Abstract
Cadmium (Cd) can harm the yield and quality of vegetables, threatening food safety. Essential microelements such as iron are crucial for plant growth and can help alleviate heavy metal stress. Recently, nanoparticles have been studied as eco-friendly solutions for mitigating heavy metal stress in plants. In the present study, iron nanoparticles (FeNPs) at 0, 100, and 300 mg/L were applied as soil drenches and foliar sprays to tomato plants under cadmium stress. A comparison was made between the application methods of FeNPs by evaluating the growth parameters of tomato plants, including shoot length (SL), root length (RL), number of branches (NB), number of leaves per plant (NL), and leaf area (LA), as well as by assessing biochemical and antioxidant enzyme parameters. In the Cd stress treatment, the protein content decreased by 24.71%, and the phenolic and flavonoid content of the tomato plants also decreased due to cadmium stress, with levels decreasing from 16.07 to 6.9 µg and from 0.36 to 0.16 µg, respectively. Compared with the soil drench, 100 mg/L FeNPs significantly improved the parameters of Cd-stressed plants when used as a foliar spray, leading to increases in shoot length, root length, fruit weight, number of fruits, number of leaves, and number of branches by 42%, 66%, 24%, 66%, 173%, and 45%, respectively. Tomato plants treated with this spray presented increased carotenoid and lycopene contents. FeNP foliar spray also reduced Cd accumulation in plant tissues. This technique shows promise in alleviating Cd stress in vulnerable vegetable plants such as tomatoes.
Keywords: Antioxidant enzymes, Cadmium stress, Iron nanoparticles, Lycopene, Tomato plant (Solanum lycopersicum L.), Stress
Subject terms: Photosynthesis, Plant physiology, Plant stress responses, Secondary metabolism, Plant sciences, Nanoparticles
Introduction
One of today’s major global concerns for agricultural products is the environmental pollution caused by heavy metals. It threatens food security and can have long-lasting impacts on the environment1. While microelements such as Co, Cu, Fe, Se, and Zn are essential for plant growth, heavy metals such as Pb and Cd can harm plant health2. Heavy metals cause two main issues that need to be considered. First, they adversely affect plant growth, health, and productivity. Second, they accumulate in crops, can enter the food chain, and can negatively affect human health. Therefore, it is crucial to find ways to reduce the uptake and translocation of heavy metals through plant organs3. Cadmium (Cd) is a heavy metal that can cause abiotic stress in plants. It is highly mobile and soluble in water and is usually found in the form of divalent ions or Cd2+4. It has no significant biological function and can be quite toxic to both plants and humans5. Cd enters the soil due to various human activities, including the addition of waste to the soil, i.e., sewage sludge, waste from the mining industry, synthetic fertilizers, and industrial waste. Rapid urbanization and industrialization in developing countries have led to increasing soil contamination with Cd. This Cd is then absorbed by plants and accumulates in plant tissues. This accumulated Cd is then passed to humans directly or indirectly as food, posing a threat to human health1,6.
When plants are exposed to cadmium (Cd) stress, they undergo various molecular, morphological, biochemical, and physiological changes to combat stress and manage their normal growth7. Heavy metals such as Cd can increase the generation of reactive oxygen species (ROS) in plants. These ROS lead to oxidative damage in plants, resulting in disruption of photosynthesis and hindering growth by compromising the defense system of plants. Numerous strategies have been developed to address heavy metal issues in agriculture. One such strategy is the use of nanotechnology, which involves the use of extremely fine elements known as nanoparticles that range in size from 1 to 100 nm in at least two dimensions8,9. NPs play a significant role in promoting plant growth, increasing yield, and improving quality. They possess unique catalytic properties10 and have demonstrated remarkable progress in recent years. These enhanced and better effects of nanomaterials are due to their small size, comparatively very high surface area-to-volume ratio, and enhanced reactivity in comparison to those of their bulk-sized materials11.
Green synthesized metal and metal oxide nanoparticles have been widely studied because of their potential as eco-friendly solutions to agricultural issues, and their use as nanofertilizers has been promoted12. The use of traditional fertilizers can have negative effects, which can be addressed by the use of metallic nanoparticles as nanofertilizers. Recent research has demonstrated that nanofertilizers can effectively increase crop growth, increase productivity and yield, and prevent nutrient loss without causing any harm to the environment or soil13.
Nanoparticles (NPs) are an essential part of nanotechnology, and their impact on plants varies depending on their characteristics, including their morphology, dimension, and method of application to crops14. NPs can help eliminate reactive oxygen species (ROS) produced under various stress situations in plants, including both biotic and abiotic stresses15. Recently, the use of metal nanoparticles in agriculture has attracted increased interest. Nanofertilizer (NF) is a new and promising area of research in agriculture. These tiny materials can help plants obtain the nutrients that they need to grow. NFs are a novel way to optimize nutrient delivery, either on their own or in combination with other fertilizers. The proper delivery of nanoparticles (NPs) inside plants is crucial for their effective function. NPs can be applied to plants through different methods, such as seed coating, soil drenching, and foliar spraying. The transportation system of plants is a possible pathway for the absorption and transport of NPs within the plant system. To estimate the effectiveness of the delivery method, the micromorphological and physiological characteristics of plants are also taken into account16.
NPs interact with plants through three main routes: (i) deposition on the plant surface (leaves, roots, or stems); (ii) penetration through the cuticle and epidermis; and (iii) transport and transformation inside plant tissues17. Once inside a plant, NPs are efficiently transported to other parts of the plant through the vascular system18. Foliar applications of various chemicals are common in agriculture e.g., gibberellic acid and benzyl adenine applied as foliar spray can significantly improve plant growth and flowering19,20.
Soil-applied fertilizers have low utilization rates because of their lower rates of adsorption and various interactions with soil complexes, along with losses due to rainfall and irrigation, which results in increased application of chemical fertilizers, leading to eutrophication21. Low-solubility fertilizers can cause poor plant growth because plants cannot obtain enough nutrients and trace elements. Furthermore, the availability of nutrients to plants is also reduced due to the binding of these nutrients as soil colloids22. Foliar-applied nanofertilizers offer several advantages over traditional soil-applied fertilizers. They are used in lesser quantities, are absorbed at plant surfaces at higher rates with the least impact on soil composition, and are proven to be cost-effective methods23. Foliar application can also provide plants with vitamins and essential elements that may be lacking in the soil24. The slow release of nano fertilizers can also help plants absorb relatively high amounts of potassium, phosphorus, and nitrogen. Furthermore, the application of NPs through foliar spraying can help plants respond better to drought stress by increasing water storage and reducing oxidative stress25. Foliar nanoparticles (NPs) can enter plants through the leaf epidermis or stomata and then travel via the apoplastic or symplastic pathways. When absorbed by plants, NPs are transported through the phloem and xylem, whereas these are stored in vacuoles and cell walls26.
The tomato (Lycopersicon esculentum) is a member of the Solanaceae family. Tomatoes are versatile fruits that are suitable for consumption in various forms, ranging from natural to processed foods. They are valuable commercial crops, especially in tropical and subtropical regions, where they are grown extensively, second only to potatoes27. Tomatoes have significant agricultural, nutritional, and culinary importance. However, they are sensitive to environmental changes that can affect their yield28. Tomatoes are a great source of vitamins and minerals that can help prevent diseases29. Overall, tomatoes are commercially important due to their versatility, nutritional value, and wide range of applications in the food industry.
Iron (Fe) is an essential microelement that is the fourth most abundant element for all organisms. It plays a crucial role in various metabolic, physiological, and biochemical processes of the plant life cycle30. Iron is one of many metalloenzymes and acts as a cofactor with various essential enzymes. Iron is also involved in the processes of chlorophyll synthesis, DNA transcription, enzyme structure, hormone production, nitrate synthesis, nitrogen fixation, photosynthesis, respiration, and RNA synthesis31. Iron deficiency is not good for both plants and animals. If plants or humans are iron deficient, their growth and development are retarded. The application of biogenic synthesized metallic NPs can increase the solubility and bioavailability of iron in plants, thereby addressing the deficiency in humans32. Studies have shown that the growth of plants is significantly influenced by Fe-NPs. The positive effects of iron nanoparticles on plants depend upon various factors, including the dose and duration of NP application, the time and type of application, and the type of plant under treatment. Crop plants treated with Fe-NPs presented enhanced physiological and morphological characteristics33. Plants absorb soil iron via IRT1, which also transports cadmium, thereby entering the food chain34. Iron and zinc effectively increase plant growth under cadmium stress35. Recent studies have shown that cadmium can impact the expression of genes related to iron-regulated transporter-like protein (ZIP) and heavy metal ATPase (HMA), both of which are associated with the plant response to cadmium stress36. Furthermore, the use of iron has been shown to alleviate cadmium toxicity in plants by improving their mineral nutrition and activating antioxidant enzymes37.
Iron deficiency in soils is transferred to plants, and their growth is negatively affected. This deficiency can also cause a slow rate of development, growth, and metabolism. Therefore, there is always a need to address the problem of iron deficiency in plants. The use of iron NPs is a smart tool to increase the bioavailability and solubility of iron, which can have significant positive effects on plant health. Several studies have shown that Fe-NPs influence the growth of plants and that their impact depends on the concentration of NPs, exposure time, form of exposure, and plant species32. Compared with those of the bulk forms of Fe salts, enhanced physiological and morphological characteristics have been demonstrated in crop plants treated with Fe-NPs33.
In previous studies, the effects of magnetic Fe3O4 NPs on tomato plants were investigated in the context of cadmium stress. These magnetic NPs caused a decrease in the toxic effects of cadmium. Iron can decrease the absorption of cadmium by roots and increase the absorption of other essential elements38. Similarly, FeO NPs mitigate the effects of salinity and cadmium stress in wheat plants. FeONPs were found to cause a decrease in Cd absorption and an increase in chlorophyll and carotenoids39. Fe3O4 NPs were also found to be effective at reducing the negative effects of other heavy metals, including lead, chromium, copper and zinc40. However, the effects of Fe3O4 NPs on A. thaliana are concentration dependent, with both positive and negative impacts observed41. Therefore, this study aimed to analyze the effects of iron nanoparticles applied through soil drenching and foliar spraying on tomato plants grown under cadmium stress. The objective of this study was to improve tomato yield and alleviate the negative effects of cadmium stress to compare the effectiveness of the mode of application of FeNPs for this purpose.
Materials and methods
Plant material
The experiment used locally grown tomato seeds (Solanum lycopersicum L.) of variety ANSAL for the present study in Lahore, Pakistan. For the green synthesis of FeONPs, black cumin seeds (Nigella sativa) were obtained from the local market.
The green synthesis of iron nanoparticles for the present study was carried out by mixing a microwave-assisted aqueous extract of Nigella sativa (30 mL) and an aqueous solution of iron chloride (10 mL of 1 mM) via a magnetic stirrer, and the resulting mixture was subsequently centrifuged. The pellets were separated, washed three times with ethanol and preserved as FeONPs for further study.
Characterization
The synthesized FeONPs were characterized by visual observation and UV‒visible spectrophotometry, scanning electron microscopy (SEM) analysis, and a particle size analyzer. A BMS UV-2600 spectrophotometer was used to observe the absorption spectra of the plant extracts and mixtures of plant extracts with salt solutions (probable nanoparticles). The sample was prepared for SEM analysis of synthesized NPs42.
The morphology of the synthesized iron nanoparticles was studied by scanning electron microscopy. A zeta-sizer was used to study the particle size, size distribution, and surface charge of the synthesized NPs. Sonicated samples of NP aliquots (1 µg/mL) were used for this purpose.
Screening experiment
A screening experiment on tomato seeds was conducted on Petri dishes to assess Cd stress toxicity. The Petri dishes with filter paper were sterilized at 120 °C for half an hour. Afterward, the seeds were transferred to the respective plates and properly labeled, and approximately 3 mL of deionized water was added. The CdCl2 concentrations used were 20, 40, 80, 100, and 200 mg/L. Iron chloride FeCl3 (100 mg/L) was also used to determine its effect without Cd stress. Each treatment had three replicates. Germination was observed daily and the germination percentage was calculated 8 days of sowing.
Experimental details
First, the seedlings were grown in late winter of 2023, and a mixture of coco-peat, moss–peat, and loamy soil in equal parts was used as the growing media. When the seedlings reached the 4–5-leaf stage, they were transferred to clay pots with 15 kg of soil each. A simple randomized block design was used for the experiments. When the seedlings were established in the pots, Cd was introduced into the pots at a concentration of 200 mg/kg of soil in form of CdCl2. After stress induction, iron oxide nanoparticles (FeO NPs) were applied as a foliar spray or soil drench, as detailed in Table 1. After 3 days of exposure to heavy metal stress, nanoparticles were applied to seedlings at a concentrations of 100 and 300 mg/L, along with their respective salts, via foliar spraying and soil drenching as per treatment details given in Table 2. Same concentrations and amounts of solution were used for both methods. The nanoparticles were dissolved in 500 mL of water via a magnetic stirrer. For the foliar spray, the pots were covered with plastic sheets to prevent the nanospray from contacting the soil. Then, using a hand sprayer, the nanocolloidal solution (pH of 6.0–7.0) were sprayed onto the plants until it started dripping from the leaves.
Table 1.
Treatment activities and details for the entire experiment.
| Sr. no. | Activities | Dates | Details |
|---|---|---|---|
| 1 | Seed germination | 14-2-2023 | Seedlings were produced in 128-cell plastic trays. Trays were filled with the mixture (coco, moss peat, and soil). Seeds were planted (one seed per plug) at 1/4th inch depth |
| 2 | Transplantation | 26-3-2023 | Transplanted the seedlings into clay pots (after 41 days of sowing) each pot had 2 seedlings |
| 3 | Cadmium stress to seedlings | 18-4-2023 | 200 mg/kg CdCl2 was applied to the soil as a stress |
| 4 | 1st nanoparticle and salt dose | 21-4-2023 | 100 and 300 mg/L FeNPs and Fe salt (foliar and soil drench 500 mL/plant) |
| 5 | 2nd nanoparticle and salt dose | 25-5-2023 | 100 and 300 mg/L FeNPs and Fe salt (foliar and soil drench 500 mL/plant) |
| 6 | Harvesting | 12-6-2023 | End of experiment |
Table 2.
Treatment details for the comparison of foliar and drench treatments on tomato plants.
| Treatments | Dose (mg/L) | |
|---|---|---|
| WT | Water | As required |
| CT | Cadmium Chloride (200 mg/kg of soil) | Once |
| DT1 | Cd + Fe-salt Cd + Fe-salt | 100 mg/L |
| DT2 | 300 mg/L | |
| DT3 | Cd + Fe-NPs Cd + Fe-NPs | 100 mg/L |
| DT4 | 300 mg/L | |
| FT1 | Cd + Fe-salt Cd + Fe-salt | 100 mg/L |
| FT2 | 300 mg/L | |
| FT3 | Cd + Fe-NPs Cd + Fe-NPs | 100 mg/L |
| FT4 | 300 mg/L | |
Growth and yield parameters
Various growth and yield parameters were selected for the present study. The growth parameters included root and shoot lengths, the number of leaves and branches, and the average leaf area/plant. The yield parameters included the number of days to fruit initiation, the average fruit weight per plant, and the number of fruits per plant.
Biochemical parameters
In the present study, the chlorophyll, carotenoid, proline, malondialdehyde, antioxidative enzyme, ascorbic acid, and phenolic contents of tomato plants grown under Cd stress and FeONP treatments were also tested via the protocols described below.
-
i.
Chlorophyll and carotenoid contents
This study involved an analysis of the photosynthetic pigments in leaves, namely, chlorophyll and carotenoids. For this purpose, 0.55 grams of plant leaves were crushed via a mortar and pestle and mixed with 5 mL of 80% acetone. Three wavelengths (470 nm, 645 nm, and 663 nm) were used to check absorption. The total chlorophyll, chlorophyll a and b, and carotenoid contents were calculated43.In the above equation, P represents the total amount of pigment (mg/L), W represents the weight of the leaves in grams, and V represents the volume of acetone.
-
ii.
Proline content
Proline is another estimator of stress levels in plant tissue; therefore, it was also measured in the present study. First, fresh leaves from each treatment were extracted via sulfosalicylic acid (2 mL). This extract was then mixed with 2 mL of ninhydrin and glacial acetic acid solutions, followed by heating (100 °C) and the addition of toluene (5 mL). The upper layer was separated, and its absorbance was checked at 528 nm for the presence of proline44.
-
iii.
Malondialdehyde (MDA) content
For MDA analysis, cold acetone (10 mL) was used to make a mixture with 2 g of plant sample from each treatment separately. The mixture was filtered. The filtrate was diluted with distilled water (15 mL). Two milliliters of this diluted plant extract was then mixed with 1 mL of TBA (0.67%) and 2 mL of TCA (20%). Here, TCA is trichloroacetic acid, and TBA is thiobarbituric acid. The mixture was then heated for 15 min at 95 °C. After heating, the mixture was cooled and centrifuged at 1500 rpm for 15 min. The sample was diluted, and its absorption at 532 and 600 nm was determined. The MDA content was calculated via the following formula45. -
iv.
Antioxidative enzyme content
The antioxidant enzyme activity of the plants from each treatment was determined to determine stress levels, as the expression of antioxidant enzymes under stress increased in the plants. To separate the enzymes, fresh leaves (1 g) were placed in 0.1 M phosphate buffer (pH 7) and 1% polyvinylpyrrolidone followed by centrifugation in a temperature-controlled centrifuge for 10 min (at 15000 rpm and 4 °C). After this, the supernatant was separated and labeled with antioxidant enzymes, i.e., superoxide dismutase (SOD), peroxidase (POX), and catalase (CAT). A total of 0.5 mL of the abovementioned antioxidant enzyme extract was added to reaction mixture A (RMA), where RMA contained 1 mL of 1% hydrogen peroxide, 1 mL of 1% pyrogallol, and 1 mL of phosphate buffer (pH 6.8). The absorbance was then measured at 420 nm every 20 s to determine the change in the absorbance capacity of the reaction mixture46, and the results are reported as the activity of the peroxidase enzyme. For the calculation of superoxide dismutase activity, the nitroblue tetrazolium method was used47. To conduct the catalase assay, 0.5 mL of hydrogen peroxide (15 mM) was mixed with 0.5 mL of enzyme extract in 1.5 mL of phosphate buffer (0.1 M). The absorbance of the reaction mixture was analyzed at 240 nm.
-
v.
Ascorbic acid content
The level of ascorbic acid in the fruit extract was also determined. The fruit extract was mixed thoroughly with oxalic acid (4%) and centrifuged. Titration was carried out by using the supernatant to react with the 2,6-dichlorophenol indophenol dye. The endpoint was noted and used for calculations48.
-
vi.
Phenolic content
A spectrophotometric assay was used to estimate the phenolics present in plant leaves under different treatments. First, 1 mg of aqueous plant extract was mixed well with 1 mL of FC (Folin and Ciocalteu) reagent, and then, 1 mL of saturated sodium carbonate was added to the mixture after 5 min. The total volume was adjusted to 10 mL with distilled water. After 90 min in the dark, the absorbance was measured at 725 nm. To construct a standard curve, gallic acid was used as a standard phenolic49.
-
vii.
Cd and iron content
To assess plant samples for iron and cadmium content, we employed the wet digestion technique, which entails the use of sulfuric acid digestion as described by Jones and Case (1990). The process commenced with the addition of 0.5 g of plant tissue (leaf or fruit) into a round-bottom flask, followed by the addition of 3.5 mL of concentrated sulfuric acid. This mixture was allowed to sit at room temperature for 30 min, after which 3.5 mL of 30% hydrogen peroxide was incorporated. The sample was then subjected to heating at 250 °C in a crucible muffle furnace for duration of 30 min. Following the cooling of the sample, 1 mL of 30% hydrogen peroxide was added repeatedly until the digestion became clear. The transparent solution was subsequently filtered through filter paper and quantitatively transferred into a 25 mL volumetric flask by adding distilled water. The concentration of iron and cadmium in the final solution was analyzed using an atomic absorption spectrometer.
Data analysis
Data from three replicates were collected, and the means were compared via one-way ANOVA via SPSS statistical software. DMRT was used as a post hoc test at the 5% level of significance.
Results and discussion
Characterization of the nanoparticles
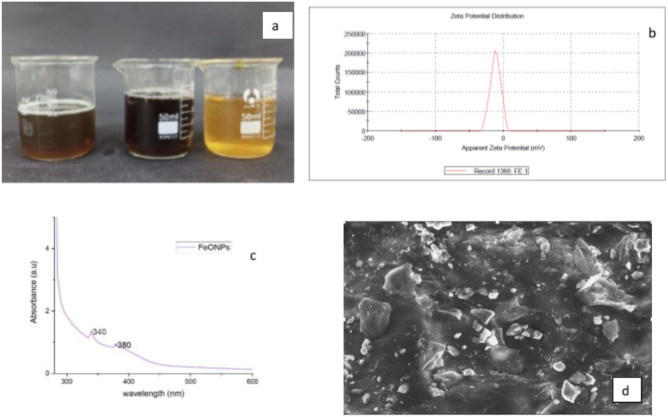
A color change from orange salt solution to dark brown was observed after mixing the plant extract and salt solution. This indicates the formation of nanoparticles (Fig. 1a). Previous studies have shown color change as an indicator. The synthesis of FeO nanoparticles was also indicated by the UV-absorbance spectrum, which showed an absorbance in the range of 300–400 nm (Fig. 1b) with a peak at 380 nm. These results are in agreement with prior studies on FeO nanoparticles50. The formation of the peak is a result of surface plasmon resonance (which occurs when the free electrons in the metal nanoparticles oscillate in response to the incident electromagnetic radiation) of FeNPs. UV‒visible spectrophotometry is a good preliminary technique for identifying the presence of nanomaterials in a solution42. The iron nanoparticles (FeNPs) synthesized in the present study had a zeta potential of − 11.8 mV, which indicates that the particles had a negative charge on the surface (Fig. 1c). Previously, various researchers reported negative zeta potentials of − 26.751, − 82.652, − 19, and − 8.7 mV for iron nanoparticles synthesized from Embilica officinalis, Urtica dioica, Tea extract, and Rhamnella gilgitica, respectively53,54. Further study of the morphology of FeO NPs via SEM analysis revealed triangular and cubical nanoparticles. These particles were also found to form aggregates (Fig. 1d). The aggregate formation or agglomeration of NPs is usually caused by H-bonding among the secondary plant metabolites that surround the surface of the green synthesized NPs. These metabolites are also responsible for specific shapes of newly synthesized NPs55.
Fig. 1.
Characterization of the synthesized nanoparticles via different techniques: (a) visual observation, (b) determination of the zeta potential of the FeNPs, (c) UV‒visible spectrophotometry, and (d) scanning electron micrograph of the iron nanoparticles.
Screening experiment
Effect of Cd stress on tomato seed germination parameters
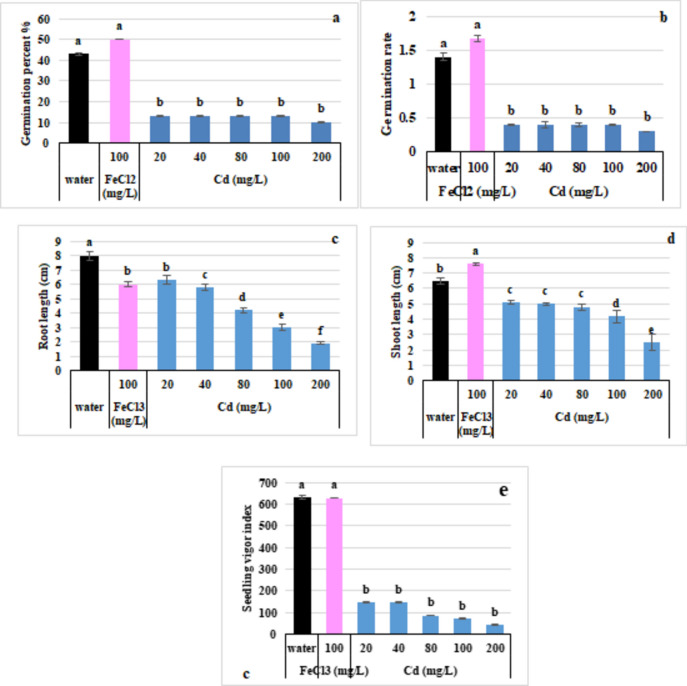
The experimental data indicated that the germination percentage decreased with increasing Cd stress. At a low concentration of Cd (20 mg/L), the percentage of germinated seedlings decreased by 13.9% compared with that of the control. As the Cd concentration increased, the percent reductions were 53, 62, 69, and 76% for 20, 40, 80, and 200 mg/L Cd, respectively, compared with those of the control, as shown in Fig. 2.
Fig. 2.
Effects of Cd and FeCl3 on seed germination parameters (a); germination percentage (b); germination rate (c); germination rate index (d); shoot length (e); and root length.
Compared with the control (water), the application of FeCl2 resulted in a 16% increase in the germination percentage and a 14% increase in the germination rate. However, there was no significant difference in the seedling vigor index compared with that of the control. Additionally, shoot length increased (16.94%) in the presence of FeCl2, as shown in Fig. 2d.
Comparison of the growth and yield responses of tomato plants under cadmium-induced stress and treated with iron nanoparticles (soil drenching and foliar spraying)
For the present study, the growth parameters chosen were shoot length (SL), root length (RL), number of branches (NB), number of leaves per plant (NL), and average leaf area (ALA). The results of the present study revealed that the use of iron nanoparticles as a soil treatment or foliar spray had a progressive influence on the growth of tomato plants under cadmium stress. As indicated in Table 3, Cd had significant adverse effects on plant growth. This study revealed that under the normal control treatment (WT), the results of the growth parameters did not significantly differ between the soil drench and foliar spray treatments. However, under cadmium stress, all the growth parameters significantly decreased. Drench application of 100 mg/L FeNPs increased RL, SL, ALA, NB, and NL by 25%, 29%, 390%, 100%, and 35.9%, respectively. Foliar sprays of FeONPs were more effective at mitigating the effects of Cd stress on the selected growth parameters of tomato plants. Furthermore, the results of yield studies under iron salt and NP treatments (by drench and foliar spray methods) under cadmium stress are shown in Table 3. Foliar spray of FeONPs increased the number of fruits/plant and fruit weight/plant by 100%, as did the number of fruits produced in plants under Cd stress and treated with water only.
Table 3.
Effects of the FeNP treatments, namely, soil drenching (SD) and foliar spraying (FS), on tomato plants under cadmium stress.
| Treatments | Root length (cm) | Shoot length | Average Leave area (m2) | Fruit weight/plant (g) | No. of fruits/plant | No. of leaves/plant | No. of branches/plant | |
|---|---|---|---|---|---|---|---|---|
| Control (Water) | WT | 6.21d ± 1.02 | 15.6e ± 2.3 | 7.7c ± 0.2 | 8.69e ± 0.21 | 0.33f ± 0.02 | 34.2d ± 4.10 | 19.3b ± 0.32 |
| Cadmium | CT | 5.33f ± 0.5 | 13.6f ± 0.4 | 3.25d ± 0.6 | 0.00f ± 0.0 | 0.00f ± 0.00 | 28.0e ± 3.0 | 14.2c ± 0.33 |
| Cd + Fe Salt | DT1 | 6.00e ± 1.0 | 21.3c ± 1.0 | 5.25cd ± 0.4 | 41.0c ± 1.0 | 6.00d ± 1.0 | 58.6c ± 2.1 | 27.0a ± 1.0 |
| Cd + Fe Salt | DT2 | 6.30d ± 0.5 | 21.0c ± 1.0 | 6.83c ± 0.5 | 42.9c ± 0.05 | 6.30d ± 0.5 | 68.3c ± 2.0 | 14.0c ± 1.0 |
| Cd + FeNPs | DT3 | 6.67d ± 1.1 | 17.6d ± 1.0 | 26.0a ± 1.0 | 62.0b ± 13 | 6.67d ± 1.1 | 33.3d ± 2.0 | 19.3b ± 0.5 |
| Cd + FeNPs | DT4 | 5.67ef ± 0.5 | 15.0e ± 1.1 | 14.8b ± 2.0 | 59.5b ± 11 | 5.67e ± 0.5 | 36.6d ± 5.0 | 20.3b ± 1.5 |
| Cd + Fe Salt | FT1 | 7.16c ± 0.7 | 23.6b ± 1.0 | 9.17bc ± 0.7 | 22.3d ± 0.2 | 7.16c ± 0.7 | 67.6c ± 3.0 | 22.0b ± 0.7 |
| Cd + Fe Salt | FT2 | 9.00b ± 1.1 | 21.0c ± 1.0 | 4.50d ± 0.5 | 30.4c ± 0.6 | 9.00b ± 1.1 | 38.3d ± 2.1 | 14.0c ± 1.0 |
| Cd + FeNPs | FT3 | 11.00a ± 1.0 | 25.0a ± 1.0 | 14.1b ± 0.7 | 77.7a ± 9.0 | 11.00a ± 1.0 | 91a ± 7.0 | 27.6a ± 1.0 |
| Cd + FeNPs | FT4 | 8.00b ± 1.1 | 18.3d ± 3.0 | 13.6b ± 0.5 | 72.6a ± 0.3 | 8.00b ± 1.1 | 81.6b ± 2.0 | 17.0bc ± 1.0 |
a,b,c,d,e,fColumns not sharing the same letter are significantly different
Previous research revealed that applying iron oxide NPs to the soil and leaves of wheat plants under Cd stress resulted in a decrease in the Cd content of grains56. Iron NPs prevent plants from absorbing cadmium. It was also reported that NPs caused a decrease in the rate of leaf electrolyte leakage and an increase in antioxidant enzyme activity caused by Cd stress. These factors improved the performance of the plants in terms of dry weight. According to a previous study, the availability of iron in the soil often becomes limiting due to its reaction with other minerals, the pH of the soil, etc. Hence, foliar spraying of Fe NPs can be a better option than soil application. In another study, Fe NPs were applied to rice plants in foliar form, and biochar was applied as a soil application during the Cd stress study. FeNPs effectively alleviate stress2.
Cadmium stress adversely affects the growth parameters of tomato plants. A decrease in growth is a significant characteristic of cadmium toxicity in plants, including tomatoes57, wheat58, and cucumber59. The reduction in growth under cadmium stress is likely due to the interaction of cadmium with cell wall pectin and the pectin methylesterase enzyme60.
Iron is a crucial element for plant growth and photosynthesis. However, if iron is deficient, it can reduce the growth rate of plants. Nano ferric oxide can be used to increase the amount of chlorophyll in plants if the appropriate amount is applied. Moreover, it is more stable in soil than other alternatives61. In a recent study, the growth of tomato plants significantly decreased when they were exposed to cadmium stress62. The addition of nano-Fe3O4 in the presence of cadmium stress has been found to significantly improve growth parameters while also reducing the harmful effects of cadmium accumulation. Similarly, it has been reported earlier that the application of nanoiron oxide in wheat leads to improved growth parameters63. It has also been reported that the use of nanoiron oxide improves the length and fresh and dry weight of tomato plants64. Previous studies have shown that foliar applications of Fe2O3 and nano fertilizers increase crop yields in plants22. Nano-Fe2O3 can also act as a nanofertilizer that promotes plant growth and improves photosynthetic efficiency65. Furthermore, iron-based nanomaterials have shown potential for protecting plants against abiotic stress. Nano-Fe3O4 alleviated the adverse effects of Cd stress on growth and yield parameters56.
The flowering phase is a crucial part of the life cycle of angiospermic plants, and it can be affected by both internal and external factors66. The present study revealed that heavy metal stress can seriously harm this stage of plants. The concentration of heavy metals in the soil can delay or even destroy the reproductive stage of plants67,68.
Iron is well known to be important for plant metabolism. Iron is a part of cytochrome and other electron donor and acceptor in the ETCs involved in respiration and photosynthesis. When it is applied as a foliar spray in nanoiron form, it is directly absorbed by the foliage and used in chloroplasts while maintaining the nutritional status of treated plants69, which helps in the growth and flowering stages of plants under stress.
Numerous studies have shown that the photosynthetic activity of leaves is significantly increased by the application of iron NPs via foliar spray. Researchers have shown that foliar spraying of iron NPs is a smart way of supplying a good amount of iron to plants without causing nutrient wastage; therefore, it could be a less expensive alternative to supplying iron. However, inconsistent results have also been reported in response to iron spray. Therefore, more research is needed. Iron compounds applied to leaves can penetrate through cracks and imperfections in the cuticle, as well as through leaf hairs, specialized epidermal cells, and stomata. The adaxial and abaxial surfaces of the leaf play a role in absorbing the applied solutions. The humidity level and water content of the leaf are also important factors that affect the absorption process70.
Estimation of the photosynthetic pigments of tomato plants (Solanum lycopersicum) in the presence of cadmium and the application of iron nanoparticles (soil drenching and foliar spraying)
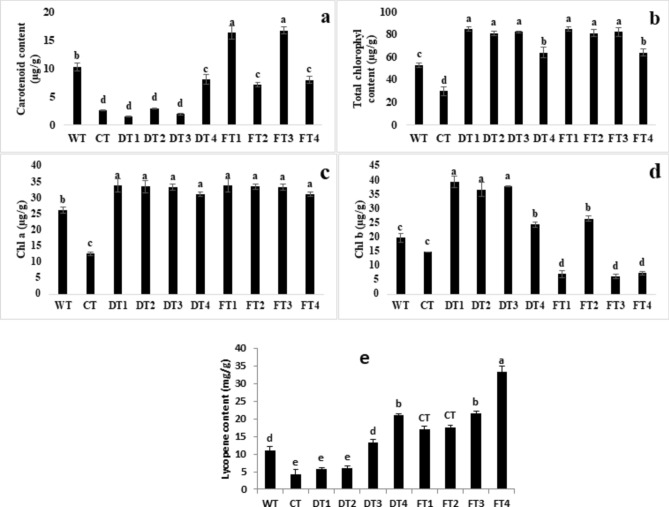
The findings revealed significant variations in the levels of photosynthetic pigments under Cd stress, with and without Fe nanoparticle treatment. The results revealed that Cd stress had adverse effects on these pigments, leading to a reduction in these pigments compared with those under normal conditions (WT treatment), as shown in Fig. 3. In contrast, soil drench treatments with FeNPs helped mitigate cadmium stress in tomato plants by increasing the total chlorophyll, chl a, and chl b contents by 180, 175, and 178%, respectively, compared with those in Cd-stressed but nontreated plants (CT). Furthermore, in the present study, the carotenoid content of leaves and the lycopene content of fruits were also studied (Fig. 3d,e). The maximum carotenoid content was detected in tomato plants treated with foliar spray of 100 mg/L FeNPs. The maximum lycopene content was detected in fruits from the foliar spray treatment with 100 mg/L FeNPs (FT3).
Fig. 3.
Effects of iron nanoparticles (FeNPs) and Fe salts on photosynthetic pigments under Cd stress (a): carotenoid content; (b): total chlorophyll content; (c): chlorophyll a; and (d): chlorophyll b (e): lycopene in tomato plants (Solanum lycopersicum).
Photosynthetic pigments serve as crucial energy sources in plant biological systems, representing a vital indicator of photosynthesis, with any changes causing parallel effects on metabolism overall71. Notably, our study revealed that FeNPs significantly increased the chlorophyll content in the soil drench DT1, as shown in Fig. 3b. Research has suggested that the impact of nanoparticles (NPs) on chlorophyll content depends on concentration72. Therefore, various concentrations of NPs play critical roles in influencing various physiological processes in plants.
As described earlier, the presence of iron causes an increase in the photosynthetic activity of plants, as iron ions compete with Cd ions. These ions have the same channels in the root cell membrane. When iron is present in excess or in an active form such as a nanoform, it defeats Cd ions. As iron in nanoform is more surface active and acts more smartly, it is considered more effective in treating Cd stress73. Iron nanoparticles release iron as micronutrients for plants. Fe participates in various physiological processes, including redox reactions, respiration, and chlorophyll biosynthesis74. FeO-NPs improved wheat plant functions, nutrient concentrations, and biomass yield under Cd and salt stresses39.
Chlorophyll is an essential molecule of plant life and is responsible for the existence of life on Earth. The presence of Cd and other metals has a deleterious effect on the chlorophyll content of leaves, resulting in destruction, and leaf yellowing, which is one of the major signs of metal toxicity. It causes a direct reduction in plant biomass and yield75. Iron NPs can predominantly increase the chlorophyll content and activity in plants such as wheat and rice in the presence of Cd stress8,76. When plants are exposed to cadmium (Cd), their contents of photosynthetic and accessory pigments decrease. However, the negative effects of Cd on plant growth can be counteracted by the addition of iron (Fe), which suggests that the supply of iron to plants can help mitigate Cd stress in plants. Lycopene is a carotenoid pigment that results in an orange‒red color in tomatoes77. The biosynthesis of carotenoids is a complex process in which various enzymes and cofactors are involved. Therefore, nutrients such as iron can directly affect carotenoid content. Under stress conditions, the carotenoid content is also increased to maintain efficient light absorption during photosynthesis78. In turn, increased lycopene can protect plants from lipid peroxidation and DNA damage79.
Estimation of antioxidants in tomato plants (Solanum lycopersicum) under cadmium-induced stress and the application of iron nanoparticles (soil drenching and foliar spraying)
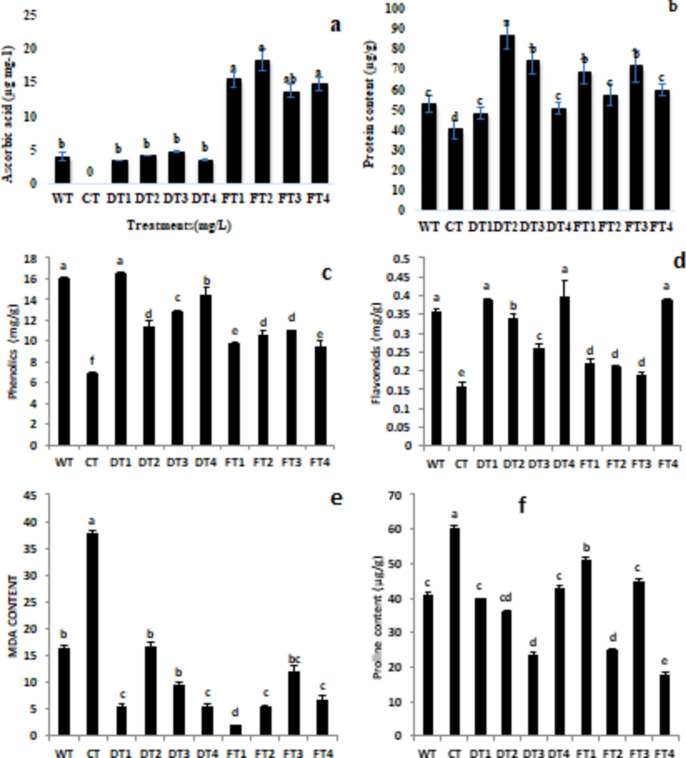
One of the major antioxidant agents, along with other minerals, is ascorbic acid (vitamin C), which removes free radicals80. Plants use enzymatic and nonenzymatic antioxidant mechanisms to detoxify the ROS produced during stress. This includes the accumulation of ascorbic acid and the activation of antioxidant enzymes81. In the course of the study, the plants that were treated with foliar sprays presented more ascorbic acid than those that were treated with soil drenching. Among the FeNP concentrations used, 300 mg/L Fe NPs resulted in the highest ascorbic acid content in fruits, whereas cadmium-induced plants did not yield any fruits during the experimental period. Similarly, the ascorbic acid content was also significantly greater in the NP-treated plants that were subjected to cadmium stress, as depicted in Fig. 3a.
Vitamin C is an antioxidant that works as a second-line defense system. It directly interacts with radicals such as O2−, H2O2, and OH. Both vitamin C and α-tocopherol help minimize the consequences of lipid peroxidation in membranes82. Decrease in ascorbic acid levels under the effect of cadmium stress in lettuce (Lactuca sativa L.). Ascorbic acid plays crucial roles in cell division, expansion, and enzyme cofactors, and participation in the photosynthetic apparatus83. It helps detoxify ROS, which are produced by stress. Moreover, ascorbic acid can directly provide electrons to tocopherol radicals and reduce lipid peroxidation, safeguarding cellular membranes84.
Furthermore, the protein content in tomato leaves also decreased by 24.71% due to cadmium stress in the CT treatment. However, the plants treated with iron salt presented the highest protein content. Moreover, the treatment of plants with Fe salt was found to be helpful in maintaining the protein content of the plant tissue by reducing the stress of cadmium, whereas the results of the FeO NPs were also comparable (Fig. 4b).
Fig. 4.
Effects of iron nanoparticles (FeNPs) and Fe salts on (a) ascorbic acid (b) protein content, (c) MDA content, (d) flavonoid content, (e) phenolic content, and (f) proline content in tomato plants (Solanum lycopersicum) under Cd stress.
In the present study, the levels of phenolic compounds and flavonoids were examined as markers of nonenzymatic antioxidants (as shown in Fig. 4c,d). The study revealed that exposure to Cd stress led to a decrease in the phenolic and flavonoid contents of tomato plants, with levels decreasing from 16.07 to 6.9 µg and from 0.36 to 0.16 µg, respectively. This study revealed that cadmium (Cd) causes oxidative stress in tomato plants. However, the introduction of iron nanoparticles (300 mg/L) improved the flavonoid content by 150% in plants under Cd stress. The results revealed no significant difference between the soil drench and foliar spray treatments, as indicated in Fig. 4d,e.
These results suggest that the use of FeNPs can help tomato plants increase their secondary metabolite content to combat the reactive oxygen species (ROS) produced by Cd stress. The control treatment WT (water) had a phenolic content of 16 µg, but it decreased to 6.9 µg in the CT (cadmium) treatment. However, the phenolic content increased to 14.59 and 16.59 µg in the FeNP- and Fe-salt-treated plants, respectively (Fig. 4d). Compared with the foliar spray treatment, the soil drench treatment resulted in better outcomes.
Hussain et al. reported that applying Fe2O3 nanoparticles to the soil and foliage of wheat plants under Cd stress led to a reduction in the rate of leaf electrolyte leakage. When plants are exposed to heavy metals, nonenzymatic antioxidants such as phenolic and flavonoid compounds act as metal chelators. They have strong redox potential and can detoxify the effects of ROS inside plant cells. They can do so by neutralizing free radicals, absorbing singlet and triplet oxygen, and deteriorating peroxides. These antioxidants act as strong scavengers of ROS and can stop the activity of enzymes that produce ROS in plant cells under stress. When plants are under natural stress, they tend to produce more secondary metabolites. NPs help plants produce more phenolic compounds and flavonoids, thus helping them fight ROS more efficiently, which explains our findings. Nanomaterials can improve plant metabolism and physiologically, plants respond to stress, but the mechanisms underlying these effects are still unknown. Flavonoids are secondary metabolites in plants with essential functions such as UV protection, signaling, and defense85–87. These compounds function as antioxidants when the dihydroxy B-ring is replaced and can be found in the endoplasmic reticulum and the nuclei of mesophilic cells. Flavonoids inhibit reactive oxygen species by forming complexes with iron and copper ions88.
In the present study, MDA content analysis of tomato plants revealed that Cd stress increased the MDA content in plants subjected to the CT treatment by 128%, which was greater than the degree of toxicity. The MDA content of the FeNP-treated and Fe salt-treated plants was significantly lower than that of the CT plants but comparable to that of the WT plants (Fig. 4e). Proline is another indicator of stress in plants and was also studied in the present study. In the present study, the proline content also increased by 50% in cadmium-stressed (CT) plants compared with that in the control (WT) plants. The lowest proline content was detected in the Fe NP-treated (FT4) plants (Fig. 4f).
MDA serves as a marker for lipid peroxidation within cellular organelles. In our study, the induction of Cd stress caused an increase in the content of MDA in the leaves of tomato plants. This suggests that the stress induced by Cd resulted in notable peroxidation of the inner membranes of the plants’ cells, leading to structural damage to the cellular membrane. However, the MDA content was significantly decreased by the application of NPs to the treated plants, thereby protecting the integrity of the cellular membranes and positively influencing growth and yield. The chelating ability of proline enables it to form bonds with metal contaminants and alleviate metal toxicity in plants89. It has been previously demonstrated that Fe3O4 NPs can help reduce the MDA levels in the shoots and roots of wheat plants under metal stress. The same results were observed for Brassica juncea roots and leaves under heavy metal stress and treatment with iron NPs40,90.
In numerous plant species, the accumulation of free proline is a common response to various biotic and abiotic stresses. This phenomenon suggests that under stress conditions, there is an increased flow of reducing equivalents into both the proline synthesis (in the cytosol) and degradation (in the mitochondria) pathways. This increased flow may allow for precise regulation of the cellular redox potential in the cytosol91,92. Our results also revealed that tomato plants exposed to Cd stress presented relatively high levels of proline, indicating that Cd toxicity enhances proline synthesis in plants to mitigate osmotic stress. Interestingly, iron has been found to alleviate salt, metal, and low-temperature stress in plants by regulating the biosynthesis of proline93. Proline is also known to act as an osmoprotectant and helps to regulate cellular metabolic activities. It helps to maintain the stability of cell membranes and to reduce the degradation of proteins and carbohydrates94. When plants are exposed to metal toxicity, their ability to absorb and transport water is negatively affected. This leads to an increase in the biosynthesis of proline, which helps to regulate the cellular osmotic balance95. Studies have shown that plants exposed to cadmium (Cd) stress tend to accumulate relatively high levels of proline96. Proline also acts as a molecular chaperone to maintain protein integrity97.
Estimation of the oxidative stress and antioxidant activities of tomato plants (Solanum lycopersicum) under cadmium-induced stress and the application of iron nanoparticles (soil drenching and foliar spraying)
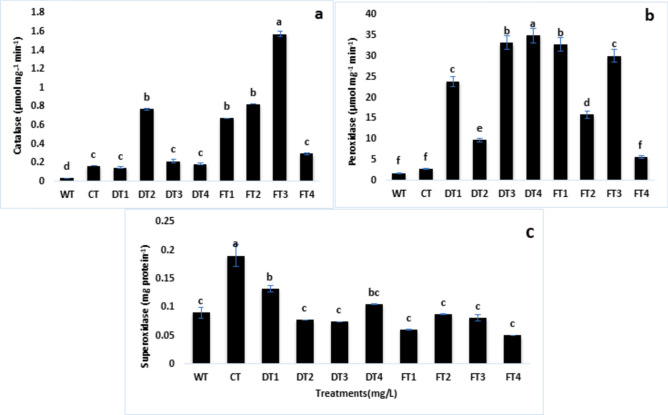
Plants subjected to Cd stress undergo oxidative stress, which is characterized by an accumulation of ROS and lipid peroxidation. To evaluate the efficacy of FeNPs in reducing oxidative damage in plants during Cd stress, it is possible to measure the activity of various antioxidant enzymes, including CAT, POX, and SOD. Figure 5 shows the results of the antioxidant enzyme studies of the tomato plants subjected to cadmium stress and FeNP treatments. The results revealed that catalase activity was greater in CT plants than in WT plants. Compared with soil drenching, foliar spraying of FeNPs and Fe salts increased catalase activity (Fig. 5a). There were no significant differences in peroxidase activity between the soil drench and foliar spray treatments (Fig. 5b). The same a trend was observed for SOD (Fig. 5c), but catalase activity was significantly greater in foliar spray-treated plants subjected to FT3.
Fig. 5.
Effects of iron nanoparticles (FeNPs) and Fe salts on (a) catalase activity, (b) peroxidase activity, and (c) superoxide dismutase activity in tomato plants (Solanum lycopersicum) under Cd stress.
Catalase is a crucial enzyme in the antioxidant system of plants, and it has a heme group in its structure. Therefore, if the plant is deficient in iron, the activity of catalase is also affected, and vice versa, when sufficient amounts of iron are provided to the plants. This means that when plants are deprived of iron and are exposed to cadmium toxicity, catalase levels decrease significantly. Iron nanoparticles are known to increase the gene expression of the CAT enzyme98. When plants face any stressful conditions, catalase levels decrease. Other studies also suggest that antioxidant enzymes are important targets for investigation in plants exposed to Fe deficiency-induced Cd toxicity99,100. In white clover leaves, CAT (catalase) activity increases during the first 14 days of drought conditions101. These previous findings suggest that catalase enzymes inhibit ROS species, and if gene expression of this enzyme is reduced, then ROS scavenging is also reduced102. However, our research revealed that in plants treated with Cd (cadmium), the CAT activity increased with the application of FeNPs (iron nanoparticles) (Fig. 4a). This finding is consistent with those of other previous studies on the combined effects of Fe and Cd on mung bean plants103, sugarcane100, rice104, barley, etc.105.
The activity of ascorbate peroxidase is reduced when there is a lack of iron. When treated with cadmium, the activity is further reduced. Ascorbate peroxidase activity is reduced in the apoplastic fluid as well as at the intracellular level106. The reason for this reduction in activity is that the APX molecule contains an iron atom that is nonheme, which is in high demand when iron is scarce. However, when iron was present under cadmium treatment, the APX activity increased (Fig. 5b). This increase in activity may be due to the limited damage caused by an increased supply of iron77.
SOD is a significant enzyme in the ascorbate–glutathione pathway. When superoxide anions are produced as ROS, they are converted to H2O2 by this enzyme. These findings indicate that SOD activity was reduced (Fig. 5c). This may be related to the findings that Fe is integrated into the negative feedback of Cd treatment to maintain redox status. The presence of iron in the feedback mechanism during cadmium treatment indicates that iron might assist in regulating the cellular response to cadmium toxicity. Specifically, it could be involved in a negative feedback loop that controls the expression of genes responsible for oxidative stress responses and metal homeostasis.
Estimation of the Cadmium and iron content of tomato plants (Solanum lycopersicum) under cadmium-induced stress and the application of iron nanoparticles (soil drenching and foliar spraying)
Results showed that highest iron content was found in the leaves of treatment FT4 and FT3. Whereas iron content of fruits was significantly higher in iron treated plants either by drench or foliar treatment as compared to WT and CT treatments. It is also evident from the results that Cd content of treatment FT4 and FT3 was significantly lower as compared to non-treated plants (CT). Iron is known to compete with cadmium for the same transporters in the plant roots and sufficient levels of iron in the soil make sure the absorption of iron instead of cadmium107. Iron reduces the absorption of cadmium in plants by changing the composition of root exudates. These exudates then bind with cadmium, making their absorption impossible by roots108. Iron is also reported to upregulate the genes for iron balance in the cells that also indirectly influence the Cd absorption by plants109. Whereas, in recent studies it has been found that iron in form of foliar application is absorbed more actively and particularly in nanoform, it is highly efficient. It can significantly improve the antioxidant system of the plant, thus adding to the plant defense system against the negative effects of absorbed cadmium110. Iron nanoparticles directly applied to the surface of plants, on leaves, is absorbed instantly and can decrease th absorption of cadmium. Foliar spray of iron also enhances the photosynthetic strength of the plants as well111 and can reduce the translocation of cadmium.
Table 4 Treatment details for the comparison of foliar and drench treatments on tomato plants for iron and cadmium content of leaves and fruits
Table 4.
Treatment details for the comparison of foliar and drench treatments on tomato plants.
| Treatments | Iron content mg/g | Cd content mg/g | ||
|---|---|---|---|---|
| Leaves | Fruits | Leaves | Fruits | |
| WT | 0.131d ± 0.001 | 0.0035b ± 0.0005 | 0.001d ± 0.000 | 0.000b ± 0.001 |
| CT | 0.091e ± 0.004 | 0.0021c ± 0.0004 | 0.015a ± 0.001 | 0.007a ± 0.004 |
| DT1 | 0.133d ± 0.003 | 0.0039ab ± 0.0003 | 0.011b ± 0.001 | 0.0001b ± 0.003 |
| DT2 | 0.135d ± 0.001 | 0.0038b ± 0.0001 | 0.011b ± 0.002 | 0.0001b ± 0.001 |
| DT3 | 0.222c ± 0.001 | 0.0041a ± 0.0001 | 0.009c ± 0.000 | 0.0001b ± 0.001 |
| DT4 | 0.245b ± 0.001 | 0.0041a ± 0.0001 | 0.010b ± 0.001 | 0.0001b ± 0.001 |
| FT1 | 0.213c ± 0.002 | 0.0044a ± 0.0001 | 0.002d ± 0.000 | 0.0001b ± 0.002 |
| FT2 | 0.220c ± 0.004 | 0.0040a ± 0.0001 | 0.001d ± 0.000 | 0.0001b ± 0.004 |
| FT3 | 0.317a ± 0.005 | 0.0042a ± 0.0002 | 0.000e ± 0.000 | 0.0001b ± 0.005 |
| FT4 | 0.345a ± 0.003 | 0.0040a ± 0.0002 | 0.000e ± 0.000 | 0.0001b ± 0.003 |
a,b,c,d,e,fColumns not sharing the same letter are significantly different
Conclusion
Iron nanoparticles effectively reduce the absorption of cadmium from the roots of tomato plants, thus mitigating the negative effects of this heavy metal. When the tomato plants were exposed to cadmium stress, the Fe-NPs improved their growth and yield parameters. The biomolecule markers and stress levels of the plants also significantly improved under the influence of the Fe-NPs. Furthermore, foliar spray treatment was more effective at increasing the growth and yield parameters of tomato plants and controlling stress markers than soil drench treatment at a conc. of 100 mg/L. This study highlights the potential of foliar applications to increase the resilience of tomato plants to Cd toxicity. Optimizing FeNP application can help improve crop health and yield in contaminated environments. Foliar spraying of iron nanoparticles should be a key practice for managing Cd stress in tomato cultivation, offering a valuable approach to sustainable agricultural practices. Future research should focus on field trials to validate the effectiveness of FeNP application under natural conditions, providing further insights into its practical applications and long-term benefits in real-world agricultural settings.
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSP2024R393), King Saud University, Riyadh, Saudi Arabia.
Author contributions
AA & TS; Experimentation and Methodology, SJ; Conceptualization and Supervision, SI; Statistical analysis, SN; Validation, AAS; Resource acquisition and Investigation, SS & MKG writing-original draft preparation. All authors read and approved the final manuscript.
Funding
Researchers Supporting Project number (RSP2024R393), King Saud University, Riyadh, Saudi Arabia.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
We declare that the manuscript reporting studies do not involve any human participants, human data or human tissues. So, it is not applicable. Our experiment follows with the relevant institutional, national, and international guidelines and legislation.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sumera Javad, Email: zif_4@yahoo.com.
Anis Ali Shah, Email: anisalibot@gmail.com.
References
- 1.Hussain, B. et al. Foliage application of selenium and silicon nanoparticles alleviates Cd and Pb toxicity in rice (Oryzasativa L.). Sci. Total Environ.712, 136497. 10.1016/j.scitotenv.2020.136497 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Rizwan, M., Ali, S., Rehman, M. Z. U. & Maqbool, A. A critical review on the effects of zinc at toxic levels of cadmium in plants. Environ. Sci. Pol. Res.26, 6279–6289. 10.1007/s11356-019-04174-6 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Panahirad, S. et al. Foliar application of chitosan-putrescine nanoparticles (CTS-Put NPs) alleviates cadmium toxicity in grapevine (Vitisvinifera L.) cv. Sultana: Modulation of antioxidant and photosynthetic status. BMC Plant Biol.23, 411. 10.1186/s12870-023-04420-7 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azimi, F., Oraei, M., Gohari, G., Panahirad, S. & Farmarzi, A. Chitosan-selenium nanoparticles (Cs–Se NPs) modulate the photosynthesis parameters, antioxidant enzymes activities, and essential oils in Dracocephalummoldavica L. under cadmium toxicity stress. Plant Phys. Biochem.167, 257–268. 10.1016/j.plaphy.2021.08.013 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Haider, F. U. et al. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf.211, 111887. 10.1016/j.ecoenv.2020.111887 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Gall, J. E., Boyd, R. S. & Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Moni. Asst.187, 1–21. 10.1007/s10661-015-4436-3 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Wang, C. et al. Effects and mechanisms of foliar application of silicon and selenium composite sols on diminishing cadmium and lead translocation and affiliated physiological and biochemical responses in hybrid rice (Oryzasativa L.) exposed to cadmium and lead. Chemosphere.251, 126347. 10.1016/j.chemosphere.2020.126347 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Rizwan, M. et al. Influence of biochar amendment and foliar application of iron oxide nanoparticles on growth, photosynthesis, and cadmium accumulation in rice biomass. J. Soils Sed.19, 3749–3759. 10.1007/s11368-019-02327-1 (2019). [Google Scholar]
- 9.Afzal, S. & Singh, N. K. Effect of zinc and iron oxide nanoparticles on plant physiology, seed quality and microbial community structure in a rice-soil-microbial ecosystem. Environ. Pollut.314, 120224. 10.1016/j.envpol.2022.120224 (2022). [DOI] [PubMed] [Google Scholar]
- 10.Afzal, S., Aftab, T. & Singh, N. K. Impact of zinc oxide and iron oxide nanoparticles on uptake, translocation, and physiological effects in Oryza sativa L.. J. Plant Grt. Reg.41, 1445–1461. 10.1007/s00344-021-10388-1 (2022). [Google Scholar]
- 11.Faizan, M. et al. Nanoparticle mediated plant tolerance to heavy metal stress: What we know?. Sustainability15, 1446. 10.3390/su15021446 (2023). [Google Scholar]
- 12.Avila-Quezada, G. D., Ingle, A. P., Golińska, P. & Rai, M. Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks. Nanotech. Rev.11, 2123–2140. 10.1515/ntrev-2022-0126 (2022). [Google Scholar]
- 13.Yoon, H. Y. et al. Synergistic release of crop nutrients and stimulants from hydroxyapatite nanoparticles functionalized with humic substances: Toward a multifunctional nanofertilizer. ACS Omega5, 6598–6610. 10.1021/acsomega.9b04354 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rastogi, A. et al. Application of silicon nanoparticles in agriculture. 3 Biotech9, 1–11. 10.1007/s13205-019-1626-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubio, L. et al. Safer-by-design flame-sprayed silicon dioxide nanoparticles: The role of silanol content on ROS generation, surface activity and cytotoxicity. Part. Fibre Toxicol.16, 1–15. 10.1186/s12989-019-0325-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dilnawaz, F., Misra, A. N. & Apostolova, E. Involvement of nanoparticles in mitigating plant’s abiotic stress. Plant Stress10, 100280. 10.1016/j.stress.2023.100280 (2023). [Google Scholar]
- 17.Su, Y. et al. Delivery, uptake, fate, and transport of engineered nanoparticles in plants: A critical review and data analysis. Environ. Sci. Nano.6, 2311–2331. 10.1039/C9EN00461K (2019). [Google Scholar]
- 18.Keller, A. A., Huang, Y. & Nelson, J. Detection of nanoparticles in edible plant tissues exposed to nano-copper using single-particle ICP-MS. J. Nano. Res.20, 1–13. 10.1007/s11051-018-4192-8 (2018). [Google Scholar]
- 19.Alzuhairi, A. K. A. & AlSheikly, A. A. Effect of chemical fertilization and foliar spraying with gibberellic acid and benzyl adenine in the growth and flowering of rose plants. Int. J. Health Sci.10.53730/ijhs.v6nS1.5683 (2022). [Google Scholar]
- 20.Tighe-Neira, R. et al. Metallic nanoparticles influence the structure and function of the photosynthetic apparatus in plants. Plant Physiol. Biochem.130, 408–417. 10.1016/j.plaphy.2018.07.024 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Raliya, R. et al. Quantitative understanding of nanoparticle uptake in watermelon plants. Front. Plant Sci.7, 198920. 10.3389/fpls.2016.01288 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Achari, G. A. & Kowshik, M. Recent developments on nanotechnology in agriculture: Plant mineral nutrition, health, and interactions with soil microflora. J. Agric. Food Chem.66, 8647–8661. 10.1021/acs.jafc.8b00691 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Meier, S. et al. Synthesis of calcium borate nanoparticles and its use as a potential foliar fertilizer in lettuce (Lactuca sativa) and zucchini (Cucurbita pepo). Plant Phys. Biochem.151, 673–680. 10.1016/j.plaphy.2020.04.025 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Alshaal, T. & El-Ramady, H. Foliar application: from plant nutrition to biofortification. Environ. Biodiv. Soil Sec.1, 71–83 (2017). [Google Scholar]
- 25.Ahmadi, S. Z., Zahedi, B., Ghorbanpour, M. & Mumivand, H. Comparative morpho-physiological and biochemical responses of Capsicumannuum L. plants to multi-walled carbon nanotubes, fullerene C60 and graphene nanoplatelets exposure under water deficit stress. BMC Plant Biol.24, 116. 10.1186/s12870-024-04798-y (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ullah, H., Li, X., Peng, L., Cai, Y. & Mielke, H. W. In vivo phytotoxicity, uptake, and translocation of PbS nanoparticles in maize (Zeamays L.) plants. Sci. Total Environ.737, 139558. 10.1016/j.scitotenv.2020.139558 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Meena, R. K. & Kumar, S. Variability, heritability and genetic advance in tomato (Solanumlycopersicum L.) genotypes. Int. J. Plant Soil Sci.35, 138–144. 10.9734/IJPSS/2023/v35i42810 (2023). [Google Scholar]
- 28.Sandoval-Ceballos, M. G. et al. The importance of conserving Mexico’s tomato agrodiversity to research plant biochemistry under different climates. Plants People Planet3, 703–709. 10.1002/ppp3.10218 (2021). [Google Scholar]
- 29.Bamaniya, B., Ali, S., Ramgiri, S., Shrivastava, A. & Bain, R. Performance of tomato hybrids for growth, yield and quality under the western track of Vindhyan Plateau of Madhya Pradesh, India. Int. J. Curr. Microbiol. Appl. Sci.8, 2226–2232 (2019). [Google Scholar]
- 30.Askary, M., Talebi, S. M., Amini, F. & Bangan, A. D. B. Effects of iron nanoparticles on Menthapiperita L. under salinity stress. Biologija10.6001/biologija.v63i1.3476 (2017). [Google Scholar]
- 31.Ning, X. et al. Research progress on iron absorption, transport, and molecular regulation strategy in plants. Front. Plant Sci.14, 1190768. 10.3389/fpls.2023.1190768 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pariona, N., Martinez, A. I., Hdz-García, H., Cruz, L. A. & Hernandez-Valdes, A. Effects of hematite and ferrihydrite nanoparticles on germination and growth of maize seedlings. Saudi J. Biol. Sci.24, 1547–1554. 10.1016/j.sjbs.2016.06.004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elanchezhian, R. et al. Morpho-physiological and biochemical response of maize (Zeamays L.) plants fertilized with nano-iron (Fe3O4) micronutrient. J. Plant Nut.40, 1969–1977. 10.1080/01904167.2016.1270320 (2017). [Google Scholar]
- 34.Spielmann, J. et al. Differential metal sensing and metal-dependent degradation of the broad spectrum root metal transporter IRT1. Plant J.112, 1252–1265. 10.1111/tpj.16010 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Elazab, D. S., Abdel-Wahab, D. A. & El-Mahdy, M. T. Iron and zinc supplies mitigate cadmium toxicity in micropropagated banana (Musa spp.). Plant Cell Tissue Organ. Cult. (PCTOC)145(2), 367–377. 10.1007/s11240-021-02013-6 (2021). [Google Scholar]
- 36.Moravčíková, D. & Žiarovská, J. The effect of cadmium on plants in terms of the response of gene expression level and activity. Plants12, 1848. 10.3390/plants12091848 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dad, F. P. et al. Influence of iron-enriched biochar on Cd sorption, its ionic concentration and redox regulation of radish under cadmium toxicity. Agriculture11, 1. 10.3390/agriculture11010001 (2020). [Google Scholar]
- 38.Rahmatizadeh, R. et al. Response of tomato plants to interaction effects of magnetic (Fe3O4) nanoparticles and cadmium stress. J. Plant Interact.14(1), 474–481. 10.1080/17429145.2019.1626922 (2019). [Google Scholar]
- 39.Manzoor, N. et al. Iron oxide nanoparticles ameliorated the cadmium and salinity stresses in wheat plants, facilitating photosynthetic pigments and restricting cadmium uptake. Sci. Total Environ.769, 145221. 10.1016/j.scitotenv.2021.145221 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Konate, A. et al. Magnetic (Fe3O4) nanoparticles reduce heavy metals uptake and mitigate their toxicity in wheat seedling. Sustainability9, 790. 10.3390/su9050790 (2017). [Google Scholar]
- 41.Bombin, S. et al. Developmental and reproductive effects of iron oxide nanoparticles in Arabidopsis thaliana. Int. J. Mol. Sci.16, 24174–24193. 10.3390/ijms161024174 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghaffar, N. et al. Metal nanoparticles assisted revival of Streptomycin against MDRS Staphylococcus aureus. PLoS ONE17, e0264588. 10.1371/journal.pone.0264588 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnon, D. & Whatley, F. Determination of total chlorophyll and carotenoids content. Crop Sci.110, 554–556 (1949). [Google Scholar]
- 44.Bates, L. S., Waldren, R. & Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil39, 205–207. 10.1007/BF00018060 (1973). [Google Scholar]
- 45.Heath, R. L. & Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys.125, 189–198. 10.1016/0003-9861(68)90654-1 (1968). [DOI] [PubMed] [Google Scholar]
- 46.Chance, B. Assay of catalase and peroxidases. In Advances in Enzymology 764–775 (1955).
- 47.Beauchamp, C. & Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem.44, 276–287. 10.1016/0003-2697(71)90370-8 (1971). [DOI] [PubMed] [Google Scholar]
- 48.Dinesh, B., Yadav, B., Reddy, R. D., Padma, A. S. & Sukumaran, M. Determination of ascorbic acid content in some Indian spices. Int. J. Curr. Microbiol. Appl. Sci.4, 864–868 (2015). [Google Scholar]
- 49.Akhtar, I., Javad, S., Ansari, M., Ghaffar, N. & Tariq, A. Process optimization for microwave assisted extraction of Foeniculum vulgare Mill using response surface methodology. J. King Saud Univ.-Sci.32, 1451–1458. 10.1016/j.jksus.2019.11.041 (2020). [Google Scholar]
- 50.Salgado, P., Márquez, K., Rubilar, O., Contreras, D. & Vidal, G. The effect of phenolic compounds on the green synthesis of iron nanoparticles (FexOy-NPs) with photocatalytic activity. Appl. Nanosci.9, 371–385. 10.1007/s13204-018-0931-5 (2019). [Google Scholar]
- 51.Kumar, R., Singh, N. & Pandey, S. Potential of green synthesized zero-valent iron nanoparticles for remediation of lead-contaminated water. Int. J. Environ. Sci. Tech.12, 3943–3950. 10.1007/s13762-015-0751-z (2015). [Google Scholar]
- 52.Ebrahiminezhad, A., Zare-Hoseinabadi, A., Berenjian, A. & Ghasemi, Y. Green synthesis and characterization of zero-valent iron nanoparticles using stinging nettle (Urtica dioica) leaf extract. Green Process. Syn.6, 469–475. 10.1515/gps-2016-0133 (2017). [Google Scholar]
- 53.Lourenço, I. M., Pieretti, J. C., Nascimento, M. H. M., Lombello, C. B. & Seabra, A. B. Eco-friendly synthesis of iron nanoparticles by green tea extract and cytotoxicity effects on tumoral and non-tumoral cell lines. Environ. Ecol. Environ.4, 261–270. 10.1007/s40974-019-00134-5 (2019). [Google Scholar]
- 54.Iqbal, J. et al. Biogenic synthesis of green and cost effective iron nanoparticles and evaluation of their potential biomedical properties. J. Mol. Struct.1199, 126979. 10.1016/j.molstruc.2019.126979 (2020). [Google Scholar]
- 55.Bibi, I. et al. Green and eco-friendly synthesis of cobalt-oxide nanoparticle: Characterization and photo-catalytic activity. Adv. Powder. Tech.28, 2035–2043. 10.1016/j.apt.2017.05.008 (2017). [Google Scholar]
- 56.Hussain, A. et al. Responses of wheat (Triticum aestivum) plants grown in a Cd contaminated soil to the application of iron oxide nanoparticles. Ecotoxicol. Environ. Saf.173, 156–164. 10.1016/j.ecoenv.2019.01.118 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Ünyayar, S., Keleş, Y. & Çekiç, F. The antioxidative response of two tomato species with different drought tolerances as a result of drought and cadmium stress combinations. Gen1, 2 (2005). [Google Scholar]
- 58.Ci, D., Jiang, D., Dai, T., Jing, Q. & Cao, W. Effects of cadmium on plant growth and physiological traits in contrast wheat recombinant inbred lines differing in cadmium tolerance. Chemosphere77, 1620–1625. 10.1016/j.chemosphere.2009.08.062 (2009). [DOI] [PubMed] [Google Scholar]
- 59.Feng, J. et al. Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (Cd) toxicity in Cucumis sativus L.. Sci. Horticult.123, 521–530. 10.1016/j.scienta.2009.10.013 (2010). [Google Scholar]
- 60.Shen, S. et al. Reduced cadmium toxicity in rapeseed via alteration of root properties and accelerated plant growth by a nitrogen-fixing bacterium. J. Hazard. Mater.449, 131040. 10.1016/j.jhazmat.2023.131040 (2023). [DOI] [PubMed] [Google Scholar]
- 61.Wang, Z., Xiao, D., Bush, R. T. & Liu, J. Coprecipitated arsenate inhibits thermal transformation of 2-line ferrihydrite: Implications for long-term stability of ferrihydrite. Chemosphere.122, 88–93. 10.1016/j.chemosphere.2014.11.017 (2015). [DOI] [PubMed] [Google Scholar]
- 62.Sun, L. et al. Mitigation mechanism of zinc oxide nanoparticles on cadmium toxicity in tomato. Front. Plant Sci.14, 1162372. 10.3389/fpls.2023.1162372 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iannone, M. F., Groppa, M. D., de Sousa, M. E., van Raap, M. B. F. & Benavides, M. P. Impact of magnetite iron oxide nanoparticles on wheat (Triticumaestivum L.) development: Evaluation of oxidative damage. Environ. Exp. Bot.131, 77–88. 10.1016/j.envexpbot.2016.07.004 (2016). [Google Scholar]
- 64.Shankramma, K., Yallappa, S., Shivanna, M. & Manjanna, J. Fe2O3 magnetic nanoparticles to enhance S. lycopersicum (tomato) plant growth and their biomineralization. App. Nanosci.6, 983–990. 10.1007/s13204-015-0510-y (2016). [Google Scholar]
- 65.Ghafariyan, M. H., Malakouti, M. J., Dadpour, M. R., Stroeve, P. & Mahmoudi, M. Effects of magnetite nanoparticles on soybean chlorophyll. Environ. Sci. Tech.47, 10645–10652. 10.1021/es402249b (2013). [DOI] [PubMed] [Google Scholar]
- 66.Yang, X., Wang, X., Wang, L. & Wei, M. Control of light environment: A key technique for high-yield and high-quality vegetable production in protected farmland. 10.4236/as.2012.37112 (2012)
- 67.Wu, Z. et al. Comparison of foliar silicon and selenium on cadmium absorption, compartmentation, translocation and the antioxidant system in Chinese flowering cabbage. Ecotoxicol. Environ. Saf.166, 157–164. 10.1016/j.ecoenv.2018.09.085 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Antoniadis, V. et al. Bioavailability and risk assessment of potentially toxic elements in garden edible vegetables and soils around a highly contaminated former mining area in Germany. J. Environ. Man.186, 192–200. 10.1016/j.jenvman.2016.04.036 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Mahmoud, A. W. M. et al. Foliar application of different iron sources improves morpho-physiological traits and nutritional quality of broad bean grown in sandy soil. Plants11, 2599. 10.3390/plants11192599 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hong, J. et al. Foliar application of nanoparticles: Mechanisms of absorption, transfer, and multiple impacts. Environ. Sci. Nano.8, 1196–1210. 10.1039/D1EN00630D (2021). [Google Scholar]
- 71.Morales-Espinoza, M. C. et al. Se nanoparticles induce changes in the growth, antioxidant responses, and fruit quality of tomato developed under NaCl stress. Molecules24, 3030. 10.3390/molecules24173030 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Falco, W. et al. Interaction between chlorophyll and silver nanoparticles: A close analysis of chlorophyll fluorescence quenching. J. Photochem. Photobiol. A Chem.299, 203–209. 10.1016/j.jphotochem.2014.12.001 (2015). [Google Scholar]
- 73.Solti, Á. et al. Impact of iron supply on the kinetics of recovery of photosynthesis in Cd-stressed poplar (Populus glauca). Ann. Bot.102, 771–782. 10.1093/aob/mcn160 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rui, M. et al. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front. Plant Sci.7, 815. 10.3389/fpls.2016.00815 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rizwan, M. et al. Cadmium stress in rice: Toxic effects, tolerance mechanisms, and management: A critical review. Environ. Sci. Pollut. Res.23, 17859–17879. 10.1007/s11356-016-6436-4 (2016). [DOI] [PubMed] [Google Scholar]
- 76.Sebastian, A. & Prasad, M. Exogenous citrate and malate alleviate cadmium stress in Oryza sativa L.: Probing role of cadmium localization and iron nutrition. Ecotoxicol. Environ. Saf.166, 215–222. 10.1016/j.ecoenv.2018.09.084 (2018). [DOI] [PubMed] [Google Scholar]
- 77.Biyani, K. et al. Dynamic role of iron supply in amelioration of cadmium stress by modulating antioxidative pathways and peroxidase enzymes in mungbean. AoB Plants11, plz005. 10.1093/aobpla/plz005 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun, D., Zhang, Z., Zhang, Y., Cheng, K.-W. & Chen, F. Light induces carotenoids accumulation in a heterotrophic docosahexaenoic acid-producing microalga Crypthecodinium sp. SUN. Biores. Tech.276, 177–182. 10.1016/j.biortech.2018.12.093 (2019). [DOI] [PubMed] [Google Scholar]
- 79.Matos, H. R., Di Mascio, P. & Medeiros, M. H. Protective effect of lycopene on lipid peroxidation and oxidative DNA damage in cell culture. Arch. Biochem. Biophys.383, 56–59. 10.1006/abbi.2000.2035 (2000). [DOI] [PubMed] [Google Scholar]
- 80.Padayatty, S. J. et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Collg. Nutr.22, 18–35. 10.1080/07315724.2003.10719272 (2003). [DOI] [PubMed] [Google Scholar]
- 81.Paciolla, C. et al. Vitamin C in plants: From functions to biofortification. Antioxidants8, 519. 10.3390/antiox8110519 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Celi, G. E. A., Gratão, P. L., Lanza, M. G. D. B. & Dos Reis, A. R. Physiological and biochemical roles of ascorbic acid on mitigation of abiotic stresses in plants. Plant Phys. Biochem.202, 107970. 10.1016/j.plaphy.2023.107970 (2023). [DOI] [PubMed] [Google Scholar]
- 83.Jibril, S. A., Hassan, S. A., Ishak, C. F. & Megat Wahab, P. E. Cadmium toxicity affects phytochemicals and nutrient elements composition of lettuce (Lactucasativa L.). Adv. Agric.2017, 1–7. 10.1155/2017/1236830 (2017). [Google Scholar]
- 84.Akram, N. A., Shafiq, F. & Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci.8, 238088. 10.3389/fpls.2017.00613 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mathesius, U. Flavonoid functions in plants and their interactions with other organisms. Plants7(2), 30. 10.3390/plants7020030 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang, Y., Cai, P., Cheng, G. & Zhang, Y. A brief review of phenolic compounds identified from plants: Their extraction, analysis, and biological activity. Nat. Prod. Commun.10.1177/1934578X211069721 (2022). [Google Scholar]
- 87.Nisar, B., Lone, R., Khan, S., Kamili, A. N. & Tahir, I. Interactive role of phenolics and PGPR in alleviating heavy metal toxicity in maize. In Plant Phenolics in Abiotic Stress Management (eds Lone, R. et al.) 235–263 (Springer Nature Singapore, Singapore, 2023). 10.1007/978-981-19-6426-8_12. [Google Scholar]
- 88.Agati, G., Azzarello, E., Pollastri, S. & Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci.196, 67–76. 10.1016/j.plantsci.2012.07.014 (2012). [DOI] [PubMed] [Google Scholar]
- 89.Nawaz, F. et al. Supplemental selenium improves wheat grain yield and quality through alterations in biochemical processes under normal and water deficit conditions. Food Chem.175, 350–357. 10.1016/j.foodchem.2014.11.147 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Praveen, A. et al. Iron oxide nanoparticles as nano-adsorbents: A possible way to reduce arsenic phytotoxicity in Indian mustard plant (Brassicajuncea L.). J. Plant Growth. Regul.37, 612–624. 10.1007/s00344-017-9760-0 (2018). [Google Scholar]
- 91.Lattanzio, V. et al. Relationship of secondary metabolism to growth in oregano (Origanumvulgare L.) shoot cultures under nutritional stress. Environ. Exp. Bot.65, 54–62. 10.1016/j.envexpbot.2008.09.002 (2009). [Google Scholar]
- 92.Verslues, P. E. & Sharma, S. Proline metabolism and its implications for plant-environment interaction. Arabidopsis Book8, e0140. 10.1199/tab.0140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hassanpouraghdam, M. B., Mehrabani, L. V. & Tzortzakis, N. Foliar application of nano-zinc and iron affects physiological attributes of Rosmarinus officinalis and quietens NaCl salinity depression. J. Soil Sci. Plant Nutr.20(2), 335–345. 10.1007/s42729-019-00111-1 (2020). [Google Scholar]
- 94.Zhang, S., Gan, Y. & Xu, B. Application of plant-growth-promoting fungi Trichoderma longibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidative defense system and gene expression. Front. Plant Sci.7, 217856. 10.3389/fpls.2016.01405 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rady, M. M. & Hemida, K. A. Modulation of cadmium toxicity and enhancing cadmium-tolerance in wheat seedlings by exogenous application of polyamines. Ecotoxicol. Environ. Saf.119, 178–185. 10.1016/j.ecoenv.2015.05.008 (2015). [DOI] [PubMed] [Google Scholar]
- 96.Zhao, H. et al. Effects of cadmium stress on growth and physiological characteristics of sassafras seedlings. Sci. Rep.11, 9913. 10.1038/s41598-021-89322-0 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Szabados, L. & Savouré, A. Proline: A multifunctional amino acid. Trend Plant Sci.15, 89–97. 10.1016/j.tplants.2009.11.009 (2010). [DOI] [PubMed] [Google Scholar]
- 98.Mazaheri-Tirani, M., Kashani, A. & Koohi-Dehkordi, M. The role of iron nanoparticles on morpho-physiological traits and genes expression (IRT 1 and CAT) in rue (Ruta graveolens). Plant Mol. Biol.110, 147–160. 10.1007/s11103-022-01292-7 (2022). [DOI] [PubMed] [Google Scholar]
- 99.Shigeoka, S. et al. Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Bot.53, 1305–1319. 10.1093/jexbot/53.372.1305 (2002). [PubMed] [Google Scholar]
- 100.Haque, A. M. et al. The Cd-induced morphological and photosynthetic disruption is related to the reduced Fe status and increased oxidative injuries in sugar beet. Plant Phys. Biochem.166, 448–458. 10.1016/j.plaphy.2021.06.020 (2021). [DOI] [PubMed] [Google Scholar]
- 101.Lee, B. et al. Water deficit-induced oxidative stress and the activation of antioxidant enzymes in white clover leaves. Biol. Plant.53, 505–510. 10.1007/s10535-009-0091-2 (2009). [Google Scholar]
- 102.Qureshi, M., Abdin, M., Qadir, S. & Iqbal, M. Lead-induced oxidative stress and metabolic alterations in Cassia angustifolia Vahl. Biol. Plant.51, 121–128. 10.1007/s10535-007-0024-x (2007). [Google Scholar]
- 103.Muneer, S., Kim, T. H. & Qureshi, M. I. Fe modulates Cd-induced oxidative stress and the expression of stress responsive proteins in the nodules of Vigna radiata. Plant Growth. Regul.68, 421–433. 10.1007/s10725-012-9731-1 (2012). [Google Scholar]
- 104.Sebastian, A. & Prasad, M. Iron-and manganese-assisted cadmium tolerance in Oryza sativa L.: Lowering of rhizotoxicity next to functional photosynthesis. Planta241, 1519–1528. 10.1007/s00425-015-2276-6 (2015). [DOI] [PubMed] [Google Scholar]
- 105.Astolfi, S. et al. Response of barley plants to Fe deficiency and Cd contamination as affected by S starvation. J. Exp. Bot.63, 1241–1250. 10.1093/jxb/err344 (2012). [DOI] [PubMed] [Google Scholar]
- 106.Hernández, J. A., Ferrer, M. A., Jiménez, A., Barceló, A. R. & Sevilla, F. Antioxidant systems and O2/H2O2 production in the apoplast of pea leaves. Its relation with salt-induced necrotic lesions in minor veins. Plant Phys.127, 817–831. 10.1104/pp.010188 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mahawar, L., Ramasamy, K. P. & Pandey, A. Iron deficiency in plants: An update on homeostasis and its regulation by nitric oxide and phytohormones. Plant Growth100, 283–299 (2023). [Google Scholar]
- 108.Connorton, J. M., Balk, J. & Celma, J. R. Iron homeostasis in plants- a brief overview. Metallomics9, 813–823 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tripathi, D. K. et al. Acquisition and homeostasis of iron in higher plants andtheir probable role in abiotic stress tolerance. Environ. Toxicol.5, 00086 (2017). [Google Scholar]
- 110.Niu, J., Liu, C. & Huang, M. Effects of foliar fertilization: A review of current status and future perspectives. J. Soil Sci. Plant Nutr.21, 104–118 (2021). [Google Scholar]
- 111.Wang, X., Deng, S. & Zhou, Y. Application of different foliar iron fertilizers for enhancing the growth and antioxidant capacity of rice and minimizing cadmium. Environ. Sci. Pollut.28, 7828–7839 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.