Extract
Chronic respiratory diseases are the third leading cause of death and affect more than 450 million people worldwide [1]. Major risk factors such as cigarette smoking have long been studied in their pathogenesis, but as the global population ages, increasing attention must now be paid to the contributory role of ageing [2]. Epidemiological evidence indicates a decline in lung health over time with lung function classically reaching its peak between 20–30 years of age and starting an inevitable descent thereafter [3]. Modern paradigms suggest that this rise and descent may occur at different rates along the lifespan, which may indicate that the links between age and lung function may be variable between individuals [4]. Deciphering how lung ageing influences the development of chronic respiratory diseases may hold powerful clues into novel therapeutics and management strategies.
Shareable abstract
Epigenetic age is a novel biomarker utilising DNA methylation profiles that can detect accelerated biological ageing. Potential uses in respiratory disease include risk stratification for vulnerable patients and prognostication for poor clinical outcomes. https://bit.ly/3ZMTAK1
Introduction
Chronic respiratory diseases are the third leading cause of death and affect more than 450 million people worldwide [1]. Major risk factors such as cigarette smoking have long been studied in their pathogenesis, but as the global population ages, increasing attention must now be paid to the contributory role of ageing [2]. Epidemiological evidence indicates a decline in lung health over time with lung function classically reaching its peak between 20–30 years of age and starting an inevitable descent thereafter [3]. Modern paradigms suggest that this rise and descent may occur at different rates along the lifespan, which may indicate that the links between age and lung function may be variable between individuals [4]. Deciphering how lung ageing influences the development of chronic respiratory diseases may hold powerful clues into novel therapeutics and management strategies.
Epigenetic regulation, including DNA methylation, evolves as the lung ages [5] and is one mechanism of ageing that may be particularly relevant for lung disease given its dynamic responsiveness to exposures over a lifetime [6]. DNA methylation, which involves the addition of methyl groups to cytosine residues (located next to guanine residues) in the DNA sequence (CpG sites), can alter gene expression, thereby potentially disrupting pathways implicated in disease pathogenesis [7, 8]. Particular DNA methylation patterns have since emerged linking ageing with common lung diseases. For instance, in patients with asthma, altered blood DNA methylation has been observed along ageing-related genes [9]. Airway epithelial cell CpG sites differentially methylated in COPD enrich specific ageing-related pathways such as longevity regulation, cellular senescence and mTOR signalling [10]. These findings underscore the role of epigenetic regulation in lung ageing and disease. How we can leverage the enormous potential of the DNA methylome to better understand and treat chronic respiratory diseases is the subject of this summary. Here, we propose that epigenetic ageing biomarkers can be utilised as powerful indicators of disease and disease progression in pulmonary medicine.
Epigenetic clocks: estimations of biological ageing
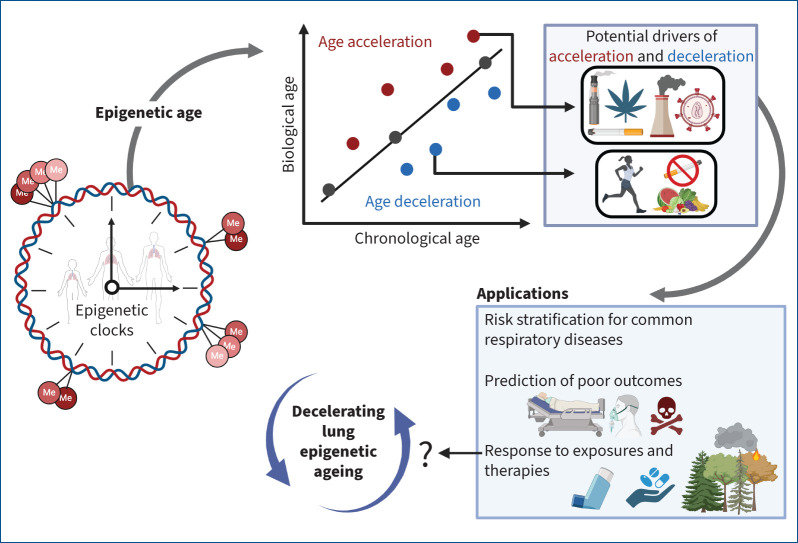
DNA methylation predictably changes over a lifespan [11], making it an attractive mechanism for quantifying biological age. Markers of epigenetic age, also known as DNA methylation or epigenetic clocks, have since been developed, with the most widely applied one created by Horvath [11]. An epigenetic clock is a composite calculation of the methylation of age-associated CpG sites calibrated in “healthy” tissues; thus, epigenetic age reflects an individual's underlying biological age. These clocks were derived by applying an elastic net regression model to select CpGs associated with chronological age. The deviation of epigenetic age from chronological age is interpreted as biological age acceleration (when epigenetic age is greater than chronological age) or deceleration (when epigenetic age is less than chronological age) (figure 1).
FIGURE 1.
Leveraging the DNA methylome for respiratory health. Epigenetic clocks measure biological ageing. The deviation of biological from chronological age (represented by the linear plot) can represent an unhealthy state. Age acceleration (red dots) can be caused by environmental factors such as smoking, vaping, pollution, and chronic viral infections. In contrast, age deceleration (blue dots) could potentially result from lifestyle changes such as smoking cessation, a healthy diet and increased physical activity. Epigenetic clocks can be used to stratify risk for respiratory diseases, predict significant clinical outcomes, and evaluate the relationship between the exposome and lung health. Efforts to understand what might slow the ageing rate down may allow for novel therapeutics for patients with chronic respiratory diseases. Figure created using BioRender.com
A summary of the most frequently used epigenetic clocks is provided in table 1. Epigenetic clocks can be categorised into two generations. The first-generation clocks were exclusively trained on chronological age and include DNAmAge, a pan-tissue clock trained on the chronological age of 51 tissues from healthy individuals [11]; DNAmAgeHannum, a blood-specific clock trained on chronological age [12]; and DNAmAgeSkinBlood, a fibroblast and blood-specific clock [13]. Second-generation clocks further capture hallmarks of ageing (e.g. chronic inflammation) along with chronological age. The most widely studied second-generation clocks include DNAmPhenoAge, which incorporates clinical measurements of phenotypic ageing (e.g. albumin, creatinine, glucose, C-reactive protein, lymphocytes, cell volume, red cell distribution, alkaline phosphatase and white blood cell counts) [14]; DNAmGrimAge (versions 1 [15] and 2 [16]), which is considered a biomarker of mortality (lifespan and healthspan) and captures inflammatory proteins associated with mortality, cigarette smoking, gender, and chronological age in its training algorithm; and DNAmTL [17], which was trained on leukocyte telomere length. As testament to their promise in the study of ageing, these clocks display stronger correlations with chronological age when compared to other ageing biomarkers such as telomere length [18], and have been shown to be associated with mortality in the general population [19].
TABLE 1.
Summary of epigenetic clocks
| Epigenetic clock | Tissue derivation | Training parameters | CpGs n | Prediction | Year |
|---|---|---|---|---|---|
| DNAmAge [11] | Pan-tissue (51 healthy tissues) | Chronological age of healthy tissue samples | 353 | Ageing rate | 2013 |
| DNAmAgeHannum [12] | Blood | Chronological age of healthy tissue samples | 71 | Ageing rate | 2013 |
| DNAmAgeSkinBlood [13] | Skin, blood and saliva | Chronological age of healthy tissue samples | 391 | Ageing rate | 2015 |
| DNAmPhenoAge [14] | Blood | Chronological age and clinical biomarkers (albumin, creatine, glucose, C-reactive protein, lymphocyte percentage, mean cell volume, red cell distribution width, alkaline phosphatase, white blood cell count) | 513 | Lifespan and health span (mortality and morbidity) | 2018 |
| DNAmGrimAge [15] | Blood | Time to death regression on chronological age, gender, CpGs estimates for smoking pack-years and seven plasma proteins (adrenomedullin levels, beta-2 microglobulin, cystatin C, growth differentiation factor 15, leptin, plasminogen activation inhibitor 1, tissue inhibitor metalloproteinase 1) | 1031 | Lifespan and health span (mortality and morbidity) | 2019 |
| DNAmGrimAge2 [16] | Blood | Time to death regression on chronological age, gender, CpGs estimates for smoking pack-years and nine plasma proteins (adrenomedullin levels, beta-2 microglobulin, cystatin C, growth differentiation factor 15, leptin, plasminogen activation inhibitor 1, tissue inhibitor metalloproteinase 1, high sensitivity C-reactive protein and haemoglobin A1C) | 1331 | Lifespan and health span (mortality and morbidity) | 2022 |
| DNAmTL [17] | Blood | Leukocyte telomeric length | 140 | Replicative history of cells and health outcomes | 2019 |
Why the specific CpGs that constitute epigenetic clock algorithms are so tightly linked with ageing is still a matter of speculation. In silico analyses have shown that the 353 CpGs that make up the original pan-tissue Horvath clock enriched cell death/survival, cellular growth/proliferation, organismal/tissue development, and cancer pathways [11]. Experiments in primary human cells designed to better understand the underlying biology of epigenetic clocks have also been performed in which various methods to induce different ageing processes were tested to determine their relative impact on epigenetic age. Indeed, not all mechanisms of ageing were linked with epigenetic age. For instance, cellular senescence, telomere attrition and genomic instability did not in fact increase epigenetic age, but impaired nutrient sensing, stem cell exhaustion, altered cell–cell communication, and mitochondrial dysfunction did [20]. Other clocks, such as DNAmGrimAge, may be linked with immune reconstitution, as thymic regeneration has been shown to decrease DNAmGrimAge [21]. Until further experiments are performed, it is conceivable that the different iterations of the epigenetic clock might also reflect different ageing mechanisms.
What accelerates the lung's epigenetic age?
As a key mechanism linking gene and environment, DNA methylation may fundamentally change depending on an individual's particular exposures (figure 1), offering insight into which risk factors may accelerate lung ageing. The impact that traditional risk factors for lung disease such as smoking and air pollution have on epigenetic age has largely been studied in peripheral blood. Toxic inhaled particles, particularly those from tobacco smoking, likely result in the acceleration of peripheral blood epigenetic age [22]. Similar observations have been made in individuals exposed to cannabis smoke [23]. While both cannabis and tobacco are likely detrimental to epigenetic ageing, the effect of the latter is greater [23], suggesting that the relative ageing risk may be specific to each exposure. Both indoor air pollution, particularly exposure to polycyclic aromatic hydrocarbons following indoor coal combustion [24], and outdoor air pollution [25, 26] have also been linked with blood epigenetic age acceleration. Studies evaluating epigenetic age in lung tissue, however, remain scarce. To date, investigations in bronchoscopy-derived lung epithelial cells demonstrate that both cigarette and e-cigarette use [27, 28], ozone exposure [29] and infection with HIV [10, 30] can significantly raise epigenetic age. Even less is known, though, about what might slow down the lung's epigenetic ageing rate and whether such deceleration could improve clinical outcomes (or might even have its own pathophysiological consequences). Cigarette smoking cessation at least appears to somewhat attenuate airway epigenetic age acceleration [28], while cannabis smoking cessation may have a similar effect in the blood [23]. Lifestyle modifications such as diet [31] and exercise [32] may also reduce epigenetic age, although studies specifically evaluating this in lung tissue have yet to be performed. Understanding the full scope of the exposome's effects on lung ageing is crucial for developing comprehensive strategies to mitigate these risks and promote strategies for respiratory well-being.
Applying the epigenetic clock to respiratory health
The striking ability of epigenetic clocks to capture biological ageing [11] and poor health outcomes [33] has led to an increased interest in their application in chronic respiratory diseases, particularly in COPD, where the greatest number of studies have been performed. Previous work has demonstrated that airway epithelial cell epigenetic age is increased in COPD, even after adjusting for confounders such as cigarette smoking [10]. Furthermore, the strong correlation between airway epithelial cell and blood epigenetic age [34] raises the possibility that the epigenetic clock could serve as an accessible blood biomarker to predict important clinical outcomes in COPD. For instance, Breen et al. [35] found that a greater increase in the difference between blood extrinsic epigenetic age (which measures both epigenetic age and age-related changes in blood cell composition) and chronological age is associated with a higher odds ratio (1.02, 95% CI 1.01–1.03; p=0.04) of incident COPD over a mean follow-up time of 7 years. Weaker, yet still significant, associations were observed between this difference and decreased forced expiratory volume in 1 s to forced vital capacity ratio. In people living with HIV, higher baseline blood DNAmGrimAge acceleration was also detected in participants who would ultimately develop airflow limitation over a 6-year follow-up period [36], demonstrating the potential of the epigenetic clock for risk stratification. For patients already with COPD, epigenetic age can also predict those at high risk for poor clinical outcomes. Short blood DNAmTL is associated with higher St George's Respiratory Questionnaire scores (p=0.026), an increased risk for an acute exacerbation of any severity (p=0.03), and hospitalisations (p=0.03) [37]. Both short blood DNAmTL (p=0.024) and higher blood DNAmGrimAge (p=0.031) are also associated with a greater risk of death over a 1-year period [38]. Although additional replication in larger (and longitudinal) cohorts are needed, these findings suggest that in the search for personalised medicine approaches for patients with COPD, epigenetic clocks could represent a novel avenue to identify those at greatest risk for clinical worsening.
Although the investigation of the epigenetic clock in other respiratory diseases remains in the nascent stages, similar epigenetic age acceleration has been reported in asthma, where a 1-year increase in blood DNAmAge at mid-childhood was associated with a 1.16 (95% CI 1.00–1.34; p=0.044) higher odds of asthma [39]. Nasal epithelial age acceleration in children is also associated with asthma, with a 10-fold increase in exhaled nitric oxide fraction accelerating epigenetic age by 1.11 years and a 10-fold increase in total IgE accelerating epigenetic age by 0.58 years [40]. Efforts to quantify the ability of epigenetic age to predict lung cancer remain mixed, though, with some studies suggesting a strong association between certain epigenetic clocks and incident lung cancer [41] and others suggesting no association at all [42]. How the epigenetic clock may be utilised in other chronic respiratory diseases, such as cystic fibrosis, bronchiectasis and pulmonary fibrosis, will require further investigation.
Transitioning the epigenetic clock from theory to practice
Epigenetic markers of ageing have shown potential as tools for understanding respiratory diseases and predicting clinical outcomes. However, several limitations must be overcome prior to their successful integration into clinical practice [43]. If, at least in COPD, epigenetic age acceleration denotes poor prognosis, we still do not yet understand what interventions might slow down and/or reverse epigenetic ageing and, moreover, whether epigenetic age deceleration can even improve these outcomes in the first place. The field is still hampered by a lack of longitudinal evaluation of epigenetic age in chronic respiratory diseases, which also means we still do not yet know how many years in epigenetic age constitutes a clinically meaningful difference for a patient. Second, the currently available epigenetic clocks were derived from an array of healthy tissues of which the lung comprised only a small proportion [11]. A reasonable assumption is that an effective epigenetic clock responsive to the inherent dynamics of respiratory diseases and their interventions may require training on much larger sample sizes reflecting a wider variety of lung diseases. Derivation of a lung-specific epigenetic clock may enhance our ability to utilise these tools more effectively in respiratory medicine. Third, further investigation on the mechanisms behind the epigenetic clocks is needed to target the specific pathways that affect lung health. Fundamentally, we still do not understand the specific biology represented by each clock and what deviations of epigenetic age might imply when it comes to aberrant lung pathology. Fourth, epigenetic age can be significantly affected by cell type composition [44]; therefore, improved deconvolution methods that can help to correct for cell type differences as well as study designs that focus on specific cell populations are still required. Finally, lung ageing is a complex multifaceted process in which DNA methylation represents only a single mechanism. Integrating epigenetic clocks with additional ageing biomarkers derived from transcriptomic, metabolomic and microbiome platforms [45] may be one approach that could boost their performance metrics and provide the necessary validation required for successful biomarker development. These strategies may be critical steps in taking what is now the promise of the epigenetic clock to its full potential as a biomarker that can diagnose, predict and manage patients with chronic respiratory diseases.
Shareable PDF
Footnotes
Conflict of interest: A.I. Hernandez Cordero reports grants from the Canadian Institutes of Health Research and payments to their institution for research from the BC Lung Foundation. J.M. Leung reports grants from the Canadian Institutes of Health Research and BC Lung Foundation (paid to institution, work outside of this manuscript), payment or honoraria for lectures, presentations, manuscript writing, or educational events from the BC Lung Foundation (payments for participation in COPD patient forums) and the University of British Columbia (payments for continuing medical education lectures), participation on data safety monitoring boards with Enhance Quality Safety, and Patient experience in Chronic Obstructive Pulmonary Disorder (EQuiP COPD) (no payments), and leadership or fiduciary roles with the Canadian Respiratory Research Network and CanCOLD Study as a member of the steering committee (no payments).
Support statement: A.I. Hernandez Cordero is supported by the Canadian Institutes of Health Research Excellence, Diversity, and an Independence Early Career Transition Award. J.M. Leung is supported by the Canada Research Chairs Program and the GlaxoSmithKline Chair in COPD. No conflicts of interest are reported.
References
- 1.GBD 2019 Chronic Respiratory Diseases Collaborators . Global burden of chronic respiratory diseases and risk factors, 1990–2019: an update from the Global Burden of Disease Study 2019. EClinicalMedicine 2023; 59: 101936. doi: 10.1016/j.eclinm.2023.101936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rojas M, Mora AL, Kapetanaki M, et al. . Aging and lung disease. Clinical impact and cellular and molecular pathways. Ann Am Thorac Soc 2015; 12: S222. doi: 10.1513/AnnalsATS.201508-484PL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J 1999; 13: 197–205. doi: 10.1183/09031936.99.14614549 [DOI] [PubMed] [Google Scholar]
- 4.Reyfman PA, Washko GR, Dransfield MT, et al. . Defining impaired respiratory health. A paradigm shift for pulmonary medicine. Am J Respir Crit Care Med 2018; 198: 440. doi: 10.1164/rccm.201801-0120PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kachroo P, Morrow JD, Vyhlidal CA, et al. . DNA methylation perturbations may link altered development and aging in the lung. Aging 2021; 13: 1742–1764. doi: 10.18632/aging.202544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skov-Jeppesen SM, Kobylecki CJ, Jacobsen KK, et al. . Changing smoking behavior and epigenetics: a longitudinal study of 4,432 individuals from the general population. Chest 2023; 163: 1565–1575. doi: 10.1016/j.chest.2022.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robertson KD. DNA methylation and human disease. Nat Rev Genet 2005; 6: 597–610. doi: 10.1038/nrg1655 [DOI] [PubMed] [Google Scholar]
- 8.Wilson AS, Power BE, Molloy PL. DNA hypomethylation and human diseases. Biochim Biophys Acta 2007; 1775: 138–162. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Yuan L, Yang M, et al. . Aberrant methylation of aging-related genes in asthma. Front Mol Biosci 2021; 8: 655285. doi: 10.3389/fmolb.2021.655285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernández Cordero AI, Yang CX, Yang J, et al. . Airway aging and methylation disruptions in HIV-associated chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2022; 206: 150–160. doi: 10.1164/rccm.202106-1440OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol 2013; 14: R115. doi: 10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hannum G, Guinney J, Zhao L, et al. . Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell 2013; 49: 359–367. doi: 10.1016/j.molcel.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horvath S, Oshima J, Martin GM, et al. . Epigenetic clock for skin and blood cells applied to Hutchinson Gilford progeria syndrome and ex vivo studies. Aging 2018; 10: 1758–1775. doi: 10.18632/aging.101508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine ME, Lu AT, Quach A, et al. . An epigenetic biomarker of aging for lifespan and healthspan. Aging 2018; 10: 573–591. doi: 10.18632/aging.101414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu AT, Quach A, Wilson JG, et al. . DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging 2019; 11: 303–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu AT, Binder AM, Zhang J, et al. . DNA methylation GrimAge version 2. Aging 2022; 14: 9484–9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu AT, Seeboth A, Tsai p-C, et al. . DNA methylation-based estimator of telomere length. Aging 2019; 11: 5895–5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearce EE, Alsaggaf R, Katta S, et al. . Telomere length and epigenetic clocks as markers of cellular aging: a comparative study. GeroScience 2022; 44: 1861–1869. doi: 10.1007/s11357-022-00586-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marioni RE, Shah S, McRae AF, et al. . DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol 2015; 16: 25. doi: 10.1186/s13059-015-0584-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabacik S, Lowe D, Fransen L, et al. . The relationship between epigenetic age and the hallmarks of aging in human cells. Nat Aging 2022; 2: 484–493. doi: 10.1038/s43587-022-00220-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fahy GM, Brooke RT, Watson JP, et al. . Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell 2019; 18: e13028. doi: 10.1111/acel.13028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui F, Tang L, Li D, et al. . Early-life exposure to tobacco, genetic susceptibility, and accelerated biological aging in adulthood. Sci Adv 2024; 10: eadl3747. doi: 10.1126/sciadv.adl3747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez Cordero AI, Li X, Yang CX, et al. . Cannabis smoking is associated with advanced epigenetic age. Eur Respir J 2024; 63: 2400458. doi: 10.1183/13993003.00458-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blechter B, Cardenas A, Shi J, et al. . Household air pollution and epigenetic aging in Xuanwei, China. Environ Int 2023; 178: 108041. doi: 10.1016/j.envint.2023.108041 [DOI] [PubMed] [Google Scholar]
- 25.Baranyi G, Deary IJ, McCartney DL, et al. . Life-course exposure to air pollution and biological ageing in the Lothian Birth Cohort 1936. Environ Int 2022; 169: 107501. doi: 10.1016/j.envint.2022.107501 [DOI] [PubMed] [Google Scholar]
- 26.Koenigsberg SH, Chang C-J, Ish J, et al. . Air pollution and epigenetic aging among Black and White women in the US. Environ Int 2023; 181: 108270. doi: 10.1016/j.envint.2023.108270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song M-A, Mori KM, McElroy JP, et al. . Accelerated epigenetic age, inflammation, and gene expression in lung: comparisons of smokers and vapers with non-smokers. Clin Epigenetics 2023; 15: 160. doi: 10.1186/s13148-023-01577-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu X, Huang Q, Javed R, et al. . Effect of tobacco smoking on the epigenetic age of human respiratory organs. Clin Epigenetics 2019; 11: 183. doi: 10.1186/s13148-019-0777-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weston WC, Bind MA, Cascio WE, et al. . Accelerated aging and altered subclinical response to ozone exposure in young, healthy adults. Environ Epigenetics 2024; 10: dvae007. doi: 10.1093/eep/dvae007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konstantinidis I, Crothers K, Kunisaki KM, et al. . HIV-associated lung disease. Nat Rev Dis Primer 2023; 9: 39. doi: 10.1038/s41572-023-00450-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawamura T, Higuchi M, Ito T, et al. . Healthy Japanese dietary pattern is associated with slower biological aging in older men: WASEDA'S health study. Front Nutr 2024; 11: 1373806. doi: 10.3389/fnut.2024.1373806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawamura T, Radak Z, Tabata H, et al. . Associations between cardiorespiratory fitness and lifestyle-related factors with DNA methylation-based ageing clocks in older men: WASEDA'S Health Study. Aging Cell 2024; 23: e13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faul JD, Kim JK, Levine ME, et al. . Epigenetic-based age acceleration in a representative sample of older Americans: associations with aging-related morbidity and mortality. Proc Natl Acad Sci USA 2023; 120: e2215840120. doi: 10.1073/pnas.2215840120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernandez Cordero AI, Yang CX, Li X, et al. . The blood DNA methylation clock GrimAge is a robust surrogate for airway epithelia aging. Biomedicines 2022; 10: 3094. doi: 10.3390/biomedicines10123094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breen M, Nwanaji-Enwerem JC, Karrasch S, et al. . Accelerated epigenetic aging as a risk factor for chronic obstructive pulmonary disease and decreased lung function in two prospective cohort studies. Aging 2020; 12: 16539–16554. doi: 10.18632/aging.103784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernández Cordero AI, Yang CX, Yang J, et al. . The relationship between the epigenetic aging biomarker “GrimAge” and lung function in both the airway and blood of people living with HIV: an observational cohort study. EBioMedicine 2022; 83: 104206. doi: 10.1016/j.ebiom.2022.104206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernández Cordero AI, Yang CX, Li X, et al. . Epigenetic marker of telomeric age is associated with exacerbations and hospitalizations in chronic obstructive pulmonary disease. Respir Res 2021; 22: 316. doi: 10.1186/s12931-021-01911-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez Cordero AI, Yang CX, Milne S, et al. . Epigenetic blood biomarkers of ageing and mortality in COPD. Eur Respir J 2021; 58: 2101890. doi: 10.1183/13993003.01890-2021 [DOI] [PubMed] [Google Scholar]
- 39.Peng C, Cardenas A, Rifas-Shiman SL, et al. . Epigenetic age acceleration is associated with allergy and asthma in children in Project Viva. J Allergy Clin Immunol 2019; 143: 2263–2270.e14. doi: 10.1016/j.jaci.2019.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cardenas A, Sordillo JE, Rifas-Shiman SL, et al. . The nasal methylome as a biomarker of asthma and airway inflammation in children. Nat Commun 2019; 10: 3095. doi: 10.1038/s41467-019-11058-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levine ME, Hosgood HD, Chen B, et al. . DNA methylation age of blood predicts future onset of lung cancer in the women's health initiative. Aging 2015; 7: 690–700. doi: 10.18632/aging.100809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michaud DS, Chung M, Zhao N, et al. . Epigenetic age and lung cancer risk in the CLUE II prospective cohort study. Aging 2023; 15: 617–629. doi: 10.18632/aging.204501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bell CG, Lowe R, Adams PD, et al. . DNA methylation aging clocks: challenges and recommendations. Genome Biol 2019; 20: 249. doi: 10.1186/s13059-019-1824-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loyfer N, Magenheim J, Peretz A, et al. . A DNA methylation atlas of normal human cell types. Nature 2023; 613: 355–364. doi: 10.1038/s41586-022-05580-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mavromatis LA, Rosoff DB, Bell AS, et al. . Multi-omic underpinnings of epigenetic aging and human longevity. Nat Commun 2023; 14: 2236. doi: 10.1038/s41467-023-37729-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This PDF extract can be shared freely online.
Shareable PDF ERJ-01257-2024.Shareable (275.9KB, pdf)