Abstract
Environmental temperature is increasing while natural populations are forced to adjust their life cycle to new conditions, resulting in the expression of new phenotypic traits. Still, the links between these new environmental conditions and the subsequent phenotypic expressions are not fully explored. Here, we conducted manipulative experiments with embryos of the marine gastropod Trophon geversianus to assess the effects of warmer temperatures upon shell form. We observed lethal effects together with alterations in the shell form (size + shape) of embryos exposed to 18°C water compared to the control temperature environment (13°C). Our results reveal that T. geversianus from Patagonian coasts growing under warm temperatures will change their phenotype by developing smaller and more elongated shells during ontogeny, as well as an expanded shell aperture, increasing their predation vulnerability. Therefore, we consider that the embryonic shell shape change could be a good biomarker of thermal stress produced at early developmental stages in marine gastropods.
Keywords: Direct development, Atlantic Patagonia, 2D geometric morphometrics, Early development stages, Thermal stress
BACKGROUND
The Earth’s climate is a dynamic system, varying naturally on different time scale ranges: multimillennial scale changes such as glacier and inter-glacial transitions, inter-decadal cycles such as North Atlantic oscillations and Pacific Decadal, interannual patterns such as ENSO (El Niño, Southern Oscillation), and also in seasonal cycles (Harley et al. 2006). Nevertheless, human activities have become an important additional component of the climate system (IPCC 2022 2021a; Wolff et al. 2020), where greenhouse gas emissions -mostly because of CO2-increase the temperature (Feely et al. 2004; IPCC 2022). The increase in CO2 and the consequent increases in global temperature are expected to cause a cascade of physical and chemical changes in marine ecosystems (Arias et al. 2021; Harley et al. 2006). In the marine environment, thermal increase was first detected in the sea surface temperature, then in the durations and frequency of marine heatwaves (Androulidakis and Krestenitis 2022). Nevertheless, the thermal increase impacts the shallow and deepwater environments (Meinen et al. 2020), producing morphological (Almada-Villela et al. 1982; Hethke et al. 2021) and physiological effects on ectothermic organisms (Pöhlmann et al. 2011; Díaz et al. 2021).
Morphologically, high temperatures demand plastic responses from communities (Hethke et al. 2021; Melatunan et al. 2013; Pigliucci 2001). Exposure to heightened temperatures during development can impact the morphology and dimensions of organisms possessing calcareous structures (Hethke et al. 2021). Furthermore, temperature is a key environmental variable in calcareous shells’ biomineralization and growth processes (Gazeau et al. 2013; Mackenzie et al. 2014). Since the shell is the anatomical structure that protects most molluscs against waves, predators, desiccation, and overheating, its modification has implications for the organism’s life across multiple dimensions (Byrne and Fitzer 2019; Melatunan et al. 2013). For example, Olson et al. (2012) showed that temperature can alter the microstructure of the shell, changing the angle and thickness of the aragonite layers. In turn, Almada-Villela et al. (1982) found a relationship between increases in shell growth as a function of temperature in bivalves, but also a decrease in hardness, making them more vulnerable to predation pressure (Mackenzie et al. 2014). Recently, negative effects in calcareous structures due to due to the acidification of the ocean by temperature increase were described as direct consequences of global warming (Bednaršek et al. 2020 2021). Any modification in the calcareous structure of invertebrates can alter their strategy of shutting themselves up tightly in their shells to survive low tide periods (Pöhlmann et al. 2011). Under scenario RCP8.5, the IPCC predicts an ocean temperature increase from 0.8 ± 0.4°C in the first scenario to 3.1 ± 0.6°C in the fifth (Collins et al. 2013; IPCC 2001a 2021b 2022). Therefore, under IPCC predictions, the thermal increase in the following years could have farreaching effects on the development of organisms with carbonate shells.
Most organisms inhabiting the intertidal and subtidal ecosystems have an external calcareous structure to shelter from the inconstant ambient conditions (Pöhlmann et al. 2011; Raffaelli and Hawkins 2012). Intertidal organisms can exhibit plastic responses in different development stages (Melatunan et al. 2013; Pigliucci 2001). Shell shape alteration was described as an early stress indicator for pollution (Primost et al. 2016 2021), thermal stress (Morley et al. 2010; Boomer et al. 2003; Kontrovitz 1987), predation (Stafford et al. 2015; Dietl et al. 2010; Bourdeau 2009) or invasive species detection (Kistner and Dybdahl 2014). In Atlantic North Patagonia, the marine gastropod Trophon geversianus (Pallas, 1776) presents a complex phenotype pattern exhibiting a site dependence on shape variation (Nieto-Vilela et al. 2021) and two ecomorphotypes related to physical and biological pressures in the intertidal and subtidal environments (Márquez et al. 2015). This specialist predator (Palomo et al. 2019) is distributed from the province of Buenos Aires to the Burdwood bank in the Namuncurá Reserve in the Southern Atlantic Ocean (Castellanos and Landoni 1993; Griffin and Pastorino 2005; Pastorino 2005) and up to 42° latitude south in the Pacific Ocean. The adult individuals of the subtidal ecomorphotype present a slender and narrower shell shape due to exposure to a less physical stressors in the environment and higher predation pressure (Márquez et al. 2015). Trophon geversianus is a gonochoristic species with internal fertilization. Despite being dioecious, the species does not present external sexual dimorphism, and the female snails differ internally by the presence of the albumin and capsule glands (Cumplido et al. 2010). Females produce egg capsules, where the embryos develop until hatching as crawling juveniles surrounded by numerous nurse eggs used as nutritional resources (Zaixso 1973). Each female can produce 18 egg capsules arranged in clusters, spending 10–25 h in attaching each one (Cumplido et al. 2010). Experiments made with intertidal egg capsules revealed an average maturation time of 120 days and an average hatching of 4 embryos 2.79 + 0.03 mm in length (Cumplido et al. 2011).
The increase in thermal stress in the oceans will trigger organisms’ plastic responses. A strategy for early detection of environmental disturbances in marine populations is to select specific responses to buffer environmental stressors (Edge et al. 2012; Bucheli and Fent 1955). Therefore, we analyzed the shell form and survival rate of embryos of the marine gastropod T. geversianus under temperatures predicted by the IPCC. We hypothesized that subtidal embryos, which usually develop in a more stable environment than intertidal ones, will modify their shell shape under higher temperatures.
MATERIALS AND METHODS
Sampling design
We carried out a single sampling event in September 2018 in Atlantic Patagonia. The selected area was the shallow subtidal zone (10 m deep) of Golfo Nuevo. A total of 150 capsules were collected from 50 different clusters (corresponding to 50 females) by scuba diving, ensuring that the encapsulated individuals were in the first cellular cleavage stage (see fig. 1A in Cumplido et al. 2011). The capsules were transferred to the laboratory and separated under a stereoscopic microscope according to the eggs’ stage of maturity (Cumplido 2008; Cumplido et al. 2011). We selected three initial maturity stages, considering the presence of nurse eggs as an indicator of the first week of the maturation stage: the initial stage, where the egg contents were not disaggregated; the intermediate stage, where the mass was condensed into small circles; and an advanced stage, where the eggs began to divide, forming irregularly shaped embryos. After that, the capsules were transferred to the aquarium for two weeks at 13°C to acclimatize. The capsules were placed in a controlled temperature chamber and individually placed inside histological cassettes in 200 ml jars with filtered seawater.
Fig. 1.
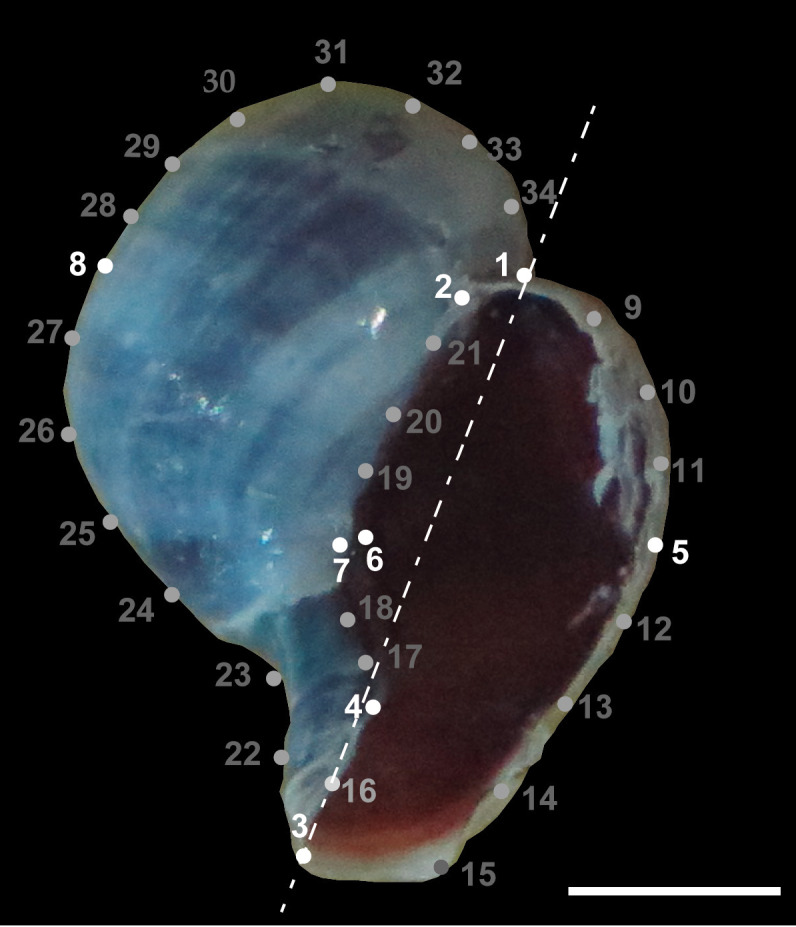
Embryonic shell of Trophon geversianus. Landmarks (LM; white) and semilandmarks (grey) configurations are used to capture the embryo’s shell shape. These include: 1-the aperture extreme of the starting point of the last whorl, 2-the union of the aperture suture with the first whorl, 3-maximum depression of columella, 4-maximum protuberance of umbilicus shell, 5-outermost tangent to the dashed line point of the lip, 6-maximum internal concavity of the aperture zone tangent with the dashed line, 7-maximum external concavity of aperture zone tangent with the dashed line and 8-maximum upper curvature of the first whorl tangent with to the dashed line, 9-11-semilandmarks slipped between LM 1 and 5, 12-15-semilandmarks slipped between LM 5 and 3, 16-semilandmark slipped between LM 3 and 4, 17-18-slipped semilandmarks between LM 4 and 7, 19-21-slipped semilandmarks between LM 7 and 2, 22-27-slipped semilandmarks between LM 3 and 8, 28-34-slipped semilandmarks between LM 8 and 1. Scale bar = 1 mm.
Temperature experiments
After the acclimatization period, we randomly selected 50 egg capsules from the total egg capsules collected (150) and assigned them to three different temperature treatments. The temperature chambers were monitored by a central control unit that switched the heaters in each container rack on or off according to the sensor readings (the sensors had a sensitivity of ± 0.1°C). In addition, data loggers were placed in each rack to keep more records of the temperatures. To assign the capsules to the treatments, we carefully considered that all maturity stages were represented in all three treatments and that egg capsules laid by the same female were assigned to different treatments. The three temperature treatments were defined based on loggers placed in the coast (Nieto-Vilela et al. 2022). The annual temperature average of Golfo Nuevo (13°C) was defined as the control temperature (T13), the naturally high-temperature treatment was the summer mean in the area of 18°C (T18), and the high projected temperature was the mean expected summer temperature of 22°C (T22) (Collins et al. 2013).
The temperature chambers containing egg capsules were placed in six racks containing 30 L of water with 25 independent jars (Supplementary data 1). The individual jars with each egg capsule remained immersed in the water with a gentle airflow (approximately one bubble per second) and continued until the juveniles hatched. Every two days, we recorded the pH, salinity, dissolved oxygen, and temperature of each treatment to maintain the water quality (Milwaukee pH 55, multiparametric sonde Hanna). Also, we changed the jar of water and externally cleaned the egg capsules with a brush weekly to evaluate the embryo state. The jars were randomly rotated around the rack in each cleaning event. At the end of the experiment, we registered the hatching time for each treatment and the number of hatched embryos from each egg capsule, and randomly selected three of them for shell shape analysis. Also, until the end of the experiment, 90 days later, we registered the number of viable and non-viable capsules as well as the number of alive and dead embryos per treatment.
Shape analysis
The three selected embryos were cleaned and orientated over a thin plastic modeling material (plasticine) layer to minimize the effects of rolling or pivoting. Afterwards, we captured their photos under a Carl Zeiss binocular magnifying glass equipped with AxioVision Rel.4.5 software (©Carl Zeiss Imaging Solutions). Over each picture, we described a configuration of 8 landmarks on the aperture face and 27 semi-landmarks to analyze the shell shape (Fig. 1). Then, the picture digitization was carried out using TPS (Thin Plate Spline; Rohlf 2010) software. First, we used the TpsRelw program (Rohlf and Marcus 1993) to calculate an iterative process of sliding semilandmarks along the contour minimizing the bending energy of the TPS function (Bookstein 1991). After sliding the semilandmarks, the total landmark configuration was superimposed by a Generalized Procrustes Analysis (Rohlf and Slice 1990) to rotate and translate the landmark configuration to a common origin and scale it to a unit of the centroid size. Finally, with the Procrustes coordinates of the obtained aligned individuals, we studied the shell shape in the MorphoJ software (Klingenberg 2011). As a proxy for shell size, we used the centroid size of the embryos, defined as the square root of the sum of the squared deviations of landmarks from the centroid (Bookstein 1991; Zelditch et al. 2004).
Statistical analysis
To compare the survival percentage among temperatures, we performed a Kaplan-Meier test with a Log-rank test (Kaplan and Meier 1958). Also, we evaluated hatching time by Analysis of Variance (ANOVA) and compared the mean number of alive embryos per treatment with a Student’s t-test. In both cases, the assumptions of homoscedasticity and normality were tested with a Levene and ShapiroWilk test, respectively. The statistical analyses were performed in GraphPad Prism 5 (CA, EE.UU.). It should be noted that to determine the survival percentage among temperatures during the experiment, the egg capsule was first evaluated qualitatively. When capsules presented an epibiotic increase, discoloration, and loss of turgor were marked as in poor condition -following the description of Cumplido 2008-we recorded the status and date. At the end of the experiment, capsule status was verified by opening and viewing their contents under a magnifying glass.
To evaluate and correct the putative allometric effect, we performed a multivariate regression of shape on centroid size variables (change in the shape associated with size increment) (Bookstein 1991; Monteiro 1999) for all treatments. To assess the independence between the shape and size variables, we performed a permutation test with 10000 rounds (Bookstein 1991; Zelditch et al. 2004). Centroid size was examined using a one-way ANOVA. Principal component analysis was carried out to arrange the individuals on maximum shape variation axes to describe this variation. Finally, we performed a discriminant analysis to find the shell shape components that maximize the separation between temperature treatments. The difference between means was tested by the T2 Hotelling test with 1000 permutations.
RESULTS
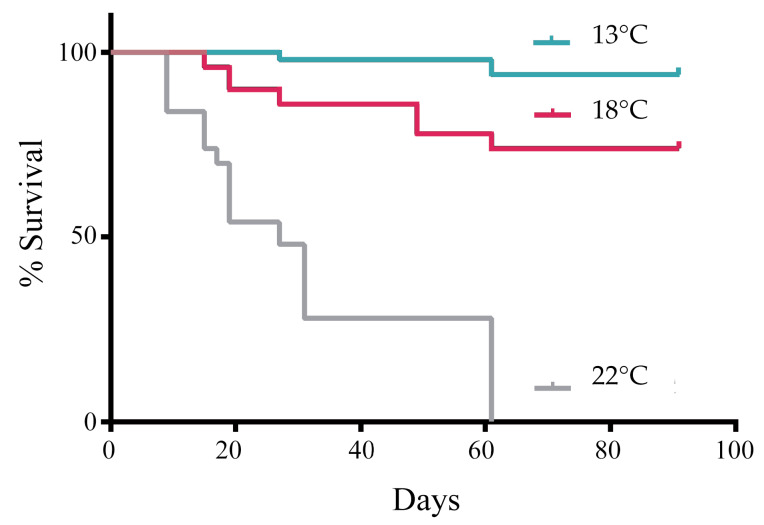
Within the 50 egg capsules placed in the control treatment and both experimental treatments, we found differences in survival numbers (Fig. 2). Forty-seven capsules from T13 were viable (95%), whereas for T18, 37 egg capsules were viable (75%). Finally, T22 was not taken into account for the shape analysis, since after 60 days of the experiment, no embryos were recorded hatching from the egg capsule. Hatching times were not different between T13 and T18 (ANOVA, F1,83 = 0.01, p = 0.92) during the length of the experiment (90 days). The first egg capsule hatched from T18 71 days after starting the experiment, and the last hatch was at 90 days from T13 and T18. The number of embryos per egg capsule was variable in each case, registering a maximum of 35 in T18 (Table 1). Although the total number of live embryos was lower in T18 than in T13, the total was lower in T18, the survival differences between them were statistically different (Mantel-Cox test p = 0.005). The lowest survival rate was recorded in T22, 50% at 25 days and 0% at 90 days (Fig. 2).
Table 1.
Total embryos in the 13°C and 18°C treatments, showing the maximums (Max.) and minimums (Min.) found in each egg capsule
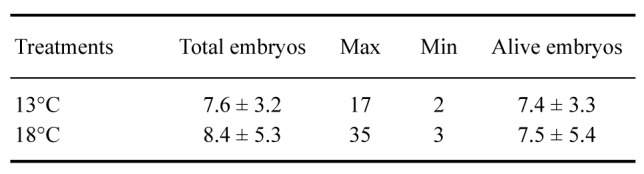
Fig. 2.
Kaplan-Meier survival curve for Trophon geversianus embryos. Temperature treatments were 13°C, 18°C and 22°C versus the experiment duration, 90 days (N = 150, 50 egg capsules for each experimental temperature). Log-rank test: 13°C vs 18°C p < 0.0001; 13°C vs 22°C p < 0.0001; 18°C vs 22°C p < 0.0001.
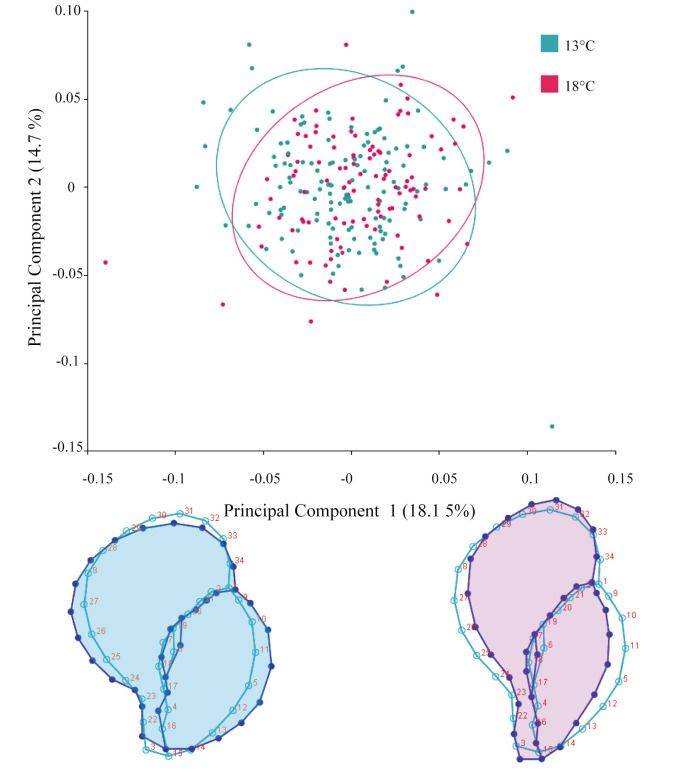
Allometry was found in the individuals from T13 and T18 (p < 0.0001). Although it explained a low percentage of the association between shape and size (4.48%), the subsequent statistical analyses used the residuals of that regression as new allometry-free shape variables. The principal component (PC) 1 was related to the opening and contour of the last whorl (Fig. 3). Individuals in the negative extreme of PC1, which was associated with a globose general shape, presented constriction of both the apex and the area of the siphonal canal, with an expansion of the opening and in the last whorl. At the positive extreme, the opposite trend was recorded, resulting in a more fusiform shape.
Fig. 3.
Dispersion plot between the first two principal components (PC1 vs PC2) with the percentage of the variation explained by each axis. The ellipses represent 90% confidence around the mean shape of the treatments. The lower graphs represent the extremes of variation with a scale factor of -0.15 and 0.15 respectively. In both cases, the deformation is shown in color compared to the consensus form of light blue polygon.
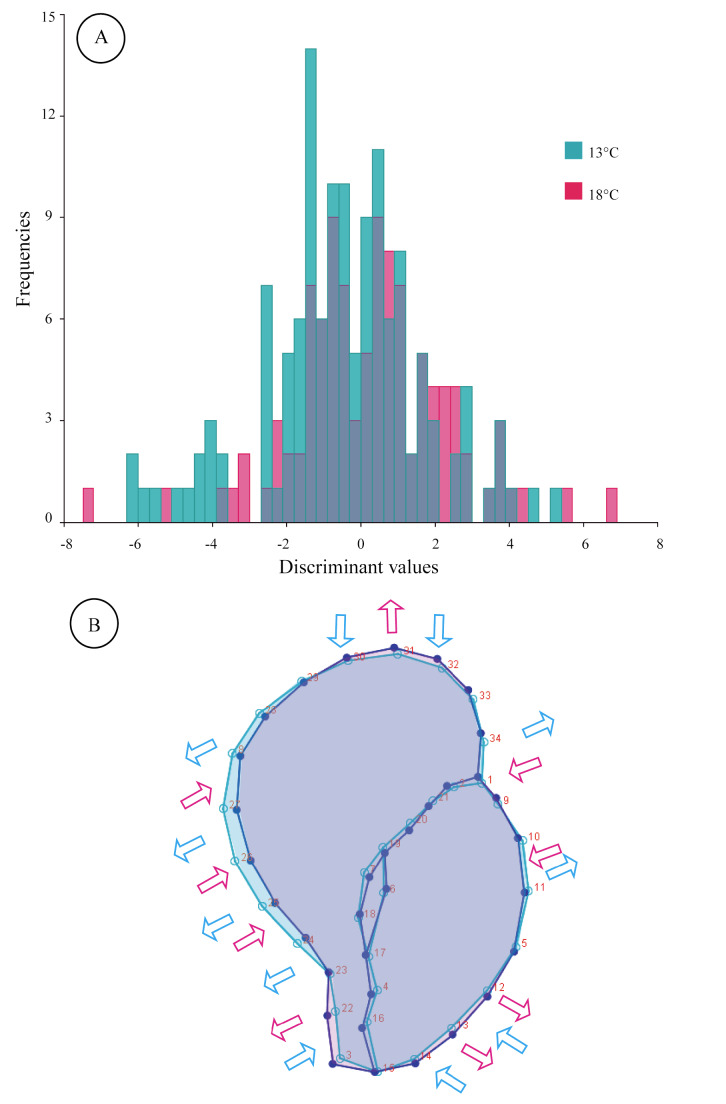
The mean shape of the embryos extracted from T13 was statistically different from that of T18 (Hotelling’s T-squared, p = 0.036). The cross-validation function indicated that the percentage of individuals correctly assigned to T13 was 77%, while 58% were correctly assigned to T18 (Fig. 4). The general shell shape appearance of the T18 embryos featured an elongated shell with an expansion of the anterior aperture and restriction on the narrowing of the contour opposite to the opening. In the case of T13 embryos, a more globose and compressed shell shape was found.
Fig. 4.
A-Frequencies of discriminant values predicted by the cross-validation test. B-The image shows the mean of the shape of each treatment overlapped, increasing the variation three times. The 13°C and 18°C shapes are shown in light blue and pink, respectively. Arrows indicate the general direction of the shell shape variation.
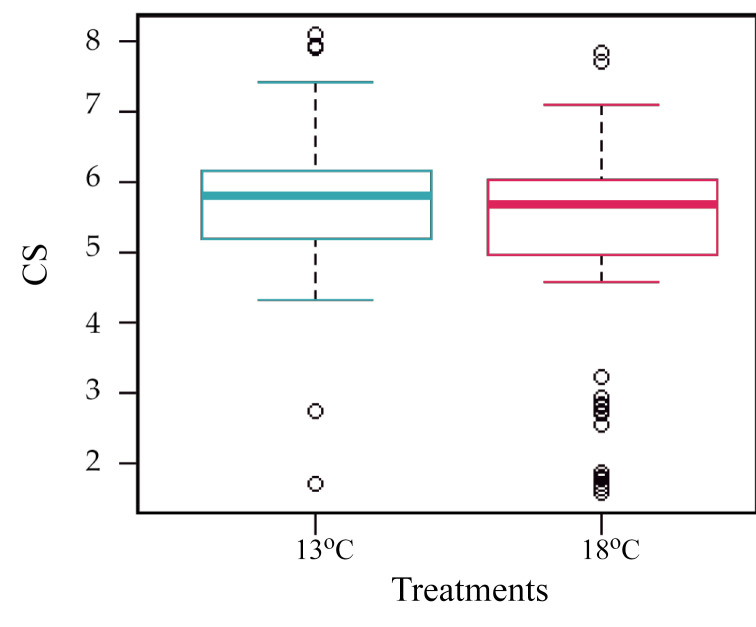
In the centroid size (CS) analysis, we found a lower CS in T18 (5.26 mm) than in T13 (5.7 mm) (ANOVA, F1,238 = 10.3, p = 0.0015). In T18, a group of individuals with sizes below the mean was observed (Fig. 5).
Fig. 5.
Centroid size (CS) means between the treatments. The differences were significant (p = 0.0015).
DISCUSSION
Water temperature is a critical environmental variable that significantly affects biomineralization processes recorded in many shelled marine molluscs (Gazeau et al. 2013) as well as their survival rate (Rawlings 1999). The present study found that exposure to higher temperature modifies the shell form (size + shape) of subtidal T. geversianus embryos, and also affects their survival rate.
We found that the embryos from subtidal environments were tolerant to temperatures as high as 18°C, but were not able to survive until hatch at 22°C. These results were similar to those described in experiments of thermal and salinity tolerance in eggs from the marine snails Bembicium nanum, Siphonaria denticulata,and Dolabrifera brazieri, which showed that the increases in temperature and salinity also increased marine snail mortality rate (Deschaseaux et al. 2010). However, they could not find a common mortality pattern, and it was determined to be specific to each species (see Table 1 in Deschaseaux et al. 2010). Mortality in capsules of the gastropod Thais haemastoma canaliculata was related to oxygen consumption. As the temperature increased, the larvae’s oxygen consumption decreased significantly (Roller and Stickle 1989). However, studies performed on adults of Kelletia kelletii demonstrated that at temperatures higher than 22°C, the oxygen consumption rate increased to 50% (Díaz et al. 2021). In the present work, the subtidal egg capsules of the T. geversianus population were unviable at temperatures of 22°C, which is 4 degrees higher than the warmest temperature recorded at the studied site. Also, in this population (subtidal population of Golfo Nuevo), a failure in the thermal defense was found in adults exposed to higher temperatures than 18°C (Nieto-Vilela et al. 2022). Hence, for subtidal populations of T. geversianus,18°C is a limit in thermal defense for adults, and the embryos are not able to survive until hatching at 22°C. Therefore, if the most extreme projections of the global warming scenario expected for 2100 occur, the summer season will be challenging for this species (RCP 8.5 see Collin et al. 2013).
The shell shape also registered variations associated with the increase in temperature. The embryos that developed in an environment with higher temperatures presented fusiform forms, with elongated shells and wide aperture zones compared to those exposed to a controlled temperature. Aperture zone variations were reported in marine snails concerning different factors and with different consequences. Smaller aperture zones were related to defense against predation in Littorina saxatilis, Nucella lamellose,and N. lapillus (Bourdeau 2009; Carvajal Rodríguez et al. 2005; Guerra-Varela et al. 2009), since a smaller inner surface in the aperture zone leaves less space for a crab attack (Johannesson 1986). Also, shell shape variations were described in early ontogeny in the muricid Acanthina monodon, with development of thicker shells in embryos from egg capsules exposed to predator treatments, and thinner shells in those exposed to water turbulence (Solas et al. 2015). The elongated shell shape in subtidal embryos of T. geversianus exposed to higher temperatures presents a more expansive anterior aperture zone. This increase in the aperture zone could affect the snail’s ability to defend against crab attacks, so the temperature rise may lead to heightened vulnerability of T. geversianus.
An implication that might be seen as beneficial is that a larger apertural facilitate improved adhesion to the substrate (Kitching et al. 1966). Larger aperture zones were identified as adaptations to avoid dislodgments in Lymnaea peregra (Lam and Calow 1988) and Littorina saxatilis (Conde-Padín et al. 2007). Nevertheless, considering that the studied population inhabits a gulf where wave energy remains relatively low, the advantage of possessing a larger aperture size becomes negligible. Hence, the notably expansive anterior aperture zone observed in subtidal embryos of T. geversianus, which were subjected to elevated temperatures as revealed in this study, could suggest a noteworthy escalation in predation vulnerability during upcoming warming scenarios without any benefit associated with the substrate attachment.
Finally, we found differences in size related to temperature increase. The shell size of organisms exposed to higher temperatures was smaller than the control, probably due to investing energy in physiological thermal defense rather than growth, a typical consequence of warmer conditions (Daufresne et al. 2009; Moore and Folt 1993; Pöhlmann et al. 2011). Research conducted on L. saxatilis demonstrated that smaller individuals faced higher predation rates from crabs (Johannesson 1986), implying that under prospective conditions, T. geversianus might encounter intensified predation pressures. Whereas thicker shells in marine snails have been previously found to be associated with higher temperatures (Doyle et al. 2010; Melatunan et al. 2013), these instances were also associated with increases in calcite and aragonite saturation (Dickson 2010). Considering the previous field studies on the species concerning shell shape variation (Márquez et al. 2015; Nieto-Vilela et al. 2021) and the phenotypic plasticity found in the early development stage provided by the present work, we consider that subtidal populations of T. geversianus will change their phenotype under future thermal increases by selecting smaller and more elongated shells during ontogeny. On the other hand, we note that embryos cannot survive to hatch at 22°C. Future inquiries will allow us to determine the consequences resulting from these phenotypic changes. Simultaneously, they will make it easier to recognize potential adjustments in their distribution, helping to improve the depth of knowledge regarding of the adaptive responsiveness of this species.
CONCLUSIONS
Under higher water temperatures, T. geversianus embryos change their phenotype and decrease their shell size. Also, we found a thermal tolerance limit for embryos at 22°C. In conclusion, the embryonic shell shape change could be used as a sentinel biomarker of thermal stress produced at early developmental stages.
Supplementary materials
Experiment diagram. Three temperature treatments in six 30 L water racks with 25 independent jars with egg capsules in histological cassettes.
Acknowledgments
We thank Julio Rúa, Nestor Ortiz and Ricardo Vera for field logistics and the staff of the CCT CONICET-CENPAT aquarium for their support during the experimental development. This work was part of the doctoral thesis of RANV at the Universidad Nacional de la Patagonia San Juan Bosco. RANV wants to thank the BIOS institution for funding training courses on climate warming effects. These courses have not only illuminated their career path but also provided the inspiration behind the current work. Finally, we would like to express gratitude to the anonymous reviewers for their invaluable comments and suggestions. This work was partially supported by PICT 2017-1750 (SG), PICT 2018-0969 (GB), PICT 201804386 (SZ), PICT2018-3197 (FM) and ANERIS project (RANV). RANV is a postdoctoral fellow of CONICET; FM, GB, SZ and SG are members of the Research Career (CONICET). Special thanks are extended to Dr. Benny K.K. Chan for his exceptionally valuable insights. This is publication #189 of the Laboratorio de Reproducción y Biología Integrativa de Invertebrados Marinos (LARBIM).
Footnotes
Authors’ contributions: Rocío Nieto-Vilela: Conceptualization, Data curation, Formal Analysis, Investigation, Writing –original draft, Writing –review and editing. Sebastián Giulianelli: Conceptualization, Funding acquisition, Validation, Writing –review and editing. Soledad Zabala: Conceptualization, Investigation, Validation, Funding acquisition, Writing –review and editing. Gregorio Bigatti: Investigation, Validation, Writing –review and editing. Federico Márquez: Conceptualization, Project administration, Investigation, Validation, Writing –review and editing.
Competing interests: All authors declare that they have no conflict of interest.
Availability of data and materials: The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Consent for publication: Not applicable.
Ethics approval consent to participate: Not applicable.
References
- Almada-Villela PC, Davenport J, Gruffydd LD. 1982. The effects of temperature on the shell growth of young Mytilus edulis. L J Exp Mar Biol Ecol 59(2-3): 275–288. doi:10.1016/0022 0981(82)90121-6.
- Androulidakis YS, Krestenitis YN. 2022. Sea surface temperature variability and marine heat waves over the Aegean, Ionian, and Cretan seas from 2008–2021. J Mar Scie Eng 10(1):42. doi:10.3390/jmse10010042.
- Arias P, Bellouin N, Coppola E, Jones R, Krinner G, Marotzke J, Naik V, Palmer M, Plattner GK, Rogelj J. 2021. Climate Change 2021: The Physical Science Basis. Contribution of Working Group14 I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Technical Summary.
- Bednaršek N, Calosi P, Feely RA, Ambrose R, Byrne M, Chan KYK, Dupont S, Padilla-Gamiño JL, Spicer JI, Kessouri F. 2021. Synthesis of thresholds of ocean acidification impacts on echinoderms. Front Mar Sci 8: 602–601. doi:10.3389/fmars.2021. https://doi.org/10.3389/fmars.2021.602601 602601.
- Bednaršek N, Feely RA, Beck MW, Alin SR, Siedlecki SA, Calosi P, Norton EL, Saenger C, Štrus J, Greeley D. 2020. Exoskeleton dissolution with mechanoreceptor damage in larval Dungeness crab related to the severity of present-day ocean acidification vertical gradients. Sci Total Environ 716: 136610. doi:10.1016/j.scitotenv.2020.136610. [DOI] [PubMed]
- Bookstein FL. 1991. Morphometric tools for landmark data. Cambridge/New York/Port Chester. Melbourne, Sydney, Cambridge University Press. ISBN 0521585988.
- Boomer I, Horne DJ, Slipper IJ. 2003. The use of ostracods in palaeoenvironmental studies, or what can you do with an ostracod shell? Pale Soc Papers 9: 153–180. doi:10.1017/S108933260000 https://doi.org/10.1017/S1089332600002199 2199.
- Bourdeau PE. 2009. Prioritized phenotypic responses to combined predators in a marine snail. Ecology 90(6):1659–1669. doi:10.1890/08-1653.1. [DOI] [PubMed]
- Bucheli TD, Fent K. 1995. Induction of cytochrome P450 as a biomarker for environmental contamination in aquatic ecosystems. Crit Rev Environ Sci Technol 25(2):201–268. doi:10.1080/10643389509388479.
- Byrne M, Fitzer S. 2019. The impact of environmental acidification on the microstructure and mechanical integrity of marine invertebrate skeletons. Conserv Physiol 7(1):coz062. doi:10.1093/conphys/coz062. [DOI] [PMC free article] [PubMed]
- Carvajal Rodríguez P, Conde-Padin P, Rolán-Alvarez E. 2005. Decomposing shell form into size and shape by geometric morphometric methods in two sympatric ecotypes of Littorina saxatilis. J Moll Stud 71(4):313–318. doi:10.1093/mollus/eyi037.
- Castellanos Z, Landoni N. 1993. Catálogo descriptivo de la malacofauna marina magallánica, Neogastropoda Muricidae y Thaisidae. Comisión de Investigaciones Científicas de la Provincia de Buenos Aires, La Plata, p. 25.
- Collins M, Knutti R, Arblaster J, Dufresne JL, Fichefet T, Friedlingstein P, Gao X, Gutowski WJ, Johns T, Krinner G, Shongwe M, Tebaldi C, Weaver AJ, Wehner M. 2013. Long-term climate change: Projections, commitments and irreversibility. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate change 2013: The physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 1029–1136.
- Conde-Padín P, Grahame JW, Rolán-Alvarez E. 2007. Detecting shape differences in species of the Littorina saxatilis complex by morphometric analysis. J Mollu Stud 73(2):147–154. doi:10.1093/mollus/eym009.
- Cumplido M. 2008. Bachelord Thesis: Estacionalidad reproductiva y Desarrollo embrionario del caracol Trophon geversianus en el intermareal de Punta Cuevas, Golfo Nuevo, Ciencias Naturales. Universidad Nacional De La Patagonia San Juan Bosco Puerto Madryn, Chubut.
- Cumplido M, Averbuj A, Bigatti G. 2010. Reproductive seasonality and oviposition induction in Trophon geversianus (Gastropoda: Muricidae) from Golfo Nuevo, Argentina. Shell Res 29(2):423–428. doi:10.2983/035.029.0219.
- Cumplido M, Pappalardo P, Fernandez M, Averbuj A, Bigatti G. 2011. Embryonic development, feeding and intracapsular oxygen availability in Trophon geversianus (Gastropoda: Muricidae). Moll Stud 77(4):429–436. doi:10.1093/mollus/eyr025.
- Daufresne M, Lengfellner K, Sommer U. 2009. Global warming benefits the small in aquatic ecosystems. Proc Natl Acad Sci 106(31):12788–12793. doi:10.1073/pnas.0902080106. [DOI] [PMC free article] [PubMed]
- Deschaseaux ES, Taylor A, Maher WA, Davis A. 2010. Cellular responses of encapsulated gastropod embryos to multiple stressors associated with climate change. J Exp Mar Biol Ecol 383(2):130–136. doi:10.1016/j.jembe.2009.12.013.
- Díaz F, Re-Araujo AD, Carpizo-Ituarte E, Garcia-Esquivel Z, Larios-Soriano E, Perez-Carrasco L, Lerma E. 2021. Thermal physiological performance and thermal metabolic scope of the whelk Kelletia kelletii (Forbes, 1850) (Gastropoda: Neptuneidae) acclimated to different temperatures. Zool Stud 60:44. doi:10.6620/ZS.2021.60-44. [DOI] [PMC free article] [PubMed]
- Dietl GP, Durham SR, Kelley PH. 2010. Shell repair as a reliable indicator of bivalve predation by shell-wedging gastropods in the fossil record. Palaeogeogr Palaeoclimatol Palaeoecol 296(12):174–184. doi:10.1016/j.palaeo.2010.07.013.
- Dickson A. 2010. Part 1: seawater carbonate chemistry. Guide to best practices for ocean acidification research and data reporting. Publication Office of the European Union, Luxembourg, pp. 17–52. doi:10.2777/66906.
- Doyle S, MacDonald B, Rochette R. 2010. Is water temperature responsible for geographic variation in shell mass of Littorina obtusata (L.) snails in the Gulf of Maine? J Exp Mar Biol Ecol 394(1-2):98–104. doi:10.1016/j.jembe.2010.07.023.
- Edge KJ, Johnston EL, Roach AC, Ringwood AH. 2012. Indicators of environmental stress: cellular biomarkers and reproductive responses in the Sydney rock oyster (Saccostrea glomerata). Ecotoxicology 21:1415–1425. doi:10.1007/s10646-012-0895-2. [DOI] [PubMed]
- Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, Fabry VJ, Millero FJ. 2004. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305(5682): 362–366. doi:10.1126/science.1097329. [DOI] [PubMed]
- Gazeau F, Parker LM, Comeau S, Gattuso JP, O’Connor WA, Martin S, Pörtner HO, Ross PM. 2013. Impacts of ocean acidification on marine shelled molluscs. Mar biol 160: 22072245. doi:10.1007/s00227-013-2219-3.
- Griffin M, Pastorino G. 2005. The genus Trophon Montfort, 1810 (Gastropoda: Muricidae) in the Tertiary of Patagonia. J Paleontology 79(2): 296–311. doi:10.1666/0022-3360(2005) 079%3C0296:TGTMGM%3E2.0.CO;2.
- Guerra-Varela J, Colson I, Backeljau T, Breugelmans K, Hughes RN, Rolán-Alvarez E. 2009. The evolutionary mechanism maintaining shell shape and molecular differentiation between two ecotypes of the dogwhelk Nucella lapillus. Evo Ecol 23:261–280. doi:10.1007/s10682-007-9221-5.
- Harley CDG, Hughes AR, Hultgren KM, Miner BG, Sorte CJB, Thornber CS, Rodriguez LF, Tomanek L, Williams SL. 2006. The impacts of climate change in coastal marine systems. Ecol Lett 9(2):228–241. doi:10.1111/j.1461-0248.2005.00871.x. [DOI] [PubMed]
- Hethke M, Weeks SC, Schöttle V, Rogers DC. 2021. Preliminary study of temperature effects on size and shape in the modern spinicaudatan Eulimnadia texana (Crustacea: Branchiopoda). Zool Stud 60:2. doi:10.1111/j.1461-0248.2005.00871.x. [DOI] [PMC free article] [PubMed]
- IPCC 2001. 2001a. Summary for Policymakers. Climate Change 2001. In: Watson R, Albritton DL, Barker T, Bashmakov I, Canziani O, Christ R, Cubasch U, Davidson O, Gitay H, Griggs D, Halsnaes K, Houghton J, House J, Kundzewicz Z, Lal M, Leary N, Magadza C, McCarthy JJ, Mitchell JB, Roberto J, Munasinghe M, Noble I, Pachauri R, Pittock B, Prather M, Richels RG, Robinson B, Sathaye J, Schneider S, Scholes R, Stocker R, Sundararaman N, Swart R, Taniguchi T, Zhou D (eds) Contribution of Working Groups I, II and III to the Third Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press. In Press. ISBN 0521 01495 6 paperback.
- IPCC 2021. 2021b. Summary for Policymakers. Climate Change 2021: The Physical Science Basis. In: Masson-Delmotte V, Zhai P, Pirani A, Connors SL, Péan C, Berger S, Caud N, Chen Y, Goldfarb L, Gomis MI, Huang M, Leitzell K, Lonnoy E, Matthews JBR, Maycock TK, Waterfield T, Yelekçi O, Yu R, Zhou B (eds) Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press. doi:10.1017/9781009157896.
- IPCC 2022. 2022. Climate Change 2022: Impacts, Adaptation and Vulnerability. In: Pörtner HO, Roberts DC, Tignor M, Poloczanska ES, Mintenbeck K, Alegría A, Craig M, Langsdorf S, Löschke S, Möller V, Okem A, Rama B (eds) Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press.
- Johannesson B. 1986. Shell morphology of Littorina saxatilis Olivi: the relative importance of physical factors and predation. J Exp Mar Biol Ecol 102(2-3): 183–195. doi:10.1016/0022-0981(86) https://doi.org/10.1016/0022-0981(86)90175-9 90175-9.
- Kaplan EL, Meier P. 1958. Nonparametric estimation from incomplete observations. J Ame Stat Assoc 53(282): 457–481. doi:10.1080/0 1621459.1958.10501452.
- Kistner EJ, Dybdahl MF. 2014. Parallel variation among populations in the shell morphology between sympatric native and invasive aquatic snails. Biol Inv 16: 2615–2626. doi:10.1007/s10530-014 0691-4.
- Kitching JA, Muntz L, Ebling FJ. 1966. The ecology of Lough Ine. XV. The ecological significance of shell and body forms in Nucella. J Anim Ecol 35:113–126. doi:10.2307/2693.
- Klingenberg CP. 2011. MorphoJ: an integrated software package for geometric morphometrics. Molec Eco Res 11:353–357. doi:10.1111/j.1755-0998.2010.02924.x. [DOI] [PubMed]
- Kontrovitz M. 1987. Ostracode shells as indicators of thermal history. GCAGS 37:383–391.
- Lam P, Calow P. 1988. Differences in the shell shape of Lymnaea peregra (Müller) (Gastropoda: Pulmonata) from lotic and lentic habitats; environmental or genetic variance? J Moll Stud 54(2):197–207. doi:10.1093/mollus/54.2.197.
- Mackenzie CL, Ormondroyd GA, Curling S, Ball RJ, Whiteley NM, Malham SK. 2014. Ocean warming, more than acidification, reduces shell strength in a commercial shellfish species during food limitation. PLoS ONE 9(1): 86764. doi:10.1371/journal. pone.0086764. [DOI] [PMC free article] [PubMed]
- Márquez F, Nieto-Vilela RA, Lozada M, Bigatti G. 2015. Morphological and behavioral differences in the gastropod Trophon geversianus associated to distinct environmental conditions, as revealed by a multidisciplinary approach. J Sea Res 95:239–247. doi:10.1016/j.seares.2014.05.002.
- Meinen CS, Perez RC, Dong S, Piola AR, Campos E. 2020. Observed ocean bottom temperature variability at four sites in the northwestern Argentine Basin: Evidence of decadal deep/abyssal warming amidst hourly to interannual variability during 2009–2019. Geophys Res Lett 47(18):e2020GL089093. doi:10.1029/2020GL089093.
- Melatunan S, Calosi P, Rundle SD, Widdicombe S, Moody AJ. 2013. Effects of ocean acidification and elevated temperature on shell plasticity and its energetic basis in an intertidal gastropod. Mar Ecol Prog Ser 472:155–168. doi:10.3354/meps10046.
- Monteiro LR. 1999. Multivariate regression models and geometric morphometrics: The search for causal factors in the analysis of shape. Sys Biol 48(1):192–199. . [DOI] [PubMed]
- Moore M, Folt C. 1993. Zooplankton body size and community structure: Effects of thermal and toxicant stress. Trends Eco Evo 8(5):178–183. doi:10.1016/0169-5347(93)90144-E. [DOI] [PubMed]
- Morley SA, Clark MS, Peck LS. 2010. Depth gradients in shell morphology correlate with thermal limits for activity and ice disturbance in Antarctic limpets. J Exp Mar Biol Ecol 390(1):1–5. doi:10.1016/j.jembe.2010.04.040.
- Nieto-Vilela RA, Márquez F, Bigatti G, Giulianelli S. 2022. Heatshock response in Patagonian gastropods. Rev Mus Argent Cienc Nat 24:187–196.
- Nieto-Vilela RA, Vrdoljak J, Giulianelli S, Bigatti G, Márquez F. 2021. Geometric morphometrics reveal complex shape variation patterns at different geographic scales in the patagonian gastropod Trophon geversianus. Evo Eco 35(5-6):705–721. doi:10.1007/s10682-021-10125-w.
- Olson IC, Kozdon R, Valley JW, Gilbert PUPA. 2012. Mollusk shell nacre ultrastructure correlates with environmental temperature and pressure. J Ame Chem Soc 134(17):7351–7358. doi:10.1021/ja210808s. [DOI] [PubMed]
- Palomo MG, Bagur M, Calla S, Cecilia M, Dalton SAS, Hawkins SJ. 2019. Biodiversity and interactions on the intertidal rocky shores of Argentina (South-West Atlantic). In: Hawkins SJ, Katrin B, Williams GA (eds) Interactions in the Marine Benthos, Oxford, UK. doi:10.1017/9781108235792.008.
- Pallas PS. 1776. Specilegia Zoologica, quibus novae imprimis et obscurae animalium species iconibus, descriptionibus atque commentariis illustrantur. Berolini, p. 15.
- Pastorino G. 2005. A revision of the genus Trophon montfort, 1810 (Gastropoda: Muricidae) from southern south America. Nautilus 119(2):55–82.
- Pigliucci M. 2001. Phenotypic plasticity: beyond nature and nurture. JHU Press.
- Pöhlmann K, Koenigstein S, Alter K, Abele D, Held C. 2011. Heatshock response and antioxidant defense during air exposure in patagonian shallow-water limpets from different climatic habitats. Cell Stress Chap 16:621–632. doi:10.1007/s12192-011-0272-8. [DOI] [PMC free article] [PubMed]
- Primost MA, Bigatti G, Márquez F. 2016. Shell shape as indicator of pollution in marine gastropods affected by imposex. Mar Freshw Res 67(12):1948–1954. doi:10.1071/MF15233.
- Primost MA, Averbuj A, Bigatti G, Márquez F. 2021. Embryonic shell shape as an early indicator of pollution in marine gastropods. Mari Environ Res 167:105283. doi:10.1016/j.marenvres.2021.105283. [DOI] [PubMed]
- Raffaelli D, Hawkins SJ. 2012. Intertidal ecology. Springer Science & Business Media, London, UK.
- Rawlings TA. 1999. Adaptations to physical stresses in the intertidal zone: the egg capsules of neogastropod molluscs. Am Zool 39(2):230–243. doi:10.1093/icb/39.2.230.
- Rohlf FJ. 2010. tpsRelw, relative warps analysis, version 1.49. Department of Ecology and Evolution, State University of New York at Stony Brook.
- Rohlf FJ, Marcus LF. 1993. A revolution in morphometrics. Trends Ecol Evol 8(4):129–132. doi:10.1016/0169-5347(93)90024-J. [DOI] [PubMed]
- Rohlf FJ, Slice DE. 1990. Extensions of the Procrustes Method for the optimal superimposition of landmarks. Syst Biol 39(1):40–59. doi:10.2307/2992207.
- Roller RA, Stickle WB. 1989. Temperature and salinity effects on the intracapsular development, metabolic rates, and survival to hatching of Thais haemastoma canaliculata (Gray) (Prosobranchia: Muricidae) under laboratory conditions. J Exp Mar Biol Ecol 125(3): 235–251. doi:10.1016/0022-0981(89) https://doi.org/10.1016/0022-0981(89)90099-3 90099-3.
- Solas MR, Hughes RN, Márquez F, Brante A. 2015. Early plastic responses in the shell morphology of Acanthina monodon (Mollusca, Gastropoda) under predation risk and water turbulence. Mar Ecol Prog Ser 527: 133–142. doi:10.3354/meps11221.
- Stafford ES, Dietl GP, Gingras MP, Leighton LR. 2015. Caedichnus, a new ichnogenus representing predatory attack on the gastropod shell aperture. Ichnos 22(2): 87–102. doi:10.1080/10420940.201 5.1031899.
- Wolff E, Fung I, Hoskins B, Mitchell J, Palmer T, Santer B, Shepherd J, Shine K, Solomon S, Trenberth K, Walsh J, Wuebbles D. 2020. Climate Change: Evidence & Causes: Update 2020. The National Academy Press. doi:10.17226/25733.
- Zaixso HE. 1973. Observaciones sobre el desove y embriología de Trophon geversianus (Pallas) 1974 (Gastropoda, Muricidae). Neotropica 19:157–163.
- Zelditch ML, Swiderski DL, Sheets HD, Fink WL. 2004. Introduction. In: Zelditch ML, Swiderski DL, Sheets HD, Fink WL (eds) Geometric Morphometrics for Biologists. Academic Press, San Diego, pp. 1–20.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experiment diagram. Three temperature treatments in six 30 L water racks with 25 independent jars with egg capsules in histological cassettes.