Abstract
Bone loss in the alveolar ridge is a factor widely studied by dentists in implant surgeries, as it poses a major challenge for aesthetic and functional recovery in patients with large maxillary bone defects. Synthetic biomaterials function as grafts designed to replace and remodel bone tissue. Calcium phosphate is a biomaterial that has good properties such as biocompatibility and bioactivity, making it a reference in bone replacement treatments. A synthetic biomaterial such as calcium phosphate can be obtained by various synthesis techniques. The microwave hydrothermal method (HTMO) is a pathway that allows changes in synthesis parameters and significantly increases the transmission efficiency of materials such as synthetic calcium phosphate derivatives. The study proposes obtaining a biomaterial for bone grafting based on calcium phosphate by the microwave HTMO and evaluating its microstructural and physicochemical characteristics. The parameters tested in this process were temperature and reaction time. The calcium phosphate particulates were obtained by the microwave HTMO at temperatures of 110°C and 130°C for 60 min and calcined at 300°C, 500°C, and 700°C. Microstructural and physicochemical characterization analyses were carried out using scanning electron microscopy, Fourier transform infrared, and X-ray diffraction. The results obtained showed the presence of more than one calcium phosphate biological interest phase, as hydroxyapatite (HA), tricalcium phosphate (β-TCP), and octacalcium phosphate (OCP), highlighting with increasing calcination temperature, the β-TCP phase becomes evident. The proposed synthesis method was efficient in obtaining a biomaterial with suitable physical and chemical characteristics, with an association of crystalline phases of biological interest related to the increase in synthesis temperature and calcination temperature.
Keywords: biomaterials, bone grafts, calcium phosphates, hydrothermal synthesis, microwave-assisted
1. Introduction
Bones, as support matrices for the body, can present different types of integration between organic and inorganic material, which produces considerable variations in mechanical properties. The ratio between the two components reflects the relationship between hardness (high inorganic content) and elasticity or resistance to fracture (low inorganic content). Besides performing these functions, bones also serve as a storage site for calcium, phosphate, and other ions, regulating their release and absorption in a controlled manner to uphold a stable concentration of these ions in bodily fluids such as interstitial fluid and blood [1–4]. The lack of bone in the alveolar ridge has been a major problem in the aesthetic-functional recovery of patients who have suffered dentoalveolar trauma, traumatic tooth extractions, congenital absence of teeth, pathologies involving the maxilla and mandible, as well as infections. Bone loss can occur due to periodontal disease, traumatic surgery, or physiological reasons due to lack of ridge function or inadequate prosthetic load [1, 2].
Much is being studied about the technological development of biomaterials to selectively influence the tissue response of the receiving bed, as bioceramics, which should lead to new bone formation by controlling the quality and quantity of bone within the receiving area [1, 2]. Synthetic biomaterials function as grafts designed to replace and remodel various types of human tissue. These synthetic grafts function as carriers of cells and various molecules that act as adjuvants in tissue repair [1, 3, 5]. Calcium phosphate derivatives are known for their biocompatibility, lack of immunogenic reactions and apparent ability to bind to host tissue. These benefits can be explained by the chemical nature of these materials, which are basically made up of calcium and phosphate ions, actively participating in the ionic balance between the biological fluid and the ceramic [2, 6, 7]. Calcium phosphate has good properties as a biomaterial, biocompatibility, and bioactivity, as well as appreciable mechanical properties, which has made it a reference in bone replacement treatments. The characteristics associated with hydrophilicity, which allows the surface to be moistened by body fluids, favor the proliferation of bone cells (osteoblasts) [7–10]. The characteristics of calcium phosphate derivatives depend on a series of factors relating to the synthesis process, such as impurities, precursor reagents, crystal morphology and size, concentration, pH, and temperature. In addition, the bioactivity of these materials also depends on the heat treatment profile for drying and sintering the material [9, 11].
Santos et al. [12] highlight the importance of the hydroxyapatite (HA) phase in bone repair, since it presents structural and chemical similarities with the bone mineral section, in addition to presenting properties such as biocompatibility, bioactivity, and osteoconductivity.
Some methods for preparing calcium phosphate synthetically can be found to literature, such as precipitation, sol-gel process, spray pyrolysis, hydrothermal synthesis, emulsion processing, and mechanochemical method. Some authors have demonstrated the use of microwave irradiation in organic chemical transformations, considering short reaction times, increased product yield, greater product purity and reduced side reactions due to microwave heating [13, 14]. The microwave heating synthesis method is used in chemical processes, especially for organic and inorganic products. In general, microwave energy goes beyond the external surface of the system, reaching its interior, allowing the reaction to occur homogeneously, reducing reaction time, in addition to not requiring postreaction cooling processes [13–16].
Leonelli and Komarneni [17] broadly developed the microwave hydrothermal process for the synthesis of inorganic materials on nanometric scale and studies demonstrated advantages such as rapid heating to the reaction temperature, increased reaction kinetics, elimination of metastable phases, and formation of new phases.
The objective of this study was to investigate the synthesis of calcium phosphate utilizing the microwave hydrothermal method (HTMO) at temperatures of 110°C and 130°C for a 60 min duration, followed by calcination at 300°C, 500°C, and 700°C, to assess the effects of time and temperature. The resulting materials were subjected to characterization using scanning electron microscopy (SEM), Fourier transform infrared (FTIR) spectroscopy, and X-ray diffraction (XRD).
2. Materials and Methods
2.1. Synthesis of Materials
The synthesis via the microwave HTMO involved dissolving Ca (OH)2 at a concentration of 1 mol/L in distilled and deionized H2O. Phosphoric acid (H3PO4) was then added to the solution in a round-bottomed flask, which was heated and continuously stirred at 90°C. Subsequently, the suspension was transferred to a Teflon reactor and subjected to the microwave hydrothermal process (HMO-100, LIEC) at temperatures of 110°C and 130°C for 60 min. After centrifugation, the resulting powders were dried in a vacuum oven and subjected to heat treatment at temperatures of 300°C, 500°C, and 700°C for 1 h. The samples were divided into 8 groups, as outlined in Table 1.
Table 1.
Groups corresponding to the synthesis and heat treatment conditions of the samples analyzed.
| Groups | Synthesis temperature/weather | Calcination |
|---|---|---|
| G1 | 110°C/60 min | Without calcination |
| G2 | 110°C/60 min | 300°C |
| G3 | 110°C/60 min | 500°C |
| G4 | 110°C/60 min | 700°C |
| G5 | 130°C/60 min | Without calcination |
| G6 | 130°C/60 min | 300°C |
| G7 | 130°C/60 min | 500°C |
| G8 | 130°C/60 min | 700°C |
2.2. Physical and Chemical Characterization
The used techniques were FTIR (Bruker Equinox 55), with an accessory for diffuse reflectance in the range of 400–4000 cm−1, XRD on a Rigaku PC-Max 2500 diffractometer with an angular scan between 10° and 60°, using CuKα radiation (λ = 1.5418 Å), voltage of 30 kV and current of 15 mA, with a speed of 0.02 degrees every 40 s and SEM Carl Zeiss, model supra 35-VP.
3. Results and Discussion
Figure 1(a) shows the diffractograms obtained by XRD of the samples synthesized at 110°C for 60 min using the microwave HTMO and calcined at 300°C, 500°C, and 700°C, which belong to groups G1, G2, G3, and G4.
Figure 1.
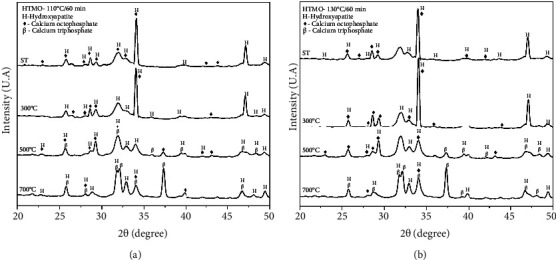
(a) X-ray diffractograms of the samples obtained by synthesizing calcium phosphate for 60 min at 110°C using the microwave hydrothermal method (HTMO). The curves represent the samples without heat treatment, and calcined at 300°C, 500°C, and 700°C. HA (hydroxyapatite), OCP (octacalcium phosphate), and TCP (tricalcium phosphate). (b) X-ray diffractograms of the samples obtained by synthesizing calcium phosphate for 60 min at 130°C using the microwave hydrothermal method (HTMO). The curves represent the samples without heat treatment and calcined at 300°C, 500°C, and 700°C. HA (hydroxyapatite), OCP (octacalcium phosphate), and TCP (tricalcium phosphate).
Figure 1(b) shows the diffractograms obtained by XRD of the samples synthesized at 130°C for 60 min using the microwave HTMO and calcined at 300°C, 500°C, and 700°C, which belong to groups G5, G6, G7, and G8.
XRD analysis showed that all synthesis temperatures, without or with heat treatment, led to the formation of HA, as well as the presence of the OCP phase, which is recognized as a precursor to the HA phase. It should also be noted that the characteristic peaks for HA show greater intensity and better resolution. This could mean that the material obtained is more crystalline. The 2θ values located within the 20° and 50° range correspond to the main peaks of the calcium phosphate phases of biological interest: HA, β-TCP, and OCP. The results found in this study can be confirmed with the work described by Lak et al. [18] and Méndez-Lozano et al. [19], who in their investigations on samples of calcium phosphate derivatives analyzed by XRD, identified HA as the main crystalline phase, evaluating it as an indication of the material's purity. Lak et al. [18] states the existence of sharp peaks and high intensity in the analysis of diffractograms confirming the formation of crystalline HA after microwave irradiation, showing differentiated XRD patterns compared to the standard file and other works that showed HA nanostructure using microwave irradiation at longer times [18–20].
This study corroborates the results presented to Santos et al. [21] among other authors, in which they demonstrate in physicochemical analyzes the formation of multiphase calcium phosphate according to the calcination temperature to which the material was exposed at temperatures above 300°C they observed the phases of amorphous phosphate (ACP 1 and 2), HAd (calcium-deficient HA phase) and HA, while at temperatures above 600°C they observed the phases HAd, HA, and β-TCP, as shown in the following reactions [12, 21, 22].
| (1) |
In the diffractograms Figures 1(a) and 1(b), the OCP phase is represented by the reflection peaks that correspond to the reflection planes that corroborate JCPDS datasheet no. 79.423. In the samples treated at 500°C and 700°C, it is evident that the OCP phase is less pronounced and exhibits lower crystallinity, while the HA and β-TCP phases are more prominent. This is indicated by peaks corresponding to specific planes, such as 200, 002, 102, 210, 211, 300, 202, 212, 130, 131, 222, 312, and 213 for HA, according to JCPDS technical file no. 731731, and peaks corresponding to planes 024, 1010, 0210, 128, 2110, 1016, 4010, 146, 1211, 404, 2116, 214, 220, and 238 for TCP, as per JCPDS technical file no. 86.1585. These results are consistent with previous studies where researchers correlated the observed peaks in diffractograms with crystallite sizes. It has been observed in the synthesis of calcium phosphate using microwave irradiation that longer synthesis times lead to an increase in crystallite size. According to the authors Natalia L [11], Lack A [18], and Méndez-Lozano [19], this result was expected since longer syntheses provide the crystal with more time for nucleation and growth and present in them analyzes a 13% increase in the size of the crystallite when compared to the methods of conventional synthesis [11, 18, 19].
The infrared analyses showed results that corroborate the XRD analyses, both for the samples without heat treatment (ST) (G1 and G5) and for the samples calcined at 300°C (G2 and G6), 500°C (G3 and G7), and 700°C (G4 and G8).
The infrared spectra obtained for the samples synthesized by the microwave HTMO at temperatures of 110°C (a) and 130°C (b) for 60 min are depicted in Figures 2(a) and 2(b), respectively. According to the FTIR analysis, the appearance of bands at 3939 cm−1, 3873 cm−1, 3783 cm−1, 3691 cm−1, 3640 cm−1, and 3572 cm−1 signifies axial stretching deformations of phosphate groups, associated with the OH group. Additionally, bands at 523 cm−1 and 694 cm−1 in the samples treated at 700°C indicate the presence of phosphate ions (PO43−), reflecting angular deformations of this group. Moreover, bands observed at 3640 cm−1, 1645 cm−1, and 1647 cm−1 in the samples treated from 300°C can be attributed to H-O-H deformation.
Figure 2.
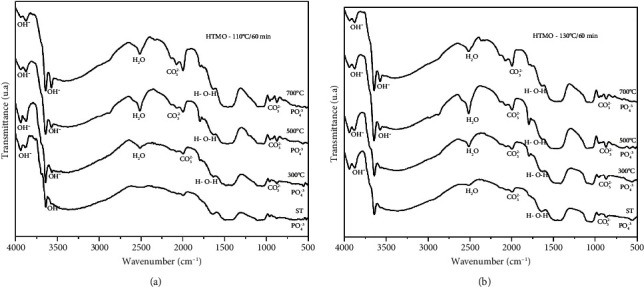
(a) Infrared spectra of the samples obtained by synthesizing calcium phosphate for 60 min at 110°C using the microwave hydrothermal method (HTMO). The curves represent the samples without heat treatment (ST) and calcined at 300°C, 500°C, and 700°C. (b) Infrared spectrum of the samples obtained by the synthesis of calcium phosphate conducted for 60 min at 130°C using the microwave hydrothermal method (HTMO). The curves represent the samples without heat treatment (ST) and calcined at 300°C, 500°C, and 700°C.
The curves indicate reduced intensity in the bands 3691 cm−1, 2142 cm−1, 1793 cm−1, 1749 cm−1, 957 cm−1, 874 cm−1, and 792 cm−1. A broad band was observed between 2700 cm−1 and 3800 cm−1, with maximum intensity around 3640 cm−1, which would reflect the combination of water and powder Ca3(PO4). The greater solubility of the ACP phase in relation to HA becomes an important characteristic for its use as a biomaterial, as it provides a higher rate of degradation in the biological environment. A soluble material allows the exchange of Ca2+ and PO4−3 ions with the biological environment, facilitating bone growth [19, 23].
The bands observed at 2513 cm−1 and 2347 cm−1 are indicative of the presence of water, while bands at 2142 cm−1, 2077 cm−1, 957 cm−1, 929 cm−1, 874 cm−1, and 792 cm−1 are associated with carbonate (CO32−) presence. Additionally, the band at 1753 cm−1 suggests the presence of carbonates, linked to Type B substitution commonly found in biologically relevant calcium phosphates [18, 21, 23]. The absorption of these bands is sensitive to the type of substitution of the CO32− group, which can be associated with CO2 from the atmosphere during the dissolution, agitation, reaction, and calcination processes, or to the formation of carbonated HA due to the possibility of substitutions occurring in the PO43− ions or hydroxyl arising from HA to the CO32− ion [12, 21, 22], as follows:
| .(2) |
The absorption of these bands is sensitive to the type of CO group substitution. In Figures 2(a) and 2(b) spectra, the functional groups corresponding to significant bands of calcium phosphate derivatives of biological interest are illustrated, as shown in Table 2.
Table 2.
Functional groups corresponding to important bands in the FTIR spectrum of calcium phosphate derivatives.
| Wavenumber (cm−1) | Groups (samples) | Functional groups |
|---|---|---|
| 523–694 | G4 e G8 | PO43− (deformation) |
| 871 | G5 a G8 | CO32− (stretch) |
| 874 | G1 a G4 | CO32− (stretch) |
| 2513 | G1 a G8 | H-O-H (stretch) |
| 1647–3640 | G2 e G6 | H2O (deformation) |
| 3572–3939 | G1 a G8 | OH− (stretch) |
Méndez-Lozano et al. [19] and Gubicza [24] discussed the presence of an extensive band between 4000 cm−1 and 3700 cm−1 in an analysis of the infrared spectrum curves, corroborating the data obtained from the XRD, analyzed at the temperatures and time of synthesis, which revealed points of low crystallinity, characteristic of a biphasic material. According to the authors, the intensity of this peak is also related to the crystallinity of the powder, relating the HA phase, and even stating that the amorphous phase of the powder is more hydrophilic than its crystalline analog [19, 24].
When comparing the results obtained by SEM analysis with the results obtained by XRD, it can be seen the materials characterized by this technique have more than one calcium phosphate phase which are of biological interest in bone regeneration, HA, β-TCP, and OCP. By analyzing Figures 3(a), 3(b), 3(c), and 3(d), it was possible to see that in the samples synthesized at 110°C for 60 min there is a predominance of agglomerates of particles tending towards a cubic shape, characteristic of less crystalline patterns, corroborating with the XRD, which indicates a more amorphous pattern [19]. However, in the samples calcined at 500°C (c) and 700°C (d), the formation of rods is observed, demonstrating an increase in the crystallinity of the material. Méndez-Lozano [19], among other authors, reports that the associated reaction time and temperature are factors that directly influence the morphology and growth direction of the HA crystal [25].
Figure 3.
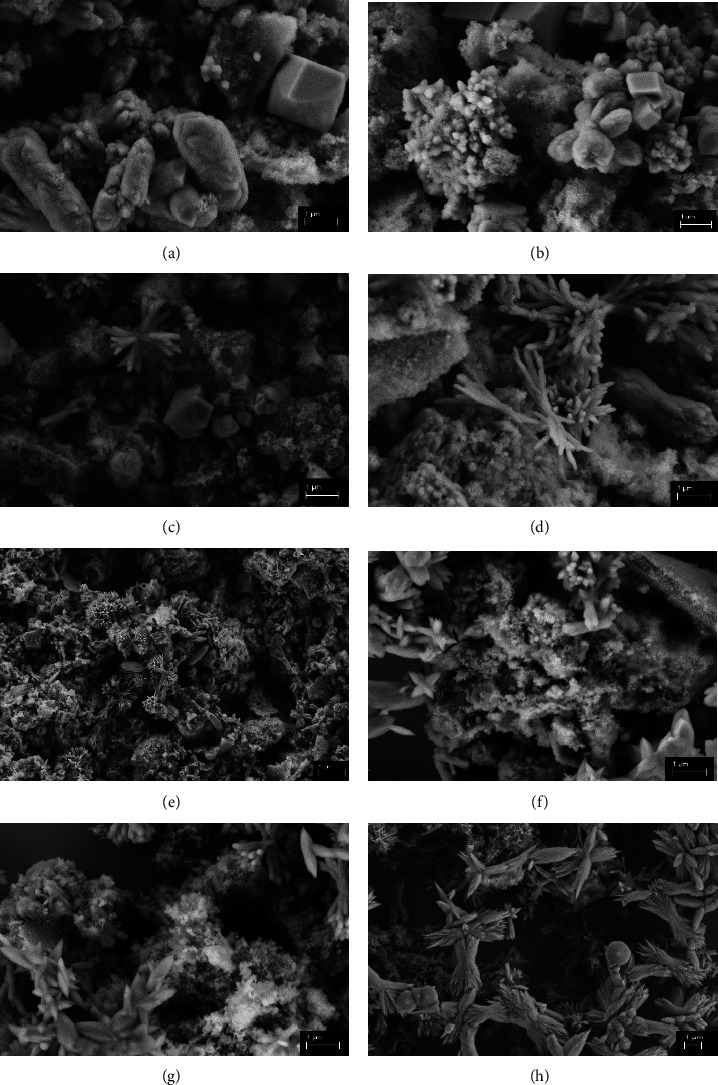
SEM micrographs of samples synthesized at 110°C (a) without heat treatment and calcined at 300°C (b), 500°C (c), 700°C (d), and 130°C (e) without heat treatment and calcined at 300°C (f), 500°C (g), and 700°C (h).
The samples synthesized at 130°C, shown in Figures 3(e), 3(f), 3(g), and 3(h), show the formation of apatite with flower-shaped agglomerates (< 5 μm), which suggests a high surface area. It is recognized that the specific surface area is dependent on the synthesis temperature, the higher the temperature, the higher the surface energy, favoring the development of the flower shape of the HA [24, 25]. In an analysis of the micrographs taken, according to the mechanism suggested by Ma [26], the nucleation of calcium ions and self-organized growth occur under hydrothermal conditions, thus forming HA with a flower-like morphology [26]. The influence of microwave radiation on the hydrothermal process accelerates the crystallization of HA and helps to obtain it in a shorter synthesis time, with a high specific surface area, as well as providing a high yield. However, the high specific surface area and average particle size depend on the power (temperature) used in the microwave and the synthesis time [26, 27]. According to Lopes, Oliveira, and Esteves [28], the morphology of calcium phosphate particles can be spheroid and needle-shaped, as observed in samples synthesized at 130°C and calcined at 500°C and 700°C. The authors also reported that HA crystals tend to form agglomerates and porous shape by varying the size of the crystallites by increasing the calcination temperature, which suggests that the samples calcined at 700°C present dispersed particles with irregular sizes Figure 3(h) [28].
However, pressure can lead to an amorphous or partially disordered phase arrangement leading to larger crystalline sizes [11, 24, 29]. These considerations justify the fact that microwave-assisted hydrothermal systems produce larger crystals [29].
The biomaterial under study showed characteristics of multiphase bioceramics, corroborating the studies by Rustom, Poellmann, and Johnson [30], who also found similar results when evaluating a biphasic calcium phosphate HA/β-TCP. They suggest that the surface of the biphasic phosphate allows osteoclasts to act in a more natural way than pure HA or β-TCP, as well as being able to bind to living bone tissue and undergo natural actions during the bone remodeling process. They point out that biphasic calcium phosphate bioceramics are promising for traumatological applications in the repair of traumatized bone tissue and for the controlled release of drugs in bone structure treatments [25, 30, 31].
At times academic and industrial researchers are challenged to seek methods that do not release waste into the environment, considered by “green chemistry principles.” As microwave irradiation is recognized as an effective and nonionizing electromagnetic energy source, it attracts attention as an environmentally friendly and reliable method for multiphase processes. Microwave energy presents considerable efficiency in various processes that require selective heating [32]. When microwave heating is applied under isothermal conditions, thermally unstable elements absorb the energy generated. The thermal distribution is balanced by the activation energy of the microwaves, which is inversely proportional to the energy of the Ca and P ions, resulting in dissociation of the CaP compound phases as the temperature increases [33].
4. Conclusion
The primary inorganic constituent of bone tissue consists of calcium phosphate derivatives interwoven within a collagen matrix. A key advantage of calcium phosphates in bone regeneration biomaterials lies in their ability to modulate biodegradability and bioactivity by combining different calcium phosphate phases. In this investigation, calcium phosphate derivatives with biological relevance were synthesized using the microwave heating method. The study explored the effects of microwave irradiation time and energy on particle formation. Our findings indicate the microwave HTMO yielded satisfactory results for producing biologically relevant calcium phosphate derivatives, underscoring its potential as a bone regeneration material. Compared to conventional techniques, microwave HTMO offers advantages such as volumetric heating, ensuring homogeneous nucleation, and rapid saturation through dissolving precipitates. Analysis via XRD, FTIR, and SEM revealed characteristics of a biphasic phosphate capable of forming strong bonds with bone tissue, facilitating bone neoformation, a critical trait of bioactive materials. Structural assessments demonstrated the purity and crystallinity of the microstructures of the calcium phosphate phases, displaying distinct and well-aligned microstructures, as confirmed by morphological analysis. This study presented results in which synthesis time/temperature by HTMO was evaluated. The results presented in the synthesis at 130°C/1 h suggest that high sintering temperatures are not required to obtain the phases of biological interest from calcium phosphate. As preliminary experiments, the material obtained at 130°C without heat treatment presented (OCP) precursor phases of HA, and when calcined at temperatures of 500°C and 700°C, phases of biological interest of calcium phosphate (HA, β-TCP) were identified, which are important characteristics from the point of view of materials science for a biomaterial indicated for bone regeneration.
Acknowledgments
The authors would like to thank the team at the Interdisciplinary Laboratory of Electrochemistry and Ceramics (LIEC) of the Chemistry Department at the Sao Carlos Federal University-São Paulo (UFSCar).
Data Availability Statement
The authors declare that data supporting the conclusions of this study are available from the corresponding author upon reasonable request, reaffirming the information mentioned in the system and has already been readapted in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Ana Elisa Vilicev Italiano designed the article and wrote the manuscript; Ricardo Luis Tranquilin and Márcio Luiz dos Santos reviewed the data analysis; Danny Omar Mendoza Marin and Luís Geraldo Vaz presented literature analysis. All authors reviewed the manuscript.
Funding
This work was carried out with support from the Coordination for the Improvement of Higher Education Personnel-Brazil (CAPES)-Financing code 001, under grant number 88887.570438/2020-00.
References
- 1.Rehfeld A., Nylander M., Karnov K. Bone Tissue. Compendium of Histology . New York, NY: Springer; 2017. [Google Scholar]
- 2.Bayani M., Torabi S., Shahnaz A., Pourali M. Main Properties of Nanocrystalline Hydroxyapatite as a Bone Graft Material in Treatment of Periodontal Defects. A Review of Literature. Biotechnology & Biotechnological Equipment . 2017;31(2):215–220. doi: 10.1080/13102818.2017.1281760. [DOI] [Google Scholar]
- 3.Khanijou M., Seriwatanachai D., Boonsiriseth K., et al. Bone Graft Material Derived from Extracted Tooth: A Review Literature. Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology . 2019;31:1–7. doi: 10.1016/j.ajoms.2018.07.004. [DOI] [Google Scholar]
- 4.Medeiros R. V. B. Evaluation of Modulators and Biomaterials for Applications Inbone Tissue Disorders . Belo Horizonte, Brazil: Institute of Biological Sciences-Federal University of Minas Gerais; 2021. [Google Scholar]
- 5.Sinhoreti M. A. C., Vitti R. P., Correr-Sobrinho L. Biomaterials in Dentistry: Currentpanorama and Future Perspectives. Revista Da Associacao Paulista de Cirurgioes Dentistas . 2013;67:256–261. [Google Scholar]
- 6.Azevedo A. G. D. S., Strecker K., Gorgulho H. F. Efeito da Temperatura em Processos de Sinterização de pós de Hidroxiapatita. Cerámica Plus . 2015;61(357):52–59. doi: 10.1590/0366-69132015613571879. [DOI] [Google Scholar]
- 7.Salma-Ancane K., Stipniece L., Irbe Z. Effect of Biogenic and Synthetic Starting Materials on the Structure of Hydroxyapatite Bioceramics. Ceramics International . 2016;42(8):9504–9510. doi: 10.1016/j.ceramint.2016.03.028. [DOI] [Google Scholar]
- 8.Lima E. K. A. D., Fernandes E. F. D. S., Pacheco N. I., Aguiar E. S., Lopes D. C., Mendes L. A. P. P. F. Uma Breve Revisão Sobre a Hidroxiapatita: Uma Biocerâmica Promissora. Research, Society and Development . 2022;11(1):p. e26411124767. doi: 10.33448/rsd-v11i1.24767. [DOI] [Google Scholar]
- 9.Hou X., Zhang L., Zhou Z., et al. Calcium Phosphate-Based Biomaterials for Bone Repair. Journal of Functional Biomaterials . 2022;13(4):p. 187. doi: 10.3390/jfb13040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laurindo C. A. H., Lepienski C. M., Amorim F. L., Torres R. D., Soares P. Mechanical and Tribological Properties of Ca/P-Doped Titanium Dioxide Layer Produced by Plasma Electrolytic Oxidation: Effects of Applied Voltage and HeatTreatment. Tribology Transactions . 2018;61(4):733–741. doi: 10.1080/10402004.2017.1404176. [DOI] [Google Scholar]
- 11.Cividattia L. N., Santos V., Urbano A., Silva A. F., Dall’Antonia L. H. Full Paper Study of the Influence of Synthesis Time in Obtaining Hydroxyapatite by Hydrothermal. Microwave . 2014;6:6–13. [Google Scholar]
- 12.Santos M. L., Riccardi C. S., Filho E. A., Guastaldi A. C. Calcium Phosphates of Biological Importance Based Coatings Deposited on Ti-15Mo Alloy Modified by Laser Beam Irradiation for Dental and Orthopedic Applications. Ceramics International . 2018;44-18:22432–22438. [Google Scholar]
- 13.Gedye R., Smith F., Westaway K., et al. The Use of Microwave Ovens for Rapidorganic Synthesis. Tetrahedron Letters . 1986;27(3):279–282. doi: 10.1016/s0040-4039(00)83996-9. [DOI] [Google Scholar]
- 14.Sözügeçer S., Bayramgil N. P. Preparation and Characterization of Polyacrylic Acid-Hydroxyapatite Nanocomposite by Microwave-Assisted Synthesis Method. Heliyon . 2021;7(6):p. e07226. doi: 10.1016/j.heliyon.2021.e07226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giguere R. J., Bray T. L., Duncan S. M., Majetich G. Application of Commercial Microwave Ovens to Organic Synthesis. Tetrahedron Letters . 1986;27(41):4945–4948. doi: 10.1016/s0040-4039(00)85103-5. [DOI] [Google Scholar]
- 16.Komarneni S. Nanophase Materials by Hydrothermal, Microwave- Hydrothermal and Microwave-Solvothermal Methods. Current Science . 2003;85:1730–1734. [Google Scholar]
- 17.Leonelli C., Komarneni S. Inorganic Syntheses Assisted by Microwave Heating. Inorganic Chemistry Journal . 2015;3(4):388–391. doi: 10.3390/inorganics3040388. [DOI] [Google Scholar]
- 18.Lak A., Mazloumi M., Mohajerani M. S., et al. Rapid Formation of Mono- Dispersed Hydroxyapatite Nanorods With Narrow-Size Distribution via Microwave Irradiation. Journal of the American Ceramic Society . 2008;91(11):3580–3584. doi: 10.1111/j.1551-2916.2008.02690.x. [DOI] [Google Scholar]
- 19.Méndez-Lozano N., Velázquez-Castillo R., Rivera-Muñoz E. M., et al. Crystal Growth and Structural Analysis of Hydroxyapatite Nanofibers Synthesized by the Hydrothermal Microwave-Assisted Method. Ceramics International . 2017;43(1):451–457. doi: 10.1016/j.ceramint.2016.09.179. [DOI] [Google Scholar]
- 20.Antunes L. Synthesis of Hydroxyapatite by the Microwave-Assisted Hydrothermalmethod . Ponta Grossa, Brazil: State University of Ponta Grossa; 2018. [Google Scholar]
- 21.Santos M. L., Riccardi C. S., Filho E. A., Guastaldi A. C. Biomimetic Calcium Phosphates-Based Coatings Deposited on Binary Ti-Mo Alloys Modified by Laser Beam Irradiation for Biomaterial/clinical Applications. MRS Advances . 2018;30-3:1711–1718. [Google Scholar]
- 22.Kanazawa T. Inorganic Phosphate Materials. Materials Science Monographs . 1989;52 [Google Scholar]
- 23.Saxena V., Shukla I., Pandey L. M. Hydroxyapatite: An Inorganic Ceramic for Biomedical Applications. Materials for biomedical engineering . 2019;8:205–249. doi: 10.1016/b978-0-12-816909-4.00008-7. [DOI] [Google Scholar]
- 24.Gubicza J. Reliability and Interpretation of the Microstructural Parameters Determined by X-Ray Line Profile Analysis for Nanostructured Materials. The European Physical Journal Special Topics . 2022;231(24):4153–4165. doi: 10.1140/epjs/s11734-022-00572-z. [DOI] [Google Scholar]
- 25.Dallabrida A. L., Camargo N. H. A., Moraes A. N., et al. Caracterização de Biocerâmica de Fosfatos de Cálcio Microestruturada em Diferentes Composições em Ovinos. Pesquisa Veterinária Brasileira . 2018;38(7):1327–1336. doi: 10.1590/1678-5150-pvb-4930. [DOI] [Google Scholar]
- 26.Ma M. G. Hierarchically Nanostructured Hydroxyapatite: Hydrothermal Synthesis, Morphology Control, Growth Mechanism, and Biological Activity. International Journal of Nanomedicine . 2012;7:1781–1791. doi: 10.2147/ijn.s29884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mary I. R., Sonia S., Viji S., Mangalaraj D., Viswanathan C., Ponpandian N. Novel Multiform Morphologies of Hydroxyapatite: Synthesis and Growth Mechanism. Applied Surface Science . 2016;361:25–32. doi: 10.1016/j.apsusc.2015.11.123. [DOI] [Google Scholar]
- 28.Lopes J. R., Oliveira J. A. C., Esteves A. A. Synthesis and Characterization of Hydroxyapatite Powders [Ca10(Po4)6 (Oh)2] Obtained from the Sol-Gel Process. Applied Geochemistry . 2015;24:1251–1260. [Google Scholar]
- 29.Sanseverino A. M. Microondas Em Síntese Orgânica. Química Nova . 2002;25(4):660–667. doi: 10.1590/s0100-40422002000400022. [DOI] [Google Scholar]
- 30.Rustom L. E., Poellmann M. J., Johnson A. J. Mineralization in Microporesof Calcium Phosphate Scaffolds. Acta Biomaterialia . 2019;83:435–455. doi: 10.1016/j.actbio.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Ebrahimi M., Botelho M. G., Dorozhkin S. V. Biphasic Calcium Phosphates Bioceramics (HA/TCP): Concept, Physicochemical Properties and the Impact of Standardization of Study Protocols in Biomaterials Research. Materials Science and Engineering: C . 2017;71:1293–1312. doi: 10.1016/j.msec.2016.11.039. [DOI] [PubMed] [Google Scholar]
- 32.Ying-Jie Z., Chen F. Microwave-Assisted Preparation of Inorganic Nanostructures in Liquid Phase. Chemical Reviews . 2014;114-12:6462–6555. doi: 10.1021/cr400366s. [DOI] [PubMed] [Google Scholar]
- 33.Sandoval W. N., Pham V., Ingle E. S., Liu P. S., Lill J. R. Applications of Microwave-Assisted Proteomics in Biotechnology. Combinatorial Chemistry & High Throughput Screening . 2007;10(9):751–765. doi: 10.2174/138620707783018504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that data supporting the conclusions of this study are available from the corresponding author upon reasonable request, reaffirming the information mentioned in the system and has already been readapted in the manuscript.


