Abstract
The number of cancer survivors worldwide is increasing every year, making secondary primary cancers (SPC) is a growing health threat. Studies have been conducted to investigate the risk of occurrence between digestive system tumors (DST) and thyroid cancer (TC). However, existing studies have tended to focus more on the risk of developing SPC and less on the impact of SPC on the survival of cancer survivors. In this study, using the Surveillance, Epidemiology, and End Results database, we aimed to explore the impact of TC on the survival of patients with DST by data between 2000 and 2018. The study employed the standardized incidence ratio to assess the relative risk of SPC, propensity score matching was conducted to mitigate confounding effects, Kaplan-Meier, Cox proportional risk model and competitive risk model were used to analyze the factors affecting the overall survival and cancer-specific survival. We furthermore explored the influence of pathological types and radiotherapy of TC on the survival of DST patients. 518,901 patients with DST only, 801 patients with TC occurring earlier than DST (TC-1st), and 744 patients with DST occurring earlier than TC (DST-1st) were included. The total incidence rate of small intestine cancer after TC was higher than that of the general population, and the incidence of TC after DST was higher than in the general population. DST patients with a history of TC had better overall survival and lower cancer-specific mortality and this difference was particularly significant in patients with DST-1st. In addition, radiotherapy for TC had no effect on cancer-specific mortality in patients with DST.
Keywords: Digestive system tumors, Thyroid cancer, Second primary cancer, Survival analysis, Competitive risk model
Subject terms: Cancer, Gastrointestinal diseases
Introduction
Digestive system tumors (DST), which mainly include gastric, esophageal, liver, pancreatic and colorectal cancers, are the leading cause of death worldwide1. According to the cancer statistics in 2020, colorectal cancer (CRC), gastric cancer (GC), liver cancer (LC) and esophageal cancer (EC) are among the top 10 cancers in the world, with 604,000 new cases of EC, more than 1,000,000 new cases of GC, 906,000 new cases of LC and 190,000 new cases of CRC2. The high morbidity and mortality rates that characterize DST warrant further study of their survival characteristics. With improved diagnostic sensitivity and advances in treatment, cancer patients are surviving longer, which, combined with the long-term side effects of chemotherapy and/or radiotherapy and the continuing influence of genetic and behavioral risk factors3,4, places these cancer survivors at increased risk of developing a second primary cancer (SPC)5–7. Long-term survivors face a variety of challenges, including the physical, psychological, medical, behavioral, and socioeconomic consequences of cancer and its treatment, underscoring the urgent need for large-scale research in this population8. Thyroid cancer (TC), the most common endocrine malignancy with high survival rates, may place survivors at higher risk of developing SPC due to their longer life expectancy, and radiotherapy for patients with first primary TC may be associated with an increased risk of digestive SPC9–11. In addition, a Japanese population-based study showed that the thyroid was also one of the three most common sites for SPC in all cancer patients12. Yang et al. also showed that breast or thyroid cancer may become the most common SPC among multiple primary cancers, and that the combination of first or second primary cancers with TC may be caused by thyroid hormone signaling4. Evidence suggests that thyroid hormone signaling components are involved in the development and progression of various cancers of the digestive tract, and many studies have also observed an increased risk of TC after different cancers of the digestive system13–18. However, the incidence and prognosis of SPC is often underestimated and overlooked due to the chronically low survival rates of DST19. Existing studies have tended to focus more on the risk of developing SPC and less on the impact of SPC on the survival of cancer survivors. At the same time, patients who develop SPC are often excluded from most cancer clinical trials or observational studies, and even less is known about their survival and associated factors20. In this study, using the Surveillance, Epidemiology, and End Results (SEER) database, we aimed to assess the survival characteristics of this population who develop TC or DST subsequent to a diagnosis of DST or TC. Additionally, the impact of TC on the survival of patients with DST was explored. The findings of this study may inform the development of more effective strategies and preventive measures.
Materials and methods
Data collection
The data on patients with DST were obtained from the SEER database (http://seer.cancer.gov), which was submitted in November 2023 by 17 healthcare organizations representing approximately 26.5% of the U.S. population. DST and TC were identified according to the International Classification of Diseases of Oncology, Third Revision, ICD-O-3 (EC: C15.0-C15.9, GC: C16.0-C16.9, SIC: C17.0-C17.3, C17.8, and C17.9, CC: C18.0-C18.9, RC: C19.9, and C20.9, HCC: C22.0, PC: C25.0-C25.3, C25.7-C25.9, and TC: C73.9). No other primary malignancies occurred in the patients with thyroid angiosarcoma in our study, and the fact that thyroid angiosarcoma is a very aggressive tumor that kills 89.3% of patients within 9 months could be a potential cause21. For this reason, it is only natural to exclude this group of patients from our study. The inclusion criteria were as follows: (1) patients diagnosed with the occurrence of DST and TC between 2000 and 2018; (2) patients aged between 18 and 80 years; (3) a minimum interval of six months between the onset of the two cancers; and (4) complete available follow-up time. The exclusion criteria included (1) survival time of 0; (2) unknown race and marital status; (3) unknown specific cause of death classification; (4) history of other malignancies; and (5) patients certified only by autopsy or death. Clinicopathologic information including sex, age, race, histologic grade, SEER stage, pathologic type, marital status, primary site, radiotherapy, surgery, chemotherapy, months of survival, survival status, and cause of death classification were collected. Patients were classified into two groups based on the sequence of occurrence of DST and TC: (1) TC occurring earlier than DST; (2) DST occurring earlier than TC.
Statistical analysis
Categorical variables are expressed as numbers and proportions (n, %), and continuous variables in this study were all non-normally distributed and expressed as medians and quartiles [M(Q1, Q3)]. The χ2/Fisher exact test was used to compare differences between categorical variables, and the Mann-Whitney U rank-sum test was used to compare differences between continuous variables. The MP-SIR algorithm of the Seer*stat program was employed to calculate the standardized incidence ratio (SIR) of second primary TC in DST and second primary DST in TC compared with a reference group representing the general population, and to explore the impact of specific treatments on the incidence of second primary cancer. Propensity score matching (PSM) was conducted to mitigate confounding effects by employing the MatchIt R package, with all covariates designated as scoring factors, a caliper value of 0.1, and a matching ratio of 1:10. Survival analyses were performed using Kaplan-Meier (KM) curves and log-rank tests. Univariate and multivariate Cox proportional risk models were employed to examine the association between overall survival (OS) and each of the variables. The Cmprsk R package was utilized to control for the competing event of death from other causes using Fine and Gray’s competing risk regression. Cancer-specific survival (CSS) was analyzed to examine the association between DST-specific death and each of the variables. All data were analyzed using R 4.4.0 and SPSS 27.0. All statistical tests were two-tailed with a cutoff p-value of 0.05 for statistical differences.
Results
Patients selection
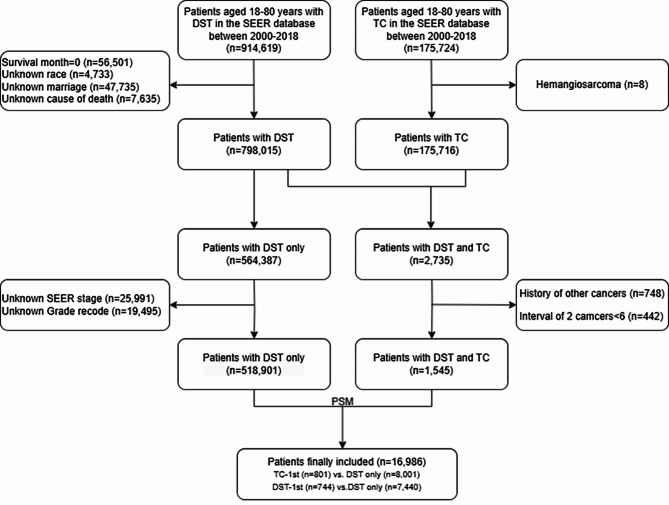
An initial selection of 914,619 patients aged 18–80 years with DST was identified from the SEER database. A total of 56,501 patients with zero months of survival, 150,589 patients with incomplete demographic and survival information, 748 patients with a history of other cancers, and 442 patients with an interval between two cancers of < 6 months were excluded. Finally, 518,901 patients with DST only, 801 patients with TC occurring earlier than DST (TC-1st), and 744 patients with DST occurring earlier than TC (DST-1st) were screened. After PSM matching, 801 patients with TC-1st and 8001 patients with primary DST, as well as 744 patients with DST-1st and 7440 patients with primary DST were included in the subsequent survival analyses. The patient selection process is shown in Fig. 1.
Fig. 1.
Flow chart of the screening process. We used propensity score matching (PSM) with a caliper value of 0.1 and a ratio of 1:10 for matching, each digestive system tumors (DST) patient with thyroid cancer(TC) was matched by all covariates with 10 patients without TC.
Characteristics of patients
Compared with patients with primary DST, in patients with TC-1st, a high percentage of patients were female, Caucasian, low grade and summary stage, surgery, married, and alive, whereas the percentage of patients with radiotherapy, chemotherapy, and death from DST was lower, and the site of DST were more often seen in the stomach, small intestine, and colon. In patients with DST-1st, patients were younger, and female, Caucasian, low grade and summary stage, adenocarcinoma, surgery, married, longer survival time and higher survival rate and similarly lower percentage of dying from DST were observed, and the site of DST were more often seen in the small bowel, colon, and rectum. Baseline characteristics of all patients are shown in Table 1.
Table 1.
Clinical characteristics of patients.
| Variables | DST-only (n = 518,901) | TC-1st (n = 801) | P-value | DST-1st (n = 744) | P-value |
|---|---|---|---|---|---|
| Sex | < 0.001 | < 0.001 | |||
| Male | 300,767(58.0%) | 250(31.2%) | 299(40.2%) | ||
| Female | 218,134(42.0%) | 551(68.8%) | 445(59.8%) | ||
| Age | 64(55–73) | 63(53-71.5) | 0.058 | 58(51–64) | < 0.001 |
| Race | 0.036 | 0.001 | |||
| White | 397,982(76.7%) | 639(79.8%) | 594 (79.8%) | ||
| Black | 64,609(12.5%) | 76(9.5%) | 60(8.1%) | ||
| Other | 56,310(10.9%) | 86(10.7%) | 90(12.1%) | ||
| Grade | < 0.001 | < 0.001 | |||
| Grade I | 49,945(9.6%) | 92(11.5%) | 101(13.6%) | ||
| Grade II | 235,849(45.5%) | 323(40.3%) | 378 (50.8%) | ||
| Grade III | 102,223(19.7%) | 129(16.1%) | 109(14.7%) | ||
| Grade IV | 8852(1.7%) | 7(0.9%) | 12(1.6%) | ||
| Unknown | 122,032(23.5%) | 250(31.2%) | 144(19.4%) | ||
| Summary stage | < 0.001 | < 0.001 | |||
| Localized | 180,247(34.7%) | 357(44.6%) | 351(47.2%) | ||
| Regional | 180,631(34.8%) | 254(31.7%) | 303(40.7%) | ||
| Distant | 135,127(26.0%) | 167(20.8%) | 70(9.4%) | ||
| Unknown | 22,896(4.4%) | 23(2.9%) | 20(2.7%) | ||
| Histology | 0.662 | 0.011 | |||
| Adenocarcinoma | 377,994 (72.8%) | 589(73.5%) | 573 (77.0%) | ||
| Other | 140,907(27.2%) | 212 (26.5%) | 171 (23.0%) | ||
| Surgery | 0.002 | 0.002 | |||
| Yes | 354,458(68.3%) | 587 (73.3%) | 685 (92.1%) | ||
| No | 164,443(31.7%) | 214 (26.7%) | 59 (7.9%) | ||
| Radiation | 0.006 | 0.469 | |||
| Yes | 93,734(18.1%) | 115(14.4%) | 142(19.1%) | ||
| None/Unknown | 425,167(81.9%) | 686(85.6%) | 602(80.9%) | ||
| Chemotherapy | 0.038 | 0.35 | |||
| Yes | 236,634(45.6%) | 336(41.9%) | 352(47.3%) | ||
| None/Unknown | 282,267(54.4%) | 465(58.1%) | 392(52.7%) | ||
| Marital status | 0.006 | < 0.001 | |||
| Married | 304,407(58.7%) | 508(63.4%) | 483(64.9%) | ||
| Not married | 214,494(41.3%) | 293(36.6%) | 261(35.1%) | ||
| The primary site | < 0.001 | < 0.001 | |||
| Esophagus | 31,302(6.0%) | 23(2.9%) | 16(2.2%) | ||
| Stomach | 58,509(11.3%) | 104(13.0%) | 61(8.2%) | ||
| Small intestine | 16,040(3.1%) | 46(5.7%) | 48(6.5%) | ||
| Colon | 224,388(43.2%) | 366(45.7%) | 374(50.3%) | ||
| Rectum | 107,103(20.6%) | 157(19.6%) | 199(26.7%) | ||
| Liver | 59,231(11.4%) | 75 (9.4%) | 33 (4.4%) | ||
| Pancreas | 22,328(4.3%) | 30(3.7%) | 13 (1.7%) | ||
| Survival months | 43(10–103) | 54(19-94.5) | 0.474 | 127(81-180.75) | < 0.001 |
| Vital statu | < 0.001 | < 0.001 | |||
| Alive | 174,177(33.6%) | 416(51.9%) | 513(69.0%) | ||
| Death | 344,724(66.4%) | 385(48.1%) | 231(31.0%) | ||
| Cause of death | < 0.001 | < 0.001 | |||
| Alive | 174,177(33.6%) | 416(51.9%) | 513(69.0%) | ||
| Digestive | 199,857(38.5%) | 197(24.6%) | 102(13.7%) | ||
| Other | 144,717(27.9%) | 188(23.5%) | 129(17.3%) |
TC, thyroid cancer; DST, digestive system tumors; TC-1st, thyroid cancer was diagnosed first, followed by subsequent diagnosis of digestive system tumors; DST-1st, digestive system tumors was diagnosed first, followed by subsequent diagnosis of thyroid cancer.
SPC incidence
As shown in Table 2, the prevalence of total DST following TC did not exhibit a statistically significant divergence from that observed in the general population. However, for the occurrence of GC after TC, the incidence rate was higher than that of the general population during the 12–59 month period (SIR 1.44, 95% CI 1.127–1.813), and the total incidence rate of SIC after TC was also higher than that of the general population (SIR 1.454, 95% CI 1.129–1.843). In contrast, a reduced risk was observed in EC (SIR 0.515, 95% CI 0.361–0.713). For first primary TC, there was an increased risk of GC and SIC and a decreased risk of EC after receiving radiotherapy; an increased risk of total DST and GC after receiving chemotherapy; and an increased risk of GC and SIC and a decreased risk of EC after surgery, which was similar to the risk after receiving radiotherapy. As illustrated in Table 3, the incidence of TC following DST was observed to be higher than that observed in the general population (SIR 1.95, 95% CI 1.85–2.05). The incidence of cancers of the esophagus (SIR 2.51, 95% CI 1.82–3.39), stomach (SIR 2.69, 95% CI 2.23–3.22), small bowel (SIR 2.88, 95% CI 2.25–3.63), colon (SIR 1.71, 95% CI 1.58–1.85), rectum (SIR 1.87, 95% CI 1.65–2.12), liver (SIR 2.10, 95% CI 1.61–2.70), and pancreas (SIR 2.71, 95% CI 2.15–3.37) were all associated with an increased risk of developing TC. For first primary DST, there was an increased risk of TC after radiotherapy for EC, GC and RC, and an increased risk of TC after chemotherapy and after surgery for all DST.
Table 2.
Standardized incidence ratio of DST after TC in different situations.
| Different time | Different treatments | |||||||
|---|---|---|---|---|---|---|---|---|
| 2-11months | 12-59months | 60-119months | 120 + months | Total | Radiotherapy | Chemotherapy | Surgery | |
| Observed SIR(95%CI) | Observed SIR(95%CI) | Observed SIR(95%CI) | Observed SIR(95%CI) | Observed SIR(95%CI) | Observed SIR(95%CI) | Observed SIR(95%CI) | Observed SIR(95%CI) | |
| DST | 167 1.167(1.00-1.36) | 621 1.02(0.94–1.11) | 528 0.99(0.91–1.08) | 309 0.95(0.84–1.06) | 1625 1.01(0.96–1.06) | 807 1.06(0.98–1.13) | 45 1.64*(1.20–2.20) | 1603 1.02(0.97–1.07) |
| EC | 6 0.95(0.35–2.07) | 8 0.30*(0.13–0.60) | 16 0.70(0.40–1.13) | 6 0.43*(0.16–0.93) | 36 0.52*(0.36–0.71) | 18 0.53*(0.31–0.83) | 1 0.74(0.02–4.14) | 35 0.51*(0.36–0.71) |
| GC | 10 0.84(0.40–1.55) | 72 1.44*(1.12–1.81) | 40 0.92(0.65–1.25) | 31 1.17(0.80–1.66) | 153 1.16(0.98–1.36) | 85 1.34*(1.07–1.66) | 8 3.598*(1.55–7.07) | 154 1.19*(1.01–1.40) |
| SIC | 5 1.25(0.41–2.92) | 21 1.22(0.75–1.86) | 26 1.66*(1.08–2.43) | 16 1.63(0.93–2.65) | 68 1.45*(1.13–1.84) | 36 1.63*(1.14–2.26) | 3 4.29(0.88–12.53) | 69 1.51*(1.17–1.91) |
| CC | 63 1.23(0.94–1.57) | 222 1.03(0.90–1.18) | 183 0.99(0.85–1.14) | 106 0.96(0.79–1.16) | 574 1.02(0.94–1.11) | 281 1.06(0.94–1.20) | 12 1.20(0.62–2.09) | 564 1.03(0.94–1.11) |
| RC | 19 1.12(0.68–1.75) | 74 1.05(0.83–1.33) | 48 0.81(0.69–1.07) | 27 0.77(0.51–1.12) | 168 0.93(0.79–1.08) | 84 0.95(0.76–1.18) | 3 1.12(0.23–3.29) | 162 0.91(0.78–1.06) |
| HCC | 12 1.04(0.54–1.81) | 53 1.07(0.80–1.40) | 35 0.79(0.55–1.10) | 12 0.44*(0.23–0.76) | 112 0.84(0.69–1.02) | 62 0.95(0.73–1.21) | 2 0.90(0.11–3.27) | 109 0.84(0.69–1.01) |
| PC | 30 1.42(0.96–2.03) | 99 1.07(0.87–1.31) | 91 1.06(0.85–1.30) | 65 1.17(0.90–1.49) | 285 1.12(0.99–1.26) | 124 1.04(0.87–1.24) | 9 1.96(0.89–3.72) | 281 1.13(1.00-1.27) |
SIR, standardized incidence ratio; 95%CI, confidence interval; DST, digestive system tumors; EC, esophageal cancer; GC, gastric cancer; SIC, small intestine cancer; CC, colon cancer; RC, rectal cancer; HCC, hepatocellular carcinoma; PC, pancreatic cancer.
* P < 0.05, compared with the natural population.
Table 3.
Standardized incidence ratio of TC after DST in different situations.
| Different time | Different treatments | |||||||
|---|---|---|---|---|---|---|---|---|
| 2-11months | 12-59months | 60-119months | 120 + months | Total | Radiotherapy | Chemotherapy | Surgery | |
| Observed SIR(95%CI) | Observed SIR(95%CI) | Observed SIR(95%CI) | Observed SIR(95%CI) | Observed SIR(95%CI) | Observed SIR(95%CI) | Observed SIR(95%CI) | Observed SIR(95%CI) | |
| DST | 438 3.77*((3.43–4.14) | 639 1.95*(1.80–2.10) | 271 1.31*(1.16–1.48) | 112 1.14(0.94–1.37) | 1460 1.95*(1.85–2.05) | 261 2.35*(2.08–2.66) | 641 2.33*(2.15–2.52) | 1230 1.97*(1.86–2.09) |
| EC | 23 5.21*(3.30–7.81) | 14 1.82(0.99–3.05) | 6 1.68(0.62–3.66) | 0 0.00(0.00-2.59) | 43 2.51*(1.82–3.39) | 35 3.43*(2.39–4.78) | 37 3.34*(2.35–4.60) | 29 3.06*(2.05–4.40) |
| GC | 53 6.02*(4.51–7.87) | 43 2.18*(1.58–2.93) | 16 1.48(0.85–2.41) | 6 1.33(0.49–2.90) | 118 2.69*(2.23–3.22) | 36 2.76*(1.93–3.82) | 70 3.09*(2.41–3.91) | 99 2.78*(2.26–3.38) |
| SIC | 19 5.60*(3.37–8.75 ) | 38 3.36*(2.38–4.61) | 10 1.39(0.66–2.55) | 5 1.63(053-3.81) | 72 2.88*(2.25–3.63) | 2 2.19(0.26–7.90) | 21 2.92*(1.81–4.46) | 73 2.92*(2.29–3.67) |
| CC | 162 3.35*(2.86–3.91) | 289 1.801*(1.60–2.02) | 128 1.18(0.98–1.40) | 55 1.04(0.79–1.36) | 634 1.71*(1.58–1.85) | 11 1.74(0.87–3.12) | 257 2.15*(1.90–2.43) | 626 1.74*(1.61–1.88) |
| RC | 63 3.76*(2.89–4.81) | 112 1.96*(1.62–2.36) | 54 1.37*(1.03–1.79) | 20 1.00(0.61–1.55) | 249 1.87*(1.65–2.12) | 163 2.27*(1.93–2.64) | 201 2.36*(2.05–2.71) | 310 1.92*(1.71–2.15) |
| HCC | 29 3.90*(2.61–5.61) | 19 1.34(0.80–2.09) | 10 1.81(0.87–3.33) | 3 1.61(0.33–4.70) | 61 2.10*(1.61–2.70) | 5 2.76(0.90–6.44) | 24 1.80*(1.15–2.67) | 33 2.12*(1.46–2.98) |
| PC | 23 2.09*(1.33–3.14) | 37 2.87*(2.02–3.95) | 15 3.56*(1.99–5.88) | 5 3.48*(1.13–8.12) | 80 2.71*(2.15–3.37) | 9 1.34(0.61–2.54) | 31 1.87*(1.27–2.66) | 60 3.64*(2.78–4.69) |
SIR, Standardized incidence ratio; 95%CI, confidence interval; DST, digestive system tumors; EC, esophageal cancer; GC, gastric cancer; SIC, small intestine cancer; CC, colon cancer; RC, rectal cancer; HCC, hepatocellular carcinoma; PC, pancreatic cancer.
* P < 0.05, compared with the natural population.
Survival analyses of OS in DST patients
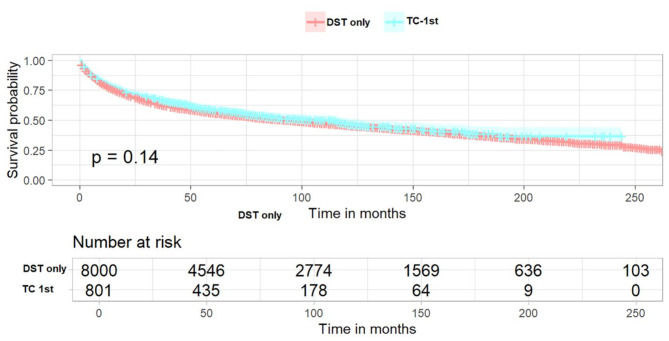
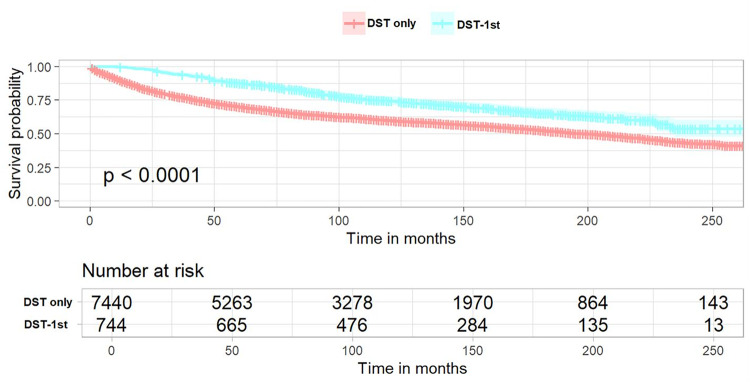
In patients with TC-1st, the KM survival curve demonstrated no statistically significant difference in OS compared to patients with primary DST (Fig. 2, log-rank p = 0.14). To identify factors associated with OS in both groups of patients, univariate and multivariate Cox regression analyses were performed. The results, as presented in Table 4, indicated that female patients, non-adenocarcinoma and occurrence of TC were independent favorable prognostic factors for survival. Conversely, age, high grade and summary stage, no surgery, no chemotherapy and being unmarried were independent unfavorable factors for survival Additionally, the primary site of DST located in the small bowel, colon and rectum was more favorable for survival compared with the disease site located in the esophagus, and the primary site of tumors located in the liver and pancreas were identified as being at a greater risk of death. In patients with DST-1st, the KM survival curve demonstrated a statistically significant improvement in OS in patients with DST-1st compared to those with DST alone (Fig. 3, log-rank p < 0.0001). The results (Table 5) showed approximately the same independent favorable and unfavorable prognostic factors as observed in TC-1st patients. Additionally, unfavorable survival was noted among black patients, while chemotherapy did not demonstrate an impact on OS. Meanwhile, the occurrence of TC was associated with a more significant reduction in all-cause mortality (HR 0.485, 95%CI 0.425–0.555 vs. HR 0.816, 95%CI 0.735–0.906).
Fig. 2.
Kaplan–Meier curves of TC-1st patients. The overall survival between DST-only patients and TC-1st patients (p = 0.14). TC-1st, thyroid cancer was diagnosed first, followed by subsequent diagnosis of digestive system tumors. DST-only, patients with digestive system tumors only.
Table 4.
Univariable and multivariable Cox analysis of overall survival in patients with TC-1st.
| Variables | Univariable analysis | Multivariable analysis | |
|---|---|---|---|
| HR (95% CI) P-value | HR (95% CI) P-value | ||
| Sex | |||
| Male | Reference | Reference | |
| Female | 0.775 (0.731–0.822) < 0.001 | 0.827 (0.778–0.878) < 0.001 | |
| Age | 1.032 (1.030–1.035) < 0.001 | 1.032 (1.029–1.035) < 0.001 | |
| Race | |||
| White | Reference | ||
| Black | 1.001 (0.913–1.096) 0.991 | ||
| Other | 1.013 (0.921–1.114) 0.789 | ||
| Grade | |||
| Grade I | Reference | Reference | |
| Grade II | 1.637 (1.447–1.851) < 0.001 | 1.268 (1.115–1.443) < 0.001 | |
| Grade III | 3.379 (2.968–3.847) < 0.001 | 1.713 (1.492–1.967) < 0.001 | |
| Grade IV | 2.714 (2.134–3.451) < 0.001 | 2.142 (1.678–2.734) < 0.001 | |
| Unknown | 2.555 (2.256–2.893) < 0.001 | 1.423 (1.251–1.618) < 0.001 | |
| Summary stage | |||
| Localized | Reference | Reference | |
| Regional | 1.802 (1.682–1.932) < 0.001 | 2.027 (1.876–2.190) < 0.001 | |
| Distant | 6.439 (5.981–6.932) < 0.001 | 5.696 (5.190–6.250) < 0.001 | |
| Unknown | 3.223 (2.735–3.798) < 0.001 | 1.523 (1.284–1.806) < 0.001 | |
| Histology | |||
| Adenocarcinoma | Reference | Reference | |
| Other | 1.191 (1.119–1.269) < 0.001 | 0.706 (0.643–0.775) < 0.001 | |
| Surgery | |||
| Yes | Reference | Reference | |
| No | 5.656 (5.326–6.006) < 0.001 | 2.782 (2.553–3.032) < 0.001 | |
| Radiation | |||
| Yes | Reference | Reference | |
| None/Unknown | 0.783 (0.726–0.845) < 0.001 | 0.794 | |
| Chemotherapy | |||
| Yes | Reference | Reference | |
| None/Unknown | 0.583 (0.551–0.616) < 0.001 | 1.228 (1.147–1.314) < 0.001 | |
| Marital status | |||
| Married | Reference | Reference | |
| Not married | 1.306 (1.233–1.383) < 0.001 | 1.225 (1.156-1.300) < 0.001 | |
| Prior thyroid cancer history | |||
| With | 0.925 (0.834–1.027) 0.144 | 0.816 (0.735–0.906) 0.009 | |
| Without | Reference | Reference | |
| The primary site | |||
| Esophagus | Reference | Reference | |
| Stomach | 0.710 (0.618–0.816) < 0.001 | 0.937 (0.811–1.081) 0.372 | |
| Small intestine | 0.236 (0.193–0.287) < 0.001 | 0.484 (0.392–0.598) < 0.001 | |
| Colon | 0.307 (0.270–0.348) < 0.001 | 0.538 (0.485–0.622) < 0.001 | |
| Rectum | 0.269 (0.235–0.309) < 0.001 | 0.520 (0.448–0.602) < 0.001 | |
| Liver | 1.031 (0.896–1.187) 0.671 | 1.921 (1.648–2.240) < 0.001 | |
| Pancreas | 2.362 (1.990–2.803) < 0.001 | 1.206 (1.007–1.445) 0.042 | |
Fig. 3.
Kaplan–Meier curves of DST-1st patients. The overall survival between DST-only patients and DST-1st patients (p < 0.0001). DST-1st, digestive system tumors was diagnosed first, followed by subsequent diagnosis of thyroid cancer. DST-only, patients with digestive system tumors only.
Table 5.
Univariable and multivariable Cox analysis of overall survival in patients with DST-1st.
| Variables | Univariable analysis | Multivariable analysis | |
|---|---|---|---|
| HR (95% CI) P-value | HR (95% CI) P-value | ||
| Sex | |||
| Male | Reference | Reference | |
| Female | 0.732 (0.684-0783) < 0.001 | 0.838 (0.780–0.899) < 0.001 | |
| Age | 1.033 (1.029–1.036) < 0.001 | 1.035 (1.032–1.038) < 0.001 | |
| Race | |||
| White | Reference | Reference | |
| Black | 1.302 (1.163–1.456) < 0.001 | 1.224 (1.092–1.373) < 0.001 | |
| Other | 0.975 (0.870–1.091) 0.655 | 0.808 (0.719–0.907) < 0.001 | |
| Grade | |||
| Grade I | Reference | Reference | |
| Grade II | 1.289 (1.138–1.460) < 0.001 | 1.211 (1.061–1.381) 0.004 | |
| Grade III | 2.566 (2.246–2.932) < 0.001 | 1.769 (1.537–2.037) < 0.001 | |
| Grade IV | 3.022 (2.338–3.907) < 0.001 | 2.105 (1.623–2.730) < 0.001 | |
| Unknown | 1.259 (1.092–1.453) 0.002 | 1.018 (0.877–1.183) 0.811 | |
| Summary stage | |||
| Localized | Reference | Reference | |
| Regional | 1.743 (1.612–1.884) < 0.001 | 1.836 (1.690–1.995) < 0.001 | |
| Distant | 6.880 (6.262–7.560) < 0.001 | 7.321 (6.612–8.108) < 0.001 | |
| Unknown | 2.170 (1.549–2.868) < 0.001 | 1.991 (1.442–2.751) < 0.001 | |
| Histology | |||
| Adenocarcinoma | Reference | Reference | |
| Other | 1.163 (1.070–1.263) < 0.001 | 0.823 (0.723–0.938) 0.004 | |
| Surgery | |||
| Yes | Reference | Reference | |
| No | 4.186 (3.766–4.653) < 0.001 | 2.564 (2.259–2.911) < 0.001 | |
| Radiation | |||
| Yes | Reference | Reference | |
| None/Unknown | 0.686 (0.634–0.742) < 0.001 | 0.146 | |
| Chemotherapy | |||
| Yes | Reference | Reference | |
| None/Unknown | 0.578 (0.540–0.619) < 0.001 | 0.369 | |
| Marital status | |||
| Married | Reference | Reference | |
| Not married | 1.287 (1.201–1.397) < 0.001 | 1.384 (1.288–1.486) < 0.001 | |
| Prior thyroid cancer history | |||
| With | 0.583 (0.510–0.666) < 0.001 | 0.485 (0.425–0.555) < 0.001 | |
| Without | Reference | Reference | |
| The primary site | |||
| Esophagus | Reference | Reference | |
| Stomach | 0.504 (0.414–0.613) < 0.001 | 0.977 (0.800-1.193) 0.819 | |
| Small intestine | 0.181 (0.141–0.232) < 0.001 | 0.441 (0.338–0.575) < 0.001 | |
| Colon | 0.231 (0.194–0.275) < 0.001 | 0.462 (0.382–0.559) < 0.001 | |
| Rectum | 0.233 (0.577–0.868) < 0.001 | 0.505 (0.416–0.612) < 0.001 | |
| Liver | 0.708 (0.111–0.345) < 0.001 | 1.993 (1.587–2.503) < 0.001 | |
| Pancreas | 0.936 (0.672–1.302) 0.693 | 1.684 (1.204–2.356) 0.002 | |
Survival analyses of CSS in DST patients
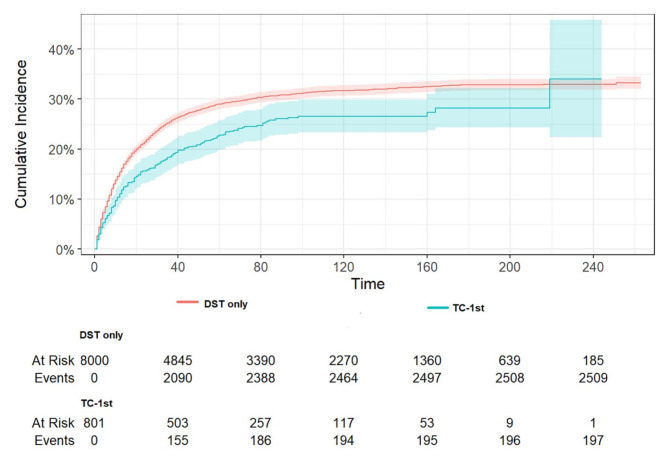
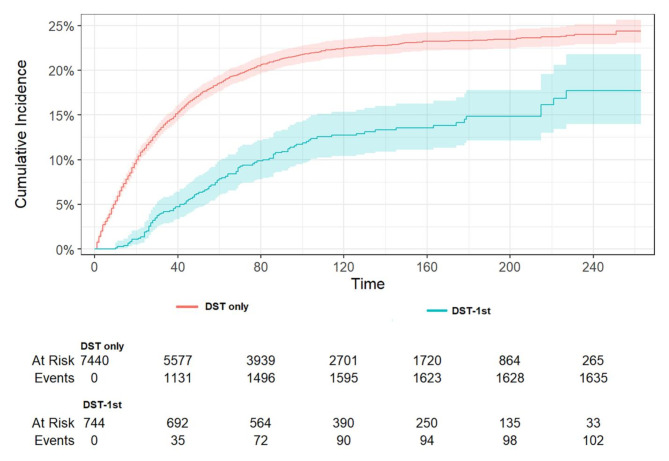
The mortality rate was found to be lower in patients with TC-1st than in patients without a history of TC (Fig. 4, p < 0.001). The results of the multivariate analysis of CSS in patients with DST, as presented in the Fine and Gray competing risk model, are displayed in Table 6. A history of TC was associated with a 0.31-fold lower DST-specific mortality (HR 0.69, 95%CI: 0.59–0.81, p < 0.001). In addition, a higher DST-specific mortality was significantly associated with age, black race, higher grade, higher stage, and no surgery, while women and no chemotherapy had a lower risk of dying from DST. For DST with a primary site in the esophagus, a lower CSS was associated with sites in the stomach, small intestine, colon, and rectum, while a higher CSS was associated with sites in the liver and pancreas. As illustrated in Fig. 5, patients with DST-1st who developed TC exhibited a significantly lower specific mortality rate compared to those who did not develop TC (p < 0.001). The occurrence of TC was associated with a 0.49-fold lower DST-specific mortality (HR 0.51, 95%CI: 0.42–0.62, p < 0.001). Thus, having TC may be a favorable prognostic factor for DST-specific mortality in patients with DST, especially when TC occurs after DST. In addition, a higher DST-specific mortality rate was significantly correlated with advanced age, higher grade, higher stage, non-adenocarcinoma, and no surgery, while patients who did not receive chemoradiotherapy had a lower risk of dying from DST. For DST with a primary site in the esophagus, a lower CSS was associated with sites in the small intestine, colon, and rectum, whereas a higher CSS was associated with sites in the liver.
Fig. 4.
The cumulative incidence of DST-specific death between DST only patients and TC-1st patients (P < 0.001). Patients with TC-1st exhibited a significantly lower specific mortality rate compared to those who did not develop TC.
Table 6.
Multivariable competing risk regression analysis of DST-specific survival in the two groups of patients.
| Variables | Multivariable analysis (TC-1st) | Multivariable analysis (DST-1st) | ||
|---|---|---|---|---|
| HR (95% CI) P-value | HR (95% CI) P-value | |||
| Sex | ||||
| Male | Reference | Reference | ||
| Female | 0.91 (0.83-1.00) 0.046 | 0.97 (0.87–1.08) 0.60 | ||
| Age | 1.01 (1.00-1.01) < 0.001 | 1.01 (1.01–1.02) < 0.001 | ||
| Race | ||||
| White | Reference | Reference | ||
| Black | 1.18 (1.03–1.35) 0.016 | 1.19 (1.00-1.43) 0.054 | ||
| Other | 1.11 (0.97–1.27) 0.130 | 0.95(0.80–1.13) 0.56 | ||
| Grade | ||||
| Grade I | Reference | Reference | ||
| Grade II | 1.68 (1.35–2.09) < 0.001 | 1.28 (1.05–1.58) 0.017 | ||
| Grade III | 2.21 (1.76–2.78) < 0.001 | 2.07 (1.66–2.57) < 0.001 | ||
| Grade IV | 1.91 (1.27–2.87) 0.002 | 1.42 (0.87–2.31) 0.16 | ||
| Unknown | 1.52 (1.22–1.90) < 0.001 | 1.11 (0.87–1.41) 0.40 | ||
| Summary stage | ||||
| Localized | Reference | Reference | ||
| Regional | 2.45 (2.18–2.76) < 0.001 | 2.45 (2.10–2.87) < 0.001 | ||
| Distant | 5.10 (4.41–5.89) < 0.001 | 8.77 (7.29–10.6) < 0.001 | ||
| Unknown | 2.18 (1.70–2.80) < 0.001 | 3.96 (2.49–6.31) < 0.001 | ||
| Histology | ||||
| Adenocarcinoma | Reference | Reference | ||
| Other | 0.90 (0.78–1.04) 0.140 | 1.24(1.02–1.51) 0.031 | ||
| Surgery | ||||
| Yes | Reference | Reference | ||
| No | 2.15 (1.90–2.43) < 0.001 | 1.76 (1.43–2.17) < 0.001 | ||
| Radiation | ||||
| Yes | Reference | Reference | ||
| None/Unknown | 0.91 (0.80–1.05) 0.190 | 0.76 (0.64–0.90) 0.002 | ||
| Chemotherapy | ||||
| Yes | Reference | Reference | ||
| None/Unknown | 0.88 (0.79–0.97) 0.013 | 0.71 (0.62–0.83) < 0.001 | ||
| Marital status | ||||
| Married | Reference | Reference | ||
| Not married | 1.04 (0.95–1.14) 0.360 | 1.05 (0.94–1.17) 0.39 | ||
| Prior thyroid cancer history | ||||
| With | 0.69 (0.59–0.81) < 0.001 | 0.51 (0.42–0.62) < 0.001 | ||
| Without | Reference | Reference | ||
| The primary site | ||||
| Esophagus | Reference | Reference | ||
| Stomach | 0.67 (0.54–0.82) < 0.001 | 0.78 (0.58–1.05) 0.10 | ||
| Small intestine | 0.24 (0.17–0.35) < 0.001 | 0.15 (0.09–0.25) < 0.001 | ||
| Colon | 0.53 (0.42–0.66) < 0.001 | 0.60 (0.45–0.82) 0.001 | ||
| Rectum | 0.23 (0.18–0.28) < 0.001 | 0.28 (0.21–0.37) < 0.001 | ||
| Liver | 1.36 (1.08–1.72) 0.010 | 1.73 (1.18–2.52) 0.005 | ||
| Pancreas | 1.40 (1.06–1.84) 0.017 | 1.68 (0.98–2.88) 0.058 | ||
Fig. 5.
The cumulative incidence of DST-specific death between DST only patients and DST-1st patients (P < 0.001). Patients with DST-1st exhibited a significantly lower specific mortality rate compared to those who did not develop TC.
Subgroup analysis of different gender and different sites of incidence
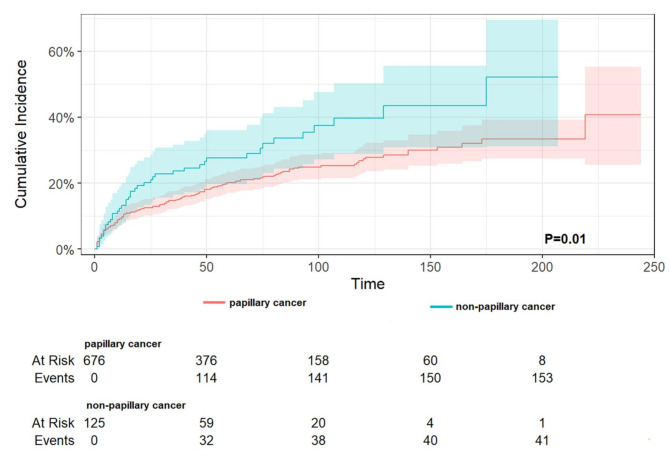
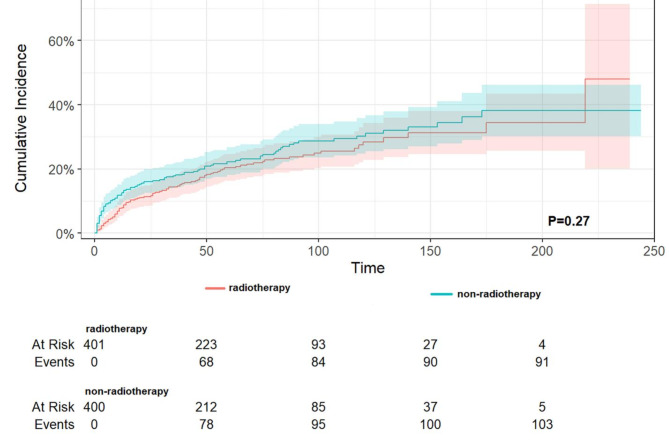
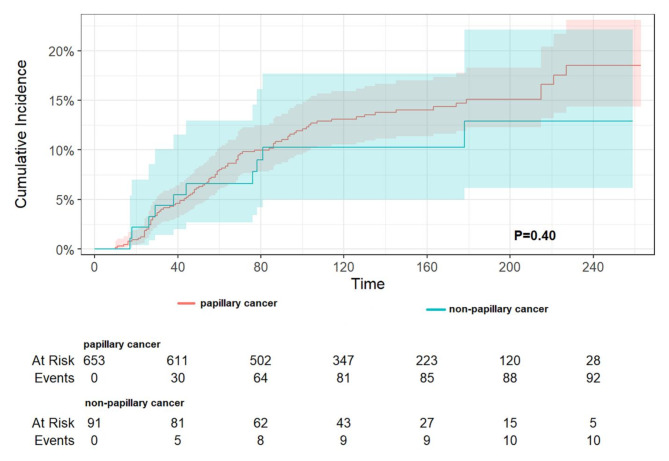
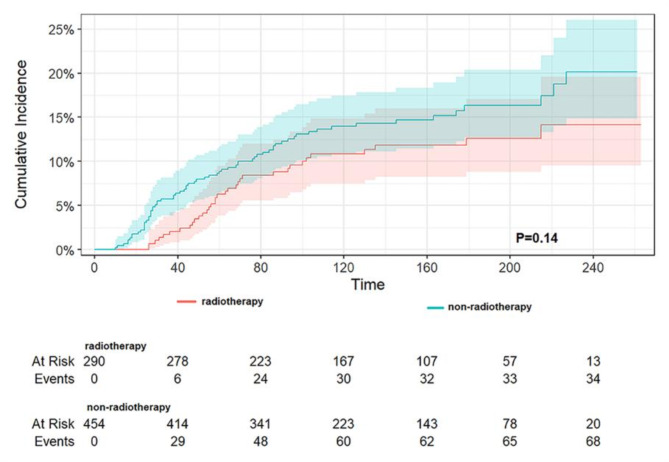
A subgroup analysis by sex (as demonstrated in Table 7) revealed that, in TC-1st, a history of TC reduced DST-specific mortality by 0.28-fold (HR 0.72, 95% CI 0.55–0.95, p = 0.020) for male patients and by 0.34-fold (HR 0.66, 95% CI 0.54–0.81, p < 0.001) for female patients. In DST-1st, a history of TC reduced DST-specific mortality by 0.56-fold (HR 0.44, 95% CI 0.32–0.61, p < 0.001) for male patients and by 0.47-fold (HR 0.53, 95% CI 0.41–0.69, p < 0.001) for female patients. A subgroup analysis of the different sites of DST demonstrated that in TC-1st (as shown in Table 8), the presence of TC significantly reduced DST-specific mortality in the stomach, colon, and liver. Conversely, in the esophagus, rectum, and pancreas, no significant differences between groups were observed, yet all also reduced DST-specific mortality. It is noteworthy that in the small intestine, the presence of TC was associated with an increase in DST-specific mortality, although this was not found to be statistically significant. Reduced cancer-specific mortality was observed only in female patients with HCC. In the small bowel (no statistically significant difference) and in the rectum, no chemotherapy was associated with lower CSS, and in all other sites, no chemotherapy was associated with higher CSS (only in the pancreas there was a statistically significant difference). In DST-1st patients (as shown in Table 9), TC was associated with a significant reduction in DST-specific mortality rates in the stomach, colon, rectum, and liver. Cancer-specific mortality was also reduced in all other sites, although this was not statistically significant. Increased DST-specific mortality was observed in female patients with gastric and pancreatic cancers. No chemotherapy was associated with lower CSS in the stomach, colon (P = 0.028), rectum and pancreas, and no chemotherapy was associated with higher CSS in the other sites (statistically different only in the liver). Additionally, the pathological type of TC and radiotherapy were examined, revealing that in TC-1st, papillary thyroid cancer had a lower specific mortality rate than non-papillary cancer (p = 0.01) (Fig. 6), and no difference was observed in the specific mortality rate between those who received radiotherapy and those who did not (p = 0.27) (Fig. 7). In DST-1st, no difference in cancer-specific mortality was found between papillary and non-papillary TC (p = 0.40) (Fig. 8), and no difference in cancer-specific mortality was found between those who received radiotherapy and those who did not (p = 0.14) (Fig. 9).
Table 7.
Effect of TC on DST-specific survival based on gender grouping.
| TC-1st | DST-1st | |
|---|---|---|
| With thyroid cancer | history (Reference: Without) | |
| Male | 0.72 (0.55–0.95) 0.020 | 0.44 (0.32–0.61) <0.001 |
| Female | 0.66 (0.54–0.81) < 0.001 | 0.53 (0.41–0.69) <0.001 |
Table 8.
Effect of TC, gender, and chemotherapy on DST-specific survival based on different primary site in patients with TC-1st.
| The primary site | Thyroid cancer (Reference: Without) | Sex (Reference: Male) | Chemotherapy (Reference: Yes) |
|---|---|---|---|
| HR (95% CI) P-value | HR (95% CI) P-value | HR (95% CI) P-value | |
| Esophagus(n = 329) | 0.58(0.32–1.07) 0.083 | 0.77(0.57–1.03) 0.078 | 1.41(0.98–2.04) 0.063 |
| Stomach(n = 997) | 0.60(0.40–0.92) 0.019 | 1.18(0.94–1.47) 0.16 | 1.12(0.85–1.47) 0.44 |
| Small intestine(n = 394) | 1.39(0.50–3.86) 0.53 | 1.73(0.65–2.90) 0.40 | 0.94(0.41–2.16) 0.89 |
| Colon(n = 4189) | 0.73(0.57–0.93) 0.011 | 0.93(0.81–1.07) 0.29 | 1.06(0.89–1.25) 0.52 |
| Rectum(n = 1813) | 0.76(0.47–1.22) 0.25 | 0.83(0.63–1.09) 0.18 | 0.57(0.37–0.88) 0.011 |
| Liver(n = 809) | 0.68(0.48–0.96) 0.029 | 0.74(0.61–0.90) 0.003 | 1.05(0.86–1.27) 0.65 |
| Pancreas(n = 270) | 0.85(0.53–1.37) 0.51 | 0.79(0.56–1.11) 0.17 | 1.43(1.09–1.88) 0.010 |
Table 9.
Effect of TC, gender, and chemotherapy on DST-specific survival based on different primary site in patients with DST-1st.
| The primary site | Thyroid cancer (Reference: Without) | Sex (Reference: Male) | Chemotherapy (Reference: Yes) |
|---|---|---|---|
| HR (95% CI) P-value | HR (95% CI) P-value | HR (95% CI) P-value | |
| Esophagus(n = 175) | 0.48(0.20–117) 0.11 | 0.75(0.47–1.19) 0.23 | 2.02(0.86–4.79) 0.11 |
| Stomach(n = 626) | 0.49(0.28–0.84) 0.010 | 1.47(1.05–2.08) 0.027 | 0.97(0.61–1.53) 0.89 |
| Small intestine(n = 389) | 0.41(0.04–3.87) 0.44 | 1.40(0.43–4.49) 0.58 | 1.22(0.42–3.55) 0.72 |
| Colon(n = 4225) | 0.52(0.40–0.67) <0.001 | 0.89(0.77–1.03) 0.11 | 0.81(0.67–0.98) 0.028 |
| Rectum(n = 2299) | 0.54(0.32–0.89) 0.017 | 0.84(0.66–1.07) 0.16 | 0.74(0.46–1.20) 0.23 |
| Liver(n = 402) | 0.26(0.12–0.58) <0.001 | 1.07(0.75–1.53) 0.70 | 1.72(1.12–2.63) 0.013 |
| Pancreas(n = 68) | 0.15(0.02–1.02) 0.053 | 3.17(1.50–6.66) 0.002 | 0.25(0.04–1.43) 0.12 |
Fig. 6.
The cumulative incidence of DST-specific death between papillary and non-papillary cancer in patients with TC-1st. In patients with TC-1st, papillary thyroid cancer had a lower specific mortality rate than non-papillary cancer (p = 0.01).
Fig. 7.
The cumulative incidence of DST-specific death between radiotherapy and non- radiotherapy of TC in patients with TC-1st. No difference was observed in the specific mortality rate between those who received radiotherapy and those who did not (p = 0.27).
Fig. 8.
The cumulative incidence of DST-specific death between papillary and non-papillary cancer in patients with DST-1st. No difference in cancer-specific mortality was found between papillary and non-papillary TC (p = 0.40).
Fig. 9.
The cumulative incidence of DST-specific death between radiotherapy and non- radiotherapy of TC in patients with DST-1st. No difference in cancer-specific mortality was found between those who received radiotherapy and those who did not (p = 0.14).
Discussion
The number of cancer survivors worldwide is increasing every year, making SPC a growing health threat. The underlying mechanisms leading to the development of SPC are largely unknown, specific genetic susceptibility22, lifestyle (e.g., obesity, smoking, and alcohol consumption)23,24, and the long-term effects of treatment for the first primary cancer25,26 are important. Survivors of TC are at increased risk of developing SPC, including gastric and colorectal cancers9, but studies have also shown that prior radiotherapy for TC is not associated with a significant difference in the risk of SPC, including DST27–29. In our study, a higher overall incidence of SIC than in the general population was observed after TC, and a higher overall incidence of GC than in the general population only in the period of 12–59 months after the occurrence of TC; in addition, a reduction in risk was observed for EC. In contrast, the overall incidence of TC after DST was higher than in the general population, and previous studies have shown an increased risk of TC in patients with different DST. We hypothesize that, possibly due to the poorer prognosis of DST compared to TC, patients after DST undergo more imaging, leading to increased detection of thyroid nodules, which in turn leads to increased diagnosis of TC. Assessing whether a thyroid nodule is cancerous is assessed by fine-needle aspiration, and the Bethesda classification system is the standard for interpreting its results, with studies suggesting that patients with the more prevalent Bethesda II nodules may be diagnosed with incidental TC even after thyroidectomy; and the most controversial Bethesda III nodules may be at a higher risk of developing malignancy than conventional wisdom suggests30,31. We should be on the lookout for both types of nodules in cancer survivors. Since SPC are one of the most serious sequelae of successful cancer treatment and either radiotherapy or chemotherapy may increase the incidence of SPC32, the effect of different treatment modalities on the incidence of SPC was further analyzed. The treatment of TC increased the incidence of GC, while radiotherapy reduced the incidence of EC; the treatment of DST has almost all increased the incidence of TC, especially after chemotherapy and surgery. The results of the studies leading to these different findings may be related to regulation of tumor growth by thyroid hormones13, the dose of radioactive and chemotherapeutic treatment, the heterogeneity among different patient populations, the environmental exposure to different carcinogens, and genetic polymorphisms33and other relevant confounding factors, and such gene-gene/gene-environment interactions are extremely complex, and the results should be interpreted with caution. Despite the presence of these confounding factors, this increased risk sheds some light on the significance of long-term surveillance of patients with DST. The latency period between the first primary cancer and the development of SPC can guide clinicians to develop appropriate treatment and monitoring strategies to improve patients’ quality of life; for patients who have developed second primary TC, monitoring the survival characteristics of these patients, as well as identifying the corresponding susceptibility genes and therapeutic targets to provide personalized treatment. As this patient population grows, it is not enough to focus on the risk of developing SPC, but also on the impact of SPC on survival.
Due to the rarity of SPC, few studies have analyzed the survival outcomes of SPC patients after their first primary cancer. In patients with two new cancers, 13% die from the primary cancer and 55% die from SPC34. Since more and more cancer survivors are dying from SPC and different SPC have different survival characteristics, monitoring for signs of SPC in the long-term follow-up of cancer survivors is very important and enables us to identify and intervene as early as possible in this subset of patients in order to reduce mortality. Some studies have shown that most patients with heterochronic primary cancers have a similar or better prognosis than patients with a single primary35, while Kim et al. showed that the 5-year survival rate of patients who developed SPC in GC was lower than that of patients who did not have SPC36. These studies analyzed the occurrence of all SPCs as a whole and did not analyze the different types of SPCs separately, which may be the reason for the conflicting results. Therefore, more studies are needed to further investigate the prognosis of different types of SPC in first primary cancer. In our study, we analyzed the effect of TC on survival in DST and further grouped the analysis according to the order of time of onset of the two cancers. It was found that DST patients with a history of TC had better overall survival and lower cancer-specific mortality. This difference was particularly significant in patients whose DST had developed earlier than their TC. Regarding the different sites of DST, TC preceded by gastric, colon, and liver cancer was a significant factor in reducing cancer-specific mortality, whereas a history of TC increased cancer-specific mortality in small bowel cancer, although there was no statistically significant difference. The occurrence of TC after gastric, colon, rectal, and liver cancers all significantly reduced cancer-specific mortality. As the study by Bian et al. showed that different prior cancer histories were associated with different survival rates in patients with GC, the exclusion and inclusion of patients with prior malignancies should be reconsidered based on the specific type of malignancy37. When EC as the SPC, the overall risk of death in this group of patients during the first 5 years after diagnosis was similar to that of patients who did not develop SPC, and patients with second primary EC should not be completely excluded from clinical trials38. The rationale for excluding patients with a history of cancer from clinical trials in pancreatic and lung cancer has also been reconsidered39,40. These findings would help to alleviate the anxiety of cancer survivors about SPC and, in the absence of adverse effects on clinical outcomes, we could consider enrolling this subset of patients with a history of prior cancer in a clinical trial to improve the completion rate and generalizability of clinical trials.
In terms of survival, some previous studies have also found prior TC to be a favorable prognostic factor for SPC, such as favoring survival in subsequent liver, pancreatic, and breast cancers41. In our study, we also observed that the presence of TC was associated with better survival, which may be due to the fact that cancer survivors may detect SPC earlier during surveillance of the primary tumor42, and that cancer survivors may change their lifestyle and dietary patterns after the diagnosis or treatment of the first primary cancer43, which may also potentially account for the better survival of SPC patients. We observed that this “protective effect” of TC was more pronounced in patients with advanced DST, and since age is an unfavorable factor for cancer mortality and was shown to be associated with lower OS and higher CSS in our study, we compared the age of patients in both groups, with a median age of 63 years in the TC-1st group and 58 years in the DST-1st group, with a significant difference between the two groups (P < 0. 001), so we hypothesized that it was related to the synergistic protective effect of younger age in the DST-1st group of patients, which favored cancer prognosis. In addition, this study showed that more patients with TC were Caucasian than those without TC, and more in the DST-1st group than in the TC-1st group. Due to the influence of sociology and other factors44, blacks have a higher mortality rate for most cancers than any other race45. This may be another potential confounding factor for the protective effect of TC on survival in patients with DST. Our study demonstrated that the absence of chemotherapy was associated with a reduced CSS. A substantial body of evidence has indicated that the utilization of systemic chemotherapy in patients with advanced cancers at the end of life is associated with diminished OS46–48. Notably, 66–83% of patients with solid tumors received chemotherapy in the final three months of life, and 20–40% received chemotherapy within the initial 30 days of death46. Since the goal of treatment for advanced metastatic cancer is usually not cure, but rather symptom control and prolonged survival, this meaning that the use of chemotherapy indicates more advanced disease. These may be the possible reasons for this result. Additionally, a higher number of patients in both groups who developed TC underwent surgery compared to those who developed DST only, and the lack of surgery was associated with higher DST-specific mortality. The higher rate of surgery in these patient populations leads us to consider surgery-related complications that may affect survival. A new biomarker, Butyrylcholinesterase, correlates with systemic levels of inflammation, and studies have shown its association with survival in patients with DST, surgical site infections, and postoperative complications such as sepsis, delirium, and so on49. This type of economical and easily accessible enzyme warrants further investigation of its clinical value in malignant tumor surgery.
Furthermore, our study examined the pathological type of TC and the impact of radiotherapy on survival in patients with DST, thereby enhancing the comprehensiveness of the results. The specific mortality rate was found to be lower in TC-1st for papillary thyroid cancer than for non-papillary cancer, and no significant difference was observed between the two in DST-1st. Chuang et al. demonstrated that beam radiation for TC was associated with an increased risk of developing digestive tract cancers, including those of the colon, rectum, and upper gastrointestinal tract10. Sawka et al. demonstrated that TC treated with radioactive iodine did not elevate the risk of cancers in the colorectal, digestive tract, stomach, and pancreatic sites27. It has been established that neither radioactive iodine treatment nor the intrinsic biological aggressiveness of differentiated TC affects the clinical characteristics of subsequent tumors. Our study also revealed no difference in specific mortality between patients who received radiotherapy and those who did not in both groups.
This study is subject to several limitations. Firstly, there are unavoidable biological and other confounding factors, in the future, develop effective epidemiologic study designs to investigate genetic susceptibility and genetic modifiers associated with the development of multiple primary cancers, and to validate the results of retrospective study by controlling for other confounding factors as much as possible through prospective clinical trials with large sample sizes. Secondly, this study was unable to investigate the causal relationship between DST and TC and the associated mechanisms, conducting Mendelian randomization and basic experiments is necessary, with a view to further elucidating the relationship between DST and TC. Thirdly, although subgroup analyses were performed in this study according to different sites of DST and different histological types of TC, different sites of DST have different histological types, different histologic types have different survival characteristics, future studies should further explore for different histological types. Fourthly, the lack of patient-specific treatment regimens, such as unknown chemotherapeutic agents and cycles, precludes further exploration of the impact of treatment regimens on survival, future studies are needed to investigate the different effects and mechanisms of action of different chemotherapy regimens in the survival of TC on DST. Finally, the results of this study are based on the SEER database, and the application to clinical practice needs to take into account the differences in different regions and populations, and thus in the future, by studying populations of more races and regions, with a view to providing evidence-based management guidelines.
The findings of this retrospective study indicated that female patients, non-adenocarcinoma, and the occurrence of TC were independent favorable prognostic factors for survival in patients with DST. Additionally, the results suggested that TC reduced the specific mortality of patients with DST, particularly in cases where DST preceded TC. Furthermore, a history of radiotherapy for TC had no significant effect on specific survival in patients with DST. This result has potentially important clinical and biological implications for the specific group of patients with DST and TC, further clarification of the relationship between DST and TC is worthwhile.
Acknowledgements
We thank all authors for their contributions, and we are grateful to all the staff in the National Cancer Institute (USA) for their contribution to the SEER program.
Abbreviations
- DST
Digestive system tumors
- SPC
Second primary cancer
- EC
Esophageal cancer
- GC
Gastric cancer
- SIC
Small intestine cancer
- CC
Colon cancer
- RC
Rectal cancer
- HCC
Hepatocellular carcinoma
- PC
Pancreatic cancer
- TC
Thyroid cancer
- SIR
Standardized incidence ratio
- PSM
Propensity score matching
- OS
Overall survival
- CSS
Cancer-specific survival
Author contributions
S.Z.: Conceptualization; data curation; formal analysis; visualization; investigation; methodology; writing-original draft. Y. Z.: Data curation; formal analysis; visualization; methodology; Y. Z.: Data curation; formal analysis; visualization; methodology; writing-original draft; funding acquisition. R.J.: Conceptualization; data curation; formal analysis; visualization; methodology; writing-review and editing; funding acquisition. All authors reviewed the manuscript.
Data availability
The data underlying this article are available in Surveillance Epidemiology and End Results (SEER) database, at https://seer.cancer.gov/data/.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ya Zheng, Email: zhengya10@126.com.
Rui Ji, Email: jir@lzu.edu.cn.
References
- 1.Li, X. et al. Abdominal obesity and digestive system cancer: A systematic review and meta-analysis of prospective studies. BMC Public. Health. 23, 2343. 10.1186/s12889-023-17275-2 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung, H. et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71, 209–249. 10.3322/caac.21660 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Liu, H., Heng, X., Tian, Y. & Yang, Z. M. Effect of prior thyroid cancer on survival of primary liver cancer: A study based on the SEER database. Sci. Rep.12, 13887. 10.1038/s41598-022-17729-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang, X. B. et al. High incidence combination of multiple primary malignant tumors of the digestive system. World J. Gastroenterol.28, 5982–5992. 10.3748/wjg.v28.i41.5982 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sung, H., Hyun, N., Leach, C. R., Yabroff, K. R. & Jemal, A. Association of First Primary Cancer with risk of subsequent primary Cancer among survivors of adult-onset cancers in the United States. Jama. 324, 2521–2535. 10.1001/jama.2020.23130 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robertson, D., Ng, S. K., Baade, P. D. & Lam, A. K. Risk of extracolonic second primary cancers following a primary colorectal cancer: A systematic review and meta-analysis. Int. J. Colorectal Dis.37, 541–551. 10.1007/s00384-022-04105-x (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bright, C. J. et al. Risk of subsequent primary neoplasms in survivors of adolescent and young adult cancer (teenage and young adult Cancer Survivor Study): A population-based, cohort study. Lancet Oncol.20, 531–545. 10.1016/S1470-2045(18)30903-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Shami, K. et al. American Cancer Society Colorectal Cancer Survivorship Care Guidelines. CA Cancer J. Clin.65, 428–455. 10.3322/caac.21286 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subramanian, S. et al. Second primary malignancy risk in thyroid cancer survivors: A systematic review and meta-analysis. Thyroid. 17, 1277–1288. 10.1089/thy.2007.0171 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Chuang, S. C. et al. Radiotherapy for primary thyroid cancer as a risk factor for second primary cancers. Cancer Lett.238: 42–52. 10.1016/j.canlet.2005.06.015(2006). [DOI] [PubMed]
- 11.Kim, M. et al. Risk factors for second primary malignancies following thyroid cancer: A nationwide cohort study. Eur. J. Endocrinol.186, 561–571. 10.1530/EJE-21-1208 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Utada, M., Ohno, Y., Hori, M. & Soda, M. Incidence of multiple primary cancers and interval between first and second primary cancers. Cancer Sci.105, 890–896. 10.1111/cas.12433 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown, A. R., Simmen, R. C. & Simmen, F. A. The role of thyroid hormone signaling in the prevention of digestive system cancers. Int. J. Mol. Sci.14, 16240–16257. 10.3390/ijms140816240 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song, J. H. et al. Secondary Primary Cancer after Primary Gastric Cancer: Literature Review and Big Data Analysis Using the Health Insurance Review and Assessment Service (HIRA) Database of Republic of Korea. Cancers (Basel). 14. (2022). 10.3390/cancers14246165 [DOI] [PMC free article] [PubMed]
- 15.Crocetti, E. et al. Risk of thyroid as a first or second primary cancer. A population-based study in Italy, 1998–2012. Cancer Med.10, 6855–6867. 10.1002/cam4.4193 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu, G. et al. Risk of second primary cancer after treatment for esophageal cancer: A pooled analysis of nine cancer registries. Dis. Esophagus. 10.1111/j.1442-2050.2011.01273.x (2012). 25: 505 – 11. [DOI] [PubMed] [Google Scholar]
- 17.Shah, B. K., Kandel, P. & Khanal, A. Second primary malignancies in Hepatocellular Cancer - A US Population-based study. Anticancer Res.36, 3511–3514. 10.21873/anticanres.16879 (2016). [PubMed] [Google Scholar]
- 18.Schonfeld, S. J., Morton, L. M., de Berrington, A., Curtis, R. E. & Kitahara, C. M. Risk of second primary papillary thyroid cancer among adult cancer survivors in the United States, 2000–2015. Cancer Epidemiol.64, 101664. 10.1016/j.canep.2019.101664 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin, L. Y., Su, X. Y., Li, W. J., Wu, J. & Zhang, H. Incidence, risk and prognosis of second primary malignancy of patients with gastric adenocarcinoma. Sci. Rep.14, 5766. 10.1038/s41598-024-56408-4 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy, C. C., Gerber, D. E. & Pruitt, S. L. Prevalence of prior Cancer among persons newly diagnosed with Cancer: An initial report from the Surveillance, Epidemiology, and end results program. JAMA Oncol.4, 832–836. 10.1001/jamaoncol.2017.3605 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kehagias, D., Kostopoulou, E., Ravazoula, P. & Panagopoulos, K. Thyroid angiosarcoma (TAS) - a rare diagnosis not to be missed. Clin. Case Rep.9 (1), 173–176. 10.1002/ccr3.3492 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch, H. T. & de la Chapelle, A. Genetic susceptibility to non-polyposis colorectal cancer. J. Med. Genet.36, 801–818 (1999). [PMC free article] [PubMed] [Google Scholar]
- 23.Bhaskaran, K. et al. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5·24 million UK adults. Lancet. 384, 755–765. 10.1016/S0140-6736(14)60892-8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braakhuis, B. J., Tabor, M. P., Kummer, J. A., Leemans, C. R. & Brakenhoff, R. H. A genetic explanation of Slaughter’s concept of field cancerization: Evidence and clinical implications. Cancer Res.63, 1727–1730 (2003). [PubMed] [Google Scholar]
- 25.Win, A. K. et al. Risks of primary extracolonic cancers following colorectal cancer in lynch syndrome. J. Natl. Cancer Inst.104, 1363–1372. 10.1093/jnci/djs351 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bassal, M. et al. Risk of selected subsequent carcinomas in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J. Clin. Oncol.24, 476–483. 10.1200/JCO.2005.02.7235 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Sawka, A. M. et al. Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: A systematic review and meta-analysis. Thyroid. 19, 451–457. 10.1089/thy.2008.0392 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Cappagli, V. et al. Nonthyroidal second primary malignancies in differentiated thyroid cancer patients: Is the incidence increased comparing to the general population and could it be a radioiodine therapy consequence? Int. J. Cancer. 147, 2838–2846. 10.1002/ijc.33116 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Kim, S. et al. Second primary malignancy risk in thyroid cancer and matched patients with and without radioiodine therapy analysis from the observational health data sciences and informatics. Eur. J. Nucl. Med. Mol. Imaging. 49, 3547–3556. 10.1007/s00259-022-05779-9 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Mulita, F. et al. Cancer rate of Bethesda category II thyroid nodules. Med. Glas (Zenica). 19(1). 10.17392/1413-21 (2022). [DOI] [PubMed]
- 31.Mulita, F. et al. Patient outcomes following surgical management of thyroid nodules classified as Bethesda category III (AUS/FLUS). Endokrynol Pol.72 (2), 143–144. 10.5603/EP.a2021.0018 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Holm, L. E. Cancer occurring after radiotherapy and chemotherapy. Int. J. Radiat. Oncol. Biol. Phys.19 (5), 1303–1308. 10.1016/0360-3016(90)90249-j (1990). [DOI] [PubMed] [Google Scholar]
- 33.Yuan, Y. et al. Risk of second primary thyroid cancer in cancer survivors. Sci Rep. 30;14(1):12478. (2024). 10.1038/s41598-024-63155-z [DOI] [PMC free article] [PubMed]
- 34.Donin, N. et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer. 122, 3075–3086. 10.1002/cncr.30164 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kudo, H. et al. Do prognoses of patients with second primary cancers differ from those of patients with no prior cancer? A population-based study. Cancer Epidemiol.80, 102218. 10.1016/j.canep.2022.102218 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Kim, C. et al. Prediction of metachronous multiple primary cancers following the curative resection of gastric cancer. BMC Cancer. 13, 394. 10.1186/1471-2407-13-394(2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bian, X. et al. The impact of a prior malignancy on outcomes in gastric cancer patients. Cancer Med.10, 1457–1470. 10.1002/cam4.3722 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leng, J. H., Qiu, H. R., Huang, Q. Z., Zhang, J. Q. & Zhou, H. Y. Recommendations for broadening eligibility criteria in esophagus cancer clinical trials: the mortality disparity of esophagus cancer as a first or second primary malignancy. J. Thorac. Dis.16, 3882–3896. 10.21037/jtd-23-1881 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He, C. B., Zhang, Y., Cai, Z. Y. & Lin, X. J. Effect of prior cancer on survival outcomes for patients with pancreatic adenocarcinoma: A propensity score analysis. 19: 509. (2019). 10.1186/s12885-019-5744-8 [DOI] [PMC free article] [PubMed]
- 40.Pruitt, S. L., Laccetti, A. L., Xuan, L., Halm, E. A. & Gerber, D. E. Revisiting a longstanding clinical trial exclusion criterion: impact of prior cancer in early-stage lung cancer. Br. J. Cancer. 116, 717–725. 10.1038/bjc.2017.27 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He, J., Wang, Y., Chen, X. M., Chen, W. & Zhou, J. Y. Value of thyroid cancer history in the prognosis of pancreatic cancer: A SEER population-based study. Sci. Rep.13, 5771. 10.1038/s41598-023-32635-z (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veach, E. et al. Race matters: analyzing the relationship between Colorectal Cancer Mortality Rates and various factors within respective racial groups. Front. Public. Health. 2, 239. 10.3389/fpubh.2014.00239 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Du, Q., Zheng, Z. Y., Wang, Y., Yang, L. & Zhou, Z. G. Genetically predicted thyroid function and risk of colorectal cancer: A bidirectional mendelian randomization study. J. Cancer Res. Clin. Oncol.149, 14015–14024. 10.1007/s00432-023-05233-9 (2023). [DOI] [PubMed] [Google Scholar]
- 44.Aizer, A. A. et al. Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. 120, 1532–1539. 10.1002/cncr.28617 (2014). [DOI] [PubMed] [Google Scholar]
- 45.Giaquinto, A. N., Miller, K. D., Tossas, K. Y., Winn, R. A. & Jemal, A. Siegel, R. L. Cancer statistics for African American/Black people 2022. CA Cancer J. Clin.72, 202–229. 10.3322/caac.21718 (2022). [DOI] [PubMed] [Google Scholar]
- 46.Lapeyre-Prost, A. et al. Chemotherapy use in end-of-life digestive cancer patients: A retrospective AGEO observational study. Clin. Res. Hepatol. Gastroenterol.45, 101709. 10.1016/j.clinre.2021.101709 (2021). [DOI] [PubMed] [Google Scholar]
- 47.Geyer, T. et al. Systemic Anticancer Treatment Near the end of life: A narrative literature review. Curr. Treat. Options Oncol.24, 1328–1350. 10.1007/s11864-023-01115-x (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheng, J. et al. Chemotherapy near the end of life for Chinese patients with solid malignancies. Oncologist. 22, 53–60. 10.1634/theoncologist.2016-0013 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verras, G. I. & Mulita, F. Butyrylcholinesterase levels correlate with surgical site infection risk and severity after colorectal surgery: A prospective single-center study. Front. Surg.11, 1379410. 10.3389/fsurg.2024.1379410 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in Surveillance Epidemiology and End Results (SEER) database, at https://seer.cancer.gov/data/.