Abstract
For systematic monitoring of radioactive nuclides in marine products, this study aimed at streamlining and simplifying the analysis method for the prominent radioisotope, strontium-90 (90Sr). The DGA chelate solid-phase extraction technique was employed for enhanced efficiency. The study focused on optimizing the necessary pretreatment procedures while minimizing the steps involving HNO3 leaching. This protocol enabled the quantitative recovery of strontium, and it facilitated a rapid analysis without the need for a time-consuming evaporation step and without waiting for secular equilibrium between 90Sr and its progeny to be reached. The method incorporating the optimized pretreatment protocol was applied to three diverse marine fish species and the accurate quantification of 90Sr at background levels in surface seawater was achieved. The method obtained concentrations in bone samples from these species that ranged from 0.036 to 0.120 mBq per kg-dry, and chemical yield values were notably high, ranging from 87.7% to 92.5%.
Introduction
Strontium-90 (90Sr) is widely acknowledged as a critical fission product in the field of radiation environmental monitoring due to its propensity to accumulate in the hard tissues of animals and the emission of high-energy beta rays by its progeny, yttrium-90 (90Y). While various conventional methods for sample preparation have been established, including coprecipitation, ion exchange, and fuming HNO3 precipitation, several pretreatment techniques also have been proposed, among which the chelating resin solid-phase extraction method [1, 2] is notable. After sample preparation, mass spectrometry methods such as Inductively Cupled Mass Spectrometry (ICP-MS) [3, 4], Thermal Ionization Mass Spectrometry (TIMS) [5–7], and Accelerator Mass Spectrometry (AMS) [8, 9] are now commonly applied for measurements. Despite its importance, the analysis of 90Sr in marine products, such as marine fish, remains challenging due to the presence of abundant amounts of calcium (Ca) and stable Sr isotopes. Ca interferes with the chemical separation of Sr. In addition, the large amounts of stable Sr result in self-absorption of beta rays during radiation measurements or they lower the 90Sr/Sr atom ratio in mass spectrometry, resulting in a quantitative limit below the detection limit.
Marine fish that are characterized by a low Sr enrichment factor (e.g. CR = 2.5) [10] require a substantial amount of bone samples for analyzing low concentrations of 90Sr at global fallout levels. The minimum detectable activity by the solvent extraction method has been reported 0.10 Bq per kg in whole body ashed sample [11], which is almost the same as the global fallout level.
Stable Sr often surpasses the maximum adsorption capacity of Sr-specified resins. The solid phase extraction using DGA resin (DGA-SPE) for 90Y separation represents a promising approach for the analysis of marine fish bone samples. DGA-SPE has been developed as a method for quantifying 90Sr via 90Y and it has been previously applied in soil and seawater matrices [12–14].
DGA-SPE effectively adsorbs Y under high concentration HNO3 conditions and it is minimally affected by the sample matrix. Moreover, a subsequent rinse with various acids enables the removal of interfering radionuclides, making it particularly suitable for the analysis of low concentrations 90Y [13, 15]. However, a drawback of DGA-SPE is its relatively slow flow rate. When handling large sample volumes, processing times are extended, which results in increased analytical uncertainties related to 90Y decay correction. Typical operations for DGA resin prepacked in small cartridges (1 ml per 2 ml) are done at flow rates ranging from 1 to 2 ml per min, with the sample volume usually restricted to ~200 ml.
In this study, the focus was on optimizing the acid dissolution pretreatment process to efficiently separate 90Y in bone samples from marine fish for DGA-SPE. Acid extraction data were acquired for different fish species and they were subsequently applied to the analysis of 90Sr using actual bone samples.
Materials and methods
Samples
Marine fish samples were collected off the Pacific coast of Japan. Two bony fishes (class Osteichthyes) (Japanese Amberjack, Seriola quinqueradiata and Yellow Goosefish, Lophius litulon) and one cartilaginous fish (class Chondrichthyes) (Red Stingray, Hemitrygon akajei) were collected.
Raw fishes were cut with a knife, and the head, viscera, and muscles were removed. The muscles remaining on the vertebral bones were scraped off with a nylon brush as much as possible. However, bone marrow remained inside of the bones. Bones were dried at 110 °C for >2 days and combusted at 450 °C in a muffle oven to get ash bone samples.
The elemental compositions of fish bones (dried) are shown in Table 1. Ca concentration was the highest in the Japanese Amberjack (15.1%) and lowest in the yellow goosefish (13.2%). Sr concentration also showed approximately a two-fold difference among fish species. The Sr concentration in seawater was ~ 0.0079‰. The Sr concentration in dried bones was 175–340 times higher than that of seawater. Sr is excluded during shell formation because Sr interferes with the formation of the calcium skeleton. The trend may also vary depending on the trophic level within the food chain. Low Sr/Ca ratios in the bone compared with the ratio in seawater (0.0192; [16]) indicate that a process occurs during bone formation in which other elements are removed during the uptake of Ca.
Table 1.
Chemical composition of fish bone samples (dry) and comparison with seawater.
| Ca | P | Na | K | Mg | Sr | Mg/Ca | Sr/Ca | |
|---|---|---|---|---|---|---|---|---|
| % | % | ‰ | ‰ | ‰ | ‰ | |||
| Japanese amberjack | 15.1 | 7.9 | 3.2 | 2.2 | 2.05 | 0.52 | 0.014 | 0.0034 |
| Red stingray | 17.9 | 10.3 | 11.2 | 4.0 | 3.69 | 0.70 | 0.021 | 0.0039 |
| Yellow goosefish | 13.2 | 7.4 | 17.7 | 5.9 | 2.27 | 1.14 | 0.017 | 0.0086 |
| Seawater (Salinity =35)* | 0.041 | 10.73 | 0.41 | 1.28 | 0.0079 | 0.31 | 0.0192 |
*from Millero et al. (2008) [16]
Reagents
Ultrapure water (>18.2 MΩ∙cm) was obtained from Milli-Q reference (Merck Millipore, USA). Preparations for all reagent and sample dilutions used the ultrapure water. HCl, HNO3 and NH4OH were available as Electro-industry Grade from Kanto Chemicals (Japan). Ultrapure Grade hydrofluoric acid (TAMAPURE AA-100, Tama Chemicals, Japan) was used to avoid contamination of Th and U series radionuclides. Purified Fe solution (1 mg/L) was prepared from FeCl3∙6H2O (Wako Chemicals, Japan) by solvent extraction with diisopropyl ether. About 2 ml DGA resin prepacked cartridges (DN-R50-S, Eichrom Technology Inc., USA) were used for chemical separation.
Apparatus
The SPE-DGA method was carried out with a vacuum box system (Eichrom Technology Inc., USA) with a diaphragm vacuum pump (LABOPORT N96, KNF, USA). Elemental analysis for acid-leached samples and evaluation for Y chemical recovery during DGA-SPE were performed by an inductively coupled plasma optical emission spectrometer (ICP-OES; SPECTROBLUE TI, Ametek, USA). Beta counting was performed by an LB-4200 2 π gas flow proportional counter (Mirion Technology, USA) to determine 90Y activity, which provided 3 mBq per sample of minimum detectable activity with a 40 h counting time.
Acid leaching process
For the acid leaching process, 40 g of an ash sample was processed. This is a sufficient amount for quantitative analysis of 90Sr at the global fallout level, considering the stable Sr concentration in the bone samples and the specific activity of open ocean seawater [14, 17].
When a significant sample mass is introduced into the HNO3 solution at one time, there is a potential risk of insoluble salts forming on the solid surface, potentially impeding the dissolution. Therefore, each ash bone sample was gradually added to 60 ml of 61% HNO3 with continuous stirring to dissolve it.
The resulting solution was decanted into a 50 ml centrifuge tube, centrifuged at 3000 rpm for 10 min, and then filtered through a 0.45 μm pore mixed cellulose ester membrane filter (47 mm in diameter). Subsequently, the residue and centrifuge tube were rinsed three times with 30 ml of 8 M HNO3, with each rinsing solution collected separately. These leachate samples were appropriately diluted and quantified using ICP-OES for the elements Ca, P, Na, K, Mg, and Sr.
Strontium-90 analysis
Secular equilibrium between 90Sr and 90Y was established during 2 weeks following the acid leaching process. The leached samples were combined to determine 90Sr activity concentration, resulting in HNO3 concentration of ~6 M. The distribution coefficient of Y to DGA resin is known to be sufficiently high with > 1 M HNO3 media [14]. This is attributed to the dissolution of calcium phosphate and carbonate component playing a role in neutralizing H+.
The sample solution was introduced onto the DGA resin cartridge with vacuum box system after adding 200 μg of stable Y as a yield tracer. Tazoe et al. [12] previously reported the chromatogram of Y separation using the 1 ml cartridge. In this study, a cartridge with a 2 ml volume was employed to account for potential inhibition of Y adsorption due to the presence of abundant Ca, phosphate, and residual organic substances.
Following the sample introduction (Y retention), the DGA resin cartridge was rinsed with 20 ml of 8 M HNO3. Interference elements and radionuclides such as Th and U were eluted using 20 ml of 8 M HCl, 40 ml of 3 M HNO3 + 0.3 M HF, and 40 ml of 0.02 M HNO3. Finally, 90Y and stable Y were eluted using 30 ml of 0.1 M HCl.
To determine chemical yield of 90Y, a 0.1 ml aliquot of the Y elution fraction was taken and diluted with 1% HNO3 for Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). Next, 1 mg of Fe was added to the remaining main portion of the Y elution fraction and Y was precipitated with Fe hydroxide by adding 1 ml of 28% NH4OH. The precipitated Y included 90Y. A mixed cellulose ester membrane filter (0.45 μm pore, 25 mm in diameter) was used to remove the Y and Fe hydroxide precipitates. After drying using an IR lamp, the filter and precipitates were encapsulated between an acrylic disc and a plastic film as a beta radiation source. A 60 h beta radiation measurement was conducted. This measurement consisted of 30 repetitions of 2 h measurements and that was sufficient to allow confirmation of 90Y decay.
Results and discussion
Results for acid leaching process of bone samples
Figure 1 shows the changes in Sr, Ca, Na, and Mg recovery yields in the leachate during the HNO3 leaching. The results of the leaching yielded over 80% recovery in the first extraction for all three fish species. Only yellow goosefish showed a slightly lower recovery rate, which may be attributed to a higher ash content. Incomplete combustion of organic substances and insoluble silicate could interfere with extraction of these elements from ash to HNO3. In the fourth extraction, all recovery rates were <1%, indicating that quantitative Sr recovery could be achieved through three extraction steps. In this study, the chemical separation of 90Y was conducted after allowing the necessary time for radiochemical equilibrium to be reached following acid dissolution. Notably, if secular equilibrium between 90Sr and 90Y is preserved post-extraction, this implies the feasibility of promptly carrying out DGA-SPE and successive beta counting. Eliminating the waiting period for secular equilibrium to be reached (2 weeks) is of paramount importance for urgent analyses in radiological emergency situations, as this time-consuming step, significantly restricts 90Sr analysis.
Figure 1.
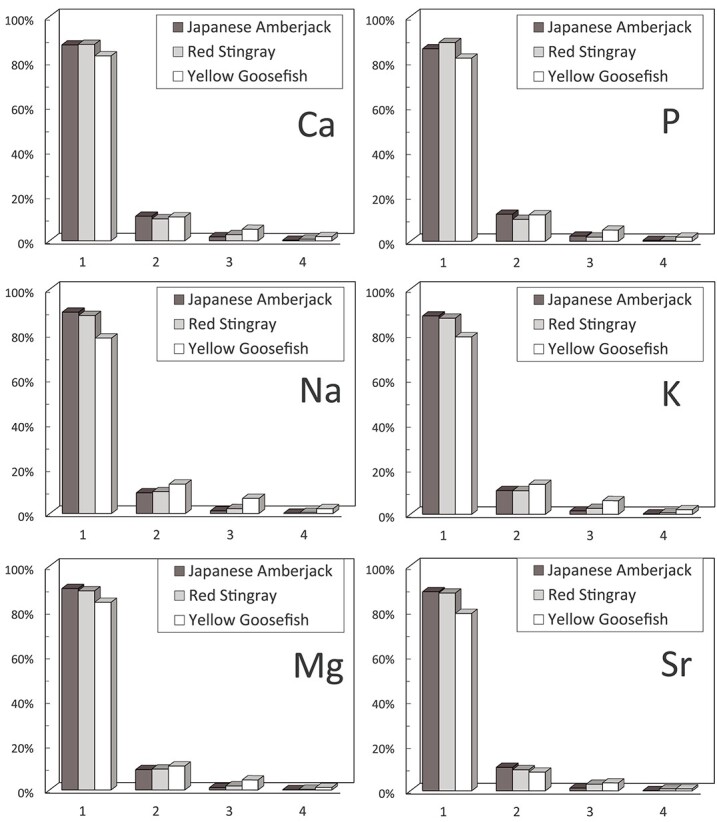
Element recovery rates in HNO3 leaching experiments for solubilizing marine fish bone samples.
Application to 90Sr analysis in marine fish samples
Table 2 summarizes the results of the 90Sr analysis method developed in this study when applied to marine fish bone samples. For Japanese Amberjack samples, 200 g of ashed bone sample was homogenized and divided into portions for duplicate analysis. The recovery rates for 90Y ranged from 87.7% to 92.5% with an average of 89.5% (± 2.1% of standard deviation). There is no precedent for the successful single-stage extraction of Y from samples containing a large amount of matrix, making this achievement significant. Furthermore, all the 90Sr concentration analysis values exceeded the minimum detectable activity concentration (MDC). The measurements for two samples of Japanese amberjack yielded results of 0.040 mBq per kg-dry (± 0.006 of combined standard uncertainty) and 0.036 (± 0.006) mBq per kg-dry, indicating a high degree of agreement. Variations were observed in other fish species, with yellow goosefish exhibiting the highest 90Sr concentration, approximately three times that of Japanese amberjack. This trend mirrored stable strontium values, as shown in Table 1, and the fluctuations in the 90Sr/stable Sr ratio were minimal. There was a possibility that Japanese amberjack might have a slightly smaller 90Sr/stable Sr ratio within the analytical error. The seawater values near Jeju Island reported by Kim et al. from 2021 to 2023 were ~ 0.72–0.78 mBq per kg at 2 m depth and 0.79–0.91 mBq per kg at 100 m depth. Based on these data and the stable Sr concentration (0.0079 g per kg at salinity of 35 [16]) in seawater, the Sr-90/stable Sr ratio is in the range of 0.09 to 0.12. However, this does not account for variations in stable Sr concentration with salinity. Nevertheless, the Sr-90/stable Sr ratio of 0.07 to 0.12 obtained in this study is consistent with the seawater values, supporting the reliability of this analytical method.
Table 2.
90Sr activity concentration in fish bone sample.
| Sample ID | 90Y yield | 90Sr concentration (mBq/kg-dry)* | MDC (mBq/kg-dry) | 90Sr/stable Sr (Bq/g)* | ||||
|---|---|---|---|---|---|---|---|---|
| Japanese Amberjack | ||||||||
| 1 | 92.5% | 0.040 | ± | 0.006 | 0.027 | 0.077 | ± | 0.012 |
| 2 | 87.7% | 0.036 | ± | 04.006 | 0.027 | 0.069 | ± | 0.012 |
| Red Stingray | 88.4% | 0.086 | ± | 0.008 | 0.032 | 0.122 | ± | 0.011 |
| Yellow Goosefish | 89.6% | 0.120 | ± | 0.007 | 0.027 | 0.105 | ± | 0.006 |
* ± (combined standard uncertainty)
Conclusions
This study focused on the minimization of HNO3 leaching as part of the pretreatment protocol to optimize the effective DGA-SPE method for the analysis of 90Sr in bone samples from marine fish. The practical applicability of this method when applied to real samples was also assessed.
A three-step HNO3 leaching process enabled the quantitative recovery of inorganic components, including strontium.
The leaching, centrifugation, and filtration processes could be completed in a single day, eliminating the need for subsequent concentration processes and allowing for direct DGA-SPE.
When applied to samples of three distinct marine fish species, the developed method exhibited high strontium recovery rates. The 90Sr analysis results ranged from 0.036 to 0.12 mBq/kg.
Disparities in concentration among fish species could be attributed to differences in Sr uptake efficiency in their bones, and the levels observed were consistent with background levels.
These findings highlight the effectiveness and efficiency of this method for 90Sr analysis in marine environments. From applications of the method valuable insights for future research in this field are expected.
Contributor Information
Hirofumi Tazoe, Institute of Radiation Emergency Medicine, Hirosaki University, 66-1 Hon-cho, Hirosaki 036-8564 Aomori, Japan.
Yosuke Amano, Fukushima Prefectural Fisheries and Marine Science Research Centre, 13-2, Matsushita, Onahama, Iwaki, Fukushima 970-0316, Japan.
Yasuo Ishida, KANSO TECHNOS CO., LTD., 3-1-1, Higashikuraji, Katano, Osaka 576-0061, Japan; Central Laboratory, Marine Ecology Research Institute, 300, Iwawada, Onjuku, Isumi, Chiba 299-5105, Japan.
Masatoshi Yamada, Institute of Radiation Emergency Medicine, Hirosaki University, 66-1 Hon-cho, Hirosaki 036-8564 Aomori, Japan; Central Laboratory, Marine Ecology Research Institute, 300, Iwawada, Onjuku, Isumi, Chiba 299-5105, Japan.
Naofumi Akata, Institute of Radiation Emergency Medicine, Hirosaki University, 66-1 Hon-cho, Hirosaki 036-8564 Aomori, Japan.
Conflict of interest statement
The authors have no conflicts of interest directly relevant to the content of this article.
Funding
This work was supported by Environmental Radioactivity Research Network Center (E-23-11).
References
- 1. Vajda N, Kim C. Determination of radiostrontium isotopes: a review of analytical methodology. Appl Radiat Isot 2010;68:2306–26. 10.1016/j.apradiso.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 2. Shao Y, Yang GS, Tazoe H. et al. A review of measurement methodologies and their applications to environmental 90Sr. J Environ Radioact 2018;192:321–33. 10.1016/j.jenvrad.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 3. Yanagisawa K, Odashima M, Matsueda M. et al. Online solid-phase extraction−inductively coupled plasma–quadrupole mass spectrometric quantification of 90Sr using 88Sr/86Sr isotope dilution method. Talanta 2022;244:123442. 10.1016/j.talanta.2022.123442. [DOI] [PubMed] [Google Scholar]
- 4. Tomita J, Takeuchi E. Rapid analytical method of 90Sr in urine sample: rapid separation of Sr by phosphate co-precipitation and extraction chromatography, followed by determination by triple quadrupole inductively coupled plasma mass spectrometry (ICP-MS/MS) Appl. Radiat Isot 2019;150:103–9. 10.1016/j.apradiso.2019.05.026. [DOI] [PubMed] [Google Scholar]
- 5. Aoki J, Wakaki S, Ishiniwa H. et al. Direct quantification of Attogram levels of strontium-90 in microscale biosamples using isotope dilution-thermal ionization mass spectrometry assisted by quadrupole energy filtering. Anal Chem 2023;95:4932–9. 10.1021/acs.analchem.2c04844. [DOI] [PubMed] [Google Scholar]
- 6. Wakaki S, Aoki J, Shimode R. et al. A part per trillion isotope ratio analysis of 90Sr/88Sr using energy-filtered thermal ionization mass spectrometry. Sci Rep 2022;12:1151. 10.1038/s41598-022-05048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kavasi N, Sahoo SK. Method for 90Sr analysis in environmental samples using thermal ionization mass spectrometry with Daly ion-counting system. Anal Chem 2019;91:2964–9. 10.1021/acs.analchem.8b05184. [DOI] [PubMed] [Google Scholar]
- 8. Sasa K, Honda M, Hosoya S. et al. A sensitive method for Sr-90 analysis by accelerator mass spectrometry. J Nucl Sci Technol 2021;58:72–9(22021). 10.1080/00223131.2020.1801530. [DOI] [Google Scholar]
- 9. Honda M, Martschini M, Marchhart O. et al. Novel 90Sr analysis of environmental samples by ion-laser InterAction mass spectrometry. Anal Sci 2022;14:2732–38. 10.1039/D2AY00604A. [DOI] [PubMed] [Google Scholar]
- 10. International Atomic Energy Agency (IAEA) . Sediment Distribution Coefficients and Concentration Factors for Biota in the Marine Environment; Technical Reports series No. 422. IAEA.
- 11. Deng FF, Lin F. Measurement of 90Sr in marine biological samples. Molecules 2022;27:3730. 10.3390/molecules27123730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maxwell SL, Culligan BK, Shaw PJ. Rapid determination of radiostrontium in large soil samples. J Radioanal Nucl Chem 2013;295:965–71. 10.1007/s10967-012-1863-2. [DOI] [Google Scholar]
- 13. Tazoe H, Obata H, Yamagata T. et al. Determination of strontium-90 from direct separation of yttrium-90 by solid phase extraction using DGA resin for seawater monitoring. Talanta 2016;152:219–27. 10.1016/j.talanta.2016.01.065. [DOI] [PubMed] [Google Scholar]
- 14. Tazoe H, Obata H, Tomita M. et al. Novel method for low level Sr-90 activity detection in seawater by combining oxalate precipitation and chelating resin extraction. Geochem J 2017;51:193–7. 10.2343/geochemj.2.0441. [DOI] [Google Scholar]
- 15. Pourmand A, Dauphas N. Distribution coefficients of 60 elements on TODGA resin: application to Ca, Lu, Hf. U and Th isotope geochemistry Talanta 2010;81:741–53. 10.1016/j.talanta.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 16. Millero FJ, Feistel R, Wright D. et al. The composition of standard seawater and the definition of the reference-composition salinity scale. Deep Sea Res I 2008;55:50–72. 10.1016/j.dsr.2007.10.001. [DOI] [Google Scholar]
- 17. Kim G, Choi SD, Lim JM. et al. Strontium-90 levels in seawater southeast of Jeju Island during 2021–2023. Mar Pollut Bull 2023;193:115258. 10.1016/j.marpolbul.2023.115258. [DOI] [PubMed] [Google Scholar]


