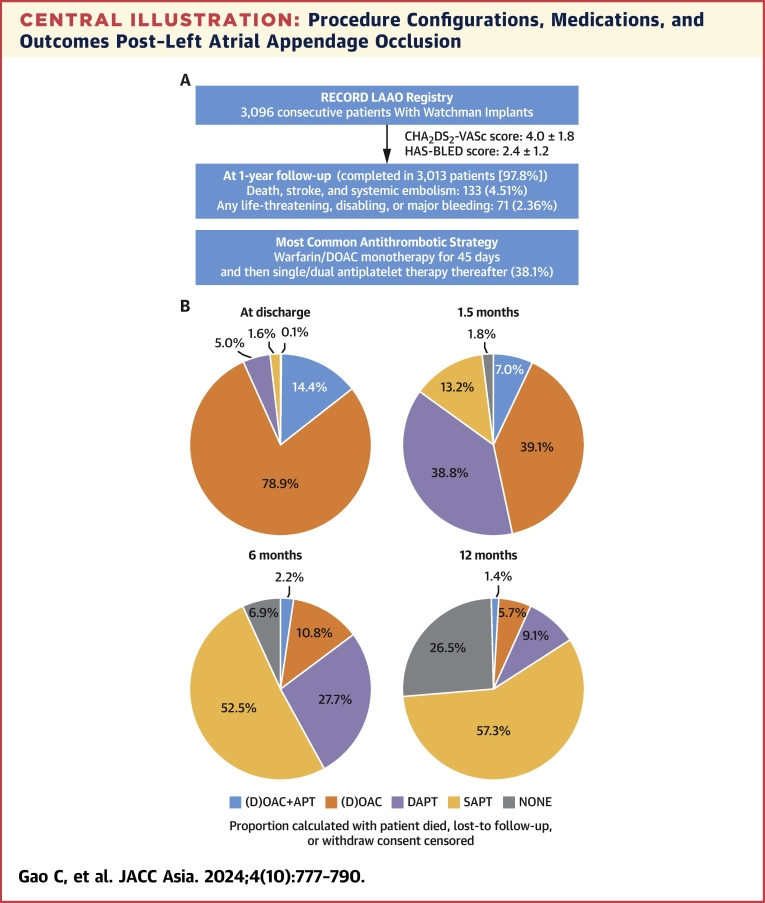
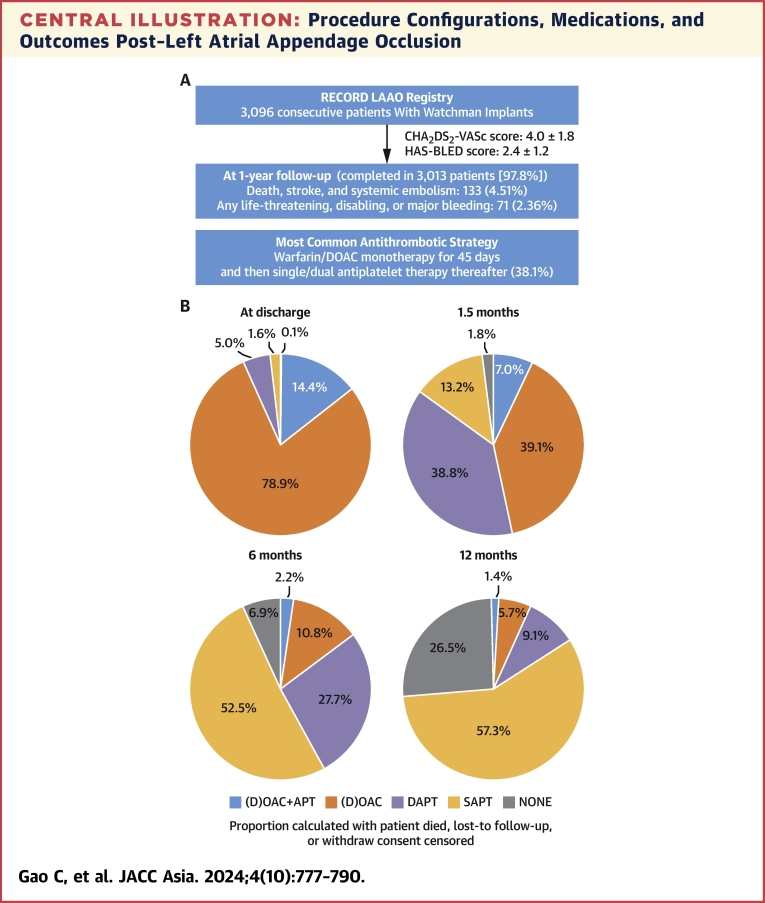
Abstract
Background
The clinical performance of left atrial appendage occlusion (LAAO) as a procedure and the long-term impact of its varied implantation configurations and anticoagulation regimens remain unclear.
Objectives
This study sought to provide data in routine practice from a prospective multicenter registry.
Methods
A total of 3,096 consecutive patients from 39 Chinese centers undergoing LAAO were enrolled between April 1, 2019, and October 31, 2020.
Results
The baseline CHA2DS2-VASc and HAS-BLED scores were 4.0 ± 1.8 and 2.4 ± 1.2, respectively; mean age was 69 ± 9 years. One-year follow-up was completed in 3,013 (97.8%) patients. The ischemic endpoint of death, stroke, and systemic embolism occurred in 133 (4.51%) patients, and life-threatening, disabling, or major bleeding occurred in 71 (2.36%) patients. After inverse probability of treatment weighting, no significant association was found between anesthesia type (moderate sedation vs general anesthesia) or image guidance (transesophageal/intracardiac echocardiography vs fluoroscopy) and ischemic or bleeding events. In 1,295 (42.0%) cases, LAAO combined with catheter ablation was associated with a significantly lower rate of death, stroke, or systemic embolism than LAAO only (3.5% vs 5.2%, inverse probability of treatment weighting HR: 0.68; 95% CI: 0.47-0.99). The most common post-LAAO antithrombotic regimen was warfarin/direct oral anticoagulant monotherapy for 45 days, followed by single-/dual-antiplatelet therapy (38.1%).
Conclusions
In Chinese centers, patients undergoing LAAO had low rates of ischemic and bleeding events at 1 year. Combining LAAO with catheter ablation was associated with a lower rate of ischemic events than LAAO only. (Registry to Evaluate Chinese Real-World Clinical Outcomes in Patients With Atrial Fibrillation Using the Watchman Left Atrial Appendage Closure Technology [RECORD]; NCT03917563)
Key Words: 1-year outcomes, antithrombotic strategies, left atrial appendage occlusion, procedural configurations
Central Illustration
Percutaneous left atrial appendage occlusion (LAAO) is a nonpharmacologic stroke prevention strategy for patients with nonvalvular atrial fibrillation (AF) who have contraindications or are unsuitable for long-term oral anticoagulation (OAC).1,2 However, the pivotal randomized controlled trials (RCT)3, 4, 5 and registries6, 7, 8, 9, 10, 11 investigating LAAO were mostly conducted in U.S. or European sites and primarily enrolled White populations (between 92% and 94%). The RECORD (Registry to Evaluate Chinese Real-World Clinical Outcomes in Patients With Atrial Fibrillation Using the Watchman Left Atrial Appendage Closure Technology; NCT03917563) was a real-world cohort study that enrolled approximately 3,000 Chinese patients, documenting the safety and efficacy profiles of LAAO as a procedure. We have reported that, at 30 days, the periprocedural rate of death, stroke, and systemic embolism was 0.5%.12
According to expert consensus on percutaneous LAAO, transesophageal echocardiography (TEE) or intracardiac echocardiography (ICE) is recommended as the standard imaging guidance.13 However, LAAO planning and guidance14,15 have evolved over time, with a greater understanding of the complex and variable anatomy of the left atrial appendage and how imaging should guide intervention. In the 30-day report of RECORD,12 we observed that LAAO was guided by fluoroscopy alone in 16.0% of cases. In addition, we also found that 42.0% underwent LAAO combined with radiofrequency ablation or cryoablation for AF (combined procedure treatment), despite the recent Society for Cardiovascular Angiography and Interventions/Heart Rhythm Society Expert Consensus Statement on LAAO,16 which suggests that combined procedures of LAAO with structural interventions or pulmonary vein isolation should not be routinely recommended because of a lack of evidence. The long-term clinical performance of LAAO as a procedure and the impact of these varied procedural configurations and treatment strategies remain debated and require further documentation.
The RCTs3,4 have stipulated the antithrombotic regimens post-LAAO and were subsequently supported by guidelines.2 Briefly, patients undergoing LAAO were discharged on warfarin and aspirin for 45 days; if there was no leak of >5 mm, antithrombotic strategies could switch to dual-antiplatelet therapy (DAPT) for 6 months, followed by aspirin thereafter. Although more than one-half of LAAO patients (57.7%) in the United States were discharged with aspirin plus (direct) OAC ([D]OAC)17 and most patients (73%) in Europe were prescribed without (D)OAC,8 RECORD observed substantial deviations from the standardized protocols: 78.9% of Chinese patients received (D)OAC monotherapy in the initial 45 days. Whether these varied regimens are associated with different long-term outcomes is unclear.
To address these gaps in knowledge, in the current study, we report the 1-year clinical outcomes of RECORD, explored their associations with procedural techniques, and investigated the anticoagulation patterns at discharge, 45 days, 6 months, and 12 months.
Methods
Study population
The study outline has been previously described in detail.12 In brief, RECORD (NCT03917563) was a multicenter, prospective cohort study that included 3,096 patients from 39 centers in China, conducted between April 1, 2019, and October 31, 2020. Consecutive patients from each participating center who received the left atrial appendage (LAA) closure device (Watchman generation 2.5) and were of legal age to provide informed consent were recruited in this registry. Patients who were participating in other trials, declined to provide informed consent, or refused to participate were excluded. Peri- and intraprocedural techniques and postprocedural medications were left to the operator’s discretion. A total of 159 operators, with varying levels of experience implanting the device, participated in the study.
This cohort study adhered to the international standards for scientific research and the principles of the Declaration of Helsinki. Central (Ethics Committee of Xijing Hospital) or local Ethics Committee approval was obtained in all participating centers. All participants provided informed consent before the procedure.
Outcomes
Events, including any death, stroke, systemic embolism (SE), transient ischemic attack, readmission, device-related thrombosis, peridevice leak, and bleeding events, were collected. The rates of device/technical/procedural success, periprocedural complications, and adverse events at 30 days post-LAAO have been reported previously.12 Death, stroke, SE, and bleeding were adjudicated by an independent Clinical Event Committee comprising 5 physicians with expertise in electrophysiology and/or interventional cardiology. The adjudication of events was based on the definitions outlined in the consensus document on percutaneous LAAO: the Munich consensus document on definitions, endpoints, and data collection requirements for clinical studies.18 Bleeding was evaluated by both the Munich consensus document criteria18 and the Bleeding Academic Research Consortium criteria.19 Anticoagulation patterns were recorded at discharge, 45 days, 6 months, and 12 months post-LAAO.
Follow-Up
Follow-up visits were scheduled at 45 days (±14 days), 6 months (±30 days), and 12 (±30 days) months after the LAAO procedure. These visits were preferably conducted onsite; however, if patients were unable or unwilling to visit the outpatient clinic, the scheduled visit could be conducted via telephone, with the exception of the 45-day and 1-year visits. Similar to the previous study,8 the timing of LAA imaging follow-up was recommended at 45-day and/or 12-month visits, based on each institution’s standard practice.
Statistical analysis
The sample size was determined by the objective of consecutively enrolling all available cases at each participating center during the 18-month study enrollment course to provide sufficiently precise estimates of rare adverse events, rather than based on power requirements for a formal hypothesis test. The initial sample size was set at 1,050. However, this target was quickly achieved, and the Steering Committee then decided to continue enrollment until the planned study period concluded. Eventually, 3,086 participants were enrolled.
Continuous variables with normal distribution are expressed as the mean ± SD and were compared using Student’s t-test, and those with a skewed distribution are described as the median (IQR) and were assessed by the Wilcoxon rank sum test. Categorical data are presented as absolute numbers and proportions and were compared using the Fisher exact test. All reported outcomes were based the first occurrence of the event and were estimated using the Kaplan-Meier method. Cox proportional hazards regression models were used to estimate HRs for each outcome at the specified timepoints. The proportional hazards assumption was checked using Schoenfeld residuals and visual assessment of log(−log) plots, and the assumption was met in all models. To reduce confounding bias related to baseline characteristics when comparing procedural configurations, 2 statistical approaches were applied: multivariable adjustment and inverse probability of treatment weighting (IPTW). The variables included in the multivariable adjustment or IPTW are provided in the Supplemental Appendix. There was no formal correction for multiple testing, taking into account the observational nature of the study.20 Analyses were performed using SAS version 9.4 (SAS Institute) and R project version 4.1 (R Foundation). A 2-sided P value of <0.05 was considered statistically significant.
Results
Baseline characteristics
From April 1, 2019, to October 31, 2020, a total of 3,569 consecutive patients planned for LAAO were screened across the 39 participating centers. After applying exclusions (Supplemental Figure 1), 3,096 participants were enrolled in the study. Of these, 3,082 participants successfully received an LAAO implant, and 3,013 (97.8%) completed the 1-year follow-up (2,778 through clinic visits and 235 through phone contact). Among them, 1,831 (65.9%) underwent computed tomography angiography (CTA) or TEE examinations.
Baseline demographics are summarized in Table 1, categorized by the occurrence of death, stroke, and systemic embolism (SE). The mean age of participants was 69.1 ± 9.4 years, and 42.5% were female. The mean CHA2DS2-VASc score was 4.0 ± 1.8, and the mean HAS-BLED score was 2.4 ± 1.2. Procedures were performed under moderate sedation in 41.6% of cases. Intraprocedure image guidance was performed using TEE in 81.0% of cases, fluoroscopy alone in 16.0%, and ICE in 3.1%. In 42.0% of cases, the percutaneous LAAO was combined with 1-stage radiofrequency ablation or cryoablation.
Table 1.
Baseline Characteristics
| Total (N = 3,082) | Death, Stroke, and SE (–) (n = 2,949) |
Death, Stroke, and SE (+) (n = 133) |
P Value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, y | 69.1 ± 9.4 | 68.93 ± 9.3 | 73.26 ± 9.2 | <0.001 |
| ≥75 y | 908 (29.5) | 842 (28.6) | 66 (49.6) | <0.001 |
| Male | 1,771 (57.5) | 1,689 (57.3) | 82 (61.7) | 0.363 |
| Body mass index, kg/m2 | 24.83 ± 3.5 | 24.85 ± 3.5 | 24.34 ± 4.0 | 0.122 |
| Heart rate, beats/min | 81.91 ± 20.4 | 81.85 ± 20.4 | 83.35 ± 22.2 | 0.408 |
| Diabetes | 714 (23.2) | 669 (22.7) | 45 (33.8) | 0.004 |
| Previous stroke or TIA | 1,411 (45.8) | 1,331 (45.1) | 80 (60.2) | 0.001 |
| Ischemic stroke or TIA | 1,373 (44.5) | 1,297 (44.0) | 76 (57.1) | 0.004 |
| Hemorrhagic stroke | 106 (3.4) | 101 (3.4) | 5 (3.8) | 0.524 |
| Hypertension | 2,120 (68.8) | 2,012 (68.2) | 108 (81.2) | 0.007 |
| Coronary artery disease | 875 (28.4) | 833 (28.2) | 42 (31.6) | 0.464 |
| Previous PCI | 326 (10.6) | 303 (10.3) | 23 (17.3) | 0.023 |
| Previous CABG | 46 (1.5) | 41 (1.4) | 5 (3.8) | 0.066 |
| Vascular diseasea | 1,674 (54.3) | 1,589 (53.9) | 85 (63.9) | 0.029 |
| Current smoker | 333 (10.8) | 318 (10.8) | 15 (11.3) | 0.923 |
| Alcohol abuse | 168 (5.5) | 163 (5.5) | 5 (3.8) | 0.494 |
| Chronic heart failure | 462 (15.0) | 429 (14.5) | 33 (24.8) | 0.002 |
| LVEF, % | 60.05 ± 8.3 | 60.12 ± 8.3 | 58.53 ± 9.6 | 0.040 |
| Abnormal thyroidal function | 132 (4.3) | 132 (4.5) | 0 (0.0) | 0.057 |
| Abnormal renal function | 72 (2.3) | 61 (2.1) | 11 (8.3) | <0.001 |
| Abnormal liver function | 51 (1.7) | 46 (1.6) | 5 (3.8) | 0.110 |
| Bleeding history or predispositionb | 313 (10.2) | 293 (9.9) | 20 (15.0) | 0.079 |
| Concomitant use of drugs | 1,034 (33.5) | 975 (33.1) | 59 (44.4) | 0.009 |
| Classification of AF | 0.743 | |||
| Paroxysmal | 1,245 (40.4) | 1,196 (40.6) | 49 (36.8) | |
| Persistent | 1,270 (41.2) | 1,211 (41.1) | 59 (44.4) | |
| Long-standing persistent (>1 y)/permanent | 567 (18.4) | 542 (18.4) | 25 (18.8) | |
| CHADS2 score | 2.3 ± 1.4 | 2.3 ± 1.4 | 3.1 ± 1.4 | <0.001 |
| CHA2DS2-VASc score | 4.0 ± 1.8 | 3.9 ± 1.8 | 4.9 ± 1.8 | <0.001 |
| HAS-BLED score | 2.4 ± 1.2 | 2.4 ± 1.2 | 3.1 ± 1.1 | <0.001 |
| ATRIA score | 6.2 ± 3.0 | 6.2 ± 3.0 | 7.6 ± 2.5 | <0.001 |
| Procedural characteristics | ||||
| Recaptured (≥2 times) before release | 252 (8.2) | 240 (8.1) | 12 (9.0) | 0.840 |
| Devices used | 3,187 (1.03 per patient) | 3,053 (1.04 per patient) | 134 (1.01 per patient) | 0.003 |
| Device size, mm | ||||
| 21 | 188 (6.1) | 180 (6.1) | 8 (6.0) | |
| 24 | 634 (20.6) | 615 (20.9) | 19 (14.3) | |
| 27 | 918 (29.8) | 877 (29.7) | 41 (30.8) | |
| 30 | 714 (23.2) | 679 (23.0) | 35 (26.3) | |
| 33 | 633 (20.5) | 603 (20.4) | 30 (22.6) | |
| Anesthesia | 0.111 | |||
| General anesthesia | 1,799 (58.4) | 1,712 (58.1) | 87 (65.4) | |
| Moderate sedation | 1,283 (41.6) | 1,237 (41.9) | 46 (34.6) | |
| Imaging guidance | 0.759 | |||
| TEE/ICE | 2,590 (84.0) | 2,480 (84.1) | 110 (82.7) | |
| Fluoroscopy | 492 (16.0) | 469 (15.9) | 23 (17.3) | |
| Combined procedures | ||||
| Radiofrequency ablation/cryoablation | 1,295 (42.0) | 1,251 (42.4) | 44 (33.1) | 0.041 |
| Cryoablation | 350 (11.4) | 337 (11.4) | 13 (9.8) | |
| Radiofrequency ablation | 945 (30.7) | 914 (31.0) | 31 (23.3) | |
| Othersc | 154 (5.0) | 150 (5.1) | 4 (3.0) | 0.383 |
Values are mean ± SD, or n (%). N = 3,082 except for body mass index (n = 2,842), LVEF (n = 2,774), CHADS2 score (n = 3,065), CHA2DS2-VASc score (n = 3,065), HAS-BLED score (n = 3,068), and ATRIA score (n = 3,068).
AF = atrial fibrillation; CABG = coronary artery bypass graft; ICE = intracardiac echocardiography; LVEF = left ventricular ejection fraction; PCI = percutaneous coronary intervention; SE = systemic embolism; TEE = transesophageal echocardiography; TIA = transient ischemic attack.
Vascular disease includes previous myocardial infarction, peripheral artery disease, or aortic plaque, defined according to the CHA2DS2-VASc score.
Bleeding history or predisposition includes previous major hemorrhage or anemia or severe thrombocytopenia, defined according to the HAS-BLED score.
Others included atrial septal defect/patent foramen ovale occlusion (n = 78), percutaneous coronary intervention/percutaneous transluminal coronary angioplasty (n = 54), pacemaker implantation (n = 8), percutaneous mitral valvuloplasty (n = 5), transcatheter aortic valve replacement (n = 2), femoral artery stent implantation (n = 1), implantation of vena cava filter (n = 1), splenic artery angiography (n = 1), renal angiography (n = 1), radiofrequency ablation of supraventricular tachycardia (n = 1), and electrocardiogram event recorder implantation (n = 1).
Clinical outcomes at 1-year follow-up
At 1 year, the composite endpoint of death, stroke, and systemic embolism occurred in 133 (4.51%) patients, consisting of 68 (2.31%) deaths, 52 (1.79%) ischemic strokes, 24 (0.82%) hemorrhagic strokes, and 4 (0.13%) systemic embolisms (Table 2). Device-related thrombus (DRT) occurred in 45 (2.48%) patients. Complete sealing was achieved in 75.5% of patients (Supplemental Table 1). The results of subgroup analyses according to demographic risk factors are shown in Supplemental Figure 2. At the 1-year follow-up, the AF recurrence rate was 27.5% in patients who had undergone 1-stage radiofrequency ablation or cryoablation.
Table 2.
Clinical Events at 1 Year After LAAO
| Events | Event Rates (95% CI) | |
|---|---|---|
| Death, stroke, systemic embolism | 133/3,082 | 4.51 (3.76-5.26) |
| Death; stroke; systemic embolism; any life-threatening, disabling, or major bleeding | 177/3,082 | 5.95 (5.09-6.80) |
| Death; stroke; systemic embolism; BARC 2, 3, or 5 bleeding | 221/3,082 | 7.42 (6.47-8.36) |
| Individual components | ||
| Death | 68/3,082 | 2.31 (1.76-2.85) |
| Cardiovascular death | 61/3,082 | 2.08 (1.56-2.60) |
| Undetermined death | 32/3,082 | 1.12 (0.73-1.50) |
| Noncardiovascular death | 7/3,082 | 0.23 (0.06-0.40) |
| Stroke | 74/3,082 | 2.54 (1.96-3.11) |
| Ischemic stroke | 52/3,082 | 1.79 (1.30-2.28) |
| Hemorrhagic stroke | 24/3,082 | 0.82 (0.49-1.15) |
| TIA | 10/3,082 | 0.34 (0.13-0.54) |
| Systemic embolism | 4/3,082 | 0.13 (0.00-0.26) |
| Readmission | 954/3,082 | 31.84 (30.15-33.49) |
| Device-related thrombus | 45/1,831 | 2.48 (1.76-3.19) |
| Incomplete sealing | 449/1,831 | 24.52 (22.57-26.56) |
| Peridevice leak of 0-5 mm | 439/1,831 | 23.98 (22.08-25.99) |
| Peridevice leak of ≥5 mm | 10/1,831 | 0.55 (0.30-1.04) |
| Bleeding | ||
| LAAO Munich consensus classification | ||
| Any life-threatening, disabling, or major bleeding | 71/3,082 | 2.36 (1.81-2.90) |
| Life-threatening or disabling bleeding | 32/3,082 | 1.09 (0.71-1.46) |
| Major bleeding | 39/3,082 | 1.27 (0.87-1.67) |
| Minor bleeding | 47/3,082 | 1.60 (1.15-2.06) |
| BARC classification | ||
| Type 2, 3, or 5 | 117/3,082 | 3.92 (3.22-4.62) |
| Type 3 or 5 | 48/3,082 | 1.61 (1.16-2.06) |
| Type 5 | 12/3,082 | 0.41 (0.18-0.65) |
| Type 3 | 36/3,082 | 1.20 (0.81-1.59) |
| Type 3a | 4/3,082 | 0.13 (0.00-0.27) |
| Type 3b | 12/3,082 | 0.39 (0.17-0.61) |
| Type 3c | 20/3,082 | 0.68 (0.38-0.97) |
| Type 2 | 69/3,082 | 2.32 (1.77-2.86) |
Event rates were provided based on Kaplan-Meier estimates. Patients who did not complete the 1-year follow-up were censored at the last contact.
BARC = Bleeding Academic Research Consortium; LAAO = left atrial appendage occlusion; TIA = transient ischemic attacks.
Impacts of the variable components of procedural performance
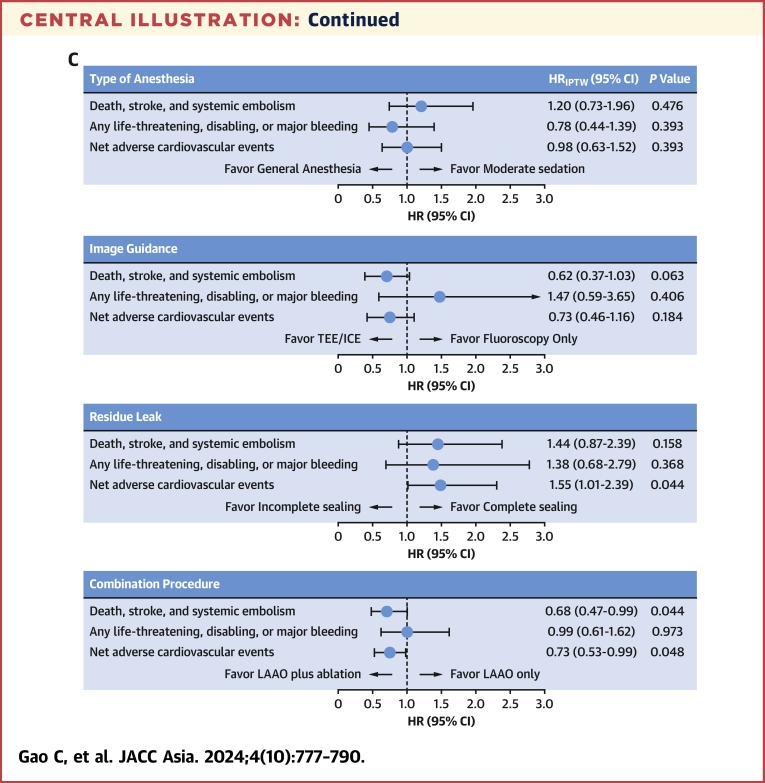
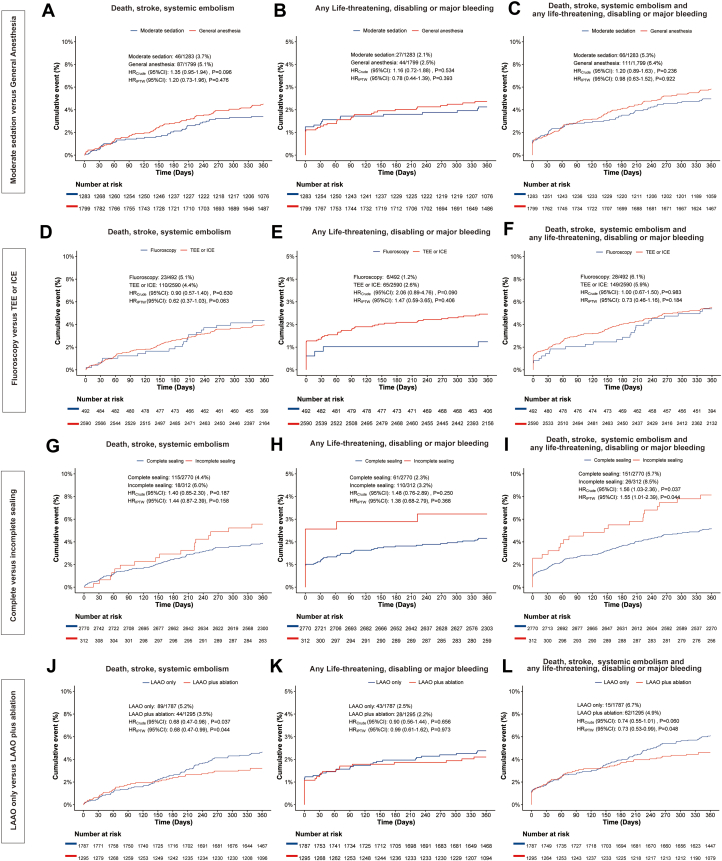
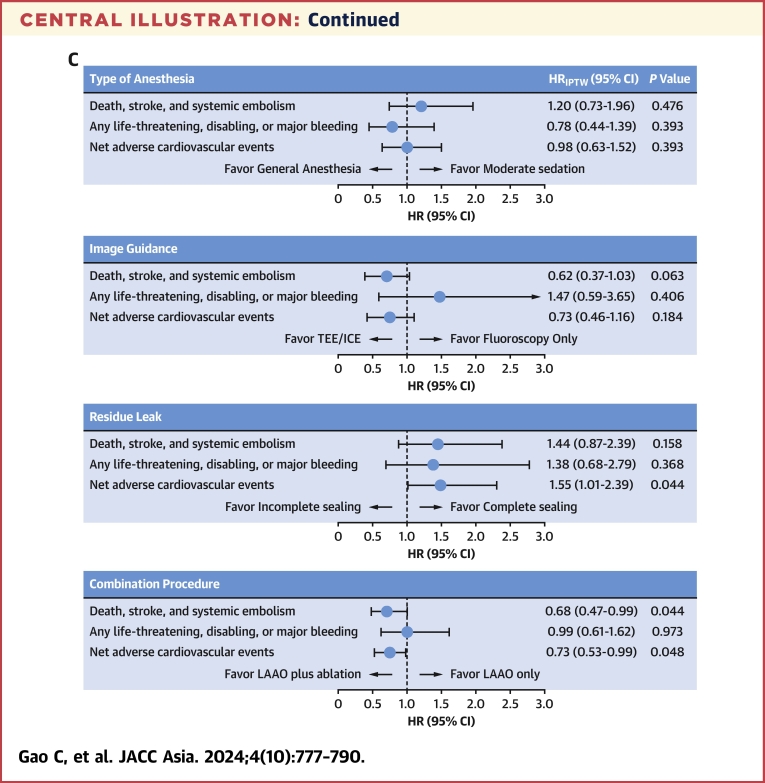
The baseline characteristics according to procedural configurations of LAAO are provided in Supplemental Table 2, and the impact of these procedural configurations on 1-year clinical outcomes are shown in Table 3, Figure 1, and the Central Illustration. There was no significant difference between general anesthesia or moderate sedation or between imaging guidance by fluoroscopy or TEE/ICE regarding the ischemic endpoint of death, stroke, or SE; or the bleeding endpoint of any life-threatening, disabling, or major bleeding; or the net adverse event by combining the ischemic and bleeding outcomes (Figures 1A to 1F). Compared with site-reported complete sealing of the LAA during the procedure, incomplete sealing was associated with a higher risk of the net adverse event, including death; stroke; SE; or life-threatening, disabling, or major bleeding (5.7% vs 8.5%; HRIPTW: 1.55; 95% CI: 1.01-2.39; P = 0.044) (Figure 1I).
Table 3.
The 1-Year Clinical Outcomes According to Procedural Configurations
| Events /Total (%) | Crude |
Multivariable Adjusted |
IPTW Adjusted |
||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Death, stroke, and systemic embolism | |||||||
| Moderate sedation | 46/1,283 (3.7) | Ref | Ref | Ref | |||
| General anesthesia | 87/1,799 (5.1) | 1.35 (0.95-1.94) | 0.096 | 1.32 (0.92-1.88) | 0.133 | 1.20 (0.73-1.96) | 0.476 |
| Fluoroscopy | 23/492 (5.1) | Ref | Ref | Ref | |||
| TEE/ICE | 110/2,590 (4.4) | 0.90 (0.57-1.40) | 0.630 | 0.83 (0.53-1.31) | 0.423 | 0.62 (0.37-1.03) | 0.063 |
| Complete sealing | 115/2,770 (4.4) | Ref | Ref | Ref | |||
| Incomplete sealing | 18/312 (6.0) | 1.40 (0.85-2.30) | 0.187 | 1.36 (0.83-2.24) | 0.223 | 1.44 (0.87-2.39) | 0.158 |
| LAAO only | 89/1,787 (5.2) | Ref | Ref | Ref | |||
| LAAO plus ablation | 44/1,295 (3.5) | 0.68 (0.47-0.98) | 0.037 | 0.67 (0.47-0.97) | 0.032 | 0.68 (0.47-0.99) | 0.044 |
| Any life-threatening, disabling, or major bleeding | |||||||
| Moderate sedation | 27/1,283 (2.1) | Ref | Ref | Ref | |||
| General anesthesia | 44/1,799 (2.5) | 1.16 (0.72-1.88) | 0.534 | 1.16 (0.72-1.87) | 0.545 | 0.78 (0.44-1.39) | 0.393 |
| Fluoroscopy | 6/492 (1.2) | Ref | Ref | Ref | |||
| TEE/ICE | 65/2,590 (2.6) | 2.06 (0.89-4.76) | 0.090 | 1.94 (0.84-4.50) | 0.121 | 1.47 (0.59-3.65) | 0.406 |
| Complete sealing | 61/2,770 (2.3) | Ref | Ref | Ref | |||
| Incomplete sealing | 10/312 (3.2) | 1.48 (0.76-2.89) | 0.250 | 1.50 (0.77-2.92) | 0.237 | 1.38 (0.68-2.79) | 0.368 |
| LAAO only | 43/1,787 (2.5) | Ref | Ref | Ref | |||
| LAAO plus ablation | 28/1,295 (2.2) | 0.90 (0.56-1.44) | 0.656 | 0.88 (0.55-1.43) | 0.613 | 0.99 (0.61-1.62) | 0.973 |
| Death; stroke; systemic embolism; any life-threatening, disabling, or major bleeding | |||||||
| Moderate sedation | 66/1,283 (5.3) | Ref | Ref | Ref | |||
| General anesthesia | 111/1,799 (6.4) | 1.20 (0.89-1.63) | 0.236 | 1.18 (0.87-1.61) | 0.277 | 0.98 (0.63-1.52) | 0.922 |
| Fluoroscopy | 28/492 (6.1) | Ref | Ref | Ref | |||
| TEE/ICE | 149/2,590 (5.9) | 1.00 (0.67-1.50) | 0.983 | 0.93 (0.62-1.4) | 0.739 | 0.73 (0.46-1.16) | 0.184 |
| Complete sealing | 151/2,770 (5.7) | Ref | Ref | Ref | |||
| Incomplete sealing | 26/312 (8.5) | 1.56 (1.03-2.36) | 0.037 | 1.55 (1.02-2.35) | 0.040 | 1.55 (1.01-2.39) | 0.044 |
| LAAO only | 115/1,787 (6.7) | Ref | Ref | Ref | |||
| LAAO plus ablation | 62/1,295 (4.9) | 0.74 (0.55-1.01) | 0.060 | 0.72 (0.53-0.99) | 0.043 | 0.73 (0.53-0.99) | 0.048 |
ICE = intracardiac echocardiography; IPTW = inverse probability of treatment weighting; LAAO = left atrial appendage occlusion; Ref = reference; TEE = transesophageal echocardiography.
Figure 1.
Impacts of the Variable Components of Procedural Performance
Kaplan-Meier curves showing the impact of various procedural components: (A to C) moderate sedation vs general anesthesia, (D to F) fluoroscopy vs TEE/ICE, (G to I) complete vs incomplete sealing, and (J to L) LAAO only vs LAAO plus ablation. These curves illustrate the composite endpoint of death, stroke, and systemic embolism; the endpoint of any life-threatening or major bleeding; or the composite endpoint of death, stroke, and systemic embolism and any life-threatening or major bleeding. ICE = intracardiac echocardiography; LAAO = left atrial appendage occlusion; TEE = transesophageal echocardiography.
Central Illustration.
Procedure Configurations, Medications, and Outcomes Post–Left Atrial Appendage Occlusion
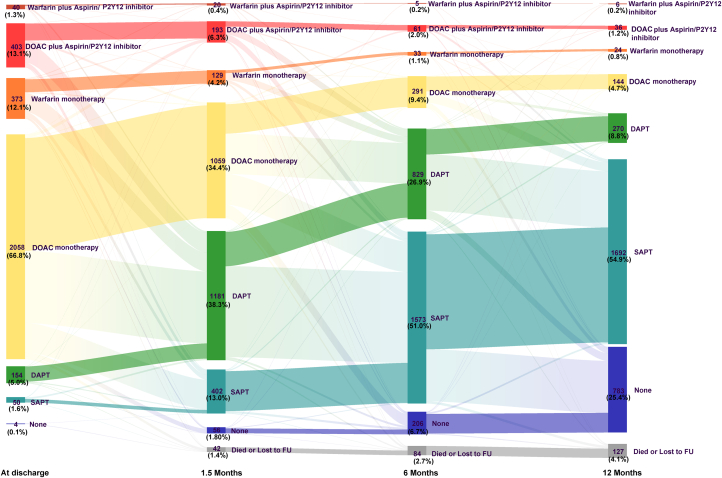
(A) Brief study flow chart. A total of 3,096 patients were included in RECORD (Registry to Evaluate Chinese Real-World Clinical Outcomes in Patients With Atrial Fibrillation Using the Watchman Left Atrial Appendage Closure Technology), and 97.8% completed the 1-year follow-up. The cumulative event rate of death, stroke, or systemic embolism occurred in 4.5% of patients at 1 year. (B) Pie charts showing the type of antithrombotic medication of patients at discharge and 1.5, 6, and 12 months postprocedure. Warfarin or DOAC monotherapy was used in ∼80% of patients at discharge, and single-antiplatelet therapy was used in ∼60% of patients at 12 months postprocedure. (C) The impact of the periprocedural configurations is shown by the forest plot. Although the type of anesthesia or the type of imaging guidance had nonsignificant impact on the prognosis of patients at 12 months postprocedure, complete sealing (in comparison with incomplete sealing) and LAAO in combination with catheter ablation (in comparison with LAAO only) were both associated with lower risk of net adverse cardiovascular events (a composite endpoint including death; stroke; systemic embolism; and any life-threatening, disabling, or major bleeding). APT = antiplatelet therapy; DAPT = dual antiplatelet therapy; (D)OAC = (direct) oral anticoagulants; FU = follow-up; ICE = intracardiac echocardiography; IPW = inverse probability of treatment weighting; LAAO = left atrial appendage occlusion; SAPT = single-antiplatelet therapy; TEE = transesophageal echocardiography.
In addition, we found that the Kaplan-Meier curve, which showed the cumulative event rate of death, stroke, or SE, began to diverge at 6 months between the strategies of combining LAAO with catheter ablation (CA) and performing LAAO only. The combined procedure strategy was associated with a significantly lower rate of death, stroke, or SE at 1 year (3.5% vs 5.2%; HRIPTW: 0.68; 95% CI: 0.47-0.99; P = 0.044) (Figure 1J). Exploratory analyses showed that LAAO plus CA in patients with AF diagnosed within 1 year (AF ≤1 year) was associated with the lowest rates of death, stroke, and SE compared with LAAO plus CA in the AF > 1-year group and LAAO only group (3.1% vs 4.0% vs 5.2%; HRadjusted vs LAAO plus CA in AF >1 year: 1.26; 95% CI: 0.69-2.28; P = 0.452; HRadjusted vs LAAO only: 1.66; 95% CI:1.01-2.71; P = 0.044) (Supplemental Table 3, Supplemental Figure 3).
Compared with the absence of DRT, the presence of DRT was associated with a 3.7-fold increase in the risk of death, stroke, and SE (11.4% vs 2.9%; HRadjusted: 3.72; 95% CI: 1.46-9.46; P = 0.006) (Supplemental Table 4, Supplemental Figure 4). The temporal relationship between DRT and the occurrence of death; stroke; SE; and any life-threatening, disabling, or major bleeding events is illustrated in Supplemental Figure 5.
Antithrombotic medications
Antithrombotic medication regimens varied among patients (Figure 2, Central Illustration). We identified 5 major patterns (mutually exclusive categories, accounting for 87.9% of the total population), presented in ascending order of the mean CHA2DS2-VASc score for each group (Table 4). The baseline characteristics of these groups are shown in Supplemental Table 5. The most common strategy (38.1%) was OAC | APT | APT, which corresponds to being discharged on warfarin or DOAC, switching to single-antiplatelet therapy or DAPT at 45 days, and continuing this regimen at the 6-month follow-up. The unadjusted rate of death; stroke; SE; or any life-threatening, disabling, or major bleeding was lowest among those treated with OAC | APT | APT (3.5%), followed by OAC + APT | APT | APT (4.5%), OAC | OAC | APT (5.0%), OAC | OAC | OAC (12.0%), and APT | APT | APT (12.3%).
Figure 2.
Medication Strategies From the Index LAAO Procedure to 1-Year Follow-Up
Sankey diagram showing the post-LAAO medication strategies. DAPT = dual-antiplatelet therapy; DOAC = direct oral anticoagulation; FU = follow-up; LAAO = left atrial appendage occlusion; SAPT = single-antiplatelet therapy.
Table 4.
The 1-Year Clinical Outcomes According to Post-LAAO Medications (Mutually Exclusive Categories)
| OAC | APT | APT (n = 1,176; 38.1%) |
OAC | OAC | APT (n = 832; 27.0%) | OAC + APT | APT | APT (n = 202; 6.6%) | OAC | OAC | OAC (n = 348; 11.3%) | APT | APT | APT (n = 150; 4.9%) | Others (n = 374; 12.1%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| OAC | DAPT | DAPT (n = 191; 6.2%) | OAC | SAPT | SAPT (n = 352; 11.4%) | OAC | DAPT | SAPT (n = 633; 20.5%) | Total | ||||||
| CHA2DS2-VASc score | 3.73 ± 1.81 | 3.76 ± 1.80 | 3.87 ± 1.75 | 3.81 ± 1.78 | 3.92 ± 1.77 | 4.16 ± 1.94 | 4.27 ± 1.82 | 4.29 ± 1.80 | 4.07 ± 1.81 |
| HAS-BLED score | 2.24 ± 1.18 | 2.26 ± 1.12 | 2.27 ± 1.14 | 2.26 ± 1.14 | 2.31 ± 1.13 | 2.56 ± 1.28 | 2.51 ± 1.11 | 2.82 ± 1.19 | 2.67 ± 1.14 |
| ATRIA score | 5.73 ± 3.01 | 5.98 ± 2.98 | 6.04 ± 3.03 | 5.97 ± 3.01 | 6.24 ± 2.97 | 6.10 ± 2.90 | 6.72 ± 2.82 | 7.02 ± 2.69 | 6.23 ± 2.85 |
| Total | |||||||||
| Death, stroke, systemic embolism | 6/191 (3.2) | 13/352 (3.9) | 14/633 (2.3) | 33/1,176 (2.9) | 23/832 (3.0) | 6/202 (3.0) | 35/348 (10.8) | 12/150 (8.3) | 24/374 (6.6) |
| Life-threatening, disabling, or major bleeding | 2/191 (1.0) | 7/352 (2.0) | 5/633 (0.8) | 14/1,176 (1.2) | 20/832 (2.5) | 5/202 (2.5) | 12/348 (3.8) | 8/150 (5.4) | 12/374 (3.3) |
| Death; stroke; systemic embolism; and any life-threatening, disabling, or major bleeding | 7/191 (3.7) | 16/352 (4.8) | 17/633 (2.7) | 40/1,176 (3.5) | 40/832 (5.0) | 9/202 (4.5) | 39/348 (12.0) | 18/150 (12.3) | 31/374 (8.5) |
| HAS-BLED score <3 | |||||||||
| Death, stroke, systemic embolism | 3/119 (2.6) | 6/208 (2.9) | 3/377 (0.8) | 12/704 (1.7) | 8/481 (1.8) | 3/97 (3.1) | 12/181 (7.2) | 0/60 (0.0) | 5/168 (3.2) |
| Life-threatening, disabling, or major bleeding | 1/119 (0.8) | 4/208 (1.9) | 4/377 (1.1) | 9/704 (1.3) | 11/481 (2.4 | 0/97 (0.0) | 3/181 (1.7) | 4/60 (6.7) | 3/168 (1.8) |
| Death; stroke; systemic embolism; and any life-threatening, disabling, or major bleeding | 4/119 (3.4) | 7/208 (3.4) | 6/377 (1.6) | 17/704 (2.4) | 18/481 (3.9) | 3/97 (3.1) | 13/181 (7.7) | 4/60 (6.7) | 8/168 (5.0) |
| HAS-BLED score ≥3 | |||||||||
| Death, stroke, systemic embolism | 3/72 (4.3) | 7/144 (5.3) | 11/256 (4.4) | 21/472 (4.7) | 15/351 (4.5) | 3/105 (2.9) | 23/167 (14.8) | 12/90 (13.7) | 19/206 (9.4) |
| Life-threatening, disabling, or major bleeding | 1/72 (1.4) | 3/144 (2.1) | 1/256 (0.4) | 5/472 (1.1) | 9/351 (2.6) | 5/105 (4.8) | 9/167 (6.0) | 4/90 (4.6) | 9/206 (4.5) |
| Death; stroke; systemic embolism; and any life-threatening, disabling, or major bleeding | 3/72 (4.2) | 9/144 (6.6) | 11/256 (4.4) | 23/472 (5.1) | 22/351 (6.5) | 6/105 (5.8) | 26/167 (16.6) | 14/90 (16.0) | 23/206 (11.3) |
Values are mean ± SD or n/N (%).
APT = antiplatelet therapy; DAPT = dual-antiplatelet therapy; LAAO = left atrial appendage occlusion; OAC = oral anticoagulant; SAPT = single-antiplatelet therapy.
Only 5.1% of patients received post-LAAO antithrombotic medications following the recommendations of the European Society of Cardiology AF guideline. Exploratory analyses showed no significant difference between the OAC | APT | APT and OAC + APT | APT | APT groups regarding ischemic events. However, in patients with a HAS-BLED of ≥3, the OAC | APT | APT regimen was associated with lower rates of life-threatening, disabling, or major bleeding events (1.1% vs 4.8%; HRadjusted: 4.55; 95% CI: 1.31-15.81; P = 0.017) (Supplemental Table 6). Compared with OAC | APT | APT, the OAC | OAC | APT regimen was associated with a higher risk of life-threatening, disabling, or major bleeding (1.2% vs 2.5%; HRadjusted: 0.50; 95% CI: 0.25-0.99; P = 0.047), whereas the risk of death, stroke, and SE was numerically similar (2.9% vs 3.0%; HRadjusted: 1.05; 95% CI: 0.61-1.78; P = 0.867) (Supplemental Table 7). The patterns of antithrombotic medication, stratified by the presence or absence of coronary artery disease, are shown in Supplemental Figure 6.
Discussion
In the real world, LAAO planning, guidance, and postprocedure antithrombotic medication strategies have evolved over time, often outpacing the guidelines21; however, supporting evidence remains limited. To the best of our knowledge, our study represents the largest real-world cohort to date investigating the impact of implantation configurations and post-LAAO medication regimens. The main findings of our study can be summarized as follows:
-
•
At 1 year, the rate of the composite endpoint of death, stroke, and SE was 4.51%, and the rate any life-threatening, disabling, or major bleeding was 2.36% in Chinese AF patients after implanting the percutaneous LAA closure device.
-
•
There was no significant association between the type of anesthesia (general anesthesia vs moderate sedation) or the modality of image guidance (TEE, ICE, or fluoroscopy) regarding the ischemic or bleeding events.
-
•
The 1-stage combination procedure of LAAO and CA was associated with a significantly lower rate of death, stroke, or SE compared with LAAO only.
-
•
The antithrombotic medication regimens post-LAAO deviated substantially from guideline recommendations. The strategy of OAC | APT | APT was applied in 38.1% of participants and was associated with the numerically lowest rate of death; stroke; SE; or life-threatening, disabling, or major bleeding.
Patient characteristics and clinical outcomes between the current and previous studies3,4,6, 7, 8, 9, 10, 11 are tabulated in Supplemental Table 8. Notably, nearly all these pivotal studies supporting the use of LAAO were conducted primarily with White populations, with other ethnic groups, including Black, Asian, Hispanic, and so on, being less represented. Compared with other ethnic groups, East Asian individuals have a unique risk-benefit tradeoff in managing stroke prevention in AF. East Asians experience reduced anti-ischemic benefits and increased bleeding risk with antithrombotic therapies, particularly intracranial bleeding, known as the “East Asian paradox.”22 Additionally, adherence to OAC is commonly suboptimal in this population.23 Consequently, East Asians with nonvalvular AF might have a greater propensity to benefit from nonpharmacologic strategies for stroke prevention, such as the use of LAA closure devices.
RECORD is the first large-scale, real-world registry documenting the safety and efficacy profiles of the LAA closure device among the East Asian population. Compared with the other studies, RECORD’s rate of all-cause death was numerically the lowest, and the rates of stroke and major bleeding were similar. The average younger age of patients enrolled in RECORD might be related to the lower death rate. Nevertheless, these findings suggest that patients at high risk of stroke (mean CHA2DS2-VASc score of 4.0) and moderate to high risk of bleeding (mean HAS-BLED score of 2.4) who underwent implantation of an LAA closure device in the East Asian population experienced relatively low rates of ischemic and bleeding events.
Expert consensus documents13 recommend intraprocedural imaging using either TEE or ICE to guide LAAO, with fluoroscopy guidance alone reserved for exceptional circumstances and performed only by experts. To reduce medical expenses, avoid the discomfort and risk of TEE, and obviate the need for general anesthesia, an increasing number of operators have applied LAAO procedures by fluoroscopy alone. However, little evidence exists regarding the long-term safety and efficacy of LAAO performed by fluoroscopy guidance alone. The Bern registry,14 which enrolled 811 participants and followed them for 5 months, showed that procedures guided by TEE had a significantly lower rate of device-related complications compared with fluoroscopy. On the other hand, other small-scale studies24,25 showed no significant differences in outcomes between TEE and fluoroscopy guidance.
In RECORD, because the number of ICE-guided LAAOs was small and previous meta-analyses have suggested that ICE guidance is as effective as TEE,26 we combined ICE and TEE guidance into 1 group and compared it with fluoroscopy guidance. After adjusting for confounding factors, we found that the rates of ischemic and bleeding endpoints were similar between the TEE/ICE group and the fluoroscopy alone group at 1 year. However, this result could also be explained by the fact that most fluoroscopy-guided LAAO procedures were conducted by expert operators, as demonstrated in our previous report.12 More careful studies of the use of fluoroscopy-guided LAAO are warranted.
In cases of symptomatic atrial fibrillation, physicians typically offer ablation for symptom relief. For patients in this category who either have a contraindication to long-term OAC or are at a high risk of major bleeding and prefer a treatment option free from anticoagulation, LAAO has emerged as a viable alternative.27 However, most studies of LAAO, such as the PROTECT-AF,3 PREVAIL,4 and PRAGUE-17,5 have excluded participants who underwent a combined procedure of LAAO with CA to focus solely on the impact of LAAO. Some small-scale registries28, 29, 30, 31 have suggested that the rate of adverse events in a combined procedure of LAAO and CA is low. However, the long-term impact of LAAO in combination with CA remains unclear. In RECORD, 1-stage LAAO combination with CA was performed in 42.0% of cases, likely because of the fact that 80.5% of operators were electrophysiologists.
Previously, we showed that these combined procedures were not associated with increased periprocedural adverse event rates.12 In the current report, we found that at 1 year, the composite rate of death, stroke, and SE was significantly lower in the combined procedure group compared with the LAAO only group. The Kaplan-Meier curves of the 2 groups started to diverge at 6 months, coinciding with the timepoint when most patients stopped (D)OAC or DAPT and switched to single-antiplatelet therapy (Figure 2).
We also found that patients with AF of ≤1 year who underwent LAAO and CA benefitted the most compared with those who underwent LAAO only or LAAO plus CA in patients with AF of >1 year. However, with currently available evidence, we cannot support the routine use of LAAO plus CA, and further dedicated randomized studies are necessary before making any recommendations. The ongoing OPTION trial (NCT03795298), which compares the effectiveness of LAAO to OAC in postablation patients with AF, will partially provide the medical community with more evidence on this issue.
Only 1 of 20 patients received the post-LAAO medication treatment in concordance with the European Society of Cardiology AF guideline.2 The most significant deviation was that (D)OAC monotherapy (78.9%) was prescribed instead of (D)OAC plus aspirin between discharge and 45 days post-LAAO. Previous analyses of the LAAO Registry of the National Cardiovascular Data Registry database10 have also shown that (D)OAC monotherapy was applied in 57.7% of U.S. patients and was associated with a lower risk of major adverse outcomes compared with aspirin plus (D)OAC. Similarly, our study demonstrated that an increased risk of bleeding was associated with adding aspirin to anticoagulation at discharge post-LAAO, particularly in patients with a HAS-BLED score of >3. As such, an RCT that removes aspirin from the list of recommended post-LAAO treatments may be warranted.
Incomplete endothelialization after LAAO may lead to device-related thrombosis and ischemic events.32 Studies have shown that complete endothelial coverage might require more than 45 days.32,33 In the EWOLUTION study, nearly 8% of LAAO patients continued OAC at the 6-month visit.8 The latest and ongoing CHAMPION-AF trial, which compares DOAC to closure, has also stipulated the use of DOAC plus aspirin or DAPT until the 3-month visit. However, such prolongation raised concerns about the safety of (D)OAC agents in a patient population at a higher risk for bleeding. Our analyses showed that extending (D)OAC to 6 months post-LAAO was not associated with a lower risk of thrombotic events; however, it significantly increased the risk of major bleeding. Balancing the risk of thrombotic events and bleeding remains a challenge. A recent study suggested that using a prolonged half-dose DOAC might be an alternative option.34
Study limitations
First, imbalances exist among the subgroups. Although statistical adjustments were made to try to estimate the true differences among groups, the inability to eliminate the impact of unmeasurable confounders produces bias that cannot be adjusted. Second, RECORD enrolled only Chinese patients; therefore, extrapolation of these results to the other ethnic groups requires cautious interpretation.
Third, because of the limited resources available, imaging follow-up was not provided free of charge. As a result, patients who underwent optimal implantation may have been reluctant to undergo routine CTA/TEE imaging follow-up. Additionally, the timing of the follow-up overlaps with the COVID-19 pandemic, which may have further contributed to patients’ reluctance to undergo the examination. As a result, only 60% of patients had TEE/CTA examinations during follow-up. The rate of DRT or incomplete sealing might be underestimated; however, with a follow-up rate of 97.8%, the vital status and serious adverse events such as stroke, SE, or major bleeding were collected robustly.
Fourth, it is worth noting that the COVID-19 pandemic may have led to an increase in mortality. However, the recent Global Burden of Disease Study 202135 revealed that in East Asia, COVID-19 accounted for only 0.4% of deaths. Finally, the device used in the current study was the generation 2.5 LAA closure device instead of the next-generation LAA closure device, which was not commercially available at the time of the study. However, the difference in adverse events between the 2 devices was believed to be mainly confined to periprocedural and in-hospital events.11,36,37
Conclusions
In Chinese centers, patients with an LAA device experienced low rates of ischemic and bleeding events at 1 year. There was no significant association between the type of anesthesia or the modality of imaging guidance with respect to ischemic or bleeding events at 1 year. The 1-stage combination procedure of LAAO and CA was associated with a significantly lower rate of ischemic events compared with performing LAAO only; however, these results should be considered exploratory and for hypothesis generation only.
Perspectives.
COMPETENCY IN PATIENT CARE: Among East Asian patients with AF at elevated risk of stroke and bleeding, percutaneous LAAO is associated with low rates of ischemic and bleeding events.
TRANSLATIONAL OUTLOOK: The implantation configurations and antithrombotic strategies adopted after LAAO are diverse. Although the type of anesthesia and modality of imaging guidance have limited impact on adverse events, we found that LAAO with catheter ablation, compared with LAAO only, might be associated with better outcomes. However, further randomized controlled trials are needed to verify this application.
Funding Support and Author Disclosures
RECORD was supported by the Boston Scientific Corporation. Boston Scientific had no role in the study design, data collection, data analyses, and interpretation of the study data, nor was it involved in the decision to publish the final manuscript. Dr Serruys has received personal consultancy fees from Sino Medical Sciences Technology, Philips/Volcano, and Xeltis outside the submitted work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank all study centers and participants in this trial, whose work made this study possible. We thank Drs Lisheng Jiang, Yixian Lin, Guodong Niu, Daxin Zhou, Xin Meng, Wei Bai, Yuan Bai, Zhiqing Jin, Qiong Huang, Gecai Chen, Dujiang Xie, Rongfang Lan, Yonghua Zhang, Songlin Zhang, Hui Huang, Yaodong Li, Yangyang Yu, Lei Jia, Yizhang Wu, Yanjie Li, Jizhe Xu, Jie Xiong, and Lifang Xie for recruiting the participants.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures as well as an expanded Methods section, please see the online version of this paper.
Appendix
References
- 1.January C.T., Wann L.S., Calkins H., et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:104–132. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Hindricks G., Potpara T., Dagres N., et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 3.Holmes D.R., Reddy V.Y., Turi Z.G., et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 4.Holmes DR Jr, Kar S., Price M.J., et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 5.Osmancik P., Herman D., Neuzil P., et al. 4-Year outcomes after left atrial appendage closure versus nonwarfarin oral anticoagulation for atrial fibrillation. J Am Coll Cardiol. 2022;79:1–14. doi: 10.1016/j.jacc.2021.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Tzikas A., Shakir S., Gafoor S., et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER Cardiac Plug. EuroIntervention. 2016;11:1170–1179. doi: 10.4244/EIJY15M01_06. [DOI] [PubMed] [Google Scholar]
- 7.Landmesser U., Schmidt B., Nielsen-Kudsk J.E., et al. Left atrial appendage occlusion with the AMPLATZER Amulet device: periprocedural and early clinical/echocardiographic data from a global prospective observational study. EuroIntervention. 2017;13:867–876. doi: 10.4244/EIJ-D-17-00493. [DOI] [PubMed] [Google Scholar]
- 8.Boersma L.V., Ince H., Kische S., et al. Efficacy and safety of left atrial appendage closure with WATCHMAN in patients with or without contraindication to oral anticoagulation: 1-year follow-up outcome data of the EWOLUTION trial. Heart Rhythm. 2017;14:1302–1308. doi: 10.1016/j.hrthm.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 9.Reddy V.Y., Mobius-Winkler S., Miller M.A., et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology) J Am Coll Cardiol. 2013;61:2551–2556. doi: 10.1016/j.jacc.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 10.Price M.J., Slotwiner D., Du C., et al. Clinical outcomes at 1 year following transcatheter left atrial appendage occlusion in the United States. JACC Cardiovasc Interv. 2022;15:741–750. doi: 10.1016/j.jcin.2022.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kar S., Doshi S.K., Sadhu A., et al. Primary outcome evaluation of a next-generation left atrial appendage closure device: results from the PINNACLE FLX Trial. Circulation. 2021;143:1754–1762. doi: 10.1161/CIRCULATIONAHA.120.050117. [DOI] [PubMed] [Google Scholar]
- 12.Su F., Gao C., Liu J., et al. Periprocedural outcomes associated with use of a left atrial appendage occlusion device in China. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.14594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glikson M., Wolff R., Hindricks G., et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion—an update. EuroIntervention. 2020;15:1133–1180. doi: 10.4244/EIJY19M08_01. [DOI] [PubMed] [Google Scholar]
- 14.Galea R., Raber L., Fuerholz M., et al. Impact of echocardiographic guidance on safety and efficacy of left atrial appendage closure: an observational study. JACC Cardiovasc Interv. 2021;14:1815–1826. doi: 10.1016/j.jcin.2021.05.042. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen-Kudsk J.E., Berti S., De Backer O., et al. Use of intracardiac compared with transesophageal echocardiography for left atrial appendage occlusion in the amulet observational study. JACC Cardiovasc Interv. 2019;12:1030–1039. doi: 10.1016/j.jcin.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 16.Saw J., Holmes D.R., Cavalcante J.L., et al. SCAI/HRS expert consensus statement on transcatheter left atrial appendage closure. JACC Cardiovasc Interv. 2023;16:1384–1400. doi: 10.1016/j.jcin.2023.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Freeman J.V., Higgins A.Y., Wang Y., et al. Antithrombotic therapy after left atrial appendage occlusion in patients with atrial fibrillation. J Am Coll Cardiol. 2022;79:1785–1798. doi: 10.1016/j.jacc.2022.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzikas A., Holmes D.R., Jr., Gafoor S., et al. Percutaneous left atrial appendage occlusion: the Munich consensus document on definitions, endpoints, and data collection requirements for clinical studies. Europace. 2017;19:4–15. doi: 10.1093/europace/euw141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehran R., Rao S.V., Bhatt D.L., et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 20.Li G., Taljaard M., Van den Heuvel E.R., et al. An introduction to multiplicity issues in clinical trials: the what, why, when and how. Int J Epidemiol. 2017;46:746–755. doi: 10.1093/ije/dyw320. [DOI] [PubMed] [Google Scholar]
- 21.Camm A.J. Leap or lag: left atrial appendage closure and guidelines. Europace. 2023 doi: 10.1093/europace/euad067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H.K., Tantry U.S., Smith S.C., Jr., et al. The East Asian paradox: an updated position statement on the challenges to the current antithrombotic strategy in patients with cardiovascular disease. Thromb Haemost. 2021;121:422–432. doi: 10.1055/s-0040-1718729. [DOI] [PubMed] [Google Scholar]
- 23.Wang C., Yang Z., Wang C., et al. Significant underuse of warfarin in patients with nonvalvular atrial fibrillation: results from the China national stroke registry. J Stroke Cerebrovasc Dis. 2014;23:1157–1163. doi: 10.1016/j.jstrokecerebrovasdis.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X., Jin Q., Kong D., et al. Comparison of fluoroscopy and transesophageal echocardiogram for intra-procedure device surveillance assessment during implantation of Watchman. Int J Cardiol. 2021;324:72–77. doi: 10.1016/j.ijcard.2020.08.070. [DOI] [PubMed] [Google Scholar]
- 25.Kleinecke C., Yu J., Neef P., et al. Clinical outcomes of Watchman vs. Amplatzer occluders for left atrial appendage closure (WATCH at LAAC) Europace. 2020;22:916–923. doi: 10.1093/europace/euaa001. [DOI] [PubMed] [Google Scholar]
- 26.Velagapudi P., Turagam M.K., Kolte D., et al. Intracardiac vs transesophageal echocardiography for percutaneous left atrial appendage occlusion: a meta-analysis. J Cardiovasc Electrophysiol. 2019;30:461–467. doi: 10.1111/jce.13820. [DOI] [PubMed] [Google Scholar]
- 27.Joglar J.A., Chung M.K., Armbruster A.L., et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2024;83(1):109–279. doi: 10.1016/j.jacc.2023.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He B., Jiang L.S., Hao Z.Y., Wang H., Miao Y.T. Combination of ablation and left atrial appendage closure as “one-stop” procedure in the treatment of atrial fibrillation: current status and future perspective. Pacing Clin Electrophysiol. 2021;44:1259–1266. doi: 10.1111/pace.14201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen M., Wang Z.Q., Wang Q.S., et al. One-stop strategy for treatment of atrial fibrillation: feasibility and safety of combining catheter ablation and left atrial appendage closure in a single procedure. Chin Med J. 2020;133:1422–1428. doi: 10.1097/CM9.0000000000000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wintgens L., Romanov A., Phillips K., et al. Combined atrial fibrillation ablation and left atrial appendage closure: long-term follow-up from a large multicentre registry. Europace. 2018;20:1783–1789. doi: 10.1093/europace/euy025. [DOI] [PubMed] [Google Scholar]
- 31.Kita K., Carlson S., Huntsinger M., Tun H., Sohn J., Doshi R.N. Safety and feasibility of combined atrial fibrillation ablation and left atrial appendage occlusion after left atrial appendage electrical isolation. J Interv Card Electrophysiol. 2020;57:43–55. doi: 10.1007/s10840-019-00603-1. [DOI] [PubMed] [Google Scholar]
- 32.Ellis C.R., Alkhouli M., Anderson J.A., Swarup V. Comparative endothelialization of amulet LAA Occluder and Watchman 2.5 LAA Device: observations from explanted hearts. JACC Clin Electrophysiol. 2022;8:828–829. doi: 10.1016/j.jacep.2022.01.024. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz R.S., Holmes D.R., Van Tassel R.A., et al. Left atrial appendage obliteration: mechanisms of healing and intracardiac integration. JACC Cardiovasc Interv. 2010;3:870–877. doi: 10.1016/j.jcin.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Della Rocca D.G., Magnocavallo M., Di Biase L., et al. Half-dose direct oral anticoagulation versus standard antithrombotic therapy after left atrial appendage occlusion. JACC Cardiovasc Interv. 2021;14:2353–2364. doi: 10.1016/j.jcin.2021.07.031. [DOI] [PubMed] [Google Scholar]
- 35.GBD 2021 Causes of Death Collaborators Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2024 doi: 10.1016/S0140-6736(24)00367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price M.J., Friedman D.J., Du C., et al. Comparative safety of transcatheter LAAO with the first-generation Watchman and next-generation Watchman FLX devices. JACC Cardiovasc Interv. 2022;15:2115–2123. doi: 10.1016/j.jcin.2022.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Galea R., Mahmoudi K., Grani C., et al. Watchman FLX vs. Watchman 2.5 in a dual-center left atrial appendage closure cohort: the WATCH-DUAL study. Europace. 2022;24:1441–1450. doi: 10.1093/europace/euac021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.