joshi H, Almgren-Bell A, Anaya EP, Todd EM, Van Dyken SJ, Seth A, McIntire KM, Singamaneni S, Sutterwala F, Morley SC. L-plastin enhances NLRP3 inflammasome assembly and bleomycin-induced lung fibrosis. Cell Rep. 2022;38:110507. doi: 10.1016/j.celrep.2022.110507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang L, Shen R, Lu Y, Xu X, Huang F. Tetrandrine alleviates inflammation and promotes macrophage M2 polarization in gouty arthritis by NF-κB-mediated Lcp1. Cell Mol Biol (Noisy-le-grand) 2024;70:205–211. doi: 10.14715/cmb/2024.70.2.29. [DOI] [PubMed] [Google Scholar]
- 31.Jiang HY, Wang ZJ. ADPGK-AS1 promotes the progression of colorectal cancer via sponging miR-525 to upregulate FUT1. Eur Rev Med Pharmacol Sci. 2020;24:2380–2386. doi: 10.26355/eurrev_202003_20505. [DOI] [PubMed] [Google Scholar]
- 32.Xing F, Li YM, Gao MM. The effect of lncRNA ADPGK-AS1 on the proliferation and apoptosis of retinoblastoma cells by targeting miR-200b-5p. Zhonghua Zhong Liu Za Zhi. 2023;45:230–237. doi: 10.3760/cma.j.cn112152-20210909-00686. [DOI] [PubMed] [Google Scholar]
- 33.Luo XF, Wu XJ, Wei X, Wang AG, Wang SH, Wang JL. LncRNA ADPGK-AS1 regulated cell proliferation, invasion, migration and apoptosis via targeting miR-542-3p in osteosarcoma. Eur Rev Med Pharmacol Sci. 2019;23:8751–8760. doi: 10.26355/eurrev_201910_19269. [DOI] [PubMed] [Google Scholar]
- 34.Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N, Yi P, Tang L, Pan Q, Rao S, Liang J, Tang Y, Su M, Luo X, Yang Y, Shi Y, Wang H, Zhou Y, Liao Q. The cancer metabolic reprogramming and immune response. Mol Cancer. 2021;20:28. doi: 10.1186/s12943-021-01316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J, Wu W, Wu M, Ding J. Long noncoding RNA ADPGK-AS1 promotes cell proliferation, migration, and EMT process through regulating miR-3196/OTX1 axis in breast cancer. In Vitro Cell Dev Biol Anim. 2019;55:522–532. doi: 10.1007/s11626-019-00372-1. [DOI] [PubMed] [Google Scholar]
- 36.Huang Z, Yang H. Upregulation of the long noncoding RNA ADPGK-AS1 promotes carcinogenesis and predicts poor prognosis in gastric cancer. Biochem Biophys Res Commun. 2019;513:127–134. doi: 10.1016/j.bbrc.2019.03.140. [DOI] [PubMed] [Google Scholar]