Abstract
Genetic variants in Folliculin interacting protein 1 (FNIP1) were recently discovered as monogenic causes for immunodeficiency and cardiomyopathy, with only a few patients diagnosed thus far. In this study, we describe a patient harboring a novel genetic variant in FNIP1 causing immunodeficiency with cardiac involvement. Clinical and immunological workups were performed. Genetic evaluation utilizing whole-exome sequencing (WES) and Sanger sequencing was conducted. The index patient (subject II-4) presented with hypertrophic cardiomyopathy, recurrent infections, and chronic diarrhea during infancy. Immune workup revealed agammaglobulinemia and a lack of B lymphocytes. Genetic evaluation identified a homozygous 13-bp duplication variant in FNIP1 (c.52_64dupGCGCCCGGCCGCG, p. Asp22GlyfsTer21) resulting in a frameshift in exon 1/18. She was treated with supplemental intravenous immunoglobulins (IVIg) with good control of sinopulmonary and gastrointestinal manifestations. Her sibling (subject II-1) had similar clinical features, along with dysmorphic facial features and hypotony, and succumbed to cardiogenic shock at the age of 2 months, prior to genetic evaluation. Diagnosis of novel immunodeficiencies promotes our understanding of the immune system, enabling genetic counseling as herein, and may assist in the development of novel medical therapies in the future. FNIP1 loss-of-function should be considered in patients presenting in infancy with cardiac manifestations along with agammaglobulinemia (and B-cell lymphopenia).
Keywords: Primary immunodeficiency, FNIP1, Cardiomyopathy
Introduction
Inborn errors of immunity (IEI) comprise a heterogeneous group of disorders of the immune system predisposing affected individuals to acquire recurrent and/or severe infections, allergy, malignancy, autoinflammation, and autoimmune manifestations (Bucciol et al. 2024; Fischer et al. 2017; Notarangelo et al. 2020). Currently, 485 genetic causes of IEI have been included by the International Union of Immunological Societies (IUIS) expert committee. These diseases encompass disorders of B-cells and/or T-cells, the innate system, NK-cells, neutrophil defects, complement disorders, immune dysregulation disorders, auto-inflammatory diseases, bone marrow failure syndromes, and syndromic IEI (McCusker et al. 2018; Tangye et al. 2022; Yu 2024).
FNIP1 loss-of-function (LOF) is a monogenic disease-causing disorder that results in hypogammaglobulinemia with syndromic features. Folliculin interacting protein 1 (FNIP1) encodes the FNIP1 protein, a regulator of adenosine monophosphate-activated protein (AMPK) and mammalian target of rapamycin (mTOR) cellular pathways (Deenick et al. 2020). FNIP1 regulates mitochondrial activity and is crucial for B-cell maturation and myocardial function in animal models (Niehues et al. 2020; Saettini et al. 2021). Animal studies conducted on neonatal hearts from knockout FNIP1 -/- mice suggest that FNIP1 may inhibit AMPK function and cause mTOR activation. These cellular processes in cardiomyocytes lead to the accumulation of glycogen, causing cardiomyopathy and conduction disorders (Siggs et al. 2016). FNIP1 LOF in humans is associated with immunodeficiency characterized by hypogammaglobulinemia, neutropenia, and recurrent infections along with cardiac involvement, including hypertrophic cardiomyopathy (HCM), tachyarrhythmias, and pre-excitation syndromes (Niehues et al. 2020; Saettini et al. 2021). Additional features reported in patients with FNIP1 LOF include myopathy, renal cysts (Deenick et al. 2020), and central nervous involvement (developmental delay, microcephaly, and others) (Moreno-Corona et al. 2023; Niehues et al. 2020). To date, seven patients with FNIP1-associated immunodeficiency have been reported in the literature, most of whom presented typical clinical features during infancy (Deenick et al. 2020; Moreno-Corona et al. 2023; Niehues et al. 2020; Park et al. 2008; Saettini et al. 2021; Yazdani et al. 2020). In this study, we aim to describe a novel genetic variant in FNIP1, causing immunodeficiency with cardiac involvement.
Methods
Patients and clinical evaluation
Clinical data were collected and reviewed from digital hospital-based data. All the procedures were performed following informed consent from the patient parents, in accordance with the ethical standards of the institutional and/or national research committees and with the current update of the Declaration of Helsinki.
Immunological evaluation
Lymphocyte markers
Cell surface marker expression of peripheral blood mononuclear cells (PBMC) was analyzed by immunofluorescent staining with monoclonal antibodies and flow cytometry (Epics V; Coulter Electronics, Hialeah, FL) (Lev et al. 2012; Somekh et al. 2019, 2021).
Genetic evaluation
Deoxyribonucleic acid (DNA) was extracted from whole blood PMBC for an index patient (subject II-4) and her mother (subject I-2). Using the SureSelect XT Human All Exon V5 + UTR or V6 + UTR kit (Agilent Technologies, USA), DNA was prepared for a generation of whole-exome sequencing (WES) libraries. Barcoded libraries were sequenced on a NextSeq 500 platform (Illumina, USA) with an average coverage depth of 100 × . Bioinformatics analysis and subsequent filtering identified rare sequence variants. Candidate variants were prioritized based on gene function and relevance to the studied phenotype. Following the WES results, familial segregation for the FNIP1 variant was performed by Sanger sequencing.
Results
Clinical evaluation
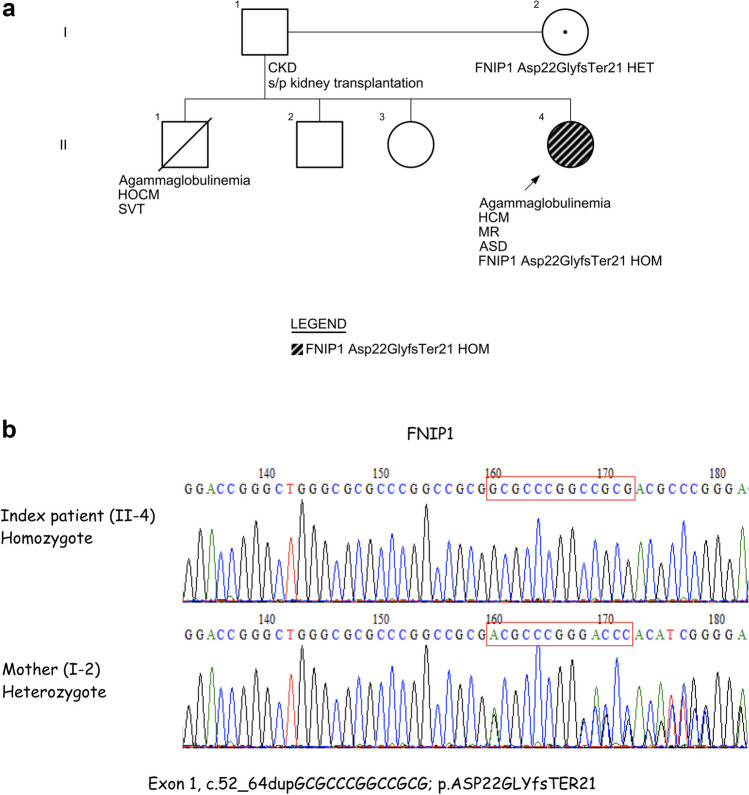
The index patient (subject II-4) was born to non-consanguineous Arab-Muslim parents (Fig. 1a). Shortly after birth, she was diagnosed with atrial-septal defect (ASD), mitral regurgitation (MR), HCM, and left ventricular hypertrophy (LVH) on transthoracic echocardiography, and cardiac computed tomography (CT). At the age of 4 months, she developed recurrent viral and bacterial infections, including recurrent sinopulmonary infections (acute otitis media and pneumonia), cellulitis, and prolonged diarrhea requiring repeated antibiotic treatments and hospitalizations. Accordingly, she developed failure to thrive (FTT). Non-typhi salmonella was isolated from stool cultures. Family history revealed a father (subject I-1) who suffered from chronic kidney disease at an early age which required allogeneic renal transplantation. Further information of his renal disease is unavailable. Her sibling (subject II-1) died in infancy.
Fig. 1.
a Pedigree of the family studied for the FNIP1 LOF. Abbreviations: Arrow, index patient; ASD, atrial septal defect; CKD, chronic kidney disease; FNIP1, Folliculin interacting protein 1; HCM, hypertrophic cardiomyopathy; HET, heterozygous; HOCM, hypertrophic obstructive cardiomyopathy; HOM, homozygote; MR, mitral regurgitation; SVT, supraventricular tachycardia. b Sanger sequencing chromatogram depicting the 13 bp duplication in FNIP1
At the age of 1 year, the patient was referred to our center for immunological and genetic evaluation. Her physical examination revealed no dysmorphic features. She did not have tonsils or palpable lymph nodes. T cell lymphocyte as well as neutrophil workups were normal (Table 1). Due to complete absence of B lymphocytes, low levels of IgG and undetectable levels of IgA and IgM, treatment with monthly intravenous immunoglobulin (IVIg) infusion was initiated, achieving normal IgG levels and exhibiting marked improvement of infections, as well as a substantial decrease in gastrointestinal symptoms. Due to cardiac involvement, she is also treated with acetylsalicylic acid, captopril, furosemide, and carnitine achieving normal growth and no physical limitations.
Table 1.
Clinical, genetic, and immunological workup
| Subject II-4 | Subject II-1 | |
|---|---|---|
| Laboratory and immunological evaluation | ||
| Immunoglobulins (normal values (%)) | ||
| IgG (540–1340 mg/dl) | 227 | 272 |
| IgA (30–188 mg/dl) | < 24 | < 24 |
| IgM (56–208 mg/dl) | < 17 | < 17 |
| IgE (0–90 IU/ml) | < 4.5 | N/A |
| Lymphocyte sub-populations (normal values (%)) | ||
| ALC (4000–10,500 cells/ mm3) (60–85%) | 10,207 | 5460 |
|
CD3+ (2600–8600 cells/ mm3) (60–85%) |
8268 (81%) | N/A |
|
CD4+ (440–1400 cells/mm3) (36–63%) |
4287 (42%) | N/A |
|
CD8+ (160–880 cells/ mm3) (15–40%) |
3675 (36%) | N/A |
| CD4 + /CD8 + (1.5–3.3) | 1.17 | N/A |
|
CD19+ (50–300 cells/ mm3) (8–22%) |
0 (0%) | N/A |
|
CD20+ (50–300 cells/ mm3) (8–22%) |
0 (0%) | N/A |
| CD56+ (6–30%) | 29% | N/A |
| CD16 + (6–30%) | 29% | N/A |
| T-cell functional tests | ||
| TCR-VB repertoire | Polyclonal | N/A |
| TREC (> 400 copies) | 10,925 | N/A |
| Neutrophils evaluation (normal values (%)) | ||
| Absolute neutrophil count (1500–8500 cells/mm3) | 5890 | 1720 |
| Dihydrorhodamine (DHR) analysis |
1. PMA-normal 2. E. coli-low |
N/A |
Abbreviations: bold font, abnormal values; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; CD, cluster of differentiation; E. coli, Escherichia coli; Ig, immunoglobulin; N/A, not available; PMA, phorbol myristate acetate; TCR, T-cell receptor; TREC, T-cell receptor excision circles
The index patient’s sibling (subject II-1) presented shortly after birth with dysmorphic facial features, hypotonia, supra-ventricular tachycardia (SVT) as well as bi-ventricular hypertrophy and hypertrophic obstructive cardiomyopathy (HOCM). He had normal lymphocyte and neutrophil counts, but profound agammaglobulinemia was found (Table 1). Normal brain, abdominal, and renal sonography were documented with no cysts. He died at 2 months old due to cardiogenic shock.
Genetic evaluation
The index patient underwent WES which identified a novel homozygous variant in FNIP1 located on chromosome 5: 131,796,857, NM_133372.3, c.52_64dupGCGCCCGGCCGCG, p. (Asp22GlyfsTer21) resulting in a frameshift of exon 1/18. According to the ACMG criteria (Richards et al. 2015), the variant is classified as likely pathogenic. The novel FNIP1 variant is a 13-bp duplication, causing a frameshift and eventually an early stop codon. The pLI score (gnomAD) was 1 (maximal score, indicating pathogenicity). Segregation studies employing Sanger sequencing identified the mother (subject I-2) as a heterozygous carrier for the identified variant (Fig. 1b). Currently, genetic data is unavailable for the father (subject I-1) and the other siblings (subjects II-2 and II-3).
Discussion
Great importance lies in the identification and characterization of novel IEI, promoting our understanding of the normal cellular biology and physiology (Kwon et al. 2023; Spivak et al. 2024). Establishing a genetic diagnosis offers numerous benefits for both patients and their families. It facilitates a better understanding of clinical manifestations, enables the development of personalized molecular and even genomic-based therapeutic strategies, and, importantly, provides patients and families with appropriate genetic counseling (Rae et al. 2018; Seleman et al. 2017; Simon et al. 2020; Somekh et al. 2024).
In this study, we describe a patient found to harbor a novel homozygous variant in FNIP1, located in exon 1, resulting in B-cell lymphopenia, agammaglobulinemia, recurrent infections, and cardiomyopathy. We could only assume that her deceased sibling (subject II-1) who exhibited a similar phenotype including profound agammaglobulinemia and severe cardiac involvement, eventually succumbing of cardiogenic shock, very likely harbored an identical genetic variant in FNIP1. A healthy mother (subject I-2) was found to harbor a heterozygous mutation. The genetic status of the father (subject I-1) was unfortunately unavailable (Fig. 1a).
The findings of the index patient and her deceased sibling are consistent with previous reports in the literature. Saettini et al. (2021) described 3 patients harboring homozygous FNIP1 genetic variants. Patient 1 harbored a homozygous nonsense FNIP1 variant (NM_133372.2; c.868C > T), patient 2, a homozygous splice site variant (c.3306 + 1G > A), and patient 3, a large deletion in exons 9 to 18 and a paternally inherited single-nucleotide variant (c.3218delT; p. Leu1073Wfs*32) causing agammaglobulinemia, neutropenia, recurrent infections along with HCM, renal cysts, and neurological disabilities. Niehues et al. (2020) described 3 patients with homozygous FNIP1 variants causing hypogammaglobulinemia, recurrent sinopulmonary and gastrointestinal infections, cardiac involvement with HCM, Wolff-Parkinson-White (WPW) syndrome, and metabolic myopathy in a single patient. Moreno‑Corona et al. (2023) described an additional FNIP1-deficient patient presenting with hypogammaglobulinemia, recurrent infections, enteropathy, HCM, and WPW. Most patients were diagnosed in childhood, whereas others were diagnosed at an older age. Cardiac involvement was the most prominent feature in some patients, while others exhibited more significant neurological manifestations. Additionally, some patients had more pronounced immunodeficiency, recurrent infections, and enteropathy. The prognosis for FNIP1 LOF patients remains uncertain, and extended follow-up is necessary for a clearer understanding (Moreno-Corona et al. 2023; Niehues et al. 2020; Saettini et al. 2021).
The index patient in our study (subject II-4) exhibited several clinical and laboratory features consistent with previously reported cases of FNIP1 LOF variants, including agammaglobulinemia, recurrent infections, chronic diarrhea, and cardiomyopathy. Neutrophil counts and function, assessed by oxidative burst with dihydrorhodamine (DHR) analysis, were normal in response to phorbol-myristate-acetate (PMA) but low in response to E. coli stimulation, in contrast to previous reports of neutropenia in other FNIP1 variants (Saettini et al. 2021), and no renal or liver cysts were found on sonography. Her sibling (subject II-1) presented with similar clinical manifestations, alongside additional features such as dysmorphic facial traits and hypotonia. Based on these findings, we believe that the homozygous novel variant in FNIP1 is the likely cause of the observed clinical phenotype.
Conclusions
This study demonstrates the significance of providing a timely genetic diagnosis of patients with IEI, for genetic counseling and appropriate treatment. FNIP1 LOF should be considered in patients presenting during infancy with cardiac manifestations along with agammaglobulinemia (and B-cell lymphopenia).
Acknowledgements
We thank the Jeffrey Modell Foundation, Israel Ministry of Health, and the Israeli Science Foundation for their support.
Author contribution
I.Sp, I.S, and R.S conceptualized the study and drafted the manuscript which was reviewed and approved by all authors. R.S treated the patient and family. A.S, A.L, and O.B performed and analyzed immune and genetic experiments.
Funding
Open access funding provided by Tel Aviv University. R.S gratefully acknowledges the funding support from the Israeli Science Foundation (ISF) under the Israel Precision Medicine Program (IPMP), grant agreement no. 3115/19 and under Personal Research Grant. I.S is funded by the Alrov scholarship and by the Sheba Research-Scientist program.
Data availability
Data is available in a repository and can be accessed via a DOI link.
Declarations
Ethics approval and consent to participate
All the procedures were performed following informed consent from the patients and first-degree relatives, in accordance with the ethical standards of the institutional and/or national research committees and with the current update of the Declaration of Helsinki.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ido Somekh and Raz Somech contributed equally to this work.
References
- Bucciol G, Delafontaine S, Meyts I, Poli C (2024) Inborn errors of immunity: a field without frontiers. Immunol Rev 322(1):15–27. 10.1111/imr.13297 [DOI] [PubMed] [Google Scholar]
- Deenick EK, Lau A, Bier J, Kane A (2020) Molecular and cellular mechanisms underlying defective antibody responses. Immunol Cell Biol 98(6):467–479. 10.1111/imcb.12345 [DOI] [PubMed] [Google Scholar]
- Fischer A, Provot J, Jais J-P, Alcais A, Mahlaoui N, Adoue D, Aladjidi N, Amoura Z, Arlet P, Armari-Alla C, Bader-Meunier B, Barlogis V, Bayart S, Beaurain B, Bertrand Y, Bienvenu B, Blanche S, Bodet D, Bonnotte B, Viallard J-F (2017) Autoimmune and inflammatory manifestations occur frequently in patients with primary immunodeficiencies. J Allergy Clin Immunol 140(5):1388-1393.e8. 10.1016/j.jaci.2016.12.978 [DOI] [PubMed] [Google Scholar]
- Kwon SS, Cho YK, Hahn S, Oh J, Won D, Shin S, Kang J-M, Ahn JG, Lee S-T, Choi JR (2023) Genetic diagnosis of inborn errors of immunity using clinical exome sequencing. Front Immunol 14:1178582. 10.3389/fimmu.2023.1178582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev A, Simon AJ, Bareket M, Bielorai B, Hutt D, Amariglio N, Rechavi G, Somech R (2012) The kinetics of early T and B cell immune recovery after bone marrow transplantation in RAG-2-deficient SCID patients. PLoS ONE 7(1):e30494. 10.1371/journal.pone.0030494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker C, Upton J, Warrington R (2018) Primary immunodeficiency. Allergy Asthma Clin Immunol 14(S2):61. 10.1186/s13223-018-0290-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Corona N, Valagussa A, Thouenon R, Fischer A, Kracker S (2023) A case report of folliculin-interacting protein 1 deficiency. J Clin Immunol 43(8):1751–1753. 10.1007/s10875-023-01559-8 [DOI] [PubMed] [Google Scholar]
- Niehues T, Özgür TT, Bickes M, Waldmann R, Schöning J, Bräsen J, Hagel C, Ballmaier M, Klusmann J, Niedermayer A, Pannicke U, Enders A, Dückers G, Siepermann K, Hempel J, Schwarz K, Viemann D (2020) Mutations of the gene FNIP1 associated with a syndromic autosomal recessive immunodeficiency with cardiomyopathy and pre-excitation syndrome. Eur J Immunol 50(7):1078–1080. 10.1002/eji.201948504 [DOI] [PubMed] [Google Scholar]
- Notarangelo LD, Bacchetta R, Casanova J-L, Su HC (2020) Human inborn errors of immunity: an expanding universe. Sci Immunol 5(49):eabb1662. 10.1126/sciimmunol.abb1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MA, Li JT, Hagan JB, Maddox DE, Abraham RS (2008) Common variable immunodeficiency: a new look at an old disease. The Lancet 372(9637):489–502. 10.1016/S0140-6736(08)61199-X [DOI] [PubMed] [Google Scholar]
- Rae W, Ward D, Mattocks C, Pengelly RJ, Eren E, Patel SV, Faust SN, Hunt D, Williams AP (2018) Clinical efficacy of a next-generation sequencing gene panel for primary immunodeficiency diagnostics. Clin Genet 93(3):647–655. 10.1111/cge.13163 [DOI] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17(5):405–424. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saettini F, Poli C, Vengoechea J, Bonanomi S, Orellana JC, Fazio G, Rodriguez FH, Noguera LP, Booth C, Jarur-Chamy V, Shams M, Iascone M, Vukic M, Gasperini S, Quadri M, BarroetaSeijas A, Rivers E, Mauri M, Badolato R, Biondi A (2021) Absent B cells, agammaglobulinemia, and hypertrophic cardiomyopathy in folliculin-interacting protein 1 deficiency. Blood 137(4):493–499. 10.1182/blood.2020006441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seleman M, Hoyos-Bachiloglu R, Geha RS, Chou J (2017) Uses of next-generation sequencing technologies for the diagnosis of primary immunodeficiencies. Front Immunol 8:847. 10.3389/fimmu.2017.00847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggs OM, Stockenhuber A, Deobagkar-Lele M, Bull KR, Crockford TL, Kingston BL, Crawford G, Anzilotti C, Steeples V, Ghaffari S, Czibik G, Bellahcene M, Watkins H, Ashrafian H, Davies B, Woods A, Carling D, Yavari A, Beutler B, Cornall RJ (2016) Mutation of Fnip1 is associated with B-cell deficiency, cardiomyopathy, and elevated AMPK activity. Proceed Nat Acad Sci 113(26). 10.1073/pnas.1607592113 [DOI] [PMC free article] [PubMed]
- Simon AJ, Golan AC, Lev A, Stauber T, Barel O, Somekh I, Klein C, AbuZaitun O, Eyal E, Kol N, Unal E, Amariglio N, Rechavi G, Somech R (2020) Whole exome sequencing (WES) approach for diagnosing primary immunodeficiencies (PIDs) in a highly consanguineous community. Clin Immunol 214:108376. 10.1016/j.clim.2020.108376 [DOI] [PubMed] [Google Scholar]
- Somekh I, Thian M, Medgyesi D, Gülez N, Magg T, Gallón Duque A, Stauber T, Lev A, Genel F, Unal E, Simon AJ, Lee YN, Kalinichenko A, Dmytrus J, Kraakman MJ, Schiby G, Rohlfs M, Jacobson JM, Özer E, Boztug K (2019) CD137 deficiency causes immune dysregulation with predisposition to lymphomagenesis. Blood 134(18):1510–1516. 10.1182/blood.2019000644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somekh I, Lev A, Barel O, Lee YN, Hendel A, Simon AJ, Somech R (2021) Exploring genetic defects in adults who were clinically diagnosed as severe combined immune deficiency during infancy. Immunol Res 69(2):145–152. 10.1007/s12026-021-09179-3 [DOI] [PubMed] [Google Scholar]
- Somekh I, Hendel A, Somech R (2024) Evolution of gene therapy for inborn errors of immunity. JAMA Pediatr 178(7):645. 10.1001/jamapediatrics.2024.1116 [DOI] [PubMed] [Google Scholar]
- Spivak I, Frizinsky S, Mandola A, Lev A, Simon AJ, Barel O, Vishnevskia-Dai V, Somech R, Somekh I (2024) Investigating concomitant RAG-2 and LRBA mutations in SCID and autoimmunity. Clin Experimental Immunol uxae083. 10.1093/cei/uxae083 [DOI] [PubMed]
- Tangye SG, Al-Herz W, Bousfiha A, Cunningham-Rundles C, Franco JL, Holland SM, Klein C, Morio T, Oksenhendler E, Picard C, Puel A, Puck J, Seppänen MRJ, Somech R, Su HC, Sullivan KE, Torgerson TR, Meyts I (2022) Human inborn errors of immunity: 2022 update on the classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol 42(7):1473–1507. 10.1007/s10875-022-01289-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani R, Habibi S, Sharifi L, Azizi G, Abolhassani H, Olbrich P, Aghamohammadi A (2020) Common variable immunodeficiency: epidemiology, pathogenesis, clinical manifestations, diagnosis, classification, and management. J Investig Allergol Clin Immunol 30(1):14–34. 10.18176/jiaci.0388 [DOI] [PubMed] [Google Scholar]
- Yu JE (2024) New primary immunodeficiencies 2023 update. Curr Opin Pediatr 36(1):112–123. 10.1097/MOP.0000000000001315 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available in a repository and can be accessed via a DOI link.