Abstract
This study examined the modifying effects of surgery status on the association between the Standard Deviation of NN Intervals (SDNN) of the heart rate variability and the post-traumatic stress disorder (PTSD) development. Participants with physical injury were recruited from a trauma center and followed for two years. Baseline assessment included SDNN and surgery status. Socio-demographic and clinical covariates were collected. PTSD was diagnosed at 3, 6, 12, and 24 months post-injury using the Clinician-Administered PTSD Scale for DSM-5. Logistic regression analyses were performed. Among 538 participants, 58 (10.8%) developed PTSD during the study, with prevalence rates of 8.4% at 3 months, 6.5% at 6 months, 4.7% at 12 months, and 2.5% at 24 months. A significant modifying effect was found that lower SDNN were significantly associated with PTSD in non-surgical patients but not in surgical patients, with significant interaction terms. This pattern was observed from 3 to 12 months but not at 24 months. Surgery-dependent associations between SDNN and PTSD development were observed, highlighting the need for tailored PTSD prevention strategies considering SDNN and surgery status.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-79049-z.
Keywords: Posttraumatic stress disorder, Surgery, Heart rate variability, SDNN, Longitudinal study
Subject terms: Psychology, Biomarkers
Introduction
Post-Traumatic Stress Disorder (PTSD) is a severe psychiatric condition that manifests through a variety of psychological and physiological symptoms. Heart Rate Variability (HRV) serves as a physiological marker reflecting autonomic nervous system function1. Generally, reduced HRV indicates diminished cardiovascular adaptability to internal and external stressors, thereby increasing susceptibility to PTSD2. Specifically, the Standard Deviation of NN intervals (SDNN) is the most prominent HRV marker, assessing overall heart rate variability and indicating general autonomic nervous system function3.
Previous research and meta-analyses have demonstrated a significant association between low SDNN and PTSD2,4–6. However, there are also studies that report no such association7–10, indicating a need for further investigation into this relationship.
Physical injuries are a substantial cause of PTSD11. Many individuals who suffer physical injuries require surgical interventions. HRV metrics, including SDNN, can be affected after surgery due to the body’s response to surgical trauma and anesthesia12. Consequently, the effect of SDNN on PTSD development might differ based on whether the patient underwent surgery. Despite its importance, research examining the impact of surgical intervention on the relationship between SDNN and PTSD is lacking.
This study aims to address this gap by conducting a two-year follow-up study in patients with physical injuries to investigate how SDNN influences PTSD development, considering the surgical intervention status.
Materials and methods
Study overview and participants
This analysis is part of the Biomarker-based Diagnostic Algorithm for Post-Traumatic Syndrome (BioPTS) study, aimed at refining PTSD diagnostic and predictive models. The protocol is detailed in a prior publication13. We prospectively enrolled patients admitted to the Trauma Center at Chonnam National University Hospital (CNUH), South Korea, for physical injuries from June 2015 to January 2021. Patients who met the following inclusion and exclusion criteria were invited to to participate in the present study. Inclusion criteria were (i) aged 18 years or older at the time of injury; (ii) hospitalized for more than 24 h following a moderate to severe physical injury (Injury Severity Score, ISS ≥ 9)14; and (iii) proficient in Korean to understand the study protocol. Exclusion criteria included: (i) moderate or severe brain injury (Glasgow Coma Scale, GCS < 10)15; (ii) injuries resulting from suicide attempts; (iii) severe physical conditions preventing comprehensive psychiatric evaluation; (iv) history of psychiatric disorders (psychotic disorders, bipolar disorder, or substance use disorders excluding depression and anxiety); (v) significant cognitive impairments due to organic or neurocognitive disorders; and (vi) pre-existing convulsive disorders or anticonvulsant use. Baseline psychiatric assessments including HRV measures, were conducted within one month of hospitalization, conducted in person and post-surgery if applicable. The mean (SD) duration from injury to baseline assessment was 8.8 (5.3) days. Follow-up evaluations were via telephone at 3, 6, 12, and 24 months post-injury, using Clinician-Administered PTSD Scale for Diagnostic and Statistical Manual of mental disorders 5th edition (DSM-5)16 (CAPS-5)17. The CNUH Institutional Review Board approved the study (CNUH 2015 − 148). Informed consent was obtained from all participants.
SDNN data collection and analysis
SDNN data were collected and analyzed using the SA-6000 HRV analyzer (Medicore Co., Seoul, Korea). Participants rested for 5 min before the test, removing any metal accessories, keeping their eyes open, and lying comfortably. To avoid biases from movement or posture changes, participants remained still, speaking and breathing naturally. Electrode sensors were placed on the insides of both wrists and the left ankle, with a 3-minute recording duration. A trained experimenter supervised the data collection to ensure protocol adherence and data accuracy. To ensure the accuracy and reliability of the data, HRV recordings were initially screened for artifacts using automated algorithms, which identified and marked discrepancies based on physiological thresholds and statistical parameters. Approximately 2.7% of inter-beat intervals (IBIs) were flagged by this automated process. Flagged IBIs were then manually reviewed by trained personnel who evaluated each potential artifact to determine whether adjustments or exclusions were necessary, adhering to standardized criteria that prioritize data integrity without compromising the natural variability in heart rate. Corrections were applied to less than 1% of the total IBIs, either by interpolation of adjacent normal intervals or by exclusion of non-physiological spikes. The SDNN (ms) parameter was derived using the Medicore HRV Analysis System in the SA-6000 device. SDNN data were analyzed as both continuous and categorical variables. The data were dichotomized using the median value due to the absence of established reference values and to ensure an equal distribution of participants across categories.
Operation status
Operation status was determined based on medical records documented by the surgeons. Patients were classified into two categories: those who received an operation and those who did not during the period between the physical injury and the baseline assessment.
Other baseline characteristics
To comprehensively evaluate factors potentially influencing PTSD development and HRV outcomes, a diverse array of variables was assessed as follows.
Socio-demographic characteristics
The following baseline socio-demographic characteristics were recorded: age, sex, duration of education, marital status (categorized as currently married or not), cohabitation status (living alone or not), and occupational state (current employed or not).
Pre-trauma characteristics
Prior histories of psychiatric disorders including depressive disorders, panic disorder, agoraphobia, social phobia, and generalized anxiety disorder, were documented. Participants’ experiences of previous lifetime traumatic events were assessed using the Life Events Checklist18, with the occurrence of at least one type of event being categorized as present for analysis purposes. Childhood abuse experiences were evaluated using the Nemesis Childhood Trauma Interview19, encompassing emotional/psychological, physical, and sexual abuse before the age of 16. A broad definition of “childhood abuse” (having at least one type of abuse) was utilized for the analysis. The prevalence of physical disorders was assessed via a questionnaire encompassing 15 systems or diseases. Smoking status was categorized as current smoker or not. Alcohol use was screened using the Alcohol Use Disorders Identification Test (AUDIT)20, where higher scores indicate more severe alcohol-related issues. Body Mass Index (BMI) was also calculated.
Trauma related characteristics
Type of accidental injury was evaluated using the Life Events Checklist18, to identify the specific type of traumatic event participants experienced. Recognizing that PTSD prevalence and symptom patterns often significantly differ between unintentional (e.g., accidents) and intentional (e.g., violent, interpersonal) traumas21, injury types were categorized into these two distinct groups. The severity of injuries sustained by participants was assessed using the ISS and GCS as described in the Eligibility criteria above, with higher ISS scores and lower GCS scores indicating more pronounced symptomatology.
Peri-trauma characteristics
During the peri-trauma period spanning from the index injury to the baseline evaluation, participants’ symptoms and functional status were assessed. PTSD symptom severity was gauged by summing the frequency and intensity scores of the 20 DSM-5 PTSD symptoms, yielding a total severity score17. Anxiety and depressive symptoms were measured using the Hamilton Anxiety Rating Scale (HAMA)22 and Hamilton Depression Rating Scale23, respectively, with higher scores indicating more severe symptoms. Baseline physiological status was checked through measurements of vital signs, including systolic and diastolic blood pressures and heart rate.
Follow-up diagnoses of PTSD with CAPS-5
The CAPS-5 is highly reliable and valid, suitable for detailed PTSD assessment, including via telephone24. To confirm PTSD, participants must meet DSM-5 criteria across several symptom clusters: one symptom from Cluster B (intrusion), one from Cluster C (avoidance), two from Cluster D (negative alterations in cognition and mood), and two from Cluster E (alterations in arousal and reactivity). The criteria for symptom duration (Cluster F) and functional significance (Cluster G) must also be met. This study’s outcome variables included any PTSD diagnosis during follow-up and PTSD presence at 3, 6, 12, and 24 months post-trauma, with the CAPS-5 specifically anchored to the traumatic injury event that led to hospitalization.
Statistical analysis
Participants included those who completed at least one follow-up after baseline, in line with DSM-5 PTSD criteria16. Baseline characteristics were categorized by PTSD development, SDNN median value (higher vs. lower), and surgery status (received vs. not received). Continuous and categorical variables were compared using t-tests or χ² tests. Covariates for further analysis were chosen based on statistical significance (P < 0.05). Logistic regression analyzed individual associations of SDNN and surgery status with PTSD development, adjusting for covariates. To assess the modifying effects of surgery on the SDNN-PTSD relationship, multinomial logistic regression with interaction terms was applied. This method was iteratively used for PTSD occurrences at each follow-up interval. All tests were two-sided with a significance level of P < 0.05. Analyses were conducted using SPSS, version 21.0.
Results
Recruitment and baseline data
The recruitment trajectory and PTSD prevalence at each follow-up interval are shown in Fig. S1. Of 1142 patients assessed at baseline, 580 (50.8%) underwent HRV evaluation. Baseline comparisons between those who completed and did not complete HRV evaluation are in Supplementary Table S1. Higher Injury Severity Scores (ISS) were significantly related to non-completion of HRV, but other variables were not. Of those who completed HRV assessment, 42 (7.4%) did not proceed to the 3-month evaluation, leaving 538 patients (92.6%) for analysis. No significant differences in baseline characteristics were found between completers and non-completers (all P-values > 0.05). Within this cohort, 58 patients (10.8%) were diagnosed with PTSD during the 24-month period. Baseline characteristic comparisons between patients with and without PTSD are in Table S2. PTSD diagnosis was significantly associated with female sex, higher education, previous psychiatric disorders, prior traumatic events, and elevated anxiety and depressive symptoms. Lower SDNN (≤ 22 ms) was significantly associated with higher age, female sex, lower education, more physical disorders, higher depressive symptoms, and higher heart rate (Table S3). Received surgery (N = 268; 49.8%) was significantly associated with higher depressive symptoms (Table 1). Eight covariates were identified for further analysis: age, sex, education, previous psychiatric disorders, prior traumatic events, number of physical disorders, depressive symptoms, and heart rate.
Table 1.
Baseline characteristics by post-injury surgical intervention status in 538 patients with physical injuries.
| All patients (N = 538) | Received surgery (N = 268) | Not received surgery (N = 270) | Statistical coefficients | P-valuea | |
|---|---|---|---|---|---|
| Socio-demographic characteristics | |||||
| Age, mean (SD) years | 57.0 (16.9) | 56.3 (17.1) | 57.8 (16.8) | t=-1.020 | 0.308 |
| Sex, N (%) female | 169 (31.4) | 94 (35.1) | 75 (27.8) | χ2 = 3.324 | 0.068 |
| Education, mean (SD) years | 10.7 (4.2) | 10.7 (4.3) | 10.7 (4.2) | t = + 0.003 | 0.997 |
| Marital status, N (%) unmarried | 178 (33.1) | 82 (30.6) | 96 (35.6) | χ2 = 1.494 | 0.222 |
| Living alone, N (%) | 79 (14.7) | 36 (13.4) | 43 (15.9) | χ2 = 0.667 | 0.414 |
| Unemployed status, N (%) | 88 (16.4) | 50 (18.7) | 38 (14.1) | χ2 = 2.065 | 0.151 |
| Pre-trauma characteristics | |||||
| Previous psychiatric disorders, N (%) | 41 (7.6) | 19 (7.1) | 22 (8.1) | χ2 = 0.214 | 0.644 |
| Previous traumatic events, N (%) | 29 (5.4) | 13 (4.9) | 16 (5.9) | χ2 = 0.305 | 0.581 |
| Any childhood abuse, N (%) | 37 (6.9) | 22 (8.2) | 15 (5.6) | χ2 = 1.479 | 0.224 |
| Physical disorders, mean (SD) numbers | 2.0 (2.1) | 1.9 (2.0) | 2.1 (2.1) | t=-1.303 | 0.193 |
| Current smoker, N (%) | 140 (26.0) | 75 (28.0) | 65 (24.1) | χ2 = 1.069 | 0.301 |
| AUDIT, mean (SD) scores | 10.2 (10.0) | 10.5 (10.2) | 9.9 (9.8) | t = + 0.641 | 0.522 |
| Body mass index, mean (SD) | 23.6 (3.5) | 23.6 (3.4) | 23.7 (3.6) | t=-0.267 | 0.789 |
| Trauma related characteristics | |||||
| Injury type, N (%) intentional | 51 (9.5) | 23 (8.6) | 28 (10.4) | χ2 = 0.501 | 0.479 |
| Injury severity score, mean (SD) scores | 14.0 (5.3) | 13.8 (5.7) | 14.2 (4.7) | t=-0.812 | 0.417 |
| Glasgow coma scale, mean (SD) scores | 14.9 (0.6) | 14.9 (0.5) | 14.9 (0.6) | t = + 0.565 | 0.572 |
| Peri-trauma assessment scales and measurements, mean (SD) | |||||
| HAMA | 4.7 (4.7) | 5.0 (4.7) | 4.4 (4.6) | t = + 1.438 | 0.151 |
| HAMD | 6.3 (5.4) | 6.8 (5.5) | 5.8 (5.3) | t = + 2.025 | 0.043 |
| Systolic blood pressure, mmHg | 119.1 (14.1) | 118.3 (13.5) | 120.0 (14.7) | t=-1.374 | 0.170 |
| Diastolic blood pressure, mmHg | 71.9 (9.2) | 71.4 (9.0) | 72.4 (9.3) | t=-1.298 | 0.195 |
| Heart rate per minute | 78.7 (11.1) | 78.7 (11.5) | 78.6 (10.6) | t = + 0.106 | 0.915 |
AUDIT alcohol use disorders identification test, HAMA Hamilton anxiety rating scale, HAMD Hamilton depression rating scale.
a t-tests or χ2 tests, as appropriate between patients who received surgical intervention and those who did not. Bold style indicates statistical significance (P < 0.05).
Individual associations
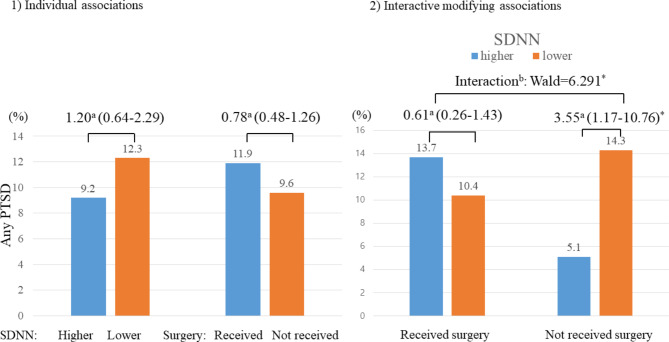
SDNN levels were not significantly associated with surgery status (Table S3). Neither SDNN levels nor surgery status was associated with any PTSD development after adjustment for covariates (left part of Fig. 1).
Fig. 1.
Individual and interactive modifying associations of SDNN and post-injury surgical intervention status with any posttraumatic stress disorder (PTSD) over 2-years in 538 patients with physical injuries. aOdds ratios (95% confidence intervals) were calculated using binary logistic regression; binteractive modifying associations were estimated using multinomial logistic regression between higher (> 22 ms) vs. lower (≤ 22 ms) SDNN and/or received vs. not received post-injury surgical interventions at baseline on development of any PTSD over 2-years, adjusted for age, sex, education, previous psychiatric disorders, previous traumatic events, number of physical disorders, scores on Hamilton Depression Rating Scale, and heart rate. *P < 0.05.
Interactive modifying associations
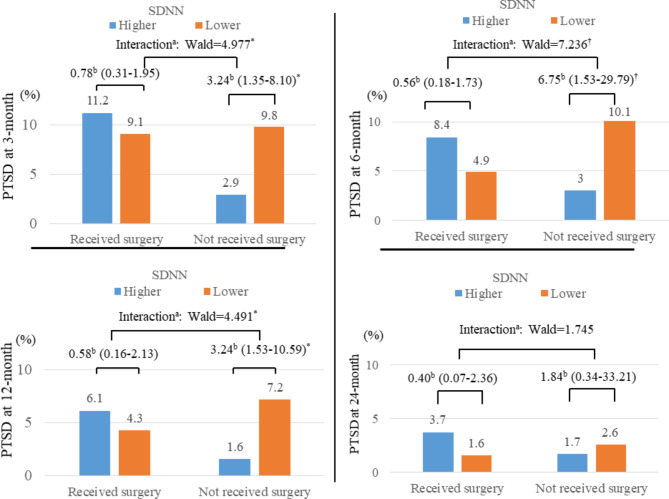
A significant modifying effect was found: lower SDNN levels were significantly associated with PTSD development in patients without surgery, but not in those who underwent surgery, with significant interaction terms (right part of Fig. 1st row of Table 2). The prevalence rates of PTSD at each follow-up interval were 8.4% at 3 months, 6.5% at 6 months, 4.7% at 12 months, and 2.5% at 24 months. The temporal relationship between SDNN levels and PTSD diagnosis over these intervals by surgery status is illustrated in Fig. 2and 2nd ~ 5th rows of Table 2. Consistent with the overarching findings, lower SDNN levels were significantly associated with PTSD diagnoses at the 3, 6, and 12-month intervals, underscored by significant interaction terms. However, this association was not evident at the 24-month evaluation. Similar interactive associations were observed when analyzing SDNN levels as continuous variables. Interactive modifying effects of SDNN on the relationships between post-injury surgical intervention and PTSD status were significant for any PTSD (Wald = 7.119; p = 0.01), PTSD at 3 months (Wald = 5.211; p = 0.012), PTSD at 6 months (Wald = 7.614; p = 0.003), and PTSD at 12 months (Wald = 4.815; p = 0.027). However, no significant association was found for PTSD at 24 months (Wald = 1.980; p = 0.306).
Table 2.
Interactive modifying effects of SDNN and post-injury surgical intervention status with posttraumatic stress disorder (PTSD) status in patients with physical injuries.
| PTSD status | Surgical intervention | SDNN | N, Patient | No. (%), PTSD | Odds ratio (95% confidence interval) | |
|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | |||||
| Any PTSD | Received | Higher (> 22 ms) | 124 | 17 (13.7) | Ref | Ref |
| Lower (≤ 22 ms) | 144 | 15 (10.4) | 0.73 (0.35–1.53) | 0.61 (0.26–1.43) | ||
| Not received | Higher (> 22 ms) | 137 | 7 (5.1) | Ref | Ref | |
| Lower (≤ 22 ms) | 133 | 19 (14.3) | 3.10 (1.26–7.63)* | 3.55 (1.17–10.76)* | ||
| PTSD at 3-month | Received | Higher (> 22 ms) | 124 | 15 (11.2) | Ref | Ref |
| Lower (≤ 22 ms) | 144 | 13 (9.1) | 0.82 (0.32–2.04) | 0.78 (0.31–1.95) | ||
| Not received | Higher (> 22 ms) | 137 | 4 (2.9) | Ref | Ref | |
| Lower (≤ 22 ms) | 133 | 13 (9.8) | 3.06 (1.19–7.86)* | 3.24 (1.35–8.10)* | ||
| PTSD at 6-month | Received | Higher (> 22 ms) | 119 | 10 (8.4) | Ref | Ref |
| Lower (≤ 22 ms) | 144 | 7 (4.9) | 0.54 (0.21–1.51) | 0.56 (0.18–1.73) | ||
| Not received | Higher (> 22 ms) | 134 | 4 (3.0) | Ref | Ref | |
| Lower (≤ 22 ms) | 130 | 13 (10.1) | 3.61 (1.15–11.38)* | 6.75 (1.53–29.79)† | ||
| PTSD at 12-month | Received | Higher (> 22 ms) | 115 | 7 (6.1) | Ref | Ref |
| Lower (≤ 22 ms) | 137 | 6 (4.3) | 0.51 (0.16–1.59) | 0.58 (0.16–2.13) | ||
| Not received | Higher (> 22 ms) | 129 | 2 (1.6) | Ref | Ref | |
| Lower (≤ 22 ms) | 125 | 9 (7.2) | 3.12 (1.04–11.88)* | 3.24 (1.53–10.59)* | ||
| PTSD at 24-month | Received | Higher (> 22 ms) | 109 | 4 (3.7) | Ref | Ref |
| Lower (≤ 22 ms) | 126 | 3 (1.6) | 0.44 (0.16–1.87) | 0.40 (0.07–2.36) | ||
| Not received | Higher (> 22 ms) | 120 | 2 (1.7) | Ref | Ref | |
| Lower (≤ 22 ms) | 118 | 3 (2.6) | 2.18 (0.26–27.99) | 1.84 (0.34–33.21) | ||
aAdjusted for age, sex, education, previous psychiatric disorders, previous traumatic events, number of physical disorders, scores on Hamilton Depression Rating Scale, and heart rate. *P < 0.05; †P < 0.01.
Fig. 2.
Interactive modifying associations of SDNN and post-injury surgical intervention status with posttraumatic stress disorder (PTSD) at 3, 6, 12, and -24months in patients with physical injuries. aInteractive modifying associations of SDNN and post-injury surgical interventions on PTSD onset were estimated using multinomial logistic regression; and bodds ratios (95% confidence intervals) were calculated using binary logistic regression for higher (> 22 ms) vs. lower (≤ 22 ms) SDNN at baseline on development of PTSD at each follow-up, adjusted for age, sex, education, previous psychiatric disorders, previous traumatic events, number of physical disorders, scores on Hamilton Depression Rating Scale, and heart rate. *P < 0.05; †P < 0.01.
Discussion
The principal findings of this two-year longitudinal study indicate that the association between lower SDNN and subsequent PTSD development was significant only in patients who did not receive surgery for their physical injury, with significant interaction terms. This pattern was consistent during the follow-up periods from 3 to 12 months post-injury but dissipated at the 24-month follow-up.
Previous research on the association between SDNN and PTSD has primarily involved cross-sectional studies of military veterans, where SDNN levels were examined based on PTSD status4,6–10. Direct comparisons with the results of this prospective study on patients with physical injuries are therefore challenging. One prior study with a similar design to ours assessed HRV at hospital admission following a traffic accident and evaluated PTSD at 2 and 6 months post-injury5. This study found that low SDNN was significantly associated with PTSD at both time points. While these findings partially align with our results, the study did not report whether the participants underwent surgery, and the sample size was relatively small, with approximately 20 participants.
Our study, with a larger cohort and long-term follow-up, reveals that the SDNN-PTSD link varies by surgical intervention status. Several mechanisms may explain these differences. Firstly, surgical interventions and perioperative care can normalize autonomic nervous system function. Surgery typically involves pain management, rehabilitation, and psychological support, stabilizing autonomic responses and HRV metrics like SDNN12. This could obscure the low SDNN-PTSD link in surgical patients, making SDNN a less effective biomarker. Secondly, surgery-induced autonomic adjustments during healing can alter HRV measures like SDNN25, masking SDNN’s predictive value for PTSD in surgical patients. Thirdly, intensive follow-up and monitoring in surgical patients can lead to early psychological intervention, reducing PTSD severity and the role of SDNN as a predictor26. Lastly, biological recovery differences between surgical and non-surgical patients, involving controlled inflammation, hormonal changes, and neuroplasticity, can disrupt the typical SDNN-PTSD associations.
Notably, the significant modifying effects of surgery status observed from 3 to 12 months post-injury dissipated by the 24-month follow-up. This can be interpreted in several ways. Firstly, the acute and subacute recovery phases involve significant physiological and psychological adjustments, influencing autonomic nervous system function and HRV metrics like SDNN27. By 24 months, these processes may have stabilized, reducing the SDNN-PTSD association. Secondly, over time, individuals may develop coping mechanisms and psychological resilience that mitigate initial autonomic dysregulation26. Thirdly, other health factors and life events might influence PTSD outcomes more significantly than initial physiological responses by 24 months. New stressors, health changes, and life circumstances can alter PTSD’s trajectory, making early biomarkers like SDNN less relevant for long-term predictions28.
A notable limitation of this study is its exclusive focus on individuals with physical injuries. While relevant to PTSD research due to the strong link between traumatic physical injuries and PTSD, the generalizability to those experiencing other trauma types remains uncertain11. Recruitment from a single trauma center aids consistency but may limit broader applicability. The HRV evaluation completion rate was 51%, with non-completion significantly associated with higher Injury Severity Scores (ISS), indicating more severely injured patients were less likely to complete HRV evaluations. This could lead to an underestimation of the HRV-PTSD association. Additionally, follow-up via telephone interviews, though validated, may lack the depth and accuracy of in-person assessments, potentially affecting data reliability24.
A principal strength of our study is its two-year longitudinal design with a large cohort. Another key advantage is the consecutive recruitment of participants from the entire population of recently injured patients, significantly reducing selection bias and ensuring a representative sample. The structured schedule of regular follow-up evaluations minimizes biases from inconsistent timing. Adherence to a rigorous research protocol ensured uniform evaluations and data collection, enhancing consistency and reliability. Additionally, collecting a wide array of potential baseline covariates for PTSD allowed for a comprehensive analytical framework. Reasonable long-term follow-up rates and analyses showing no evidence of selective attrition further bolster the credibility and reliability of our findings.
In conclusion, our study offers new insights into PTSD etiology, demonstrating surgery-dependent associations between SDNN and PTSD development, along with temporal variations in patients with physical injuries. Identifying individuals at elevated risk based on surgery status enables more precisely tailored interventions, potentially reducing PTSD onset or severity. Clinically, our findings highlight the value of incorporating SDNN measures and surgery status into PTSD diagnostic and treatment plans, emphasizing stress response regulation and coping strategy enhancement. Future research should generalize these results across diverse trauma populations and multi-centers, and investigate the underlying biological mechanisms linking SDNN alterations to PTSD development.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Conceptualization: J-MK. Methodology: J-MK, H-JK, JWK, SWK, ISS. Conuction of the study: J-MK, H-JK, JWK, HJ, JCK, JKJ, SWK, ISS. Statistical analysis and Interpretation: J-MK, H-JK. Writing -original draft preparation: J-MK, H-JK. Writing -review and editing: J-MK, H-JK. JWK, HJ, JCK, JKJ, JYL, SWK, ISS. Resources: J-MK. Supervision: J-MK, H-JK. JWK, HJ, JCK, JKJ, JYL, SWK, ISS. All authors contributed to the article and approved the submitted version.
Funding
The study was funded by a grant of National Research Foundation of Korea Grant [NRF-2020R1A2C2003472] and [NRF-2020M3E5D9080733]. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Data availability
The data that support the findings of study are available from the corresponding author (J-M Kim) upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics statement
This study was approved by the Chonnam National University Hospital Institutional Review Board (CNUH 2015 − 148) and complied with all provisions of the Declaration of Helsinki.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jae-Min Kim and Hee-Ju Kang contributed equally as first authors.
Contributor Information
Jae-Min Kim, Email: jmkim@chonnam.ac.kr.
Jae-Kyun Ju, Email: jkju@chonnam.ac.kr.
References
- 1.Schwerdtfeger, A. R. et al. Heart Rate Variability (HRV): from brain death to resonance breathing at 6 breaths/minute. Clin. Neurophysiol.131, 676–693. 10.1016/j.clinph.2019.11.03 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Schneider, M. & Schwerdtfeger, A. Autonomic dysfunction in posttraumatic stress disorder indexed by heart rate variability: a meta-analysis. Psychol. Med.50, 1937–1948. 10.1017/S003329172000207X (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaffer, F. & Ginsberg, J. P. An overview of heart rate variability metrics and norms. Front. Public. Health5, 258. 10.3389/fpubh.2017.00258 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee, E. A. D. & Theus, S. A. Lower heart rate variability associated with military sexual trauma rape and posttraumatic stress disorder. Biol. Res. Nurs.14, 412–418. 10.1177/1099800412454453 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Shaikh al arab, A. et al. Temporal analysis of heart rate variability as a predictor of post traumatic stress disorder in road traffic accidents survivors. J. Psychiatr Res.46, 790–796. 10.1016/j.jpsychires.2012.02.006 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Park, J. E. et al. Heart rate variability of chronic posttraumatic stress disorder in the Korean veterans. Psychiatry Res.255, 72–77. 10.1016/j.psychres.2017.05.011 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Agorastos, A. et al. Diminished vagal activity and blunted diurnal variation of heart rate dynamics in posttraumatic stress disorder. Stress16, 300–310. 10.3109/10253890.2012.751369 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Minassian, A. et al. Heart rate variability characteristics in a large group of active-duty marines and relationship to post-traumatic stress. Psychosom. Med.76, 292. 10.1097/PSY.0000000000000056 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brady, R. E. et al. Effect of symptom over-reporting on heart rate variability in veterans with posttraumatic stress disorder. J. Trauma. Dissoc. 16, 551–562. 10.1080/15299732.2015.1021505 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Meyer, P. W. et al. Heart rate variability in patients with post-traumatic stress disorder or borderline personality disorder: Relationship to early life maltreatment. J. Neural Transm. 123 (9), 1107–18 (2016). [DOI] [PubMed]
- 11.Bryant, R. A. et al. The psychiatric sequelae of traumatic injury. Am. J. Psychiatry. 167, 312–320. 10.1176/appi.ajp.2009.09050617 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Grässler, B., Thielmann, B., Böckelmann, I. & Hökelmann, A. Effects of different exercise interventions on heart rate variability and cardiovascular health factors in older adults: a systematic review. Eur. Rev. Aging Phys. Act.18, 24. 10.1186/s11556-021-00278-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim, J. W. et al. Development of a biomarker-based diagnostic algorithm for Posttraumatic Syndrome after Physical Injury: design of the BioPTS Study. Psychiatry Investig. 14, 513–517. 10.4306/pi.2017.14.4.513 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker, S. P., O’Neil, B., Haddon, W. & Long, W. B. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J. Trauma14, 187–196 (1974). [PubMed] [Google Scholar]
- 15.Teasdale, G. & Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet2 (7872), 81–84 (1974). [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 5th edn (American Psychiatric Publishing, 2013).
- 17.Weathers, F. W. et al. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). http://www.ptsd.va.gov/professional/assessment/adult-int/caps.asp (2017).
- 18.Jenewein, J., Wittmann, L., Moergeli, H., Creutzig, J. & Schnyder, U. Mutual influence of posttraumatic stress disorder symptoms and chronic pain among injured accident survivors: a longitudinal study. J Trauma Stress 22 (6), 540-8. 10.1002/jts.20453 (2009). [DOI] [PubMed]
- 19.De Graaf, R., Bijl, R. V., Smit, F., Vollebergh, W. A. M. & Spijker, J. Risk factors for 12-month comorbidity of mood, anxiety, and substance use disorders: findings from the Netherlands Mental Health Survey and Incidence Study. Am. J. Psychiatry. 159, 620–629 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Saunders, J. B., Aasland, O. G., Babor, T. F., de la Fuente, J. R. & Grant, M. Development of the Alcohol Use disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption. II Addict.88, 791–804. 10.1111/j.1360-0443.1993.tb02093.x (1993). [DOI] [PubMed] [Google Scholar]
- 21.Geoffrion, S. et al. Systematic review and Meta-analysis on Acute stress disorder: Rates following different types of traumatic events. Trauma Violence Abuse23 (1), 213–223 (2022). [DOI] [PubMed] [Google Scholar]
- 22.Hamilton, M. The assessment of anxiety states by rating. Br. J. Med. Psychol.32, 50–55. 10.1111/j.2044-8341.1959.tb00467.x (1959). [DOI] [PubMed] [Google Scholar]
- 23.Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 23, 56–62. 10.1136/jnnp.23.1.56 (1960). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aziz, M. & Kenford, S. Comparability of telephone and face-to-face interviews in assessing patients with posttraumatic stress disorder. J. Psychiatr. Pract.10, 07–313. 10.1097/00131746-200409000-00004 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Wynter-Blyth, V. & Moorthy, K. Prehabilitation: preparing patients for surgery. BMJ358, j3702. 10.1136/bmj.j3702 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Villa, G. et al. Effects of psychological interventions on anxiety and pain in patients undergoing major elective abdominal surgery: a systematic review. Perioper Med.9, 38. 10.1186/s13741-020-00169-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skou, S. T., Juhl, C. B., Hare, K. B., Lohmander, L. S. & Roos, E. M. Surgical or non-surgical treatment of traumatic skeletal fractures in adults: systematic review and meta-analysis of benefits and harms. Syst. Rev.9, 179. 10.1186/s13643-020-01424-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armenta, R. F. et al. Longitudinal trajectories of comorbid PTSD and depression symptoms among U.S. service members and veterans. BMC Psychiatry19, 396. 10.1186/s12888-019-2375-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of study are available from the corresponding author (J-M Kim) upon reasonable request.