Abstract
Flesh flies (Diptera: Sarcophagidae) exhibit a wide range of feeding habits including necrophagy, coprophagy, kleptoparasitism, parasitism, and predation. Among them are species of Sarcophaga Meigen belonging to the subgenera Baranovisca Lopes and Mehria Enderlein that are specialized predators of spider eggs. These flies hover around spider webs and lay their larvae on the spider egg sac. While progress has been made on the taxonomy of Baranovisca and Mehria in recent decades, our knowledge about their biology, prey selection, and distribution remains limited, restricting our understanding of the evolutionary dynamics of Sarcophagidae-Araneae interactions. Here, we describe and illustrate the first record of S. (M.) lorosa Hall preying on egg sacs of Metepeira galatheae (Thorell) (Araneae: Araneidae) in Chile. The taxonomy of S. (M.) lorosa is revised, with two new junior synonyms proposed: Weyrauchimyia ruficauda Lopes and Tibana, syn. nov., and Arachnidomyia travassosi Tibana and Mello, syn. nov. Furthermore, we present an annotated catalog that comprehensively reviews the existing records of spider egg-predating Sarcophagidae, and provide an overview of the evolution of Sarcophagidae-Araneae interactions. Our catalog includes information on at least four species of Baranovisca and 10 species of Mehria that have been documented as preying on eggs from species of various spider families, such as Araneidae, Cheiracanthiidae, Clubionidae, Philodromidae, Salticidae, and Tetragnathidae. These records cover all biogeographical regions except the Afrotropical. Our results enhance our understanding of the evolution of Sarcophagidae-Araneae interactions.
Keywords: Araneidae, Coevolution, Egg sacs, Metepeira, Oviposition strategy
BACKGROUND
Flesh flies (Diptera: Sarcophagidae) are often considered as necrophagous or coprophagous flies, although there are several lineages with parasitoid or predatory habits (Pape 1996; Pape and Dahlem 2010). The subfamily Miltogramminae contains a large clade of kleptoparasitic species (Piwczyński et al. 2017), while parasitoid and predatory larvae are frequently found in species of the subfamilies Paramacronychiinae and Sarcophaginae, attacking mostly invertebrates such as insects, snails, millipedes, earthworms, and spiders, but even producing myiasis in several species of vertebrates (Aldrich 1914; Pape 1996). For instance, the largest genus of the family, Sarcophaga Meigen, includes approximately 890 species classified in several subgenera worldwide (Buenaventura et al. 2017), some of which are adapted to parasitism or predation.
Sarcophaga larvae of the subgenera Baranovisca Lopes and Mehria Enderlein are specialized predators of spider eggs; the females hover around and fly through the spider web, trying to reach the egg sac, where they lay one larva per attack (Lubin 1974; Hieber and Uetz 1990; Rayor and Uetz 1990; Hieber et al. 2002). The larva then enters the egg sac and eats the eggs, thus completing its development. Some flies of the families Bombyliidae, Chloropidae, Ephydridae, and Phoridae, and wasps of the family Ichneumonidae are also specialized parasitoids or predators of spider eggs (Sobczak et al. 2012; Fritzén and Sääksjärvi 2016; Villanueva-Bonilla et al. 2016; Gillung and Borkent 2017; Riccardi and Pádua 2021; Souza-Santiago et al. 2023). However, in some cases, the incidence of flesh flies is much higher than that of the other parasitoids/predators (Hieber and Uetz 1990).
The subgenus Baranovisca includes six species distributed in the Australasian and Oriental regions, while Mehria includes 15 species distributed in the Nearctic, Neotropical, and Palaearctic regions (Pape 1996). Although the taxonomy of these species has been improved with detailed descriptions and illustrations during the final decades of the last century (Lopes 1946 1959 1981 1985 1989; Cantrell 1981; Tibana and Mello 1992), information about their biology, prey, and distribution is restricted to a few species, which hinders a deeper understanding of the evolution of Sarcophagidae-Araneae interactions. Most records of flesh flies preying on spider eggs were recently listed (Gillung and Borkent 2017), but not all were included.
Here, we record for the first time the interaction of Sarcophaga (Mehria) lorosa Hall preying on egg sacs of Metepeira galatheae (Thorell) (Araneae: Araneidae) during a desert bloom in the Copiapó and Huasco provinces from the Atacama region, Chile (Chávez et al. 2019). The taxonomy and nomenclature of S. (M.) lorosa is revised, including notes on type material and new distribution records. Furthermore, we propose two new junior synonyms: Weyrauchimyia ruficauda Lopes and Tibana, syn. nov., and Arachnidomyia travassosi Tibana and Mello, syn. nov. Finally, we present an annotated catalog that comprehensively reviews the existing records of spider egg-predating Sarcophagidae and provide an overview of the evolution of Sarcophagidae-Araneae interactions.
MATERIALS AND METHODS
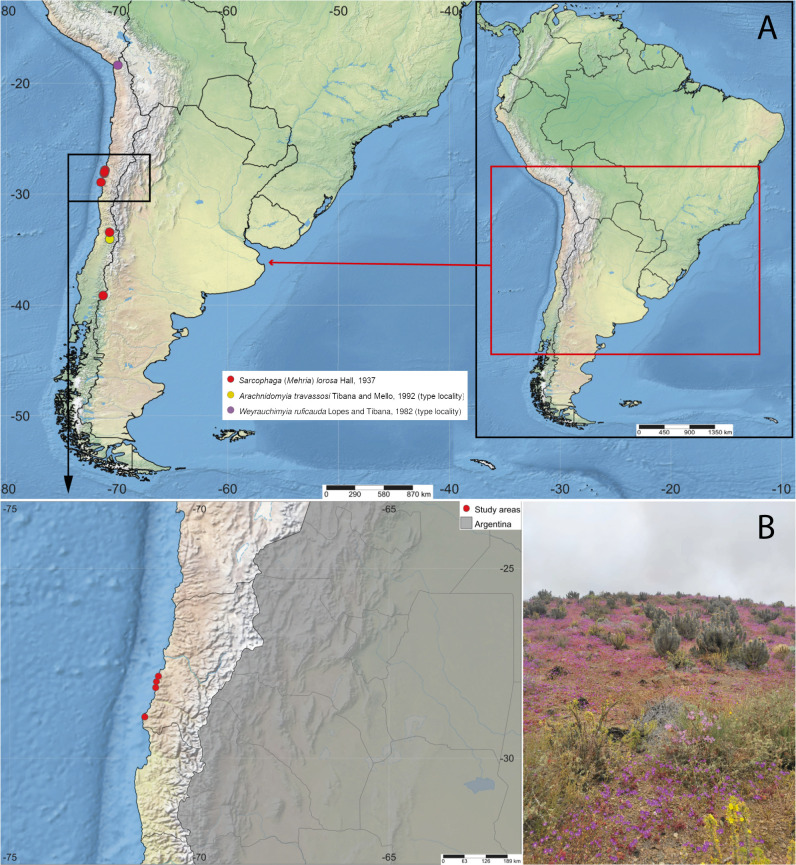
Metepeira galatheae adults (n = 20: 5 ♂, 15 ♀) (Fig. 1A, B) and egg sacs (n = 21) (Fig. 1C) were manually collected at four localities from two provinces in the Atacama region during a desert bloom (16–17 November 2022): Copiapó Province: Sector Totoral (27°50'14"S, 71°05'05"W); and Huasco Province: Parque Nacional Llanos del Challe (28°08'55"S, 71°09'24"W), Quebrada Honda (27°59'22,30"S, 71°08'14"W), and Parque Eolico Cabo Leones (28°55'14"S, 71°26'38"W) (Fig. 2B). The collected egg sacs and spiders were deposited in Falcon tubes, closed with voile, and transported to the Laboratorio de Ecología de Abejas of the Universidad Católica del Maule (UCM), Talca, Chile. All egg sacs and spiders were kept separately in Falcon tubes at room temperature (27–30°C).
Fig. 1.
Record of Sarcophaga (Mehria) lorosa Hall (Diptera: Sarcophagidae) in Metepeira galatheae (Thorell) (Araneae: Araneidae). A–C, Female, male, and egg sac of M. galatheae, respectively; D, Puparium of S. (M.) lorosa.
Fig. 2.
A, Distribution map of Sarcophaga (Mehria) lorosa Hall, highlighting the type localities of the junior synonyms Arachnidomyia travassosi Tibana and Mello, syn. nov., and Weyrauchimyia ruficauda Lopes and Tibana, syn. nov.; B, Study areas in theAtacama region, Chile, during a desert bloom. Map created with SimpleMappr (https://www.simplemappr.net/).
Specimens of S. (M.) lorosa were identified based on examination of the holotype male deposited in the Natural History Museum (NHMUK) in London, United Kingdom. Additional material of S. (M.) lorosa was obtained from the Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” (MACN), Buenos Aires, Argentina. Morphological terminology follows Cumming and Wood (2017). Fly vouchers were deposited in UCM (5 ♂ and 1 ♀), and spider vouchers in UCM (13 ♀) and UNAP (5 ♂ and 2 ♀). Metepeira galatheae specimens were identified by Dr. Andres Taucare-Rios de la Universidad Arturo Prat (UNAP), Iquique, Chile.
The catalog follows a format similar to that presented by Guimarães (1977) and Arnaud (1978). Sarcophagidae species are listed alphabetically according to their respective biogeographical regions, with valid names and junior synonyms accompanied by authorship. Published records of egg predation are given with the name of the fly followed by respective spider prey, author, year of publication, page number, locality, and notes on the record. Comments and emendations regarding taxonomy, nomenclature or reliability of the record are included in square brackets. Secondary references citing only previous records were not included. The classification of Sarcophagidae follows Pape (1996), and the classification of spiders follows Gloor et al. (2017).
Digital images were taken using a Leica MC170 HD digital camera attached to a Leica S8AP0 stereomicroscope with an LED illumination dome (Kawada and Buffington 2016). Multiple layers were stacked using the software Helicon Focus A8.1.0.0. Distributional data of S. (M.) lorosa were obtained directly from the labels and plotted on a map using SimpleMappr (Shorthouse 2010). When necessary, coordinates were estimated using Global Gazetteer Version 2.3.
RESULTS
Records of Sarcophaga (Mehria) lorosa in egg sacs of Metepeira galatheae
A total of 21 egg sacs of M. galatheae were collected and reared. Six specimens of S. (M.) lorosa emerged from two egg sacs (Fig. 4): four flies (4 ♂) emerged between 27–28 November 2022 from one egg sac collected on 16 November 2022, and two flies (1 ♂ and 1 ♀) emerged on 30 November 2022 from one egg sac collected on 16 November 2022. We verified that no flies emerged from the remaining 19 egg sacs and manually opened them to search for fly puparia. Nineteen hatched puparia were found in seven egg sacs (Fig. 1D): Two egg sacs with one puparium, three egg sacs with two puparia, one egg sac with five puparia, and one egg sac with six puparia. One egg sac had a puparium and a dead specimen of S. (M.) lorosa. Therefore, approximately 42% of the egg sacs had been attacked by S. (M.) lorosa.
TAXONOMY
Sarcophagidae Macquart, 1834
Subfamily Sarcophaginae Macquart, 1835
Genus Sarcophaga Meigen, 1824
Subgenus Mehria Enderlein, 1928
Sarcophaga (Mehria) lorosa Hall, 1937
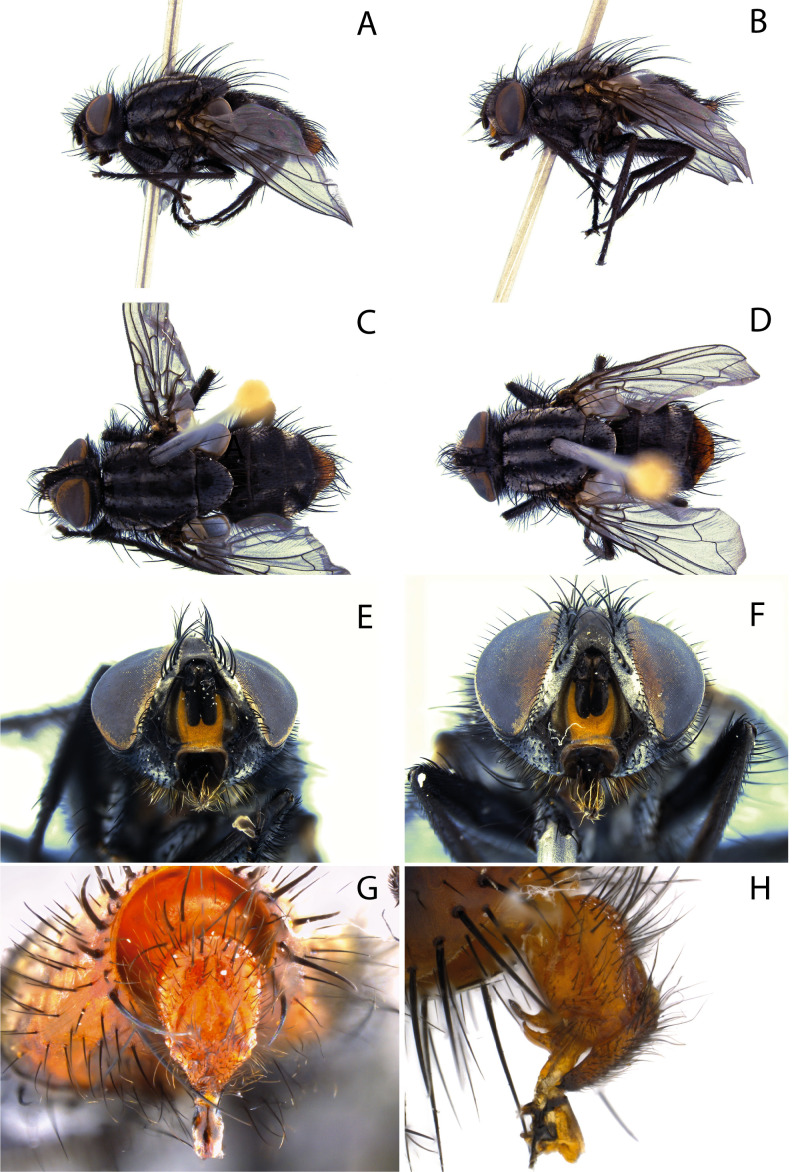
Sarcophaga lorosa Hall, 1937: 367. Holotype male (NHMUK), examined (Fig. 3A, C, E). Type locality: Chile, Santiago.
Weyrauchimyia ruficauda Lopes and Tibana, 1982: 142. Holotype male (MNRJ, lost). Type locality: Chile, Arica y Parinacota [as Tarapacá], Lluta. [Junior secondary homonym of Sarcophaga ruficauda Zetterstedt, 1838.]. Syn. nov.
Arachnidomyia travassosi Tibana and Mello, 1992: 293. Holotype male (CNC), examined (Fig. 3B, D, F). Type locality: Chile, O’Higgins, La Leonera. Syn. nov.
Material examined: Argentina: 1 ♂ (MACN): Neuquén, Parque Nacional Lanín, Ñorquinco, S 39°09,073 W 71°15,475, trampa Malaise, 9.i.2013, Olea, Mulieri and Patitucci leg.; Chile: 5 ♂, 1 ♀ (UCM): Huasco Province, Parque Nacional Llanos del Challe, 28°08'55"S, 71°09'24"W, reared from Metepeira galatheae egg sacs, 27–30.xi.2022, D.G. Pádua et al. leg.
Diagnosis: Distinguished from the other New World species of subgenus Mehria by the following morphological features: (1) face with deep goldenyellow pruinosity, (2) wing vein R1 with setae, (3) abdominal tergite 5 reddish or yellow, and (4) genital segments (syntergosternite 7+8 and epandrium) reddish. Phallic morphology most similar to S. (M.) guyanensis Lopes and S. (M.) lindae Lopes, but separable from S. (M.) lindae by the median stylus of similar length to the lateral styli and its vesica shorter than width of distiphallus; and separable from S. (M.) guyanensis by the shape of juxta slightly folded dorsoapically, not rounded (see Lopes 1946: 125, fig. 15).
Males (Fig. 4A, C, E, G, H): Body length: 7–8 mm. Wing length: 5.5–6 mm (n = 6).
Fig. 3.
Holotype male of Sarcophaga lorosa Hall (NHMUK) and holotype male of Arachnidomyia travassosi Tibana and Mello (CNC), respectively. A, B: Lateral habitus and labels; C, D: Dorsal habitus; E, F: Head in frontal view.
Fig. 4.
arcophaga (Mehria) lorosa Hall specimens reared from egg sacs of Metepeira galatheae (Thorell). A, C, E, G, H: Lateral habitus, dorsal habitus, head in frontal view, terminalia in posterior view, and terminalia in lateral view of male, respectively; B, D, F: Lateral habitus, dorsal habitus, and head in frontal view of female, respectively.
Head (Fig. 4E): Parafacial, fronto-orbital plate, and postocular orbits with silvery gray pruinosity; frontal vitta blackish; face with deep golden-yellow pruinosity. Facial ridge setose on lower half; frontoorbital plate and parafacial with row of setulae close to eye, parafacial with additional row of setulae in lower half. Frons 0.15–0.18 head width at level of ocellar triangle. Antenna black; first flagellomere approximately twice pedicel length; arista short plumose on proximal half. Frontal setae 10–12, well-developed, row reaching level of apex of pedicel; rows of frontal setae parallel for most of their length, diverging at the level of antennal insertion. Two reclinate orbital setae; proclinate orbital setae absent; ocellar setae developed and proclinate; outer vertical seta undifferentiated from postocular setae. Gena with silver pruinosity, covered with black setulae; genal groove blackish; postgena with silver pruinosity, covered with black setulae. Palpus, prementum, and labella blackish.
Thorax (Fig. 4A, C): Black with silverygray pruinosity. Acrostichal setae 2+1; dorsocentral setae 2+3; intra-alar setae 1+2; supra-alar setae 2+3; postpronotal setae 3–4; notopleural setae 4. Postalar wall setulose. Postalar callus with 2 setae. Proepisternum bare. Katepisternal setae 3. Scutellum with a pair of basal and subapical setae; apical setae present; discal setae absent. Wing: Tegula black; basicosta yellowish; veins brown. Costal spine not developed; third costal sector setulose ventrally; vein R1 with setae dorsally; vein R4+5 with setulae dorsally from base to crossvein r-m. Cell r4+5 open at wing margin. Legs: Black, except for brown tarsi. Mid femur without ctenidium; mid tibia with 1 median anterior seta; posterior femur with anterodorsal row, and anteroventral row with stronger setae on median surface; posterior tibia with three dorsal setae, one anterior row, and three ventral setae. Tarsal claws long, subequal to length of tarsomere 5.
Abdomen (Fig. 4A, C): Syntergite 1+2 to tergite 4 black with silvery-gray pruinosity; tergite 5 reddish. Tergites 2 and 3 without median marginal setae; tergite 4 with complete row of marginal setae; tergite 5 with row of marginal setae. Sternite 5 slightly reddish and V-shaped, with fringe of setae along the median margin of each arm.
Terminalia (Fig. 4G, H): Syntergosternite 7+8 and epandrium reddish. Syntergosternite 7+8 with three pairs of setae. Cercus apically curved and pointed. Pregonite with rounded and well-sclerotized apex, postgonite with short setulae. Phallus with vesica bifid, short; lateral stylus well-sclerotized; juxta rounded apically, slightly folded dorsoapically.
Female (Fig. 4B, D, F): Differs from male as follows: Body length: 8 mm. Wing length: 6.5 mm (n = 1). Frons 0.27 head width at level of ocellar triangle. Frontal setae 8; reclinate setae 2, with posterior seta lateroclinate; proclinate orbital setae 2; inner vertical setae parallel; outer vertical seta about 2/3 inner vertical seta. Tarsal claws shorter than tarsomere 5.
Distribution: Argentina (Neuquén) and Chile (Arica y Parinacota, Atacama, O’Higgins, Región Metropolitana de Santiago) (Fig. 2A).
Remarks: We propose W. ruficauda and A. travassosi as junior synonyms of S. lorosa Hall. Both nominal species share the above enumerated external characters of color of face pruinosity, wing vein R1, abdominal tergite 5, and genitalia with S. lorosa. They also share a short, bifid vesica, and juxta slightly folded dorsoapically, which are here considered to provide a conspecific match. Pape (1996) transferred W. ruficauda to Sarcophaga and proposed A. travassosi as its synonym, keeping S. (M.) travassosi (Tibana and Mello) as the valid name because of the secondary homonymy between S. (M.) ruficauda (Lopes and Tibana) and Sarcophaga ruficauda Zetterstedt. Unfortunately, the holotype male of W. ruficauda was lost in the fire that consumed most of the MNRJ entomological collection (Cunha 2018; Escobar 2018).
Further clarification is required regarding the type locality of A. travassosi. Pape (1996) recorded the city of La Leonera in the Biobío region as the type locality. However, the holotype label explicitly states the city La Leonera in the O’Higgins region (Fig. 3B), which is more than 400 km north of La Leonera in the Biobío region. The exact locality can be verified by consulting the works of the Chilean entomologist Luis Enrique Peña, the collector of the holotype. Peña (1974) listed the type locality of several insects collected by him and included two localities in O’Higgins: La Leonera and Cerro Poqui. Cerro Poqui is a hill located at the border of the regions O’Higgins and Región Metropolitana de Santiago, which shows that Peña used O’Higgins as a reference to the O’Higgins region. Thus, there is no reason to consider that Peña was not accurate when recording La Leonera in the O’Higgins region.
The golden-yellow pruinosity on the face of S. (M.) lorosa is not commonly seen in Sarcophagidae and is not present in Nearctic and Palaearctic species of Mehria. In the Neotropical region, only S. (M.) insularis Lopes has the face and frontal vitta slightly goldenyellow (Lopes 1946), but lacks setae on vein R1.
Based on the specimens examined here, the distribution of S. (M.) lorosa seems to be restricted to southwestern South America, specifically on the western side of the Andes Mountains (Fig. 2A).
Annotated catalog of records of spider eggpredating Sarcophagidae
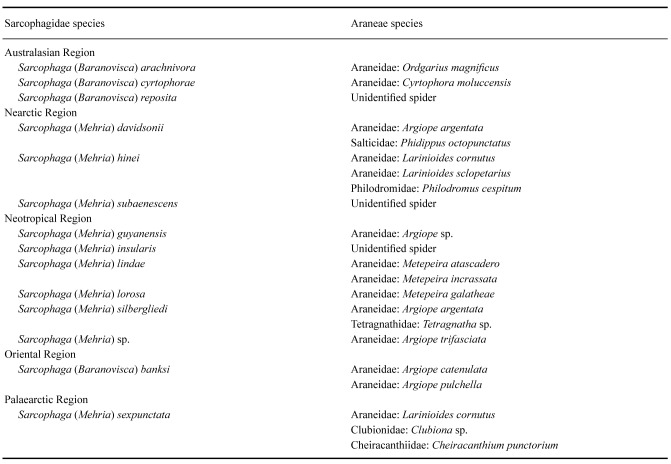
Four species of the subgenus Baranovisca have been recorded as egg predators from at least four species of Araneidae; and 10 species of the subgenus Mehria have been recorded from at least 13 species of the families Araneidae, Cheiracanthiidae, Clubionidae, Philodromidae, Salticidae, and Tetragnathidae (Table 1). Records are from all biogeographical regions except the Afrotropical.
Table 1.
List of spider egg-predating Sarcophagidae and their respective prey according to biogeographical regions. Details about records, localities, and respective references can be found in the catalog in the main text
Australasian Region
Sarcophaga (Baranovisca) arachnivora Lopes
From egg sacs of Ordgarius magnificus (Rainbow).
Recorded by Lopes (1985: 51, as B. arachnivora) as a parasite in egg sacs of Dicrostichus magnificus Rainbow [= O. magnificus (Rainbow)]) in Hornsby Heights, New South Wales, Australia.
Sarcophaga (Baranovisca) cyrtophorae Cantrell
From egg sacs of Cyrtophora moluccensis (Doleschall).
Recorded by Lubin (1974: 329, as an unidentified Sarcophagidae [identified later by Yefremova and Lubin (2020)]) as preying on eggs of C. moluccensis (Doleschall) at Wau Ecology Institute, Wau, Morobe, Papua New Guinea; by Cantrell (1980: 42, as Parasarcophaga reposita Lopes [misidentification, see Cantrell (1986)]) as preying on eggs of C. moluccensis in Brisbane, Queensland, Australia; by Cantrell (1981: 29, as P. reposita Lopes [misidentification, see Cantrell (1986)]) as preying on eggs of C. moluccensis in Queensland, Australia, and Papua New Guinea [locality not specified]; by Cantrell (1986: 3, as Parasarcophaga cyrtophorae) as preying on eggs of C. moluccensis in Brisbane, Queensland, Australia; and by Yefremova and Lubin (2020: 3) as preying on eggs of C. moluccensis at Wau Ecology Institute, Wau, Morobe, Papua New Guinea.
Sarcophaga (Baranovisca) reposita Lopes
From egg sacs of an unidentified spider.
Recorded by Lopes (1959: 65, as Parasarcophaga (Rosellea) reposita) as preying on eggs of an unidentified spider in Sidney, New South Wales, Australia.
Nearctic Region
Sarcophaga (Mehria) davidsonii Coquillett
From egg sacs of Argiope argentata (Fabricius) and Phidippus octopunctatus (Peckham and Peckham).
Recorded by Coquillett (1892: 24) as preying on eggs of Phidippus opifex (McCook) [= Attus opifex McCook = Attus octopunctatus Peckham and Peckham = Phidippus octopunctatus (Peckham and Peckham)] in Los Angeles, Los Angeles County, California, United States of America; and by Davidson (1894: 269) as preying on eggs of A. argentata (Fabricius) in Santa Catalina Island and Redondo Beach, Los Angeles County, California, United States of America.
Sarcophaga (Mehria) hinei Aldrich
From egg sacs of Larinioides cornutus (Clerck), L. sclopetarius (Clerck), and Philodromus cespitum (Walckenaer).
Recorded by Auten (1925: 244) as preying on eggs of Aranea frondosa Comstock [as Walckenaer, error] [= Araneus cornutus Clerck = L. cornutus (Clerck)], Epeira sclopetaria (Clerck) [= Araneus sclopetarius = L. sclopetarius], and Philodromus canadensis Emerton [= Aranea cespitum Walckenaer = P. cespitum (Walckenaer)]) in Put-In-Bay region, Ottawa County, Ohio, United States of America.
Remarks: Gillung and Borkent (2017) listed these records for S. (M.) sexpunctata (Fabricius) as a senior synonym of S. (M.) hinei,following the classification in Systema Dipterorum (Evenhuis and Pape 2022). However, this synonymy has never been published and both nominal species are considered as valid in Pape (1996). They also included P. aureolus (Clerck) as prey of S. (M.) hinei as a record made by Auten (1925), but there is no record of this spider species in the original article.
Sarcophaga (Mehria) subaenescens Aldrich
From egg sacs of an unidentified spider.
Recorded by Aldrich (1925: 28) as preying on eggs of an unidentified spider in Somerville, Somerset County, New Jersey, United States of America.
Unidentified Sarcophaga Meigen
From egg sacs of Larinioides cornutus (Clerck).
Recorded by Kaston and Jenks (1937: 161, as Sarcophaga sp. (hinei?)) as preying on eggs of Epeira cornuta (Clerck) [= Araneus cornuta Clerck = L. cornutus (Clerck)] in West Haven, New Haven County, Connecticut, United States of America.
Neotropical Region
Sarcophaga (Mehria) guyanensis Lopes
From egg sacs of unidentified Argiope Audouin.
Recorded by Lopes (1946: 126, as Arachnidomyia guyanensis) as preying on eggs of unidentified Argiope Audouin in Berbice, Guyana.
Sarcophaga (Mehria) insularis Lopes
From egg sacs of an unidentified spider.
Recorded by Lopes (1946: 125, as Arachnidomyia insularis) as preying on eggs of an unidentified spider [as an epeirid spider] in Haiti [locality not specified].
Sarcophaga (Mehria) lindae Lopes
From egg sacs of Metepeira atascadero Piel and M. incrassata Pickard-Cambridge.
Recorded by Lopes (1989: 1098, as Arachnidomyia lindae) as preying on eggs of M. cf. atascadero Piel and M. incrassata Pickard-Cambridge in Fortin de las Flores, Veracruz, Mexico; and by Hieber and Uetz (1990: 146, as A. lindae) as preying on eggs of M. atascadero and M. incrassata in San Miguel de Allende, Guanajuato, and Fortin de las Flores, Veracruz, Mexico.
Sarcophaga (Mehria) lorosa Hall
From egg sacs of Metepeira galatheae (Thorell).
New record by Gudin et al. as preying on eggs of M. galatheae (Thorell) in Copiapó and Huasco, Atacama, Chile.
Sarcophaga (Mehria) silbergliedi Lopes
From egg sacs of Argiope argentata (Fabricius) and unidentified Tetragnatha Latreille.
Recorded by Lopes (1981: 310, as Arachnidomyia silbergliedi) as preying on eggs of unidentified Tetragnatha Latreille in Madden Lake, Canal Zone, Panama; and by Miranda et al. (2020: 10) as preying on eggs of A. argentata (Fabricius) in Cerro Azul, Panama City, Panama District, Panama.
Unidentified Sarcophaga Meigen
From egg sacs of Argiope trifasciata (Forsskål)
Recorded by Armas and Alayón García (1986: 116, as unidentified Sarcophagula Wulp [misidentification]) as a parasitoid in egg sacs of A. trifasciata (Forsskål) in San Antonio de los Baños, La Habana, Cuba.
Remarks: Sarcophagula is a subgenus of Tricharaea Thomson including mostly saprophagous and coprophagous species (Pape and Dahlem 2010; Patitucci et al. 2015). Therefore, the specimens recorded in egg sacs of A. trifasciata were misidentified and probably belong to Sarcophaga,subgenus Mehria.
Oriental Region
Sarcophaga (Baranovisca) banksi Senior-White
From egg sacs of Argiope catenulata (Doleschall) and A. pulchella Thorell.
Recorded by Prakash and Pandian (1978: 210) as preying on eggs of A. pulchella Thorell in Idumban Pond, Ayakudi, Tamil Nadu, India; and by Shinonaga and Barrion (1980: 538, as Pierretia litsingeri Shinonaga and Barrion [= S. banksi Senior-White]) as preying on eggs of A. catenulata (Doleschall) in Laguna, Luzon, Philippines.
Palaearctic Region
Sarcophaga (Mehria) sexpunctata (Fabricius)
From egg sacs of Cheiracanthium punctorium (Villers), unidentified Clubiona Latreille, and Larinioides cornutus (Clerck).
Recorded by Bertkau (1880: 332, as unidentified Tachina Meigen [misidentification; identified later by Mik (1890: 153) as Sarcophaga clathrata Meigen = S. (M.) sexpunctata, and confirmed by Herting (2017)]) as preying on eggs of Epeira cornuta (Clerck) [= Araneus cornuta Clerck = L. cornutus (Clerck)] in Bonn, North Rhine-Westphalia, Germany; by Kryger (1910: 267, as unidentified Sarcophaga Meigen [identified later by Lundbeck (1927: 181) as S. clathrata = S. (M.) sexpunctata]) as preying on eggs of Epeira cornuta (Clerck) [= Araneus cornuta Clerck = L. cornutus (Clerck)] in Gribskov and Hareskoven, Zealand, Denmark; by Grunin (1964: 71, as Thyrsocnema clathrata = S. (M.) punctata) as preying on eggs of unidentified Clubiona Latreille in Ural, Russia; by Finch (2005: 2345) as preying on eggs of L. cornutus in Oldenburg, Lower Saxony, Germany; and by Krehenwinkel et al. (2016: 1235) aspreying on eggs of C. punctorium (Villers) in Italy [locality not specified].
DISCUSSION
Spider egg-predating Sarcophagidae show diverse prey selection. Although the majority of records are from Araneidae, S. (M.) davidsonii, S. (M.) hinei, S. (M.) sexpunctata, and S. (M.) silbergliedi attack prey from different families of Araneomorphae (Table 1). Consequently, prey choice does not seem to be correlated with a particular spider taxon. Previous studies by Lubin (1974) and Hieber and Uetz (1990) demonstrated a positive correlation between the rate of predation and colony size in the social spiders C. moluccensis and M. incrassata, respectively. However, it is worth noting that prey choice is not exclusively associated with social spiders, as most of the records reviewed here are on solitary species.
Prey choice by predating flies may be determined by the conspicuousness and exposure level of spider egg sacs. The diversity in the shape and structure of egg sacs has been considered a significant adaptation to evade or reduce the success of parasitoids and predators (Austin 1985; Hieber 1992; Cloudsley-Thompson 1995). Hieber (1992) showed that predators that burrow into spider egg sacs, such asfly larvae, achieve higher success rates when attacking loosely woven or flocculated egg sacs than for those with denser coverings. This evidence indicates that egg sacs serve as a protective barrier against parasitoids and predators, requiring them to overcome this barrier to reach the eggs. Furthermore, in the case of the social spider C. moluccensis, Lubin (1974) observed that females of S. (B.) cyrtophorae closely inspected only webs with egg sacs within the entire colony, suggesting that these flies are attracted by the presence of conspicuous egg sacs. The egg sacs of spider species attacked by Sarcophagidae are typically conspicuous and are placed in freely exposed areas by the spider: They can be found attached to the spider web, as observed in species such as A. argentata, A. pulchella, C. moluccensis, and M. incrassata (Lubin 1974; Prakash and Pandian 1978; Hieber and Uetz 1990; Miranda et al. 2020); hanging from leaves, as in A. catenulata and O. magnificus (Todd 1988; Brown and Henderson 2019); or found attached to the vegetation, like in P. octopunctatus (Edwards 2004).
Although the structure and position of egg sacs provide valuable insights into the prey selection of spider egg-predating flies, the evolutionary pathways that led to this association in the Sarcophaga subgenera Baranovisca and Mehria remain unclear because of the absence of a phylogenetic framework. A few Mehria species have been sampled in phylogenetic studies aimed at elucidating the relationships within the subgenera of Sarcophaga (Giroux et al. 2010; Piwczyński et al. 2014; Buenaventura et al. 2017; Buenaventura and Pape 2017). However, the monophyly of Mehria has not been confirmed. On the other hand, Baranovisca species have never been sampled in phylogenetic studies of Sarcophagidae. Buenaventura (2021) proposed the first phylogenetic hypothesis of Sarcophagidae based on proteinencoding ultraconserved elements, revealing that S. (M.) sexpunctata is strongly supported as the basal taxon within a clade that is sister to the subgenus Pandelleana Rohdendorf, confirming previous findings (Piwczyński et al. 2014). In this context, larvae of Mehria and Pandelleana share similar predatory habits, aslarvae of Pandelleana species are predators of lizard eggs (Pape and Arribas 1999). While this phylogenetic position may indicate a shared predatory habit in the most recent common ancestor of Mehria and Pandelleana, a larger sampling of both Mehria and Baranovisca species is required to confirm the robustness of these relationships and to clarify the evolution of Sarcophagidae-Aranae interactions.
Despite this, some evidence indicates that the interactions between spider egg-predating Sarcophagidae and their prey might be a product of coevolution. Hieber et al. (2002) documented a set of stereotyped behaviors exhibited by S. (M.) lindae and M. incrassata females as a reaction to each other. As soon as the fly approached the web, the spider started to swing its legs towards the fly and protect the egg sac, grooming it with its chelicerae and pedipalps. In response, the fly tried to lure the spider out of the egg sac by landing on the hub or signal line of the web and mimicking the vibrations of captured prey. Moreover, the spider can distinguish the wing-beat vibrations of S. (M.) lindae from those of other flies. Although these behavioral patterns suggest that the interaction between these two species is a result of coevolution, further studies involving diverse populations and other species of spider egg-predating Sarcophagidae are required to determine the degree of specialization in the relationship between predator and prey.
Lastly, literature records mention spider eggpredating Sarcophagidae either as a parasitoid or as a predator. Hieber et al. (2002) provided an interesting perspective on the nature of this ecological interaction based on observations of the ovi/larviposition behavior of S. (M.) lindae in M. incrassata. They argued that the behavior of these flies resembles that of a parasitoidhost system, as the female approaches the egg sac as a suitable host for larval development. However, the fly larvae feed on spider eggs within the sac, which is best described as predatory behavior. Buenaventura (2021) estimated the diversification of larval feeding habits in Sarcophagidae, considering the parasitoid habit as a variation of predation. Although the habits are interconnected, a more precise classification may be necessary to explain the transition between parasitic and predatory habits across the diverse lineages of Sarcophagidae.
CONCLUSIONS
The evolution of Sarcophagidae-Araneae interactions still has much to be clarified, but some hypotheses discussed here can guide future studies. The subgenus Mehria includes 14 valid species, with S. (M.) lorosa as the sole species distributed in southwestern South America, specifically on the western side of the Andes Mountains. At least four Baranovisca and 10 Mehria species have been documented in the families Araneidae, Cheiracanthiidae, Clubionidae, Philodromidae, Salticidae, and Tetragnathidae. Prey selection by spider egg-predating Sarcophagidae does not seem to be correlated with a particular spider taxon; rather, the conspicuousness and exposure level of spider egg sacs may offer a more plausible explanation for prey selection by female flies. Larger sampling of Baranovisca and Mehria species in phylogenetic studies is required to confirm the validity of both subgenera and to estimate the origin of this predatory behavior in Sarcophagidae.
Acknowledgments
We want to thank Marco Antonio Menezes, Museu Nacional, Rio de Janeiro, Brazil, and the staff of the library of the Instituto de Biociências da Universidade de São Paulo, Brazil, for kindly helping with access to references; Andres O. Taucare-Rios, Universidad Arturo Prat, Chile, for identifying the specimens of Metepeira galatheae; Jimmy Cabra-García, Universidad del Valle, Colombia, for help with spider classification; and two anonymous reviewers for valuable comments and suggestions on an earlier version of the manuscript. FMG thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, proc. 152937/2022-6) for financial support. AMM (as principal investigator) and ROA (as coinvestigator) thank the Agencia Nacional de Investigación y Desarrollo (ANID), Chile, for supporting this study through FONDECYT Regular project nº 1221879. Images and data of the holotype of S. lorosa Hall (Fig. 3A, C, E) were provided by the Natural History Museum (2014), Specimens (from Collection specimens) [Photograph], Natural History Museum, Licensed under CC-BY-4.0 (https://creativecommons.org/licenses/by/4.0), https://data.nhm. ac.uk/dataset/collection-specimens/resource/05ff2255c38a-40c9-b657-4ccb55ab2feb. Images and data of the holotype of S. travassosi Tibana and Mello (Fig. 3B, D, F) were provided by the Canadian National Collection of Insects, Arachnids, and Nematodes (CNC), ©Her Majesty The Queen in Right of Canada, as represented by the Minister of Agriculture and Agri-Food, licensed under the Open Government Licence –Canada.
List of abbreviations
- CNC
Canadian National Collection of Insects, Ottawa, Canada.
- MACN
Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires, Argentina.
- MNRJ
Museu Nacional, Rio de Janeiro, Brazil.
- NHMUK
Natural History Museum, London, United Kingdom.
- UCM
Laboratorio de Ecología de Abejas, Talca, Chile.
- UNAP
Universidad Arturo Prat, Iquique, Chile.
Footnotes
Authors’ contributions: Specimen collection: DGP, BCR, AMM, and ROA. Taxonomy: FMG and PRM.
Revision of records: FMG. Production of digital images: DGP, BCR, and FMG. Original draft: FMG. All authors reviewed and edited the manuscript and approved the submitted version.
Competing interests: The authors declare that there are no competing interests.
Availability of data and materials: Holotypes of Sarcophaga lorosa Hall and Arachnidomyia travassosi Tibana and Mello are deposited in Natural History Museum, London, United Kingdom, and Canadian National Collection of Insects, Ottawa, Canada, respectively. Additional material examined of S. (Mehria) lorosa is deposited in Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Buenos Aires, Argentina, and Laboratorio de Ecología de Abejas, Talca, Chile.
Consent for publication: Not applicable.
Ethics approval consent to participate: Not applicable.
References
- Aldrich JM. 1914. The economic relations of the Sarcophagidae. Econ Entomol 8:242–246. doi:10.1093/jee/8.2.242.
- Aldrich JM. 1925. New Diptera or two-winged flies in the United States National Museum. Proc U S Natl Mus 66:1–36. doi:10.5479/si.00963801.66-2555.1.
- Armas LF de, Alayón García G. 1986. Depredadores y parasitoides de Argiope trifasciata (Araneae: Araneidae) en el sur de La Habana. Cienc Biol (La Habana) 16:114–117.
- Arnaud PH. 1978. A host-parasite catalog of North American Tachinidae (Diptera). U S D A Misc Publ 1319:1–860.
- Austin AD. 1985. The function of spider egg sacs in relation to parasitoids and predators, with special reference to the Australian fauna. J Nat Hist 19:359–376. doi:10.1080/00222938500770261.
- Auten M. 1925. Insects associated with spider nests. Ann Entomol Soc Am 18:240–250. doi:10.1093/aesa/18.2.240.
- Bertkau P. 1880. Verzeichnis der bisher bei Bonn beobachteten Spinnen. Verh Natur Ver Preuss Rheinl Westfalens 37:215–343.
- Brown GR, Henderson CL. 2019. First record of the grass cross spider (Argiope catenulata) of the family Araneidae in Australia. North Territ Nat 29:98–102.
- Buenaventura E. 2021. Museomics and phylogenomics with proteinencoding ultraconserved elements illuminate the evolution of life history and phallic morphology of flesh flies (Diptera: Sarcophagidae). BMC Ecol Evo 21: 70. doi:10.1186/s12862-021 01797-7. [DOI] [PMC free article] [PubMed]
- Buenaventura E, Pape T. 2017. Multilocus and multiregional phylogeny reconstruction of the genus Sarcophaga (Diptera, Sarcophagidae). Mol Phylogenet Evol 107:619–629. doi:10.1016/j.ympev.2016.12.028. [DOI] [PubMed]
- Buenaventura E, Whitmore D, Pape T. 2017. Molecular phylogeny of the hyperdiverse genus Sarcophaga (Diptera: Sarcophagidae), and comparison between algorithms for identification of rogue taxa. Cladistics 33:109–133. doi:10.1111/cla.12161. [DOI] [PubMed]
- Cantrell BK. 1980. A sarcophagid predator of spider eggs. News Bull Entomol Soc Queensland 8:42–44.
- Cantrell BK. 1981. Redescription of Parasarcophaga reposita Lopes (Diptera: Sarcophagidae). Aust Entomol 8:29–35.
- Cantrell BK. 1986. Notes on the taxonomy and biology of species of Parasarcophaga Johnston and Tiegs and Baranovisca Lopes (Diptera: Sarcophagidae) associated with spiders in eastern Australia. Aust Entomol 13:1–10.
- Chávez RO, Moreira-Muñoz A, Galleguillos M, Olea M, Aguayo J, Latín A. 2019. GIMMS NDVI time series reveal the extent, duration, and intensity of “blooming desert” events in the hyperarid Atacama Desert, Northern Chile. Int J Appl Earth Obs Geoinf 76:193–203. doi:10.1016/j.jag.2018.11.013.
- Cloudsley-Thompson JL. 1995. A review of the anti-predator devices of spiders. Bull Br Arachnol Soc 10:81–96.
- Coquillett DW. 1892. The dipterous parasite of Melanoplus devastator in California. Insect Life 5:22–24.
- Cumming JM, Wood DM. 2017. 3. Adult morphology and terminology. In: Kirk-Spriggs AH, Sinclair BJ (eds) Manual of Afrotropical Diptera -Volume 1. Introductory chapters and keys to Diptera families, Suricata 4. South African National Biodiversity Institute, Pretoria, South Africa.
- Cunha MB da. 2018. Um museu em chamas: o caso do Museu Nacional do Rio de Janeiro. RICI 12:1–3.
- Davidson A. 1894. Concerning spider-egg parasites. Insect Life 6:268–269.
- Edwards GB. 2004. Revision of the jumping spiders of the genus Phidippus (Araneae: Salticidae). Collect Arthropods 11:1–158.
- Enderlein G. 1928. Klassifikation der Sarcophagiden. SarcophagidenStudien I. Arch Klassif Phylogenet Entomol 1:1–56.
- Escobar H. 2018. In a ‘foretold tragedy,’ fire consumes Brazil museum. Science 361:960–960. doi:10.1126/science.361.6406.960. [DOI] [PubMed]
- Evenhuis NL, Pape T. 2022. Systema Dipterorum, Version 4.2.2. Available at: http://www.diptera.org/.Accessed 30 June 2023.
- Finch O. 2005. The parasitoid complex and parasitoid‐induced mortality of spiders (Araneae) in a Central European woodland. J Nat Hist 39:2339–2354. doi:10.1080/00222930502005720.
- Fritzén NR, Sääksjärvi IE. 2016. Spider silk felting—functional morphology of the ovipositor tip of Clistopyga sp. (Ichneumonidae) reveals a novel use of the hymenopteran ovipositor. Biol Lett 12:20160350. doi:10.1098/rsbl.2016.0350. [DOI] [PMC free article] [PubMed]
- Gillung JP, Borkent CJ. 2017. Death comes on two wings: a review of dipteran natural enemies of arachnids. J Arachnol 45:1–19. doi:10.1636/JoA-S-16-085.1.
- Giroux M, Pape T, Wheeler TA. 2010. Towards a phylogeny of the flesh flies (Diptera: Sarcophagidae): Morphology and phylogenetic implications of the acrophallus in the subfamily Sarcophaginae. Zool J Linn Soc 158: 740–778. doi:10.1111/j.1096-3642.2009. https://doi.org/10.1111/j.1096-3642.2009.00561.x 00561.x.
- Gloor D, Nentwig W, Blick T, Kropf C. 2017. World Spider Catalog. Version 24. Natural History Museum Bern. Available at: http://wsc.nmbe.ch. Accessed 13 Mar. 2023.
- Grunin KY. 1964. On the biology and distribution of certain Sarcophaginae (Diptera, Sarcophagidae) in the USSR. Entomol Óbozrenie 43:71–79. (in Russian)
- Guimarães JH. 1977. Host-parasite and parasite-host catalogue of South American Tachinidae (Diptera). Arq Zool 28:1–131. doi:10.11606/issn.2176-7793.v28i3p1-131.
- Hall DG. 1937. Sarcophaginae. In: Diptera of Patagonia and south Chile. Part 7. British Museum (Natural History), London.
- Herting B. 2017. A critical revision of host records of Palearctic Tachinidae (Diptera) until 1937. Stuttg Beitr Naturkd A 10:41–173. doi:10.18476/sbna.v10.a3.
- Hieber C, Wilcox S, Boyle J, Uetz G. 2002. The spider and fly revisited: ploy-counterploy behavior in a unique predator-prey system. Behav Ecol Sociobiol 53: 51–60. doi:10.1007/s00265 002-0547-2.
- Hieber CS. 1992. Spider cocoons and their suspension systems as barriers to generalist and specialist predators. Oecologia 91:530–535. doi:10.1007/BF00650327. [DOI] [PubMed]
- Hieber CS, Uetz GW. 1990. Colony size and parasitoid load in two species of colonial Metepeira spiders from Mexico (Araneae: Araneidae). Oecologia 82:145–150. doi:10.1007/BF00323527. [DOI] [PubMed]
- Kaston BJ, Jenks GE. 1937. Dipterous parasites of spider egg sacs. Bull Brooklyn Entomol Soc 32:160–165.
- Kawada R, Buffington ML. 2016. A scalable and modular dome illumination system for scientific microphotography on a budget. PLoS ONE 11:e0153426. doi:10.1371/journal.pone.0153426. [DOI] [PMC free article] [PubMed]
- Krehenwinkel H, Rödder D, Năpăruş-Aljančič M, Kuntner M. 2016. Rapid genetic and ecological differentiation during the northern range expansion of the venomous yellow sac spider Cheiracanthium punctorium in Europe. Evol Appl 9:1229–1240. doi:10.1111/eva.12392. [DOI] [PMC free article] [PubMed]
- Kryger JP. 1910. Snyltere i Edderkoppeæg. Entomol Medd 8:257–285. (in Danish)
- Lopes H de S. 1946. Novos sarcofagídeos neotrópicos representados na coleção do “Imperial Institute of Entomology” (Diptera, Sarcophagidae). Rev Bras Biol 6:117–131.
- Lopes H de S. 1959. A review of Australian Sarcophagidae (Diptera). Stud Entomol 2:33–67.
- Lopes H de S. 1981. Two new species of Sarcophagidae (Diptera) living on Arthropods. Rev Bras Entomol 25:307–312.
- Lopes H de S. 1985. New genus of Sarcophagidae (Diptera) based on an Australian species living on spider egg cases. Aust Entomol 12:51–53.
- Lopes H de S. 1989. On Arachnidomyia (Diptera, Sarcophagidae) with a new species associated to Metepeira spp. (Arachnida, Araneida). Rev Bras Biol 49:1093–1099.
- Lopes H de S, Tibana R. 1982. Sarcophagid flies of Tarapaca, north of Chile (Diptera). Rev Bras Biol 4:135–145.
- Lubin YD. 1974. Adaptive advantages and the evolution of colony formation in Cyrtophora (Araneae: Araneidae). Zool J Linn Soc 54:321–339. doi:10.1111/j.1096-3642.1974.tb00806.x.
- Lundbeck W. 1927. Diptera Danica: Genera and species of flies hitherto found in Denmark. Part 7. Platypezidae, Tachinidae. G.E.C. Gad, Copenhagen.
- Macquart P-J-M. 1834. Insectes diptères du nord de la France. Tome V. Athéricères: créophiles, oestrides, myopaires, conopsaires, scénopiniens, céphalopsides. Lille.
- Macquart P-J-M. 1835. Histoire naturelle des insectes. Diptères. Tome deuxième. Paris. doi:10.5962/bhl.title.14274.
- Meigen JW. 1824. Systematische Beschreibung der bekannten europäischen zweiflügeligen Insekten. Schulz-Wundermann, Hamm.
- Mik J. 1890. Dipterologische Miscellen XVI. Wiener Entomol Ztg 9:153–158.
- Miranda R, Santos-Murgas A, Quintero-A D, Abrego-L JC. 2020. Insectos asociados a ovisacos de Argiope argentata (Fabricius, 1775) (Arachnida: Araneae) en Panamá. Intropica 15:8–15. doi:10.21676/23897864.3280.
- Pape T. 1996. Catalogue of the Sarcophagidae of the world (Insecta: Diptera). Associated Publishers, Gainsville, Florida, USA.
- Pape T, Arribas OJ. 1999. Sarcophaga protuberans Pandellé -an Old World predator of lizard eggs (Diptera: Sarcophagidae; Reptilia: Lacertidae). Stud Dipterol 6:73–78.
- Pape T, Dahlem GA. 2010. Sarcophagidae (flesh flies). In: Brown VB, Borkent A, Cumming JM et al. (eds) Manual of Central American Diptera Volume 2. National Research Council of Canada, Ottawa, Ontario, Canada.
- Patitucci LD, Mulieri PR, Domínguez MC, Mariluis JC. 2015. An inventory of saprophagous Calyptratae (Insecta: Diptera) in urban green spaces of Buenos Aires City. Rev Mus Argent Cienc Nat 17:97–107. doi:10.22179/REVMACN.17.385.
- Peña LEG. 1974. Los tipos de insectos de la colección Luis E. Peña G. Bol Soc Biol Concepc 47:259–282.
- Piwczyński M, Szpila K, Grzywacz A, Pape T. 2014. A large-scale molecular phylogeny of flesh flies (Diptera: Sarcophagidae). Syst Entomol 39:783–799. doi:10.1111/syen.12086.
- Piwczyński M, Pape T, Deja-Sikora E et al. 2017. Molecular phylogeny of Miltogramminae (Diptera: Sarcophagidae): Implications for classification, systematics and evolution of larval feeding strategies. Mol Phylogenet Evol 116:49–60. doi:10.1016/j.ympev.2017.07.001. [DOI] [PubMed]
- Prakash RN, Pandian TJ. 1978. Energy flow from spider eggs through dipteran parasite and hymenopteran hyperparasite populations. Oecologia 33:209–219. doi:10.1007/BF00344849. [DOI] [PubMed]
- Rayor LS, Uetz GW. 1990. Trade-offs in foraging success and predation risk with spatial position in colonial spiders. Behav Ecol Sociobiol 27:77–85. doi:10.1007/BF00168449.
- Riccardi PR, Pádua DG. 2021. First record of egg sac predation of the fly Pseudogaurax cingulatus Sabrosky (Diptera, Chloropidae) upon spider Tetragnatha sp. (Araneae, Tetragnathidae) in northern Brazil. Pap Avulsos Zool 61: 1–5. doi:10.11606/1807 0205/2021.61.04.
- Shinonaga S, Barrion AT. 1980. A new species of sarcophagid fly parasitic in the egg sac of the spider, Argiope catenulata (Doleschall) in the Philippines. Kontyû 48:537–539.
- Shorthouse DP. 2010. SimpleMappr, an online tool to produce publication-quality point maps. Available at: https://www. simplemappr.net. Accessed 15 Feb. 2023.
- Sobczak JF, Loffredo APS, Sobczak JCMSM. 2012. First record of egg sac predation of the wasp Tromatobia sp. Foster, 1869 (Hymenoptera: Ichneumonidae) upon Araneus omnicolor (Keyserling, 1893) (Araneae: Araneidae). Rev Iber Aracnol 20:113–115.
- Souza-Santiago BK, Messas YF, de Pádua DG, Santos AJ, Vasconcellos-Neto J. 2023. Taking care of the enemy: egg predation by the Darwin wasp Tromatobia sp. (Ichneumonidae) on the cobweb spider Chrysso compressa (Araneae, Theridiidae). J Hymenopt Res 95:103–112. doi:10.3897/jhr.95.97029.
- Tibana R, Mello CA. 1992. Uma nova espécie de Arachnidomyia Townsend, 1934. Mem Inst Oswaldo Cruz 87:293–297. doi:10.1590/S0074-02761992000500055.
- Todd DV. 1988. An illustrated guide to the genera of orb-weaving spiders in Australia. Mem Queensl Mus 25:273–332.
- Villanueva-Bonilla GA, Onody HC, Santos BF, Vasconcellos-Neto J. 2016. First record of egg sac predation on a wall crab spider Selenopidae (Araneae) by the wasp Camera lunavenatrix sp. n. (Ichneumonidae, Cryptinae). J Hymenopt Res 49:65–79. doi:10.3897/JHR.49.7862.
- Yefremova ZA, Lubin Y. 2020. Tachinobia repanda (Hymenoptera: Eulophidae) from egg sacs of a colonial spider, Cyrtophora moluccensis (Araneae: Araneidae) in Papua New Guinea. J Insect Sci 20:12. doi:10.1093/jisesa/ieaa104. [DOI] [PMC free article] [PubMed]
- Zetterstedt JW. 1838. Sectio tertia. Diptera. Dipterologis Scandinaviae amicis et popularibus carissimus. In: Zetterstedt JW (ed) Insecta lapponica (1838–1840). Leopold Voss, Lipsiae [= Leipzig]. doi:10.5962/bhl.title.8242.