Abstract
Background
Traumatic brain injury is a common health problem with significant effect on quality of life. Each year in the USA approximately 0.56% of the population suffer a head injury, with a case fatality rate of about 40% for severe injuries. These account for a high proportion of deaths in young adults. In the USA, 2% of the population live with long‐term disabilities following head injuries. The major causes are motor vehicle crashes, falls, and violence (including attempted suicide). Hyperbaric oxygen therapy (HBOT) is the therapeutic administration of 100% oxygen at environmental pressures greater than 1 atmosphere absolute (ATA). This involves placing the patient in an airtight vessel, increasing the pressure within that vessel, and administering 100% oxygen for respiration. In this way, it is possible to deliver a greatly increased partial pressure of oxygen to the tissues. HBOT can improve oxygen supply to the injured brain, reduce the swelling associated with low oxygen levels and reduce the volume of brain that will ultimately perish. It is, therefore, possible that adding HBOT to the standard intensive care regimen may reduce patient death and disability. However, a concern for patients and families is that using HBOT may result in preventing a patient from dying only to leave them in a vegetative state, entirely dependent on medical care. There are also some potential adverse effects of the therapy, including damage to the ears, sinuses and lungs from the effects of the pressure and oxygen poisoning, so the benefits and risks of the therapy need to be carefully evaluated.
Objectives
To assess the effects of adjunctive HBOT for traumatic brain injury.
Search methods
We searched CENTRAL, MEDLINE, EMBASE, CINAHL and DORCTHIM electronic databases. We also searched the reference lists of eligible articles, handsearched relevant journals and contacted researchers. All searches were updated to March 2012.
Selection criteria
Randomised studies comparing the effect of therapeutic regimens which included HBOT with those that did not, for people with traumatic brain injury.
Data collection and analysis
Three authors independently evaluated trial quality and extracted data.
Main results
Seven studies are included in this review, involving 571 people (285 receiving HBOT and 286 in the control group). The results of two studies indicate use of HBOT results in a statistically significant decrease in the proportion of people with an unfavourable outcome one month after treatment using the Glasgow Outcome Scale (GOS) (relative risk (RR) for unfavourable outcome with HBOT 0.74, 95% CI 0.61 to 0.88, P = 0.001). This five‐point scale rates the outcome from one (dead) to five (good recovery); an 'unfavourable' outcome was considered as a score of one, two or three. Pooled data from final follow‐up showed a significant reduction in the risk of dying when HBOT was used (RR 0.69, 95% CI 0.54 to 0.88, P = 0.003) and suggests we would have to treat seven patients to avoid one extra death (number needed to treat (NNT) 7, 95% CI 4 to 22). Two trials suggested favourably lower intracranial pressure in people receiving HBOT and in whom myringotomies had been performed. The results from one study suggested a mean difference (MD) with myringotomy of ‐8.2 mmHg (95% CI ‐14.7 to ‐1.7 mmHg, P = 0.01). The Glasgow Coma Scale (GCS) has a total of 15 points, and two small trials reported a significant improvement in GCS for patients treated with HBOT (MD 2.68 points, 95%CI 1.84 to 3.52, P < 0.0001), although these two trials showed considerable heterogeneity (I2 = 83%). Two studies reported an incidence of 13% for significant pulmonary impairment in the HBOT group versus 0% in the non‐HBOT group (P = 0.007).
In general, the studies were small and carried a significant risk of bias. None described adequate randomisation procedures or allocation concealment, and none of the patients or treating staff were blinded to treatment.
Authors' conclusions
In people with traumatic brain injury, while the addition of HBOT may reduce the risk of death and improve the final GCS, there is little evidence that the survivors have a good outcome. The improvement of 2.68 points in GCS is difficult to interpret. This scale runs from three (deeply comatose and unresponsive) to 15 (fully conscious), and the clinical importance of an improvement of approximately three points will vary dramatically with the starting value (for example an improvement from 12 to 15 would represent an important clinical benefit, but an improvement from three to six would leave the patient with severe and highly dependent impairment). The routine application of HBOT to these patients cannot be justified from this review. Given the modest number of patients, methodological shortcomings of included trials and poor reporting, the results should be interpreted cautiously. An appropriately powered trial of high methodological rigour is required to define which patients, if any, can be expected to benefit most from HBOT.
Keywords: Humans, Brain Injuries, Brain Injuries/mortality, Brain Injuries/therapy, Glasgow Coma Scale, Hyperbaric Oxygenation, Hyperbaric Oxygenation/adverse effects, Hyperbaric Oxygenation/methods, Intracranial Pressure, Randomized Controlled Trials as Topic, Treatment Outcome, Tympanic Membrane, Tympanic Membrane/surgery
Plain language summary
Does hyperbaric oxygen therapy improve the survival and quality of life in patients with traumatic brain injury?
Traumatic brain injury is a major cause of death and disability. Not all damage to the brain occurs at the moment of injury; a reduction of the blood flow and oxygen supply to the brain can occur afterwards and cause further secondary brain damage that is itself an important cause of avoidable death and disability. In the early stages after injury, it is therefore important that efforts are made to minimise secondary brain damage to provide the best chances of recovery.
Hyperbaric oxygen therapy (HBOT) has been proposed as a treatment for minimising secondary brain damage by improving the oxygen supply to the brain. Patients undergoing HBOT are placed inside a specially designed chamber in which 100% oxygen is delivered at a greater than normal atmospheric pressure. It is sometimes used as a treatment to increase the supply of oxygen to the injured brain in an attempt to reduce the area of brain that will die.
The effectiveness of HBOT on the recovery of brain‐injured patients is uncertain. There is also concern regarding potential adverse effects of the therapy, including damage to the ears, sinuses and lungs from the effects of pressure, temporary worsening of short‐sightedness, claustrophobia and oxygen poisoning.
In an attempt to address the uncertainty surrounding the use of HBOT, the authors of this review identified all studies which were randomied controlled trials investigating the effects of HBOT in traumatically brain‐injured people of all ages.
The authors found seven eligible studies involving 571 people. The combined results suggest that HBOT reduces the risk of death and improves the level of coma; however, there is no evidence that these survivors have an improved outcome in terms of quality of life. It is possible, therefore, that the overall effect of hyperbaric oxygen is to make it more likely that people will survive with severe disability after such injuries. The authors conclude that the routine use of HBOT in brain‐injured patients cannot be justified by the findings of this review.
Due to the small number of trials with a limited number of people, it is not possible to be confident in the findings. Further large, high quality trials are required to define the true extent of benefit from HBOT.
Summary of findings
Summary of findings for the main comparison. Hyperbaric oxygen therapy for acute traumatic brain injury.
| Hyperbaric oxygen therapy for acute traumatic brain injury | ||||||
| Patient or population: Patients with acute traumatic brain injury Settings: Patients admitted to acute neurosurgical intensive care Intervention: Hyperbaric oxygen therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Hyperbaric Oxygen Therapy | |||||
| Death at any time in trial period case mortality count Follow‐up: 2 to 52 weeks | Study population | RR 0.69 (0.54 to 0.88) | 385 (4 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 414 per 1000 | 286 per 1000 (224 to 364) | |||||
| Low risk population | ||||||
| 160 per 1000 | 110 per 1000 (86 to 141) | |||||
| High risk population | ||||||
| 500 per 1000 | 345 per 1000 (270 to 440) | |||||
| Favourable outcome GOS <9 or similar Follow‐up: 1 to 12 months | Study population | RR 1.94 (0.92 to 4.08)2 | 380 (4 studies) | |||
| 337 per 1000 | 654 per 1000 (310 to 1000) | |||||
| Low risk population | ||||||
| 100 per 1000 | 194 per 1000 (92 to 408) | |||||
| High risk population | ||||||
| 500 per 1000 | 970 per 1000 (460 to 1000) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Two of the contributing trials are more than 20 years old and assessed as at high risk of bias in some dimensions 2 Note that this is the RR for a favourable outcome with HBOT. In the main text this has been presented as the RR of an unfavourable outcome in order to maintain consistency in the graphical direction of effects through all outcomes.
Background
Description of the condition
Traumatic brain injury (TBI) is a significant cause of premature death and disability. Despite the deployment of increasingly sophisticated therapy at the scene (including early intubation) and increased monitoring in hospital, the mortality rate remains at about 40% in severe TBI (Murray 1999). A meta‐analysis of studies on severe TBI in 2010 confirmed that case fatality rates have not decreased since 1990 (Stein 2010). Each year, there are at least 10 million new head injuries worldwide and these account for a high proportion of deaths in young adults (Alexander 1992; Thurman 1999). In the USA there are more than 50,000 deaths due to traumatic brain injury each year, and a recent population‐based survey in the USA estimated the incidence rate per 100,000 person‐years at 558, or 0.56% (95% confidence interval (CI) 528 to 590) (Leibson 2011). The major causes of TBI in high income countries are motor vehicle crashes (50%), falls (38%) and violence (including attempted suicide) (4%) (figures from a prospective survey in the Netherlands, Adriessen 2011). Prevention strategies, including restraints for vehicle occupants, are now legally enforced in many countries. However, while road death rates are falling in most industrialised countries they are rising in many rapidly motorising countries, particularly in Asia. For example, road death rates per head in China are already similar to those in the USA, although far fewer people own motor vehicles (Roberts 1995). Head injuries are associated with long‐term disability in many patients. In the USA, for example, 2% of the population (a total of 5.3 million people) are living with disability as a result of TBI (Thurman 1999) and this places considerable medical, social and financial burden on both families and health systems (Fearnside 1997).
The pathophysiology of brain injury has a primary and secondary component. At the time of impact there is a variable degree of irreversible damage to the neurological tissue (primary injury). Following this, a chain of events occurs in which there is ongoing injury to the brain through oedema, hypoxia and ischaemia secondary to raised tissue or intracranial pressure, release of excitotoxic levels of excitatory neurotransmitters (for example glutamate) and impaired calcium homeostasis (Fiskum 2000; Tymianski 1996) (secondary injury).
Therapy focuses on prevention or minimisation of secondary injury by ensuring adequate oxygenation, haemodynamics, control of intracranial hypertension, and strategies to reduce cellular injury. A number of therapies, including barbiturates, calcium channel antagonists, steroids, hyperventilation, mannitol, hypothermia and anticonvulsants have been the topic of previous Cochrane reviews, though none has shown unequivocal efficacy in reducing poor outcomes (Alderson 2008; Langham 2005; Roberts 2012; Roberts 2009; Schierhout 2003; Sydenham 2009).
Description of the intervention
Hyperbaric oxygen therapy (HBOT) is a further adjunctive therapy that has been proposed to improve outcomes in acute brain injury. HBOT is the therapeutic administration of 100% oxygen at environmental pressures greater than 1 atmosphere absolute (ATA). This involves placing the patient in an airtight vessel, increasing the pressure within that vessel, and administering 100% oxygen for respiration. In this way, it is possible to deliver a greatly increased partial pressure of oxygen to the tissues. Typically, treatments involve pressurisation to between 1.5 and 3.0 ATA, for periods between 60 and 120 minutes, one or more times daily.
How the intervention might work
Since the 1960s, there have been reports that HBOT improves outcomes following brain trauma (Fasano 1964). Administration of HBOT is based on the observation that hypoxia following closed head trauma is an integral part of the secondary injury described above. Hypoxic neurons performing anaerobic metabolism results in acidosis and an unsustainable reduction in cellular metabolic reserve (Muizelaar 1989). As the hypoxic situation persists, the neurons lose their ability to maintain ionic homeostasis, and free oxygen radicals accumulate and degrade cell membranes (Ikeda 1990; Siesjo 1989). Eventually, irreversible changes result in unavoidable cell death. When ischaemia is severe enough, these changes occur rapidly, but there is some evidence that the effects can occur over a period of days (Robertson 1989). This gives some basis to the assertion that a therapy designed to increase oxygen availability in the early period following TBI may improve long‐term outcomes. HBOT is also thought to reduce tissue oedema by an osmotic effect (Hills 1999), and any agent that has a positive effect on brain swelling following trauma might also contribute to improved outcomes. On the other hand, oxygen in high doses is potentially toxic to normally perfused tissue, and the brain is particularly at risk (Clark 1982). For this reason, it is appropriate to postulate that in some TBI patients HBOT may do more harm, through the action of increased free oxygen radical damage, than good through the restoration of aerobic metabolism.
Why it is important to do this review
Despite 40 years of interest in the delivery of HBOT in TBI patients, little clinical evidence of effectiveness exists. HBOT has been shown to reduce both intracranial pressure (ICP) and cerebrospinal fluid pressure (CSFP) in brain‐injured patients (Hayakawa 1971; Sukoff 1982), improve grey matter metabolic activity on single photon emission computed tomography (SPECT) scan (Neubauer 1994) and improve glucose metabolism (Holbach 1977). Some studies suggest that any effect of HBOT may not be uniform across all brain‐injured patients. For example, Hayakawa demonstrated that cerebrospinal fluid pressure (CSFP) rebounded to higher levels following HBOT than at pre‐treatment estimation in some patients, while others showed persistent reductions (Hayakawa 1971). It is possible that HBOT has a positive effect in a subgroup of patients with moderate injury but not in those with extensive cerebral injury. Furthermore, repeated exposure to hyperbaric oxygen may be required to attain consistent changes (Artru 1976a). Clinical reports have attributed a wide range of improvements to the utilisation of HBOT including cognitive and motor skills, improved attention span and increased verbalisation (Neubauer 1994; Sukoff 1982). These improvements are, however, difficult to ascribe to any single treatment modality because HBOT was most often applied in conjunction with intensive supportive and rehabilitative therapies.
HBOT is associated with some risk of adverse effects, including damage to the ears, sinuses and lungs from the effects of pressure, temporary worsening of short‐sightedness, claustrophobia and oxygen poisoning. Although serious adverse events are rare, HBOT cannot be regarded as an entirely benign intervention. Further, it is conceivable that the addition of HBOT might improve survival from serious brain injury without improving the proportion of those who survive with a useful functional level, while at the same time increasing overall costs of therapy. For a number of reasons, therefore, the administration of HBOT for TBI patients remains controversial.
Objectives
The aim of this review is to assess the evidence for the benefit or harm of adjunctive HBOT in the treatment of acute TBI. We compared intensive treatment regimens including adjunctive HBOT against similar regimens excluding HBOT. Where regimens differed significantly between studies, this is clearly stated and the implications discussed.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials that compared the effect of treatment for acute TBI where HBOT administration was included with the effect of similar treatment in the absence of HBOT.
Types of participants
Any person admitted to an intensive care or intensive neurosurgical facility with an acute TBI following blunt trauma.
Types of interventions
HBOT administered in a compression chamber between pressures of 1.5 ATA and 3.5 ATA and treatment times between 30 minutes and 120 minutes at least once. We accepted any standard treatment regimen designed to maximise brain protection and promote recovery from TBI. We did not include studies in which comparator interventions were not undertaken in a specialised acute care setting.
Types of outcome measures
Studies were eligible for inclusion if they reported any of the following outcome measures at any time. We expected the timing of outcome evaluations to vary between studies. In general, our aim was to group outcomes into three stages for analysis: early (immediately after treatment course), medium‐term (four to eight weeks after treatment), and longer‐term (six months or longer).
Primary outcomes
Functional outcome (defined as 'unfavourable' if: Glasgow Outcome Score (GOS) of one, two, or three, described as 'dead', 'vegetative state' or 'severely disabled')
Mortality (where there were multiple times recorded, we chose final follow‐up)
Secondary outcomes
Activities of daily living (ADL)
Intracranial pressure (ICP)
Magnetic resonance imaging (MRI) or computed tomography (CT) evidence of lesion resolution or size of persistent defect
Progress of Glasgow Coma Scale (GCS)
Adverse events as a result of HBOT
Cost‐effectiveness
Search methods for identification of studies
We aimed to capture both published and unpublished studies. All languages were considered. We contacted study authors if there was any ambiguity about the published data.
Electronic searches
We searched:
Cochrane Central Register of Controlled Trials (CENTRAL) (2012, March 30th) in The Cochrane Library;
MEDLINE (Ovid SP) (1950 to March 2012);
EMBASE (Ovid SP) (1980 to March 2012);
CINAHL (EBSCOhost) (1982 to March 2012);
DORCTHIM, the Database of Randomised Trials in Hyperbaric Medicine (www.hboevidence.com) (March 2012).
The searches were last updated in March 2012. The search strategy was adapted, where necessary, for each database. The EMBASE and MEDLINE (Ovid) strategies are presented in Appendix 1.
Searching other resources
In addition, we made a systematic search for relevant controlled trials in specific hyperbaric literature sources (to March 2012) as follows.
We contacted experts in the field and leading hyperbaric therapy centres (as identified by personal communication and searching the Internet) for additional relevant data in terms of published or unpublished randomised trials).
We handsearched relevant hyperbaric textbooks (Kindwall, Jain, Marroni, Bakker, Bennett and Elliot), journals (Undersea and Hyperbaric Medicine, Hyperbaric Medicine Review, South Pacific Underwater Medicine Society (SPUMS) Journal, European Journal of Hyperbaric Medicine and Aviation, Space and Environmental Medicine Journal) and conference proceedings (Undersea and Hyperbaric Medical Society, SPUMS, European Undersea and Baromedical Society, International Congress of Hyperbaric Medicine) published since 1980.
We contacted authors of relevant studies to request details of unpublished or ongoing investigations.
Data collection and analysis
Studies were entered into a bibliographic software package (Reference Manager 5.1). Review Manager version 5 was used for data analysis (RevMan).
Selection of studies
One author (MB) was responsible for handsearching and the identification of appropriate studies for consideration. Two authors (MB and BJ) examined the electronic search results to identify possible eligible studies. All comparative clinical trials identified were retrieved in full and reviewed independently by three authors, two with content expertise with HBOT and two with content expertise in treating acute TBI (BT practices in both areas). In addition, one of the authors (MB) has expertise in clinical epidemiology.
Assessment of risk of bias in included studies
Using the data extraction form developed for this review, each author extracted relevant data and this was compared and discussed until consensus was achieved and a recommendation made for inclusion or exclusion from the review. We entered details in a risk of bias table for each included study, indicating the method of allocation, adequacy of concealment of allocation, blinding status of participants and outcome observers, and how patient attrition was handled. We considered these factors for possible sensitivity analysis. All data extracted reflected the original allocation group, where possible, to allow an intention‐to‐treat analysis. We identified dropouts where this information was given.
Measures of treatment effect
We used an intention‐to‐treat analysis to make comparisons, where possible. Comparisons reflected efficacy in the context of randomised trials rather than true effectiveness in any particular clinical context.
For proportions (dichotomous outcomes), we used relative risks (RRs) with 95% confidence intervals (CI). A statistically significant difference between the experimental intervention and control intervention was assumed if the 95% CI of the RR did not include the value 1.0. To analyse scale scores, mean differences or standardised mean differences with 95% CIs were calculated. A statistically significant difference was defined as existing if the 95% CI did not include a zero MD.
As an estimate of the clinical relevance of any difference between the experimental intervention and the control intervention, we calculated the number needed to treat (NNT) and number needed to harm (NNH) with 95% CI where appropriate.
Assessment of heterogeneity
We explored heterogeneity and performed subgroup analyses as outlined below. We assessed statistical heterogeneity using the I2 statistic and considered the appropriateness of pooling and meta‐analysis according to the guidance in the Cochrane Handbook (Higgins 2011).
Data synthesis
We used a fixed‐effect model where there was no evidence of significant heterogeneity between studies, and a random‐effects model when such heterogeneity was likely.
For the primary outcome, the proportion of participants with an unfavourable functional outcome (for example GOS) was dichotomised. The Glasgow Outcome Scale is a five‐point scale, from one (dead) to five (good recovery). We included participants with a good recovery or moderate disability in the 'favourable' outcome group, while those who were severely disabled, remained in a vegetative state or died were included in the 'unfavourable' outcome group. The RR for an unfavourable outcome with HBOT was established using the intention‐to‐treat data of the HBOT versus the control group.
We intended to present cost‐effectiveness data as it was described in the study report.
Subgroup analysis and investigation of heterogeneity
Where we found appropriate data, we considered subgroup analysis based on:
age, adults versus children;
dose of oxygen received (pressure, time and length of treatment course);
nature of the comparative treatment modalities;
severity of injury;
nature of injury on CT scan.
Sensitivity analysis
We planned to perform sensitivity analyses for missing data and study quality (based on the use of a reliable method of allocation concealment).
Missing data
We employed sensitivity analyses by using different approaches to impute missing data. The best‐case scenario assumed that none of the originally enrolled patients missing from the primary analysis in the treatment group had the negative outcome of interest, whilst all those missing from the control group did. The worst‐case scenario was the reverse.
Study quality
If appropriate, we also planned to conduct a sensitivity analysis by study quality, based on the presence or absence of a reliable random sequence method, concealment of allocation, and blinding of participants or outcome assessors.
Results
Description of studies
Results of the search
Combining both our original search and the updates in January 2009 and March 2012, our searches retrieved a total of 962 citations. After removal of duplicates we screened 428 citations, from which we retained 45 papers for examination of their abstracts (of which 12 were new citations since the last search). Examination confirmed 14 were case reports or case series, 10 were reviews without new data, two were animal studies, two were editorial commentary, one a letter and one an account of a planned trial. These reports were excluded, leaving 15 possible randomised comparative trials. These papers were examined in full. After appraisal of the full reports we excluded two publications as preliminary or secondary reports containing no data additional to that in the final publication (Ren 2001b; Rockswold 1985), three were trials that dealt with non‐acute head injury (Lin 2008; Shi 2003; Shi 2006), two were case series (Belokurov 1988; Gossett 2010) and one was a plan for a potential trial (Helms 2011). The other seven trials were included in the review (Artru 1976; Holbach 1974; Ren 2001a; Rockswold 1992; Xie 2007; Rockswold 2010; Mao 2010). See the study flow diagram (Figure 1).
1.
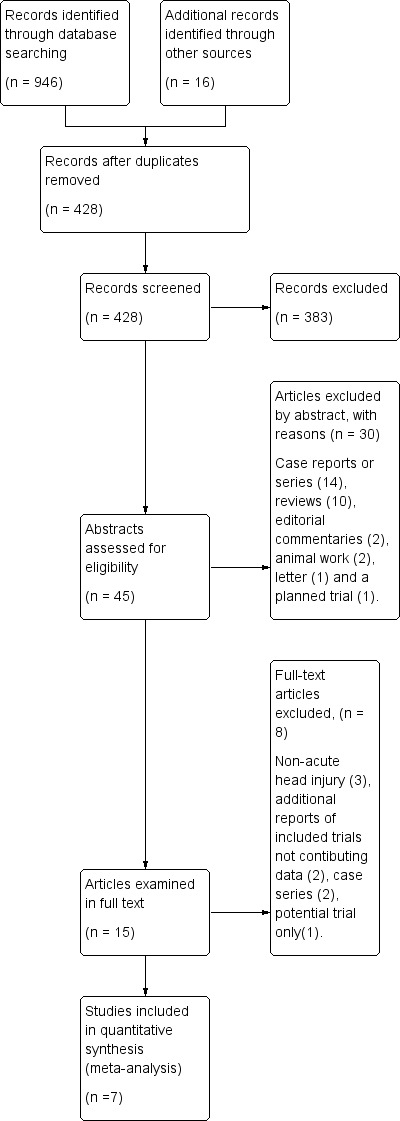
Figure 1. Study flow diagram.
Included studies
The included trials were published between 1974 (Holbach 1974) and 2010 (Mao 2010; Rockswold 2010), and the review authors are aware of a further trial being organised by Rockswold based on the information obtained from that group's most recent trial. In total, these trials included data on 571 participants, 285 receiving HBOT and 286 control (see table 'Characteristics of included studies').
The dose of oxygen per treatment session and for the total course of treatment varied between studies. The lowest dose administered was 1.5 ATA for 60 minutes daily (Holbach 1974; Rockswold 2010) while the highest dose was 2.5 ATA for 40 to 60 minutes 10 times in four days (Ren 2001a). All authors used between 1.5 and 2.5 ATA as the maximum oxygen pressure. The total number of individual treatment sessions varied from three (Rockswold 2010) to between 30 and 40 (Ren 2001a).
No trial administered a sham treatment and only Rockswold 1992 attempted any concealment by blinding the outcome assessor. All trials included participants with severe closed head injury. Five trials specified a GCS grade on admission (Mao 2010: GCS < 8; Ren 2001a and Rockswold 2010: GCS < 9; Rockswold 1992: GCS < 10; Xie 2007: GCS 3 to 12), one specified the severity of coma using the Jouvet Scale (Artru 1976; Jouvet 1960) and the remaining trial stated that the patients were comatose on admission (Holbach 1974). Specific exclusion criteria varied between trials but open head injuries and participants with other than isolated head trauma were excluded when any criteria were specified.
All trials compared a standard intensive treatment regimen to the same regimen with the addition of HBOT. Reported details of the standard regimen are given in 'Characteristics of included studies'.
The follow‐up periods varied from immediately following the course of therapy (Holbach 1974; Rockswold 2010; Xie 2007), to three months (Mao 2010), six months (Ren 2001a), one year (Artru 1976) and 1.5 years (Rockswold 1992). All included studies reported at least one clinical outcome of interest. Of the outcomes identified above, the trials reported data on both primary outcomes (good functional outcome and mortality) but only the Glasgow Coma Score, intracranial pressure and adverse events from the secondary outcomes of interest.
Other outcomes (including non‐clinical) that were reported included: survival time (Holbach 1974), duration of coma (Artru 1976), GCS before and after treatment (Mao 2010; Ren 2001a), brain‐stem auditory evoked potentials (BAEP) and short‐latency somatosensory evoked potentials (SSEP) (Rockswold 1992), serum C‐reactive protein (Xie 2007), brain electric activity mapping (BEAM) (Ren 2001a), electroencephalograph (EEG) (Mao 2010) and a range of markers of cerebral oxygenation and metabolism (Rockswold 2010).
Risk of bias in included studies
Each study was assessed using the risk of bias tables presented in the 'Characteristics of included studies' table. In general, these studies were small and carried a significant risk of bias. The major potential sources of bias are discussed below. The assessments for each included study are summarised in Figure 2 and Figure 3.
2.
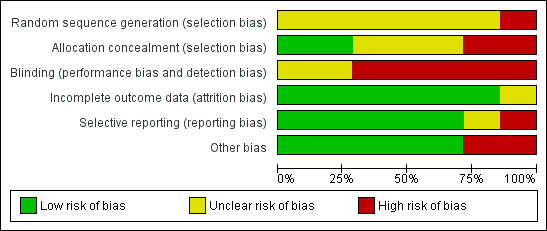
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. Seven studies are included in this review.
3.
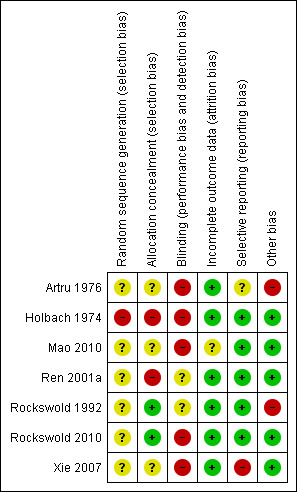
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Randomisation
Allocation concealment was not adequate in any of the studies, being inadequate in Holbach 1974 and unclear in the remaining studies. Randomisation procedures were not described in any of the studies. They were unlikely to have been truly random for Holbach 1974, where the selection method was described as 'every second patient was treated with HBOT'. None of the studies provided a clear indication that the investigators were unable to predict the prospective group to which a participant would be allocated.
Patient baseline characteristics
All patients had suffered a head injury. Three of the studies closely defined the entry criteria as those patients with an isolated, closed head injury and a specified GCS: Rockswold 1992 (GCS < 10), Ren 2001a and Rockswold 2010 (GCS < 9), Xie 2007 (GCS 3 to 12). All trials enrolled patients with acute TBI, but only Xie 2007 stated the time between injury and enrolment (24 hours). Rockswold 1992 mentioned a case enrolled at day 29 following acute clinical deterioration such as to satisfy the entry criteria. Artru 1976 assessed injury severity according to a scale described by Jouvet 1960 and reported that there was no statistical difference in the mean score between groups (HBOT group mean 9.39, control mean 9.59). We have not been able to review the characteristics of this scale. Holbach 1974 admitted comatose patients but did not state a specific measure of injury severity or the period of time prior to enrolment. Holbach described the patients as having 'mid‐brain symptomatology' but did not define this term, while Artru stratified patients on enrolment to one of nine categories (brain stem contusion, bilateral frontal contusion, acute subdural haematoma, frontotemporal contusion, infratemporal haematoma, epidural haematoma, hydrocephaly, subdural hygroma and cribriform plate defect). Holbach 1974 excluded patients who died within the first 48 hours, but it is not clear whether these patients were enrolled and then withdrawn or were simply ineligible for entry.
Blinding
Neither the participants (who were unaware of their surroundings) nor treating staff were blinded as to allocation, and no study employed a sham hyperbaric exposure. Rockswold 1992 calculated GOS for each participant using a neurologist who was unaware of the treatment group to which the patient was allocated.
Patients lost to follow‐up
Rockswold 1992 reported that two participants were lost to follow‐up from the control group, and these participants did not appear in the analysis. None of the remaining studies suffered any losses to follow‐up or reported any violation of allocated treatment (Artru 1976; Holbach 1974; Ren 2001a; Xie 2007). However, Ren stated that participants who died were excluded from the study (numbers not given). It is not clear if these participants were entered and then excluded or all died before enrolment on day three. Sensitivity analysis in this review involved making best and worse case analyses to examine potentially important effects on outcome where the Rockswold study contributed patients.
Intention‐to‐treat analysis
Rockswold 1992 specifically described participants who did not receive HBOT as being analysed in the intended group, but participants lost to follow‐up were excluded from analysis. Rockswold 2010 analysed patients by intention to treat even when HBOT could not be given. No other trial mentioned this strategy, but neither were there any losses to follow‐up or violations of the protocol reported.
Effects of interventions
See: Table 1
Primary outcomes
1. Proportion of participants with an unfavourable functional outcome
Proportion of participants with an unfavourable functional outcome at end of the treatment period to four weeks
Analysis 1.1 Two trials reported this outcome using the five‐point GOS (Artru 1976; Holbach 1974). We included participants with a good recovery or moderate disability (GOS four or five) in the 'favourable' outcome group, while those who were severely disabled, remained in a vegetative state or died (GOS one to three) were included in the 'unfavourable' outcome group. These trials enrolled 159 participants (42% of the total participants in this review), with 80 (50%) allocated to standard treatment plus HBOT and 79 (50%) to standard therapy alone. There was a statistically significant decrease in the proportion of participants with an unfavourable outcome following HBOT (the RR of a poor outcome with HBOT was 0.74, 95% CI 0.61 to 0.88, P = 0.001, I2 =0%).
1.1. Analysis.
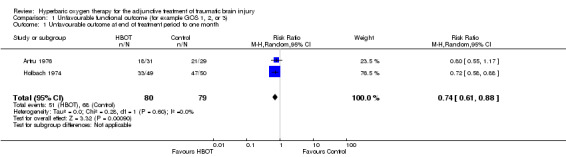
Comparison 1 Unfavourable functional outcome (for example GOS 1, 2, or 3), Outcome 1 Unfavourable outcome at end of treatment period to one month.
Proportion of participants with an unfavourable functional outcome at six months
Analysis 1.2 Only one trial reported this outcome (Ren 2001a), involving 55 patients (14% of the total participants in this review), with 35 (64%) randomised to standard therapy with HBOT and 20 (36%) to standard therapy alone. There was a significant decrease in the proportion of participants with an unfavourable outcome following HBOT (RR for unfavourable outcome with HBOT 0.24, 95% CI 0.11 to 0.54).
1.2. Analysis.
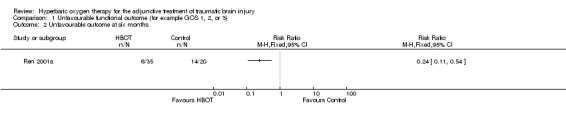
Comparison 1 Unfavourable functional outcome (for example GOS 1, 2, or 3), Outcome 2 Unfavourable outcome at six months.
Proportion of participants with an unfavourable functional outcome at one year
Analysis 1.3, Analysis 1.4, Analysis 1.5 Only one trial reported this outcome (Rockswold 1992), involving 168 patients (44% of the total participants in this review) with 84 randomised to each arm. There was no difference in the proportion of participants with an unfavourable outcome following HBOT (RR 0.98, 95% CI 0.71 to 1.34). This result was not sensitive to the allocation of the two dropouts in the control group (best case RR 1.05, 95%CI 0.76 to 1.46; worst case RR 1.00, 95% CI 0.73 to 1.37).
1.3. Analysis.
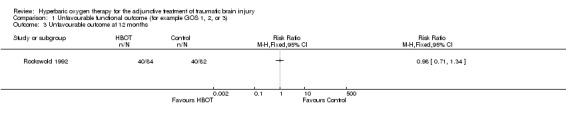
Comparison 1 Unfavourable functional outcome (for example GOS 1, 2, or 3), Outcome 3 Unfavourable outcome at 12 months.
1.4. Analysis.
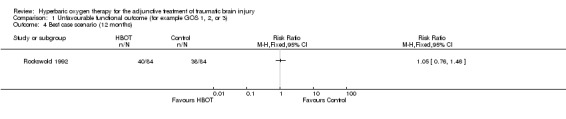
Comparison 1 Unfavourable functional outcome (for example GOS 1, 2, or 3), Outcome 4 Best case scenario (12 months).
1.5. Analysis.
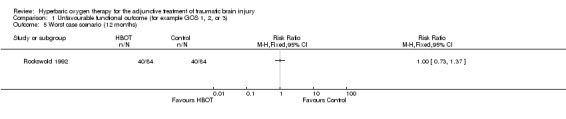
Comparison 1 Unfavourable functional outcome (for example GOS 1, 2, or 3), Outcome 5 Worst case scenario (12 months).
Proportion of patients with an unfavourable outcome at final assessment
Analysis 1.6, Analysis 1.7, Analysis 1.8 This comparison pooled all trials regardless of the time at which final assessment was made. All four trials reported this outcome at some time (Holbach 1974 at 12 days, Ren 2001a at 6 months, Artru 1976 and Rockswold 1992 at 1 year), involving all 382 participants: 199 were randomised to standard therapy plus HBOT, 183 to standard therapy alone. There was no significant change in the proportion of participants with unfavourable functional outcome following the application of HBOT (RR for unfavourable outcome with HBOT 0.71, 95% CI 0.50 to 1.01, P = 0.06). Heterogeneity accounted for a substantial proportion of the variability between studies (I2 = 75%), so this result was achieved using a random‐effects model. This result was not sensitive to the allocation of dropouts in the Rockswold trial (best case RR 0.72, 95% CI 0.49 to 1.02, P = 0.07; worst case RR 0.71, 95% CI 0.50 to 1.00, P = 0.05).
1.6. Analysis.
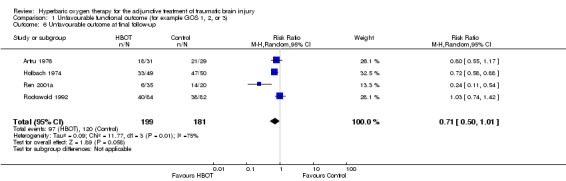
Comparison 1 Unfavourable functional outcome (for example GOS 1, 2, or 3), Outcome 6 Unfavourable outcome at final follow‐up.
1.7. Analysis.
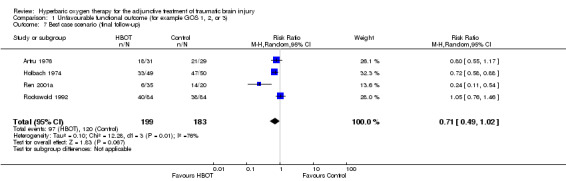
Comparison 1 Unfavourable functional outcome (for example GOS 1, 2, or 3), Outcome 7 Best case scenario (final follow‐up).
1.8. Analysis.
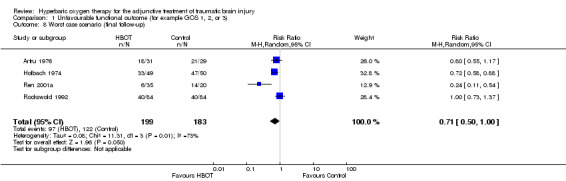
Comparison 1 Unfavourable functional outcome (for example GOS 1, 2, or 3), Outcome 8 Worst case scenario (final follow‐up).
Analysis 1.9 Subgroup analysis by treatment pressure did not suggest any advantage to either high or low pressure treatment (2.5 ATA RR for unfavourable outcome 0.46, 95% CI 0.14 to 1.58, P = 0.22; 1.5 ATA RR 0.84, 95% CI 0.57 to 1.24, P = 0.38).
1.9. Analysis.
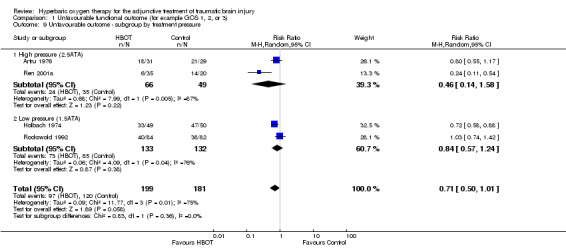
Comparison 1 Unfavourable functional outcome (for example GOS 1, 2, or 3), Outcome 9 Unfavourable outcome ‐ subgroup by treatment pressure.
2. Proportion of participants dying
Mortality reported at any time
Analysis 2.1, Analysis 2.2, Analysis 2.3 This outcome pooled all trials regardless of the time at which final assessment was made. Three trials reported this outcome at some time (Holbach 1974 at 12 days, Artru 1976 and Rockswold 1992 at 12 months) whilst Xie 2007 reported that all patients reached final follow‐up at 10 days. These trials involved 387 participants (87.6% of the total); 194 (50%) were randomised to standard therapy plus HBOT and 193 to standard therapy alone. Rockswold 1992 contributed 51% of the participants to this analysis. There was a significantly reduced chance of dying in the HBOT group. The RR of dying if given HBOT was 0.69 (95% CI 0.54 to 0.88, P = 0.003). Heterogeneity between studies was low (I2 = 0%) and there was no significant effect exerted by allocation of participants who dropped out of the Rockswold trial (best case RR 0.69, 95% CI 0.54 to 0.89; worst case RR 0.67, 95% CI 0.53 to 0.87). The absolute risk difference of 15% was significant and the NNT to avoid one death by applying HBOT was 7 (95% CI 4 to 22).
2.1. Analysis.
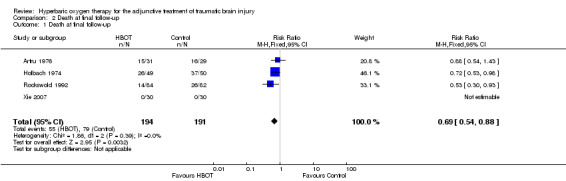
Comparison 2 Death at final follow‐up, Outcome 1 Death at final follow‐up.
2.2. Analysis.
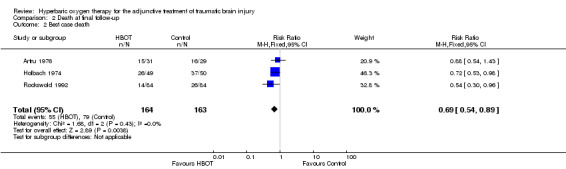
Comparison 2 Death at final follow‐up, Outcome 2 Best case death.
2.3. Analysis.
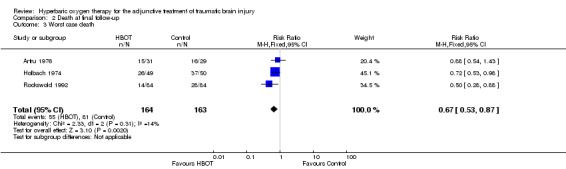
Comparison 2 Death at final follow‐up, Outcome 3 Worst case death.
Secondary outcomes
1. Activities of daily living (ADL)
No trials reported any data on this outcome.
2. Intracranial pressure
Analysis 3.1 Two trials reported results on this outcome (Rockswold 1992; Rockswold 2010), involving 215 patients (44% of the total): 110 randomised to HBOT and 105 to standard care. Twelve patients from Rockswold 1992 did not contribute data to this analysis, five in the standard care plus HBOT group and seven receiving standard care alone. The result was complicated by a change in the protocol during the conduct of Rockswold 1992. The results could not be pooled because Rockswold 2010 did not report standard deviations for the measured differences in intracranial pressure (ICP). For Rockswold 1992, while overall there was no difference in the mean maximum ICP between the two groups (MD 4.2 mmHg lower with HBOT, 95% CI ‐9.4 to +0.9 mmHg), the authors noted higher than expected ICP in the HBOT patients and performed pre‐treatment myringotomy in the last 46 participants in the HBOT group (data analysed for 42). Comparing the standard care alone group with the HBOT participants with and without myringotomy, there was a significant lowering of ICP with HBOT plus myringotomy (MD with myringotomy ‐8.20 mmHg, 95% CI ‐14.68 to ‐1.72 mmHg, P = 0.01; without myringotomy MD +2.7 mmHg, 95% CI ‐5.87 to +11.27 mmHg, P = 0.54).
3.1. Analysis.
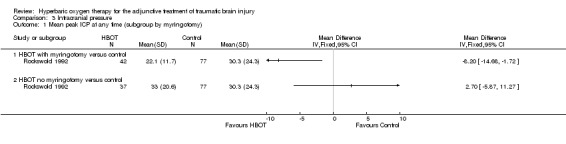
Comparison 3 Intracranial pressure, Outcome 1 Mean peak ICP at any time (subgroup by myringotomy).
Rockswold 2010 reported a mean drop in ICP following HBOT of 0.25 mmHg and an increase over the same period in the standard treatment group of 0.67 mmHg, and reported this difference to be statistically significant (P = 0.001).
3. MRI or CT evidence of lesion resolution or size of persistent defect
No trials reported any data on this outcome.
4. Improvements in GCS
Analysis 4.1 Three trials contributed results to this outcome (Mao 2010; Ren 2001a; Xie 2007), involving 175 patients (31% of the total): 95 randomised to HBOT and 80 to standard therapy. These trials suggested a statistically significant improvement in Glasgow Coma Score (GCS) in the group that received HBOT. Ren 2001a did not contribute to the pooled result as this trial did not report the variability in the mean data. The GCS was significantly higher after completion of therapy in the group that received HBOT (MD 2.68, 95% CI 1.84 to 3.52, P < 0.0001, I2 = 83%).
4.1. Analysis.
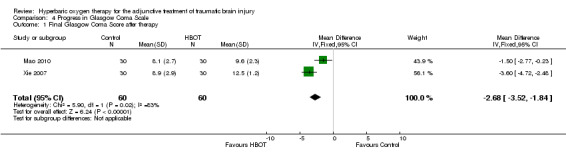
Comparison 4 Progress in Glasgow Coma Scale, Outcome 1 Final Glasgow Coma Score after therapy.
In the two trials that reported the relevant data, the mean GCS was similar at enrolment (Xie 2007: HBOT group 8.2, SD 2.2 versus control 8.1, SD 2.1; Mao 2010: HBOT group 6.0, SD 1.1 versus control 6.3, SD 1.3) but apparently improved after the completion of therapy in both groups (Xie 2007: HBOT 12.5, SD 1.2 versus control 8.9, SD 2.9; Mao 2010: HBOT 9.6, SD 2.3 versus control 8.1, SD 2.7). Both reported statistically significant improvements over time in the HBOT group but not the standard treatment group. These trials did not report the proportion of participants with an unfavourable outcome at that time, and have not contributed data to the analyses of functional outcome 1.1 or 1.4. Ren 2001a reported on the mean improvement in GCS after therapy in both groups (the standard treatment plus HBOT mean GCS increased from 5.1 to 14.6; standard treatment alone mean GCS increased from 5.3 to 9.5).
5. Adverse events
No trials reported on any adverse effects in relation to standard therapeutic measures.
Pulmonary effects of HBOT
Analysis 5.1 Two trials contributed results to this outcome (Artru 1976; Rockswold 1992), involving 228 patients (60% of the total): 115 randomised to standard therapy plus HBOT, and 113 to standard therapy alone. Rockswold reported 10 patients in the HBOT group with rising oxygen requirements and infiltrates on chest x‐ray, while Artru reported five patients with respiratory symptoms including cyanosis and hyperpnoea so severe as to imply 'impending hyperoxic pneumonia' and for whom HBOT was ceased. Overall, therefore, 15 patients (13% of those receiving HBOT) had severe pulmonary complications while no such complications were reported in the standard therapy arm. This difference was significant (RR 15.57, 95% CI 2.11 to 114.72, P = 0.007). There was no indication of heterogeneity between trials (I2 = 0%) and this analysis suggested that we might expect to treat eight patients with HBOT in order to cause this adverse effect in one individual (NNH 8, 95% CI 5 to 15).
5.1. Analysis.
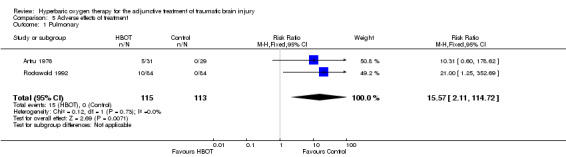
Comparison 5 Adverse effects of treatment, Outcome 1 Pulmonary.
Neurological oxygen toxicity with HBOT
Analysis 5.2 Only one trial reported on this outcome (Rockswold 1992), involving 168 patients (44% of the total): 84 randomised to each arm. Rockswold reported two patients in the HBOT arm having an isolated generalised seizure (2.3%) and none in the control arm. This difference was not statistically significant (RR for seizure with HBOT 5.0, 95% CI 0.24 to 102.6, P = 0.3).
5.2. Analysis.
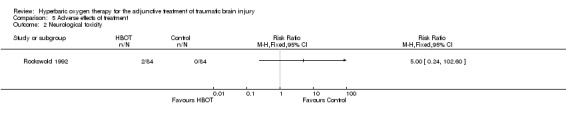
Comparison 5 Adverse effects of treatment, Outcome 2 Neurological toxicity.
Middle ear barotrauma with HBOT
Analysis 5.3 Only one trial reported on this outcome (Rockswold 1992), involving 168 patients (44% of the total): 84 randomised to each arm. Rockswold reported two patients in the HBOT arm having a haemotympanum (2.3%) and none in the control arm. This difference was not statistically significant (RR for haemotympanum with HBOT 5.0, 95% CI 0.24 to 102.6, P = 0.3).
5.3. Analysis.
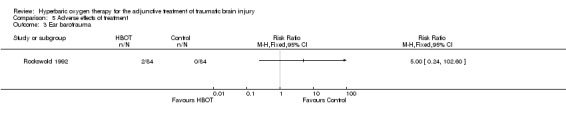
Comparison 5 Adverse effects of treatment, Outcome 3 Ear barotrauma.
6. Cost‐effectiveness
No trials attempted to estimate the cost‐effectiveness of therapy.
Discussion
Summary of main results
We found no evidence that HBOT improves functional outcome or ability to perform activities of daily living following severe head injury, although the evidence does suggest an improvement in survival with the use of HBOT. The only two trials measuring the proportion of patients with functional outcomes in the short term were both reported in the 1970s (Holbach 1974 and Artru 1976), and together suggest increased unfavourable outcomes with HBOT (RR 0.74, 95% CI 0.61 to 0.88; P = 0.001). Pooled analysis of functional outcome at one year and drawing on two further studies do not suggest any advantage from HBOT (RR 0.71, 95% CI 0.49 to 1.03; P = 0.07).
The two trials looking at ICP as a proxy for beneficial effects also suggest that ICP was lower immediately following HBOT when patients had received myringotomy tubes. These tubes avoid middle ear barotrauma on compression, a highly painful and stimulating condition that might be expected to raise ICP regardless of the underlying brain injury. Indeed, in Rockswold 1992 the treatment protocol was altered to include myringotomy because of the unexpected finding of increased ICP in the HBOT group. The ICP of the subsequent patients that received HBOT was 8.2 mmHg lower and this difference is of potential clinical importance given that the normal ICP is 7 to 15 mmHg and pressures of 20 to 25 mmHg require urgent treatment (Steiner 2006). Two trials also suggested that the Glasgow Coma Score was significantly improved following HBOT, but did not report any functional assessment. A subgroup analysis by study pressure did not suggest any significant advantage in outcome whether a higher (2.5 ATA) or lower (1.5 ATA) pressure was used. Given the high heterogeneity between trials, this result should be interpreted with extreme caution.
More convincingly, the risk of death in the HBOT group was significantly lower than in the control group (RR 0.69, 95% CI 0.54 to 0.88). This analysis suggests that we would need to treat seven patients with HBOT in order to avoid one death (NNT 7, 95% CI 4 to 22) and was not sensitive to the allocation of dropouts. Given the small number of participants and generally poor quality of these trials, this result needs to be interpreted with caution. Taken together, however, these two primary outcome analyses suggest that while survival may be positively influenced by the addition of HBOT, there is little to suggest these patients have better functional outcomes.
The two trials measuring GCS as an outcome also suggested an improvement following HBOT but they analysed these data from a nominal scale as if it were a continuous variable, by comparing the mean value in the two groups. It is not clear that this approach is appropriate and the outcome is difficult to interpret clinically. This scale runs from three (deeply comatose and unresponsive) to 15 (fully conscious), and the clinical importance of an improvement of approximately three points will vary dramatically with the starting value (for example an improvement from 12 to 15 would represent an important clinical benefit, but an improvement from three to six would leave the patient with a severe and highly dependent impairment remaining).
Overall completeness and applicability of evidence
This review has included data from seven trials, and we believe these represent all randomised human trials in this area, both published and unpublished, at the time of searching the databases.
As is common with small trials, the incidence of adverse effects was poorly assessed by the studies included in this review. Rockswold 1992 and Artru 1976 reported a 13% incidence of severe pulmonary compromise with the application of HBOT (NNH 8, 95% CI 5 to 15). However, the other five trials did not report any such cases. It is not clear if this constitutes a true difference in incidence or a publication bias. HBOT is regarded as a relatively benign intervention. There are few major adverse effects (pulmonary barotrauma, drug reactions, injuries or death related to chamber fire) and the report of a 13% incidence of significant pulmonary compromise is surprising and may indicate a complication associated specifically with severe head injury when exposed to hyperbaric oxygen.
There are a number of more minor complications that may commonly occur. Visual disturbance, usually a reduction in visual acuity secondary to conformational changes in the lens, is very commonly reported; perhaps as many as 50% of those having a course of 30 treatments (Khan 2003). While most patients recover spontaneously over a period of days to weeks, a small proportion of patients continue to require correction to restore sight to pre‐treatment levels. None of the trials included in this review reported visual changes. The second most common adverse effect associated with HBOT is aural barotrauma. Barotrauma can affect any air‐filled cavity in the body (including the middle ear, lungs and respiratory sinuses) and occurs as a direct result of compression. Aural barotrauma is by far the most common as the middle ear air space is small, largely surrounded by bone and the sensitive tympanic membrane, and it usually requires active effort by the patient in order to inflate the middle ear through the eustachian tube on each side. Barotrauma is thus not a consequence of HBOT directly but rather of the physical conditions required to administer it. Most episodes of barotrauma are mild, easily treated or recover spontaneously and do not require the therapy to be abandoned. Only Rockswold reported any cases of middle ear barotrauma (two in the HBOT arm). Less commonly, HBOT may be associated with acute neurological toxicity manifesting as seizure. Again, Rockswold reported two such occurrences in the HBOT arm.
Quality of the evidence
Only seven trials with 571 participants were available for evaluation using our planned comparisons, and meta‐analysis was not appropriate or possible for a number of these. Other problems for this review were the poor methodological quality of many of these trials; variability in entry criteria and the nature and timing of outcomes; and poor reporting of both outcomes and methodology. In particular, there is a possibility of bias due to different mechanisms and severity of injury on entry to these small trials, as well as from non‐blinded management decisions in all trials. We had planned a sensitivity analysis based on the presence or absence of allocation concealment but no trials were reliably concealed in this regard.
These trials were published over a 36‐year period, up to 2010, and from a wide geographical area. We had planned to perform subgroup analyses with respect to age, dose of oxygen received (pressure, time and length of treatment course), nature of the comparative treatment modalities, severity of injury, and the nature of injury on CT scan. However, the paucity of eligible trials and poor reporting suggested that the majority of these analyses would not be informative, and we only performed subgroup analysis with respect to treatment pressure for the proportion of individuals achieving a good outcome at any time. Patient inclusion criteria were not standard, and poorly reported in some trials. No standard severity index was employed uniformly across these trials, no standard injury pattern was established, and only Rockswold 1992, Rockswold 2010, Xie 2007, Mao 2010 and Ren 2001a described the time at which the inclusion criteria were applied. There was significant variation both in oxygen dose during an individual treatment session and in the number of sessions administered to each patient. While subgroup analysis by treatment pressure suggested that those treated at 2.5 ATA did significantly better than those treated at 1.5 ATA, this result should be treated with extreme caution given the heterogeneity between the lower pressure trials and the observation that the estimated risk of a poor outcome was actually lower in the low pressure group. While all trials used some form of 'standard' intensive therapy, these comparator therapies were generally poorly described and could not form the basis for a meaningful subgroup analysis.
Potential biases in the review process
All of these findings are subject to a potential publication bias. While we have made every effort to locate further unpublished data, it remains possible that this review is subject to a positive publication bias, with generally favourable trials more likely to achieve reporting. With regard to long‐term outcomes following HBOT and any effect on the quality of life for these patients, we have not located any relevant data.
Agreements and disagreements with other studies or reviews
A search revealed six publications reviewing the evidence for hyperbaric oxygen therapy in acute traumatic brain injury since 2000. All were in broad agreement with our findings. An early systematic review undertaken by the Alternative Therapy Evaluation Committee for the Insurance Corporation of British Columbia concluded "the scientific literature up to August 2001 does not support the use of hyperbaric oxygen for traumatic brain injuries...." (Alternative Therapy Committee 2003). Later reviews have given guarded support to HBOT, with recommendations for further high‐quality trials in this area. A systematic review without meta‐analysis in 2004 concluded there was insufficient evidence to recommend HBOT for acute brain injury without better evidence (McDonagh 2004). Qualitative reviews from the Rockswold group and others have all made similar conclusions (Adamides 2006; Ali Wali 2005; Rockswold 2007), while the most recent review relied heavily on the conclusions from our own review (Meyer 2010).
Authors' conclusions
Implications for practice.
There is limited evidence that HBOT reduces the chance of dying and improves the GCS following a traumatic brain injury. Although there is some evidence of improvements in GCS, there is little evidence that more survivors have a good outcome. Thus, the routine adjunctive use of HBOT in these patients cannot be justified by this review. The small number of studies, the modest numbers of patients, and the methodological and reporting inadequacies of the primary studies included in this review demand a cautious interpretation of the findings.
Implications for research.
Given the findings of improved survival and GCS with the use of HBOT in acute traumatic head injury, there is a case for large randomised trials of high methodological rigour in order to define the true extent of benefit from the administration of HBOT. Specifically, more information is required on the subset of disease severity or classification most likely to benefit from this therapy, and the oxygen dose that is most appropriate. Any future trials would need to consider in particular:
appropriate sample sizes with power to detect expected differences;
careful definition and selection of target patients;
appropriate range of oxygen doses per treatment session (pressure and time);
appropriate and carefully defined comparator therapy;
use of an effective sham therapy;
effective and explicit blinding of outcome assessors and neurosurgeons or intensives;
appropriate outcome measures including all those listed in this review;
careful elucidation of any adverse effects;
the cost‐utility of the therapy.
What's new
| Date | Event | Description |
|---|---|---|
| 10 July 2012 | New citation required and conclusions have changed | The search has been updated to 9 March 2012, and two new studies have been added. Graphical representations of the 'Risk of bias', a 'Summary of findings' table and a 'Study flow diagram' have been added. The conclusions have changed (minor). |
| 9 March 2012 | New search has been performed | The search for studies has been updated to 9 March 2012. Two new studies have been included. |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 18 February 2009 | New search has been performed | One new trial has been included in this update. The conclusions are unchanged. |
| 30 June 2008 | Amended | Converted to new review format. |
| 1 April 2006 | New search has been performed | May 2006 The searches were updated in April 2006; no new studies for inclusion were identified. |
Acknowledgements
We acknowledge the assistance provided by the Cochrane Injuries Group, and particularly of Katharine Ker and Paul Chinnock, in the production of this review.
Appendices
Appendix 1. Search strategy
MEDLINE (Ovid) (1950 to March 2012)
1. exp head injuries‐penetrating 2. exp head injuries‐closed 3. exp coma‐post head injury 4. exp craniocerebral trauma 5. head or crani$ or capitis or brain$ or forebrain$ or skull$ or hemisphere or intracran$ or orbit$ 6. injur$ or trauma$ or lesion$ or damage$ or wound$ or destruction$ or oedema$ edema$ or fracture$ or contusion$ or concus$ or commotion$ or pressur$ 7. 5 and 6 8. diffuse axonal injur$ 9. 1 or 2 or 3 or 4 or 7 or 8 10. exp hyperbaric oxygenation 11. (high$) adj3 (pressure or tension$) 12. hyperbaric$ 13. oxygen$ 14. 12 or 13 15. 14 and 11 16. HBO or HBOT 17. multiplace chamber$ 18. monoplace chamber$ 19. 10 or 15 or 16 or 17 or 18 20. 9 and 19
EMBASE (Ovid) (1980 to March 2012)
1. exp head injury/ 2. (head or cerebr$ or crani$ or capitis or brain$ or forebrain$ or skull$ or hemisphere or intracran$ or orbit$).mp. 3. (injur$ or trauma$ or lesion$ or damag$ or wound$ or destruction$ or oedema$ or edema$ or fracture$ or contusion$ or concus$ or commotio$ or pressur$).mp 4. 2 and 3 5. diffuse axonal injur$.mp. 6. 1 or 4 or 5 7. exp coma/ 8. 6 or 7 9. exp hyperbaric oxygen/ 10. (high adj5 (pressur$ or oxygen$)).mp. 11. hyperbaric$.mp. 12. 10 or 11 13. oxygen$.mp. 14. 12 and 13 15. (HBO or HBOT).mp. 16. multiplace chamber$.mp. 17. monoplace chamber$.mp. 18. 9 or 14 or 15 or 16 or 17 19. 8 and 18
Data and analyses
Comparison 1. Unfavourable functional outcome (for example GOS 1, 2, or 3).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Unfavourable outcome at end of treatment period to one month | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.61, 0.88] |
| 2 Unfavourable outcome at six months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Unfavourable outcome at 12 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Best case scenario (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Worst case scenario (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Unfavourable outcome at final follow‐up | 4 | 380 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.50, 1.01] |
| 7 Best case scenario (final follow‐up) | 4 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.49, 1.02] |
| 8 Worst case scenario (final follow‐up) | 4 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.50, 1.00] |
| 9 Unfavourable outcome ‐ subgroup by treatment pressure | 4 | 380 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.50, 1.01] |
| 9.1 High pressure (2.5ATA) | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.14, 1.58] |
| 9.2 Low pressure (1.5ATA) | 2 | 265 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.57, 1.24] |
Comparison 2. Death at final follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death at final follow‐up | 4 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.54, 0.88] |
| 2 Best case death | 3 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.54, 0.89] |
| 3 Worst case death | 3 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.53, 0.87] |
Comparison 3. Intracranial pressure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean peak ICP at any time (subgroup by myringotomy) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 HBOT with myringotomy versus control | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 HBOT no myringotomy versus control | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Progress in Glasgow Coma Scale.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Final Glasgow Coma Score after therapy | 2 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐2.68 [‐3.52, ‐1.84] |
Comparison 5. Adverse effects of treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pulmonary | 2 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.57 [2.11, 114.72] |
| 2 Neurological toxicity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Ear barotrauma | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Artru 1976.
| Methods | Randomised controlled trial with stratification. No blinding reported. 60 patients, 31 HBOT, 29 control. Inclusion depended on availability of hyperbaric chamber. | |
| Participants | Patients with closed head injury and coma. Stratified in 9 subgroups of severity and pathology. | |
| Interventions | HBOT 2.5 ATA for 1 hour daily for 10 days, followed by 4 days rest and repeat if not responding. Standard care included hyperventilation and frusemide. | |
| Outcomes | Death, unfavourable outcome, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in the text: "Afterwards, the patient was selected randomly.... for OHP therapy or standard therapy". |
| Allocation concealment (selection bias) | Unclear risk | Not stated, although quite probable given the methodology: "Once he was admitted to the study, a patient was assigned to one of nine subgroups.....Afterwards, the patient was selected randomly.... for OHP therapy or standard therapy". |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not described and appears unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes for consideration were not clearly stated. |
| Other bias | High risk | 60 of a potential 185 patients were entered. Entry to study depended on availability of the chamber. |
Holbach 1974.
| Methods | Quasi‐randomised, unblinded trial. 99 patients, 31 HBOT, 29 control. | |
| Participants | Patients with a history of closed head injury and who are comatose with 'acute midbrain syndrome'. | |
| Interventions | HBOT at 1.5 ATA daily ‐ time of each sessions and total number of sessions unknown. Standard care given to both groups described as 'usual intensive care regimen'. | |
| Outcomes | Complete recovery, mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Appears to have been a sequential allocation: "every second patient received hyperbaric oxygenation" . |
| Allocation concealment (selection bias) | High risk | No evidence of concealment and it seems unlikely: "every second patient received hyperbaric oxygenation". |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding and appears unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No other obvious potential for bias. |
Mao 2010.
| Methods | Randomised controlled trial. No blinding reported. 60 patients with 30 allocated to each group. | |
| Participants | Patients with severe craniocerebral injuries, GCS <8 within 24 hours of injury, aged between 11 and 68 years. | |
| Interventions | The conventional treatment group received the usual medical and or surgical management as usual in the study institution. HBO group started HBO when clinically stable (mean 12 days), consisting of a mean 29 daily treatments. | |
| Outcomes | GCS and GOS. They also measured EEG changes. | |
| Notes | Translated from the original Chinese. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated: "The subjects are randomly allocated". |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No mention of blinding and this was unlikely given there is no mention of sham therapy, |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There is no mention of any loss of subjects to follow‐up. |
| Selective reporting (reporting bias) | Low risk | No evidence of planned outcome measures that are not reported. |
| Other bias | Low risk | No evidence of other bias operating. |
Ren 2001a.
| Methods | Randomised controlled trial. No blinding reported. 55 patients, 35 HBOT, 20 control. | |
| Participants | Patients with closed head injury admitted with GCS < 9. Randomised on day 3 post‐admission after condition stabilised. Death in first 3 days therefore excluded. | |
| Interventions | HBOT at 2.5 ATA for a total of 400 to 600 minutes every 4 days, repeated 3 or 4 times. Standard care included dehydration, steroids and antibiotics. | |
| Outcomes | Favourable GOS, change in GCS. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described in text, groups unbalanced (20 v 35): "Fifty‐five severe brain injury patients were randomly divided into two groups". |
| Allocation concealment (selection bias) | High risk | No evidence of concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No evidence of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All flagged outcomes reported. |
| Other bias | Low risk | No other evident source of bias. |
Rockswold 1992.
| Methods | Randomised, controlled trial. Observers ("medical assessors") blinded, but not patients or carers. | |
| Participants | Patients with a history of closed head injury with GCS of < 10 for > 6 hours and < 24 hours. | |
| Interventions | HBOT at 1.5 ATA for 1 hour every 8 eight hours for 2 weeks or until death or waking (average number of treatments = 21). Standard care described as 'intensive neurosurgical care according to a comprehensive protocol' was delivered to both groups. | |
| Outcomes | Favourable functional outcome (GOS 1 or 2), mortality, intra‐cranial pressure and adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated: "random assignment of the patient'. |
| Allocation concealment (selection bias) | Low risk | Possible and although not clearly described, consent prior to randomisation: "After eligibility and the GCS score were established, informed consent was obtained. Random assignment of the patient.....then occurred". |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Observers were blinded but not carers or patients, main outcomes relatively hard: "Outcome was assessed by blinded independent examiners". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Secondary interim outcomes had significant losses to follow‐up. Only two control patients were lost to all follow‐up: "Two control patients were lost to follow‐up...therefore the 12‐month outcome analyses are based on ... a total of 166 patients". |
| Selective reporting (reporting bias) | Low risk | All flagged outcomes were reported. |
| Other bias | High risk | Change in protocol for myringotomy during the course has affected some outcomes (discussed in this review). |
Rockswold 2010.
| Methods | Randomised, unblinded trial to test the feasibility of proposed larger RCT to come. | |
| Participants | Adult patients with acute traumatic brain injury (within 24 hrs) and GCS <9 admitted to a neurosurgical intensive care setting. | |
| Interventions | Two control groups: Intensive neurosurgical care according to current guidelines of Brain Trauma Foundation and 100% oxygen at 1 ATA for 3 hours daily (normobaric arm in this study) and intensive neurosurgical care without extra oxygen (standard care arm). Experimental group: As above plus 100% oxygen at 1.5 ATA for one hour daily for three days. |
|
| Outcomes | Multiple measures of brain metabolism, including oxygenation, oxygen consumption, lactate etc. Also intracranial pressure (relevant for this review). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, but no description of details: "Twenty‐six patients were randomised to the HBO2 group..." |
| Allocation concealment (selection bias) | Low risk | Probably concealment at entry: "After study eligibility and a GCS score were established, informed consent was obtained from each participant. Randomiszation occurred immediately after consenting..." |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attempt at blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients entered are accounted for with only minor data loss. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported by intention to treat. |
| Other bias | Low risk | No clear other bias. |
Xie 2007.
| Methods | Randomised but no evidence of blinding. | |
| Participants | 60 patients 24 hours after head injury (confirmed with C/T or MRI) and with GCS between 3 and 12. No major chest or abdominal trauma or disease. | |
| Interventions | Standard neurosurgical care including ICP control, neurosurgical procedures and antibiotics. Addition of HBOT at between 2 and 2.5 ATA for 70 to 80 minutes daily for 10 days. | |
| Outcomes | Glasgow Coma Score. | |
| Notes | Main purpose of the study was to document changes in the serum level of the inflammatory marker, C‐reactive protein. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in the paper: "All the subjects were randomly divided ...with 30 in each group". |
| Allocation concealment (selection bias) | Unclear risk | Not described in the paper. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No apparent blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were complete. |
| Selective reporting (reporting bias) | High risk | Authors signalled 'effectiveness of therapy' as an outcome but this is not given in the paper. |
| Other bias | Low risk | No other obvious potential source of bias. |
ATA ‐ Atmospheres Absolute GCS ‐ Glasgow Coma Score GOS ‐ Glasgow Outcome Score HBOT ‐ Hyperbaric Oxygen Therapy
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Belokurov 1988 | Not a comparative trial. |
| Gossett 2010 | Not a comparative trial. |
| Helms 2011 | A plan for a potential future trial. |
| Lin 2008 | Not a trial of acute head injury (average delay 27.5 days). |
| Ren 2001b | No additional data presented ‐ same study as Ren 2001a. |
| Rockswold 1985 | Preliminary results only. No data presented that were additional to the full report. |
| Shi 2003 | Included only patients at least 3 months after head injury. |
| Shi 2006 | Included only patients with chronic head injury. |
Contributions of authors
Bennett: conception, principal author, search strategy, identification of trials, critical appraisal and data extraction. Content expert on hyperbaric medicine and clinical epidemiology.
Jonker: co‐author, critical appraisal and data extraction. Content expert on brain trauma.
Trytko: co‐author, data extraction. Content expert on hyperbaric medicine and intensive care.
Sources of support
Internal sources
Prince of Wales Hospital, Sydney, Australia.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Artru 1976 {published data only}
- Artru F, Chacornac R, Deleuze R. Hyperbaric oxygenation for severe head injuries. Preliminary results of a controlled study. European Neurology 1976;14(4):310‐8. [DOI] [PubMed] [Google Scholar]
Holbach 1974 {published data only}
- Holbach KH, Wassmann H, Kolberg T. Improved reversibility of the traumatic midbrain syndrome using hyperbaric oxygen. Acta Neurochirurgica (Wien) 1974;30(3‐4):247‐56. [PUBMED: 4432786] [DOI] [PubMed] [Google Scholar]
Mao 2010 {published data only}
- Mao J‐H, Sun Z‐S, Xiang Y. Observation of curative effects of hyperbaric oxygen for treatment on severe craniocerebral injury. Journal of Clinical Neurology 2010;23(5):386‐8. [Google Scholar]
Ren 2001a {published data only}
- Ren H, Wang W, Ge Z. Glasgow coma scale, brain electrical activity mapping and Glasgow outcome score after hyperbaric oxygen treatment of severe brain injury. Chinese Journal of Traumatology 2001;4(4):239‐41. [PUBMED: 11835741] [PubMed] [Google Scholar]
Rockswold 1992 {published data only}
- Rockswold GL, Ford SE, Anderson DC, Bergman TA, Sherman RE. Results of a prospective randomized trial for treatment of severely brain‐injured patients with hyperbaric oxygen. Journal of Neurosurgery 1992;76(6):929‐34. [PUBMED: 1588426] [DOI] [PubMed] [Google Scholar]
Rockswold 2010 {published data only}
- Rockswold SB, Rockswold GL, Zaun DA, Zhang X, Cerra CE, Bergman TA, Liu J. A prospective, randomized clinical trial to compare the effect of hyperbaric to normobaric hyperoxia on cerebral metabolism, intracranial pressure, and oxygen toxicity in severe traumatic brain injury. Journal of Neurosurgery 2010;112:1080‐94. [DOI] [PubMed] [Google Scholar]
Xie 2007 {published data only}
- Xie Z, Zhuang M, Lin L, Xu H, Chen L, Hu L. Changes of plasma C‐reactive protein in patients with craniocerebral injury before and after hyperbaric oxygenation: a randomly controlled study. Neural Regeneration Research 2007;2(5):314‐7. [Google Scholar]
References to studies excluded from this review
Belokurov 1988 {published data only}
- Belokurov YM, Golland AV, Kochetov Kh A. Hyperbaric oxygenation in hypoxic brain injuries. Khirurgiya 1988;64(8):104‐6. [PubMed] [Google Scholar]
Gossett 2010 {published data only}
- Gossett WA, Rockswold GL, Rockswold SB, Adkinson CD, Bergman TA, Quickel RR. The safe treatment, monitoring and management of severe traumatic brain injury patients in a monoplace chamber. Undersea and Hyperbaric Medicine 2010;37(1):35‐48. [PubMed] [Google Scholar]
Helms 2011 {published data only}
- Helms A, Evans AW, Chu J, Sahgal A, Ostrowski R, Sosiak T, Wolf G, Gillett J, Whelan H. Hyperbaric oxygen for neurologic indications‐‐action plan for multicenter trials in: stroke, traumatic brain injury, radiation encephalopathy & status migrainosus. Undersea and Hyperbaric Medicine 2011;38(5):309‐19. [PubMed] [Google Scholar]
Lin 2008 {published data only}
- Lin JW, Tsai JT, Lee LM, Lin CM, Hung CC, Hung KS, et al. Effect of hyperbaric oxygen on patients with traumatic brain injury. Acta Neurochirurgica 2008;101 Suppl:145‐9. [DOI] [PubMed] [Google Scholar]
Ren 2001b {published data only}
- Ren H, Wang W, Ge Z. Clinical, Glasgow coma scale, brain electric earth map, endothelin and transcranial ultrasonic doppler findings after hyperbaric oxygen treatment for severe brain injury. Chinese Medical Journal (English) 2001;114(4):387‐90. [PUBMED: 11835741] [PubMed] [Google Scholar]
Rockswold 1985 {published data only}
- Rockswold GL, Ford SE. Preliminary results of a prospective randomized trial of treatment of severely brain‐injured patients with hyperbaric oxygen. Minnesota Medical Journal 1985;68(7):533‐5. [PubMed] [Google Scholar]
Shi 2003 {published data only}
- Shi XY, Tang ZQ, Xiong B, Bao JX, Sun D, Zhang YQ, et al. Cerebral perfusion SPECT imaging for assessment of the effect of hyperbaric oxygen therapy on patients with postbrain injury neural status. Chinese Journal of Traumatology 2003;6(6):346‐9. [PUBMED: 14642054] [PubMed] [Google Scholar]
Shi 2006 {published data only}
- Shi XY, Tang ZQ, Sun D, He XJ. Evaluation of hyperbaric oxygen treatment of neuropsychiatric disorders following traumatic brain injury. Chinese Medical Journal 2006;119(23):1978‐82. [PubMed] [Google Scholar]
Additional references
Adamides 2006
- Adamides AA, Winter CD, Lewis PM, Cooper DJ, Kossmann T, Rosenfeld JV. Current controversies in the management of patients with severe traumatic brain injury. Australia and New Zealand Journal of Surgery 2006;76(3):163‐74. [DOI] [PubMed] [Google Scholar]
Adriessen 2011
- Andriessen TMJC, Horn J, Franschman G, Naalt J, Haitsma I, Jacobs B, Steyerberg EW, Vos PE. Epidemiology, severity classification, and outcome of moderate and severe traumatic brain injury: A prospective multicenter study. Journal of Neurotrauma 2011;28(10):2011‐34. [DOI: 10.1089/neu.2011.2034] [DOI] [PubMed] [Google Scholar]
Alderson 2008
- Alderson P, Roberts I. Corticosteroids for acute traumatic brain injury. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD000196.pub2] [DOI] [PubMed] [Google Scholar]
Alexander 1992
- Alexander E. Global Spine and Head Injury Prevention Project (SHIP). Surgical Neurology 1992;38(6):478‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ali Wali 2005
- Al‐Waili NS, Butler GJ, Beale J, Abdullah MS, Hamilton RW, Lee BY, Lucus P, Allen MW, Petrillo RL, Carrey Z, Finkelstein M. Hyperbaric oxygen in the treatment of patients with cerebral stroke, brain trauma, and neurologic disease. Advances in Therapeutics 2005;22(6):659‐78. [DOI] [PubMed] [Google Scholar]
Alternative Therapy Committee 2003
- Alternative Therapy Evaluation Committee for the Insurance Corporation of British Columbia. A review of the scientific evidence on the treatment of traumatic brain injuries and strokes with hyperbaric oxygen. Brain Injury 2003;17(3):225‐36. [DOI] [PubMed] [Google Scholar]
Artru 1976a
- Artru F, Philippon B, Gau F, Berger M, Deleuze R. Cerebral blood flow, cerebral metabolism and cerebrospinal fluid biochemistry in brain‐injured patients after exposure to hyperbaric oxygen. European Neurology 1976;14(5):351‐64. [DOI] [PubMed] [Google Scholar]
Clark 1982
- Clark JM. Oxygen toxicity. In: Bennett PB, Elliott DH editor(s). The Physiology and Medicine of Diving. 3rd Edition. London: Bailliere, Tindall and Cox, 1982:200‐38. [Google Scholar]
Fasano 1964
- Fasano VA, Nunno T, Urciolo R, Lombard G. First observation on the use of oxygen under high pressure for the treatment of traumatic coma. In: Boerema, Brummelkamp, Meigne editor(s). Clinical Application of Hyperbaric Oxygen. Amsterdam: Elsevier, 1964:168‐73. [Google Scholar]
Fearnside 1997
- Fearnside MR, Gurka JA. The challenge of traumatic brain injury. Medical Journal of Australia 1997;167(6):293‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fiskum 2000
- Fiskum G. Mitochondrial participation in ischemic and traumatic neural cell death. Journal of Neurotrauma 2000;17(10):843‐55. [DOI] [PubMed] [Google Scholar]
Hayakawa 1971
- Hayakawa T, Kanai N, Kuroda R. Response of cerebrospinal fluid pressure to hyperbaric oxygenation. Journal of Neurology, Neurosurgery and Psychiatry 1971;34(5):580‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011 [Computer program]
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011].. The Cochrane Collaboration. The Cochrane Collaboration, 2011.
Hills 1999
- Hills BA. A role of oxygen‐induced osmosis in hyperbaric oxygen therapy. Medical Hypotheses 1999;52(3):259‐63. [DOI] [PubMed] [Google Scholar]
Holbach 1977
- Holbach KH, Caroli A, Wassmann H. Cerebral energy metabolism in patients with brain lesions of normo‐ and hyperbaric oxygen pressures. Journal of Neurology 1977;217(1):17‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ikeda 1990
- Ikeda Y, Long DM. The molecular basis of brain injury and brain edema: the role of oxygen free radicals. Neurosurgery 1990;27(1):1‐11. [DOI] [PubMed] [Google Scholar]
Jouvet 1960
- Jouvet DJ. Semiological study of persistent troubles with the conscience. The physiopathological bases. [Etudes semiologiques des troubles prolonges de la conscience. Ses bases physiopathologiques]. Lyon Medecine 1960;201:1401‐20. [Google Scholar]
Khan 2003
- Khan B, Evans AW, Easterbrook M. Refractive changes in patients undergoing hyperbaric oxygen therapy: a prospective study. Undersea and Hyperbaric Medicine 2003;24 Suppl:9. [Google Scholar]
Langham 2005
- Langham J, Goldfrad C, Teasdale G, Shaw D, Rowan K. Calcium channel blockers for acute traumatic brain injury. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD000565] [DOI] [Google Scholar]
Leibson 2011
- Leibson CL, Brown AW, Ransom JE, Diehl NN, Perkins PK, Mandrekar J, Malec JF. Incidence of traumatic brain injury across the full disease spectrum: a population‐based medical record review study. Epidemiology 2011;22(6):836‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonagh 2004
- McDonagh M, Helfand M, Carson S, Russman BS. Hyperbaric oxygen therapy for traumatic brain injury: a systematic review of the evidence. Archives of Physical Medicine and Rehabilitation 2004;85(7):1198‐204. [DOI] [PubMed] [Google Scholar]
Meyer 2010
- Meyer MJ, Megyesi J, Meythaler J, Murie‐Fernandez M, Aubut JA, Foley N, Salter K, Bayley M, Marshall S, Teasell R. Acute management of acquired brain injury part I: an evidence‐based review of non‐pharmacological interventions. Brain Injury 2010;24(5):694‐705. [DOI] [PubMed] [Google Scholar]
Muizelaar 1989
- Muizelaar JP. Cerebral blood flow, cerebral blood volume and cerebral metabolism after severe head injury. In: Becker DP, Gudeman SK editor(s). Textbook of Head Injury. Philadelphia: WB Saunders, 1989:221‐40. [Google Scholar]
Murray 1999
- Murray GD, Teasdale GM, Braakman R, Cohadon F, Dearden M, Iannotti F, Karimi A, Lapierre F, Maas A, Ohman J, Persson L, Servadei F, Stocchetti N, Trojanowski T, Unterberg A. The European Brain Injury Consortium survey of head injuries. Acta Neurochirurgica 1999;141:223‐6. [DOI] [PubMed] [Google Scholar]
Neubauer 1994
- Neubauer RA, Gottlieb SF, Pevsner NH. Hyperbaric oxygen for treatment of closed head injury. Southern Medical Journal 1994;87(9):933‐6. [DOI] [PubMed] [Google Scholar]
RevMan [Computer program]
- The Nordic Cochrane Centre. Review Manager (RevMan). Version 5.1. Copenhagen: The Cochrane Collaboration, 2011.
Roberts 1995
- Roberts I. Letter from Chengdu: China takes to the roads. BMJ 1995;310(6990):1311‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 2009
- Roberts I, Schierhout G. Hyperventilation therapy for acute traumatic brain injury. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD000566] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 2012
- Roberts I, Sydenham E. Barbiturates for acute traumatic brain injury. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD000033.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Robertson 1989
- Robertson CS, Narayan RK, Gokaslan ZL, Pahwa R, Grossman RG, Caram P Jr, et al. Cerebral arteriovenous oxygen difference as an estimate of cerebral blood flow in comatose patients. Journal of Neurosurgery 1989;70(2):222‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rockswold 2007
- Rockswold SB, Rockswold GL, Defillo A. Hyperbaric oxygen in traumatic brain injury. Neurology Research 2007;29(2):162‐72. [DOI] [PubMed] [Google Scholar]
Schierhout 2003
- Schierhout G, Roberts I. Anti‐epileptic drugs for preventing seizures following acute traumatic brain injury. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD000173] [DOI] [PubMed] [Google Scholar]
Siesjo 1989
- Siesjo BK, Agardh CD, Bengtsson F. Free radicals and brain damage. Cerebrovascular and Brain Metabolism Review 1989;1:165‐211. [MEDLINE: ] [PubMed] [Google Scholar]
Stein 2010
- Stein SC, Georgoff P, Meghan S, Mizra K, Sonnad SS. 150 years of treating severe traumatic brain injury: a systematic review of progress in mortality. Journal of Neurotrauma 2010;27:1343–53. [DOI] [PubMed] [Google Scholar]
Steiner 2006
- Steiner LA, Andrews PJ. Monitoring the injured brain: ICP and CBF. British Journal of Anaesthesia 2006;97(1):26‐38. [DOI: ] [DOI] [PubMed] [Google Scholar]
Sukoff 1982
- Sukoff MH, Ragatz RE. Hyperbaric oxygenation for the treatment of acute cerebral edema. Neurosurgery 1982;10(1):29‐38. [PubMed] [Google Scholar]
Sydenham 2009
- Sydenham E, Roberts I, Alderson P. Hypothermia for traumatic head injury. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD001048.pub4] [DOI] [PubMed] [Google Scholar]
Thurman 1999
- Thurman DJ, Alverson C, Browne D, et al. Traumatic brain injury in the United States: a report to congress. US Department of health and Human Services, National Centre for Injury Prevention and Control 1999.
Tymianski 1996
- Tymianski M, Tator CH. Normal and abnormal calcium homeostasis in neurons: a basis for the pathophysiology of traumatic and ischemic central nervous system injury. Neurosurgery 1996;38(6):1176‐95. [DOI] [PubMed] [Google Scholar]


