ABSTRACT
There are well‐established relationships between aging and neurodegenerative changes, and between aging and hearing loss. The goal of this study was to determine how structural brain aging is influenced by hearing loss. Human Connectome Project Aging data were analyzed, including T1‐weighted Magnetic Resonance Imaging (MRI) and Words in noise (WIN) thresholds (n = 623). Freesurfer extracted gray and white matter volume, and cortical thickness, area, and curvature. Linear regression models targeted (1) interactions between age and WIN threshold and (2) correlations with WIN threshold adjusted for age, both corrected for false discovery rate (pFDR < 0.05). WIN threshold moderated age‐related increase in volume in bilateral inferior lateral ventricles, with a higher threshold associated with increased age‐related ventricle expansion. Age‐related differences in the occipital cortex also increased with higher WIN thresholds. When controlling for age, high WIN threshold was correlated with reduced cortical thickness in Heschl's gyrus, calcarine sulcus, and other sensory regions, and reduced temporal lobe white matter. Older volunteers with poorer hearing and cognitive scores had the lowest volume in left parahippocampal white matter. These results suggest that better hearing is associated with reduced age‐related differences in medial temporal lobe, while better hearing at any age is associated with greater cortical tissue in auditory and other sensory regions. Future longitudinal studies are needed to assess the causal nature of these relationships, but these results indicate interventions that preserve or protect hearing function may combat some neurodegenerative changes in aging.
Keywords: aging, hearing loss, morphometry, temporal cortex
We studied anatomical MRIs (Magnetic Resonance Imaging) from the Human Connectome Project and found that older adults with better hearing had reduced signs of aging in medial temporal lobe, a part of the brain implicated in age‐related dementia. For example, a common sign of brain aging, increased ventricle size, was less apparent in the medial temporal lobes of older adults with better hearing.
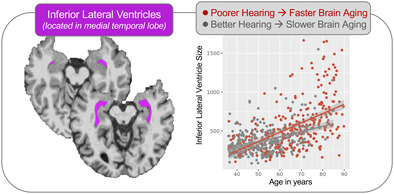
Summary.
Poorer hearing was associated with increased age‐related ventricle expansion in medial temporal lobes and reduced temporal lobe white matter at any age.
Poorer hearing was associated with thinner cortex in Heschl's gyrus thickness, calcarine sulcus, and other sensory regions.
Preserving hearing may reduce brain aging in medial temporal lobe.
1. Introduction
There are established hallmarks of brain aging on the macro‐scale, including cortical and subcortical atrophy, increased ventricle size, changes in cerebral perfusion, and other specific markers associated with age‐related neurodegenerative disease (Cole 2020; Fjell et al. 2013; Frangou et al. 2022; Juttukonda et al. 2021). Though these overall patterns of change are well characterized, there is sufficient variability to suggest that not all people experience these changes at the same rate or to the same degree (Cox and Deary 2022). Understanding why some people's brains age more or faster than others could help identify risk factors and interventions to promote healthy brain aging.
Hearing loss is also a well‐established hallmark of aging. Age‐related hearing loss is associated with stiffening of outer hair cells in the cochlea, cumulative otologic injury from loud sounds or drugs, and other factors causing loss of peripheral input from the inner ear to the central auditory system. Hearing loss is associated with social, occupational, cognitive, and mental health impacts, including increased risk of dementia (Killeen, Zhou, and Ehrlich 2023; Lin et al. 2013; Stevenson et al. 2022) and depression (Li et al. 2014; Parravano et al. 2021). Yet, hearing loss also occurs in younger adults (with risk increasing in recent years (Dillard et al. 2022)), and may impact the brain independently of age in some brain systems, while accelerating the pace of age‐related changes in others. Therefore, studying the impact of hearing on the brain and on brain aging appears critical.
Hearing loss has been linked to structural differences in the central auditory system, including reduced gray and white matter in the temporal lobe (Armstrong et al. 2019; Eckert et al. 2012; Eckert, Vaden, and Dubno 2019; Li et al. 2023), including Heschl's gyrus, the location of core/primary auditory cortex (Eckert, Vaden, and Dubno 2019; Lin et al. 2014; Peelle et al. 2011). Differences in other brain systems have also been identified, sometimes interpreted as compensatory changes (e.g., frontal cortex (Husain et al. 2011; Khan et al. 2021; Koops, de Kleine, and van Dijk 2020; Melcher, Knudson, and Levine 2013; Qian et al. 2017; Yang et al. 2014)). However, it is unclear whether some of these effects are related to hearing loss or aging (or both) in these studies because age is not always included as a covariate in statistical models, and sample size and age ranges may also be limited.
The Human Connectome Project (HCP) offers a unique opportunity to address this issue on a much larger scale, with high‐quality, well‐characterized multimodal Magnetic Resonance Imaging (MRI) datasets collected across the lifespan (Elam et al. 2021; Harms et al. 2018). The HCP did not select for or exclude hearing loss during recruitment and did not include clinical audiometry or standard assessments of peripheral hearing like pure‐tone thresholds. However, these datasets do include a basic hearing test as part of an extended cognitive/perceptual test battery, the Words in noise (WIN) task (Barch et al. 2013). The WIN task assesses speech reception threshold in noise (specifically, monosyllabic words in multispeaker babble). There are reasons to assume that elevated WIN thresholds could reflect hearing difficulties arising from peripheral hearing loss in the HCP cohorts. For example, WIN thresholds correlate with pure‐tone thresholds assessed via air conduction even in normal hearing (Holmes and Griffiths 2019), though the strength of this relationship depends on the population studied and the manner of WIN task administration (Fitzgerald et al. 2023; Holmes and Griffiths 2019; Humes 2021; Kam and Fu 2020; Leaver 2024; Vermiglio et al. 2020). Peripheral hearing loss is also more common than central dysfunction alone (i.e., in the absence of peripheral loss; ~25 vs. 13% of adults), respectively (Lin, Niparko, and Ferrucci 2011; Lisan et al. 2022; Quaranta et al. 2014; Spankovich et al. 2018). Furthermore, elevated speech reception and speech‐in‐noise thresholds are common in age‐related hearing loss (Cunningham and Tucci 2017), and yet are better explained by peripheral hearing loss than cognitive factors (Akeroyd 2008; Humes and Roberts 1990; van Rooij and Plomp 1992). Taken together, this suggests that WIN threshold, a common screener for peripheral loss, could be most reflective of peripheral hearing function in the HCP and similar datasets. However, central auditory processing disorder and “hidden” hearing loss (C. Kohrman et al. 2020) cannot be ruled out in the absence of comprehensive clinical audiometry.
In the present study, we analyzed relationships between brain structure, hearing loss, and age using the HCP Aging dataset. Our goal was to explore instances where hearing function moderated the effects of aging on brain structure (i.e., a WIN‐by‐age interaction, or “moderation analysis” on brain structure). In addition, we hypothesized that hearing loss could affect the brain at any age, particularly in auditory cortex, and therefore also identified instances where hearing loss correlated with brain structure while controlling for age (i.e., main effect of WIN threshold). Brain structure was measured comprehensively using Freesurfer pipelines on T1‐weighted MRI scans, and included the volume of ventricles, subcortical structures, and cortical gray and white matter, as well as cortical thickness, curvature, and surface area. Hearing loss was assessed using WIN task threshold, and exploratory analyses used the Montreal Cognitive Assessment (MoCA) to address the influence of cognitive status on relationships between hearing function, age, and brain morphometry.
2. Materials & Methods
2.1. Participants and Data
Data for this analysis were taken from the HCP Aging dataset (Bookheimer et al. 2019). Data were downloaded in August 2023 from the NIMH Data Archive, and reflect data release 2.0. In the HCP Aging study, participants underwent an MRI protocol, a National Institutes of Health (NIH) Toolbox battery including the WIN task, and other assessments at four sites: Washington University St. Louis, University of Minnesota, Massachusetts General Hospital, and University of California, Los Angeles. Ages ranged from 36 to 90+ years. In this download, 725 participants had structural MRI data and MoCA scores (Nasreddine et al. 2005), while 631 had NIH Toolbox WIN data. For the current analysis, we retained complete cases (i.e., data from participants with both MRI and WIN data) with age below 90 years. Participants above 90 years were coded as the same age in the HCP dataset (1200 months) and thus were excluded from this analysis due to potential influence of inaccurate age data on statistical model fit (n = 8 of complete cases). This yielded 623 complete cases for analysis.
2.2. NIH Toolbox WIN Task
During the WIN task, volunteers were asked to repeat common monosyllabic words presented unilaterally (i.e., separately to each ear), spoken by one target speaker along with multispeaker babble background noise (Zecker et al. 2013). The signal‐to‐noise ratio (SNR) of target speaker to noise was varied (24, 20, 16, 12, 8, 4, 0 dB SNR), and 6 trials were presented at each SNR. In this task, the experimenter records spoken responses from the volunteer using a tablet device, and sounds are played through over‐ear headphones (e.g., Sennheiser 280 Pro) with tablet volume set at a “comfortable level.” For MRI analyses, we used WIN threshold as reported by NIH Toolbox averaged over both ears.
2.3. MRI Acquisition & Preprocessing
MRI imaging was performed at all four sites using the same hardware, a Siemens 3T Prisma (Harms et al. 2018). The current study analyzed T1‐weighted structural scans, though diffusion, perfusion, and functional MRI data are also available from the HCP‐A dataset (Bookheimer et al. 2019). Freesurfer's reconall pipeline (Version 7.20.0, (Dale, Fischl, and Sereno 1999)) was used to extract gray and white matter volume, as well as cortical thickness, area, and curvature using standard aseg, aparc.2009s, and wmparc atlases (546 total regions; (Destrieux et al. 2010; Fischl et al. 2002; Fischl and Dale 2000)). Generally speaking, metrics that are expected to decrease with age include gray and white matter volume, cortical thickness, and (perhaps to a lesser extent, (Winkler et al. 2018)) cortical surface area, while ventricle volume and mean cortical curvature are expected to increase with age, though some regions may deviate from this general pattern (Salat et al. 2004). Each metric type (gray matter volume, white matter volume, cortical thickness, cortical area, and cortical curvature) was harmonized across study sites applying neuroCombat separately for each metric type (Fortin et al. 2017) in R (https://www.r‐project.org). NeuroCombat uses an empirical Bayesian approach originally developed to mitigate batch effects in genomics (Johnson, Li, and Rabinovic 2007), and has been successfully applied to structural and functional MRI metrics in a variety of contexts (Cetin Karayumak et al. 2019; Fortin et al. 2017; Fortin et al. 2018; Radua et al. 2020; Yu et al. 2018). Outliers greater than 4 standard deviations above or below the sample mean were excluded from analysis (1 participant removed for 115 metrics; 2–8 for an additional 40 metrics).
2.4. Statistical Analyses
All statistical analyses were completed in R (https://www.r‐project.org). To test for relationships amongst WIN threshold, site, demographic, and other variables, Pearson's correlation, Student's t‐test, analysis of variance, or Chi‐squared tests were used as appropriate. For MRI analyses, linear regression models measured relationships between brain morphometry (dependent variable), WIN thresholds, and age (both linear factors) adjusted for participant sex (categorical factor). Two statistical models were applied. The first model was a moderation analysis targeting an interaction between age and WIN threshold, with the goal of identifying instances where age‐related differences in brain morphometry differed across WIN thresholds (i.e., how brain aging is impacted by hearing function). A second, separate model targeted the main effects of WIN threshold while controlling for age and sex (i.e., how hearing function impacts brain structure independent of age). For both models, effect size is reported as partial r 2 (partial_r 2 function, (Cinelli and Hazlett 2020)), an estimate of the unique variance in freesurfer metric explained by each model term (i.e., WIN, age, or interaction). Two statistical thresholds were used for each model: false discovery rate q < 0.05 across all 546 metrics, and uncorrected p < 0.05 for auditory cortical regions. For main effects meeting these statistical criteria for model two, interaction effects from the moderation model are also reported. For moderation or main effects p FDR < 0.05, an exploratory analysis tested for a triple interaction between WIN threshold, age, and MoCA score adjusted for sex, p uncorr < 0.05.
3. Results
3.1. Participant Characteristics and WIN Threshold
Age and sex did not differ across sites (F(3,619) = 2.34, p = 0.07 and χ 2(3) = 2.79, p = 0.42, respectively), though the University of California, Los Angeles (UCLA) cohort was slightly younger (Table 1). Reported WIN threshold differed across sites (F(3,619) = 8.83, p = 0.00005), with the Minnesota cohort having slightly higher thresholds (p TukeyHSD < 0.001 for all). As expected, WIN threshold correlated with age (r = 0.55, p = 0.37 x 10−51) (Figure 1). WIN threshold was also slightly higher on average in males (t(621) = 4.03, p = 0.00006; mean difference [95% Confidence Interval (CI)] = 1.40[0.68] dB SNR) and for left ear stimuli (t(622) = 2.66, p = 0.008; mean difference [95% CI] = 0.33[0.24] dB SNR).
TABLE 1.
Demographic and clinical information by site.
| MGH | UCLA | UMinn | WashU | |
|---|---|---|---|---|
| Sample size | 125 | 130 | 186 | 182 |
| Age, mean (SD) yrs | 60.44 (15.47) | 56.08 (12.76) | 59.62 (14.76) | 59.34 (14.47) |
| Sex, females/males | 64/61 | 78/52 | 106/80 | 109/73 |
| WIN threshold right ear, mean (SD) dB SNR | 8.62 (3.75) | 8.59 (4.45) | 10.31 (5.53) | 8.29 (3.98) |
| WIN threshold left ear, mean (SD) dB SNR | 8.6 (3.99) | 8.6 (3.69) | 10.76 (5.4) | 8.98 (4.37) |
Abbreviations: MGH, Massachusetts General Hospital; SD, standard deviation; UCLA, University of California, Los Angeles; UMinn, University of Minnesota; WashU, Washington University in St. Louis; WIN, words in noise.
FIGURE 1.
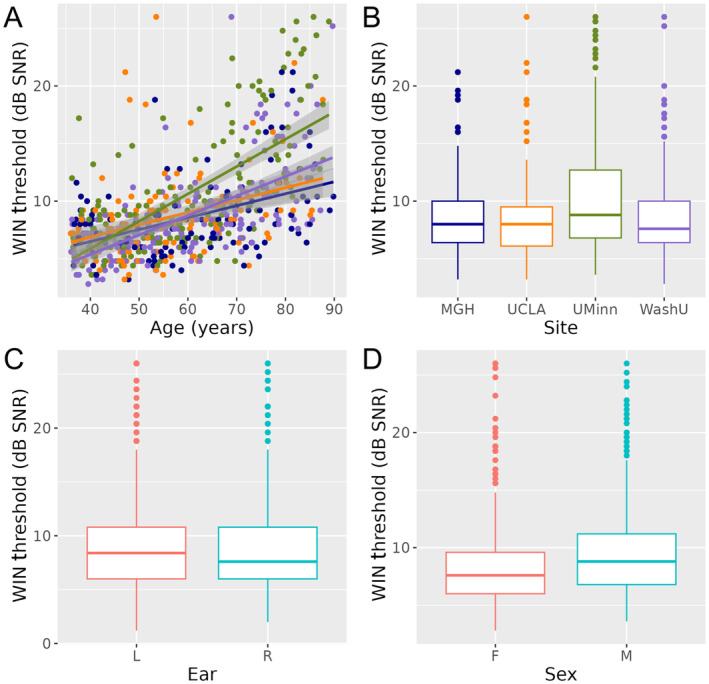
WIN Threshold differs over age, study site, ear, and sex. (A) Scatter plot displays WIN Threshold and age for each volunteer, with color reflecting study site (MGH blue, UCLA orange, UMinn green, WashU purple). Linear regression lines are fitted for each site, with shading reflecting standard error. (B–D) Boxplots display WIN Threshold across sites, ear, and sex at birth, respectively. Abbreviations: dB, decibel; F, female; L, left; M, male; R, right; SNR, signal‐to‐noise ratio; WIN, words in noise.
3.2. Hearing Function Statistically Moderates Effects of Age on Brain Structure
In MRI analyses of the effects of hearing loss on age‐related differences in brain structure, interactions between WIN threshold and age were noted bilaterally in inferior lateral ventricles, such that the rate of age‐related increase in volume was greater in people with higher WIN thresholds (Figure 2A, Table 2). WIN‐by‐age interactions were also present in occipital cortex, where the rate of age‐related thinning was greater in people with higher WIN thresholds (Figure 2B, Table 2). This included several left lateral occipital regions, left cuneus, and bilateral occipital pole. In anterior cingulate cortex thickness, an interaction showed the opposite pattern: age‐related decreases were more pronounced in people with better WIN thresholds.
FIGURE 2.
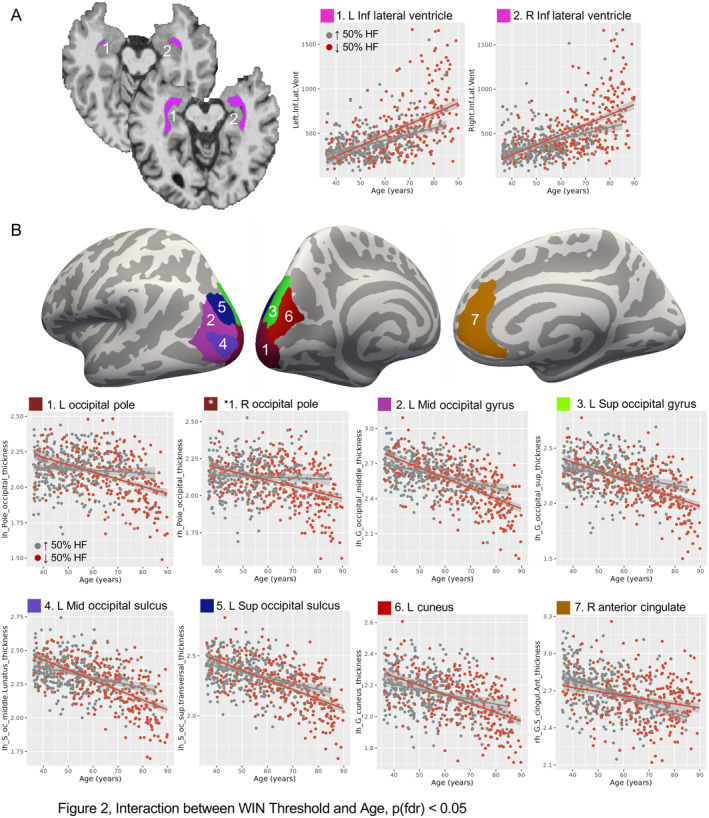
WIN Threshold moderates age‐related effects on brain structure. (A) Left and right inferior lateral ventricles exhibited a WIN‐by‐age interaction. Location is displayed in two representative volunteers with typical (top) and enlarged (bottom) ventricles for reference. Scatter plots at right show ventricle volume (mm3) and age for each volunteer, with linear regression fit displayed separately for the highest 50% of WIN thresholds (hearing loss, red) and lowest 50% of WIN thresholds (better hearing, gray). Shading reflects standard error. Note that WIN threshold was binarized for display purposes only; statistics used full range of WIN thresholds. (B) Cortical thickness in occipital regions and right anterior cingulate cortex also showed WIN‐by‐age interactions, and are displayed on a template cortical surface (fsaverage). Scatter plots display cortical thickness (mm) and age for each volunteer, with regression lines plotted as in (A) Abbreviations: Inf, inferior; L, left; Mid, middle; R, right; Sup, superior; WIN, words in noise.
TABLE 2.
WIN‐by‐age interactions on brain structure, p FDR < 0.05.
| Region, measure | Effect | β | β SE | t | df | p | p FDR | Partial r 2 |
|---|---|---|---|---|---|---|---|---|
| L inferior lateral ventricle, volume | WIN*Age | 0.043 | 0.012 | 3.711 | 611 | 0.0002 | 0.02 | 0.022 |
| WIN | −30.868 | 9.809 | −3.147 | 611 | 0.002 | 0.24 | 0.016 | |
| Age | 0.428 | 0.117 | 3.654 | 611 | 0.0003 | 0.000000 | 0.021 | |
| R inferior lateral ventricle, volume | WIN*Age | 0.043 | 0.012 | 3.626 | 614 | 0.0003 | 0.02 | 0.021 |
| WIN | −29.607 | 10.094 | −2.933 | 614 | 0.003 | 0.12 | 0.014 | |
| Age | 0.376 | 0.120 | 3.123 | 614 | 0.0019 | 0.000000 | 0.016 | |
| L cuneus gyrus, thickness | WIN*Age | −0.00003 | 0.00001 | −4.368 | 618 | 0.00001 | 0.002 | 0.030 |
| WIN | 0.023 | 0.006 | 3.724 | 618 | 0.0002 | 0.18 | 0.022 | |
| Age | −0.00006 | 0.00007 | −0.874 | 618 | 0.38 | 0.000000 | 0.001 | |
| L middle occipital gyrus, thickness | WIN*Age | −0.00004 | 0.00001 | −5.204 | 618 | 0.000000 | 0.00007 | 0.042 |
| WIN | 0.031 | 0.007 | 4.379 | 618 | 0.00001 | 0.09 | 0.030 | |
| Age | −0.00015 | 0.00008 | −1.785 | 618 | 0.07 | 0.000000 | 0.005 | |
| L superior occipital gyrus, thickness | WIN*Age | −0.00004 | 0.00001 | −4.010 | 618 | 0.00007 | 0.006 | 0.025 |
| WIN | 0.02523 | 0.00778 | 3.244 | 618 | 0.001 | 0.09 | 0.017 | |
| Age | −0.0002 | 0.00009 | −1.635 | 618 | 0.10 | 0.000000 | 0.004 | |
| L occipital pole, thickness | WIN*Age | −0.00004 | 0.00001 | −5.644 | 618 | 0.000000 | 0.00001 | 0.049 |
| WIN | 0.033 | 0.007 | 4.944 | 618 | 0.000001 | 0.17 | 0.038 | |
| Age | 0.0002 | 0.00008 | 1.971 | 618 | 0.05 | 0.000000 | 0.006 | |
| R occipital pole, thickness | WIN*Age | −0.00003 | 0.00001 | −4.067 | 618 | 0.00005 | 0.01 | 0.026 |
| WIN | 0.021 | 0.006 | 3.274 | 618 | 0.001 | 0.09 | 0.017 | |
| Age | 0.00009 | 0.00008 | 1.177 | 618 | 0.24 | 0.000005 | 0.002 | |
| L middle occipital sulcus and lunatus, thickness | WIN*Age | −0.00003 | 0.00001 | −4.587 | 617 | 0.00001 | 0.001 | 0.033 |
| WIN | 0.024 | 0.006 | 3.807 | 617 | 0.0002 | 0.09 | 0.023 | |
| Age | −0.00016 | 0.00007 | −2.119 | 617 | 0.03 | 0.000000 | 0.007 | |
| L sup occipital sulcus and transversal, thickness | WIN*Age | −0.00003 | 0.00001 | −3.921 | 617 | 0.0001 | 0.01 | 0.024 |
| WIN | 0.022 | 0.007 | 3.199 | 617 | 0.001 | 0.11 | 0.016 | |
| Age | −0.00024 | 0.00008 | −2.830 | 617 | 0.005 | 0.000000 | 0.013 | |
| R anterior cingulate gyrus and sulcus, thickness | WIN*Age | 0.00003 | 0.00001 | 3.350 | 618 | 0.0009 | 0.05 | 0.018 |
| WIN | −0.023 | 0.008 | −2.813 | 618 | 0.005 | 0.25 | 0.013 | |
| Age | −0.001 | 0.000 | −7.412 | 618 | 0.000000 | 0.000000 | 0.082 |
Note: Values listed as 0.000000 are less than 0.000001.
Abbreviation: WIN, words in noise.
3.3. Effects of Hearing Function on Brain Structure Controlling for Age
The main effects of WIN threshold independent of age were noted in many temporal lobe regions (Figure 3, Table 3). This included cortical thickness in the left Heschl's gyrus, where high WIN threshold was associated with less tissue. Higher WIN threshold was similarly associated with less volume in left entorhinal and parahippocampal white matter, as well as right middle temporal and fusiform white matter. Calcarine sulcus thickness also showed a similar pattern, though a significant WIN‐by‐age interaction was also noted in this metric (t(618) = −3.07, p = 0.002, partial r 2 = 0.015). In right rectus gyrus (medial orbitofrontal cortex) thickness and left mid‐posterior cingulate cortex curvature, positive correlations were present, where poorer performance associated with higher morphometry measures. Of all these structures, only calcarine sulcus thickness showed a WIN‐by‐age interaction puncorr < 0.05 (Table S1).
FIGURE 3.
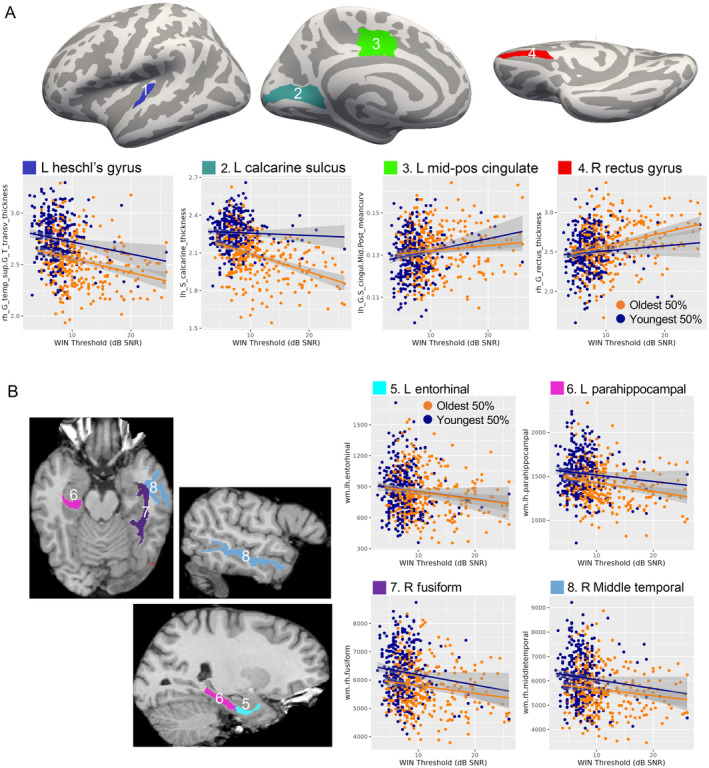
WIN threshold correlates with brain structure independently of age‐related change. (A) Regions with thickness or curvature significantly correlated with WIN threshold are displayed on a template cortical surface (top). Scatter plots display cortical thickness (mm) or mean curvature and WIN threshold for each volunteer, with linear regression lines shown for the top 50% oldest and 50% youngest ages. As in Figure 2, age was binarized for display only. (B) Regions where volume significantly correlated with WIN threshold are displayed on a single subject at left. Scatter plots are displayed at right as in (A) Abbreviations: Inf, inferior; L, left; Mid‐Pos, Mid‐posterior; R, right; WIN, words in noise.
TABLE 3.
Main effects of words in noise threshold on brain structure, p FDR < 0.05.
| Region, measure | Effect | β | β SE | t | df | p | p FDR | Partial r 2 |
|---|---|---|---|---|---|---|---|---|
| L heschl's gyrus, thickness | WIN | −0.009 | 0.002 | −3.644 | 619 | 0.0003 | 0.037 | 0.021 |
| Age | −0.001 | 0.00006 | −11.162 | 619 | 0.00000 | 0.00000 | 0.168 | |
| L entorhinal white matter, volume | WIN | −8.530 | 2.414 | −3.533 | 619 | 0.0004 | 0.037 | 0.020 |
| Age | −0.022 | 0.059 | −0.371 | 619 | 0.711 | 0.746 | 0.000 | |
| L paraphippocampal white matter, volume | WIN | −8.197 | 2.341 | −3.502 | 618 | 0.000 | 0.037 | 0.019 |
| Age | −0.340 | 0.058 | −5.904 | 618 | 0.00000 | 0.00000 | 0.053 | |
| R fusiform white matter, volume | WIN | −30.667 | 8.676 | −3.535 | 619 | 0.0004 | 0.037 | 0.020 |
| Age | −1.448 | 0.214 | −6.775 | 619 | 0.00000 | 0.00000 | 0.069 | |
| R middle temporal white matter, volume | WIN | −32.812 | 9.433 | −3.478 | 619 | 0.001 | 0.037 | 0.019 |
| Age | −1.389 | 0.232 | −5.978 | 619 | 0.00000 | 0.00000 | 0.055 | |
| L mid pos cingulate gyrus & sulcus, mean curvature | WIN | 0.0004 | 0.0001 | 3.571 | 619 | 0.0004 | 0.037 | 0.020 |
| Age | 0.00000 | 0.00000 | −0.585 | 619 | 0.559 | 0.614 | 0.001 | |
| L calcarine sulcus, thickness | WIN | −0.006 | 0.002 | −3.713 | 619 | 0.0002 | 0.037 | 0.022 |
| Age | −0.001 | 0.00004 | −14.500 | 619 | 0.00000 | 0.00000 | 0.254 | |
| R rectus gyrus, thickness | WIN | 0.011 | 0.003 | 3.723 | 618 | 0.0002 | 0.037 | 0.022 |
| Age | 0.0003 | 0.0001 | 3.495 | 618 | 0.001 | 0.001 | 0.019 |
Note: Values listed as 0.00000 are less than 0.00001.
Abbreviation: WIN, words in noise.
For completeness, we also report metrics exhibiting main effects of age controlling for hearing loss. Such effects were present across many measures and can be reviewed in Table S2.
3.4. Exploratory Analysis of Auditory Cortex
In exploratory analyses of auditory cortex, two Heschl's gyrus metrics showed modest WIN‐by‐age interactions, including right hemisphere white matter and left hemisphere curvature. In both cases, age‐related decreases were modestly less pronounced in volunteers with poorer hearing (puncorr < 0.05; Table 4 top, Figure 4A). Several metrics exhibited modest main effect of WIN threshold (puncorr < 0.05, Table 4 bottom, Figure 4B), including negative correlations with cortical thickness in right Heschl's gyrus, the entire left temporal plane (i.e., Heschl's gyrus, Heschl's sulcus, planum temporale, planum polare) and left lateral superior temporal gyrus. White matter in right superior temporal cortex and bilateral temporal pole were also negatively correlated with WIN threshold independent of age, while left planum temporale curvature showed the opposite pattern (more curvature with poorer hearing).
TABLE 4.
Auditory regions showing WIN‐by‐age or main effects of WIN, p < 0.05.
| Analysis | Region, measure | Effect | β | β SE | t | df | p | p FDR | Partial r 2 |
|---|---|---|---|---|---|---|---|---|---|
| WIN*age | R heschl's gyrus white matter, volume | WIN*Age | 0.017 | 0.007 | 2.518 | 617 | 0.012 | 0.308 | 0.010 |
| WIN | −15.281 | 5.780 | −2.644 | 617 | 0.008 | 0.686 | 0.011 | ||
| Age | −0.096 | 0.069 | −1.396 | 617 | 0.163 | 0.145 | 0.003 | ||
| L heschl's gyrus, mean curvature | WIN*Age | 0.00000 | 0.00000 | 2.835 | 618 | 0.005 | 0.185 | 0.013 | |
| WIN | −0.002 | 0.001 | −2.468 | 618 | 0.014 | 0.495 | 0.010 | ||
| Age | 0.000 | 0.00001 | −4.442 | 618 | 0.00001 | 0.0002 | 0.031 | ||
| WIN | L heschl's sulcus, thickness | WIN | −0.005 | 0.003 | −1.981 | 619 | 0.048 | 0.222 | 0.006 |
| Age | −0.001 | 0.000 | −15.747 | 619 | 0.000 | 0.000 | 0.286 | ||
| R heschl's gyrus, thickness | WIN | −0.006 | 0.002 | −2.588 | 619 | 0.010 | 0.111 | 0.011 | |
| Age | −0.001 | 0.000 | −10.475 | 619 | 0.00000 | 0.00000 | 0.151 | ||
| L lateral STG, thickness | WIN | −0.005 | 0.002 | −2.260 | 619 | 0.024 | 0.163 | 0.008 | |
| Age | −0.001 | 0.000 | −14.635 | 619 | 0.000 | 0.000 | 0.257 | ||
| L anterior STG, thickness | WIN | −0.008 | 0.003 | −2.591 | 619 | 0.010 | 0.111 | 0.011 | |
| Age | −0.001 | 0.000 | −9.725 | 619 | 0.00000 | 0.00000 | 0.133 | ||
| L posterior STG, curvature | WIN | 0.0004 | 0.0002 | 2.282 | 619 | 0.023 | 0.161 | 0.008 | |
| Age | −0.00001 | 0.00000 | −2.050 | 619 | 0.041 | 0.061 | 0.007 | ||
| R STG white matter, volume | WIN | −30.912 | 9.694 | −3.189 | 619 | 0.002 | 0.063 | 0.016 | |
| Age | −0.767 | 0.239 | −3.213 | 619 | 0.001 | 0.003 | 0.016 | ||
| L temporal pole white matter, volume | WIN | −3.281 | 1.175 | −2.791 | 619 | 0.005 | 0.092 | 0.012 | |
| Age | −0.017 | 0.029 | −0.578 | 619 | 0.564 | 0.618 | 0.001 | ||
| R temporal pole white matter, volume | WIN | −2.390 | 1.201 | −1.990 | 619 | 0.047 | 0.221 | 0.006 | |
| Age | −0.069 | 0.030 | −2.333 | 619 | 0.020 | 0.032 | 0.009 |
Note: Values listed as 0.00000 are less than 0.00001.
Abbreviation: WIN, words in noise.
FIGURE 4.
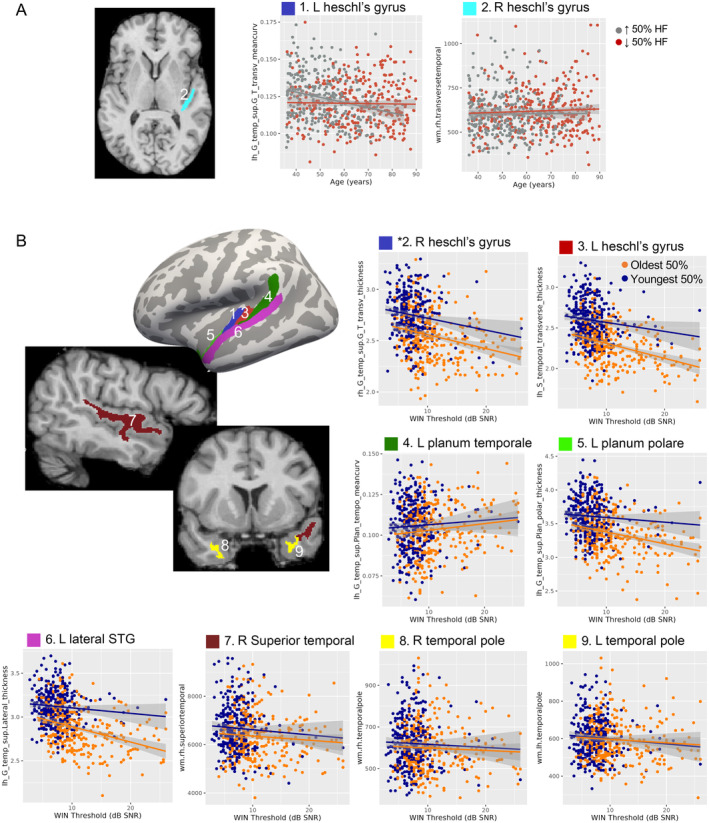
Exploratory analyses targeted auditory regions. (A) A region showing WIN‐by‐age interaction puncorr < 0.05 is displayed at right, with scatterplots displayed as in Figure 2. (B) Regions exhibiting main effects of WIN threshold independent of age puncorr < 0.05 are displayed at left on template cortical surface (thickness, mean curvature) and representative subject (volume). Scatter plots at right and bottom are displayed as in Figure 3. Abbreviations: L, left; R, right; STG, superior temporal gyrus; WIN, words in noise.
3.5. Exploratory Analysis of Hearing and Cognitive Function
In the current sample, mean MoCA total score was 26.37 (SD = 2.54) and 225 of 623 volunteers had a score consistent with mild cognitive impairment (i.e., < 26, (Nasreddine et al. 2005)). For freesurfer metrics meeting statistical criterion pFDR < 0.05 for either model described above, exploratory analyses measured a triple interaction between age, hearing loss, and total MoCA score (puncorr < 0.05; Table S3). Of these, only left parahippocampal white matter was significant (t(613) = 2.18, p = 0.03, partial r 2 = 0.008; Figure 5A). Here, the slope of age‐related volume decrease was steepest in volunteers with poorer hearing and cognitive scores (i.e., higher WIN threshold and lower MoCA score). These interactions were not present for any other metric, though bilateral inferior lateral ventricles were both puncorr < 0.10. To complement these exploratory analyses, we also tested a linear model of mean WIN threshold, with independent variables age, sex, and MoCA score as factors of interest (site was a nuisance factor). In this model, all three factors explained a significant amount of variance in WIN threshold, with age having the largest effect size (partial r2 = 0.29, t(616) = 16.03, p < 2e−16; Figure 5B), and small effect sizes for sex (partial r 2 = 0.04, t(616) = 4.22, p = 0.00002) and MoCA (partial r 2 = 0.03, t(616) = −5.31, p = 0.0000002; Figure 5C).
FIGURE 5.
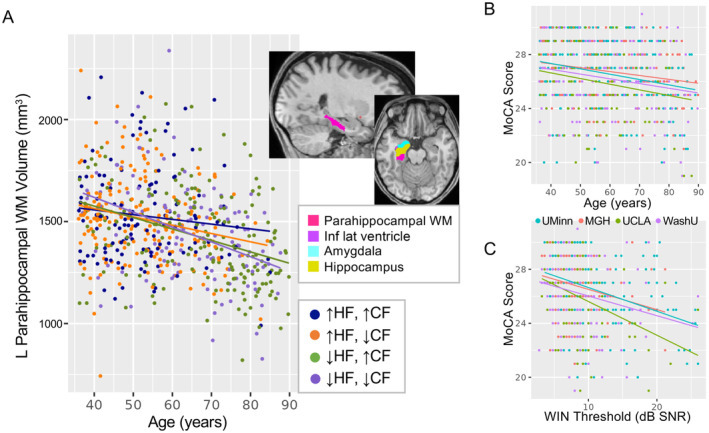
Exploratory analysis of cognitive function. (A) Left parahippocampal white matter (pink in inset at upper right) showed a triple interaction between WIN threshold, age, and MoCA score puncorr < 0.05. Scatter plot displays white matter volume (mm3) and age for each volunteer. For visualization only, WIN threshold and MoCA scores were binarized (top and bottom 50%), and regression lines are shown for each group. (B and C) Scatter plots display relationships between MoCA score, age, and WIN threshold. Color reflects cohort. Abbreviations: ↑, top half of scores; ↓, bottom half of scores; CF, cognitive function (MoCA score); HF, hearing function (WIN threshold); Inf, inferior; L, left; Lat, lateral; MoCA, Montreal Cognitive Assessment; WIN, words in noise; WM, white matter.
4. Discussion
In the current study, we demonstrated that hearing function and age have both interacting and independent relationships with macro‐anatomical brain features measured with MRI. In moderation analyses, poorer hearing function was associated with bigger age‐related differences, including increased ventricle size near medial temporal lobe structures. In primary auditory cortex and other sensory cortical regions, poorer hearing function correlated with reduced tissue content independent of age, suggesting that hearing loss may be linked to sensory cortical tissue loss at any age (or that having less sensory cortical tissue makes one more vulnerable to the effects of hearing loss). Volunteers with poorer hearing and cognitive scores also tended to show steeper age‐related reductions in left parahippocampal white matter. Taken together, these results suggest that hearing loss could be a modifiable risk factor in brain aging, particularly in medial temporal lobe structures affected by age‐related neurodegenerative conditions like Alzheimer's disease. However, longitudinal studies designed and powered to address the complexities of hearing loss are needed to directly assess causal relationships amongst hearing, brain aging, and age‐related cognitive decline.
4.1. Potential Impacts of Hearing Loss on Brain Aging
Many neuroimaging studies have reported relationships between hearing loss and brain structure and function. The current results replicate some of these findings, including correlations between Heschl's gyrus and gyrus rectus gray matter and hearing scores while controlling for the effects of age (Eckert et al. 2012; Eckert, Vaden, and Dubno 2019; Husain et al. 2011; Koops, de Kleine, and van Dijk 2020; Lin et al. 2014; Melcher, Knudson, and Levine 2013; Peelle et al. 2011). We also report some novel findings, including an association between poorer hearing scores and reduced cortical thickness in primary visual cortex (i.e., calcarine sulcus), which could be interpreted as sensory deficits in one system impacting primary sensory cortex in other systems. This interpretation may also apply to findings in the gyrus rectus and mid‐cingulate, which have been linked to olfaction (Rolls and Baylis 1994) and somatosensation/pain (Sikes and Vogt 1992; Wager et al. 2013), respectively. Although age‐related differences were also apparent in these regions, hearing loss did not influence the rate of these trajectories. However, WIN‐by‐age interactions were present in left primary visual cortex and left visual association areas in lateral occipital cortex, suggesting that hearing loss may exacerbate age‐related cortical thinning in these visual areas (or vice versa). WIN threshold also explained relatively more residual variance in lateral occipital cortex regions than age when controlling for the interaction term (Table 2). Taken together, these results suggest that hearing function may impact sensory cortices in all modalities, perhaps due to auditory deafferentation and/or loss of crossmodal cortico‐cortical connections, and that these effects may be more pronounced in visual cortex in older adults. However, it is important to note that longitudinal studies are better suited to address causal relationships amongst these factors.
This point regarding causality is particularly salient when assessing whether our findings implicate hearing loss as a causal factor in anatomical changes in medial temporal lobe, which is heavily implicated in age‐related cognitive impairment and dementias. In the current study, hearing function statistically moderated age‐related expansion of bilateral inferior lateral ventricles, located adjacent to entorhinal and parahippopcampal cortex, amygdala, and hippocampus. Ventricle expansion is a well‐established biomarker of brain aging (Fujita et al. 2023; Irimia 2021), and our current finding suggests that hearing loss associates with accelerated aging specifically in the medial temporal lobe. Given that the majority of volunteers in the current HCP Aging cohort likely had normal hearing (e.g., < 6–10 dB SNR, (Humes 2021; Leaver 2024)) or mild loss, medial temporal lobe ventricles could be particularly sensitive to the impacts of hearing function on age‐related changes in this region. Indeed, pure‐tone thresholds predicted overall ventricle expansion and white matter loss measured just ~2.5 years later in volunteers ~65 years old, suggesting that hearing loss may precede age‐related ventricle expansion (Eckert, Vaden, and Dubno 2019).
We also noted correlations between WIN threshold and medial temporal lobe white matter in left entorhinal and parahippocampal cortex in the current study. This is consistent with previous studies reporting correlations between hearing loss and entorhinal and parahippocampal gray matter, as well as hippocampus and amygdala volume (Armstrong et al. 2019; Li et al. 2023; Rudner et al. 2019). Notably, WIN threshold also correlated with gray matter volume in bilateral amygdala and hippocampus when controlling for age in our study, though these effects did not meet our strict correction for multiple comparisons correction (Table S2). Though effects of age were also present in these structures, WIN‐by‐age interactions were not, suggesting that the pace of age‐related differences in these structures were not impacted by hearing function in this cohort of healthy adults. Future studies including a wider range of hearing and cognitive function might be more sensitive to these relationships. Indeed, exploratory analyses indicated that age‐related atrophy left parahippocampal white matter might be greatest in volunteers with both lower hearing and cognitive scores in our study. This is consistent with Li et al. 2023, who reported that gray matter in a small subregion of left parahippocampal gyrus mediated relationships between hearing and cognitive function in this same cohort (Li et al. 2023). These effects are also compatible with Armstrong et al. 2019, who reported that hearing loss measured at age ~45 years predicted lower gray matter volume measured at age ~65 in right hippocampus and left entorhinal cortex (Armstrong et al. 2019). So, although the current study is cross‐sectional, evidence from longitudinal studies suggests that hearing loss could be detectable before macro‐anatomical tissue loss brain aging in medial temporal lobe regions implicated in age‐related cognitive impairment.
However, though detectable hearing loss may precede brain aging measured with structural MRI, this does not necessarily mean that hearing loss causes brain aging. It is also possible that forms of brain aging not detectable on MRI (e.g., DNA methylation or other molecular changes (Horvath et al. 2012)) could cause and/or exacerbate the functional impacts of hearing loss. Auditory perception in a natural environment is exceedingly more complex than auditory sensation assessed with pure‐tone audiometry or the WIN task and relies on brain systems that analyze speech sounds, voice identity and inflection, separate sounds of interest from background noise, and so on (Peelle and Wingfield 2016; Wayne and Johnsrude 2015). Therefore, it is possible that age‐related atrophy and other changes in superior and/or medial temporal lobe make it more difficult to hear or compensate for hearing loss, thus worsening performance on pure‐tone and/or words‐in‐noise detection thresholds during audiometric examinations, or on cognitive examinations. It is likely that causal relationships amongst hearing loss, brain aging, and age‐related cognitive decline may not be unidirectional, and may instead mutually interact in different ways over time (Wayne and Johnsrude 2015), and there are a number of other cogent reviews on this topic (Griffiths et al. 2020; Johnson et al. 2021; Wayne and Johnsrude 2015; Whitson et al. 2018). Yet, regardless of the precise causal mechanisms, the idea that early intervention with hearing aids or other assistive devices in midlife could delay brain aging remains compelling, especially if those interventions could delay the onset of age‐related dementias or ameliorate their functional impact (Lin et al. 2023). An alternate point of intervention could be to bolster attentional compensation strategies in hearing loss, which could be reflected by greater anterior cingulate cortex thickness in older volunteers with hearing loss in the current study (Pezzoli et al. 2024). Longitudinal neuroimaging or other studies that measure brain aging in the same cohort over time are needed to assess both mechanistic causality, particularly those including comprehensive audiometric evaluations.
4.2. WIN Task Performance as a Potential Measure of Peripheral Hearing Loss
In the current study, we assumed that WIN task performance most likely reflected the effects of peripheral hearing loss in the HCP Aging cohort, where hearing loss was not exclusionary (to our understanding). Indeed, prevalence of hearing loss of central origin is estimated to be much less than hearing loss with peripheral origin (Lin, Niparko, and Ferrucci 2011; Lisan et al. 2022; Quaranta et al. 2014; Spankovich et al. 2018). Previous studies have also noted correlations between WIN and pure‐tone thresholds in typical populations (Fitzgerald et al. 2023; Holmes and Griffiths 2019; Humes 2021; Kam and Fu 2020; Leaver 2024; Vermiglio et al. 2020), and that speech thresholds in older adults are better explained by peripheral hearing than cognitive assessments (Akeroyd 2008; Humes and Roberts 1990; van Rooij and Plomp 1992). When administered without adjusting output volume to accommodate hearing loss in each volunteer, tablet‐based WIN tasks can indeed be a quick, low‐burden way of assessing hearing in large studies like the HCP, UK Biobank, and clinical trials (Leaver 2024; Vermiglio et al. 2020), particularly when hearing is not the target of study.
Yet, it is widely understood that difficulty hearing in noisy environments can indicate central auditory dysfunction, independently of (or in conjunction with) peripheral hearing loss. Difficulty hearing in noise can occur in older adults without measurable peripheral hearing loss (Dubno, Horwitz, and Ahlstrom 2002; Helfer and Freyman 2008; Schoof and Rosen 2014), where tracking speech with competing talkers (vs. other types of noise) may be particularly affected (Helfer and Freyman 2008; Rajan and Cainer 2008; Schoof and Rosen 2014; Tun, O'Kane, and Wingfield 2002). In people with peripheral hearing loss, amplification does not always improve hearing in noise, though directionality settings may be under‐utilized (Davidson, Marrone, and Souza 2022). However, it is unclear why such hearing in noise difficulties arise, and few therapies are available if amplification strategies fail. Therefore, there is a clear need to understand the brain bases of hearing in noise difficulties, and our study and others using commonly available datasets from the HCP, UK Biobank, ADNI, and others do not include comprehensive audiometry and are not able to address these nuances. So, although analyzing WIN task performance in these and similar datasets can improve our understanding of how hearing function impacts brain aging, large‐scale studies combining full audiometry, neuroimaging, and other measures are needed.
One pattern of results noted in the current study may be relevant speech perception. When controlling for age, WIN threshold explained a significant amount of variance in structural metrics in the temporal lobe. Notably, effects in superior temporal regions tended to occur in gray matter in the left hemisphere, and in white matter in the right hemisphere. It is well established that speech perception relies predominantly (though not exclusively) on left superior temporal regions (Leaver and Rauschecker 2010; Scott and Johnsrude 2003). However, our results also suggest that right hemisphere white matter connections may also be important for typical speech perception and/or compensatory strategies in difficult hearing situations (e.g., using prosodic or timbre cues when decoding noisy speech). Given the limitations of the current study, it is difficult to say definitively that this pattern is the result of difficulty hearing speech in noise versus peripheral hearing loss. However, it would be interesting to dissociate the impacts of central speech hearing difficulties and peripheral loss on brain structure and function in these populations in future studies (Holmes and Griffiths 2019).
4.3. Limitations
As with any study, there are limitations that should be considered when interpreting the current results. Perhaps most importantly, it is important to reiterate that the goal of the HCP Aging study was to characterize healthy brain aging, and so the current study was not designed a priori to study hearing loss or cognitive impairment. So, although the current data include a range of hearing and cognitive scores, a study that includes fuller variability on these measures with a more balanced number of volunteers with hearing loss and/or cognitive impairment might be more sensitive to the types of effects we sought to identify here. Similarly, though the WIN task is very likely to approximate peripheral hearing loss in this sample, studies including full audiometric assessment are needed to dissociate contributions of central versus peripheral hearing function. Despite these limitations, the current study and others like it provide evidence to support a role for hearing loss in brain aging in the medial temporal lobe elsewhere, motivating future studies of hearing loss to promote healthy brain aging.
5. Conclusions
These findings provide evidence that age‐related differences in brain morphometry are statistically moderated by hearing function in the HCP Aging cohorts. In particular, poorer hearing correlated with age‐related increased volume in bilateral inferior ventricles and with increased thinning in occipital structures. Additionally, even when controlling for age, WIN threshold explained variations in several temporal regions, including thinning of the left Heschl's gyrus. These findings are consistent both with hearing‐related changes to auditory structures and with changes to brain structures associated with other sensory systems. Our results also provide additional evidence linking age‐related tissue loss in the left parahippocampal cortex with both hearing loss and poorer cognitive scores (Li et al. 2023), going further by demonstrating that hearing loss may be a key driving factor in brain aging in this region, though longitudinal studies are needed to determine causality. Taken together, these findings offer support for early interventions such as hearing aids to delay age‐related changes to brain structures. Such interventions could prove especially valuable insofar as hearing loss has also been shown to be correlated with cognitive function (Lin et al. 2013; Stevenson et al. 2022; Whitson et al. 2018). Future research in this area should focus on the causal relationship of these associations, particularly longitudinal studies that assess the progression of hearing loss and cognitive function in tandem with changes to brain morphometry. These future studies may also benefit from the use of functional neuroimaging modalities, such as arterial spin labelling MRI, which may be more sensitive to differences in persons at risk for cognitive impairment (Okonkwo et al. 2014). Ideally, these studies should also include participants with clinically defined hearing loss or cognitive decline, to confirm these findings in target populations. Taken together, our results indicate the potential utility of such longitudinal studies in developing an understanding of the associations between hearing loss, brain structure, and cognitive decline, and that protecting hearing may be important for brain health.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Table S1WIN‐by‐Age Interaction Statistics For Metrics Showing Main Effects of WIN Threshold p FDR < 0.05. Table S2. Structural Metrics Showing Main Effect of Age Threshold p FDR < 0.05. Table S3. Win‐by‐Age‐by‐MoCA Interactions in metrics meeting Meeting p FDR < 0.05 for target Target effects Effects (Tables 2&3).
Acknowledgements
This work was supported by the National Institutes of Health (NIH) under award number R21DC015880 to Dr. Leaver. Data used in the preparation of this manuscript were obtained from the National Institute of Mental Health (NIMH) Data Archive (NDA). NDA is a collaborative informatics system created by the NIH to provide a national resource to support and accelerate research in mental health. Dataset identifier(s): HCP Aging Release 2.0 funded by U01AG052564 and U01AG052564‐S1 (to the HCP Aging PIs). This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or of the Submitters submitting original data to NDA.
Funding: This work was supported by the National Institutes of Health, R21DC015880.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- Akeroyd, M. A. 2008. “Are Individual Differences in Speech Reception Related to Individual Differences in Cognitive Ability? A Survey of Twenty Experimental Studies With Normal and Hearing‐Impaired Adults.” International Journal of Audiology 47: S53–S71. [DOI] [PubMed] [Google Scholar]
- Armstrong, N. M. , An Y., Doshi J., et al. 2019. “Association of Midlife Hearing Impairment With Late‐Life Temporal Lobe Volume Loss.” JAMA Otolaryngology. Head & Neck Surgery 145: 794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch, D. M. , Burgess G. C., Harms M. P., et al. 2013. “Function in the Human Connectome: Task‐fMRI and Individual Differences in Behavior.” NeuroImage 80: 169–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer, S. Y. , Salat D. H., Terpstra M., et al. 2019. “The Lifespan Human Connectome Project in Aging: An Overview.” NeuroImage 185: 335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- C. Kohrman, D. , Wan G., Cassinotti L., and Corfas G.. 2020. “Hidden Hearing Loss: A Disorder With Multiple Etiologies and Mechanisms.” Cold Spring Harbor Perspectives in Medicine 10: a035493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin Karayumak, S. , Bouix S., Ning L., et al. 2019. “Retrospective Harmonization of Multi‐Site Diffusion MRI Data Acquired With Different Acquisition Parameters.” NeuroImage 184: 180–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinelli, C. , and Hazlett C.. 2020. “Making Sense of Sensitivity: Extending Omitted Variable Bias.” Journal of the Royal Statistical Society, Series B: Statistical Methodology 82: 39–67. [Google Scholar]
- Cole, J. H. 2020. “Multimodality Neuroimaging Brain‐Age in UK Biobank: Relationship to Biomedical, Lifestyle, and Cognitive Factors.” Neurobiology of Aging 92: 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, S. R. , and Deary I. J.. 2022. “Brain and Cognitive Ageing: The Present, and Some Predictions (…About the Future).” Aging Brain 2: 100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, L. L. , and Tucci D. L.. 2017. “Hearing Loss in Adults.” New England Journal of Medicine 377: 2465–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale, A. M. , Fischl B., and Sereno M. I.. 1999. “Cortical Surface‐Based Analysis. I. Segmentation and Surface Reconstruction.” NeuroImage 9: 179–194. [DOI] [PubMed] [Google Scholar]
- Davidson, A. , Marrone N., and Souza P.. 2022. “Hearing Aid Technology Settings and Speech‐In‐Noise Difficulties.” American Journal of Audiology 31: 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destrieux, C. , Fischl B., Dale A., and Halgren E.. 2010. “Automatic Parcellation of Human Cortical Gyri and Sulci Using Standard Anatomical Nomenclature.” NeuroImage 53: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillard, L. K. , Arunda M. O., Lopez‐Perez L., Martinez R. X., Jiménez L., and Chadha S.. 2022. “Prevalence and Global Estimates of Unsafe Listening Practices in Adolescents and Young Adults: A Systematic Review and Meta‐Analysis.” BMJ Global Health 7: e010501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubno, J. R. , Horwitz A. R., and Ahlstrom J. B.. 2002. “Benefit of Modulated Maskers for Speech Recognition by Younger and Older Adults With Normal Hearing.” Journal of the Acoustical Society of America 111: 2897–2907. [DOI] [PubMed] [Google Scholar]
- Eckert, M. A. , Vaden K. I., and Dubno J. R.. 2019. “Age‐Related Hearing Loss Associations With Changes in Brain Morphology.” Trends Hear 23: 2331216519857267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert, M. A. , Cute S. L., Vaden K. I., Kuchinsky S. E., and Dubno J. R.. 2012. “Auditory Cortex Signs of Age‐Related Hearing Loss.” JARO 13: 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam, J. S. , Glasser M. F., Harms M. P., et al. 2021. “The Human Connectome Project: A Retrospective.” NeuroImage 244: 118543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. , and Dale A. M.. 2000. “Measuring the Thickness of the Human Cerebral Cortex From Magnetic Resonance Images.” Proceedings of the National Academy of Sciences of the United States of America 97: 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. , Salat D. H., Busa E., et al. 2002. “Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain.” Neuron 33: 341–355. [DOI] [PubMed] [Google Scholar]
- Fitzgerald, M. B. , Gianakas S. P., Qian Z. J., Losorelli S., and Swanson A. C.. 2023. “Preliminary Guidelines for Replacing Word‐Recognition in Quiet With Speech in Noise Assessment in the Routine Audiologic Test Battery.” Ear and Hearing 44: 1548–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell, A. M. , Westlye L. T., Grydeland H., et al. 2013. “Critical Ages in the Life Course of the Adult Brain: Nonlinear Subcortical Aging.” Neurobiology of Aging 34: 2239–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin, J.‐P. , Parker D., Tunç B., et al. 2017. “Harmonization of Multi‐Site Diffusion Tensor Imaging Data.” NeuroImage 161: 149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin, J.‐P. , Cullen N., Sheline Y. I., et al. 2018. “Harmonization of Cortical Thickness Measurements Across Scanners and Sites.” NeuroImage 167: 104–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangou, S. , Modabbernia A., Williams S. C. R., et al. 2022. “Cortical Thickness Across the Lifespan: Data From 17,075 Healthy Individuals Aged 3‐90 Years.” Human Brain Mapping 43: 431–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, S. , Mori S., Onda K., et al. 2023. “Characterization of Brain Volume Changes in Aging Individuals With Normal Cognition Using Serial Magnetic Resonance Imaging.” JAMA Network Open 6: e2318153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths, T. D. , Lad M., Kumar S., et al. 2020. “How Can Hearing Loss Cause Dementia?” Neuron 108: 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms, M. P. , Somerville L. H., Ances B. M., et al. 2018. “Extending the Human Connectome Project Across Ages: Imaging Protocols for the Lifespan Development and Aging Projects.” NeuroImage 183: 972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer, K. S. , and Freyman R. L.. 2008. “Aging and Speech‐On‐Speech Masking.” Ear and Hearing 29: 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes, E. , and Griffiths T. D.. 2019. “‘Normal’ Hearing Thresholds and Fundamental Auditory Grouping Processes Predict Difficulties With Speech‐In‐Noise Perception.” Scientific Reports 9: 16771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath, S. , Zhang Y., Langfelder P., et al. 2012. “Aging Effects on DNA Methylation Modules in Human Brain and Blood Tissue.” Genome Biology 13: R97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes, L. E. 2021. “Factors Underlying Individual Differences in Speech‐Recognition Threshold (SRT) in Noise Among Older Adults.” Frontiers in Aging Neuroscience 13: 702739. 10.3389/fnagi.2021.702739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes, L. E. , and Roberts L.. 1990. “Speech‐Recognition Difficulties of the Hearing‐Impaired Elderly.” Journal of Speech, Language, and Hearing Research 33: 726–735. [DOI] [PubMed] [Google Scholar]
- Husain, F. T. , Medina R. E., Davis C. W., et al. 2011. “Neuroanatomical Changes due to Hearing Loss and Chronic Tinnitus: A Combined VBM and DTI Study.” Brain Research 1369: 74–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia, A. 2021. “Cross‐Sectional Volumes and Trajectories of the Human Brain, Gray Matter, White Matter and Cerebrospinal Fluid in 9473 Typically Aging Adults.” Neuroinformatics 19: 347–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, J. C. S. , Marshall C. R., Weil R. S., Bamiou D.‐E., Hardy C. J. D., and Warren J. D.. 2021. “Hearing and Dementia: From Ears to Brain.” Brain 144: 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, W. E. , Li C., and Rabinovic A.. 2007. “Adjusting Batch Effects in Microarray Expression Data Using Empirical Bayes Methods.” Biostatistics 8: 118–127. [DOI] [PubMed] [Google Scholar]
- Juttukonda, M. R. , Li B., Almaktoum R., et al. 2021. “Characterizing Cerebral Hemodynamics Across the Adult Lifespan With Arterial Spin Labeling MRI Data From the Human Connectome Project‐Aging.” NeuroImage 230: 117807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam, A. C. S. , and Fu C. H. T.. 2020. “Screening for Hearing Loss in the Hong Kong Cantonese‐Speaking Elderly Using Tablet‐Based Pure‐Tone and Word‐In‐Noise Test.” International Journal of Audiology 59: 301–309. [DOI] [PubMed] [Google Scholar]
- Khan, R. A. , Sutton B. P., Tai Y., Schmidt S. A., Shahsavarani S., and Husain F. T.. 2021. “A Large‐Scale Diffusion Imaging Study of Tinnitus and Hearing Loss.” Scientific Reports 11: 23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen, O. J. , Zhou Y., and Ehrlich J. R.. 2023. “Objectively Measured Visual Impairment and Dementia Prevalence in Older Adults in the US.” JAMA Ophthalmology 141: 786–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koops, E. A. , de Kleine E., and van Dijk P.. 2020. “Gray Matter Declines With Age and Hearing Loss, but Is Partially Maintained in Tinnitus.” Scientific Reports 10: 21801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver, A. M. , and Rauschecker J. P.. 2010. “Cortical Representation of Natural Complex Sounds: Effects of Acoustic Features and Auditory Object Category.” Journal of Neuroscience 30: 7604–7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver, A. M. 2024. “Perceptual and Cognitive Effects of Focal Transcranial Direct Current Stimulation in Tinnitus.” Neuromodulation. Published ahead of print, October 11, 2024. 10.1016/j.neurom.2024.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C.‐M. , Zhang X., Hoffman H. J., Cotch M. F., Themann C. L., and Wilson M. R.. 2014. “Hearing Impairment Associated With Depression in US Adults, National Health and Nutrition Examination Survey 2005–2010.” JAMA Otolaryngology. Head & Neck Surgery 140: 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R. , Miao X., Han B., and Li J.. 2023. “Cortical Thickness of the Left Parahippocampal Cortex Links Central Hearing and Cognitive Performance in Aging.” Annals of the New York Academy of Sciences 1522: 117–125. [DOI] [PubMed] [Google Scholar]
- Lin, F. R. , Niparko J. K., and Ferrucci L.. 2011. “Hearing Loss Prevalence in the United States.” Archives of Internal Medicine 171: 1851–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, F. R. , Pike J. R., Albert M. S., et al. 2023. “Hearing Intervention Versus Health Education Control to Reduce Cognitive Decline in Older Adults With Hearing Loss in the USA (ACHIEVE): A Multicentre, Randomised Controlled Trial.” Lancet 402: 786–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, F. R. , Yaffe K., Xia J., et al. 2013. “Hearing Loss and Cognitive Decline in Older Adults.” JAMA Internal Medicine 173: 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, F. R. , Ferrucci L., An Y., et al. 2014. “Association of Hearing Impairment With Brain Volume Changes in Older Adults.” NeuroImage 90: 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisan, Q. , Goldberg M., Lahlou G., et al. 2022. “Prevalence of Hearing Loss and Hearing Aid Use Among Adults in France in the CONSTANCES Study.” JAMA Network Open 5: e2217633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher, J. R. , Knudson I. M., and Levine R. A.. 2013. “Subcallosal Brain Structure: Correlation With Hearing Threshold at Supra‐Clinical Frequencies (>8 kHz), but Not With Tinnitus.” Hearing Research 295: 79–86. [DOI] [PubMed] [Google Scholar]
- Nasreddine, Z. S. , Phillips N. A., Bédirian V., et al. 2005. “The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment.” Journal of the American Geriatrics Society 53: 695–699. [DOI] [PubMed] [Google Scholar]
- Okonkwo, O. C. , Xu G., Oh J. M., et al. 2014. “Cerebral Blood Flow Is Diminished in Asymptomatic Middle‐Aged Adults With Maternal History of Alzheimer's Disease.” Cerebral Cortex 24: 978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parravano, M. , Petri D., Maurutto E., et al. 2021. “Association Between Visual Impairment and Depression in Patients Attending Eye Clinics: A Meta‐Analysis.” JAMA Ophthalmology 139: 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle, J. E. , and Wingfield A.. 2016. “The Neural Consequences of Age‐Related Hearing Loss.” Trends in Neurosciences 39: 486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle, J. E. , Troiani V., Grossman M., and Wingfield A.. 2011. “Hearing Loss in Older Adults Affects Neural Systems Supporting Speech Comprehension.” Journal of Neuroscience 31: 12638–12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzoli, S. , Giorgio J., Martersteck A., Dobyns L., Harrison T. M., and Jagust W. J.. 2024. “Successful Cognitive Aging Is Associated With Thicker Anterior Cingulate Cortex and Lower Tau Deposition Compared to Typical Aging.” Alzheimer's & Dementia 20: 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, Z. J. , Chang P. D., Moonis G., and Lalwani A. K.. 2017. “A Novel Method of Quantifying Brain Atrophy Associated With Age‐Related Hearing Loss.” NeuroImage: Clinical 16: 205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaranta, N. , Coppola F., Casulli M., et al. 2014. “The Prevalence of Peripheral and Central Hearing Impairment and Its Relation to Cognition in Older Adults.” Audiology & Neuro‐Otology 19, no. Suppl 1: 10–14. [DOI] [PubMed] [Google Scholar]
- Radua, J. , Vieta E., Shinohara R., et al. 2020. “Increased Power by Harmonizing Structural MRI Site Differences With the ComBat Batch Adjustment Method in ENIGMA.” NeuroImage 218: 116956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan, R. , and Cainer K. E.. 2008. “Ageing Without Hearing Loss or Cognitive Impairment Causes a Decrease in Speech Intelligibility Only in Informational Maskers.” Neuroscience 154: 784–795. [DOI] [PubMed] [Google Scholar]
- Rolls, E. T. , and Baylis L. L.. 1994. “Gustatory, Olfactory, and Visual Convergence Within the Primate Orbitofrontal Cortex.” Journal of Neuroscience 14: 5437–5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner, M. , Seeto M., Keidser G., Johnson B., and Rönnberg J.. 2019. “Poorer Speech Reception Threshold in Noise Is Associated With Lower Brain Volume in Auditory and Cognitive Processing Regions.” Journal of Speech, Language, and Hearing Research 62: 1117–1130. [DOI] [PubMed] [Google Scholar]
- Salat, D. H. , Buckner R. L., Snyder A. Z., et al. 2004. “Thinning of the Cerebral Cortex in Aging.” Cerebral Cortex 14: 721–730. [DOI] [PubMed] [Google Scholar]
- Schoof, T. , and Rosen S.. 2014. “The Role of Auditory and Cognitive Factors in Understanding Speech in Noise by Normal‐Hearing Older Listeners.” Frontiers in Aging Neuroscience 6: 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, S. K. , and Johnsrude I. S.. 2003. “The Neuroanatomical and Functional Organization of Speech Perception.” Trends in Neurosciences 26: 100–107. [DOI] [PubMed] [Google Scholar]
- Sikes, R. W. , and Vogt B. A.. 1992. “Nociceptive Neurons in Area 24 of Rabbit Cingulate Cortex.” Journal of Neurophysiology 68: 1720–1732. [DOI] [PubMed] [Google Scholar]
- Spankovich, C. , Gonzalez V. B., Su D., and Bishop C. E.. 2018. “Self Reported Hearing Difficulty, Tinnitus, and Normal Audiometric Thresholds, the National Health and Nutrition Examination Survey 1999‐2002.” Hearing Research 358: 30–36. [DOI] [PubMed] [Google Scholar]
- Stevenson, J. S. , Clifton L., Kuźma E., and Littlejohns T. J.. 2022. “Speech‐In‐Noise Hearing Impairment Is Associated With an Increased Risk of Incident Dementia in 82,039 UK Biobank Participants.” Alzheimer's & Dementia 18: 445–456. [DOI] [PubMed] [Google Scholar]
- Tun, P. A. , O'Kane G., and Wingfield A.. 2002. “Distraction by Competing Speech in Young and Older Adult Listeners.” Psychology and Aging 17: 453–467. [DOI] [PubMed] [Google Scholar]
- van Rooij, J. C. G. M. , and Plomp R.. 1992. “Auditive and Cognitive Factors in Speech Perception by Elderly Listeners. III. Additional Data and Final Discussion.” Journal of the Acoustical Society of America 91: 1028–1033. [DOI] [PubMed] [Google Scholar]
- Vermiglio, A. J. , Soli S. D., Freed D. J., and Fang X.. 2020. “The Effect of Stimulus Audibility on the Relationship Between Pure‐Tone Average and Speech Recognition in Noise Ability.” Journal of the American Academy of Audiology 31: 224–232. [DOI] [PubMed] [Google Scholar]
- Wager, T. D. , Atlas L. Y., Lindquist M. A., Roy M., Woo C.‐W., and Kross E.. 2013. “An fMRI‐Based Neurologic Signature of Physical Pain.” New England Journal of Medicine 368: 1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne, R. V. , and Johnsrude I. S.. 2015. “A Review of Causal Mechanisms Underlying the Link Between Age‐Related Hearing Loss and Cognitive Decline.” Ageing Research Reviews 23: 154–166. [DOI] [PubMed] [Google Scholar]
- Whitson, H. E. , Cronin‐Golomb A., Cruickshanks K. J., et al. 2018. “American Geriatrics Society and National Institute on Aging Bench‐To‐Bedside Conference: Sensory Impairment and Cognitive Decline in Older Adults.” Journal of the American Geriatrics Society 66: 2052–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler, A. M. , Greve D. N., Bjuland K. J., et al. 2018. “Joint Analysis of Cortical Area and Thickness as a Replacement for the Analysis of the Volume of the Cerebral Cortex.” Cerebral Cortex 28: 738–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, M. , Chen H.‐J., Liu B., et al. 2014. “Brain Structural and Functional Alterations in Patients With Unilateral Hearing Loss.” Hearing Research 316: 37–43. [DOI] [PubMed] [Google Scholar]
- Yu, M. , Linn K. A., Cook P. A., et al. 2018. “Statistical Harmonization Corrects Site Effects in Functional Connectivity Measurements From Multi‐Site fMRI Data.” Human Brain Mapping 39: 4213–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecker, S. G. , Hoffman H. J., Frisina R., et al. 2013. “Audition Assessment Using the NIH Toolbox.” Neurology 80: S45–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1WIN‐by‐Age Interaction Statistics For Metrics Showing Main Effects of WIN Threshold p FDR < 0.05. Table S2. Structural Metrics Showing Main Effect of Age Threshold p FDR < 0.05. Table S3. Win‐by‐Age‐by‐MoCA Interactions in metrics meeting Meeting p FDR < 0.05 for target Target effects Effects (Tables 2&3).
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


