Abstract
In this paper, using a 20 L spherical explosive device and a Hartmann device, we carried out explosion suppression experiments on 19 and 30 μm aluminum powders (500 g/m3) with different concentrations of the new explosive suppressants (MCM41@CS-APP) and CaCO3 and elaborated on the suppression mechanism of the explosion of MCM41@CS-APP on aluminum powder. The experimental results show that when the concentration of the explosion suppressor is 50 g/m3, the maximum explosion pressure (Pmax) produced by the explosion of mixed dust is higher than that of the explosion of aluminum powder, and with the increase of the concentration of the deflagration suppressant, the Pmax of the mixed dust decreases. When the concentrations of MCM41@CS-APP and CaCO3 reached 400 g/m3, the Pmax of the mixed dust (Al = 19 μm) was 0.133 and 0.364 MPa, which decreased by 81.3% and 48.9%, respectively. The Pmax of the mixed dust (Al = 30 μm) was not significant. Both detonation inhibitors inhibited the explosion of aluminum powder; the detonation duration of Al/MCM41@CS-APP is shorter; there are fewer aluminum particles in the product; and the initial oxidation temperature of aluminum powder is higher.
1. Introduction
Aluminum (Al) is an important raw metal material widely used in the metal manufacturing and metallurgy fields. Since the 21st century, the rapid development of the finishing industry, especially in the polishing and grinding processes of Al products, has generated large amounts of Al dust. As the Al dust accumulates to a certain concentration in a limited space and encounters an ignition source, it may explode or cause severe consequences. Therefore, Al dust explosion prevention is of great significance, and the explosion suppression characteristics and mechanism are the basis for Al dust explosion suppression. Al dust explosion suppression has been extensively studied, and great progress has been made.1−3
Due to the extremely high activity of Al, different materials including inert gas, fine water mist, and solid powder antiexplosion agents (phosphate, carbonate, etc.) have been studied in depth for their explosion suppression effects, and their suppression characteristics and laws are explored.4−7 To evaluate the dust explosion suppression abilities of NaHCO3 and NH4H2PO4, Amrogowicz et al., respectively, injected the suppressants into a container and measured the sum of the explosion lower limits.8 They found that the explosion suppression effect of NH4H2PO4 was better than that of NaHCO3. Jiang et al. revealed by observing the flame shape of Al dust deflagration that the maximum flame speed decreased with the increase of the inhibitor concentration, and the inhibition effect of NH4H2PO4 was better than that of NaHCO3.9 Zhang and Bi et al. studied the suppression effects of CO2 and H2 on the explosion of Al dust in a closed cuboid space and found that their suppression mechanisms were different, and the suppression effect of CO2 was significantly better than that of H2.10 Zhang et al. developed the ATH/ZB composite explosion inhibitor by physical/chemical synthesis.11 The minimum inhibitory concentrations of ATH and ZB alone for the aluminum dust explosion were 1000 g/m3, while that of ATH/ZB was only 600 g/m3. They also studied the suppression mechanism of NZB on the Al dust explosion and demonstrated that the suppression performance of NZB was better than that of ZB2335.12 Dai et al. compared the Al dust explosion suppression abilities of NaH2PO4, (NH4)2HPO4, NH4H2PO4, KHCO3, and NaHCO3 using a 20 L spherical explosion device and found that 40% NaH2PO4 could completely suppress the explosion of Al dust, while 50% (NH4)2HPO and 50% NH4H2PO4 showed obvious suppression effects.13 Ke and Meng et al. obtained the KH2PO4/SiO2 composite deflagration suppressant by mixing and grinding the two raw materials and explored its suppression effect in a Hartmann tube.14 The results revealed that the flame propagation of Al powder deflagration could be effectively suppressed with 60% KH2PO4/SiO2 and completely suppressed with 90% KH2PO4/SiO2. Deng et al. measured the explosion degrees of Al/ABC, Al/MCA, and Al/ABC/MCA mixtures, respectively, using a vertical explosion tube device and analyzed the explosion suppression effects of different suppressant powders.15 The results showed that the suppression effect of ABC was the worst, followed by ABC/MCA, and that of MCA was the best.
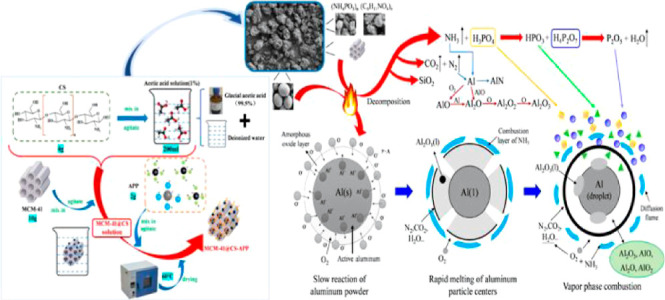
Composite powder explosion suppressants exhibit synergistic effects, leading to superior explosion suppression performance compared to that of single powder suppressants. At present, the study of the development of composite solid explosion suppressants and the study of their suppression performances are urgently needed. Due to its large specific surface area, good thermal stability, and high porosity,16,17 mesoporous SiO2 has been rapidly developed in the fields of catalysis, adsorption, and biomedicine.18−20 Ammonium polyphosphate (APP) and chitosan (CS) are good flame retardants,21−24 where APP serves as the acid and gas source of the flame retardant system, and CS serves as the carbon source. Herein, a new mesoporous composite explosion powder suppressant, MCM41@CS-APP, was prepared by functionally modifying the surface of mesoporous SiO2.25 Since CaCO3 has a good inhibitory effect on dust explosion,26−28 the article used MCM41@CS-APP and CaCO3 to carry out the explosion inhibition experiment of aluminum powder to obtain the effect of the two on the explosion pressure and combustion flame of aluminum powder. Based on the scanning electron microscopy (SEM), thermogravimetric analysis (TGA), and X-ray photoelectron spectroscopy (XPS) analyses of the explosion products, the inhibition performance and inhibition mechanism of MCM41@CS-APP on the explosion of aluminum powder were obtained, which not only improved the study on the Al dust explosion suppression by the composite solid explosion suppressant but also provided theoretical support for the development of effective prevention and control techniques and equipment for Al dust explosion accidents.
2. Experiments
2.1. Materials
2.1.1. Al Powder
The Al dust explosion accident occurred in Jiangsu Kunshan Zhongrong Metal Products Co., Ltd. in 2014 was taken as the research background. Based on the site conditions, spherical Al powders with particle sizes of 19 and 30 μm (Henan Yuanyang Powder Technology Co., Ltd.) were selected as the research subjects. The physical properties of the Al powders are shown in Table 1.
Table 1. Physical Properties of 19 μm/30 μm Al Powders.
| elemental
composition |
||||||
|---|---|---|---|---|---|---|
| appearance | Al (%) | Fe (%) | Cu (%) | Si (%) | H2O (%) | |
| 19 μm | silver gray, no aggregations | 99.78 | 0.1018 | 0.0012 | 0.0422 | 0.02 |
| 30 μm | silver gray, no aggregations | 99.64 | 0.1037 | 0.0019 | 0.0527 | 0.03 |
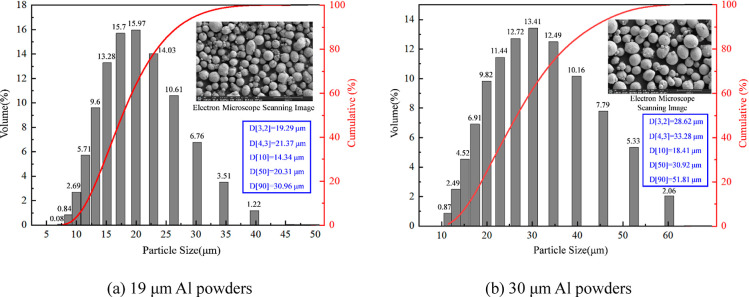
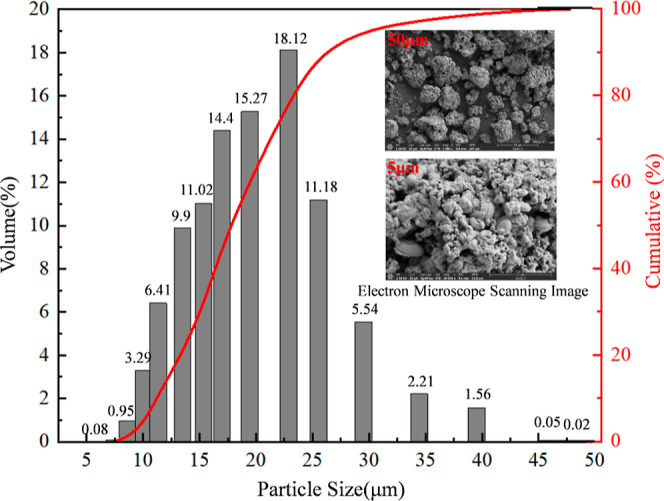
The particle size distributions of the 19 and 30 μm Al powders were measured with a laser diffractometer. As shown in Figure 1, the particle sizes of the 19 μm Al powder mainly fell in the range of 15.1–22.9 μm, and its Sauter mean diameter D(2,3) and De Brouckere mean diameter D(3,4) were measured to be 19.3 and 21.3 μm, respectively.
Figure 1.
Particle size distributions and SEM images of Al powders.
2.1.2. CaCO3
CaCO3 was purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). Its physical properties are listed in Table 2. Figure 2 shows the scanning electron microscopy image of CaCO3.
Table 2. Physical Properties of CaCO3.
| appearance | melting point | decomposition temperature | density | water solubility |
|---|---|---|---|---|
| white powder | 1339 °C | 825–896 °C | 2.93 g/cm3 (25 °C) | insoluble |
Figure 2.
SEM images of CaCO3.
2.1.3. MCM41@CS-APP
The MCM-41 molecular sieve was provided by Nankai University Catalyst Co., Ltd. (Nanjing, China). Its physical properties are listed in Table 3.
Table 3. Physical Properties of MCM-41.
| proportion of silicon | relative crystallinity | pore diameter | specific surface area |
|---|---|---|---|
| 100% | ≥95% | 3.8 nm | 1180 m2/g |
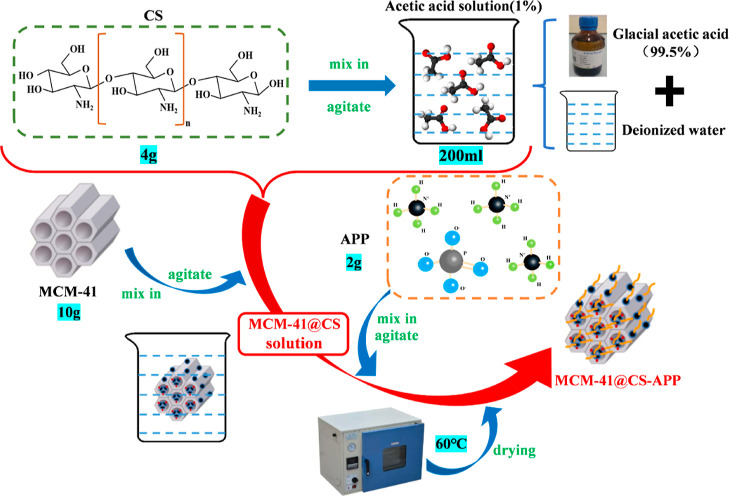
To synthesize MCM41@CS-APP, 4 g of CS was added into 200 mL of 1% acetic acid solution (2 mL of glacial acetic acid + 200 mL of deionized water) and stirred until completely dissolved. Ten g of MCM-41 molecular sieve was added into the CS solution, and the mixture was vigorously stirred for 1 h. The pH of the suspension was adjusted to 13 with 0.05 mol/L NaOH. Two g of APP powder was added into the suspension and stirred thoroughly. The suspension was centrifuged, and the residue was washed with deionized water until it became neutral and dried under vacuum at 60 °C to obtain MCM41@CS-APP.29
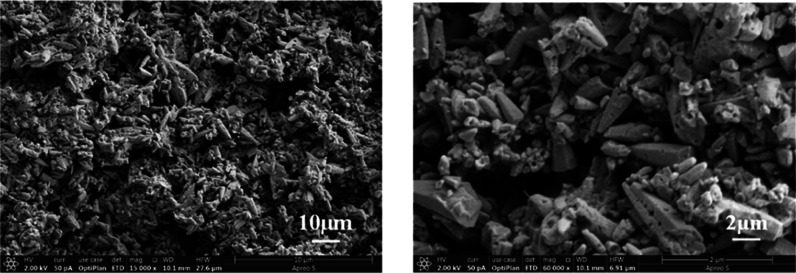
Figure 3 shows the schematic diagram for the synthesis of MCM41@CS-APP, and its microscopic morphology is presented in Figure 4.
Figure 3.
Schematic diagram of the synthesis of MCM41@CS-APP.
Figure 4.
Particle size distributions and SEM images of MCM41@CS-APP.
2.2. Experimental Equipment
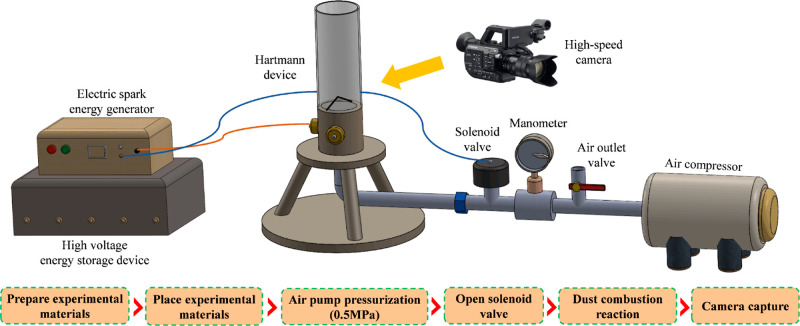
To explore the Al dust explosion suppression mechanism of the mesoporous composite explosion suppressant, the pressures and flame evolutions during the deflagration of Al powder and mixed dust were tested using a 20 L spherical explosion apparatus and a dust cloud energy measurement system as recommended by international standard ISO 6184-130 and the national standard GB/T 16425.31
2.2.1. 20 L Spherical Explosion Apparatus
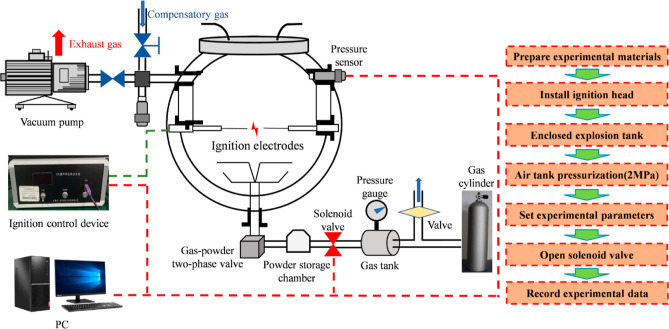
The 20 L spherical explosion apparatus used for the explosion experiments was mainly composed of an explosion chamber, a powder diffuser, a pneumatic control system, an ignition device, and a data acquisition and control system. Figure 5 shows a schematic diagram of the apparatus structure. The chemical igniters of zirconium, barium nitrate, and barium peroxide have a weight ratio of 4:3:3, and the energy release of 0.48 g chemical igniter corresponds to 2 kJ. After mixing the experimental dust evenly, it was spread evenly into the powder storage chamber. The explosion chamber was evacuated to 0.06 MPa, and the dust storage chamber was pressurized to 2 MPa. Once the operation parameters were set by the computer control software and gas distribution was calculated and set by the gas distribution system, the start valve was open. The explosion pressure evolutions were measured by a pressure sensor installed in the vessel wall and recorded by a data acquisition system for each run. These data yielded values of the maximum explosion pressure (Pmax) and maximum rate of pressure rise (dP/dtmax).
Figure 5.
Schematic diagram of 20 L spherical explosion apparatus.
2.2.2. Dust Cloud Energy Measurement System
The dust cloud energy measurement system was mainly composed of a Hartmann tube, an electric spark energy generator, an air compressor, and a solenoid valve. Figure 6 shows the schematic diagram of the system. The dust was placed under the Hartmann tube and adjusted to the position of the two electrodes to make them vertical and facing each other. The pressure was raised to 0.5 MPa with the air pump. The solenoid valve was turned on to blow the dust into the cylindrical glass tube. The spark energy generator was turned on to output an ignition energy of 20 J. The flame evolution of the dust cloud during the combustion process was recorded with a high-speed camera.
Figure 6.
Schematic diagram of the dust cloud energy measurement system.
3. Explosion Experiments of Al Powder and Mixed Dusts
3.1. Explosion Overpressure of Al Powder
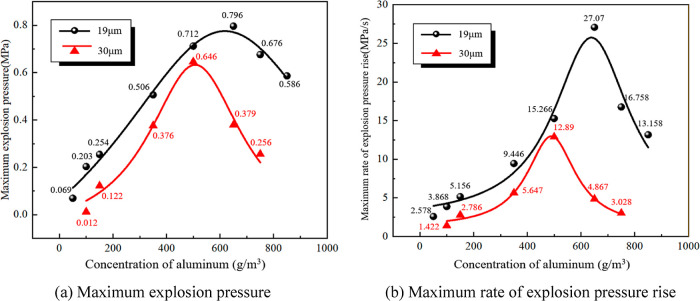
In order to determine the optimal explosion concentration of aluminum powder, explosion experiments were conducted on the 19 and 30 μm Al powders at concentrations in the ranges of 50–750 and 50–850 g/m3, respectively, and the aluminum powder at each concentration was subjected to three explosion tests. Their maximum explosion pressures (Pmax) and the maximum rates of explosion pressure rise (dP/dtmax) are shown in Figure 7.
Figure 7.
Maximum explosion pressures and maximum rates of explosion pressure rise of the two Al powders at different concentrations.
The Pmax of 19 and 30 μm Al powders both increase first and then decrease with the increase of concentration (Figure 7a). The highest Pmax = 0.796 MPa of 19 μm Al powder is obtained at a concentration of 650 g/m3, while the Pmax of 30 μm Al powder reaches the maximum of 0.646 MPa at a concentration of 500 g/m3. Their dP/dtmax values also show the same increasing and decreasing trends with the increase of concentration (Figure 7b). The highest dP/dtmax of the 19 μm Al powder is 27.07 MPa/s, and that of the 30 μm Al powder is 12.89 MPa/s. Smaller particle sizes are conducive to oxygen diffusion,32 and their larger surface areas provide larger contact areas with oxygen. Therefore, at the same concentration, the Al powder with the smaller particle sizes causes more violent explosions.33,34
Based on the analyses above and the capacity of the experimental equipment, the Al powder concentration was set to 500 g/m3 in the subsequent experiments.
3.2. Effects of the Explosion Suppressant on Explosion Overpressure
With the concentration of Al powder at 500 g/m3, the concentration of CaCO3 in the mixed dust was varied to 50, 100, 200, 300, 400, and 500 g/m3, and that of MCM41@CS-APP was varied to 50, 100, 200, 300, and 400 g/m3, respectively. The maximum explosion pressures generated by these mixed dusts are listed in Figure 8.
Figure 8.
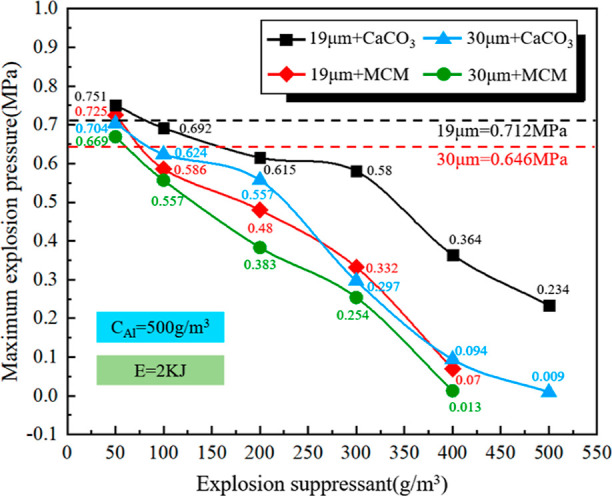
Maximum explosion pressures of Al/MCM41@CS-APP and Al/CaCO3 mixed dusts containing different concentrations of the suppressant.
As shown in Figure 8, at the fixed aluminum powder concentration, Pmax shows downward trends with the increases of the concentrations of MCM41@CS-APP and CaCO3. At the same suppressant concentration, the Pmax produced by the reaction of the Al powder (19 μm)/suppressant is higher than that of the Al powder (30 μm)/suppressant (except for at the suppressant concentration of 50 g/m3), suggesting that the explosive power of Al powder with a smaller particle size is greater. As 50 g/m3 of MCM41@CS-APP was added, the Pmax of the Al powders increased to 0.725 and 0.669 MPa, respectively, higher than those generated by the pure Al powders. The same phenomenon is observed as 50 g/m3 of CaCO3 is added. These observations indicate that low concentrations of the suppressant can promote the explosion of Al dust. Amyotte et al. analyzed this phenomenon and believed that the decomposition of the low concentration of the suppressant in the high-temperature environment could enhance the explosion.35,36 Both MCM41@CS-APP and CaCO3 significantly inhibited the explosion of aluminum powder when the concentration of the suppressant reached 400 g/m3. The Pmax of the mixed dust (Al = 19 μm) was 0.133 and 0.364 MPa, which decreased by 81.3% and 48.9%, respectively. The Pmax of the mixed dust (Al = 30 μm) was not significant.
In addition, the aluminum powder particle size is unchanged; adding the same concentration of detonation inhibitor, Al/MCM@CS-APP-produced Pmax is lower than that produced by Al/CaCO3, preliminary indication that the detonation inhibition effect of MCM41@CS-APP is significantly better than that of CaCO3.
4. Experimental Study on Flame Evolution during Deflagration
4.1. Flame Evolution during Al Powder Deflagration
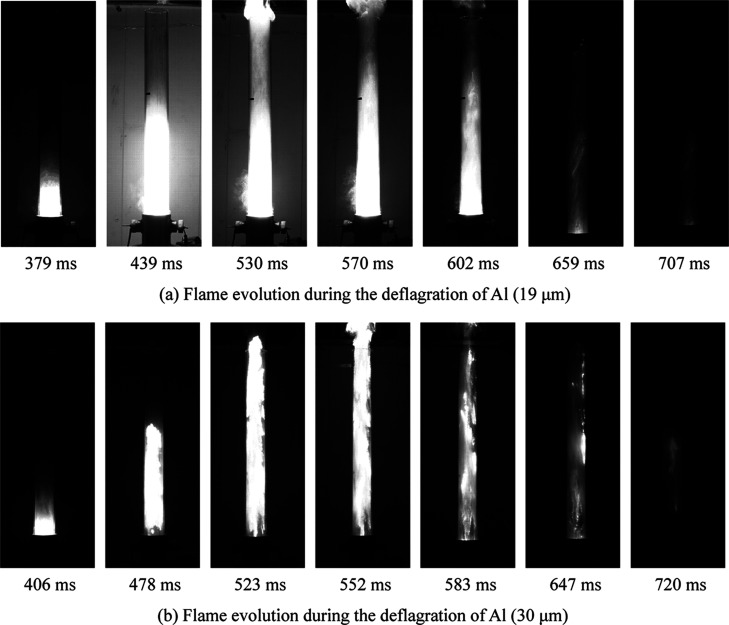
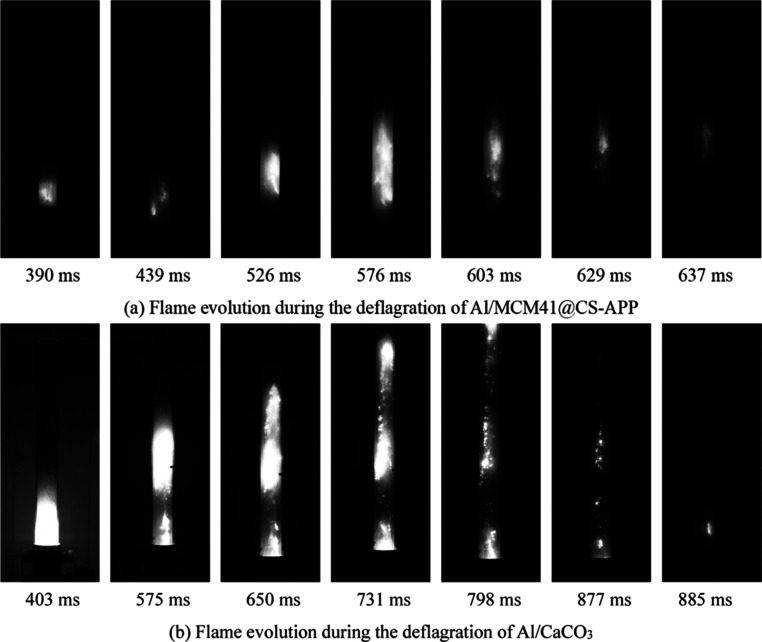
Figure 9 shows the flame evolution during the deflagration of 19 and 30 μm aluminum powders (500 g/m3) recorded by the dust cloud ignition energy measurement system.
Figure 9.
Flame evolution during the deflagration of Al powder.
As shown in Figure 9a, under a pressure of 0.5 MPa, the Al powder quickly fills into the Hartmann tube. At 379 ms, the Al dust is ignited, and then the flame spreads upward along the Hartmann tube. At 530 ms, the flame rushes out of the tube and develops most violently. During the period of 530–602 ms, the flame near the tube mouth gradually disappears because of the reaction of Al dust in the upper part of the tube ends. Meanwhile, the relative high concentration of Al powder in the middle and lower parts of the tube maintains the flame at the bottom, and the disappearance of the flame is relatively slow. At 707 ms, the deflagration of the Al powder is over, and the flame is no longer observed. The deflagration of the 19 μm Al powder lasts 328 ms. As observed from Figure 9a, 30 μm aluminum powder combustion lasted for 314 ms, and the initial combustion moment of 30 μm aluminum powder is 27 ms later, which indicates that the aluminum powder with a smaller particle size is easier to ignite.
4.2. Flame Evolution during Mixed Dust Deflagration
4.2.1. Flame Evolution during Mixed Dust Deflagration (Al = 19 μm)
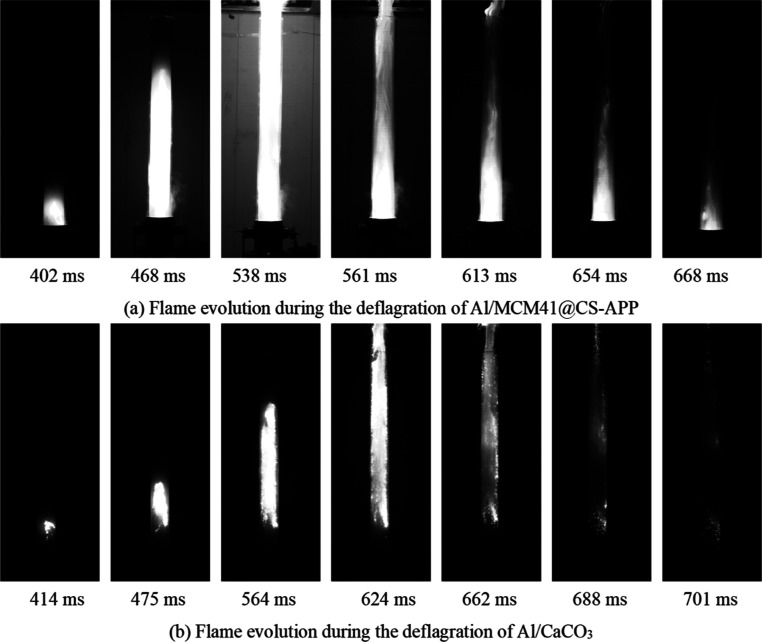
Based on the deflagration flame evolution of 19 μm Al powder, 200 and 400 g/m3 of MCM41@CS-APP and CaCO3 were, respectively, mixed with the Al powder. The deflagration flame evolution laws of the four mixed dusts are obtained, as shown in Figures 10 and 11.
Figure 10.
Flame evolutions during the deflagrations of Al/MCM41@CS-APP (200 g/m3) and Al/CaCO3 (200 g/m3).
Figure 11.
Flame evolutions during the deflagrations of Al/MCM41@CS-APP (400 g/m3) and Al/CaCO3 (400 g/m3).
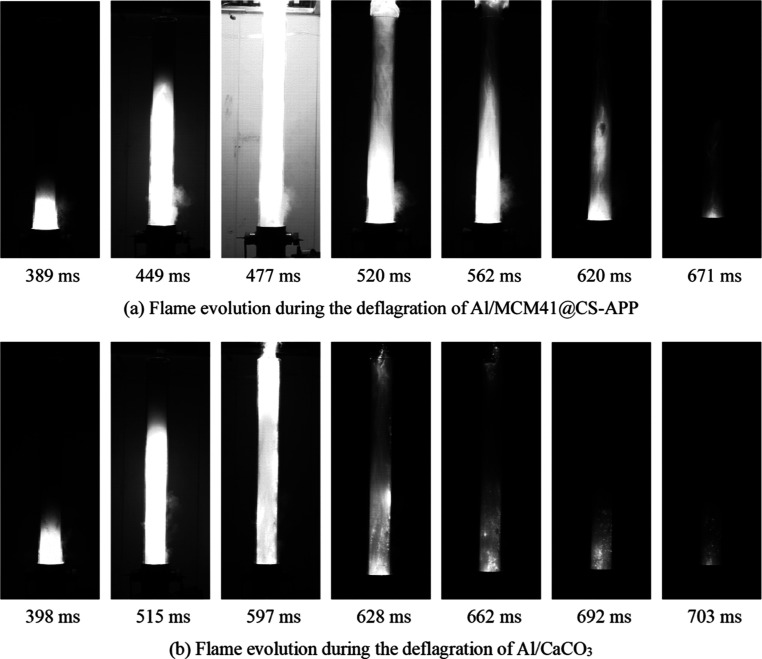
In Figure 10a, the fire of mixed dust Al/MCM41@CS-APP appeared at 389 ms, which was 10 ms later than the initial reaction time of Al/MCM41@CS-APP compared to Figure 9a; the reaction degree of Al/MCM41@CS-APP reached the maximum at 477 ms; and the combustion of the mixed dust was near the end at 671 ms. Its reaction process is 282 ms, which is 46 ms shorter than the reaction maintenance time of aluminum powder. As shown in Figure 10b, Al/CaCO3 starts to react at 398 ms, which is later than the initial reaction time of aluminum powder, and its reaction process duration is 305 ms, which is 23 ms shorter than the reaction maintenance time of aluminum powder.
In Figure 11, the degree of flame development of the two mixtures of dusts was significantly reduced after addition of 400 g/m3 of the deflagration suppressant to the aluminum powder. In Figure 11a, the initial reaction moment of Al/MCM41@CS-APP was unchanged, but the flame development to the optimum extent was delayed by 99 ms compared with that in Figure 10a, and the flame extinguished more quickly.
4.2.2. Flame Evolution during Mixed Dust Deflagration (Al = 30 μm)
Based on the deflagration flame evolution of 30 μm Al powder, 200 and 400 g/m3 of MCM41@CS-APP and CaCO3 were, respectively, mixed with the Al powder. The deflagration flame evolution laws of the four mixed dusts are obtained, as shown in Figures 12 and 13.
Figure 12.
Flame evolutions during the deflagrations of Al/MCM41@CS-APP (200 g/m3) and Al/CaCO3 (200 g/m3).
Figure 13.
Flame evolutions during the deflagrations of Al/MCM41@CS-APP (400 g/m3) and Al/CaCO3 (400 g/m3).
As shown in Figure 12a, the Al/MCM41@CS-APP reaction process is 266 ms, which is 48 ms shorter than the reaction sustaining time of aluminum powder (30 μm). As Figure 12b shows, the Al/CaCO3 reaction process is 287 ms, and the reaction sustaining time also appears to be scaled down. In Figure 13, the flame development of the mixed dust was substantially weakened by adding 400 g/m3 of the deflagration suppressant to the aluminum powder. As displayed in Figure 13a, Al/MCM41@CS-APP reacted at 429 ms, and its reaction process reached 113 ms. In Figure 13b, the degree of reaction of Al/CaCO3 was also significantly weakened, a stronger flame appeared in the 419–452 ms, and its initial reaction moment and the end of the reaction moment were advanced.
According to the above experiments, it is observed that:
-
(1)
The concentration of suppressor is constant, and the larger the particle size of the aluminum powder, the shorter the reaction time of the mixed dust, and the weaker the flame development.
-
(2)
Further verified that the inhibition of the burning of aluminum powder by MCM41@CS-APP is significantly better than that of CaCO3, which is reflected in three aspects: the flame produced by Al/MCM41@CS-APP in the optimum degree is obviously weaker than that by Al/CaCO3; adding the same concentration of MCM41@CS-APP and CaCO3, the reaction time of Al/MCM41@CS-APP is much smaller than that of Al/CaCO3, and the high-concentration MCM41@CS-APP shows significantly superior suppression effects in terms of flame brightness and flame height when compared to CaCO3 (see Figure 14).
Figure 14.
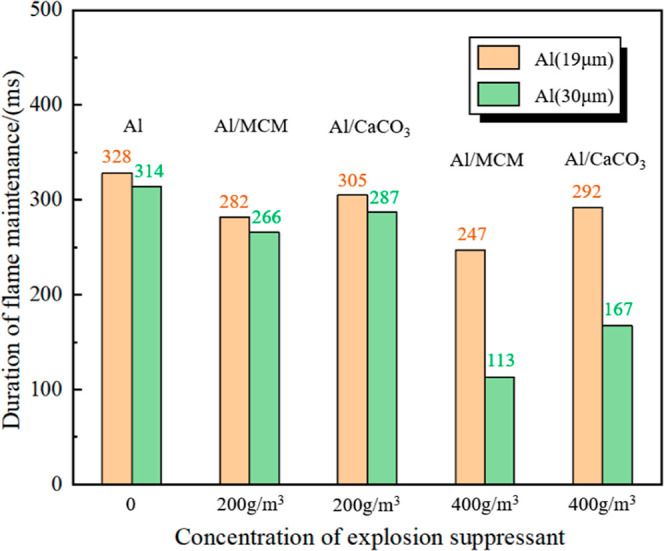
Durations of the deflagrations of different mixed dusts.
5. Explosion Residue Analysis
5.1. Physical Form Analysis
5.1.1. Microanalysis (SEM)
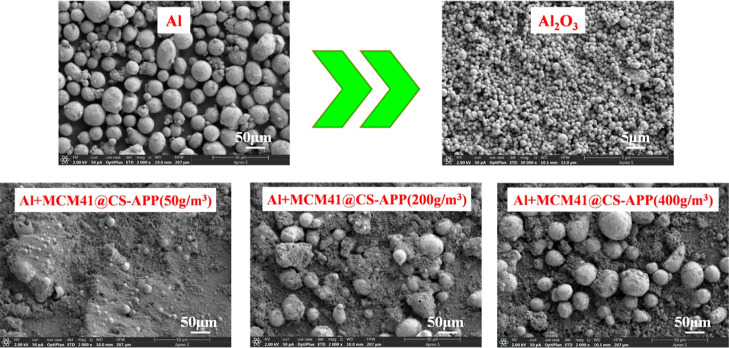
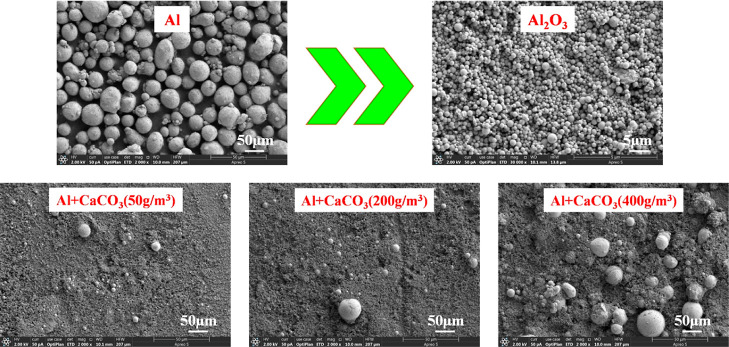
The explosion residues of 19 μm Al powder and Al powder/suppressant (50, 200, and 400 g/m3) mixed dusts were characterized by SEM for their morphologies. The results are shown in Figures 15 and 16.
Figure 15.
SEM images of the explosion residues of Al (19 μm) powder and Al/MCM41@CS-APP mixed dusts.
Figure 16.
SEM images of the explosion residues of Al (19 μm) powder and Al/CaCO3 mixed dusts.
As can be seen from Figure 15, the particle size of Al2O3 produced from the explosion of Al powder is significantly smaller than the particle size of the Al powder. After 50 g/m3 of MCM41@CS-APP was added to the 500 g/m3 Al powder, different sizes of Al2O3 particles were observed in the explosion residue. As the MCM41@CS-APP concentration increased to 200 and 400 g/m3, the number of Al particles drastically increased in the explosion residue, indicating that MCM41@CS-APP can greatly inhibit the explosion of Al powder at those doses.
Similarly, after 50 and 200 g/m3 CaCO3 are respectively added to the Al powder, the SEM images reveal more Al2O3 particles and fewer Al particles in the explosion residues (Figure 16). As the CaCO3 concentration increased to 400 g/m3, more Al particles were found in the explosion residue, and the explosion suppression effect of CaCO3 at this concentration is relatively obvious.
5.1.2. Macroanalysis
A uniform Al2O3 film is observed on the chamber wall after the Al powder explosion (Figure 17a). As 50 g/m3 of MCM41@CS-APP is added, a layer of off-white substance is found at the lower part of the chamber, and there are flocculent reactants on the ignition electrode (Figure 17b). The explosion residue of the mixed dust containing 200 g/m3 MCM41@CS-AP at the bottom of the chamber is dark gray (Figure 17c), indicating that a dense layer of product of MCM41@CS-AP itself is formed at high temperature as more MCM41@CS-APP is added.37 This dense layer prevents the reaction of a considerable amount of Al powder. At the MCM41@CS-APP concentration of 400 g/m3, a dense white substance is formed on the surface of the aluminum powder due to the reaction of MCM41@CS-APP, which prevents the reaction of aluminum powder.
Figure 17.
Images of the explosion residues of Al powder and Al/MCM41@CS-APP mixed dusts.
These SEM results and the explosion residues can be explained by the fact that the APP in MCM41@CS-APP decomposes in the high-temperature environment, which releases strong acids, such as polyphosphoric acid and pyrophosphoric acid.38−40 These strong acids tend to dehydrate the surface of organic substances such as CS in the suppressant to form a colloidal carbon layer. Meanwhile, the gases including water vapor and NH3 released from the thermal decomposition of APP and CS foam the carbon layer to form a porous colloidal carbon layer, which greatly hinders the direct contact between Al powder and oxygen, thereby suppressing the combustion and explosion of Al powder.
5.2. Thermogravimetric Analysis
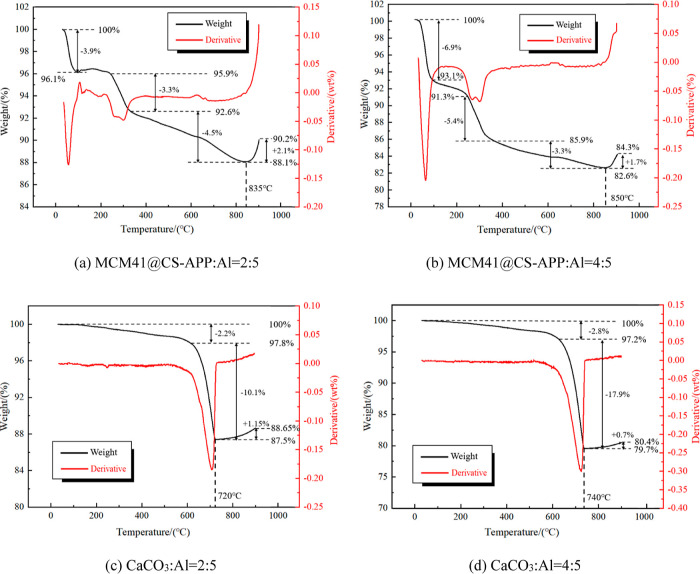
In the above section, the suppression effects of MCM41@CS-APP and CaCO3 were qualitatively analyzed by observing the morphologies of the mixed dust before and after explosion. In order to further understand their explosion suppression effects, TGA was conducted on Al powder (19 μm), MCM41@CS-APP, CaCO3, and mixed dusts with CaCO3/Al = 2:5, CaCO3/Al = 4:5, MCM41@CS-APP/Al = 2:5, and MCM41@CS-APP/Al = 4:5, respectively, in a nitrogen atmosphere. Figure 18 shows the TG curves of Al powder, MCM41@CS-APP, and CaCO3. The TG and derivative TG curves of the mixed dusts are shown in Figure 19.
Figure 18.
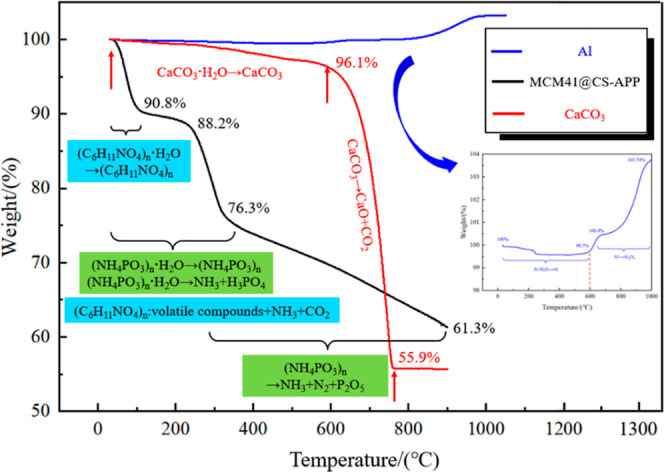
TG curves of Al powder (19 μm), MCM41@CS-APP, and CaCO3.
Figure 19.
TG and derivative TG curves of Al/CaCO3 and Al/MCM41@CS-APP with different ratios.
From room temperature to 600 °C, trace moisture in aluminum powder is evaporated (Figure 18). As the temperature increased to over 600 °C, the weight of Al powder slightly rose due to the oxidation with air that produced amorphous alumina. The weight rapidly increases and then becomes constant as the temperature increases to over 800 °C. At this stage, the Al powder vigorously reacts to form Al2O3, but due to the incomplete reaction, the TG curve rises slowly from room temperature to 600 °C, mainly due to the evaporation of adsorbed water.41 The TG curve declines dramatically as temperature increases to over 600 °C; CaCO3 is decomposed in the high-temperature environment to form CaO and a large amount of CO2.42,43 The TG curve of MCM41@CS-APP is relatively complex. The adsorbed water in MCM41@CS-APP is evaporated as heated from room temperature to 320 °C. With the further increase of temperature, the APP in the suppressant is decomposed to release NH3 and H3PO4. As the CS in the suppressant is heated, the dehydration of the sugar ring and the degradation of the polymer unit generate a certain amount of NH3. From 320 to 900 °C, the decomposition of APP continues to produce NH3, as well as N2, CO2, and P2O5.44,45
As can be seen from Figure 19, the larger the proportion of the suppressant, the smaller the final mass percentage of mixed dust and the higher the initial oxidation temperature of Al powder. As shown in Figure 19a,b, when MCM41@CS-APP/Al = 2:5/4:5, the aluminum powder in the mixed dust started to oxidize at 835 and 850 °C, and the mass of Al/MCM41@CS-APP decreased by 9.8% and 15.7% at 900 °C, respectively. As Figure 19c shows, when CaCO3/Al = 2:5, at 620–720 °C, the thermal decomposition of CaCO3 made the mass of dust decrease by 10.1%; when the temperature reached 720 °C, the oxidation reaction of aluminum made the mass of dust begin to rise; and at 900 °C, the mass of Al/CaCO3 decreased by 11.35%. As depicted in Figure 19d, when CaCO3/Al = 4:5, the aluminum powder in Al/CaCO3 began to oxidize and react at 740 °C, and the mass of Al/CaCO3 decreased by 19.6% at 900 °C.
5.3. X-ray Photoelectron Spectroscopy
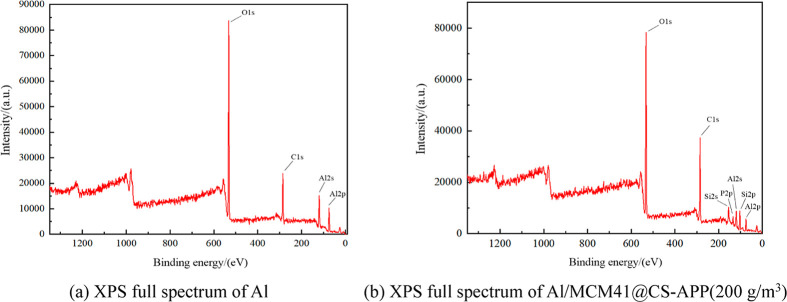
In order to analyze the substances that may be present in the explosion products of the mixed dust, 200 g/m3 of MCM41@CS-APP was added to the aluminum powder, and the full XPS spectra of Al and Al (500 g/m3)/MCM41@CS-APP (200 g/m3) were obtained after the explosion.
As shown in Figure 20a, after XPS analysis of the alumina produced by the explosion of aluminum powder, only three elements, namely, O, C, and Al, appeared in the full spectrum. In Figure 20b, after adding 200 g/m3 of MCM41@CS-APP to the aluminum powder, three elements, P, Si, and N, appeared newly.
Figure 20.
XPS full spectrum.
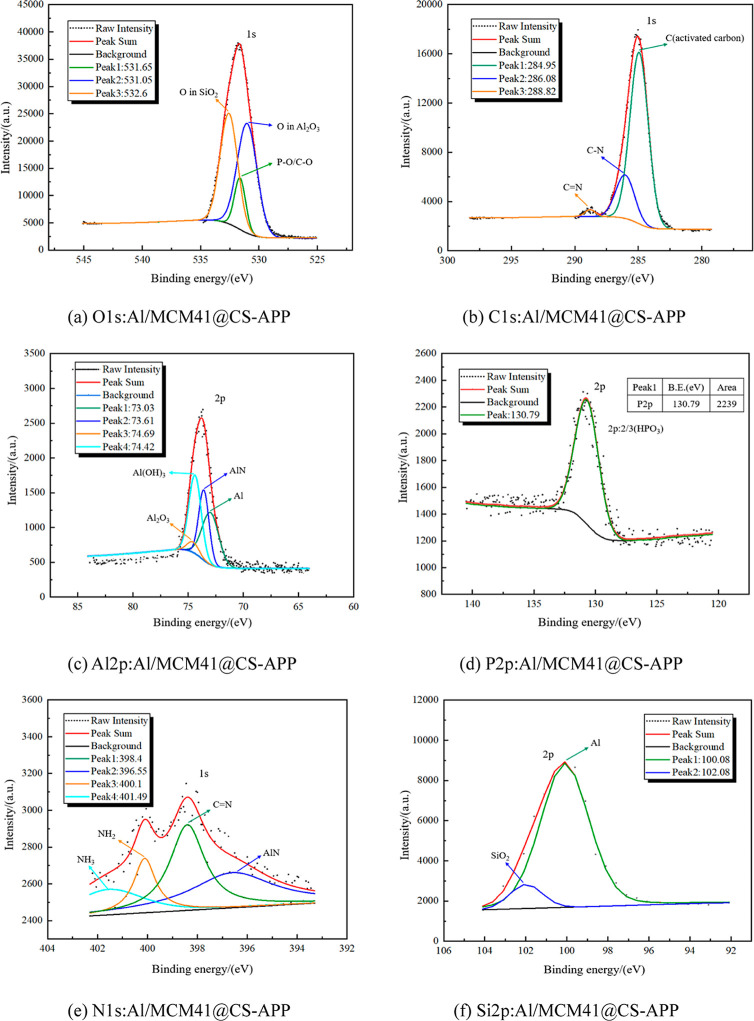
The XPS full spectrum of O 1s, C 1s, Al 2p, Si 2p, P 2p, and N 1s was obtained after peak splitting of the different elements in the product, as shown in Figure 21.
Figure 21.
XPS full spectrum of O 1s, C 1s, Al 2p, P 2p, N 1s, and Si 2p in Al/MCM41@CS-APP explosion products.
After the addition of MCM41@CS-APP, three peaks appeared in the O 1s spectra of the explosion products after the peak splitting process, corresponding to Al2O3, P–O bond, C–O bond, and O element in SiO2 (Figure 21a). As shown in Figure 21b, three peaks appear in the C 1s spectra with C elements originating from activated carbon, the C–N bond, and the C=N bond.46 As illustrated in Figure 21c, four peaks in the Al 2p spectra of the explosion products were obtained after the peak splitting process, and after analysis, it was concluded that the four peaks corresponded to Al, AlN, Al(OH)3, and Al2O3, respectively. Under the inhibition effect of MCM41@CS-APP, most of the explosion products of Al/MCM41@CS-APP consisted of alumina particles and some unreacted aluminum particles. Al(OH)3 may also be present. After the addition of MCM41@CS-APP at a concentration of 200 g/m3, the P element was considered to belong to phosphoric acid and other compounds such as P2O5, and the N element was derived from AlN, NH2, NH3, and C=N bonding.47 In addition, the selected molecular sieve (MCM-41) specification is all-silicon, which combines Si with O to form SiO2 in high-temperature environments.48
6. Results and Discussion
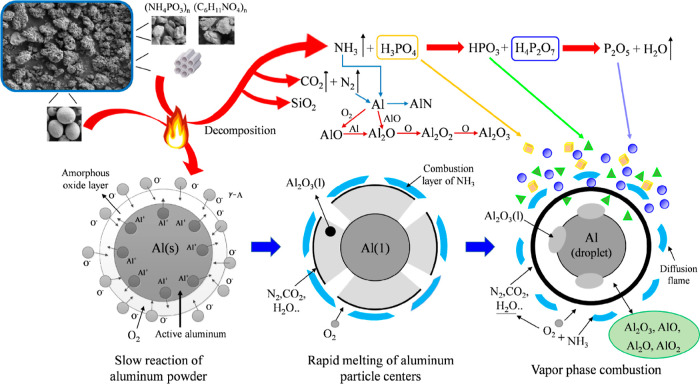
Through the XPS analysis of Al/MCM41@CS-APP explosion products, combined with SEM analysis and TGA, it was obtained that the inhibitory effect of MCM41@CS-APP on the explosion of aluminum powder was reflected in the condensation phase and gas phase. From the condensed-phase aspect, the APP loaded by MCM-41 is thermally decomposed to phosphoric acid and phosphates with CS as the carbon source, expanding and carbonizing to form a dense carbon layer under the action of acid and gas sources.49−52 The charcoal layer can effectively prevent the penetration of oxygen and the diffusion of the combustible gas, which inhibits the oxidation and combustion of Al powder to great extents. From the gas-phase aspect, the amino group in CS is converted into NH3 at the high temperatures, and the reaction of APP produces large amounts of inert gases, such as NH3, CO2, and N2. These gas products dilute O2 and take the heat produced from the combustion away. The free radicals, PO• and HPO•, generated from APP at the high temperatures can capture the active free radicals, such as H•, HO•, O•, and so on, as shown below, which makes it very difficult to continue the combustion chain reaction.53−55
According to the above analysis, the suppression mechanism of aluminum powder explosion by MCM41@CS-APP is obtained, as shown in Figure 22.
Figure 22.
Schematic diagram of the mechanism of inhibition of aluminum particle explosion by MCM41@CS-APP.
7. Conclusions
In this paper, the inhibition effect of MCM41@CS-APP and CaCO3 on the explosion flame and explosion pressure of aluminum powder (500 g/m3) was investigated; the inhibition performance of MCM41@CS-APP and CaCO3 on the explosion of aluminum powder was compared in various aspects such as flame development, pressure changes, and initial material analysis; and the inhibition mechanisms of MCM41@CS-APP on the explosion of aluminum powder were obtained through SEM, TG, and XPS analyses, and the following conclusions were obtained:
-
(1)
The Pmax of the mixed dust decreased continuously with the increase of the deflagration suppressant concentration, and the decreasing trend of Al/MCM41@CS-APP was more significant. When the concentrations of MCM41@CS-APP and CaCO3 reached 400 g/m3, the Pmax of mixed dust (Al = 19 μm) was 0.133 and 0.364 MPa, which decreased by 81.3% and 48.9%, respectively; the Pmax of mixed dust (Al = 30 μm) was not significant.
-
(2)
The particle size of aluminum powder has an impact on the explosion effect. Aluminum powder with a smaller particle size burns more strongly and has a longer combustion process. Adding the same concentration of the deflagration suppressant, Al/MCM41@CS-APP produced a significantly weaker flame optimum than that of Al/CaCO3, and the burning time of Al/MCM41@CS-APP was shorter.
-
(3)
The larger the proportion of the suppressant, the smaller the final mass percentage of mixed dust and the higher the initial oxidation temperature of Al powder; when CaCO3/Al = 2:5, MCM41@CS-APP/Al = 2:5, CaCO3/Al = 4:5, and MCM41@CS-APP/Al = 4:5, the initial oxidation reaction temperatures of aluminum are 720, 835, 740, and 850 °C, respectively.
-
(4)
Under the high-temperature environment, APP and CS in MCM41@CS-APP react synergistically to generate a gelatinous carbon layer; APP and CS molecules also release a large number of inert gases; and the free radicals generated by APP, such as PO• and HPO•, are able to capture reactive free radicals. The inhibition of the explosion of aluminum powders by MCM41@CS-APP has been described in the condensation phase and the gas phase.
As a self-developed solid powder suppressant, the preliminary results show that it has a good suppressant effect (compared with the conventional solid suppressant calcium carbonate). In order to explore whether MCM41@CS-APP has application value, it will be considered in the future to compare its explosion suppression performance with NH4H2PO4 or MCA materials under different initial conditions.
Acknowledgments
This work was supported by the Key Project of the Natural Science Foundation of Shandong Province (project no. ZR2022ME183) and the National Natural Science Foundation of China (project no. 52374215).
The authors declare no competing financial interest.
References
- Dufaud O.; Traoré M.; Perrin L.; Chazelet S.; Thomas D. Experimental investigation and modelling of aluminum dusts explosions in the 20 L sphere. J. Loss Prev. Process. Ind. 2010, 23 (2), 226–236. 10.1016/j.jlp.2009.07.019. [DOI] [Google Scholar]
- Beckstead M. W.; Liang Y.; Pudduppakkam K. V. Numerical Simulation of Single Aluminum Particle Combustion (Review). Combust. Explos. Shock Waves 2005, 41 (6), 622–638. 10.1007/s10573-005-0077-0. [DOI] [Google Scholar]
- Vignes A.; Krietsch A.; Dufaud O.; Santandréa A.; Perrin L.; Bouillard J. Course of explosion behaviour of metallic powders-From micron to nanosize. J. Hazard. Mater. 2019, 379, 120767. 10.1016/j.jhazmat.2019.120767. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Fang X.; Shu C.; Wang Q.; Sheng Y.; Jiang J.; Sun Y.; Sheng Z. Minimum ignition temperatures and explosion characteristics of micron-sized aluminium powder. J. Loss Prev. Process. Ind. 2020, 64, 104076. 10.1016/j.jlp.2020.104076. [DOI] [Google Scholar]
- Dastidar A.; Amyotte P. Determination of Minimum Inerting Concentrations for Combustible Dusts in a Laboratory-Scale Chamber. Process Saf. Environ. Prot. 2002, 80 (6), 287–297. 10.1205/095758202321154916. [DOI] [Google Scholar]
- Jérôme T.; Vingerhoets J.; et al. Suppression of metal dust deflagrations. J. Loss Prev. Process. Ind. 2015, 36, 244–251. 10.1016/j.jlp.2015.02.011. [DOI] [Google Scholar]
- Mintz K. J.; Bray M. J.; Zuliani D. J.; Amyotte P.; Pegg M. Inerting of fine metallic powders. J. Loss Prev. Process. Ind. 1996, 9 (1), 77–80. 10.1016/0950-4230(95)00043-7. [DOI] [Google Scholar]
- Amrogowicz J.; Kordylewski W. Effectiveness of dust explosion suppression by carbonates and phosphates. Combust. Flame 1991, 85 (3–4), 520–522. 10.1016/0010-2180(91)90155-5. [DOI] [Google Scholar]
- Jiang H.; Bi M.; Gao W. Flame inhibition of aluminum dust explosion by NaHCO3 and NH4H2PO4. Combust. Flame 2019, 200, 97–114. 10.1016/j.combustflame.2018.11.016. [DOI] [Google Scholar]
- Zhang S.; Bi M.; Jiang H.; Gao W. Suppression effect of inert gases on aluminum dust explosion. Powder Technol. 2021, 388, 90–99. 10.1016/j.powtec.2021.04.073. [DOI] [Google Scholar]
- Zhang T.; Jiang H.; Shang S.; Zhang K.; Gao W. Synthesis of aluminum hydroxide/Zinc borate composite inhibitor and its inhibition effect on aluminum dust explosion. Chem. Eng. Sci. 2022, 248 (Part B), 117204. 10.1016/j.ces.2021.117204. [DOI] [Google Scholar]
- Zhang T.; Jiang H.; Zhang K.; Zhu C.; Xue C.; Zhang Z.; Xu J.; Gao W. Inhibition effect of aluminum dust explosions by one novel zinc borate. Chem. Eng. Sci. 2022, 255, 117682. 10.1016/j.ces.2022.117682. [DOI] [Google Scholar]
- Dai L. L.; Hao L.; Kang W.; Xu W.; Shi N.; Wei H. Inhibition of different types of inert dust on aluminum powder explosion. Chin. J. Chem. Eng. 2020, 28 (7), 1941–1949. 10.1016/j.cjche.2020.02.021. [DOI] [Google Scholar]
- Ke Y.; Meng X. B.; Pan Z. C.; Wang Z.; Zhang Y. S. Effect and mechanism of KH2PO4/SiO2 composite powder in inhibiting aluminum dust deflagration. Explos. Shock Waves 2021, 42 (6), 062101-1. [Google Scholar]
- Deng J.; Jiao Q. U.; Qiu-hong W.; Yu-feng Y. Inhibition of ABC/MCA powder on explosion characteristics of aluminum metal powder. J. Xi’an Univ. Eng. Sci. Technol. 2020, 40 (1), 18–23. [Google Scholar]
- Jiang S. D.; Bai Z. M.; Tang G.; Song L.; Stec A. A.; Hull T. R.; Hu Y.; Hu W. Z. Synthesis of mesoporous silica@Co-Al layered double hydroxide spheres: layer-by-layer method and their effects on the flame retardancy of epoxy resins. ACS Appl. Mater. Interfaces 2014, 6 (16), 14076–14086. 10.1021/am503412y. [DOI] [PubMed] [Google Scholar]
- Qian Y.; Wei P.; Jiang P.; Li Z.; Yan Y.; Ji K. Aluminated mesoporous silica as novel high-effective flame retardant in polylactide. Compos. Sci. Technol. 2013, 82, 1–7. 10.1016/j.compscitech.2013.03.019. [DOI] [Google Scholar]
- Zhu H.; Liang C.; Yan W.; Overbury S. H.; Dai S. Preparation of highly active silica-supported Au catalysts for CO oxidation by a solution-based technique. J. Phys. Chem. B 2006, 110 (22), 10842–10848. 10.1021/jp060637q. [DOI] [PubMed] [Google Scholar]
- Rafia R.; Gul A. R.; Lee I. G.; Baek S. H.; Kailasa S. K.; Iqbal N.; Cho E. J.; Lee M.; Park T. J. Photo-induced reactions for disassembling of coloaded photosensitizer and drug molecules from upconversion-mesoporous silica nanoparticles: An effective synergistic cancer therapy. Mater. Sci. Eng., C 2020, 110, 110545. 10.1016/j.msec.2019.110545. [DOI] [PubMed] [Google Scholar]
- Li C.; Zhang G.; Yuan B. Exceptional performance of flame-retardant polyurethane foam: the suppression effect on explosion pressure and flame propagation of methane-air premixed gas. J. Mater. 2023, 16 (24), 7602. 10.3390/ma16247602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar M. N.; Raghavendra G. M.; Vijaykumar B. V. D.; Patil K.; Seo J.; Jung-il S. Synthesis of a novel compound based on chitosan and ammonium polyphosphate for flame retardancy applications. Cellulose 2019, 26, 8801–8812. 10.1007/s10570-019-02671-y. [DOI] [Google Scholar]
- Malucelli G. Flame-Retardant Systems Based on Chitosan and Its Derivatives: State of the Art and Perspectives. Molecules 2020, 25 (18), 4046. 10.3390/molecules25184046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.; Gu X.; Jin X.; Sun J.; Zhang S. The effect of chitosan on the flammability and thermal stability of polylactic acid/ammonium polyphosphate biocomposites. Carbohydr. Polym. 2017, 157, 1586–1593. 10.1016/j.carbpol.2016.11.035. [DOI] [PubMed] [Google Scholar]
- Hu S.; Song L.; Pan H.; Hu Y. Thermal properties and combustion behaviors of chitosan based flame retardant combining phosphorus and nickel. Ind. Eng. Chem. Res. 2012, 51 (9), 3663–3669. 10.1021/ie2022527. [DOI] [Google Scholar]
- Khan D.; Shaily Synthesis and catalytic applications of organo-functionalized MCM-41 catalyst: A review. Appl. Organomet. Chem. 2023, 37 (3), e7007 10.1002/aoc.7007. [DOI] [Google Scholar]
- Lv P.; Liu Z.; Zhao J.; Pang L. Inerting effect of CaCO3 powder on flame spread of wood dust layer. J. Loss Prev. Process. Ind. 2023, 82, 105000. 10.1016/j.jlp.2023.105000. [DOI] [Google Scholar]
- Yuan G.; Kuai N.; Liu L.; Huang W. Experiment investigation on the inhibition effectiveness of CaCO3 on dust explosions. Fire Sci. Technol. 2014, 33 (2), 128–130. [Google Scholar]
- Wu Y.; Yuan J.; Kuai N.; Huang W. Experimental study on the effect of carbonate on dust explosion pressure in confined space. China Saf. Sci. J. 2010, 20, 92–96. [Google Scholar]
- Chen Z.; Jiang J.; Yu Y.; Zhang Q.; Chen T.; Ni L. Layer-by-layer assembled diatomite based on chitosan and ammonium polyphosphate to increase the fire safety of unsaturated polyester resins. Powder Technol. 2020, 364, 36–48. 10.1016/j.powtec.2020.01.037. [DOI] [Google Scholar]
- Li G.; Yang H.; Yuan C.; Eckhoff R. K. A catastrophic aluminium-alloy dust explosion in China. J. Loss Prev. Process. Ind. 2016, 39, 121–130. 10.1016/j.jlp.2015.11.013. [DOI] [Google Scholar]
- International Organization for Standardization . Explosion Protection Systems Part 1: Determination of Explosion Indices of Combustible Dusts in Air, 1985. ISO 6184-1.
- Standardization Administration of the People’s Republic of China . Determination for Minimum Explosive Concentration of Dust Clouds, 2018. GB/T 16425-2018.
- Zhang J.; Ren X.; Jia H.; Cui Z.; Xiong N.; Xiao B.; Han X. Effect of particle size distribution on explosion intensity of aluminum powder. Process Saf. Prog. 2023, 42 (1), 186–193. 10.1002/prs.12422. [DOI] [Google Scholar]
- Kim W.; Saeki R.; Ueno Y.; Johzaki T.; Endo T.; Choi K. Effect of particle size on the minimum ignition energy of aluminum powders. Powder Technol. 2023, 415, 118190. 10.1016/j.powtec.2022.118190. [DOI] [Google Scholar]
- Jiang P. Z.; Fan Tang G.; Wang Y. C.; Han Y. Explosive property and combustion kinetics of grain dust with different particle sizes. Heliyon 2020, 6, e03457 10.1016/j.heliyon.2020.e03457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastidar A. G.; Amyotte P. R.; Going J.; Chatrathi K. Flammability limits of dusts: minimum inerting concentrations. Process Saf. Prog. 1999, 18 (1), 56–63. 10.1002/prs.680180111. [DOI] [Google Scholar]
- Yang D.; Zhou M.; Ye D.; et al. A Flexible and Breathable Ammonium Polyphosphate/Chitosan/Mwcnt Doped Pedot: Pss Coating Decorated Carbon Cloth for High-Efficiency Flame Retardancy and Electromagnetic Interference Shielding Applications. Surface. Interfac. 2024, 51, 104786. 10.1016/j.surfin.2024.104786. [DOI] [Google Scholar]
- Gu J.; Zhang G.; Dong S.; Zhang Q. y.; Kong J. Study on preparation and fire-retardant mechanism analysis of intumescent flame-retardant coatings. Surf. Coat. Technol. 2007, 201 (18), 7835–7841. 10.1016/j.surfcoat.2007.03.020. [DOI] [Google Scholar]
- Zhang T.; Zhang Z.; Zhu C.; Gao W. Inhibition effects of aluminum dust explosions by various kinds of ammonium polyphosphate. J. Loss Prev. Process. Ind. 2023, 83, 105083. 10.1016/j.jlp.2023.105083. [DOI] [Google Scholar]
- Alongi J.; Carosio F.; Malucelli G. Layer by layer complex architectures based on ammonium polyphosphate, chitosan and silica on polyester-cotton blends: flammability and combustion behaviour. Cellulose 2012, 19, 1041–1050. 10.1007/s10570-012-9682-8. [DOI] [Google Scholar]
- Johnson C. E.; Fallis S.; Chafin A. P.; Groshens T. J.; Higa K. T.; Ismail I. M. K.; Hawkins T. W. Characterization of Nanometer- to Micron-Sized Aluminum Powders: Size Distribution from Thermogravimetric Analysis. J. Propul. Power 2007, 23 (4), 669–682. 10.2514/1.25517. [DOI] [Google Scholar]
- Galan I.; Glasser F. P.; Andrade C. Calcium carbonate decomposition. J. Therm. Anal. Calorim. 2013, 111, 1197–1202. 10.1007/s10973-012-2290-x. [DOI] [Google Scholar]
- Li Y.-N.; Jiao F.; Wen L. Research on the Effects of CaCO3 on Minimum Ignition Energy of Metal Dust. J. North Univ. China 2014, 35 (05), 594–598. 10.3969/j.issn.1673-3193.2014.05.019. [DOI] [Google Scholar]
- Amyotte P. R. Solid inertants and their use in dust explosion prevention and mitigation. J. Loss Prev. Process. Ind. 2006, 19, 161–173. 10.1016/j.jlp.2005.05.008. [DOI] [Google Scholar]
- Huang C.; Yuan B.; Zhang H.; Zhao Q.; Li P.; Chen X.; Yun Y.; Chen G.; Feng M.; Li Y. Investigation on thermokinetic suppression of ammonium polyphosphate on sucrose dust deflagration: Based on flame propagation, thermal decomposition and residue analysis. J. Hazard. Mater. 2021, 403, 123653. 10.1016/j.jhazmat.2020.123653. [DOI] [PubMed] [Google Scholar]
- Castrovinci A.; Camino G.; Drevelle C.; Duquesne S.; Magniez C.; Vouters M. Ammonium polyphosphate–aluminum trihydroxide antagonism in fire retarded butadiene–styrene block copolymer. Eur. Polym. J. 2005, 41 (9), 2023–2033. 10.1016/j.eurpolymj.2005.03.010. [DOI] [Google Scholar]
- Dante R. C.; Sanchez-Arevalo F. M.; Chamorro-Posada P.; Vazquez-Cabo J.; Huerta L.; Lartundo-Rojas L.; Santoyo-Salazar J.; Solorza-Feria O. Supramolecular intermediates in the synthesis of polymeric carbon nitride from melamine cyanurate. J. Solid State Chem. 2015, 226, 170–178. 10.1016/j.jssc.2015.02.018. [DOI] [Google Scholar]
- Candela-Noguera V.; Alfonso M.; Amorós P.; et al. In-depth study of factors affecting the formation of MCM-41-type mesoporous silica nanoparticles[J]. Microporous Mesoporous Mater. 2024, 363, 112840. 10.1016/j.micromeso.2023.112840. [DOI] [Google Scholar]
- Chen X. X.; Fang F.; Du T.; et al. Preparation and properties of chitosan-potassium alginate flame retardant coating via layer-by-layer self-assembly technology. Polym. Mater. Sci. Eng. 2016, 32 (7), 121–124. [Google Scholar]
- Xiao Y.; Zheng Y.; Wang X.; Chen Z.; Xu Z. Preparation of a chitosan-based flame-retardant synergist and its application in flame-retardant polypropylene. J. Appl. Polym. Sci. 2014, 131 (19), 40845. 10.1002/app.40845. [DOI] [Google Scholar]
- Jie B.; Baoxia X.; Yang Y.; et al. Flame Retardancy and Smoke Suppression of Intumescent Flame Retardant PP With Chitosan/Ammonium Phosphate. Eng. Plast. Appl. 2017, 45 (7), 119–123. [Google Scholar]
- Fang F.; Zhang X.; Meng Y.; Gu Z.; Bao C.; Ding X.; Li S.; Chen X.; Tian X. Intumescent flame retardant coatings on cotton fabric of chitosan and ammonium polyphosphate via layer-by-layer assembly. Surf. Coat. Technol. 2015, 262, 9–14. 10.1016/j.surfcoat.2014.11.011. [DOI] [Google Scholar]
- Wang G.; Yang J. Thermal degradation study of fire resistive coating containing melamine polyphosphate and dipentaerythritol. Prog. Org. Coat. 2011, 72 (4), 605–611. 10.1016/j.porgcoat.2011.07.001. [DOI] [Google Scholar]
- Zhang L. L.; Liu A. H. Synergistic Effect and Application of Phosphorus/Silicon Flame Retardant. China Plast. Ind. 2008, 29, 203–204. [Google Scholar]
- Lu H.; Ma X.; Hongxia Y. Recent progress in phosphorus flame retardants. N. Chem. Mater. 2001, 29 (12), 7–10. [Google Scholar]