Abstract
Background
This is an update of a Cochrane Review first published in The Cochrane Library in Issue 1, 2005 and previously updated in 2007 and 2009.
Idiopathic sudden sensorineural hearing loss (ISSHL) is common and has a significant effect on quality of life. Hyperbaric oxygen therapy (HBOT) may improve oxygen supply to the inner ear and result in an improvement in hearing.
Objectives
To assess the benefits and harms of HBOT for treating ISSHL and/or tinnitus.
Search methods
We searched the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL); PubMed; EMBASE; Database of Randomised Trials in Hyperbaric Medicine (DORCTHIM); CINAHL; Web of Science; BIOSIS Previews; Cambridge Scientific Abstracts; ICTRP and additional sources for published and unpublished trials. The date of the most recent search was 2 May 2012, following previous searches in 2009, 2007 and 2004.
Selection criteria
Randomised studies comparing the effect on ISSHL and tinnitus of HBOT and alternative therapies.
Data collection and analysis
Three authors evaluated the quality of trials using the 'Risk of bias' tool and extracted data from the included trials.
Main results
Seven trials contributed to this review (392 participants). The studies were small and of generally poor quality. Pooled data from two trials did not show any significant improvement in the chance of a 50% increase in hearing threshold on pure‐tone average with HBOT (risk ratio (RR) with HBOT 1.53, 95% confidence interval (CI) 0.85 to 2.78, P = 0.16), but did show a significantly increased chance of a 25% increase in pure‐tone average (RR 1.39, 95% CI 1.05 to 1.84, P = 0.02). There was a 22% greater chance of improvement with HBOT, and the number needed to treat (NNT) to achieve one extra good outcome was 5 (95% CI 3 to 20). There was also an absolute improvement in average pure‐tone audiometric threshold following HBOT (mean difference (MD) 15.6 dB greater with HBOT, 95% CI 1.5 to 29.8, P = 0.03). The significance of any improvement in tinnitus could not be assessed.
There were no significant improvements in hearing or tinnitus reported for chronic presentation (six months) of ISSHL and/or tinnitus.
Authors' conclusions
For people with acute ISSHL, the application of HBOT significantly improved hearing, but the clinical significance remains unclear. We could not assess the effect of HBOT on tinnitus by pooled analysis. In view of the modest number of patients, methodological shortcomings and poor reporting, this result should be interpreted cautiously. An appropriately powered trial is justified to define those patients (if any) who can be expected to derive most benefit from HBOT.
There is no evidence of a beneficial effect of HBOT on chronic ISSHL or tinnitus and we do not recommend the use of HBOT for this purpose.
Keywords: Humans; Hyperbaric Oxygenation; Auditory Threshold; Chronic Disease; Hearing Loss, Sensorineural; Hearing Loss, Sensorineural/therapy; Hearing Loss, Sudden; Hearing Loss, Sudden/therapy; Randomized Controlled Trials as Topic; Tinnitus; Tinnitus/therapy; Treatment Outcome
Plain language summary
Hyperbaric oxygen for sudden hearing loss and tinnitus (ringing in the ears) of unknown cause
Idiopathic sudden sensorineural hearing loss (ISSHL) is common and often results in permanent hearing loss. It therefore has a high impact on the well‐being of those affected. Tinnitus (abnormal persistent noises or ringing in the ear) is similarly common and often accompanies the hearing loss. Although the cause of these complaints is not clear, they may be related to a lack of oxygen secondary to a vascular problem not yet identified. Hyperbaric oxygen therapy (HBOT) involves breathing pure oxygen in a specially designed chamber and it is sometimes used as a treatment to increase the supply of oxygen to the ear and brain in an attempt to reduce the severity of hearing loss and tinnitus.
We found some evidence from seven small trials of generally poor quality, that hearing may be improved in people with ISSHL and possibly that tinnitus may also be improved. This may only be true if HBOT is used within two weeks of the onset of problems and there is no evidence that HBOT can help people who have been deaf for some months. Further research is needed.
Summary of findings
Summary of findings for the main comparison. Hyperbaric oxygen therapy for idiopathic sudden sensorineural hearing loss or tinnitus.
| Hyperbaric oxygen therapy for idiopathic sudden sensorineural hearing loss or tinnitus | ||||||
| Patient or population: patients with idiopathic sudden sensorineural hearing loss or tinnitus Settings: outpatients Intervention: hyperbaric oxygen therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Hyperbaric oxygen therapy | |||||
| Mean improvement in pure‐tone average hearing threshold across all frequencies Decibels Scale from: 0 to 120 Follow‐up: median 4 weeks | The mean improvement in pure‐tone average hearing threshold across all frequencies ranged across control groups from ‐0.7 to 22.3 decibels1 | The mean improvement in pure‐tone average hearing threshold across all frequencies in the intervention groups was 15.6 higher (1.5 to 29.8 higher) | 169 (4 studies) | ⊕⊕⊕⊝ moderate2 | ||
| More than 50% improvement in pure‐tone average threshold Risk ratio Follow‐up: median 4 weeks | Study population | RR 1.53 (0.85 to 2.78) | 114 (2 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 360 per 1000 | 551 per 1000 (306 to 1000) | |||||
| Low‐risk population | ||||||
| 250 per 1000 | 382 per 1000 (213 to 695) | |||||
| High‐risk population | ||||||
| 450 per 1000 | 688 per 1000 (383 to 1000) | |||||
| More than 25% improvement in pure‐tone average threshold Risk ratio Follow‐up: median 4 weeks | Study population | RR 1.39 (1.05 to 1.84) | 114 (2 studies) | ⊕⊕⊕⊝ moderate4 | ||
| 560 per 1000 | 778 per 1000 (588 to 1000) | |||||
| Low‐risk population | ||||||
| 500 per 1000 | 695 per 1000 (525 to 920) | |||||
| High‐risk population | ||||||
| 600 per 1000 | 834 per 1000 (630 to 1000) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 This is the range of mean changes in all studies included. 2 Likely due to increasing absolute improvements in hearing thresholds with worsening deafness on presentation, rather than between studies. 3 Only two studies contributed to this outcome, with a total of 114 patients. 4 No explanation was provided.
Background
This is an update of a Cochrane Review first published in The Cochrane Library in Issue 1, 2005 and previously updated in 2007 and 2009.
Description of the condition
Idiopathic sudden sensorineural hearing loss (ISSHL) is an acute hearing impairment, with an incidence of about 8 to 15 per 100,000 of the population per year (Stokroos 1996a). Although the aetiology and pathophysiology remain unclear (Haberkamp 1999), ISSHL is most commonly defined as a greater than 30 dB sensorineural hearing loss occurring in at least three contiguous audiometric frequencies over 72 hours or less (Hughes 1996). Tinnitus can be described as the perception of sound in the absence of external acoustic stimulation, and in many cases it is associated with some degree of hearing loss, particularly in those individuals who have been exposed to excessive noise. The incidence is probably around 10% to 20% of adults in developed countries (ATA 2001; Coles 1990). For the patient it may be trivial or it may become a debilitating illness (Luxon 1993). Sufferers from tinnitus hear a noise that apparently arises from the ears or within the head and may be continuous or intermittent. Brief episodes of tinnitus are probably normal, and clinically significant tinnitus is usually defined by applying one of several classification systems proposed (Dauman 1992; Stephens 1991).
The onset of ISSHL is abrupt in many patients, therefore a vascular cause has been suggested (Belal 1980) but other possibilities include viral infection, autoimmune disease and inner ear membrane rupture (Thurmond 1998; Yoon 1990). The cause of tinnitus is equally obscure, although it is often associated with ISSHL ‐ up to 90% of patients suffering with ISSHL also complain of tinnitus (Parnes 1997). The most widely discussed theories include excessive or abnormal spontaneous activity in the auditory system and in related cerebral areas (Kaltenbach 2000) and abnormal processing of a signal generated in the auditory system with 'feedback' (Jastreboff 1990). Recent work confirms that a broad multimodal network of neurons, often operating from a site remote to that of the initial pathology, is involved in generating and sustaining the tinnitus perception in some forms of the disorder (Cacace 2003). Tinnitus has, in fact, been compared to chronic pain of central origin in some regards, and when symptoms are severe, tinnitus can be associated with major depression, anxiety and other psychological disturbances, leading to a progressive deterioration of quality of life (Sullivan 1992; Sullivan 1994).
Treatments for ISSHL have mostly been designed to improve the blood circulation and oxygenation of the inner ear and include vasodilators, plasma expanders, steroids, anticoagulants, diuretics, contrast dye and antivirals. None have been proven of benefit in large randomised trials or meta‐analyses. A recent Cochrane Review found insufficient evidence to demonstrate the effectiveness of vasodilators for ISSHL (Agarwal 2009). Assessment of the effectiveness of therapy is further complicated by a high rate of spontaneous recovery, as much as 65% in some studies (Mattox 1977), and the very variable periods for which hearing loss has been present before the institution of therapy. While the impact of therapy will vary with individual circumstances, we have selected a 50% return of hearing following therapy as a clinically significant improvement when considering appropriate power for included studies in this review. Specific therapies for tinnitus have tended to focus on the impact of the noise on quality of life and mood, and include antidepressants, anticonvulsants and benzodiazepines, or on trying to mask the noise itself with white noise generators. A variety of psychotherapeutic and 'habituation' programmes are also advocated to help the sufferer deal with the problem (Noell 2003). A Cochrane Review of antidepressants for tinnitus has been published (Baldo 2006).
Description of the intervention
Hyperbaric oxygen therapy is a further, usually adjunctive, therapy that has been proposed to improve both ISSHL and tinnitus. This is the therapeutic administration of 100% oxygen at environmental pressures greater than one atmosphere absolute (ATA). Administration involves placing the patient in an airtight vessel, increasing the pressure within that vessel, and administering 100% oxygen for respiration. In this way it is possible to deliver a greatly increased partial pressure of oxygen to the tissues. Typically, treatments involve pressurisation to between 1.5 and 3.0 ATA for periods between 60 and 120 minutes once or twice daily. A typical course will involve 20 to 40 such treatments.
How the intervention might work
Hyperbaric oxygen therapy was first reported to improve the outcome following ISSHL and tinnitus in the late 1960s by both French and German workers (translations unavailable at present). The administration of hyperbaric oxygen is based on the argument that both hearing loss and tinnitus may result from an hypoxic event in the cochlear apparatus, and that hyperbaric oxygen therapy may be able to reverse that oxygen deficit (Lamm 1998). Despite more than 30 years of interest in the delivery of hyperbaric oxygen therapy in these patients, however, little clinical evidence exists for the assertion that such an intervention improves outcome.
Why it is important to do this review
Hyperbaric oxygen therapy is associated with some risk of adverse effects including damage to the ears, sinuses and lungs from the effects of pressure, temporary worsening of short‐sightedness, claustrophobia and oxygen poisoning. Although serious adverse events are rare, hyperbaric oxygen therapy cannot be regarded as an entirely benign intervention.
Objectives
To assess the evidence for the benefit of hyperbaric oxygen therapy in the treatment of both acute and chronic sensorineural hearing loss and/or tinnitus.
We compared treatment regimens including hyperbaric oxygen against similar regimens excluding hyperbaric oxygen. Where regimens differed significantly between studies, we clearly stated this and discussed the implications. We made all comparisons using an intention‐to‐treat analysis where possible and they reflect efficacy in the context of randomised trials rather than true effectiveness in any particular clinical context. Specifically, we wished to address the following.
Does the administration of hyperbaric oxygen to people with idiopathic sensorineural hearing loss (whether early or late presentation) result in an increase in the proportion attaining a useful improvement in hearing? We also intended to investigate both binaural hearing recovery and speech discrimination recovery where possible.
Does the administration of hyperbaric oxygen to people with tinnitus (whether early or late presentation) result in an increase in the proportion experiencing a useful reduction in tinnitus?
Methods
Criteria for considering studies for this review
Types of studies
Randomised and pseudo‐randomised controlled trials that compared the effect of treatment for either acute or chronic idiopathic sensorineural hearing loss and/or tinnitus where hyperbaric oxygen administration is included, with the effect of similar treatment in the absence of hyperbaric oxygen. We considered studies irrespective of allocation concealment or blinding status.
Types of participants
Any adult with acute onset sensorineural hearing loss and/or tinnitus of any duration.
Types of interventions
Trials using hyperbaric oxygen administered in a compression chamber above 1.2 ATA and for treatment times between 30 and 120 minutes on at least one occasion were eligible. The comparator group was somewhat diverse. We accepted any standard treatment regimen designed to maximise hearing loss recovery or reduction in tinnitus, or where the comparator was designed to improve quality of life for appropriate patients. Subgroup analysis was considered to evaluate the impact of different comparator strategies.
Types of outcome measures
Studies were eligible for inclusion if they reported any of the following outcome measures at any time.
Primary outcomes
1. Acute ISSHL: pure‐tone audiometric documented change in hearing in response to treatment. 2. Chronic ISSHL: pure‐tone audiometric documented change in hearing in response to treatment. 3. Acute ISSHL: relief of tinnitus. Subjective assessment of tinnitus level. 4. Chronic ISSHL: relief of tinnitus. Subjective assessment of tinnitus level.
Secondary outcomes
5. Activities of daily living (ADL). 6. Subjective or objective improvements in depression or mood disturbance. 7. Hearing handicap inventory change (and similar tool for tinnitus). 8. Adverse events associated with hyperbaric oxygen therapy and comparators.
Search methods for identification of studies
We conducted systematic searches for randomised controlled trials. There were no language, publication year or publication status restrictions. The date of the last search was 2 May 2012, following previous searches in 2009, 2007 and 2004.
Electronic searches
We searched the following databases from their inception for published, unpublished and ongoing trials: the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, 2012, Issue 4); PubMed; EMBASE; Database of Randomised Trials in Hyperbaric Medicine (DORCTHIM); AMED; CINAHL; LILACS; KoreaMed; IndMed; PakMediNet; CAB Abstracts; Web of Science; BIOSIS Previews; ISRCTN; ClinicalTrials.gov; ICTRP; Google Scholar and Google.
We modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011)). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, we searched PubMed, TRIPdatabase, The Cochrane Library and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. We searched for conference abstracts using the Cochrane Ear, Nose and Throat Disorders Group Trials Register.
In the original searches in 2004, we contacted experts in the field and leading hyperbaric therapy centres (as identified by personal communication and searching the Internet) and asked for additional relevant data in terms of published or unpublished randomised trials. In 2004 we also handsearched relevant hyperbaric textbooks (Kindwall, Jain, Marroni, Bakker, Bennett and Elliot), journals (Undersea and Hyperbaric Medicine, Hyperbaric Medicine Review, South Pacific Underwater Medicine Society (SPUMS) Journal, European Journal of Hyperbaric Medicine and Aviation, Space and Environmental Medicine Journal) and conference proceedings (Undersea and Hyperbaric Medical Society, SPUMS, European Undersea and Baromedical Society, International Congress of Hyperbaric Medicine) published since 1980.
Data collection and analysis
Selection of studies
Two authors (MB and JL) were responsible for handsearching and identification of appropriate studies for consideration. Three authors (MB, TK and PY) examined the electronic search results and identified studies that may have been relevant and these studies were entered into a bibliographic software package (Review Manager 5 (RevMan 2011)) when any of the authors considered the study might satisfy the inclusion criteria. At the 2009 update, a fourth author was added who was able to translate and appraise articles in German (MP). We retrieved all comparative clinical trials identified by this process with the assistance of the Cochrane Advanced Reviewer Support Service of the Australasian Cochrane Centre and the three authors reviewed them independently, two with content expertise in sensorineural hearing loss and tinnitus, one with content expertise in hyperbaric oxygen. In addition, two of the authors (MB and MP) have expertise in clinical epidemiology. Authors recorded data using the data extraction form developed for this review.
Where reporting methods differed between trials for the same outcome, we attempted to contact the principal authors to request further data. Our intention was to convert reported data to a form that enabled meta‐analysis, however no suitable further data were forthcoming from any author.
Data extraction and management
Each author extracted relevant data and agreed on the trial characteristics included in the 'Risk of bias' table. In addition, we ranked studies on sample size and identified those with sufficient power to determine the clinically important effect for which the trial was designed. All data extracted reflected original allocation group where possible to allow an intention‐to‐treat analysis. We identified drop‐outs where this information was given.
Assessment of risk of bias in included studies
Two authors (MB and MP) undertook assessment of the risk of bias of the included trials independently, with the following taken into consideration, as guided by the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011):
sequence generation;
allocation concealment;
blinding;
incomplete outcome data;
selective outcome reporting; and
other sources of bias.
We used the Cochrane ‘Risk of bias’ tool in RevMan 5 (RevMan 2011), in which each of these domains is described as reported in the trial and then a judgement assigned about the adequacy of each entry: low, high and unclear (or unknown) risk of bias.
Data synthesis
For proportions (dichotomous outcomes), we used risk ratio (RR). We used a fixed‐effect model where there was no evidence of significant heterogeneity between studies (see below), and employed a random‐effects model when such heterogeneity was likely. We undertook all analyses with RevMan 5 software.
Primary outcomes
1. There were two approaches to improvement in hearing loss analysis depending on the nature of the data presented:
a) Proportion of participants with good hearing loss resolution (e.g. pure‐tone average (PTA) improvement > 20 dB). We dichotomised participants into good outcome and poor outcome. We established the RR for good outcome with hyperbaric oxygen therapy using the intention‐to‐treat data of the hyperbaric oxygen therapy versus the control group. As an estimate of the statistical significance of a difference between experimental interventions and control interventions we calculated RR for benefit using hyperbaric oxygen therapy with 95% confidence intervals (CI). We assumed a statistically significant difference between experimental intervention and control intervention if the 95% CI of the RR did not include the value 1.0. As an estimate of the clinical relevance of any difference between experimental intervention and control intervention we calculated the number needed to treat to benefit (NNTB) and number needed to treat to harm (NNTH) with 95% CI as appropriate.
b) Comparison of the difference between the mean change in PTA in each group, hyperbaric oxygen versus non‐hyperbaric oxygen. We compared the mean differences (MD) in hearing loss recovery between hyperbaric oxygen and control groups using RevMan 5. We defined a statistically significant difference as existing if the 95% CI did not include a zero MD.
2. Relief of tinnitus was treated similarly to 1) above.
Secondary outcomes
3. Activities of daily living (ADL). The mean differences (MD) in ADL between hyperbaric oxygen and control groups were to be compared as in 1b) above.
4. Depression and mood disturbance. Methods were to depend on the nature of the data as in 1b) above.
5. Adverse events. We considered dichotomous data for adverse events (number of patients with adverse events versus number of patients without them in both groups) in the hyperbaric oxygen groups of the included studies.
Subgroup analysis and investigation of heterogeneity
Where appropriate data existed, we considered subgroup analysis based on:
1. time between onset and therapy ‐ early versus late presentation for treatment in the trial; 2. aetiology of the ISSHL or tinnitus; 3. dose of oxygen received (pressure, time and length of treatment course); 4. nature of the comparative treatment modalities; 5. severity of hearing loss and/or tinnitus.
We explored heterogeneity and performed subgroup analyses when appropriate. We estimated statistical heterogeneity using the I2 statistic and gave consideration to the appropriateness of pooling and meta‐analysis.
Sensitivity analysis
We intended to perform sensitivity analyses for missing data and study quality.
Missing data
We employed sensitivity analyses using different approaches to imputing missing data. The best‐case scenario assumed that none of the originally enrolled patients missing from the primary analysis in the treatment group had the negative outcome of interest whilst all those missing from the control group did. The worst‐case scenario was the reverse.
Study quality
If appropriate, we intended to conduct a sensitivity analysis by study quality based on our estimate of the risk of bias from the 'Risk of bias' tables and an assessment of adequate sample size to detect the clinically important difference in outcome for which the study was designed.
Results
Description of studies
At the initial search, we identified 91 publications apparently dealing with the use of HBOT for the treatment of ISSHL, tinnitus or both. Initial examination confirmed 23 were case reports or case series, 23 were reviews without new data, 12 were dealing with a different condition (acoustic trauma) and 12 were non‐random comparative studies. We excluded these reports. At the 2009 update, further information was available for three studies. Wang 2000 and Blagovesh 1990 were translated and confirmed not to report a randomised comparison of HBOT versus an alternative therapeutic strategy. We excluded these two trials. Pilgramm 1985 was translated and accepted into the analysis. After appraisal of the 21 full reports we excluded three further reports as reviews without new data, three as comparative trials where all groups received HBOT, four as non‐random comparative trials with historical controls or sequential treatment, and two as case series (see table Characteristics of excluded studies). The other seven trials were included in the review (Cavallazzi 1996; Fattori 2001; Hoffmann 1995a; Hoffmann 1995b; Pilgramm 1985; Schwab 1998; Topuz 2004).
A further search of all resources was repeated in February 2011 and May 2012. We found 121 citations of which seven were possible randomised trials. Two were in planning with no reported data (Bennett 2010; Barthelemy 2002) (see Characteristics of ongoing studies), one was an early report of an included trial (Hoffmann 1995a) and four have been unattainable for full appraisal to date (see Studies awaiting classification). Therefore this update has located no further trials for inclusion. The full study flow diagram is given for all searches combined in Figure 1.
1.
Study flow diagram.
The included trials were published between 1985 and 2004, and the authors are unaware of any ongoing randomised controlled trials in the area. In total, these trials include data on 392 participants, 207 receiving HBOT and 185 control (one participant was lost without information on allocation). The largest (Pilgramm 1985) accounts for 22% of cases (see table Characteristics of included studies).
Both the dose of oxygen per treatment session and for the total course of treatment varied between studies. The lowest dose administered was 1.5 ATA for 45 minutes daily for 15 days (Hoffmann 1995a; Hoffmann 1995b), while the highest dose was 2.5 ATA for 90 minutes daily for 25 days (Topuz 2004). All authors used between 1.5 and 2.5 ATA as a maximum oxygen pressure and the total number of individual treatment sessions varied from 10 (Fattori 2001; Pilgramm 1985; Schwab 1998) to 25 (Topuz 2004).
All trials except Hoffmann 1995a and part of Pilgramm 1985 included participants with acute hearing loss, with or without tinnitus. Hoffmann 1995b accepted only patients who had not improved after two weeks of pharmacological therapy, Fattori 2001 accepted patients untreated within 48 hours of hearing loss, while Schwab 1998 and Topuz 2004 accepted patients up to two weeks after loss. Cavallazzi 1996 did not define entry criteria. Hoffmann 1995a and Pilgramm 1985 were the only trials to examine the effect of HBOT on chronic presentation and these trial accepted participants with up to one year of hearing loss. There was little information on exclusion criteria. Schwab 1998 specifically excluded candidates with contra‐indications to therapy, Fattori 2001 specifically excluded candidates with a probable cause for deafness such as acoustic trauma and Pilgramm 1985 specifically excluded patients with probable causes and those who were experiencing spontaneous recovery.
Comparator regimens differed between trials. Schwab 1998, Cavallazzi 1996, Pilgramm 1985 and Topuz 2004 compared HBOT to a multimodal pharmacological approach, while Fattori 2001 used a vasodilator alone. Hoffmann 1995a (chronic ISSHL) compared HBOT to a sham treatment and Hoffmann 1995b (acute ISSHL) compared HBOT to no treatment. Details of comparator therapies are given in the table Characteristics of included studies.
The follow‐up periods varied from immediately following the treatment course (Cavallazzi 1996; Hoffmann 1995a) to 10 days (Fattori 2001), four weeks (Pilgramm 1985; Topuz 2004) and three months (Hoffmann 1995b; Schwab 1998). All included studies reported at least one clinical outcome of interest. Of the outcomes identified above, these trials reported data on both primary outcomes (pure‐tone audiometric documented change in hearing and relief of tinnitus) but none of the secondary outcomes of interest.
Other outcomes (including non‐clinical) reported by Fattori 2001 included: auditory evoked potentials, videonystagmography, static posturography, neurological examination, doppler echography, magnetic resonance imagery and computed tomography. No other trials reported additional outcomes.
Patient baseline characteristics
All participants had suffered ISSHL, tinnitus or both. Six of the studies defined a time‐based entry criteria (Fattori 2001 48 hours; Hoffmann 1995b, Schwab 1998 and Topuz 2004 two weeks; Hoffmann 1995a six months, Pilgramm 1985 14 days (acute presentation) and up to one year (chronic presentation)). All trials required no specific prior therapy except Hoffmann 1995b, where all participants had failed to respond to two weeks of pharmacological therapy in hospital. Only Schwab 1998 and Topuz 2004 defined a degree of hearing loss as a requirement for entry (at least 20 dB loss in one or more frequencies and 30 dB loss in three frequencies respectively). Cavallazzi 1996, Fattori 2001 and Topuz 2004 stratified participants on entry for severity of hearing loss. While all trials included participants with ISSHL, only Schwab 1998 and Cavallazzi 1996 specifically identified individuals with tinnitus in the absence of hearing loss.
Risk of bias in included studies
In general the methodology of these trials was poorly reported. Some details are given in the 'Risk of bias' tables. The number of studies was small with relatively little variation in the risk of bias, so we did not use study quality as a basis for sensitivity analysis.
Allocation
Allocation concealment was not adequate in any of the studies. Randomisation procedures were not described in any of the studies, except Pilgramm 1985 where a computer‐generated sequence was employed. Allocation may not have been truly random for Cavallazzi 1996, where the allocation method was not clearly described. For none of the studies is there a clear indication that the investigators were unable to predict the prospective group to which a participant would be allocated.
Blinding
Only Hoffmann 1995a described sham therapy with blinding of participants to the allocated therapy. No trial described blinding of investigators or outcome assessors.
Incomplete outcome data
Schwab 1998 did not report results for seven participants with ISSHL and 11 with tinnitus. This trial enrolled 31 participants with both ISSHL and tinnitus, and 43 with one diagnosis or the other. It is not clear how many of the losses were individuals with both diagnoses, making an intention‐to‐treat analysis problematic. Six patients dropped out of the trial and were not reported in the outcomes in Pilgramm 1985. None of the remaining studies suffered any losses to follow‐up, or reported any violation of allocated treatment. As neither Schwab 1998 nor Pilgramm 1985 reported any dichotomous outcomes, we have not performed sensitivity analysis making best and worst‐case analyses to examine potentially important effects of these losses on outcome.
Selective reporting
There was no evidence of selective reporting in any of the included trials.
Other potential sources of bias
There was no evidence of other sources of bias in any of the included trials.
Effects of interventions
See: Table 1
Primary outcomes
1. Acute idiopathic sudden sensorineural hearing loss (ISSHL): pure‐tone audiometric change in hearing (comparison 01)
All trials reported on this outcome, but there were a variety of reporting methods that limited the possibility of pooling those results.
1.1 Proportion of participants with greater than 50% return of hearing at end of therapy
See Analysis 1.1.
1.1. Analysis.
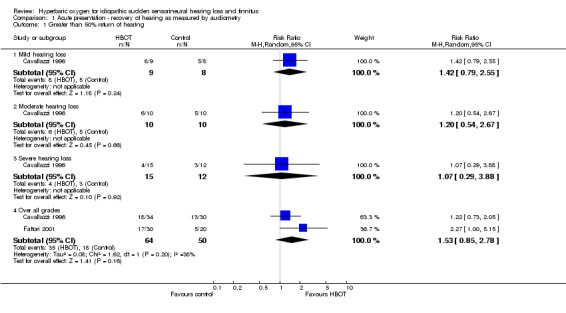
Comparison 1 Acute presentation ‐ recovery of hearing as measured by audiometry, Outcome 1 Greater than 50% return of hearing.
Two trials reported this outcome (Cavallazzi 1996; Fattori 2001), involving 114 participants (29% of the total participants in this review). Cavallazzi 1996 contributed 64 participants and Fattori 2001 50 participants. There was no statistically significant increase in the proportion of participants with more than 50% improvement in pure‐tone average (PTA) assessed hearing loss over four frequencies following hyperbaric oxygen therapy (HBOT) (risk ratio (RR) of improvement with HBOT was 1.53, 95% confidence interval (CI) 0.85 to 2.78, P = 0.16). There was moderate heterogeneity between trials (I2 = 38.2%) and therefore we used a random‐effects model to calculate the pooled estimate.
Cavallazzi 1996 gave results stratified by severity of hearing loss at enrolment. There were no statistically significant differences reported, however there was a trend suggested toward greater treatment effect with less severe presentation (RR for improvement of 50% with HBOT in mild hearing loss 1.42, 95% CI 0.79 to 2.55, P = 0.24; moderate loss 1.2, 95% CI 0.54 to 2.67, P = 0.66; severe loss 1.07, 95% CI 0.29 to 3.88, P = 0.92).
1.2 Proportion of participants with greater than 25% return of hearing at end of therapy
See Analysis 1.2.
1.2. Analysis.
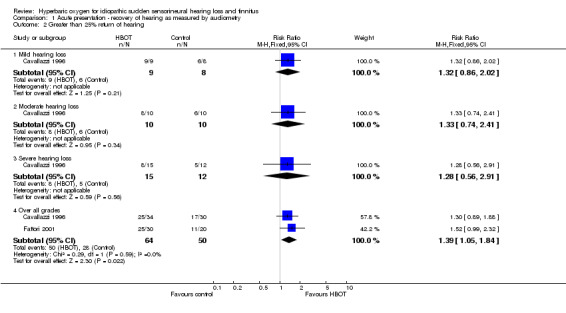
Comparison 1 Acute presentation ‐ recovery of hearing as measured by audiometry, Outcome 2 Greater than 25% return of hearing.
Two trials reported this outcome (Cavallazzi 1996; Fattori 2001), involving 114 participants (29% of the total participants in this review). Cavallazzi 1996 contributed 64 participants and Fattori 2001 50 participants. There was a statistically significant increase in the proportion of participants with more than 25% improvement in PTA assessed hearing loss over four frequencies following HBOT (RR of improvement with HBOT was 1.39, 95% CI 1.05 to 1.84, P = 0.02). There was no evidence of significant heterogeneity between trials (I2 = 0%) and therefore we used a fixed‐effect model to calculate the pooled estimate. The absolute risk difference of 22% is statistically significant, with a number needed to treat to achieve one extra good outcome of 5 (95% CI 3 to 20).
Cavallazzi 1996 gave results stratified by severity of hearing loss at enrolment. There were no statistically significant differences reported (RR for improvement of 25% with HBOT in mild hearing loss 1.32, 95% CI 0.86 to 2.02, P = 0.21; moderate loss 1.33, 95% CI 0.74 to 2.41, P = 0.34; severe loss 1.28, 95% CI 0.56 to 2.91, P = 0.56).
1.3 Mean improvement in PTA as a percentage of baseline
See Analysis 1.3.
1.3. Analysis.
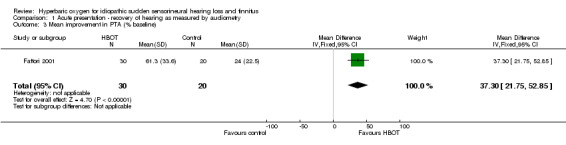
Comparison 1 Acute presentation ‐ recovery of hearing as measured by audiometry, Outcome 3 Mean improvement in PTA (% baseline).
Only one trial contributed results to this outcome (Fattori 2001), involving 50 participants (13% of the total), 30 (60%) randomised to HBOT and 20 (40%) to control. There was a mean improvement in PTA of 61% with the application of HBOT, versus an improvement of 24% in control participants, and this difference was statistically significant (mean difference (MD) 37% in favour of HBOT, 95% CI 22% to 53%).
1.4 Proportion of participants with absolute improvement in PTA more than 20 dB
See Analysis 1.4.
1.4. Analysis.
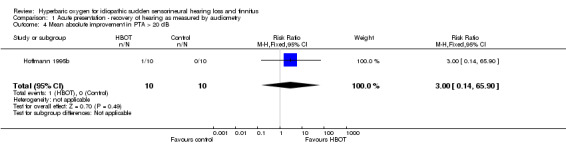
Comparison 1 Acute presentation ‐ recovery of hearing as measured by audiometry, Outcome 4 Mean absolute improvement in PTA > 20 dB.
Only one trial contributed results to this outcome (Hoffmann 1995b), involving 20 participants (5% of the total), 10 randomised to both HBOT and control. Only one patient improved and that individual was in the HBOT arm. There was no significant increase in the proportion of participants with more than 20 dB return of hearing following the application of HBOT (RR 3.0, 95% CI 0.14 to 65.9, P = 0.49).
1.5 Mean improvement in hearing over all frequencies (dB)
See Analysis 1.5.
1.5. Analysis.
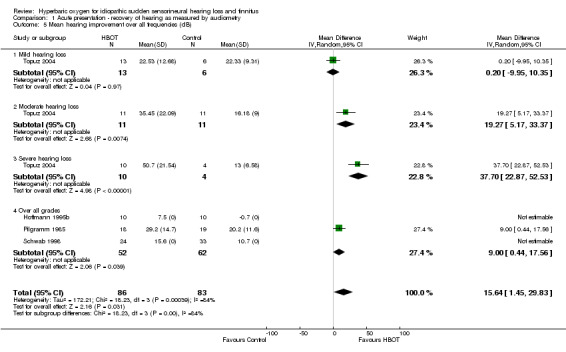
Comparison 1 Acute presentation ‐ recovery of hearing as measured by audiometry, Outcome 5 Mean hearing improvement over all frequencies (dB).
Four trials reported on this outcome (Hoffmann 1995b; Pilgramm 1985; Schwab 1998; Topuz 2004), involving 169 participants (43% of the total). Whilst Hoffmann 1995b and Schwab 1998 reported a greater mean improvement with HBOT, they did not report standard deviations and could not contribute to this analysis. Therefore only Pilgramm 1985 and Topuz 2004 (91 participants) contributed to the analysis. Over all participants in these trials, there was a statistically significant improvement with HBOT compared to control (MD 15.6 dB greater with HBOT, 95% CI 1.5 to 29.8, P = 0.03). There was evidence of significant heterogeneity between these trials (I2 = 84%) and this analysis uses a random‐effects model. Stratified analysis for severity on enrolment may account for some of the observed heterogeneity. There was a statistically significant improvement in those with severe hearing loss at enrolment (14 participants, MD 37.7 dB, 95% CI 22.9 to 52.5, P < 0.0001) and moderate hearing loss (22 participants, MD 19.3, 95% CI 5.2 to 33.4, P = 0.007), but not for mild hearing loss (19 participants, MD 0.2, 95% CI ‐10.0 to 10.4, P = 0.97). See Figure 2.
2.
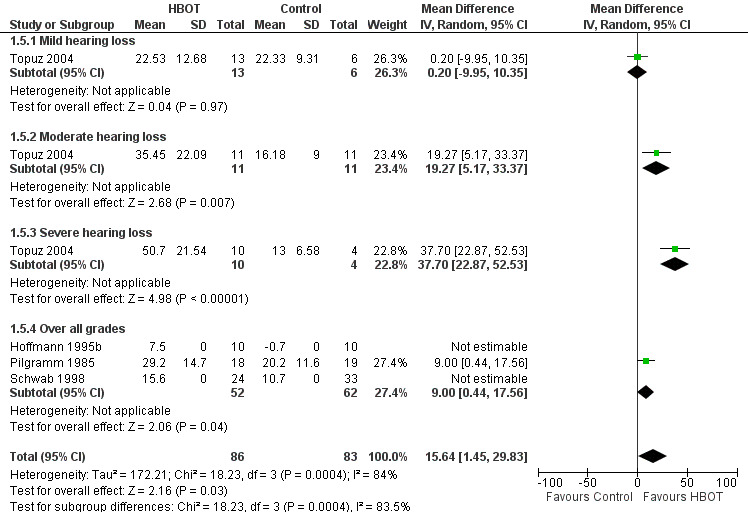
Forest plot of comparison: 1 Acute presentation ‐ recovery of hearing as measured by audiometry, outcome: 1.5 Mean hearing improvement over all frequencies (dB).
2. Chronic ISSHL: pure‐tone audiometric changes in hearing (comparison 02)
2.1 Proportion of participants with improvement in PTA
See Analysis 2.1.
2.1. Analysis.
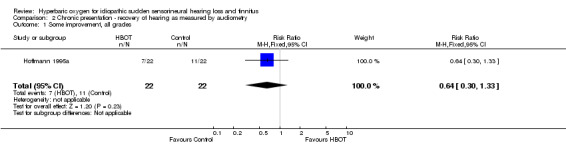
Comparison 2 Chronic presentation ‐ recovery of hearing as measured by audiometry, Outcome 1 Some improvement, all grades.
Only one trial contributed results to this outcome (Hoffmann 1995a), involving 44 participants (11% of the total), 22 randomised to each arm (HBOT and control). More individuals in the control group showed some improvement in hearing (seven versus 11), but the difference was not statistically significant (RR for improvement with HBOT 0.64, 95% CI 0.30 to 1.33, P = 0.23).
2.2 Mean improvement in hearing over all frequencies (dB)
See Analysis 2.2.
2.2. Analysis.
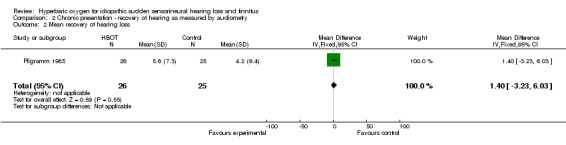
Comparison 2 Chronic presentation ‐ recovery of hearing as measured by audiometry, Outcome 2 Mean recovery of hearing loss.
Only one trial contributed results to this outcome (Pilgramm 1985), involving 51 participants (13% of the total), 26 randomised to HBOT and 25 to medical treatment alone. There were no significant differences between groups (MD 1.4 dB in favour of the HBOT group, 95% CI ‐3.2 to 6.0, P = 0.55).
3. Acute tinnitus: relief of tinnitus (comparison 03)
3.1 Mean improvement in tinnitus score
See Analysis 3.1.
3.1. Analysis.

Comparison 3 Acute presentation ‐ improvement of tinnitus, Outcome 1 Mean change in tinnitus score (0 to 10 scale).
Two trials reported on this outcome (Hoffmann 1995b; Schwab 1998), involving 53 participants (14% of the total). Schwab 1998 contributed 33 participants and Hoffmann 1995b 20 participants. While both trials reported a greater mean improvement in tinnitus (using a visual analogue scale between 0 and 10) in the HBOT arm than the control (3.1 and 0.4 units respectively), neither trial reported standard deviation around those means, making pooled analysis impossible.
3.2 Proportion of participants with improvement in tinnitus
See Analysis 3.2.
3.2. Analysis.
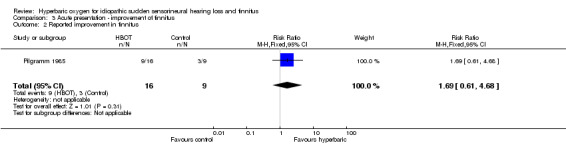
Comparison 3 Acute presentation ‐ improvement of tinnitus, Outcome 2 Reported improvement in tinnitus.
Only one trial contributed results to this outcome (Pilgramm 1985), involving 25 (out of 37 with acute ISSHL) participants with tinnitus (6% of the total), 16 randomised to HBOT and nine to medical therapy. More individuals in the HBOT group responded to therapy, but the difference was not significant (RR for improvement with HBOT 1.7, 95% CI 0.6 to 4.7, P = 0.31).
4. Chronic tinnitus: relief of tinnitus (comparison 04)
4.1 Proportion of participants with improvement in tinnitus score
See Analysis 4.1.
4.1. Analysis.
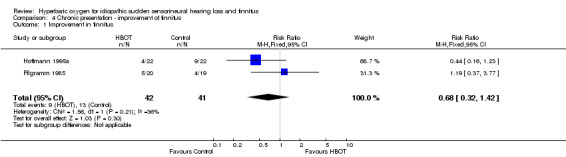
Comparison 4 Chronic presentation ‐ improvement of tinnitus, Outcome 1 Improvement in tinnitus.
Two trials contributed results to this outcome (Hoffmann 1995a; Pilgramm 1985), involving 83 participants (21% of the total), 42 randomised to HBOT and 41 to control. Individuals in the control group were more likely to show improvement, but the difference was not statistically significant (RR for improvement with HBOT 0.68, 95% CI 0.32 to 1.42, P = 0.3).
Secondary outcomes
5. Activities of daily living (ADL)
No trials reported any data on this outcome.
6. Subjective or objective improvements in depression or mood disturbance
No trials reported any data on this outcome.
7. Hearing handicap inventory change (and similar tool for tinnitus)
No trials reported any data on this outcome.
8. Adverse events associated with hyperbaric oxygen therapy and comparators
No trials reported any data on adverse events in a systematic way. Pilgramm 1985 reported that three participants suffered middle ear barotrauma and were withdrawn from HBOT, and that a further three were unable to complete HBOT because of confinement anxiety. It is not clear if these participants are included at analysis, either on an intention‐to‐treat or treatment received basis.
Three of these trials had low power to detect clinically significant differences in the main outcome of interest (a 50% improvement in average pure‐tone hearing loss or subjective tinnitus score) and three (Hoffmann 1995a; Pilgramm 1985; Schwab 1998) had > 80% power to detect a clinically significant improvement in hearing (> 20 dB more absolute improvement in HBOT group from the control group estimates). No trial reported a formal power or sample size calculation. Details are given in the table Characteristics of included studies.
Discussion
This review has included data from seven trials and we believe these represent all randomised human trials in this area, both published and unpublished, at the time of searching the databases. We found limited evidence that hyperbaric oxygen therapy (HBOT) improves hearing when applied as an early treatment in idiopathic sudden sensorineural hearing loss (ISSHL). There was some indication from the analysis of pooled data from two trials (Cavallazzi 1996; Fattori 2001) that HBOT increases the proportion of patients gaining more than 25% improvement in hearing, while one of those trials (Fattori 2001) suggested that there was a greater mean improvement in pure‐tone average (PTA) as a percentage of baseline following HBOT. Four trials also suggested improvements in mean hearing measured in decibels following HBOT (Hoffmann 1995b; Pilgramm 1985; Schwab 1998; Topuz 2004), with some evidence that more severely affected patients will improve most with the application of HBOT. We found no evidence that HBOT was useful in those individuals with long‐standing hearing loss or tinnitus of unknown aetiology.
Only seven trials with 392 participants were available for evaluation using our planned comparisons, and meta‐analysis was not appropriate or possible for a number of these. Other problems for this review were the poor methodological quality of many of these trials (see Risk of bias in included studies), variability and poor reporting of entry criteria, the variable nature and timing of outcomes, and poor reporting of both outcomes and methodology. In particular, given the high rate of spontaneous recovery from ISSHL, there is a possibility of bias due to different times to entry in these small trials, as well as from non‐blinded management decisions in all trials. The conclusions of this review are therefore to be interpreted with great caution.
These trials were published over a 19‐year period up to 2004, and are from a wide geographical area. We had planned to perform subgroup analyses with respect to the time between onset and therapy, the putative aetiology of the ISSHL or tinnitus, the dose of oxygen received (pressure, time and length of treatment course) and the nature of the comparative treatment modalities. None of these strategies were appropriate in the small number of pooled analyses. In particular, the Hoffmann 1995b trial, which differed significantly in that these authors admitted only participants who had failed to respond to two weeks of intensive multiple pharmacotherapy, did not contribute to any pooled analysis. Response rates stratified by severity of hearing loss on presentation were reported by Cavallazzi 1996 and Topuz 2004. Whilst Topuz 2004 suggested a trend to greater treatment effect in those more severely affected, this is not the case for the patients treated by Cavallazzi 1996. We have not subjected any possible trend to formal statistical testing. Patient inclusion criteria were not standard and were poorly reported in some trials. No standard severity scale was employed across these trials, and the time to entry varied from within 48 hours for Fattori 2001 to two weeks for Hoffmann 1995b, Pilgramm 1985, Schwab 1998 and Topuz 2004.
Pooling of data for clinical outcomes of interest could only be performed with respect to the proportion of patients showing an audiometric improvement in hearing of 50% or 25% from baseline to the end of therapy, and the absolute improvement in each group measured in dB. While the chance of a 50% improvement was not significantly increased following HBOT, the chance of a 25% improvement in hearing was (risk ratio (RR) 1.39, 95% confidence interval (CI) 1.05 to 1.84, P = 0.02). Heterogeneity did not seem to be an issue (I2 = 0%). This analysis suggests that we would need to treat five patients with HBOT in order to improve one person's hearing by 25% (NNT 5, 95% CI 3 to 20). Given the small number of participants and generally poor quality of these trials, this result needs to be interpreted with caution. Further, the clinical significance of a 25% improvement in hearing from baseline is not clear and will depend greatly on the starting level of impairment. No trial in this review has estimated any functional improvement. With respect to absolute improvements in pure‐tone audiometric thresholds, our analysis suggests a greater average improvement with HBOT of 15.6 dB more than control (95% CI 1.5 to 29.8, P = 0.03), and that this improvement may depend on the severity of hearing loss at presentation.
Three trials reported on improvements in tinnitus for patients with an early presentation (Hoffmann 1995b; Pilgramm 1985; Schwab 1998). While Schwab and Hoffmann both reported improvement in mean visual analogue scores for patients receiving HBOT, neither group of authors reported standard deviations around the mean and the significance of these changes is not clear. Pilgramm reported a non‐significant increase in the proportion of patients with improvements in tinnitus following HBOT (RR 1.7, P = 0.31). There was no suggestion that HBOT had a positive influence on chronic presentation of tinnitus in the two trials that reported this outcome (Hoffmann 1995a; Pilgramm 1985).
None of these trials systematically reported adverse effects with HBOT or control therapies, although Pilgramm 1985 did report six participants who were withdrawn from HBOT with either aural barotrauma or confinement anxiety. HBOT is regarded as a relatively benign intervention. There are few major adverse effects (pulmonary barotrauma, drug reactions, injuries or death related to chamber fire). There are a number of more minor complications that may occur commonly. Visual disturbance, usually reduction in visual acuity secondary to conformational changes in the lens, is very commonly reported ‐ perhaps in as many as 50% of those having a course of 30 treatments (Khan 2003). While the great majority of patients recover spontaneously over a period of days to weeks, a small proportion of patients continue to require correction to restore sight to pre‐treatment levels. The second most common adverse effect associated with HBOT is barotrauma. Barotrauma can affect any air‐filled cavity in the body (including the middle ear, lungs and respiratory sinuses) and occurs as a direct result of compression. Aural barotrauma is by far the most common as the middle ear air space is small, largely surrounded by bone and the sensitive tympanic membrane, and usually requires active effort by the patient in order to inflate the middle ear through the Eustachian tube on each side. Barotrauma is thus not a consequence of HBOT directly, but rather of the physical conditions required to administer it. Most episodes of barotrauma are mild, easily treated or recover spontaneously and do not require the therapy to be abandoned. Less commonly, HBOT may be associated with acute neurological toxicity manifesting as seizure.
While we have made every effort to locate further unpublished data, it remains possible that this review is subject to a positive publication bias, with generally favourable trials more likely to achieve reporting. With regard to long‐term outcomes following HBOT and any effect on the quality of life for these patients, we have located no relevant data.
Authors' conclusions
Implications for practice.
There is limited evidence from methodologically poor studies that hyperbaric oxygen therapy (HBOT) improves hearing in patients with idiopathic sudden sensorineural hearing loss (ISSHL) who present within two weeks of hearing loss, and some indication that HBOT might improve tinnitus presenting in the same time frame. However, there is no evidence that any improvement is functionally important. Thus, the routine use of HBOT in these patients cannot be justified by this review. The small number of studies, the modest numbers of patients, and the methodological and reporting inadequacies of the primary studies included in this review demand a cautious interpretation. Moreover, this review does not give any information regarding the safety of HBOT for these patients.
Implications for research.
Given the findings of improved hearing with the use of HBOT in these patients, there is a case for large randomised trials of high methodological rigour in order to define the true extent of benefit (if any) from the administration of HBOT. Specifically, more information is required on the subset of disease severity and time of presentation most likely to be associated with a benefit from this therapy. The effect of differing oxygen dosage and effect of other therapies administered simultaneously is not known. Any future trials would need to consider in particular:
appropriate sample sizes with power to detect expected differences;
careful definition and selection of target patients;
appropriate range of oxygen doses per treatment session (pressure and time) as well as total number of treatments;
appropriate and carefully defined comparator therapy;
use of an effective sham therapy;
effective and explicit blinding of outcome assessors;
appropriate outcome measures including all those listed in this review;
careful elucidation of any adverse effects; and
the cost‐utility of the therapy.
What's new
| Date | Event | Description |
|---|---|---|
| 10 July 2012 | New citation required but conclusions have not changed | Seven further citations found but excluded after full‐text review. We amended the 'Risk of bias' tables with changes to risk assessment of several studies in order to conform with the relevant section of the Cochrane Handbook for Systematic Reviews of Interventions. We also added a 'Summary of findings' table and a PRISMA flow diagram. |
| 10 July 2012 | New search has been performed | New searches run. |
History
Protocol first published: Issue 2, 2004 Review first published: Issue 1, 2005
| Date | Event | Description |
|---|---|---|
| 16 September 2009 | New search has been performed | New searches completed and one new study included. 'Risk of bias' tables completed. Two studies previously not classified are now excluded. |
| 27 October 2008 | Amended | Converted to new review format. |
| 14 November 2006 | New citation required and conclusions have changed | Substantive amendment. One new study included. |
Acknowledgements
We acknowledge the assistance of the Cochrane Advanced Reviewer Support Service provided by the Australasian Cochrane Centre in undertaking literature location and reference management. We would also like to thank Jenny Bellorini, Carolyn Doree and the editors of the Cochrane Ear, Nose and Throat Group for their invaluable advice and guidance.
Appendices
Appendix 1. Search strategies
| CENTRAL | EMBASE (Ovid) | CINAHL (Ovid) | AMED (Ovid) |
| #1 HYPERBARIC OXYGENATION single term (MeSH) #2 oxygen* #3 HBOT #4 HBO #5 #1 or #2 or #3 or #4 #6 HEARING LOSS, SUDDEN single term (MeSH) #7 HEARING LOSS, SENSORINEURAL single term (MeSH) #8 sudden* #9 #7 and #8 #10 sshl #11 snhl #12 ishl #13 isshl #14 issnhl #15 ssnhl #16 (sudden near hearing) #17 (sudden near deaf*) #18 #6 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 #19 TINNITUS single term (MeSH) #20 tinnitus #21 #19 or #20 #22 #18 or #21 #23 #5 and #22 | #1 exp HYPERBARIC OXYGEN/ #2 oxygen$.tw. #3 HBOT.tw. #4 HBO.tw. #5 or/1‐4 #6 exp SUDDEN DEAFNESS/ #7 exp PERCEPTION DEAFNESS/ #8 sudden$.tw. #9 7 and 8 #10 sshl.tw. #11 snhl.tw. #12 ishl.tw. #13 isshl.tw. #14 issnhl.tw. #15 ssnhl.tw. #16 (sudden adj3 hearing).tw. #17 (sudden adj3 deaf$).tw. #18 or/6,9‐17 #19 TINNITUS/ #20 tinnitus.tw. #21 (ear adj1 (buzz$ or ring$)).tw. #22 or/19‐21 #23 18 or 22 #24 5 and 23 | #1 exp HYPERBARIC OXYGENATION/ #2 oxygen$.tw. #3 HBOT.tw. #4 HBO.tw. #5 or/1‐4 #6 exp HEARING LOSS, SENSORINEURAL/ #7 sudden$.tw. #8 6 and 7 #9 sshl.tw. #10 snhl.tw. #11 ishl.tw. #12 isshl.tw. #13 issnhl.tw. #14 ssnhl.tw. #15 (sudden adj3 hearing).tw. #16 (sudden adj3 deaf$).tw. #17 or/8‐16 #18 TINNITUS/ #19 tinnitus.tw. #20 (ear adj1 (buzz$ or ring$)).tw. #21 or/18‐20 #22 17 or 21 #23 5 and 22 | #1 exp HYPERBARIC OXYGEN/ #2 oxygen$.tw. #3 HBOT.tw. #4 HBO.tw. #5 or/1‐4 #6 sshl.tw. #7 snhl.tw. #8 ishl.tw. #9 isshl.tw. #10 issnhl.tw. #11 ssnhl.tw. #12 (sudden adj3 hearing).tw. #13 (sudden adj3 deaf$).tw. #14 or/6‐13 #15 TINNITUS/ #16 tinnitus.tw. #17 15 or 16 #18 5 and (14 or 17) |
| Cochrane ENT Disorders Group Trials Register | PubMed | Web of Science/BIOSIS Previews (Web of Knowledge) | ICTRP |
| (hyperbaric OR hbo* OR oxygen* OR o2) AND (sudden OR sshl OR snhl OR ishl OR isshl OR issnhl OR ssnhl OR tinnit*) | #1 "Hyperbaric Oxygenation"[Mesh] #2 hyperbaric [tiab] OR HBOT [tiab] #3 #1 OR #2 #4 "Hearing Disorders"[Mesh] #5 sudden* [tiab] #6 #4 AND #5 #7 (sudden AND (hearing [tiab] OR deaf* [tiab] OR hypoacusis [tiab])) #8 sshl [tiab] OR snhl [tiab] OR ishl [tiab] OR isshl [tiab] OR issnhl [tiab] OR ssnhl [tiab] #9 "Tinnitus"[Mesh] #10 tinnit* [tiab] #11 (ear [ti] AND (buzz* [ti] or ring* [ti] OR roar* [ti] OR click* [ti] OR puls* [ti])) #12 #6 OR #7 OR #8 OR #9 OR #10 OR #11 #13 #3 AND #12 | #1 TS=(hyperbaric OR hbot OR oxygen* OR o2) #2 TS=(tinnit*) #3 TS=(hearing or deaf* or sshl OR snhl OR ishl OR isshl OR issnhl OR ssnhl) #4 #3 OR #2 #5 #4 AND #1 | sudden AND hyperbaric OR sudden AND hbo* OR hearing AND hyperbaric OR hearing AND hbo* OR hearing AND oxygen* OR tinnitus AND hyperbaric OR tinnitus AND hbo* OR tinnitus AND oxygen* |
Data and analyses
Comparison 1. Acute presentation ‐ recovery of hearing as measured by audiometry.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Greater than 50% return of hearing | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Mild hearing loss | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.79, 2.55] |
| 1.2 Moderate hearing loss | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 1.2 [0.54, 2.67] |
| 1.3 Severe hearing loss | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.29, 3.88] |
| 1.4 Over all grades | 2 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.85, 2.78] |
| 2 Greater than 25% return of hearing | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Mild hearing loss | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.86, 2.02] |
| 2.2 Moderate hearing loss | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.74, 2.41] |
| 2.3 Severe hearing loss | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.56, 2.91] |
| 2.4 Over all grades | 2 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.05, 1.84] |
| 3 Mean improvement in PTA (% baseline) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 37.3 [21.75, 52.85] |
| 4 Mean absolute improvement in PTA > 20 dB | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.14, 65.90] |
| 5 Mean hearing improvement over all frequencies (dB) | 4 | 169 | Mean Difference (IV, Random, 95% CI) | 15.64 [1.45, 29.83] |
| 5.1 Mild hearing loss | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐9.95, 10.35] |
| 5.2 Moderate hearing loss | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 19.27 [5.17, 33.37] |
| 5.3 Severe hearing loss | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 37.7 [22.87, 52.53] |
| 5.4 Over all grades | 3 | 114 | Mean Difference (IV, Random, 95% CI) | 9.0 [0.44, 17.56] |
Comparison 2. Chronic presentation ‐ recovery of hearing as measured by audiometry.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Some improvement, all grades | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.30, 1.33] |
| 2 Mean recovery of hearing loss | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐3.23, 6.03] |
Comparison 3. Acute presentation ‐ improvement of tinnitus.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in tinnitus score (0 to 10 scale) | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Reported improvement in tinnitus | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.61, 4.68] |
Comparison 4. Chronic presentation ‐ improvement of tinnitus.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in tinnitus | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.32, 1.42] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cavallazzi 1996.
| Methods | Pseudo‐randomised controlled trial | |
| Participants | 64 participants with a diagnosis of ISSHL, time course unknown Stratified into mild, moderate, severe and 'deep' | |
| Interventions | Control (30): multiple‐drug therapy consisting of heparin, betamethasone, nicotinic acid, flunarizine, citidine, phosphocholine, dextran, vitamins, neurotropic and antiviral drugs (doses not given) HBOT (34): pharmacotherapy as for control group plus oxygen at 2.5 ATA for 60 minutes daily for 15 sessions over 3 weeks |
|
| Outcomes | PTA recovery, stratified into percentage improvement shown at 4 strata of severity at presentation | |
| Notes | Rank 2 for sample size (see Data extraction and management): power to detect significant difference in proportion with 50% recovery of hearing is < 80%. Further details requested from authors but no reply to date | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not clearly described and may not have been truly random "Sixty‐two patients were included in this study and divided into two groups..." |
| Allocation concealment (selection bias) | Unclear risk | No mention of whether or not they attempted to conceal allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded for any party |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | Apparently reported main outcomes as planned |
| Other bias | Low risk | No obvious source of bias |
Fattori 2001.
| Methods | Randomised controlled trial | |
| Participants | 50 participants with ISSHL referred within 48 hours. Stratified into mild, moderate and severe. | |
| Interventions | Control (20): vasodilator therapy: 10‐day course iv 200 mg/day buflomedil in 250 ml physiological solution. No sham treatment. HBOT (30): 10 once‐daily treatments breathing 100% oxygen at 2.2 ATA for 90 minutes |
|
| Outcomes | PTA recovery, stratified into percentage improvement shown at 3 strata of severity at presentation Mean PTA recovery |
|
| Notes | Rank 4 for sample size: power to detect an improvement in the proportion of patients achieving a 50% return of hearing from 25% (control) to 50% (HBOT) is less than 80% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clearly described "Thirty of these patients were randomly assigned to undergo treatment with HOT and 20 were selected for treatment with intravenous vasodilation." |
| Allocation concealment (selection bias) | Unclear risk | No description of any attempt to conceal the allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of any party |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | No indication of missing outcomes |
| Other bias | Low risk | No obvious other biases |
Hoffmann 1995a.
| Methods | Randomised controlled trial | |
| Participants | 44 participants with ISSHL for more than 6 months | |
| Interventions | Control (22): air breathing at 1.5 ATA for 45 minutes daily, 5 days each week for 3 weeks HBOT (22): 100% oxygen breathing at 1.5 ATA on the same schedule as controls |
|
| Outcomes | Improved hearing and tinnitus | |
| Notes | Rank 2 for sample size (chronic hearing loss): power > 80% to detect an increase in proportion of participants with significant return of hearing | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not clear "forty‐five patients....were randomised into a HBO group and a control group" |
| Allocation concealment (selection bias) | Unclear risk | No mention of any attempt to conceal allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and outcome assessors blind "In order to exclude uncontrolled placebo effects, the control group were dealt completely in the same manner as the HBO group ...in the chamber." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient lost, no explanation |
| Selective reporting (reporting bias) | Low risk | No indication of missing outcomes |
| Other bias | Low risk | No obvious other sources of bias |
Hoffmann 1995b.
| Methods | Randomised controlled trial | |
| Participants | 20 participants with ISSHL, with or without tinnitus. All participants had no improvement after 14 days of pharmacological treatment with hydroxyethyl starch, pentoxifylline and cortisone. | |
| Interventions | Control (10): no treatment HBOT (10): 100% oxygen at 1.5 ATA for 45 minutes daily, 5 days each week for 2 to 4 weeks (10 to 20 sessions) |
|
| Outcomes | Audiometry at 3 months, subjective tinnitus scale | |
| Notes | Rank 6 for sample size: power to detect mean hearing improvement of 20 dB more in active group than control < 80% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No clear statement of sequence generation "20 patients were randomised " |
| Allocation concealment (selection bias) | Unclear risk | No mention of concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding for any party "...an untreated control group..." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No apparent losses to follow‐up, numbers not clearly reported |
| Selective reporting (reporting bias) | Low risk | No indication of missing outcomes |
| Other bias | Low risk | No obvious other sources of bias |
Pilgramm 1985.
| Methods | Randomised controlled trial | |
| Participants | 1. Acute hearing loss (< 14 days since onset). 37 patients with hearing loss and/or tinnitus, 18 allocated to HBOT group and 19 to control. 2. Chronic hearing loss (14 days to 1 year since onset). 51 patients with hearing loss and/or tinnitus; 26 allocated to HBOT, 25 to control. |
|
| Interventions | Control: vasodilator therapy: 500 ml of 10% dextran‐40 and sorbitol 5% daily for 14 days, plus daily naphtidrofuryl hydogenaxalate 600 mg and vitamin B orally HBOT: as above, plus 60 minutes daily breathing 100% oxygen at 2.5 ATA for 10 days |
|
| Outcomes | Improvement in average hearing threshold by audiometry and improvement in tinnitus. Both assessed at 4 weeks from presentation. | |
| Notes | Translated in detail by MP Rank 5 for sample size (acute hearing loss): power to detect mean hearing improvement of 20 dB more in active group than control > 80% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence "All patients were randomized into a specific treatment group by computer and were maintained in these groups." |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | No description of blinding for any party |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 individuals withdrew from treatment but it is not clear if they contributed to the analysis |
| Selective reporting (reporting bias) | Low risk | No indication of missed outcomes |
| Other bias | Low risk | No other obvious source of bias |
Schwab 1998.
| Methods | Randomised controlled trial | |
| Participants | 75 participants with sudden hearing loss with at least 20 dB loss in 1 or more frequencies and/or tinnitus, seen within 2 weeks and without any prior therapy | |
| Interventions | Control (38): no treatment HBOT (37): 100% oxygen at 1.5 ATA for 45 minutes daily, 5 days each week for 2 to 4 weeks (10 to 20 sessions) |
|
| Outcomes | Audiometric hearing and tinnitus improvement | |
| Notes | Rank 1 for sample size: power > 80% to detect an increase in proportion of participants with significant return of hearing | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of allocation sequence "75 patients were randomly assigned to one of two treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | No mention of attempt to conceal allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attempt to blind any party |
| Incomplete outcome data (attrition bias) All outcomes | High risk | This trial did not report results for seven participants with ISSHL and 11 with tinnitus. No strategy for dealing with this was identified |
| Selective reporting (reporting bias) | Low risk | No indication of missed outcomes |
| Other bias | Low risk | No other obvious source of bias |
Topuz 2004.
| Methods | Randomised controlled trial | |
| Participants | 55 participants with ISSHL seen within 2 weeks and without any prior therapy | |
| Interventions | Control (21): prednisone 1 mg/kg/day/2 weeks, rheomacrodex 500 ml/day/5 days, diazepam 5 mg bd and pentoxiphylline 200 mg bd HBOT (30): as above plus oxygen at 2.5 ATA for 90 minutes to 25 treatments in 3 weeks |
|
| Outcomes | Mean PTA recovery (dB) | |
| Notes | Rank 3 for sample size:power to detect an improvement in the proportion of patients achieving a 50% return of hearing from 25% (control) to 50% (HBOT) is less than 80% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No indication of the method of sequence generation "Fifty‐one hospitalized patients were prospectively grouped at random..." |
| Allocation concealment (selection bias) | Unclear risk | No indication of an attempt at allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | No attempt to blind any party |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss to follow‐up. All patients reported to contribute to outcomes. |
| Selective reporting (reporting bias) | Low risk | No indication of missing outcomes |
| Other bias | Low risk | No other obvious source of bias |
ATA: atmosphere absolute bd: twice a day HBOT: hyperbaric oxygen therapy ISSHL: idiopathic sudden sensorineural hearing loss iv: intravenous PTA: pure‐tone average
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Blagovesh 1990 | ALLOCATION: Not a random comparison of HBOT versus an alternative |
| Cuixia 2008 | ALLOCATION: Non‐random controlled study |
| Dauman 1993 | ALLOCATION: Randomised controlled trial PARTICIPANTS: Patients with sudden hearing loss INTERVENTIONS: Both groups received hyperbaric oxygen |
| Dundar 2007 | ALLOCATION: No indication of randomisation and included post‐viral hearing loss |
| Joachims 1978 | ALLOCATION: Case series only |
| Lamm 1995 | ALLOCATION: Review only, no new data |
| Lamm 1998 | ALLOCATION: Review only, no new data |
| Li 2010 | ALLOCATION: Not a random comparison of HBOT versus an alternative |
| Lina 2010 | ALLOCATION: Not a random comparison of HBOT versus an alternative |
| Sano 1988 | ALLOCATION: Case series |
| Sparacia 2003 | ALLOCATION: Randomised controlled trial PARTICIPANTS: Patients with sudden hearing loss INTERVENTIONS: All patients received hyperbaric oxygen |
| Tisch 2000 | ALLOCATION: Review, no new data |
| Wang 2000 | ALLOCATION: Not a randomised comparison |
| Xiao 1986 | ALLOCATION: Randomised controlled trial PARTICIPANTS: Patients with sudden hearing loss INTERVENTION: All patients received hyperbaric oxygen |
HBOT: hyperbaric oxygen therapy
Characteristics of ongoing studies [ordered by study ID]
Barthelemy 2002.
| Trial name or title | Hyperbaric oxygen in the treatment of sudden deafness after failure of previous medical treatment start up of a multicentric, prospective and randomised study |
| Methods | Randomised controlled trial |
| Participants | Adult patients with acute sudden sensorineural hearing loss and failed medical therapy for 2 weeks |
| Interventions | Hyperbaric oxygen therapy 10 treatments, 1 per day, 2.5 ATA, 100% O2 (10 to 15 minutes compression on air, 70 minutes of oxygen breathing, 10 minutes of decompression on air) |
| Outcomes | Mean hearing loss compared to good ear, mean gain, VAS for tinnitus |
| Starting date | 2002 |
| Contact information | Nil given |
| Notes | Slow recruitment |
Bennett 2010.
| Trial name or title | Hyperbaric oxygen for hearing loss and tinnitus |
| Methods | Randomised controlled trial |
| Participants | Adult patients with acute sudden sensorineural hearing loss (within 2 weeks) |
| Interventions | Hyperbaric oxygen therapy daily at 2.0 ATA for 120 minutes to 20 treatments |
| Outcomes | Percentage hearing loss return (% of starting level on PTA), absolute return (decibels), Amsterdam Hearing Inventory, VAS for tinnitus |
| Starting date | 23 July 2010 |
| Contact information | m.bennett@unsw.edu.au |
| Notes | Slow recruitment (personal correspondence) |
ATA: atmosphere absolute PTA: pure‐tone average VAS: visual analogue scale
Differences between protocol and review
At the 2009 update of this review we adopted the new Cochrane 'Risk of bias' tool for quality assessment of included studies.
Contributions of authors
Bennett: conceived review, primary author of all sections, handsearching, critical appraisal and statistics.
Kertesz: assistance with text, content expert in otorhinolaryngology, critical appraisal of selected articles.
Yeung: assistance with text, content expert in otorhinolaryngology, critical appraisal of selected articles.
Perleth: translation of German language studies, assistance with critical appraisal, interpretation of results and assistance with text.
Lehm: assistance with handsearching and preparation of text, context expert in hyperbaric medicine.
Sources of support
Internal sources
-
Department of Diving and Hyperbaric Medicine, Australia.
Use of non‐clinical time and hardware supply.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Cavallazzi 1996 {published data only}
- Cavallazzi G, Pignataro L, Capaccio P. Italian experience in hyperbaric oxygen therapy for idiopathic sudden sensorineural hearing loss. Proceedings of the International Joint Meeting on Hyperbaric and Underwater Medicine. Bologna: Grafica Victoria, 1996:647‐9.
Fattori 2001 {published data only}
- Fattori B, Berrettini S, Casani A, Nacci A, Vito A, Iaco G. Sudden hypoacusis treated with hyperbaric oxygen therapy. Ear Nose and Throat Journal 2001;80(9):655‐60. [MEDLINE: ] [PubMed] [Google Scholar]
Hoffmann 1995a {published data only}
- Desloovere C, Knecht R, Bohmer D, Hoffmann G. Hyperbaric oxygen therapy by chronic tinnitus ‐ a double‐blind study [Hyperbare Sauerstofftherapie bei chronischem Tinnitus ‐ Eine Doppelblind‐Studie]. European Archives of Oto‐Rhino‐Laryngology 1993;Suppl 2:225‐6. [Google Scholar]
- Hoffmann G, Bohmer D, Desloovere C. Hyperbaric oxygenation as a treatment of chronic forms of inner ear hearing loss and tinnitus. Proceedings of the Eleventh International Congress on Hyperbaric Medicine. Flagstaff, Az: Best Publishing, 1995:141‐5.
Hoffmann 1995b {published data only}
- Hoffmann G, Bohmer D, Desloovere C. Hyperbaric oxygenation as a treatment for sudden deafness and acute tinnitus. Proceedings of the Eleventh International Congress on Hyperbaric Medicine. Flagstaff, Az: Best Publishing, 1995:146‐51.
Pilgramm 1985 {published data only}
- Pilgramm M, Lamm H, Schumann K. Hyperbaric oxygen therapy in sudden deafness [Zur hyperbaren Sauerstofftherapie beim Hörsturz]. Laryngologie, Rhinologie, Otologie 1985;64(7):351‐4. [PubMed] [Google Scholar]
Schwab 1998 {published data only}
- Schwab B, Flunkert C, Heermann R, Lenarz T. HBO in the therapy of cochlear dysfunctions ‐ first results of a randomized study. EUBS Diving and Hyperbaric Medicine, Collected manuscripts of XXIV Annual Scientific Meeting of the European Underwater and Baromedical Society. Stockholm: EUBS, 1998:40‐2.
Topuz 2004 {published data only}
- Topuz E, Yigit O, Cinar U, Seven H. Should hyperbaric oxygen be added to treatment in idiopathic sudden sensorineural hearing loss?. European Archives of Oto‐Rhino‐Laryngology 2004;261(7):393‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Blagovesh 1990 {published data only}
- Blagoveshchenskaia NS. Treatment and prevention of acute sensorineural hearing loss. Vestnik Otorinolaringologii 1990;6:4‐12. [PubMed] [Google Scholar]
Cuixia 2008 {published data only}
- Cuixia X, Xinli Z, Lin W. Clinical observation of hyperbaric oxygen and ginaton in the treatment of sudden deafness. 16th International Congress on Hyperbaric Medicine. 2008.
Dauman 1993 {published data only}
- Dauman R, Poisot D, Cros AM, Zennaro O, Bertrand B, Duclos JY, et al. Sudden hearing loss: comparative randomized study of two modalities of hyperbaric oxygen therapy in association with naftidrofuryl [Surdites brusques: etude comparative randomisee de deux modes d'administration de l'oxygenotherapie hyperbare associee au naftidrofuryl]. Revue de Laryngologie 1993;114(1):53‐8. [PubMed] [Google Scholar]
Dundar 2007 {published data only}
- Dundar K, Gumus T, Ay H, Yetiser S, Ertugrul E. Effectiveness of hyperbaric oxygen on sudden sensorineural hearing loss: prospective clinical research. Journal of Otolaryngology 2007;36(1):32‐7. [DOI] [PubMed] [Google Scholar]
Joachims 1978 {published data only}
- Joachims HZ, Monies‐Chass I, Eliachar I. Hyperbaric oxygen in the treatment of sudden deafness. Harefuah 1978;95(7):202‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Lamm 1995 {published data only}
- Lamm H. The influence of hyperbaric oxygen therapy on tinnitus and hearing loss in acute and chronic inner ear damage. Oto‐Rhino‐Laryngologia Nova 1995;5:3‐4. [SN 1014‐8221] [Google Scholar]
Lamm 1998 {published data only}
- Lamm K, Lamm H, Arnold W. Effect of hyperbaric oxygen therapy in comparison to conventional or placebo therapy or no treatment in idiopathic sudden hearing loss, acoustic trauma, noise‐induced hearing loss and tinnitus. A literature survey. Advances in Otorhinolaryngology 1998;54:86‐99. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Li 2010 {published data only}
- Li Z, Yang H, Xiao Y, Wu Y, Yang J, Li Z. Curative effect analysis of hyperbaric oxygen and medication in the treatment of sudden hearing loss. China Modern Medicine 2010;8:1. [Google Scholar]
Lina 2010 {published data only}
- Lina H, Zhaowen Y, Yuqin D. An effective appraisal of hyperbaric oxygen combined with drugs therapy for sudden deafness. Journal of Shantou University Medical College 2001;1:1. [Google Scholar]
Sano 1988 {published data only}
- Sano H, Okamoto M, Hirayama M, Ono Y, Nitta M. Hearing recovery in sudden deafness with profound hearing loss. Nippon Jibiinkoka Gakkai Kaiho 1988;101(6):836‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sparacia 2003 {published data only}
- Sparacia B, Sparacia G. Hyperbaric oxygen therapy in treatment of sudden deafness. Acta Medica Mediterranea 2003;19(2):95‐102. [SN 0393‐6384] [Google Scholar]
Tisch 2000 {published data only}
- Tisch M, Maier H. Acute tinnitus ‐ reperfusion therapy versus hyperbaric oxygen therapy. Notfall Medizin 2000;26:1‐2. [SN 0341‐2903] [Google Scholar]
Wang 2000 {published data only}
- Wang W, Xu R, Lu R, Yan Y. A reappraisal of hyperbaric oxygenation effect and study on serum malondialdehyde and superoxide dismutase in patients with sudden deafness. Zhonghua Er Bi Yan Ke Za Zhi 2000;35(5):356‐8. [PubMed] [Google Scholar]
Xiao 1986 {published data only}
- Xiao ZX. Comparative analysis of the therapeutic effect of HBO and HBO combined with vasodilator agents in 200 cases of deafness. Journal of Hyperbaric Medicine 1986;1:192‐3. [Google Scholar]
References to studies awaiting assessment
Assenova 2012 {published data only}
- Assenova K. Hyperbaric oxygenation for treatment of hearing loss and tinnitus. 83rd Annual Meeting of the German Society of Oto‐Rhino‐Laryngology, Head and Neck Surgery. 2012.
Gui‐lian 2009 {published data only}
- Gui‐lian J. Clinical observation of batroxobin combined acupuncture point and hyperbaric oxygen to treat sudden deafness. Hebei Medicine 2009; Vol. 6.
Nie 2007 {published data only}
- Nie C, Tang M. Hyperbaric oxygen therapeutics in treatment of sudden hearing loss. Chinese Journal of Rehabilitation Medicine 2007;6:1. [Google Scholar]
Qiao 2006 {published data only}
- Qiao X, Zhang H. Clinical observation of hyperbaric oxygen and ginaton in the treatment of sudden deafness. Journal of Otolaryngology and Ophthalmology of Shandong University 2006; Vol. 5.
References to ongoing studies
Barthelemy 2002 {published data only}
- Barthélémy A, Germonpré P, Heiden C, Jansen E, Rocco M. Hyperbaric oxygen in the treatment of sudden deafness after failure of previous medical treatment start up of a multicentric, prospective and randomised study. http://gtuem.praesentiert‐ihnen.de/tools/literaturdb/project2/pdf/2002_P04%20Barthelemy.pdf 2002.
Bennett 2010 {published data only}
- Bennett MH, Kertesz T, Lehm JP. Hyperbaric oxygen therapy for sudden sensorineural hearing loss. http://www.anzctr.org.au/trial_view.aspx?id=308282 2010 (accessed 11 July 2012). [ACTRN12609000619246]
Additional references
Agarwal 2009
- Agarwal L, Pothier DD. Vasodilators and vasoactive substances for idiopathic sudden sensorineural hearing loss. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003422.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
ATA 2001
- American Tinnitus Association. Website of the American Tinnitus Association. http://www.ata.org/ (accessed April 2004).
Baldo 2006
- Baldo P, Doree C, Lazzarini R, Molin P, McFerran DJ. Antidepressants for patients with tinnitus. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003853.pub2] [DOI] [PubMed] [Google Scholar]
Belal 1980
- Belal A. Pathology of vascular sensorineural hearing impairment. Laryngoscope 1980;90:1831‐9. [DOI] [PubMed] [Google Scholar]
Cacace 2003
- Cacace AT. Expanding the biological basis of tinnitus: crossmodal origins and the role of neuroplasticity. Hearing Research 2003;175(1‐2):112‐32. [DOI] [PubMed] [Google Scholar]
Coles 1990
- Coles DA, Davis AC. Tinnitus: its epidemiology and management. 14th Danavox Jubilee Foundation, Copenhagen. 1990.
Dauman 1992
- Dauman R, Tyler RS. Some considerations on the classification of tinnitus. Proceedings of the Fourth International Tinnitus Seminar, Bordeaux. 1992:225‐9.
Haberkamp 1999
- Haberkamp TJ, Tanyeri HM. The management of idiopathic sudden sensorineural hearing loss. American Journal of Otolaryngology 1999;20:587‐92. [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hughes 1996
- Hughes GB, Freedman MA, Haberkamp TJ, Guay ME. Sudden sensorineural hearing loss. Otolaryngologic Clinics of North America 1996;29:393‐405. [PubMed] [Google Scholar]
Jastreboff 1990
- Jastreboff PJ. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neuroscience Research 1990;8(4):221‐54. [DOI] [PubMed] [Google Scholar]
Kaltenbach 2000
- Kaltenbach JA. Neurophysiologic mechanisms of tinnitus. Journal of the American Academy of Audiology 2000;11(3):125‐37. [PubMed] [Google Scholar]
Khan 2003
- Khan B, Evans AW, Easterbrook M. Refractive changes in patients undergoing hyperbaric oxygen therapy: a prospective study. Undersea and Hyperbaric Medicine 2003;24(Suppl):9. [Google Scholar]
Luxon 1993
- Luxon LM. Tinnitus: its causes, diagnosis and treatment. BMJ 1993;306:1490‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mattox 1977
- Mattox DE, Simmons FB. Natural history of sudden sensorineural hearing loss. Annals of Otolaryngology, Rhinolaryngology and Laryngology 1977;86(4):463‐80. [DOI] [PubMed] [Google Scholar]
Noell 2003
- Noell CA, Meyerhoff WL. Tinnitus. Diagnosis and treatment of this elusive symptom. Geriatrics 2003;58(2):28‐34. [PubMed] [Google Scholar]
Parnes 1997
- Parnes SM. Current concepts in the clinical management of patients with tinnitus. European Archives of Otorhinolaryngology 1997;254:406‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Stephens 1991
- Stephens D, Hetu R. Impairment, disability and handicap in audiology: towards a consensus. Audiology 1991;30:185‐200. [DOI] [PubMed] [Google Scholar]
Stokroos 1996a
- Stokroos RJ, Albers FW, Cauwenberge P. Diagnosis and treatment of idiopathic sudden sensorineural hearing loss (ISSHL): a survey in the Netherlands and Flanders. Acta Otorhinolaryngologica Belgica 1996;50:237‐45. [PubMed] [Google Scholar]
Sullivan 1992
- Sullivan M, Katon WJ, Russo J, Dobie R, Sakai C. Somatization, co‐morbidity, and the quality of life: measuring the effect of depression upon chronic medical illness. Psychiatric Medicine 1992;10(3):61‐76. [PubMed] [Google Scholar]
Sullivan 1994
- Sullivan M, Katon W, Russo J, Dobie R, Sakai C. Coping and marital support as correlates of tinnitus disability. General Hospital Psychiatry 1994;16(4):259‐66. [DOI] [PubMed] [Google Scholar]
Thurmond 1998
- Thurmond M, Amedee RG. Sudden sensorineural hearing loss: etiologies and treatments. Journal of the Louisiana State Medical Society 1998;150(5):200‐3. [PubMed] [Google Scholar]
Yoon 1990
- Yoon TH, Paparella MM, Schachern PA, Alleva M. Histopathology of sudden hearing loss. Laryngoscope 1990;100(7):707‐15. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bennett 2007
- Bennett MH, Kertesz T, Perleth M, Yeung P. Hyperbaric oxygen for idiopathic sudden sensorineural hearing loss and tinnitus. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD004739.pub3] [DOI] [PubMed] [Google Scholar]


