Abstract
The scarcity of suitable high-throughput screening technology for hydrogen sulfide (H2S) donors has hampered the discovery of H2S donors. In this study, a long-lived cyclometalated iridium complex was rationally designed as a mitochondria-targeted H2S probe to monitor the real-time dynamic change of H2S. By using the time-resolved emission spectroscopy (TRES) technique, an anti-interference high-throughput screening system was developed to monitor H2S in living cells with decreased false negative results. As a proof-of-concept, three natural products were identified as potential H2S donors from a natural product library using the developed TRES probe. Notably, the discovery of allicin and diallyl trisulfide demonstrated the feasibility of this screening platform, while garlic-derived allyl methyl sulfide was explored as a H2S donor candidate. The results were further validated by a commercial assay. We anticipate this high-throughput platform could facilitate the discovery of H2S donors by discriminating the endogenous interfering fluorescence from biological systems.
Subject terms: Drug discovery, Chemical biology, Fluorescent probes
H2S donors in living cells are essential for modulating H2S levels and have been proposed to be relevant for managing hepatic disorders, but conventional platforms to screen for H2S donors are plagued by interference by endogenous background fluorescence signals. Here, the authors develop a luminogenic probe—based on an Ir(III) complex with a 1,10-phenanthroline-5,6-dione moiety—capable of selective response to mitochondrial H2S, and set up an anti-interference high-throughput screening system capable of distinguishing target signals from complex background autofluorescence in living cells.
Introduction
Hydrogen sulfide (H2S) is endogenous gasotransmitter that mainly originates from either enzymatic or non-enzymatic biochemical reactions, and it can also be produced by intracellular sulfur stores. Studies indicated that H2S is highly associated with hepatic functions and the direct targeting of H2S or its downstream enzymes is an effective strategy for managing hepatic disorders1–3. Many researchers use H2S donors (H2S-releasing small molecules) as primary tools to modulate cellular H2S levels and have been reported to exert outstanding anti-cancer effects including hepatoblastoma4,5. Allicin (diallyl thiosulfinate) is derived from garlic and can produce H2S via non-enzymatic reactions6. Accumulating evidences suggest that allicin can reduce the risk of cancer incidence7,8. S-propargyl-cysteine (SPRC) is a structural analog of broccoli extract, which can induce endogenous H2S production in mammalian cells. Recently, SPRC was found to suppress tumor growth via inducing apoptosis and arresting cell cycle both in vitro and in vivo9. Sulforaphane has been reported to upregulate the targeting enzyme thioredoxin reductase 1 in H2S releasing process of human cancer cells, which was likely to regulate its anti-cancer effects by H2S production10,11. Even though bioactive constituents and their derivatives offer a rich source of bioactive scaffolds for H2S donors discovery12,13, two primary challenges persist in this field. Firstly, there is a notable lack of H2S-depleted control compounds, which limits the ability to conclusively interpret experimental results and identify H2S donors that selectively induce apoptosis in cancer cells while minimizing cytotoxicity to normal cells. Secondly, existing drug market lacks suitable H2S donors that can be selectively activated by specific cellular conditions14.
Fluorescent organic molecules are essential in developing molecular sensors for various analytes. Luminescent transition metal complexes, particularly those with a d⁶ electronic configuration like ruthenium(II), osmium(II), and rhenium(I), have gained significant attention. Recently, iridium(III) polypyridine complexes have emerged as promising molecular sensors due to their intense, tunable, and long-lived visible emissions. Unlike other complexes, iridium(III) polypyridines can exhibit diverse emissive states, including ³MLCT, ³IL, ³LLCT, and ³SBLCT, which can be modulated by ligand selection or environmental changes. This versatility has led to growing interest in using these complexes as probes for chemical and biological molecules15–19. Despite these advances, the development of H2S probes for high-throughput screening remains limited, posing a critical challenge in the search for H2S donors within living cells. Various H2S probes have been designed by coupling specific H2S-mediated chemical reactions with fluorescent outputs20,21. However, a significant challenge in probe development is the potential for false-negative results, often due to the strong fluorescent background noise inherent to endogenous cellular environments. This issue is further exacerbated in high-throughput screening applications, where rapid and accurate H2S detection is essential. Consequently, the lack of specialized high-throughput screening technologies for H2S detection impedes the exploration and identification of new H2S donors, particularly from underexplored natural products and their derivatives.
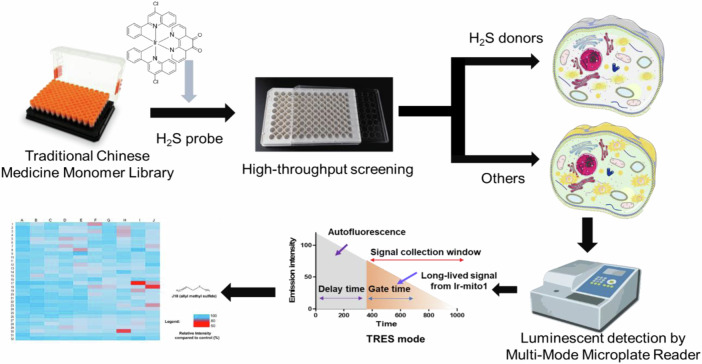
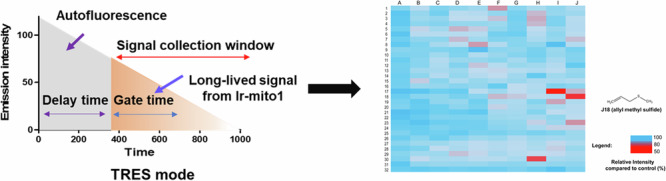
Time-resolved emission spectroscopy (TRES) technique can eliminate fluorescent background noise signals by setting recording the output signal after the specific decay gate of noise signal22,23. Transition metal complexes exert a distinguishing long lifetime even in an auto-fluorescent background, which enables them to be ideal agents for discriminating background fluorescence interference24,25, and further development of TRES-based high-throughput screening technologies26. In our previous study, we have successfully designed a long-lived iridium complex-based probe and then integrated it with lysosomotropic compounds for the construction of a TRES technique-based high-throughput screening platform23. In this study, we introduced a 1,10-phenanthroline-5,6-dione moiety to the iridium complex as the mitochondria-targeted responsive unit of H2S to monitor the H2S donor-induced H2S alteration in mitochondria. By integrating with the TRES technique, a H2S donor-embedded high-throughput screening system in living cells was developed. As a proof-of-concept, a TRES screening platform based on transition metal complexes was employed to evaluate 320 natural products sourced from the Traditional Chinese Medicine Monomer Library. This approach aimed to identify compounds with potential anti-cancer properties through their ability to modulate H2S levels within mitochondria. Out of the screened compounds, three compounds were found to specifically mediate mitochondrial H2S levels in cancer cells, highlighting their potential as innovative therapeutic agents. This discovery underscores the effectiveness of the TRES-based platform in identifying biologically active compounds from natural sources, paving the way for further research and development in targeted cancer therapies.
Results and discussion
Principle of the iridium(III) complex-based TRES system
The principle of the H2S donors high-throughput screening system is to integrate TRES technique with a H2S targeting moiety containing iridium(III) complex (Ir-mito1) (Scheme 1). Previous literature reported that 6,12-dihydroxyperylene-1,7-dione (DPD) could selectively respond to H2S27. In the present study, a 1,10-phenanthroline-5,6-dione moiety was introduced to the parent iridium(III) complex scaffold to endow its selective response to H2S. In the presence of H2S donors, the concentration of mitochondria H2S would increase and further react with the 1,10-phenanthroline-5,6-dione moiety of iridium(III) complex Ir-mito1, triggering the luminescence quenching of the long-lived signal of Ir-mito1. Integrating with the TRES technique, the long-lived luminescence signal of Ir-mito1 can be distinguished from the endogenous short-lived interfering background fluorescence. Therefore, the TRES-based H2S donors screening system can be used for the high-throughput screening of H2S donors as well as discriminating the endogenous interfering fluorescence in living cells.
Scheme 1.
Long-lived iridium(III) complex-based high-throughput screening platform for H2S donor discovery. Schematic diagram of the H2S donors screening system.
Design of the mitochondria-targeted H2S probe
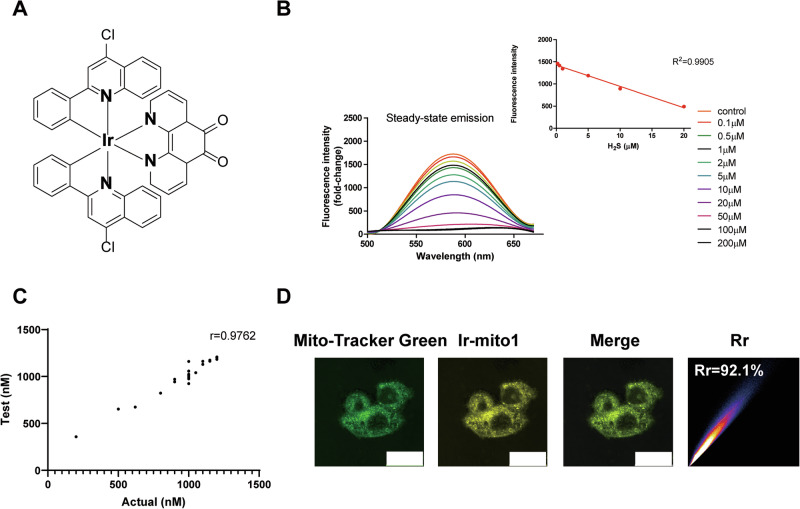
To develop the H2S probe, we rationally designed and synthesized an iridium(III) complex (Ir-mito1) by integrating it with a H2S-responsive moiety entitled 1,10-phenanthroline-5,6-dione (Fig. 1A). The synthetic details are provided in the Supplementary Methods, with additional nuclear magnetic resonance (NMR) spectra presented in Figs. S1, 2. With Ir-mito1 in hand, we investigated the photophysical properties of Ir-mito1. The photophysical properties results indicated that Ir-mito1 presented a maximal emission and excitation wavelength at 608 nm and 355 nm respectively, and exerted a large Stokes shift of 253 nm which is beneficial to prevent its self-quenching (Figure S3). To evaluate the response of Ir-mito1 to H2S, Ir-mito1 was treated with different concentrations of H2S. The emission intensity of Ir-mito1 was decreased with a good linearity (R2 = 0.9905) in the presence of increasing H2S concentration (Fig. 1B). The potential mechanism underlying the sensing response of Ir-mito1 to H2S was investigated using liquid chromatography-mass spectrometry (LC-MS) analysis (Figure S4). The results indicate that the carbonyl group on Ir-mito1 undergoes the reduction reaction to generate hydroxyl group in PBS buffer. Additionally, single-blinded experiments further confirmed the efficacy of Ir-mito1 in detecting H2S, with a correlation coefficient of r = 0.9762 (Fig. 1C). To evaluate the location of Ir-mito1, we next explored the sub-cellular localization of Ir-mito1 in cells. The results revealed that Ir-mito1 presented a high level of co-localization with mitochondria (Pearson’s correlation coefficient (Rr) = 0.92) (Fig. 1D). As mitochondria are the primary sites of cellular metabolism for H2S28–30, targeting these organelles has emerged as a promising strategy for the discovery and development of effective H2S donors31. In this study, the incorporation of an H₂S-targeting moiety on Ir-mito1 likely facilitated its mitochondrial localization. Owing to its H₂S sensitivity and precise co-localization with mitochondrial H₂S, Ir-mito1 exhibits strong potential for real-time monitoring of mitochondrial H₂S levels in living cells.
Fig. 1. Ir-mito1 based high-throughput screening platform for H2S donors discovery.
A The structures of Ir-mito1. B The fluorescence spectra of Ir-mito1 (5 μM) in PBS buffer solution with different concentration of H2S. C Response of Ir-mito1 to different concentrations of H2S, displaying the Pearson’s correlation coefficient r value for the test versus actual concentration. D The co-localization of Ir-mito1 with mitochondria in celluo. The Pearson’s correlation coefficient (Rr) of Ir-mito1 with mitochondria were calculated. Scale bar = 25 μm.
H2S response and selectivity of Ir-mito1 in vitro
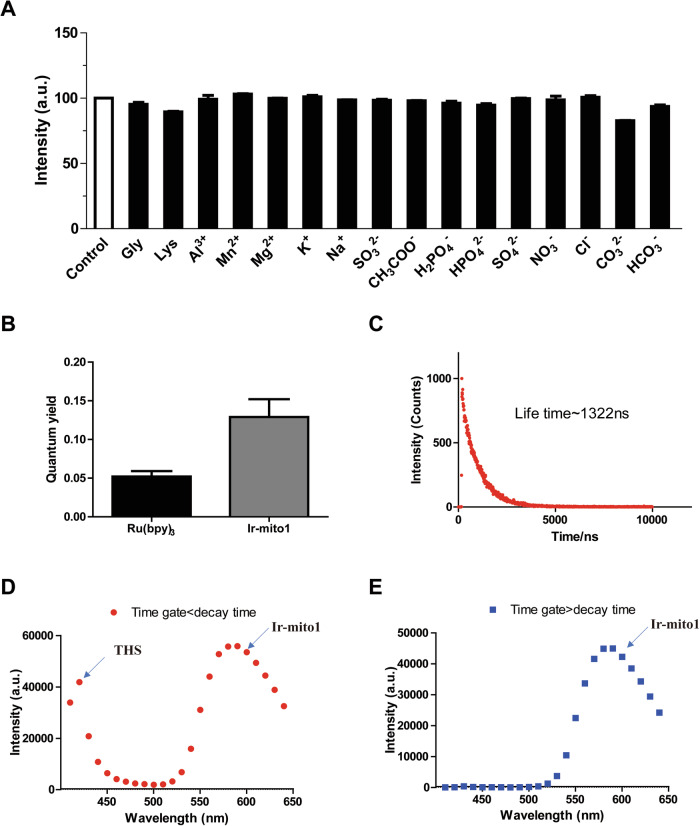
To evaluate the selectivity of Ir-mito1, we next explored the response of Ir-mito1 to different common interfering substances. The results showed that Ir-mito1 responded to H2S with a strong luminescent signal reduction. However, only negligible response could be recorded at the challenge to the other common interfering substances, including metal ions, anions, and common amino acids (Fig. 2A). These results suggested that Ir-mito1 is a promising mitochondria-targeted H2S probe with an “ON-OFF” signal output.
Fig. 2. The photophysical properties of Ir-mito1.
A Luminescence intensity of Ir-mito1 (10 μM) in various potentially interfering species including amino acids, anions or cations (20 μM), n = 3. B Quantum yields of Ir-mito1 and a standard reference solution [Ru(bpy)3][PF6]2 (10 μM) in PBS, n = 3. C Emission decay curves of complex Ir-mito1 (10 μM) in PBS buffer. D Time-resolved spectra of Ir-mito1 (10 μM) and the nuclear dye THS (10 μM) in cells with time gate set to shorter than the THS decay time. E Time-resolved spectra of Ir-mito1 (10 μM) and the nuclear dye THS (10 μM) in cells with time gate set to longer than the THS decay time.
The photophysical properties of Ir-mito1 in vitro
To demonstrate the photophysical properties of Ir-mito1 in vitro, the quantum yield and lifetime of Ir-mito1 were evaluated. The results revealed that Ir-mito1 exerts a larger quantum yield (ca. 0.151) compared with the standard transition metal complex [Ru(bpy)3][PF6]2 (bpy = 2,2′-bipyridine) (0.045) (Fig. 2B), and possesses a long-lived lifetime (ca. 1.3 μs) (Fig. 2C) that enable it to distinguish background fluorescence (lifetime <0.1 μs) using TRES. Thioflavin S (THS) is a typical commercial organic fluorophore that can be utilized as a model matrix interferent to simulate the autofluorescence of biological samples. In the TRES experiment, two signals from THS and Ir-mito1 would be observed when the decay gate was shorter than the decay time of organic dye THS. In contrast, when the decay gate was set at longer than the decay time of THS, only the emission signal from Ir-mito1 was observed (Fig. 2D,E). The results indicated Ir-mito1 can be further explored in cellulo mitochondria H2S detection experiments.
Biocompatibility of Ir-mito1 in cellulo
H2S is excessively produced in various cancers, where tumor tissues can be unambiguously distinguished by H2S probes. Meanwhile, the good biocompatibility of the developed sensing platform is important for the development of cell-based high-throughput screening technology31,32. To develop an iridium-based probe for the high-throughput screening of H2S donors in living cells, we tested the biocompatibility of Ir-mito1 to trace mitochondria H2S in cellulo. Our findings revealed that the Ir-mito1 probe exhibits minimal cytotoxicity, as demonstrated in Figure S5, and its emission signal correlates with H2S levels, detailed in Figure S6A. Moreover, the probe displayed photostability comparable to that of a commercial dye under identical conditions over a duration of 15 min, as shown in Figure S6B. These attributes highlight Ir-mito1’s potential as an effective tool for monitoring mitochondrial H2S in biological research.
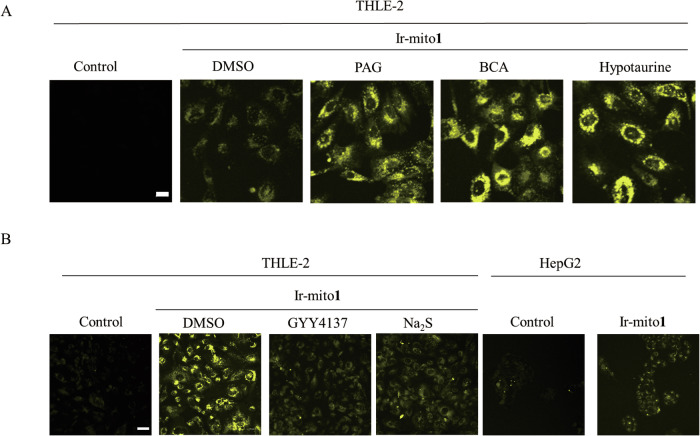
H2S plays a pivotal role in hepatic functions, and targeting H2S or its downstream enzymes has been recognized as a viable strategy for managing hepatic disorders1–3. The β-Cyano-l-alanine synthase (BCA) from Spinacia oleracea catalyzes the formation of S-substituted l-cysteines and functions as a reversible inhibitor of cystathionine gamma-lyase (CSE) by transiently modifying its apoenzyme33,34. DL-propargyl glycine (PAG) serves as an irreversible CSE inhibitor, reducing cellular H2S levels35,36. GYY4137, originally developed in the late 1950s as a vulcanization accelerator for natural rubber, was later recognized as the first slow-releasing H2S donor with vasorelaxant activity37. To assess the endogenous release of H2S in living cells, our study employed these commercially available inhibitors to diminish the production of endogenous H2S. We observed that treatment with BCA or PAG led to an increase in the luminescence emitted by the Ir-mito1 probe in THLE-2 cells. Similarly, the introduction of the H2S scavenger hypotaurine38 also resulted in enhanced luminescence of Ir-mito1 in THLE-2 cells (Fig. 3A). Conversely, exposure to the H2S donors GYY4137 or Na2S reduced the luminescence of Ir-mito1 in THLE-2 cells (Fig. 3B). Notably, elevated H2S concentrations have been reported in the blood of patients with hepatic or colorectal cancer compared to healthy individuals39. Fig. 3B illustrates that Ir-mito1 can effectively distinguish between hepatic cancer and normal liver cells by monitoring variations in H2S levels. Collectively, these findings underscore the potential of Ir-mito1 as a biocompatible probe suitable for real-time monitoring of mitochondrial H2S fluctuations in living cells, and its application in differentiating hepatic cancer from normal liver tissue.
Fig. 3. Confocal imaging of Ir-mito1 in living cells.
A Confocal imaging of THLE-2 cells treated with Ir-mito1 (1 μM, λex/λemi = 405/500−600 nm) with PAG (1 mM), BCA (10 μM) and Hypotaurine (1 mM) for 24 h. Luminescence was detected. Scale bar = 20 μm. B Confocal imaging of THLE-2 or HepG2 cells treated with Ir-mito1 (1 μM, λex/λemi = 405/500−600 nm) with GYY4137 (1 mM) for 24 h or Na2S (1 μM) for 1 h. Luminescence was detected. Scale bar = 50 μm.
H2S donors screening assay based on TRES
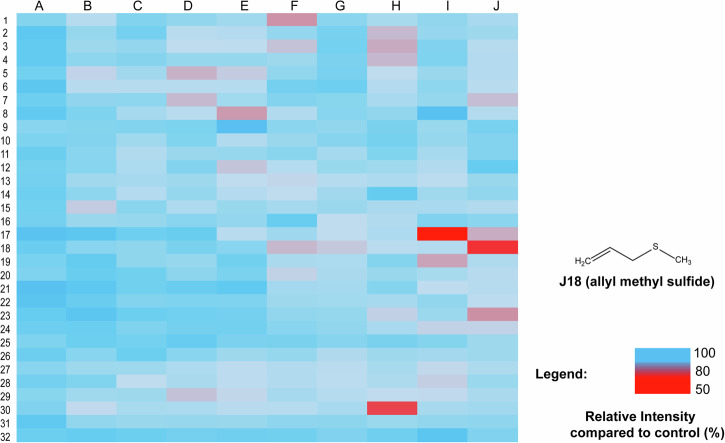
The long-lived lifetime of Ir-mito1 enables it can be utilized to distinguish background autofluorescence when the time gate is longer than the fluorescence decay time40. To demonstrate the validation of Ir-mito1 in TRES mode, we evaluated the response of Ir-mito1 under TRES mode and commercial H2S probe (WSP-1)41 in steady-state emission mode. The results showed that Ir-mito1 shows a much more sensitive response to H2S compared with the commercial H2S probe (Figure S7). This indicates that Ir-mito1 in TRES mode is superior for screening H2S donors compared to the commercial H2S probe. The possible reason is because Ir-mito1 can overcome the endogenous interference from short-lived autofluorescence when the time gate is longer than the fluorescence decay time. The proof of concept was demonstrated by using Ir-mito1 based TRES technology for high-throughput screening of H2S donors based on 320 natural products (10 μM) (Fig. 4). From the TRES-based screening, three potential H2S donors (allicin, diallyl trisulfide, and allyl methyl sulfide) were identified to enable the increase of mitochondria H2S. Encouragingly, allicin and diallyl trisulfide have been previously reported as H2S donors4, while allyl methyl sulfide acts is a H2S donor candidate. To confirm the results, the mitochondria H2S change upon treating with these compounds was evaluated by a commercial H2S probe (WSP-1) (Figure S8), which showed consistent results as the developed probe Ir-mito1 in this study. Furthermore, the cytotoxicity results of allyl methyl sulfide further validated its anti-cancer efficacy as an H2S donor candidate against hepatic cancer (Figure S9). Taken together, these results demonstrate the practical application of the luminescent iridium(III) complex-based TRES platform for the high-throughput screening of H2S donors.
Fig. 4. H2S donors screening assay based on TRES technique.
After being treated with the 320 natural products (10 μM) from the Traditional Chinese Medicine Monomer Library or DMSO in cells for 1 h, Ir-mito1 probe (10 μM) was further incubated for another 1 h. The luminescence signal output in cells was recorded using the TRES technique. The luminescence signal of Ir-mito1 in different treatment groups was compared with the DMSO control group. The means of the results are calculated from three independent experiments.
Conclusion
Given that mitochondria are the primary sites of H2S metabolism, targeting these organelles has emerged as a promising strategy for developing effective H2S donors. In this study, we designed and synthesized a long-lived iridium(III)-based complex, Ir-mito1, which incorporates a mitochondria-targeting and H2S-responsive moiety, 1,10-phenanthroline-5,6-dione. This complex serves as a mitochondria-targeted H2S probe, enabling the tracing of mitochondrial H2S in living cells. The extended lifetime of Ir-mito1 allows it to effectively differentiate from background autofluorescence when the time gate exceeds the fluorescence decay time. These properties make Ir-mito1 particularly well-suited for accurate and high-throughput screening of H2S donors in living cells. Leveraging the selective response to mitochondrial H2S and the long-lived lifetime of Ir-mito1, we integrated it with TRES technology to facilitate high-throughput screening of H2S donors in living cells. A library of 320 natural products was used to validate the proof-of-concept for this iridium(III)-based TRES platform. Our screening identified two natural products previously reported as H2S donors and highlighted allyl methyl sulfide as a potential H2S donor. The long-lived lifetime of Ir-mito1 offers significant advantages in avoiding endogenous autofluorescent interference, suggesting that this study could pave the way for developing more TRES-based luminescent screening platforms in living cells.
Methods
Confocal imaging
Cells were cultured in a glass-bottomed dish for 24 h. The cells were treated with Ir-mito1 (1 μM) for an additional 1 h. Afterwards, the luminescence imaging was evaluated. To detect mitochondria H2S alteration by a H2S donor, cells were incubated with Na2S (10 μM) for 1 h, and successively treated with Ir-mito1 (1 μM) for another 1 h. Luminescence imaging was recorded using a Carl Zeiss LSM880 confocal laser scanning microscope system with an excitation wavelength of 405 nm.
H2S donor screening
Cells were treated with the natural products (10 μM) for 1 h. Afterwards, the cells were next treated with Ir-mito1 (1 μM) for an additional 1 h. The luminescence of Ir-mito1 was recorded by a Multi-Mode Microplate Reader. The time gate of detection was set with a delay time of 200 μs42.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Nos. 82200403, 22301201, China), Natural Science Foundation of Sichuan Province (2022NSFSC1266), Tianfu Emei Young Science & Technology Talents from Sichuan Province (No. 2618).
Author contributions
Conceptualization, C. Wu and K.J. Wu; Methodology, C. Wu, K.J.Wu and C.H. Leung; Software, W. Sun, J.M. Sun and C. Lu; Validation, C. Wu and K.J.Wu; Formal analysis, C. Wu and K.J. Wu; Investigation, J.M. Sun, K.J. Wu and C. Wu; Resources, N.Sun, C.H. Leung and Y. Li; Writing—original draft preparation, C. Wu and K.J.Wu; Writing—review and editing, N. Sun, C. Wu and K.J.Wu; Supervision, C.H. Leung, K.J.Wu, N.Sun and C. Wu; Project administration, K.J.Wu and N.Sun; Funding acquisition, C. Wu, K.J.Wu, Y. Li and N.Sun. All authors have read and agreed to the published version of the manuscript.
Peer review
Peer review information
Communications Chemistry thanks Hui Chao, Maria Strianese, and Run Zhang for their contribution to the peer review of this work.
Data availability
All data are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ning Sun, Email: sunning@jiangnan.edu.cn.
Chung‐Hang Leung, Email: duncanleung@um.edu.mo.
Yan Li, Email: keliyanust@163.com.
Chun Wu, Email: ccwuchem@hotmail.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s42004-024-01332-x.
References
- 1.Wang, R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev.92, 791–896 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Sun, H.-J., Wu, Z.-Y., Nie, X.-W., Wang, X.-Y. & Bian, J.-S. Implications of hydrogen sulfide in liver pathophysiology: Mechanistic insights and therapeutic potential. J. Adv. Res.27, 127–135 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szabó, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov.6, 917–935 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Song, Z. J. et al. Hydrogen sulfide donors in research and drug development. MedChemComm5, 557–570 (2014). [Google Scholar]
- 5.Zhao, X. et al. Biothiol-triggered H 2 S release from a near-infrared fluorescent H 2 S donor promotes cutaneous wound healing. Acta Mater. Med.1, 476–485 (2022).
- 6.Searcy, D. G. & Lee, S. H. Sulfur reduction by human erythrocytes. J. Exp. Zool.282, 310–322 (1998). [DOI] [PubMed] [Google Scholar]
- 7.Bat-Chen, W., Golan, T., Peri, I., Ludmer, Z. & Schwartz, B. Allicin purified from fresh garlic cloves induces apoptosis in colon cancer cells via Nrf2. Nutr. Cancer62, 947–957 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Tang, G., Wu, L., Liang, W. & Wang, R. Direct stimulation of KATP channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol. Pharmacol.68, 1757–1764 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Kaium, M. et al. H 2 S donor, S-propargyl-cysteine, increases CSE in SGC-7901 and cancer-induced mice: evidence for a novel anti-cancer effect of endogenous H 2 S? PLoS One6, e20525 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye, L. et al. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin. Chim. acta316, 43–53 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Wang, W. et al. Sulforaphane, erucin, and iberin up-regulate thioredoxin reductase 1 expression in human MCF-7 cells. J. Agric. Food Chem.53, 1417–1421 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Wu, K.-J., Wang, W., Wang, H.-M. D., Leung, C.-H. & Ma, D.-L. Interfering with S100B–effector protein interactions for cancer therapy. Drug Discov. Today25, 1754–1761 (2020). [DOI] [PubMed]
- 13.Wu, K.-J. et al. Synthesis and evaluation of dibenzothiophene analogues as Pin1 inhibitors for cervical cancer therapy. ACS omega4, 9228–9234 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao, Y. & Pluth, M. D. Hydrogen sulfide donors activated by reactive oxygen species. Angew. Chem.128, 14858–14862 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo, K. K.-W., Li, S. P.-Y. & Zhang, K. Y. Development of luminescent iridium (III) polypyridine complexes as chemical and biological probes. N. J. Chem.35, 265–287 (2011). [Google Scholar]
- 16.Chen, Y. et al. A ratiometric fluorescent probe for rapid detection of hydrogen sulfide in mitochondria. Angew. Chem.125, 1732–1735 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Yagdi, E., Cerella, C., Dicato, M. & Diederich, M. Garlic-derived natural polysulfanes as hydrogen sulfide donors: friend or foe? Food Chem. Toxicol.95, 219–233 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Liu, C. et al. A Ruthenium (II) complex-based probe for colorimetric and luminescent detection and imaging of hydrogen sulfide in living cells and organisms. Anal. Chim. Acta1145, 114–123 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Wu, K. J. et al. Simultaneous blocking of the pan‐RAF and S100B pathways as a synergistic therapeutic strategy against malignant melanoma. J. Cell. Mol. Med.25, 1972–1981 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strianese, M. & Pellecchia, C. Fluorescent Probes for H 2 S Detection: Metal‐Based Approaches. Hydrogen Sulfide: Chemical Biology Basics, Detection Methods, Therapeutic Applications, and Case Studies9, 203–233 (2022).
- 21.Fosnacht, K. G. & Pluth, M. D. Activity-based fluorescent probes for hydrogen sulfide and related reactive sulfur species. Chem. Rev.124, 4124–4257 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Easter, J. H., DeToma, R. P. & Brand, L. Nanosecond time-resolved emission spectroscopy of a fluorescence probe adsorbed to L-alpha-egg lecithin vesicles. Biophys. J.16, 571–583 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu, K.-J. et al. Time-resolved luminescent high-throughput screening platform for lysosomotropic compounds in living cells. ACS Sensors6, 166–174 (2020). [DOI] [PubMed]
- 24.Wu, K.-J. et al. Aliphatic Group-Tethered Iridium complex as a theranostic agent against malignant melanoma metastasis. ACS Appl. Bio Mater.3, 2017–2027 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Wu, C. et al. Structure-guided discovery of a luminescent theranostic toolkit for living cancer cells and the imaging behavior effect. Chem. Sci.11, 11404–11412 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen, Y., Qiao, L., Ji, L. & Chao, H. Phosphorescent iridium (III) complexes as multicolor probes for specific mitochondrial imaging and tracking. Biomaterials35, 2–13 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Chen, J.-P. et al. Perylenequinone-based “turn on” fluorescent probe for hydrogen sulfide with high sensitivity in living cells. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc.218, 206–212 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Fu, M. et al. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc. Natl Acad. Sci.109, 2943–2948 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caliendo, G., Cirino, G., Santagada, V. & Wallace, J. L. Synthesis and biological effects of hydrogen sulfide (H2S): development of H2S-releasing drugs as pharmaceuticals. J. medicinal Chem.53, 6275–6286 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Szabo, C. et al. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br. J. Pharmacol.171, 2099–2122 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, R. et al. Aggregation enhanced responsiveness of rationally designed probes to hydrogen sulfide for targeted cancer imaging. J. Am. Chem. Soc.142, 15084–15090 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Pak, Y. L. et al. Mitochondria-targeted reaction-based fluorescent probe for hydrogen sulfide. Anal. Chem.88, 5476–5481 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Ikegami, F., Takayama, K., Tajima, C. & Murakoshi, I. Purification and properties of β-cyano-L-alanine synthase from Spinacia oleracea. Phytochemistry27, 2011–2016 (1988). [Google Scholar]
- 34.Asimakopoulou, A. et al. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE). Br. J. Pharmacol.169, 922–932 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dam, V. P., Scott, J. L., Ross, A. & Kinobe, R. T. Inhibition of cystathionine gamma-lyase and the biosynthesis of endogenous hydrogen sulphide ameliorates gentamicin-induced nephrotoxicity. Eur. J. Pharmacol.685, 165–173 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Go, Y.-M., Lee, H.-R. & Park, H. H 2 S inhibits oscillatory shear stress-induced monocyte binding to endothelial cells via nitric oxide production. Mol. cells34, 449–455 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose, P., Dymock, B. W. & Moore, P. K. GYY4137, a novel water-soluble, H2S-releasing molecule. Methods Enzymol.554, 143–167 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Amist, N. & Singh, N. in Hydrogen Sulfide in Plant Biology 87–102 (Elsevier, 2021).
- 39.Wu, L. et al. H2S-activatable near-infrared afterglow luminescent probes for sensitive molecular imaging in vivo. Nat. Commun.11, 446 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu, Z. et al. Cell-penetrating peptides transport noncovalently linked thermally activated delayed fluorescence nanoparticles for time-resolved luminescence imaging. J. Am. Chem. Soc.140, 17484–17491 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Le Trionnaire, S. et al. The synthesis and functional evaluation of a mitochondria-targeted hydrogen sulfide donor,(10-oxo-10-(4-(3-thioxo-3 H-1, 2-dithiol-5-yl) phenoxy) decyl) triphenylphosphonium bromide (AP39). MedChemComm. 5, 728–736 (2014). [Google Scholar]
- 42.O’Riordan, T. C., Zhdanov, A. V., Ponomarev, G. V. & Papkovsky, D. B. Analysis of intracellular oxygen and metabolic responses of mammalian cells by time-resolved fluorometry. Anal. Chem.79, 9414–9419 (2007). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available from the corresponding author upon reasonable request.