Abstract
Background: Allergy immunotherapy (AIT), a treatment approach for allergic rhinitis (AR), is recognized for its potential to modify the disease course beyond mere symptom relief. Interleukin-36γ (IL-36γ), a key player in immune responses, has been implicated in promoting eosinophilic inflammation in AR by activating eosinophils. We aimed to investigate the effect of IL-36γ on group II lymphoid cell (ILC2) in AR patients who underwent sublingual immunotherapy (SLIT).
Methods: Twenty-four AR patients were enrolled and administered with SLIT. Serum proteins of IL-36γ, interleukin-5 (IL-5), and interleukin-13 (IL-13) during SLIT were quantitatively assessed using enzyme-linked immunosorbent assay (ELISA). The proportion of ILC2 was determined by flow cytometry. Sorted ILC2s were stimulated by IL-36γ and ILC2 cell differentiation, and type II cytokines expression were examined.
Results: SLIT treatment decreased the serum protein levels of IL-36γ, IL-5, IL-13, and the proportion of ILC2 significantly. IL-36γ suppressed the proliferation of ILC2 by inhibiting the levels of ILC2 transcription factor. IL-36γ also inhibited IL-5 and IL-13 expression from ILC2.
Conclusion: The changes of IL-36γ during SLIT were related to the inhibited function of ILC2, implying that IL-36γ may be used as a new biomarker for monitoring the efficacy of SLIT in AR.
Keywords: allergic rhinitis, IL-36γ, sublingual immunotherapy
1. Introduction
Allergic rhinitis (AR), a prevalent condition characterized by eosinophilic inflammation of the nasal mucosa, is accompanied by the infiltration of T-help 2 (Th2) cells and excessive mucus production, significantly impacting patients' quality of life [1].
Allergy immunotherapy (AIT) is believed to alter the disease's progression, reduce reliance on medication, and maintain therapeutic benefits even after cessation of treatment [2]. AIT included either subcutaneously (SCIT) or sublingually (SLIT), both of which have been proven effective and safe in numerous studies [3–5]. Previous studies found that AIT regulates the immune balance by shifting the immune response from a Th2-dominated inflammation to T-help 1 (Th1) inflammation [6]. Meanwhile, the eosinophilic inflammation was alleviated after SLIT accompanied by elevated Immunoglobulin G4 (IgG4) levels [6]. Interleukin-36γ (IL-36γ) on group II innate lymphoid cells (ILC2s), known for their role in allergy through the secretion of Th2 cytokines, have also been found to be inhibited by AIT in AR patients [7–9].
IL-36γ has been proven to play an important role in bridging innate and adaptive immunity [10]. For example, IL-36γ is upregulated and involved in neutrophil infiltration in psoriasis by activating the endothelium and promoting lymphocyte recruitment [11, 12]. In chronic rhinosinusitis, IL-36γ production and activation may enhance neutrophilic inflammation. Our previous research has demonstrated that IL-36γ is also involved in eosinophilic inflammation in AR, activating eosinophils [13].
Given these insights, we aim to delve into the dynamics and effects of IL-36γ during SLIT in AR patients.
2. Methods
2.1. Patients
A retrospective study was conducted at our hospital, spanning from August 2020 to August 2022. A total of 24 adult participants with a confirmed diagnosis of AR were recruited for this study. The diagnosis of AR was established in accordance with the guidelines outlined in the AR and its Impact on Asthma (ARIA) document. The diagnostic criteria included typical allergic manifestations, such as rhinorrhea, nasal pruritus, sneezing, nasal obstruction, a positive skin-prick test (SPT) result, and/or the presence of serum-specific immunoglobulin E (IgE) antibodies reactive to Dermatophagoïdes farina, either singly or in conjunction with Dermatophagoïdes pteronyssinus. Participants were excluded if they had a history of asthma, nasal polyposis, immunodeficiency, severe systemic illnesses, or sensitivities to allergens other than D. farina or D. pteronyssinus.
For SLIT, D. farinae drops (manufactured by Zhejiang Wolwo, China) were administered. The dosage regimen involved a gradual escalation of concentrations, ranging from 1 to 333 μg/mL, over a 3-week period, adhering to the recommended schedule of 1, 2, 3, 4, 6, 8, and 10 drops of formulations numbered 1 through 3, respectively, each week for 3 consecutive weeks. During the maintenance phase, commencing from the 4th week onward, a constant dose of 333 μg/mL (administered as three drops) was given until the completion of the therapeutic course.
2.2. Efficacy Evaluation
Allergic symptoms were recorded and graded as follows: 0, no symptoms; 1, slight symptoms; 2, moderate symptoms; 3, severe symptoms. The medication usage was scored as follows: 1, oral antihistamine or nasal antihistamine; 2, intranasal corticosteroid. The patients who obtained a 30% reduction in symptom and medication scores than baseline scores were enrolled in the response group.
2.3. Flow Cytometry and Sorting for ILC2
Peripheral blood mononuclear cells (PBMCs) were isolated through density-gradient centrifugation using Lymphoprep (Fresenius Kabi Norge AS, Oslo, Norway) from heparinized leucocyte-enriched buffy coats. The obtained PBMCs were subsequently cultured at a concentration of 2 × 106 cells/mL in 24-well plates, supplemented with Roswell Park Memorial Institute (RPMI) 1640 medium containing 5% human albumin (AB) serum, 5 mmol/L glutamine, and a solution of penicillin and streptomycin (all reagents were sourced from Invitrogen, with the exception of the serum which was obtained from Sigma–Aldrich). PBMCs were stained by lineage markers (cluster of differentiation 3 [CD3], CD14, CD16, CD19, CD20, CD56, FcεR1α) and antibodies to chemoattractant receptor homologous molecule (CRTH2) and CD127 antibody. The ILC2 cells (Lin−CRTH2+CD127+ cells) were purified using FACSAria (BD Biosciences).
To examine the impact of IL-36γ on these purified ILC2 cells, various concentrations of IL-36γ (ranging from 10 to 100 ng/mL) were introduced into the culture system. To quantify the proliferative response of the ILC2 cells to these stimuli, we employed a tritium-labeled thymidine incorporation assay, which allowed us to measure the extent of cell division and, thus, the proliferative capacity of the ILC2 population under different conditions.
2.4. Real-Time Polymerase Chain Reaction (PCR) Analysis
RNA was extracted from ILC2 with RNeasy kit (Venlo). The expression of GATA binding protein 3 (GATA3) (5′-GCGGGCTCTATCACAAAATGA-3′, 5′-GCTCTCCTGGCTGCAGACAGC-3′) and receptor-related orphan receptor α (RORα) (5′-AAGGAGCCAGAAGGGATGAAC-3′, 5′-GGAACA ACAGACGCCAGTAAG-3′) were detected using an ABI 7300 System (Applied Biosystems). The messenger RNA (mRNA) levels of the detected genes were normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). This normalization process was achieved through the comparative ΔCt method.
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
The expression of IL-36γ protein was detected by ELISA kits (R&D Systems, DY2320-05). The detection limit of IL-36γ was 18.8 pg/mL.
2.6. Statistical Analysis
All data are presented as the medians and interquartile ranges. The nonparametric Mann–Whitney U test was done for comparison between the two groups, except additional note. One-way analysis of variance (ANOVA) followed by Dunnett's test was performed for comparison among more than two groups. Spearman rank correlation analysis was used for correlation analysis. A P < 0.05 was defined as statistically significant.
3. Results
3.1. SLIT Decreases IL-36γ Expression and Proportion of ILC2
The demographic information of patients is summarized in Table 1. SLIT administration resulted in a significant reduction in both symptom severity and medication usage scores (Table 2). After 2 years' SLIT, the serum protein levels of IL-36γ, interleukin-5 (IL-5), interleukin-13 (IL-13), and the proportion of ILC2 were inhibited significantly (Table 3). The protein levels of IL-36γ, IL-5, IL-13, and the proportion of ILC2 in the responsive group were significantly lower than in the unresponsive group (Table 4). The IL-36γ expression was significantly correlated with the ILC2 ratio.
Table 1.
Demographic characteristics of AR patients.
| Characteristics | SLIT group |
|---|---|
| Number | 24 |
| Sex (male:female) | 13:11 |
| Age (months) | 28.9 ± 11.6 |
| Baseline symptoms (TNSS) | 9.9 ± 2.8 |
Abbreviations: AR, allergic rhinitis; SLIT, sublingual immunotherapy; TNSS, total nasal symptom score.
Table 2.
Decreased symptom and medication score before and after 2 years' SLIT.
| Scores | Baseline | Two years' SLIT |
|---|---|---|
| Symptom score | 7.1 ± 2.1 | 2.2 ± 0.8∗ |
| Medication score | 6.0 ± 1.9 | 3.4 ± 1.0∗ |
| Total score | 12.8 ± 3.6 | 5.6 ± 2.2∗ |
Abbreviation: SLIT, sublingual immunotherapy.
∗ Compared with baseline, P < 0.05.
Table 3.
Decreased cytokine levels and ILC2 proportion after 2 years' SLIT.
| Characteristics | Baseline | Two years' SLIT |
|---|---|---|
| IL-36γ (pg/mL) | 956.3 ± 315.2 | 413.2 ± 159.8∗ |
| IL-5 (pg/mL) | 22.5 ± 8.9 | 11.3 ± 5.4∗ |
| IL-13 (pg/mL) | 295.1 ± 78.5 | 75.3 ± 21.6∗ |
| Proportion of ILC2 (%) | 0.13 ± 0.05 | 0.03 ± 0.01∗ |
Abbreviations: IL-5, interleukin-5; IL-13, interleukin-13; IL-36γ, interleukin-36γ; ILC2, IL-36γ on group II lymphoid cell; SLIT, sublingual immunotherapy.
∗ Compared with baseline, P < 0.05.
Table 4.
Comparison of scores and cytokine levels between responsive and unresponsive groups.
| Characteristics | Responsive group | Unresponsive group |
|---|---|---|
| Symptom score | 1.8 ± 0.6∗ | 5.9 ± 2.4 |
| Medication score | 2.8 ± 1.1∗ | 5.6 ± 1.8 |
| Total score | 4.7 ± 1.7∗ | 10.5 ± 3.3 |
| IL-36γ (pg/mL) | 377.5 ± 159.1∗ | 838.1 ± 347.8 |
| IL-5 (pg/mL) | 9.6 ± 4.8∗ | 24.1 ± 9.3 |
| IL-13 (pg/mL) | 68.2 ± 23.9∗ | 273.4 ± 81.6 |
| Proportion of ILC2 (%) | 0.02 ± 0.01∗ | 0.14 ± 0.04 |
Abbreviations: IL-5, interleukin-5; IL-13, interleukin-13; IL-36γ, interleukin-36γ; ILC2, IL-36γ on group II lymphoid cell.
∗ Compared with baseline, P < 0.05.
3.2. IL-36γ Regulated ILC2 Proliferation and Function
In vitro experiments revealed that IL-36γ exerts a suppressive effect on ILC2 proliferation by downregulating the expression of key transcription factors, GATA3, and retinoid acid RORα. Furthermore, the levels of IL-5 and IL-13 secreted by IL-36γ-stimulated ILC2s were significantly reduced compared to unstimulated controls, as illustrated in Figure 1.
Figure 1.
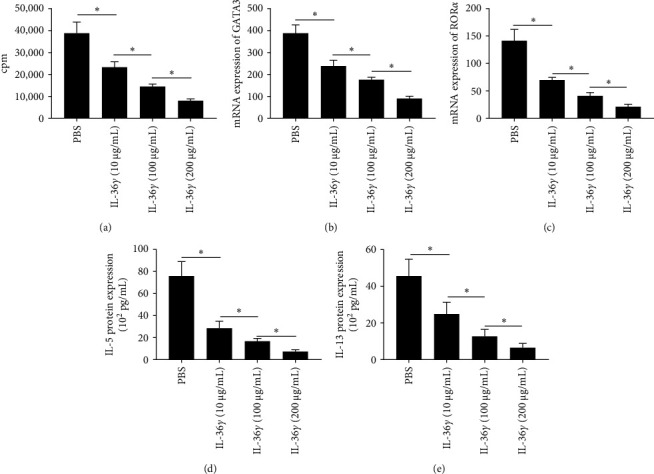
IL-36γ regulated ILC2 proliferation and function: (A) IL-36γ decreased ILC2 proliferation determined by tritiated thymidine incorporation. (B, C) The mRNA levels of GATA3 and RORα by ILC2. (D, E) The protein expression of IL-5 and IL-13 by ILC2. ∗P < 0.05. cpm, counts per minute; GATA3, GATA binding protein 3; IL-5, interleukin-5; IL-13, interleukin-13; IL-36γ, interleukin-36γ; ILC2, IL-36γ on group II lymphoid cell; mRNA, messenger RNA; PBS, phosphate-buffered saline; RORα, receptor-related orphan receptor α.
4. Discussion
AIT has emerged as an effective therapeutic approach for managing AR and asthma [14, 15]. Several studies have revealed a transition from a Th2-dominated response to a Th1-prevalent pattern subsequent to SLIT treatment, and this process involves complex immune regulatory mechanisms. For example, Ciprandi's study suggested that a decrease in the expression of human leukocyte antigen (HLA)-G, HLA-A, HLA-B, and HLA-C molecules appears to be intricately linked to one of the postulated mechanisms underlying the efficacy of SLIT, particularly its ability to redirect the immune system's response from a Th2 cytokine secretion pattern towards a Th1-oriented one [16].
Recently, studies suggested that AIT can also reduce the proportion of ILC2 in house dust mite (HDM) or grass pollen-sensitized AR patients [8, 9]. Moreover, AIT also reduced IL-13 expression by ILC2 in AR patients during the pollen season, as well as seasonal symptoms [8]. Mitthamsiri's study found a significant reduction of circulating ILC2 and elevation of ILC1 after AIT, which is similar to the proportion as seen in controls [17]. However, one study failed to demonstrate a change in ILC2 proportions in grass pollen-allergic AR patients following AIT [18].
In our study, we also found that SLIT treatment decreased ILC2 proportion in HDM-sensitized AR patients. Moreover, the serum levels of IL-36γ, IL-5, IL-13, and ILC2 proportion in the responsive group were significantly lower compared with the unresponsive group, suggesting that alleviation of symptoms during SLIT may be related to decreased proportion of ILC2.
IL-36γ, a member of the IL-1 cytokine family, has been well-established as a key mediator in various inflammatory reactions [10]. Its receptor, IL-36R, is expressed by a diverse array of immune and non-immune cells, including dendritic cells, T cells [19–23], endothelial cells [24], keratinocytes [25, 26], intestinal epithelial cells, and fibroblasts. Elevated IL-36γ expression has been implicated in the pathogenesis of inflammatory bowel disease (IBD) [27, 28] and asthma models, where it is induced in bronchial epithelial cells upon exposure to viral infections, smoke, or allergens [29, 30].
To substantiate this relationship, we conducted in vitro experiments demonstrating that IL-36γ directly inhibits ILC2 proliferation and modulates the expression of key transcription factors. Furthermore, IL-36γ suppressed the production of type II cytokines by ILC2s, highlighting its direct effect on ILC2 function.
Interestingly, our previous study suggested that the upregulation of IL-36γ in nasal epithelial cells is facilitated by IL-17, IL-25, and IL-33 [13]. Similarly, in normal bronchial epithelial cells, IL-36γ expression can be enhanced in response to double-stranded RNA, IL-1β, tumor necrosis factor-α (TNF-α), and IL-17 stimuli [31]. It is universally acknowledged that IL-17, IL-25, and IL-33 can promote the proliferation and function of ILC2, but on the other hand, they induce epithelial cells to produce IL-36γ, which in turn inhibits the proliferation and function of ILC2 cells, thus forming a negative feedback loop.
The disparate responsiveness to these stimuli may stem from the varied expression patterns of IL-36R within the nasal cavity. Intriguingly, allergen exposure, specifically to D. pteronyssinus group 1 (Der p1) from HDM, significantly upregulates IL-36R expression on eosinophils isolated from AR patients' peripheral blood and neutrophils purified from healthy individuals [13]. These findings underscore the potential significance of IL-36γ as a crucial pathway in modulating AR inflammation, particularly through its regulatory effects on eosinophils, which are key effector cells in allergic inflammatory responses.
5. Conclusion
Our study is the first to demonstrate a relationship between changes in IL-36γ levels during SLIT and the inhibition of ILC2 function. Our findings propose IL-36γ as a novel biomarker for SLIT efficacy, offering a potential predictive tool for personalized treatment strategies in AR management.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
No funding was received for this research.
References
- 1.Brozek J. L., Bousquet J., Baena-Cagnani C. E., et al. Allergic Rhinitis and Its Impact on Asthma (ARIA) Guidelines: 2010 Revision. Journal of Allergy and Clinical Immunology . 2010;126(3):466–476. doi: 10.1016/j.jaci.2010.06.047. [DOI] [PubMed] [Google Scholar]
- 2.Burks A. W., Calderon M. A., Casale T., et al. Update on Allergy Immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL Consensus Report. Journal of Allergy and Clinical Immunology . 2013;131(5):1288–1296.e3. doi: 10.1016/j.jaci.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 3.Abramson M. J., Puy R. M., Weiner J. M. Injection Allergen Immunotherapy for Asthma. Cochrane Database of Systematic Reviews . 2010;4(8) doi: 10.1002/14651858.CD001186.pub2.Cd001186 [DOI] [PubMed] [Google Scholar]
- 4.Meadows A., Kaambwa B., Novielli N., et al. A Systematic Review and Economic Evaluation of Subcutaneous and Sublingual Allergen Immunotherapy in Adults and Children With Seasonal Allergic Rhinitis. Health Technol Assess . 2013;17(27) doi: 10.3310/hta17270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yukselen A., Kendirli S. G., Yilmaz M., et al. Effect of One-Year Subcutaneous and Sublingual Immunotherapy on Clinical and Laboratory Parameters in Children With Rhinitis and Asthma: A Randomized, Placebo-Controlled, Double-Blind, Double-Dummy Study. International Archives of Allergy and Immunology . 2012;157(3):288–298. doi: 10.1159/000327566. [DOI] [PubMed] [Google Scholar]
- 6.Kappen J. H., Durham S. R., Veen H. I.’t, Shamji M. H. Applications and Mechanisms of Immunotherapy in Allergic Rhinitis and Asthma. Therapeutic Advances in Respiratory Disease . 2017;11(1):73–86. doi: 10.1177/1753465816669662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neill D. R., Wong S. H., Bellosi A., et al. Nuocytes Represent a New Innate Effector Leukocyte that Mediates Type-2 Immunity. Nature . 2010;464(7293):1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lao-Araya M., Steveling E., Scadding G. W., et al. Seasonal Increases in Peripheral Innate Lymphoid Type 2 Cells are Inhibited by Subcutaneous Grass Pollen Immunotherapy. Journal of Allergy and Clinical Immunology . 2014;134(5):1193–1195.e4. doi: 10.1016/j.jaci.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 9.Fan D. C., Wang X. D., Wang C. S., et al. Suppression of Immunotherapy on Group 2 Innate Lymphoid Cells in Allergic Rhinitis. Chinese Medical Journal . 2016;129(23):2824–2828. doi: 10.4103/0366-6999.194642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W., Yu X., Wu C., Jin H. IL-36γ Inhibits Differentiation and Induces Inflammation of Keratinocyte via Wnt Signaling Pathway in Psoriasis. International Journal of Medical Sciences . 2017;14(10):1002–1007. doi: 10.7150/ijms.20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Erme A. M., Wilsmann-Theis D., Wagenpfeil J., et al. IL-36γ (IL-1F9) Is a Biomarker for Psoriasis Skin Lesions. Journal of Investigative Dermatology . 2015;135(4):1025–1032. doi: 10.1038/jid.2014.532. [DOI] [PubMed] [Google Scholar]
- 12.Carrier Y., Ma H. L., Ramon H. E., et al. Inter-Regulation of Th17 Cytokines and the IL-36 Cytokines in Vitro and in Vivo: Implications in Psoriasis Pathogenesis. Journal of Investigative Dermatology . 2011;131(12):2428–2437. doi: 10.1038/jid.2011.234. [DOI] [PubMed] [Google Scholar]
- 13.Qin X., Liu M., Zhang S., Wang C., Zhang T. The Role of IL-36γ and Its Regulation in Eosinophilic Inflammation in Allergic Rhinitis. Cytokine . 2019;117:84–90. doi: 10.1016/j.cyto.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Calderón M. A., Bousquet J., Canonica G. W., et al. Guideline Recommendations on the use of Allergen Immunotherapy in House Dust Mite Allergy: Time for a Change? Journal of Allergy and Clinical Immunology . 2017;140(1):41–52. doi: 10.1016/j.jaci.2017.01.049. [DOI] [PubMed] [Google Scholar]
- 15.Wise S. K., Lin S. Y., Toskala E., et al. International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis. International Forum of Allergy & Rhinology . 2018;8(2):108–352. doi: 10.1002/alr.22073_c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciprandi G., Contini P., Murdaca G., DeAmici M., Gallina A. M., Puppo F. Soluble Serum HLA-G and HLA-A, -B, -C Molecules in Patients With Seasonal Allergic Rhinitis Exposed to Pollens. International Immunopharmacology . 2009;9(9):1058–1062. doi: 10.1016/j.intimp.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Mitthamsiri W., Pradubpongsa P., Sangasapaviliya A., Boonpiyathad T. Decreased CRTH2 Expression and Response to Allergen Re-Stimulation on Innate Lymphoid Cells in Patients With Allergen-Specific Immunotherapy. Allergy, Asthma & Immunology Research . 2018;10(6):662–674. doi: 10.4168/aair.2018.10.6.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lombardi V., Beuraud C., Neukirch C., et al. Circulating Innate Lymphoid Cells Are Differentially Regulated in Allergic and Nonallergic Subjects. Journal of Allergy and Clinical Immunology . 2016;138(1):305–308. doi: 10.1016/j.jaci.2015.12.1325. [DOI] [PubMed] [Google Scholar]
- 19.Ngo V. L., Abo H., Maxim E., et al. A Cytokine Network Involving IL-36γ, IL-23, and IL-22 Promotes Antimicrobial Defense and Recovery From Intestinal Barrier Damage. Proceedings of the National Academy of Sciences . 2018;115(22):E5076–E5085. doi: 10.1073/pnas.1718902115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harusato A., Abo H., Ngo V. L., et al. IL-36γ Signaling Controls the Induced Regulatory T Cell–Th9 Cell Balance via NFκB Activation and STAT Transcription Factors. Mucosal Immunology . 2017;10(6):1455–1467. doi: 10.1038/mi.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vigne S., Palmer G., Lamacchia C., et al. IL-36R Ligands are Potent Regulators of Dendritic and T Cells. Blood . 2011;118(22):5813–5823. doi: 10.1182/blood-2011-05-356873. [DOI] [PubMed] [Google Scholar]
- 22.Foster A. M., Baliwag J., Chen C. S., et al. IL-36 Promotes Myeloid Cell Infiltration, Activation, and Inflammatory Activity in Skin. The Journal of Immunology . 2014;192(12):6053–6061. doi: 10.4049/jimmunol.1301481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vigne S., Palmer G., Martin P., et al. IL-36 Signaling Amplifies Th1 Responses by Enhancing Proliferation and Th1 Polarization of Naive CD4+ T Cells. Blood . 2012;120(17):3478–3487. doi: 10.1182/blood-2012-06-439026. [DOI] [PubMed] [Google Scholar]
- 24.Bridgewood C., Fearnley G. W., Berekmeri A., et al. IL-36γ Is a Strong Inducer of IL-23 in Psoriatic Cells and Activates Angiogenesis. Frontiers in Immunology . 2018;9 doi: 10.3389/fimmu.2018.00200.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernández-Santana Y. E., Leon G., St Leger D., Fallon P. G., Walsh P. T. Keratinocyte Interleukin-36 Receptor Expression Orchestrates Psoriasiform Inflammation in Mice. Life Science Alliance . 2020;3(4) doi: 10.26508/lsa.201900586.e201900586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldstein J. D., Bassoy E. Y., Caruso A., et al. IL-36 Signaling in Keratinocytes Controls Early IL-23 Production in Psoriasis-Like Dermatitis. Life Science Alliance . 2020;3(6) doi: 10.26508/lsa.202000688.e202000688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishida A., Hidaka K., Kanda T., et al. Increased Expression of Interleukin-36, a Member of the Interleukin-1 Cytokine Family, in Inflammatory Bowel Disease. Inflammatory Bowel Diseases . 2016;22(2):303–314. doi: 10.1097/MIB.0000000000000654. [DOI] [PubMed] [Google Scholar]
- 28.Kanda T., Nishida A., Takahashi K., et al. Interleukin(IL)-36α and IL-36γ Induce Proinflammatory Mediators From Human Colonic Subepithelial Myofibroblasts. Frontiers in Medicine . 2015;2 doi: 10.3389/fmed.2015.00069.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedrich M., Tillack C., Wollenberg A., Schauber J., Brand S. IL-36γ Sustains a Proinflammatory Self-Amplifying Loop With IL-17C in Anti-TNF–Induced Psoriasiform Skin Lesions of Patients With Crohn’s Disease. Inflammatory Bowel Diseases . 2014;20(11):1891–1901. doi: 10.1097/MIB.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 30.Walsh P. T., Fallon P. G. The Emergence of the IL-36 Cytokine Family as Novel Targets for Inflammatory Diseases. Annals of the New York Academy of Sciences . 2018;1417(1):23–34. doi: 10.1111/nyas.13280. [DOI] [PubMed] [Google Scholar]
- 31.Chustz R. T., Nagarkar D. R., Poposki J. A., et al. Regulation and Function of the IL-1 Family Cytokine IL-1F9 in Human Bronchial Epithelial Cells. American Journal of Respiratory Cell and Molecular Biology . 2011;45(1):145–153. doi: 10.1165/rcmb.2010-0075OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


