Key Points
Question
Is vagus nerve preservation during distal gastrectomy for early gastric cancer using intraoperative neurophysiological monitoring and indocyanine green labeling feasible, and is it superior to conventional distal gastrectomy?
Findings
In this randomized clinical trial of 264 patients with early-stage distal gastric cancer, vagus nerve preservation resulted in significantly less postoperative gastroparesis compared to vagus nerve resection (0.8% vs 7.6%), lower incidence of gallstone formation (0% vs 6.8%), and better quality of life.
Meaning
Vagus nerve preservation using intraoperative neurophysiological monitoring and indocyanine green labeling during distal gastrectomy for early gastric cancer is safe and achieved favorable effects for patients compared with vagus nerve resection.
Abstract
Importance
Radical gastric cancer surgery can cause functional and physiological disorders due to the resection of perigastric vagus nerves. Few studies have used intraoperative neurophysiological monitoring and indocyanine green (ICG) labeling to preserve the perigastric vagus nerve and to evaluate the corresponding effects.
Objective
To assess the feasibility and effects of vagus nerve preservation using neurophysiologic monitoring and ICG labeling during laparoscopic distal gastrectomy in patients with early distal gastric cancer.
Design, Setting, and Participants
This open-label, prospective randomized clinical trial initially enrolled 285 patients with clinical stage cT1N0M0 distal gastric cancer from May 2022 to May 2023. This trial was conducted at Qilu Hospital of Shandong University in Jinan, China, and enrolled patients aged 18 to 80 years with histologically proven gastric adenocarcinoma scheduled for distal gastrectomy. The final follow-up examination was performed May 1, 2024.
Interventions
Eligible participants were randomly assigned 1:1 to vagus nerve preservation distal gastrectomy (VPG) or vagus nerve resection distal gastrectomy (VRG).
Main Outcomes and Measures
The primary outcome was the incidence of postsurgical gastroparesis. Secondary outcomes included postoperative gallstone formation, quality of life, morbidity, mortality, overall survival, and disease-free survival up to 12 months postoperatively. All analyses were based on both intention-to-treat and per-protocol analyses.
Results
Of 264 patients included in the intention-to-treat analysis, the median (IQR) patient age was 58.0 (52.0-67.0) years, and 67 patients (25.4%) were female. Both the VPG and VRG groups included 132 patients. Postoperative gastroparesis occurred in 1 patient (0.8%) in the VPG group and in 10 patients (7.6%) in the VRG group. Gallstones developed in 0 patients in the VPG group and in 9 patients (6.8%) in the VRG group. As assessed by mean (SD) score on the 30-item European Organization for Research and Treatment of Cancer Quality of Life Questionnaire, the VRG group experienced more nausea and vomiting at 6 months postsurgery (19.38 [7.62]) than the VPG group (17.15 [9.21]) (P = .03) and had significantly higher rates of persistent appetite loss, reflux symptoms, and eating difficulties at both 6 months and 12 months than the VPG group. Differences in postoperative complications and metastasis were not significant.
Conclusions and Relevance
Neurophysiologic monitoring and ICG labeling during distal laparoscopic gastrectomy for vagus nerve preservation in patients with early distal gastric cancer are safe and feasible. Preserving the perigastric vagus nerve may retain the function of the remnant stomach and improve quality of life.
Trial Registration
Chictr.org.cn Identifier: ChiCTR2200059489
This randomized clinical trial assesses the feasibility and effects of vagus nerve preservation using neurophysiologic monitoring and indocyanine green labeling during laparoscopic distal gastrectomy in patients with early distal gastric cancer at 1 hospital in Jinan, China.
Introduction
As a high-prevalence tumor, especially in East Asia, Eastern Europe, and South America, gastric cancer is a globally life-threatening disease. Partially due to the development of endoscopic instruments and government-guided screening programs, the number and proportion of patients with early gastric cancer (EGC) are rising, and gastric cancer–associated mortality has substantially reduced. Despite the progress that has been made in the understanding of gastric cancer biology, radical surgery with standard lymph node dissection is still the preferred method for curative intent. However, conventional gastrectomy usually results in a low postoperative quality of life (QOL) known as postgastrectomy syndrome, due to altered form and function of the stomach. Since the 5-year survival rate of EGC has reached 90%, the focus of interest is thus shifting to the functional outcome and QOL.
Among the existing functional preserving gastric surgeries, the vagus nerve preservation surgery for distal gastrectomy is attracting significant attention. Previous studies have mainly focused on the associations between hepatic branch preservation and the incidence of gallbladder stone formation after surgery. However, the preservation of the celiac branch or other perigastric branches of the vagus nerves is relatively understudied and controversies regarding its benefit remain This controversy over the benefit of vagus nerve preservation can be attributed in part to the possibility of unnoticed injuries to the vagus nerve. Due to the lack of specific markers during the surgery and the celiac branches being located relatively close to the left gastric artery, the identification and functional preservation of the vagus nerve is challenging. Moreover, the preservation of the celiac branch possibly hinders thorough lymph node dissection around the left gastric artery.
Intraoperative neurophysiologic monitoring (IONM) has been applied in thyroid and spinal surgeries for decades. Previous studies have confirmed this technique in determining the de-innervation or preservation of perigastric vagus nerves during gastrectomy surgery. In addition, as a novel and noticeable intraoperative navigation technology, indocyanine green (ICG) fluorescence–guided laparoscopic gastric surgery enables better tumor localization, more accurate identification of sentinel lymph nodes (LNs), and an increased number of harvest LNs. Thus, the combination of ICG fluorescence and IONM may facilitate not only reliable nerve protection, but also accurate identification and thorough lymph node dissection. A systematic review revealed that among lymph node stations 1 to 7, those along the lesser gastric curvature (station 3) exhibited the highest rate of metastases; thus, we intraoperatively basin resected the station 3 LNs and ICG-positive station 1 LNs, followed by examination via frozen section. Patients without metastasis in the resected lymph nodes were enrolled, and a randomized clinical trial was conducted to assess the feasibility and effect of vagus nerve preservation surgery.
Methods
Study Design
This open-label, prospective randomized clinical trial was performed at Qilu Hospital of Shandong University in Jinan, China, from May 2022 to May 2023. The follow-up period ended on May 1, 2024. The study protocol was approved by the ethics committee of Qilu Hospital of Shandong University and is available in Supplement 1. Written informed consent was obtained from all of the participants based on sufficient acknowledgment of the trial’s procedure. A data and safety monitoring committee reviewed the data. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines for randomized clinical trials.
Study Population
The inclusion criteria for this study were (1) patients were 18 to 80 years old with histologically proven gastric adenocarcinoma at cT1N0M0 staging, assessed according to the eighth edition of the American Joint Committee on Cancer tumor, node, metastasis classification; (2) patients were scheduled for distal gastrectomy with D1/D1+ lymphadenectomy and possible for R0 surgery by this procedure; and (3) patients were able to tolerate general anesthesia. Patients who had undergone prior endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) were considered eligible if they met the following criteria: (1) pathological examination indicated the necessity for further gastrectomy; (2) distal gastrectomy could be conducted within 3 months after EMR or ESD; and (3) no perforation or other serious complications occurred during the preceding EMR or ESD procedures. Patients were excluded if they met the following criteria: (1) allergic to iodine or specific contrast agents; (2) had recurrent gastric cancer; (3) with lymph node metastasis, distant metastasis, or direct invasion of the pancreas, spleen, or other organs nearby in the preoperative examinations; (4) had received neoadjuvant or adjuvant chemotherapy treatment; (5) suffering from other serious diseases, including cardiovascular, respiratory, kidney, or liver disease, complicated by poorly controlled hypertension, diabetes, mental disorders, or diseases; (6) need for combined organ resection due to aggression of gastric cancer of other diseases; or (7) need for concurrent surgeries due to other surgical diseases.
Randomization
Individuals who met all inclusion criteria underwent surgery, and ICG injections were performed under gastroscopy 1 day before surgery. The randomization to receive vagus nerve preservation distal gastrectomy (VPG) or vagus nerve resection distal gastrectomy (VRG) (1:1 ratio) was performed after a full exploration of the abdomen and confirmation that no metastasis occurred in the basin dissection of station 3 LNs and ICG-positive station 1 LNs by intraoperative cryosection. The data center used SAS version 9.2 (SAS Institute) to produce sequential numbers, which were requested by the chief surgeons. These numbers ranged from 1 to 260 and corresponded to the intervention assignments. An independent masked coordinator (J.G.) performed the follow-up and the surgeons were masked to treatment assignments during all postoperative follow-up visits.
Interventions
After randomization, patients were operated on by 2 surgeons with experience of more than 50 laparoscopic gastrectomies who are also proficient in performing mini-laparotomy and totally laparoscopic gastrectomies. In the VRG group, the resection range and lymph node dissection were followed by the Japanese Gastric Cancer Treatment Guidelines 2018 (fifth edition), while in the VPG group, the hepatic branches of the anterior vagal nerve were preserved, and ICG-negative station 1 LNs were retained to protect the anterior and posterior gastric branches of the vagus nerve. IONM was used to identify and preserve the nerve. Nasogastric tubes were routinely placed and were removed after surgery when evidence of bowel function returned. The diagrams and photographs depicting vagus nerve protection using IONM are available in eFigures 1 and 2 in Supplement 2.
Follow-Up
The masked trial coordinator recorded all patient symptoms and outcomes every day during hospitalization. The patients were followed up for 12 months, and follow-up was achieved for all patients after surgery. The follow-up schedule was 2 weeks, 1 month, and every 3 months in the first year after surgery. Routine physical examination and laboratory tests, including blood cell count, carcinoembryonic antigen, carbohydrate antigen 19-9, cancer antigen 125, carbohydrate antigen 72-4, and alpha-fetoprotein, were performed on each visit. Chest and abdominal computed tomography (CT) scanning were performed starting from 1 month postoperatively.
Outcome Measurements
The study’s primary end point was the incidence of postsurgical gastroparesis (PSG) within 12 months postoperation.
PSG was diagnosed using the following procedure: (1) nasogastric tube drainage volume of more than 800 mL per day or the presence of nasogastric tubes 10 days postoperatively. Then, upper gastrointestinal radiography and/or gastroscopy were performed to verify the existence of delayed stomach emptying and the absence of any physical obstructions impeding gastric outflow; (2) no apparent irregularities in the balance of fluids and electrolytes; (3) no hidden medical condition, such as hypothyroidism or choroiditis, that could be a potential cause of PSG; and (4) no ongoing medication treatment that could impact the contractile function of smooth muscles. It was hypothesized that the incidence of PSG would be decreased in the VPG group. The secondary end points were the comparison of postoperative gallstone formation, QOL, morbidity, mortality, overall survival, and disease-free survival between the 2 groups.
Scores on the European Organization for Research and Treatment of Cancer Quality of Life (EORTC QLQ) 30-item core questionnaire (EORTC QLQ-C30), version 3, and the EORTC QLQ 22-item stomach cancer–specific questionnaire (EORTC QLQ-STO22) were assessed before surgery and at 6 and 12 months after surgery.
For the EORTC QLQ-C30, these assessments included 4 items: (1) the global health status scale, an evaluation of overall health and well-being; (2) functional scales, which included 5 different scales assessing physical, role, emotional, cognitive, and social functioning; (3) symptom scales, which included 3 symptom scales to gauge the severity of fatigue, pain, nausea, and vomiting; and (4) single items, which are individual questions that cover specific issues, such as dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties.
The EORTC QLQ-STO22 questionnaire was assessed separately, with a focus on the following gastric cancer–related aspects: (1) gastric cancer–related scales, which involved 5 different scales that assessed dysphagia, eating restriction, pain, reflux, and anxiety and (2) single items, which included 4 individual items that examined issues like dry mouth, body image, taste changes, and hair loss.
On the EORTC QLQ-C30, items for each domain were averaged to obtain a mean score, which was then standardized to a 0 to 100 scale per EORTC guidelines. Higher scores on functioning and global health scales indicated better health, whereas higher scores on symptom scales indicated worse symptoms.
Sample Size
Based on a retrospective study, the incidence of PSG in laparoscopic radical gastrectomy was 6.9%, and it was assumed that the preservation of the vagus nerve would prevent the occurrence of PSG; thus, the minimum sample size to detect a difference with 80% statistical power (α = .05, 2-sided test) was 106 per group. Taking the dropout rate of 15% into account, at least 125 patients per group were needed. The sample size was calculated using nQuery Advisor version 7.0 (Statistical Solutions).
Statistical Analysis
Data were analyzed using SPSS version 27.0 (IBM). Continuous variables were tested for normality using the Kolmogorov-Smirnov test, and variables that met normality were represented by mean and SD; otherwise, variables were represented by the median with interquartile range. Categorical variables were given as numbers and percentages. When comparing 2 groups for continuous variables, unpaired t tests and adjusted unpaired t tests were used for normally distributed data with equal variances or missing variance, while the Mann-Whitney U test was applied for non-normally distributed data. Categorical variables were compared using χ2 tests or Fisher exact tests. Both intention-to-treat (ITT) and per-protocol analyses were conducted. All statistical tests were 2-sided, and a P value less than .05 was considered statistically significant.
Results
Study Population
The study flowchart is summarized in Figure 1. From May 2022 to May 2023, 285 patients were identified as having EGC in Qilu Hospital of Shandong University in Jinan, China, and were initially identified as eligible for the study. Among these patients, 12 patients declined to participate, 9 patients had positive station 3 or station 1 LNs, and the remaining 264 patients were regarded as the ITT population and randomly assigned 1:1 to the VPG and VRG groups. Two patients underwent conversion, 3 had concurrent surgery, and 3 underwent total gastrectomy. After follow-up, 128 patients from each group were considered in the per-protocol analysis. The baseline characteristics of the patients are shown in Table 1. Median (IQR) patient age was 58.0 (52.0-67.0) years, and 67 patients (25.4%) were female. The baseline characteristics of the patients who were pathologically diagnosed as pT1N0M0 were further analyzed in eTable 1 in Supplement 2.
Figure 1. CONSORT Flow Diagram.
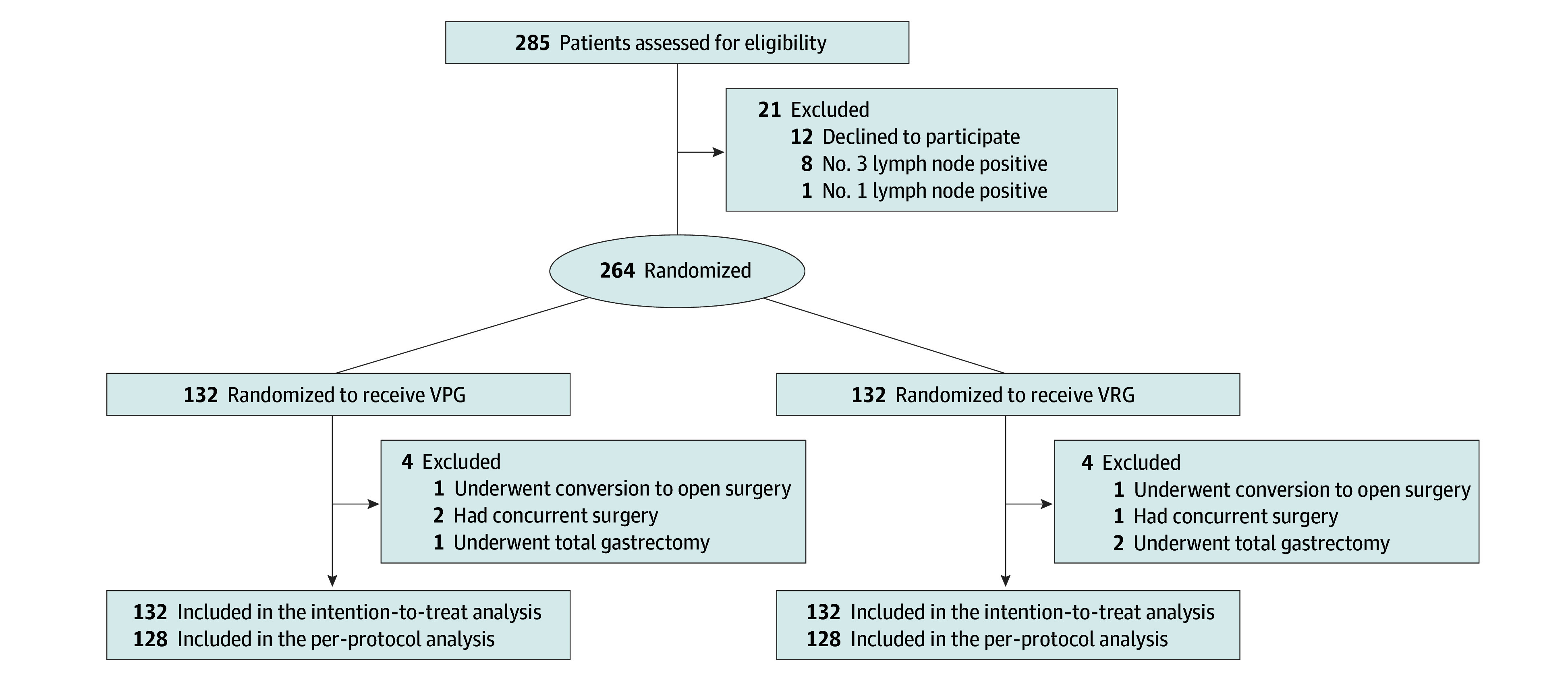
VPG indicates vagus nerve preservation distal gastrectomy; VRG, vagus nerve resection distal gastrectomy.
Table 1. Baseline Characteristics of Patients.
| Variable | Patients, No. (%) | |||
|---|---|---|---|---|
| Intention-to-treat analysis | Per-protocol analysis | |||
| VPG (n = 132) | VRG (n = 132) | VPG (n = 128) | VRG (n = 128) | |
| Sex | ||||
| Female | 32 (24.2) | 35 (26.5) | 31 (24.2) | 34 (26.6) |
| Male | 100 (75.8) | 97 (73.5) | 97 (75.8) | 94 (73.4) |
| Age, median (IQR), y | 58.5 (53.0-68.0) | 58.0 (52.0-66.0) | 58.0 (52.8-68.3) | 58.0 (52.0-66.3) |
| BMI, mean (SD)a | 24.86 (3.29) | 24.88 (3.52) | 24.87 (3.21) | 24.90 (3.58) |
| Smoking | ||||
| Yes | 45 (34.1) | 57 (43.2) | 44 (34.4) | 55 (43.0) |
| No | 87 (65.9) | 75 (56.8) | 84 (65.6) | 73 (57.0) |
| Alcohol consumption | ||||
| Yes | 34 (25.8) | 38 (28.8) | 32 (25.0) | 36 (28.1) |
| No | 98 (74.2) | 94 (71.2) | 96 (75.0) | 92 (71.9) |
| ASA score | ||||
| I | 31 (23.5) | 41 (31.1) | 30 (23.4) | 41 (32.0) |
| II | 94 (71.2) | 83 (62.9) | 91 (71.1) | 80 (62.5) |
| III | 7 (5.3) | 8 (6.1) | 7 (5.5) | 7 (5.5) |
| Comorbidities | ||||
| Diabetes | 23 (17.4) | 37 (28.0) | 23 (18.0) | 37 (28.9) |
| CHD | 16 (12.1) | 19 (14.4) | 16 (12.5) | 19 (14.8) |
| Hypertension | 27 (20.5) | 32 (24.2) | 27 (21.1) | 32 (25.0) |
| Marital status | ||||
| Married | 119 (90.2) | 118 (89.4) | 115 (89.8) | 114 (89.1) |
| Unmarried | 13 (9.8) | 14 (10.6) | 13 (11.2) | 14 (11.9) |
| Education | ||||
| ≤Primary | 24 (18.2) | 24 (18.2) | 24 (18.8) | 24 (18.8) |
| High school | 56 (42.4) | 55 (41.7) | 54 (42.2) | 53 (41.4) |
| University or college | 52 (39.4) | 53 (40.2) | 50 (39.0) | 51 (39.8) |
| Working status | ||||
| Employed | 72 (54.5) | 70 (53.0) | 70 (54.7) | 68 (53.1) |
| Unemployed | 60 (45.5) | 62 (47.0) | 58 (45.3) | 60 (46.9) |
| Tumor size, mean (SD), mm | 21.97 (13.41) | 20.60 (12.46) | 22.1 (13.6) | 20.6 (12.6) |
| pT stage | ||||
| T1a | 69 (52.3) | 76 (57.6) | 67 (52.3) | 74 (57.8) |
| T1b | 55 (41.7) | 54 (40.9) | 53 (41.4) | 52 (40.6) |
| T2 | 5 (3.8) | 2 (1.5) | 5 (3.9) | 2 (1.6) |
| T3 | 3 (2.3) | 0 | 3 (2.3) | 0 |
| pN stage | ||||
| N0 | 114 (86.4) | 117 (88.6) | 110 (85.9) | 113 (88.3) |
| N1 | 12 (9.1) | 13 (9.8) | 12 (9.4) | 13 (10.2) |
| N2 | 5 (3.8) | 2 (1.5) | 5 (3.9) | 2 (1.6) |
| N3 | 1 (0.8) | 0 | 1 (0.8) | 0 |
| Histology | ||||
| Differentiated | 67 (50.8) | 57 (43.2) | 65 (50.8) | 56 (43.8) |
| Undifferentiated | 65 (49.2) | 75 (56.8) | 63 (49.2) | 72 (56.3) |
| Anastomosis method | ||||
| Mini-laparotomy | 53 (40.2) | 59 (44.7) | 49 (38.3) | 55 (43.0) |
| Total laparoscopic | 79 (59.8) | 73 (55.3) | 79 (61.7) | 73 (57.0) |
| Reconstruction type | ||||
| Billroth I | 2 (1.5) | 3 (2.3) | 2 (1.6) | 3 (2.3) |
| Billroth II | 129 (97.7) | 127 (96.2) | 126 (98.4) | 125 (97.7) |
| Roux-en-Y | 1 (0.8) | 2 (1.5) | 0 | 0 |
| Postoperative AC | ||||
| Yes | 18 (13.6) | 14 (10.6) | 18 (14.1) | 14 (10.9) |
| No | 114 (86.4) | 118 (89.4) | 110 (85.9) | 114 (89.1) |
Abbreviations: AC, adjuvant chemotherapy; ASA, American Society of Anesthesiologists; BMI, body mass index; CHD, coronary heart disease; VPG, vagus nerve preservation distal gastrectomy; VRG, vagus nerve resection distal gastrectomy.
Calculated as weight in kilograms divided by height in meters squared.
Surgical Outcomes
In the ITT analyses, the mean (SD) operation time of the VPG group (196.10 [23.59] minutes) was significantly longer than that of the VRG group (179.08 [21.11] minutes). However, the estimated mean (SD) blood loss (VPG, 75.89 [50.21] mL vs VRG, 78.62 [51.81] mL) did not differ significantly. No significant differences were found regarding the postoperative complications in terms of bleeding, anastomosis leakage, and pancreatic fistula (VPG, 3.1% vs VRG, 2.4%). No surgery-related death occurred in either group. The surgical outcomes in the per-protocol analysis (Table 2) and pathologically diagnosed pT1N0M0 populations showed similar differences (eTable 2 in Supplement 2).
Table 2. Surgical Outcomes of Vagus Nerve Preservation (VPG) and Vagus Nerve Resection (VRG) Distal Gastrectomy.
| Surgical outcome | Patients, No. (%) | |||||
|---|---|---|---|---|---|---|
| Intention-to-treat analysis | Per-protocol analysis | |||||
| VPG (n = 132) | VRG (n = 132) | P value | VPG (n = 128) | VRG (n = 128) | P value | |
| Operation time, mean (SD), min | 196.10 (23.59) | 179.08 (21.11) | <.001 | 195.03 (23.49) | 177.72 (19.50) | <.001 |
| Blood loss, mean (SD), mL | 75.89 (50.21) | 78.62 (51.81) | .66 | 71.89 (20.51) | 74.32 (13.28) | .26 |
| Surgeon | ||||||
| A | 94 (71.2) | 86 (65.2) | .29 | 91 (71.1) | 83 (64.8) | .28 |
| B | 38 (28.8) | 46 (34.8) | 37 (28.9) | 45 (35.2) | ||
| Postoperative complications | ||||||
| Bleeding | 1 (0.8) | 1 (0.8) | .57 | 1 (0.8) | 1 (0.8) | .57 |
| Pancreatic fistula | 3 (2.3) | 1 (0.8) | 3 (2.3) | 1 (0.8) | ||
| Anastomotic leakage | 0 | 1 (0.8) | 0 | 1 (0.8) | ||
| Postoperative gastroparesis | 1 (0.8) | 10 (7.6) | .006 | 1 (0.8) | 10 (7.8) | .006 |
| Gallstone | 0 | 9 (6.8) | .007 | 0 | 9 (7.0) | .003 |
| Postoperative hospital stays, mean (SD), d | 8.12 (2.25) | 9.20 (4.04) | .02 | 8.10 (2.25) | 9.52 (3.73) | .003 |
| Regular diet, mean (SD), wk | 6.81 (1.14) | 7.59 (2.59) | .001 | 6.77 (1.05) | 7.56 (2.60) | .002 |
| Metastasis | 0 | 1 (0.8) | >.99 | 0 | 1 (0.8) | >.99 |
| Overall survival | 132 (100) | 132 (100) | NA | 128 (100) | 128 (100) | NA |
Abbreviation: NA, not applicable.
PSG
The median (IQR) follow-up duration on the last follow-up date (May 1, 2024) was 16 (12-24) months. No significant gastroparesis was observed before surgery or after postoperative adjuvant chemotherapy. After 1-year follow-up, in the VRG group, 3 patients experienced PSG within 7 days after surgery, 6 patients experienced PSG within 8 to 30 days after surgery, and 1 patient experienced PSG within 1 to 12 months after surgery. In the VPG group, 1 patient developed PSG 13 days after surgery. All patients recovered from PSG and resumed a regular diet; however, patients in the VRG group took a longer mean (SD) time to recovery (7.59 [2.59] weeks) than those in the VPG group (6.81 [1.14] weeks; P = .001). Data on the duration of PSG are shown in eTable 3 in Supplement 2.
Gallstone Formation
No gallstones were found on CT scanning before surgery, and no stones were detected in the bile duct or intrahepatic duct throughout the study period. Zero patients in the VPG group and 9 of 132 patients in the VRG group (6.8%) developed gallstones within 12 months after gastrectomy. Data regarding the proportions of patients who developed gallstones at different time points can be found in eTable 4 in Supplement 2.
QOL
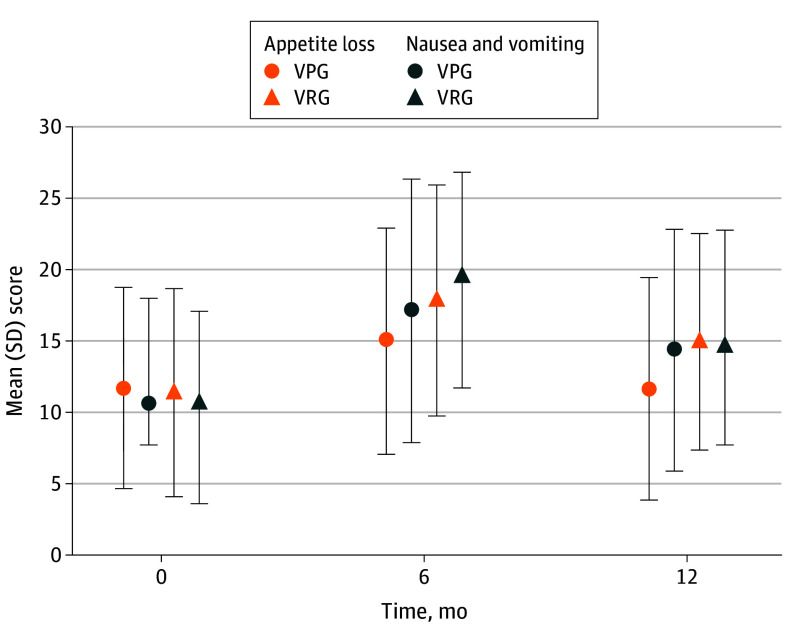
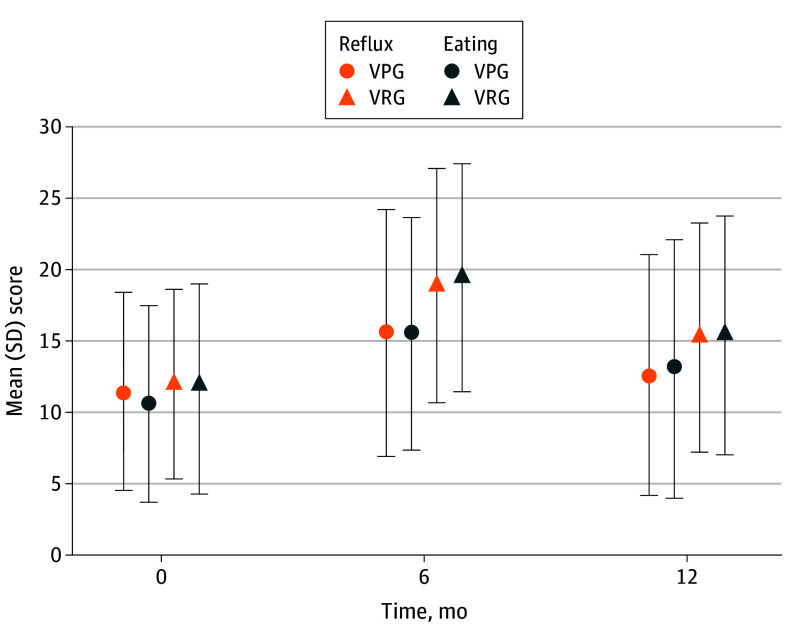
No significant difference was observed in the preoperative QOL scores. Regarding the mean (SD) EORTC QLQ-C30 scores, the VRG group showed significant appetite loss symptoms (VRG, 17.87[8.10] vs VPG, 15.02 [7.92]; P = .004) and nausea and vomiting symptoms (VRG, 19.38 [7.62] vs VPG, 17.15 [9.21]; P = .03) at 6 months after surgery. Nausea and vomiting changed overtime, with no difference observed between the two groups at 12 months postoperation. However, a significant appetite loss was observed in the VRG group (14.97 [7.56] vs VPG, 11.66 [7.77]; P = .001; Figure 2). No statistical difference was found between the 2 groups regarding the functioning scales, fatigue, pain, dysponea, insomnia, constipation, diarrhea, and financial difficulties (eFigures 3-5 in Supplement 2). Regarding gastric cancer-specific QOL in eFigures 6 and 7 in Supplement 2, no obvious difference were found between the 2 groups concerning dysphagia, pain, anxiety, dry mouth, taste, body image, and hair loss. However, in the VRG group, higher reflux symptoms (VRG, 18.90 [8.19] vs VPG, 15.57 [8.63]; P = .001), and eating symptoms (VRG, 19.42 [8.01] vs VPG, 15.51 [8.17]; P < .001) were observed 6 months after surgery. Though the symptoms were alleviated in both groups over time, persistent higher reflux symptoms (VRG, 15.27 [8.07] vs VPG, 12.59 [8.42]; P = .009) and eating symptoms (VRG, 15.45 [8.40] vs VPG, 13.07 [9.09]; P = .03) existed in the VRG group compared with the VPG group 12 months postoperation (Figure 3). Per-protocol analysis showed similar differences as the ITT analysis (eTables 5 and 6 in Supplement 2). The populations with or without postoperative chemotherapy are analyzed in eTables 7 and 8 in Supplement 2.
Figure 2. The Appetite Loss and Nausea and Vomiting Scales of the European Organization for Research and Treatment of Cancer Quality of Life 30-Item Core Questionnaire (EORTC QLQ-C30).
VPG indicates vagus nerve preservation distal gastrectomy; VRG, vagus nerve resection distal gastrectomy.
Figure 3. The Reflux and Eating Scales of the European Organization for Research and Treatment of Cancer Quality of Life 22-Item Stomach Cancer–Specific Questionnaire (EORTC QLQ-STO22).
VPG indicates vagus nerve preservation distal gastrectomy; VRG, vagus nerve resection distal gastrectomy.
Discussion
Vagus nerve-preservation gastrectomy has garnered considerable interest in recent years. Studies suggest that hepatic branch preservation reduces postoperative gallstone formation. However, these studies were limited by their retrospective nature and small sample sizes. The benefits of celiac branch preservation remain controversial, with some speculating that successful vagus nerve preservation during the procedure could influence outcomes. Additionally, safeguarding the vagus nerve while performing a thorough lymph node dissection increases the surgical complexity.
To reduce the surgical difficulty and ensure the consistency of the surgical techniques, IONM and ICG labeling were used during the surgery. To the best of our knowledge, this is the first study to investigate the role of preserving the anterior and posterior gastric branches of vagus nerve during distal gastrectomy.
PSG is known to worsen pain, delay adjuvant chemotherapy, and increase the risk of tumor recurrence and metastasis. As a multifactorial disease, PSG is influenced by various factors, with perigastric vagus nerve preservation being just 1 contributing element. To assess the impact of vagus nerve preservation on PSG, excluding other factors, patients with ECG without severe preoperative comorbidities were selected as the study population. Consistent with previous studies, PSG incidence was 7.6% in the VRG group and decreased to 0.8% with VPG.
Another common complication after gastrectomy is gallstone formation. A meta-analysis found that gallstone occurred in 296 of 1558 patients (19.0%) after distal gastrectomy. Meanwhile, 64.7% of the gallstones were detected within 1 year postgastrectomy. In this study, gallstone incidence was 0% in the VPG group and 6.8% in the VRG group. Consistent with the meta-analysis, resection of the hepatic branch of the vagus nerve was strongly and consistently related to gallstone formation.
Concerning the QLQ-C30, a decline in the overall QOL was observed 6 months postoperatively, irrespective of the preservation of the vagus nerve. Both groups showed gradual improvement over time; however, the VPG group had better scores in appetite loss, nausea, and vomiting symptoms. These improvements may relate to remnant gastric fundus motility and ghrelin secretion, both regulated by the perigastric vagus nerve. For QLQ-STO22, the persistent higher reflux symptoms in VRG group imply a possibility of reflux esophagitis occurring 6 and 12 months after surgery.
Regarding the safety of the procedure, IONM was safely applied to identify perigastric nerves. The study team has extensive experience with ICG fluorescence-guided laparoscopic gastrectomy. Combining these techniques is feasible and safe, and the procedure showed no inferiority compared with conventional laparoscopic surgery. One patient showed hepatic metastasis in VRG, while no recurrence or metastasis occurred in the VPG group after 1-year follow-up. Moreover, station 3 LNs have a much higher metastasis rate compared with station 1 LNs, and no metastasis in station 3 LNs and ICG-positive station 1 LNs is the premise of preserving the ICG-negative station 1 LNs.
Limitations
Several limitations should be noted in our study. First, all patients were from 1 single Chinese high-volume gastric cancer center, and whether this technique can be generalized to other institutions or other ethnicities should be further validated. Second, the follow-up time was relatively short. However, PSG usually occurs within 1 month postoperation. In addition, 64.7% of the gallstones were detected within 1 year postgastrectomy. Thus, a minimum 1-year follow-up period is sufficient for exploring the main study end points. An extended follow-up period will be performed to evaluate the effects on the tumor prognosis. Third, while consistent with previous studies, PSG diagnosis primarily relies on symptoms and upper gastrointestinal radiography, as no universally accepted criterion standard exists. Fourth, the diagnosis of gallstones primarily relies on CT examinations, with a lower positivity rate compared to ultrasound or magnetic resonance imaging.
Conclusions
These results showed that delicately preserving the perigastric vagus nerve by using both IONM and ICG labeling significantly reduced the incidence of PSG and gallstone formation and improved patient QOL within a 1-year follow-up period. Studies involving a larger and more diverse patient population, with longer follow-up periods, are needed to explore the safety and feasibility of this technique in the future.
Trial Protocol
eTable 1. Baseline Characteristics of Patients Pathlogically Diagnosed as pT1N0M0
eTable 2. Surgical Outcomes of Patients Pathlogically Diagnosed as pT1N0M0
eTable 3. Information on the Duration of PSG After Gastrectomy in Intention-to-Treat Analysis
eTable 4. The Proportion of Patients Developing Gallstones Within 3, 6, 9, and 12 Months After Gastrectomy in Intention-to-Treat Analysis
eTable 5. Quality of Life and Functional Outcome of Patients Followed by VPG and VRG Before Surgery, and 6, and 12 Months After Surgery in Intention-to-Treat Analysis
eTable 6. Quality of Life and Functional Outcome of Patients Followed by VPG and VRG Before Surgery, and 6, and 12 Months After Surgery in Per-Protocol Analysis
eTable 7. Quality of Life and Functional Outcomes in Patients Receiving Postoperative Adjuvant Chemotherapy Followed by VPG and VRG Before Surgery, and 6, and 12 Months After Surgery in Intention-to-Treat Analysis
eTable 8. Quality of Life and Functional Outcomes in Patients Not Receiving Postoperative Adjuvant Chemotherapy Followed by VPG and VRG Before Surgery, and 6, and 12 Months After Surgery in Intention-to-Treat Analysis
eFigure 1. Diagram For Perigastric Vagus Nerve and the Preservation of Vagus Nerve During Distal Gastrectomy
eFigure 2. The Preservation of the Vagus Nerve During Distal Gastrectomy
eFigure 3. The Functional Scales of the EORTC QLQ-C30
eFigure 4. The Fatigue, Pain, Dysponea, and Insomnia Scales of the EORTC QLQ-C30
eFigure 5. The Constipation, Diarrhea, and Financial Difficulties Scales of the EORTC QLQ-C30
eFigure 6. The Dysphagia, Pain, and Anxiety Scales of the EORTC QLQ-STO22
eFigure 7. The Dry Mouth, Taste, Body Image, and Hair Loss Scales of the EORTC QLQ-STO22
Data Sharing Statement
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Hatta W, Tsuji Y, Yoshio T, et al. Prediction model of bleeding after endoscopic submucosal dissection for early gastric cancer: BEST-J score. Gut. 2021;70(3):476-484. doi: 10.1136/gutjnl-2019-319926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magyar CTJ, Rai A, Aigner KR, et al. Current standards of surgical management of gastric cancer: an appraisal. Langenbecks Arch Surg. 2023;408(1):78. doi: 10.1007/s00423-023-02789-5 [DOI] [PubMed] [Google Scholar]
- 4.Okubo K, Arigami T, Matsushita D, et al. Evaluation of postoperative quality of life by PGSAS-45 following local gastrectomy based on the sentinel lymph node concept in early gastric cancer. Gastric Cancer. 2020;23(4):746-753. doi: 10.1007/s10120-020-01047-7 [DOI] [PubMed] [Google Scholar]
- 5.Kakeji Y, Ishikawa T, Suzuki S, et al. ; Registration Committee of the Japanese Gastric Cancer Association . A retrospective 5-year survival analysis of surgically resected gastric cancer cases from the Japanese Gastric Cancer Association nationwide registry (2001-2013). Gastric Cancer. 2022;25(6):1082-1093. doi: 10.1007/s10120-022-01317-6 [DOI] [PubMed] [Google Scholar]
- 6.Kojima K, Yamada H, Inokuchi M, Kawano T, Sugihara K. Functional evaluation after vagus-nerve-sparing laparoscopically assisted distal gastrectomy. Surg Endosc. 2008;22(9):2003-2008. doi: 10.1007/s00464-008-0016-8 [DOI] [PubMed] [Google Scholar]
- 7.Wang CJ, Kong SH, Park JH, et al. Preservation of hepatic branch of the vagus nerve reduces the risk of gallstone formation after gastrectomy. Gastric Cancer. 2021;24(1):232-244. doi: 10.1007/s10120-020-01106-z [DOI] [PubMed] [Google Scholar]
- 8.Ando S, Tsuji H. Surgical technique of vagus nerve-preserving gastrectomy with D2 lymphadenectomy for gastric cancer. ANZ J Surg. 2008;78(3):172-176. doi: 10.1111/j.1445-2197.2007.04396.x [DOI] [PubMed] [Google Scholar]
- 9.Furukawa H, Ohashi M, Honda M, et al. Preservation of the celiac branch of the vagal nerve for pylorus-preserving gastrectomy: is it meaningful? Gastric Cancer. 2018;21(3):516-523. doi: 10.1007/s10120-017-0776-8 [DOI] [PubMed] [Google Scholar]
- 10.Huang Y, Mu GC, Qin XG, Chen ZB, Lin JL, Zeng YJ. Study of celiac artery variations and related surgical techniques in gastric cancer. World J Gastroenterol. 2015;21(22):6944-6951. doi: 10.3748/wjg.v21.i22.6944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malik R, Linos D. Intraoperative neuromonitoring in thyroid surgery: a systematic review. World J Surg. 2016;40(8):2051-2058. doi: 10.1007/s00268-016-3594-y [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez AA, Jeyanandarajan D, Hansen C, Zada G, Hsieh PC. Intraoperative neurophysiological monitoring during spine surgery: a review. Neurosurg Focus. 2009;27(4):E6. doi: 10.3171/2009.8.FOCUS09150 [DOI] [PubMed] [Google Scholar]
- 13.Kong SH, Kim SM, Kim DG, et al. Intraoperative neurophysiologic testing of the perigastric vagus nerve branches to evaluate viability and signals along nerve pathways during gastrectomy. J Gastric Cancer. 2019;19(1):49-61. doi: 10.5230/jgc.2019.19.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyashiro I, Kishi K, Yano M, et al. Laparoscopic detection of sentinel node in gastric cancer surgery by indocyanine green fluorescence imaging. Surg Endosc. 2011;25(5):1672-1676. doi: 10.1007/s00464-010-1405-3 [DOI] [PubMed] [Google Scholar]
- 15.Chen QY, Xie JW, Zhong Q, et al. Safety and efficacy of indocyanine green tracer-guided lymph node dissection during laparoscopic radical gastrectomy in patients with gastric cancer: a randomized clinical trial. JAMA Surg. 2020;155(4):300-311. doi: 10.1001/jamasurg.2019.6033 [DOI] [PubMed] [Google Scholar]
- 16.Sposito C, Maspero M, Conalbi V, et al. Impact of indocyanine green fluorescence imaging on lymphadenectomy quality during laparoscopic distal gastrectomy for gastric cancer (Greeneye): an adaptative, phase 2, clinical trial. Ann Surg Oncol. 2023;30(11):6803-6811. doi: 10.1245/s10434-023-13848-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jong MHS, Gisbertz SS, van Berge Henegouwen MI, Draaisma WA. Prevalence of nodal metastases in the individual lymph node stations for different T-stages in gastric cancer: a systematic review. Updates Surg. 2023;75(2):281-290. doi: 10.1007/s13304-022-01347-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenkman HJF, Claassen L, Hannink G, et al. Learning curve of laparoscopic gastrectomy: a multicenter study. Ann Surg. 2023;277(4):e808-e816. doi: 10.1097/SLA.0000000000005479 [DOI] [PubMed] [Google Scholar]
- 19.Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24(1):1-21. doi: 10.1007/s10120-020-01042-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng H, Zhou D, Jiang X, Ding W, Lu L. Incidence and risk factors for postsurgical gastroparesis syndrome after laparoscopic and open radical gastrectomy. World J Surg Oncol. 2013;11(1):144. doi: 10.1186/1477-7819-11-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Z, Zhao X, Qiu S, Liu N, Li P, Zhou S. Risk factor analysis of gastroparesis syndrome in 2652 patients with radical distal gastrectomy. J Gastrointest Surg. 2023;27(8):1568-1577. doi: 10.1007/s11605-022-05538-z [DOI] [PubMed] [Google Scholar]
- 22.Hiramatsu Y, Kikuchi H, Takeuchi H. Function-preserving gastrectomy for early gastric cancer. Cancers (Basel). 2021;13(24):6223. doi: 10.3390/cancers13246223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Li Y. Related factors of postoperative gallstone formation after distal gastrectomy: a meta-analysis. Indian J Cancer. 2017;54(1):43-46. doi: 10.4103/ijc.IJC_91_17 [DOI] [PubMed] [Google Scholar]
- 24.Kim SM, Cho J, Kang D, et al. A randomized controlled trial of vagus nerve-preserving distal gastrectomy versus conventional distal gastrectomy for postoperative quality of life in early stage gastric cancer patients. Ann Surg. 2016;263(6):1079-1084. doi: 10.1097/SLA.0000000000001565 [DOI] [PubMed] [Google Scholar]
- 25.Cai Z, Lin H, Li Z, et al. A prediction nomogram for postoperative gastroparesis syndrome in right colon cancer: a retrospective study. Langenbecks Arch Surg. 2023;408(1):148. doi: 10.1007/s00423-023-02885-6 [DOI] [PubMed] [Google Scholar]
- 26.Fletcher R, Swanström LL. Postsurgical gastroparesis. In: McCallum RW, Parkman HP, eds. Gastroparesis. Academic Press; 2020:255-263. [Google Scholar]
- 27.Shafi MA, Pasricha PJ. Post-surgical and obstructive gastroparesis. Curr Gastroenterol Rep. 2007;9(4):280-285. doi: 10.1007/s11894-007-0031-2 [DOI] [PubMed] [Google Scholar]
- 28.Chen XD, Mao CC, Zhang WT, et al. A quantified risk-scoring system and rating model for postsurgical gastroparesis syndrome in gastric cancer patients. J Surg Oncol. 2017;116(4):533-544. doi: 10.1002/jso.24691 [DOI] [PubMed] [Google Scholar]
- 29.Fukagawa T, Katai H, Saka M, Morita S, Sano T, Sasako M. Gallstone formation after gastric cancer surgery. J Gastrointest Surg. 2009;13(5):886-889. doi: 10.1007/s11605-009-0832-8 [DOI] [PubMed] [Google Scholar]
- 30.Travagli RA, Anselmi L. Vagal neurocircuitry and its influence on gastric motility. Nat Rev Gastroenterol Hepatol. 2016;13(7):389-401. doi: 10.1038/nrgastro.2016.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perelló M, Cornejo MP, De Francesco PN, Fernandez G, Gautron L, Valdivia LS. The controversial role of the vagus nerve in mediating ghrelin’s actions: gut feelings and beyond. IBRO Neurosci Rep. 2022;12:228-239. doi: 10.1016/j.ibneur.2022.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei M, Liang Y, Wang L, et al. Clinical application of indocyanine green fluorescence technology in laparoscopic radical gastrectomy. Front Oncol. 2022;12:847341. doi: 10.3389/fonc.2022.847341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orsenigo E, Tomajer V, Di Palo S, et al. Sentinel node mapping during laparoscopic distal gastrectomy for gastric cancer. Surg Endosc. 2008;22(1):118-121. doi: 10.1007/s00464-007-9385-7 [DOI] [PubMed] [Google Scholar]
- 34.Kim YW, Min JS, Yoon HM, et al. Laparoscopic sentinel node navigation surgery for stomach preservation in patients with early gastric cancer: a randomized clinical trial. J Clin Oncol. 2022;40(21):2342-2351. doi: 10.1200/JCO.21.02242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kosuga T, Tsujiura M, Nakashima S, Masuyama M, Otsuji E. Current status of function-preserving gastrectomy for gastric cancer. Ann Gastroenterol Surg. 2021;5(3):278-286. doi: 10.1002/ags3.12430 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Baseline Characteristics of Patients Pathlogically Diagnosed as pT1N0M0
eTable 2. Surgical Outcomes of Patients Pathlogically Diagnosed as pT1N0M0
eTable 3. Information on the Duration of PSG After Gastrectomy in Intention-to-Treat Analysis
eTable 4. The Proportion of Patients Developing Gallstones Within 3, 6, 9, and 12 Months After Gastrectomy in Intention-to-Treat Analysis
eTable 5. Quality of Life and Functional Outcome of Patients Followed by VPG and VRG Before Surgery, and 6, and 12 Months After Surgery in Intention-to-Treat Analysis
eTable 6. Quality of Life and Functional Outcome of Patients Followed by VPG and VRG Before Surgery, and 6, and 12 Months After Surgery in Per-Protocol Analysis
eTable 7. Quality of Life and Functional Outcomes in Patients Receiving Postoperative Adjuvant Chemotherapy Followed by VPG and VRG Before Surgery, and 6, and 12 Months After Surgery in Intention-to-Treat Analysis
eTable 8. Quality of Life and Functional Outcomes in Patients Not Receiving Postoperative Adjuvant Chemotherapy Followed by VPG and VRG Before Surgery, and 6, and 12 Months After Surgery in Intention-to-Treat Analysis
eFigure 1. Diagram For Perigastric Vagus Nerve and the Preservation of Vagus Nerve During Distal Gastrectomy
eFigure 2. The Preservation of the Vagus Nerve During Distal Gastrectomy
eFigure 3. The Functional Scales of the EORTC QLQ-C30
eFigure 4. The Fatigue, Pain, Dysponea, and Insomnia Scales of the EORTC QLQ-C30
eFigure 5. The Constipation, Diarrhea, and Financial Difficulties Scales of the EORTC QLQ-C30
eFigure 6. The Dysphagia, Pain, and Anxiety Scales of the EORTC QLQ-STO22
eFigure 7. The Dry Mouth, Taste, Body Image, and Hair Loss Scales of the EORTC QLQ-STO22
Data Sharing Statement