This case-case-time-control study evaluates the association between gabapentinoids and the risk of hip fractures among older adults in Australia.
Key Points
Question
Is the use of gabapentinoids associated with risk of hip fracture, and does the risk differ across age, frailty, and kidney function?
Findings
In this case-case-time-control study including 28 293 patients hospitalized for hip fractures in Australia, gabapentinoid use was associated with an increased risk of hip fracture, especially in patients who were frail and had chronic kidney disease.
Meaning
These findings suggest that in addition to the known risk associated with kidney impairment, gabapentinoids should be used with caution among patients at risk of hip fractures, especially those who are frail.
Abstract
Importance
The increased use of gabapentinoids has been most pronounced in older people who are also susceptible to hip fractures.
Objective
To investigate the overall association between gabapentinoids and the risk of hip fractures and the stratified association across age groups, frailty status, and history of chronic kidney disease.
Design, Setting, and Participants
This was a case-case-time-control study in patients hospitalized for hip fracture in Victoria, Australia, between March 1, 2013, and June 30, 2018, with at least 1 prescription for a gabapentinoid before fracture. Conditional logistic regression was used to estimate the odds ratio (OR) and 95% CI for gabapentinoid dispensing in the index (1-60 days prefracture) compared with the reference (121-180 days prefracture) period. To adjust for the underlying time trend in gabapentinoid use, each index case was matched with up to 5 controls, selected from future cases of the same age and sex. Subgroup analyses were conducted in subgroups with or without chronic kidney disease (CKD), frailty scores less than 5, and frailty scores 5 and above. Frailty was computed using the Hospital Frailty Risk Score (HFRS). Data were analyzed from November 2023 to April 2024.
Exposure
Gabapentinoids (pregabalin or gabapentin).
Main Outcome and Measure
Hip fracture.
Results
Of 28 293 patients hospitalized for hip fractures, 2946 (1752 [59.5%] aged ≥80 years; 2099 [71.2%] female) were dispensed a gabapentinoid before hip fracture. Gabapentinoid dispensing was associated with increased odds of hip fractures (OR, 1.96; 95% CI, 1.66-2.32). After adjusting for the exposure-time trend and concomitant use of other central nervous system medications, the odds of hip fractures remained elevated (OR, 1.30; 95% CI, 1.07-1.57). The association between gabapentinoid dispensing and hip fracture was higher in patients with HFRS 5 and above (OR, 1.75; 95% CI, 1.31-2.33) and CKD (OR, 2.41; 95% CI, 1.65-3.52).
Conclusions and relevance
In this case-case-time-control study of Australian residents hospitalized for hip fracture, gabapentinoid use was associated with an increased risk of hip fractures, especially in patients who were frail or had chronic kidney disease. In addition to the known risk associated with kidney impairment, frailty status may be an important risk factor when considering use of gabapentinoids.
Introduction
The exponential increase in gabapentinoid use has been particularly pronounced among older adults, especially after the expansion of the indication to include various painful syndromes (eg, neuropathic pain and fibromyalgia).1,2,3,4 By 2018, gabapentin was the sixth most dispensed medication by volume in the US,5 with utilization continuing to increase until 2021.2 A corresponding 8-fold increase in prescriptions for gabapentinoids in Australia was observed from 2012 to 2018, with 1 in 7 Australians aged 80 and older prescribed a gabapentinoid across this period.4 Pregabalin remains within the 10 most subsidized medications by volume in Australia in 2023.6
Gabapentinoids have been marketed as a safer alternative to opioids for the treatment of neuropathic pain.7,8 This may be partly responsible for decreasing opioid and increasing gabapentin use in US veterans between 2015 and 2019.9 However, more evidence is needed regarding gabapentinoids’ adverse drug event (ADE) profile in older people. Gabapentinoids are actively transported across the blood-brain barrier and inhibit neurotransmitter release via multiple pathways.7,10 This explains why gabapentinoids have efficacy for various central nervous system (CNS) disorders, including seizures and neuropathic pain.7,10 It is also why gabapentinoids have CNS ADEs including somnolence, dizziness, gait disturbance, and balance disorder.11,12 These adverse events may increase the risk of falls and fractures in older people. Hip fractures are associated with the highest costs to individuals and health systems among all fragility fractures.13,14 One in 25 people aged 80 years or older experience a hip fracture each year,15 with 1 in 4 dying within 12 months.14 However, there is limited evidence regarding the possible association between gabapentinoids and hip fractures.
Selection of an appropriate medication can be informed by knowledge about patient factors that are associated with an increased risk of ADEs.16 Frailty and kidney impairment are 2 important considerations when prescribing for older people and may occur together or separately.17,18,19 Frailty is increasingly recognized as a useful clinical parameter for risk stratification.20 Frailty has also been associated with an increased risk of hip fractures.21,22,23,24 However, no studies have investigated whether frailer patients dispensed gabapentinoids are at greater risk of hip fractures than less frail patients. Similarly, gabapentinoids are predominately excreted through the kidneys and have a prolonged elimination half-life in patients with kidney impairment. No studies have investigated whether patients with chronic kidney disease (CKD) are at increased risk of hip fracture associated with gabapentinoids. The objective of our study was to investigate the overall association between gabapentinoids and the risk of hip fractures and the stratified association across age groups, frailty status, and history of CKD.
Methods
The study was approved by Australian Institute of Health and Welfare (AIHW) ethics committee and Monash University human research ethics committee. Informed consent was waived by all data custodians as data were deidentified. The study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.25
Data Sources
We extracted data from 4 linked administrative datasets: the Victorian Admitted Episodes Dataset (VAED), the Victorian Emergency Minimum Dataset (VEMD), Pharmaceutical Benefits Scheme (PBS), and the National Death Index (NDI). The VAED contains information on all public and private hospitalizations in Victoria, the second most populous state in Australia. Information available includes patient demographic and diagnostic data. The VEMD contains information on all emergency visits to the hospitals. The PBS dataset contains information on all medications subsidized by the Australian government and dispensed at community pharmacies, outpatient clinics or at hospital discharge for Australian residents. The NDI dataset contains all deaths registered across Australia. Data were available from July 1, 2006, to June 30, 2018, for all datasets. Details on the datasets have been published previously.14,26,27
Study Population
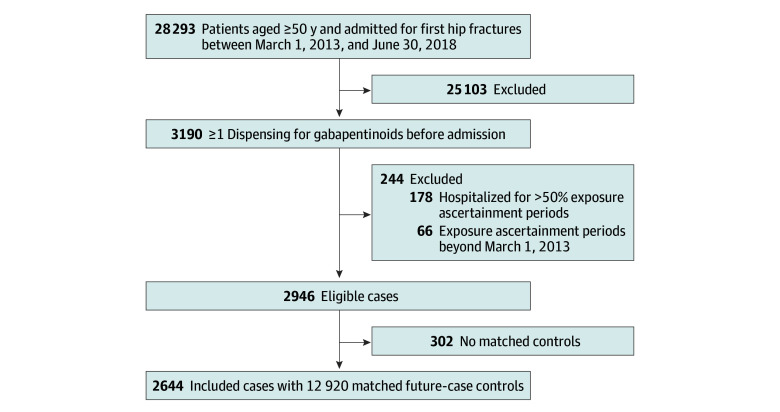
Our study included all patients aged 50 years or older admitted for first hip fracture (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Australian Modification [ICD-10-AM] codes S72.0-S72.2) between March 1, 2013, and June 30, 2018, with at least 1 dispensing of gabapentinoids (World Health Organization Anatomical Therapeutic Chemical Classification, Australian Modification [ATC] codes N02BG and N03AX) ever recorded before the fracture. First hip fracture was defined as an absence of hip fracture in the 5 years before their admission and the date of admission was defined as the index date. The cohort of patients with first hip fracture has been described previously.14 We also excluded anyone whose exposure assessment period started before the pregabalin was first PBS-subsidized on March 1, 2013.4 Furthermore, we excluded patients hospitalized for more than 50% of their exposure assessment period because PBS data do not capture inpatient dispensings.
Study Design
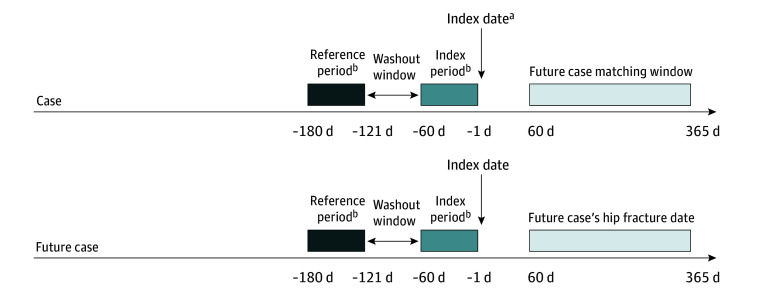
The case-case-time-control analysis consisted of 2 case-crossover analyses, (1) the case case-crossover analysis and (2) the future-case-control case-crossover analysis (Figure 1).28,29,30 Case-crossover is a self-controlled design that compares exposure to the drug of interest within the same patient across different time periods (index period immediately before event vs a reference period before the index period), thereby controlling for confounders that are stable across the time periods by design.30 Since only patients who have exposure to the drug of interest (gabapentinoids) contribute to case-crossover analyses, we restricted the study population to cases (patients with hip fracture) who had prior exposure to gabapentinoids. Self-controlled study designs are advantageous in the presence of unmeasured patient characteristics (eg, bone health, muscle strength, nutritional status, and mobility status) that would confound conventional study designs, such as cohort and case-control.31,32
Figure 1. Schematic Representation of Case-Case-Time-Control Study Design.
aThe exclusion window was 5 years to 1 day before the index date. The comorbidities assessment window was 5 years before the index date to the baseline assessment window (day 0). The frailty assessment window was 2 years to 1 day before the index date.
bIndex and reference periods were the exposure assessment periods for gabapentinoids and time-varying covariates (ie antidepressants, antipsychotics, benzodiazepines, and opioids).
In the case case-crossover analysis, the odds of dispensing gabapentinoids during the index period (1 day to 60 days preceding the index date) were compared with the odds of dispensing in the reference period (121 to 180 days preceding the index date). Medication exposure was ascertained from dispensings recorded in the PBS dataset. A 60-day period duration was chosen as each dispensing on the PBS is typically for a 1-month supply. This was in accordance with a previous study on utilization of gabapentinoids in Australia.33 A 60-day gap (days 61 to 120 before the index date) was applied between index period and reference period as a washout period to minimize any carryover effect from leftover medications in the reference period. Dispensing was assessed only during the index and reference, but not the washout period. The role of the washout period was to space apart the index and reference periods to avoid contamination from any leftover medications carrying over from reference period to index period.
Case-crossover analysis may produce biased results in the presence of increasing time trends in population drug use or when the drug of interest is used chronically.30,34 Since previous research suggests increasing use of gabapentinoids during our study period and around one-third of Australian patients are persistent gabapentinoid users,33 we implemented a case-case-time-control study design that uses future cases as controls.29 This design has been shown to control for time trends in exposure and persistent user bias in case-crossover analyses.35
The future-case-control case-crossover analysis considers all cases before their event (future cases) to be part of the at-risk population eligible as controls, approximating case-only incidence density sampling.36 The use of future cases as present controls also minimizes bias from different baseline risks across cases and controls, as the controls were sampled from soon-to-be cases with more comparable risks than external controls.28,29,30 Risk-set sampling was used to match up to 5 future cases for each case as controls, based on age (±5 years for ages <85 or ≥85) and sex. To ensure comparable risks, controls were further restricted to patients with hip fractures occurring 60 to 365 days after the matched case index dates. Cases with index dates after May 1, 2018, were precluded from our matched analysis due to unavailability of future cases for matching.
Subgroup Analyses
Patient frailty status was assessed using the Hospital Frailty Risk Score (HFRS). The HFRS is a validated deficit accumulation frailty score computed as a weighted sum of 109 diagnoses recorded within 2 years before the index date.37 Variation in susceptibility to gabapentinoids by frailty status was assessed by conducting subgroup analyses in those with low frailty risk (HFRS<5) and high frailty risk (HFRS≥5).26,37 To assess the modifying effects of age and CKD, analyses were conducted separately in subgroups of patients aged younger than 80 years or 80 years or older, as well as patients with or without CKD. CKD was defined as a diagnosis for CKD or kidney failure (according to AIHW) within 5 years before the index date (eTable 1 in Supplement 1).38
Time-Varying Covariates
The use of other CNS-active fall-risk-increasing medications could confound the association between gabapentinoids and hip fracture. Therefore, we adjusted for concomitant dispensing of antidepressants (ATC N06A), antipsychotics (ATC N05A), benzodiazepines (ATC N03AE, N05BA, and N05CD) and opioids (ATC N02A).39,40,41
Comorbidities Ascertainment
Comorbidities were ascertained from the diagnoses recorded in the VAED and VEMD dataset. Comorbidities were defined as any diagnosis recorded in the 2 datasets within 5 years before the index date (eTable 1 in Supplement 1).
Statistical Analysis
Baseline characteristics of cases and future-case-controls were characterized to assess the comparability of cases and controls. The comparability of baseline characteristics was assessed using standardized mean difference (SMD). Characteristics with SMDs less than 0.1 were considered similar. The odds ratio (OR) and 95% CI between gabapentinoid dispensing and hip fractures were estimated using a logistic regression conditioned on an individual. The time-varying covariates were added to the model as covariates. The case-case-time-control OR was estimated using the interaction term between exposure status and an indicator for cases vs controls. To assess the robustness of the results, additional sensitivity analyses were conducted by varying the lengths of time periods. The lengths of exposure assessment periods (index and reference periods) were altered from 60 days to 30 and 90 days, while the length of washout window was altered from 60 days to 30, 90, 120, 150, and 180 days. A statistically significant difference between subgroups was defined as nonoverlapping 95% CIs. All analyses were performed using SAS version 9.4 (SAS Institute) and R version 4.0.0 (R Project for Statistical Computing). Data were analyzed from November 2023 to April 2024.
Results
Characteristics of Study Population
Overall, 28 293 patients (18 759 [66%] aged ≥80 years; 19 357 [69%] female) were hospitalized for first hip fractures in Victoria, Australia, from March 1, 2013, to June 30, 2018. Of these, 3190 patients were dispensed gabapentinoids before being admitted. Most patients used pregabalin (2995 patients [93.9%]). After excluding patients with incomplete data or who were hospitalized for more than 50% of the exposure ascertainment period, 2946 patients (1752 [59.5%] aged ≥80 years; 2099 [71.2%] female) were eligible for inclusion in the main analysis. In the analysis, 2644 patients were matched with at least 1 future case as a control (Figure 2). Most of the cases were female (1887 patients [71.4%]). More than half were 80 years or older (1579 patients [59.7%]). The cases and future cases were comparable in terms of age, sex, and comorbidities (Table 1).
Figure 2. Study Flow Diagram.
Table 1. Characteristics of All Eligible Patients, Patients Included, and Matched Future-Case-Controls.
| Characteristic | Patients, No. (%) | Standardized mean difference | ||
|---|---|---|---|---|
| All eligible cases (N = 2946) | Cases included (n = 2644) | Matched controls (n = 12 920) | ||
| Age, y | 0.039 | |||
| 50-59 | 128 (4.3) | 109 (4.1) | 441 (3.4) | |
| 60-69 | 318 (10.8) | 281 (10.6) | 1356 (10.5) | |
| 70-79 | 748 (25.4) | 675 (25.5) | 3290 (25.5) | |
| 80-84 | 605 (20.5) | 536 (20.3) | 2667 (20.6) | |
| ≥85 | 1147 (38.9) | 1043 (39.4) | 5166 (40.0) | |
| Sex | 0.020 | |||
| Male | 847 (28.8) | 757 (28.6) | 3583 (27.7) | |
| Female | 2099 (71.2) | 1887 (71.4) | 9337 (72.3) | |
| HFRS | 0.002 | |||
| <5 | 1634 (55.5) | 1456 (55.1) | 7128 (55.2) | |
| ≥5 | 1312 (44.5) | 1188 (44.9) | 5792 (44.8) | |
| Comorbidities | ||||
| Anxiety | 234 (7.9) | 210 (7.9) | 1029 (8.0) | 0.001 |
| Arthritis | 609 (20.7) | 546 (20.7) | 2755 (21.3) | 0.017 |
| Alcohol use disorder | 107 (3.6) | 99 (3.7) | 462 (3.6) | 0.009 |
| Cancer | 578 (19.6) | 514 (19.4) | 2616 (20.2) | 0.020 |
| Cardiovascular disease | 1887 (64.1) | 1687 (63.8) | 8464 (65.5) | 0.036 |
| Cerebrovascular disease | 299 (10.1) | 273 (10.3) | 1366 (10.6) | 0.008 |
| Chronic kidney disease | 915 (31.1) | 811 (30.7) | 4301 (33.3) | 0.056 |
| COPD/asthma | 416 (14.1) | 376 (14.2) | 1826 (14.1) | 0.003 |
| Delirium | 471 (16.0) | 424 (16.0) | 2074 (16.1) | <0.001 |
| Dementia | 129 (4.4) | 116 (4.4) | 630 (4.9) | 0.023 |
| Depression or other affective disorders | 192 (6.5) | 175 (6.6) | 830 (6.4) | 0.008 |
| Diabetes | 773 (26.2) | 704 (26.6) | 3484 (27.0) | 0.008 |
| Epilepsy/seizures | 88 (3.0) | 77 (2.9) | 351 (2.7) | 0.012 |
| Fall | 988 (33.5) | 881 (33.3) | 4455 (34.5) | 0.025 |
| Gastroesophageal reflux disease | 292 (9.9) | 262 (9.9) | 1235 (9.6) | 0.012 |
| Osteoporosis | 865 (29.4) | 776 (29.3) | 3820 (29.6) | 0.005 |
| Peptic ulcer disease | 106 (3.6) | 100 (3.8) | 468 (3.6) | 0.008 |
| Previous vertebral fracture | 178 (6.0) | 155 (5.9) | 954 (7.4) | 0.061 |
| Visual disturbances and blindness | 100 (3.4) | 89 (3.4) | 413 (3.2) | 0.010 |
| Time-varying exposure | ||||
| Gabapentinoids | ||||
| Index period | 1313 (44.6) | 1208 (45.7) | 5927 (45.9) | 0.004 |
| Index period only | 425 (14.4) | 402 (15.2) | 1343 (10.4) | 0.144 |
| Reference period | 1107 (37.6) | 1011 (38.2) | 5499 (42.6) | 0.088 |
| Reference period only | 219 (7.4) | 205 (7.8) | 915 (7.1) | 0.026 |
| Antidepressants | ||||
| Index period | 1287 (43.7) | 1163 (44.0) | 4811 (37.2) | 0.138 |
| Reference period | 1191 (40.4) | 1077 (40.7) | 4672 (36.2) | 0.094 |
| Antipsychotics | ||||
| Index period | 180 (6.1) | 164 (6.2) | 582 (4.5) | 0.075 |
| Reference period | 147 (5.0) | 135 (5.1) | 519 (4.0) | 0.052 |
| Benzodiazepines | ||||
| Index period | 667 (22.6) | 609 (23.0) | 2647 (20.5) | 0.062 |
| Reference period | 648 (22.0) | 584 (22.1) | 2667 (20.6) | 0.035 |
| Opioids | ||||
| Index period | 1491 (50.6) | 1352 (51.1) | 5374 (41.6) | 0.192 |
| Reference period | 1329 (45.1) | 1208 (45.7) | 5195 (40.2) | 0.111 |
Abbreviation: COPD, chronic obstructive pulmonary disease; HFRS, Hospital Frailty Risk Score.
Odds of Hip Fractures
Among the 2644 matched cases, 607 had crossover in exposure across the index and reference periods (ie, discordant exposure pattern), with 402 dispensed gabapentinoids in the index period only (60 days to 1 day before index admission) and 205 dispensed in the reference period only (180 to 121 days before index admission). Gabapentinoid dispensing was associated with increased odds of hip fracture (OR, 1.96; 95% CI, 1.66-2.32). For the controls, 1343 were dispensed gabapentinoids in the index period only and 915 in the reference period only, estimating an underlying time-trend of more gabapentinoid dispensing during the index period (OR, 1.47; 95% CI, 1.35-1.60). The time-trend adjusted case-case-time-control OR was 1.34 (95% CI, 1.11-1.61), and further adjustment for time-varying dispensing of other CNS-active medications resulted in an OR of 1.30 (95% CI, 1.07-1.57) (Table 2).
Table 2. Results of Case-Case-Time-Control Analyses for Main and Subgroup Analyses.
| Characteristic | Patients, No. | Odds ratio (95% CI) | |
|---|---|---|---|
| Exposed during index period only | Exposed during reference period only | ||
| Overall | |||
| Case crossover | 402 | 205 | 1.96 (1.66-2.32) |
| Control crossover | 1343 | 915 | 1.47 (1.35-1.60) |
| Case-case-time-control | NA | NA | 1.34 (1.11-1.61) |
| Adjusted case-case-time-controla | NA | NA | 1.30 (1.07-1.57) |
| Age | |||
| <80 y | |||
| Case crossover | 167 | 84 | 1.99 (1.53-2.58) |
| Control crossover | 542 | 368 | 1.47 (1.29-1.68) |
| Case-case-time-control | NA | NA | 1.35 (1.00-1.81) |
| Adjusted case-case-time-controla | NA | NA | 1.34 (1.00-1.80) |
| ≥80 y | |||
| Case crossover | 235 | 121 | 1.94 (1.56-2.42) |
| Control crossover | 801 | 547 | 1.46 (1.31-1.63) |
| Case-case-time-control | NA | NA | 1.33 (1.04-1.69) |
| Adjusted case-case-time-controla | NA | NA | 1.25 (0.98-1.61) |
| Frailty | |||
| HFRS: <5 | |||
| Case crossover | 206 | 115 | 1.79 (1.43-2.25) |
| Control crossover | 641 | 464 | 1.38 (1.23-1.56) |
| Case-case-time-control | NA | NA | 1.29 (1.00-1.68) |
| Adjusted case-case-time-controla | NA | NA | 1.26 (0.98-1.64) |
| HFRS: ≥5 | |||
| Case crossover | 192 | 89 | 2.16 (1.68-2.77) |
| Control crossover | 492 | 415 | 1.19 (1.04-1.35) |
| Case-case-time-control | NA | NA | 1.82 (1.37-2.42) |
| Adjusted case-case-time-controla | NA | NA | 1.75 (1.31-2.33) |
| Without CKD | |||
| Case crossover | 283 | 157 | 1.80 (1.48-2.19) |
| Control crossover | 932 | 591 | 1.58 (1.42-1.75) |
| Case-case-time-control | NA | NA | 1.14 (0.92-1.43) |
| Adjusted case-case-time-controla | NA | NA | 1.13 (0.90-1.41) |
| With CKD | |||
| Case crossover | 116 | 47 | 2.47 (1.76-3.46) |
| Control crossover | 283 | 294 | 0.96 (0.82-1.13) |
| Case-case-time-control | NA | NA | 2.56 (1.76-3.73) |
| Adjusted case-case-time-controla | NA | NA | 2.41 (1.65-3.52) |
Abbreviations: CKD, chronic kidney disease; HFRS, Hospital Frailty Risk Score; NA, not applicable.
Adjusted for time-varying exposure to antidepressants, antipsychotics, benzodiazepines, and opioids.
Subgroup Analyses
Among those with CKD (1036 patients) or high frailty risk (1519 patients), 780 had both CKD and high frailty risk. Higher odds of hip fracture were found among patients with high frailty risk (OR, 1.75; 95% CI, 1.31-2.33) than among patients with low frailty risk (OR, 1.26; 95% CI, 0.98-1.64). Subgroup analysis for patients with and without chronic kidney diseases demonstrated that the risk of hip fractures associated with gabapentinoid dispensing was significantly higher among patients with CKD (OR, 2.41; 95% CI, 1.65-3.52) than those without CKD (OR, 1.13; 95% CI, 0.90-1.41). However, when stratified by age group, the higher risk of hip fractures remained similar across different age groups without reaching statistical significance due to smaller sample sizes (50-79 years old: OR, 1.34; 95% CI, 1.00-1.80; ≥80 years old: OR, 1.25; 95% CI, 0.98-1.61) (Table 2; eTable 2 in Supplement 1).
Sensitivity Analyses
The characteristics of cases and controls for each sensitivity analysis were similarly comparable (eTables 3-6 in Supplement 1). Results remained similar across sensitivity analyses, from the most restrictive analysis that used the shortest exposure assessment periods and washout period of 30 days (adjusted case-case-time-control OR, 1.40; 95% CI, 1.15-1.71) to the sensitivity analysis of the longest exposure assessment periods of 90 days and washout period of 180 days (adjusted case-case-time-control OR, 1.45; 95% CI, 1.23-1.70) (eTable 7 in Supplement 1).
Discussion
In this population-based self-controlled case-case-time-control study, we found gabapentinoid dispensing was associated with higher odds of hip fracture. The association was similar across age groups but higher in those who were frailer or had CKD. Our study suggests that frailty and CKD may be important factors for clinicians to consider when prescribing gabapentinoids.
Our results highlight that patients had 30% increased odds of hip fractures within 60 days of gabapentinoid dispensing. In comparison, previous case-crossover studies on hip fractures demonstrated 124% increased odds with antidepressant use,42 47% increased odds with antipsychotic use,43 55% to 75% increased odds with benzodiazepine use,44 and 62% increased odds with opioid use.43 However, these case-crossover studies did not include any control case-crossover analyses to account for possible bias arising from temporal trends in medication dispensing.42,43,44 A systematic review in 202045 reported association with hip fractures for psychotropic medications ranging from a pooled OR of 1.33 in sedatives to 2.36 in antiparkinsonian drugs. Recent systematic reviews reported similar increased odds of hip fractures in benzodiazepine users with a pooled OR of 1.32 across case-control studies,46 and in tramadol users with a pooled hazard ratio of 1.32 across cohort studies.47 Our results suggest that gabapentinoids may be associated with a similar risk of hip fracture as other fall-risk-increasing medications.48 In addition to previous studies on antiseizure medications and fragility fractures,49,50,51,52 our study quantifies the specific risk associated with gabapentinoids. Such specificity is useful as fragility fractures vary widely in terms of treatment and prognosis, wherein hip fractures entail the largest morbidity and mortality burden.13,53 In light of increasing gabapentinoid prescribing, our results have important implications for clinicians and policy makers.
To our knowledge, this is the first study to highlight that gabapentinoids are associated with higher odds of hip fracture in patients who are frail. This substantiates existing evidence that frail people are more susceptible to adverse drug events.17,54 Previous studies have shown that frailty score calculators could be successfully integrated into electronic health records to aid clinicians in decision-making at point-of-care.55,56,57 Our findings suggest a potential utility of frailty risk scores as a risk stratification tool and the importance of assessing risks of patients individually before prescribing gabapentinoids.
Our study also reports higher odds of hip fractures in patients with CKD who were dispensed gabapentinoids. In contrast, clinical trials on gabapentinoids have reported no increased risk of hip fractures.11,12,58 A recent systematic review suggested higher risks of adverse drug events (eg, lethargy, drowsiness, dizziness, fall, and fractures) among patients with CKD.59 However, specific studies on the association between hip fractures and gabapentinoids in CKD are scarce, with mainly 1 large observational study finding an increased risk of hip fracture in patients using high-dose gabapentinoids while receiving hemodialysis.59,60,61 American Geriatrics Society Beers Criteria recommend dosage adjustment in patients with moderate and/or severe kidney impairment who are prescribed gabapentinoids. This is because gabapentinoids could accumulate in patients with kidney impairment and cause CNS ADEs.39 The higher risk we observed may be due to insufficient dosage adjustment or residual risk remaining after dose adjustment of gabapentinoids for patients with CKD; however, we were unable to investigate the modifying effect of doses in our study.62 Further studies examining the risk of hip fractures associated with different dosages of gabapentinoids among patients with different degrees of kidney impairment or dialysis status are needed.
Strengths and Limitations
This study has several strengths. Our study used a self-controlled study design that inherently eliminates confounding from variations across individuals (eg, baseline frailty and kidney function). The unidirectional sampling of the reference period for exposure also avoided bias arising from deprescribing of gabapentinoids after hip fractures. Additionally, our case-case-time-control design used future cases to account for temporal trend and persistent user bias, 2 common sources of bias in case-crossover analyses.29,34 This was a population-based study using administrative databases, thus minimizing the risk of selection bias. The similar results from our various sensitivity analyses also assures the robustness of our results.
Our study also has several limitations. We analyzed medication dispensing and could not ascertain whether dispensed medications were taken by the patients, nor the dosages taken. Case-crossover is better suited to assess acute drug effects than long-term or cumulative effects. While most adverse drug effects of gabapentinoids related to falls (eg, dizziness and somnolence) were reported within 1 to 2 weeks of gabapentinoid initiation,11 gabapentinoids may also have long-term adverse effects on bone health resulting from interference with calcium homeostasis.63 Our results may, therefore, underestimate the overall effect of gabapentinoids on hip fractures. We could not conduct subgroup analyses for individual gabapentinoids due to the small number of patients dispensed gabapentin. However, a previous head-to-head study suggested gabapentin and pregabalin were associated with a similar risk of fragility fractures.52 While self-controlled design accounts for time-invariant confounders across individuals, the design cannot account for time-varying confounders within individuals (eg, acute pain episodes), so residual confounding is still possible. However, the only reimbursed indication for pregabalin (used by 95% of patients in this study) in Australia is neuropathic pain. It is unlikely that patients experienced substantial progression in overall severity of neuropathic pain across our prespecified assessment periods.4,64 While HFRS has been validated for use with administrative data, it is based on ICD-10-AM diagnostic codes assigned during hospital episodes; therefore, it may not capture all dimensions of frailty. We also did not have laboratory results, thus we relied on diagnoses recorded in hospitalizations to define CKD. Finally, our study population included residents of 1 state in Australia. While we do not expect that gabapentinoid effects will differ across different countries and populations, caution is needed before transporting our results to other settings, especially those with other indications for gabapentinoids. Future studies in other populations are needed to confirm our findings.
Conclusions
In this population-based case-case-time-control study of Australian adults hospitalized for first hip fracture, the use of gabapentinoids was associated with a higher risk of hip fractures, especially in patients who were frailer or with chronic kidney disease. In addition to the known risk associated with kidney impairment, frailty status may be an important risk factor when considering use of gabapentinoids.
eTable 1. Diagnostic codes for comorbidities
eTable 2. Characteristics of patients included and matched future-case controls for subgroup analyses for patients with low frailty risk (Hospital Frailty Risk Score [HFRS]<5), high frailty risk (HFRS≥5), without chronic kidney disease (CKD) and with CKD
eTable 3. Characteristics of patients included and matched future-case controls for sensitivity analyses with exposure assessment period (index and reference periods) of 60 days and washout periods of 90, 120, 150, and 180 days
eTable 4. Characteristics of patients included and matched future-case controls for sensitivity analyses with exposure assessment period (index and reference periods) of 30 days and washout periods of 30, 60, and 90 days
eTable 5. Characteristics of patients included and matched future-case controls for sensitivity analyses with exposure assessment period (index and reference periods) of 30 days and washout periods of 120, 150, and 180 days
eTable 6. Characteristics of patients included and matched future-case controls for sensitivity analyses with exposure assessment period (index and reference periods) of 90 days and washout periods of 90, 120, 150, and 180 days
eTable 7. Results of case-case-time-control analyses for sensitivity analyses
Data Sharing Statement
REFERENCES
- 1.Chan AYL, Yuen ASC, Tsai DHT, et al. Gabapentinoid consumption in 65 countries and regions from 2008 to 2018: a longitudinal trend study. Nat Commun. 2023;14(1):5005. doi: 10.1038/s41467-023-40637-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johansen ME, Maust DT. Update to gabapentinoid use in the United States, 2002-2021. Ann Fam Med. 2024;22(1):45-49. doi: 10.1370/afm.3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashworth J, Bajpai R, Muller S, et al. Trends in gabapentinoid prescribing in UK primary care using the Clinical Practice Research Datalink: an observational study. Lancet Reg Health Eur. 2023;27:100579. doi: 10.1016/j.lanepe.2022.100579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaffer AL, Busingye D, Chidwick K, Brett J, Blogg S. Pregabalin prescribing patterns in Australian general practice, 2012-2018: a cross-sectional study. BJGP Open. 2021;5(1):bjgpopen20X101120. doi: 10.3399/bjgpopen20X101120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.IQVIA Institute for Human Data Science . Medicine use and spending in the U.S.—a review of 2018 and outlook to 2023. 2019. Accessed October 9. 2024. https://www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023
- 6.Australian Government Department of Health and Aged Care . Pharmaceutical Benefits Scheme (PBS) expenditure and prescriptions report 1 July 2022 to 30 June 2023. 2023. Accessed October 9. 2024. https://www.pbs.gov.au/statistics/expenditure-prescriptions/2022-2023/PBS-Expenditure-prescriptions-report-2022-23.pdf
- 7.Goodman CW, Brett AS. A clinical overview of off-label use of gabapentinoid drugs. JAMA Intern Med. 2019;179(5):695-701. doi: 10.1001/jamainternmed.2019.0086 [DOI] [PubMed] [Google Scholar]
- 8.Landefeld CS, Steinman MA. The Neurontin legacy–marketing through misinformation and manipulation. N Engl J Med. 2009;360(2):103-106. doi: 10.1056/NEJMp0808659 [DOI] [PubMed] [Google Scholar]
- 9.Burke RE, Pelcher L, Tjader A, et al. Central nervous system-active prescriptions in older veterans: trends in prevalence, prescribers, and high-risk populations. J Gen Intern Med. 2023;38(16):3509-3516. doi: 10.1007/s11606-023-08250-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chincholkar M. Gabapentinoids: pharmacokinetics, pharmacodynamics and considerations for clinical practice. Br J Pain. 2020;14(2):104-114. doi: 10.1177/2049463720912496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freynhagen R, Serpell M, Emir B, et al. A comprehensive drug safety evaluation of pregabalin in peripheral neuropathic pain. Pain Pract. 2015;15(1):47-57. doi: 10.1111/papr.12146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiffen PJ, Derry S, Bell RF, et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6(6):CD007938. doi: 10.1002/14651858.CD007938.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Budhia S, Mikyas Y, Tang M, Badamgarav E. Osteoporotic fractures: a systematic review of U.S. healthcare costs and resource utilization. Pharmacoeconomics. 2012;30(2):147-170. doi: 10.2165/11596880-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 14.Leung MTY, Marquina C, Turner JP, Ilomäki J, Tran T, Bell JS. Hip fracture incidence and post-fracture mortality in Victoria, Australia: a state-wide cohort study. Arch Osteoporos. 2023;18(1):56. doi: 10.1007/s11657-023-01254-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng JN, Zhang CG, Li BH, Zhan SY, Wang SF, Song CL. Global burden of hip fracture: The Global Burden of Disease Study. Osteoporos Int. 2024;35(1):41-52. doi: 10.1007/s00198-023-06907-3 [DOI] [PubMed] [Google Scholar]
- 16.Anderson PA, McLachlan AJ, Abdel Shaheed C, Gnjidic D, Ivers R, Mathieson S. Deprescribing interventions for gabapentinoids in adults: a scoping review. Br J Clin Pharmacol. 2023;89(9):2677-2690. doi: 10.1111/bcp.15798 [DOI] [PubMed] [Google Scholar]
- 17.Hilmer SN, Gnjidic D. Prescribing for frail older people. Aust Prescr. 2017;40(5):174-178. doi: 10.18773/austprescr.2017.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell JS, Blacker N, Leblanc VT, et al. Prescribing for older people with chronic renal impairment. Aust Fam Physician. 2013;42(1-2):24-28. [PubMed] [Google Scholar]
- 19.Kennard A, Glasgow N, Rainsford S, Talaulikar G. Frailty in chronic kidney disease: challenges in nephrology practice. A review of current literature. Intern Med J. 2023;53(4):465-472. doi: 10.1111/imj.15759 [DOI] [PubMed] [Google Scholar]
- 20.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365-1375. doi: 10.1016/S0140-6736(19)31786-6 [DOI] [PubMed] [Google Scholar]
- 21.Ensrud KE, Ewing SK, Taylor BC, et al. ; Study of Osteoporotic Fractures Research Group . Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007;62(7):744-751. doi: 10.1093/gerona/62.7.744 [DOI] [PubMed] [Google Scholar]
- 22.Middleton R, Poveda JL, Orfila Pernas F, et al. Mortality, falls, and fracture risk are positively associated with frailty: a SIDIAP cohort study of 890 000 patients. J Gerontol A Biol Sci Med Sci. 2022;77(1):148-154. doi: 10.1093/gerona/glab102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy CC, Ioannidis G, Rockwood K, et al. A frailty index predicts 10-year fracture risk in adults age 25 years and older: results from the Canadian Multicentre Osteoporosis Study (CaMos). Osteoporos Int. 2014;25(12):2825-2832. doi: 10.1007/s00198-014-2828-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li G, Thabane L, Papaioannou A, Ioannidis G, Levine MA, Adachi JD. An overview of osteoporosis and frailty in the elderly. BMC Musculoskelet Disord. 2017;18(1):46. doi: 10.1186/s12891-017-1403-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandenbroucke JP, von Elm E, Altman DG, et al. ; STROBE initiative . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147(8):W163-W194. doi: 10.7326/0003-4819-147-8-200710160-00010-w1 [DOI] [PubMed] [Google Scholar]
- 26.Leung MTY, Turner JP, Marquina C, Ilomäki J, Tran T, Bell JS. Trajectories of oral bisphosphonate use after hip fractures: a population-based cohort study. Osteoporos Int. 2024;35(4):669-678. doi: 10.1007/s00198-023-06974-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung MTY, Turner JP, Marquina C, Ilomäki J, Tran T, Bell JS. Effect of oral bisphosphonate drug holiday on mortality following hip fracture. J Clin Endocrinol Metab. 2024:dgae272. doi: 10.1210/clinem/dgae272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang WC, Lai EC. Future-case control crossover analysis for adjusting bias in case crossover studies. BMJ. 2023;382:2136. doi: 10.1136/bmj.p2136 [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Linkletter C, Maclure M, et al. Future cases as present controls to adjust for exposure trend bias in case-only studies. Epidemiology. 2011;22(4):568-574. doi: 10.1097/EDE.0b013e31821d09cd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133(2):144-153. doi: 10.1093/oxfordjournals.aje.a115853 [DOI] [PubMed] [Google Scholar]
- 31.Viswanathan M, Reddy S, Berkman N, et al. Screening to prevent osteoporotic fractures: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(24):2532-2551. doi: 10.1001/jama.2018.6537 [DOI] [PubMed] [Google Scholar]
- 32.Inoue T, Maeda K, Nagano A, et al. Undernutrition, sarcopenia, and frailty in fragility hip fracture: advanced strategies for improving clinical outcomes. Nutrients. 2020;12(12):3743. doi: 10.3390/nu12123743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaffer AL, Brett J, Buckley NA, Pearson SA. Trajectories of pregabalin use and their association with longitudinal changes in opioid and benzodiazepine use. Pain. 2022;163(5):e614-e621. doi: 10.1097/j.pain.0000000000002433 [DOI] [PubMed] [Google Scholar]
- 34.Hallas J, Pottegård A, Wang S, Schneeweiss S, Gagne JJ. Persistent user bias in case-crossover studies in pharmacoepidemiology. Am J Epidemiol. 2016;184(10):761-769. doi: 10.1093/aje/kww079 [DOI] [PubMed] [Google Scholar]
- 35.Huang HC, Li WC, Tadrous M, et al. Evaluating the use of methods to mitigate bias from non-transient medications in the case-crossover design: a systematic review. Pharmacoepidemiol Drug Saf. 2023;32(9):939-950. doi: 10.1002/pds.5649 [DOI] [PubMed] [Google Scholar]
- 36.Greenland S, Thomas DC. On the need for the rare disease assumption in case-control studies. Am J Epidemiol. 1982;116(3):547-553. doi: 10.1093/oxfordjournals.aje.a113439 [DOI] [PubMed] [Google Scholar]
- 37.Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775-1782. doi: 10.1016/S0140-6736(18)30668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Australian Institute of Health and Welfare . Chronic kidney disease: Australian facts. 2024. Accessed October 9, 2024. https://www.aihw.gov.au/reports/chronic-kidney-disease/chronic-kidney-disease/contents/about
- 39.By the 2023 American Geriatrics Society Beers Criteria® Update Expert Panel . American Geriatrics Society 2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2023;71(7):2052-2081. doi: 10.1111/jgs.18372 [DOI] [PubMed] [Google Scholar]
- 40.Olopoenia A, Camelo-Castillo W, Qato DM, et al. Adverse outcomes associated with concurrent gabapentin, opioid, and benzodiazepine utilization: a nested case-control study. Lancet Reg Health Am. 2022;13:100302. doi: 10.1016/j.lana.2022.100302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tesfaye S, Sloan G, Petrie J, et al. ; OPTION-DM trial group . Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): a multicentre, double-blind, randomised crossover trial. Lancet. 2022;400(10353):680-690. doi: 10.1016/S0140-6736(22)01472-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Groot MC, Candore G, Uddin MJ, et al. Case-only designs for studying the association of antidepressants and hip or femur fracture. Pharmacoepidemiol Drug Saf. 2016;25(suppl 1):103-113. doi: 10.1002/pds.3850 [DOI] [PubMed] [Google Scholar]
- 43.Leach MJ, Pratt NL, Roughead EE. Psychoactive medicine use and the risk of hip fracture in older people: a case-crossover study. Pharmacoepidemiol Drug Saf. 2015;24(6):576-582. doi: 10.1002/pds.3785 [DOI] [PubMed] [Google Scholar]
- 44.Requena G, Logie J, Martin E, et al. Do case-only designs yield consistent results across design and different databases? A case study of hip fractures and benzodiazepines. Pharmacoepidemiol Drug Saf. 2016;25(Suppl Suppl 1):79-87. doi: 10.1002/pds.3822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mortensen SJ, Mohamadi A, Wright CL, et al. Medications as a risk factor for fragility hip fractures: a systematic review and meta-analysis. Calcif Tissue Int. 2020;107(1):1-9. doi: 10.1007/s00223-020-00688-1 [DOI] [PubMed] [Google Scholar]
- 46.Xu C, Leung JCN, Shi J, Lum DH, Lai FTT. Sedative-hypnotics and osteoporotic fractures: a systematic review of observational studies with over six million individuals. Sleep Med Rev. 2024;73:101866. doi: 10.1016/j.smrv.2023.101866 [DOI] [PubMed] [Google Scholar]
- 47.Bahardoust M, Mousavi S, Mozafari JK, et al. Association of tramadol use with risk of hip fractures in patients with osteoarthritis: a systematic review and meta-analysis of observational studies. Int J Orthop Trauma Nurs. 2024;52:101078. doi: 10.1016/j.ijotn.2023.101078 [DOI] [PubMed] [Google Scholar]
- 48.Seppala LJ, Petrovic M, Ryg J, et al. STOPPFall (Screening Tool of Older Persons Prescriptions in older adults with high fall risk): a Delphi study by the EuGMS Task and Finish Group on fall-risk-increasing drugs. Age Ageing. 2021;50(4):1189-1199. doi: 10.1093/ageing/afaa249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohara E, Bando Y, Yoshida T, Ohara M, Kirino Y, Iihara N. Central nervous system agent classes and fragility fracture risk among elderly Japanese individuals in a nationwide case-crossover design study. Biol Pharm Bull. 2020;43(2):340-347. doi: 10.1248/bpb.b19-00737 [DOI] [PubMed] [Google Scholar]
- 50.Jetté N, Lix LM, Metge CJ, Prior HJ, McChesney J, Leslie WD. Association of antiepileptic drugs with nontraumatic fractures: a population-based analysis. Arch Neurol. 2011;68(1):107-112. doi: 10.1001/archneurol.2010.341 [DOI] [PubMed] [Google Scholar]
- 51.Pisa F, Reinold J, Lavikainen P, et al. Hip fracture risk in antiepileptic drug initiators and non-initiators with Alzheimer’s disease. Clin Epidemiol. 2021;13:295-307. doi: 10.2147/CLEP.S278306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jørgensen EB, Overgaard LK, Folkestad L, Damkier P, Hallas J, Henriksen DP. The risk of fragility fractures following initiation of gabapentin and pregabalin: a Danish, nationwide, high-dimensional propensity score-matched cohort study. Basic Clin Pharmacol Toxicol. 2023;132(5):384-391. doi: 10.1111/bcpt.13825 [DOI] [PubMed] [Google Scholar]
- 53.Harvey N, Dennison E, Cooper C. Osteoporosis: impact on health and economics. Nat Rev Rheumatol. 2010;6(2):99-105. doi: 10.1038/nrrheum.2009.260 [DOI] [PubMed] [Google Scholar]
- 54.Cullinan S, O’Mahony D, O’Sullivan D, Byrne S. Use of a frailty index to identify potentially inappropriate prescribing and adverse drug reaction risks in older patients. Age Ageing. 2016;45(1):115-120. doi: 10.1093/ageing/afv166 [DOI] [PubMed] [Google Scholar]
- 55.Stow D, Matthews FE, Barclay S, et al. Evaluating frailty scores to predict mortality in older adults using data from population based electronic health records: case control study. Age Ageing. 2018;47(4):564-569. doi: 10.1093/ageing/afy022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khanna AK, Motamedi V, Bouldin B, et al. Automated electronic frailty index-identified frailty status and associated postsurgical adverse events. JAMA Netw Open. 2023;6(11):e2341915. doi: 10.1001/jamanetworkopen.2023.41915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sepehri K, Braley MS, Chinda B, et al. A computerized frailty assessment tool at points-of-care: development of a standalone electronic comprehensive geriatric assessment/frailty index (eFI-CGA). Front Public Health. 2020;8:89. doi: 10.3389/fpubh.2020.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Otsuki T, Higuchi T, Yamazaki T, Okawa E, Okada K, Abe M. Efficacy and safety of pregabalin for the treatment of neuropathic pain in patients undergoing hemodialysis. Clin Drug Investig. 2017;37(1):95-102. doi: 10.1007/s40261-016-0464-1 [DOI] [PubMed] [Google Scholar]
- 59.Lambourg E, Colvin L, Guthrie G, Walker H, Bell S. Analgesic use and associated adverse events in patients with chronic kidney disease: a systematic review and meta-analysis. Br J Anaesth. 2022;128(3):546-561. doi: 10.1016/j.bja.2021.08.035 [DOI] [PubMed] [Google Scholar]
- 60.Vangala C, Niu J, Montez-Rath ME, et al. Hip fracture risk among hemodialysis-dependent patients prescribed opioids and gabapentinoids. J Am Soc Nephrol. 2020;31(6):1325-1334. doi: 10.1681/ASN.2019090904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ishida JH, McCulloch CE, Steinman MA, Grimes BA, Johansen KL. Gabapentin and pregabalin use and association with adverse outcomes among hemodialysis patients. J Am Soc Nephrol. 2018;29(7):1970-1978. doi: 10.1681/ASN.2018010096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Australian Medicines Handbook (online). Australian Medicines Handbook Pty Ltd. Accessed October 9, 2024. https://amhonline.amh.net.au/
- 63.Reyes Fernandez PC, Wright CS, Warden SJ, Hum J, Farach-Carson MC, Thompson WR. Effects of gabapentin and pregabalin on calcium homeostasis: implications for physical rehabilitation of musculoskeletal tissues. Curr Osteoporos Rep. 2022;20(6):365-378. doi: 10.1007/s11914-022-00750-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Velzen M, Dahan A, Niesters M. Neuropathic pain: challenges and opportunities. Front Pain Res (Lausanne). 2020;1:1. doi: 10.3389/fpain.2020.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Diagnostic codes for comorbidities
eTable 2. Characteristics of patients included and matched future-case controls for subgroup analyses for patients with low frailty risk (Hospital Frailty Risk Score [HFRS]<5), high frailty risk (HFRS≥5), without chronic kidney disease (CKD) and with CKD
eTable 3. Characteristics of patients included and matched future-case controls for sensitivity analyses with exposure assessment period (index and reference periods) of 60 days and washout periods of 90, 120, 150, and 180 days
eTable 4. Characteristics of patients included and matched future-case controls for sensitivity analyses with exposure assessment period (index and reference periods) of 30 days and washout periods of 30, 60, and 90 days
eTable 5. Characteristics of patients included and matched future-case controls for sensitivity analyses with exposure assessment period (index and reference periods) of 30 days and washout periods of 120, 150, and 180 days
eTable 6. Characteristics of patients included and matched future-case controls for sensitivity analyses with exposure assessment period (index and reference periods) of 90 days and washout periods of 90, 120, 150, and 180 days
eTable 7. Results of case-case-time-control analyses for sensitivity analyses
Data Sharing Statement