Abstract
Juglans regia L. is a well‐known therapeutic plant in Nepal, employed in traditional medicine for treating liver ailments. This study aimed to evaluate the in vivo and in silico liver‐protective effects of J. regia extract using a carbon tetrachloride (CCl4)‐induced hepatic damage rat model. Healthy male rats were randomly divided into six groups: normal control (distilled water 10 mL/kg), toxic control (distilled water 10 mL/kg), standard test (silymarin 100 mg/kg), and three groups receiving oral J. regia extracts (125, 250, and 500 mg/kg/day) for seven days. On the eighth day, carbon tetrachloride (CCl4) was administered intraperitoneally (i.p.) (1.5 mL/kg in 1:1 olive oil ratio for all groups, except the normal control). Rats were sacrificed on the ninth day, and blood was collected retro‐orbitally for liver blood injury tests and histopathological studies. Molecular docking was performed against cytochrome P450 2E1 (CYP450 2E1) enzyme for 16 selected phytoconstituents. J. regia, at doses of 125, 250, and 500 mg/kg, significantly reduced liver enzyme levels (alanine aminotransferase, alkaline phosphatase, direct bilirubin, and total bilirubin), while increasing serum albumin. Histological analysis revealed mitigation of carbon tetrachloride (CCl4)‐induced liver injury, reducing fatty degeneration and necrosis. Molecular docking supported the findings, with Beta‐sitosterol and Betulinic acid exhibiting the best binding affinity of −9.2 and −9.1 kcal/mol, respectively. In conclusion, result suggests that J. regia showed dose‐dependent hepatoprotective activity in CCl4‐induced hepatotoxicity and it could be utilized as a promising hepatoprotective agent. This study suggests the hepatoprotective potential of J. regia bark extracts, emphasizing the need for further clinical validation.
Keywords: carbon tetrachloride, hepatoprotective, Juglans regia L., molecular docking, serum biochemical parameters
This study evaluated the liver‐protective effects of Juglans regia L. extract using a carbon tetrachloride (CCl4)‐induced hepatic damage rat model, demonstrating significant reductions in liver enzyme levels and bilirubin, along with histological evidence of liver injury mitigation. Molecular docking analysis highlighted Beta‐sitosterol and Betulinic acid as potential bioactive compounds, suggesting the hepatoprotective potential of J. regia bark extracts and indicating the necessity for additional clinical validation.
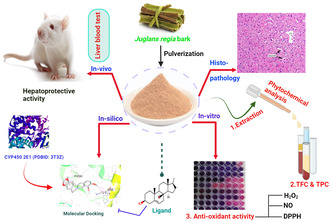
1. INTRODUCTION
The liver is an essential organ that plays a major role in the body's ability to maintain physiological homeostasis, fight disease, maintain metabolic processes, store vitamins, metabolize drugs, and protect the body from xenobiotics and toxic substances. It also secretes bile and is involved in the metabolism of biomolecules, which include proteins, carbohydrates, and fats (Real et al., 2019; Song et al., 2024; Ugwu & Suru, 2021).
Two million deaths per year are caused by liver disease, which also accounts for 4% of all deaths globally (1 out of every 25 deaths); men make up about two thirds of liver‐related deaths. Acute hepatitis accounts for a lesser percentage of deaths, with complications from cirrhosis and hepatocellular carcinoma being the main causes of death. The most prevalent causes of cirrhosis globally are alcohol consumption, nonalcoholic fatty liver disease (NAFLD), and viral hepatitis (Devarbhavi et al., 2023; Younossi et al., 2023). According to research, paracetamol overdose is linked to nearly 40% of drug‐associated liver problems (Cohen et al., 2024; Omotayo et al., 2015). For example, among the top 10 main causes of death globally are drug‐induced hepatic damage and chronic liver disorders, necessitating safe and efficient treatments (Pandey et al., 2023).
There are a lot of contemporary medications on the market, but only a small number of herbal remedies have been proven to help treat liver disease. These medications strengthen the liver's protective capabilities, restore damaged hepatic cells, and enhance liver effectiveness (Maqbool et al., 2019). Based on experimental verification, natural bioactive components generated from plant secondary metabolites have been announced as valuable alternatives for predicting and mitigating hepatotoxic effects and their chronic consequences (Pandey et al., 2023).
Natural products rich in antioxidants are utilized in traditional systems of medicine for treating liver disease due to its effectiveness and safety profile (Amin & Nagy, 2009; Pandey et al., 2013; Souid et al., 2024). Walnut fruits and leaves contain phenolic compounds, vitamins, and carotenoids that show antioxidant activity, so this prevents the attack from free harmful radicals and oxidative stress‐induced liver damage (Aydın et al., 2015). Walnut tree bark (Juglans regia L.) is a common medicinal plant known as ‘Okhar’ in Nepal, prescribed by the folk medicine systems to treat liver disease, which could be due to their anti‐inflammatory and blood‐purifying activities (Al‐Nadaf et al., 2024; Asha et al., 2010). Juglans regia L. (J. regia) bark was selected for the phytochemical analysis, antioxidant and in vivo/in silico hepatoprotective activity based on its traditional uses. A well‐established experimental rat model of hepatic damage caused by carbon tetrachloride (CCl4) is used to examine the hepatoprotective efficacy and basic molecular mechanisms of new medicines (El‐Beltagi et al., 2024; Kourkoumpetis & Sood, 2019; Yan et al., 2018). Additionally, molecular docking approach is used to establish the interaction of ligands with particular receptor. Overall, our study aim to evaluate the in vivo hepatoprotective activity of Juglans regia L. extract using a carbon tetrachloride‐induced hepatic damage rat model is confirmed by in silico studies. Furthermore, we have selected the main phytochemicals of Juglans regia L. bark as ligand and cytochrome P450 2E1 (CYP450 2E1) as a receptor to establish the hepatoprotective interaction of phytoconstituents with the hepatic enzyme.
2. EXPERIMENTAL
2.1. Chemicals and reagents
Distilled water (Thomas Scientific, India), dimethyl sulfoxide (DMSO) (Fischer Scientific, India), absolute ethanol (Changshu Hongshen Fine Chemical Co. Ltd., China), Carbon tetrachloride (Loba Chemie Pvt. Ltd, India), 10% formalin (Fischer Scientific, India), diethyl ether (Fischer Scientific, India), paraffin wax (Fischer Scientific, India), hematoxylin (Alpha Chemika, Mumbai, India), xylene, eosin (Vizag Chemicals, India), standard drug the silymarin (Ponjim Huacheng Pharma, China), assay kits for liver biomarker (Accurex Biomedical Pvt. Ltd., India), and 2,2‐diphenyl‐1‐picrylhydrazyl (Wako Pure Chemical Co. Ltd, Japan) were used. All analytical‐grade chemicals and reagents were used in this study.
2.2. Plant material
Fresh J. regia bark weighing about 505 g was harvested during November and the plant was identified by a taxonomist at the Laboratory of National Herbarium and Plants, Nepal, and a voucher specimen (PUCD‐2021‐32) was preserved for future use. The collected barks were cleaned with water and were shade‐dried until absolutely dry. The dry barks about 300 g were ground into powder using an electric grinder. The moisture content of J. regia bark was 41.0%.
2.3. Experimental animals
Swiss albino rats of either sex (female for oral acute toxicity and male for the main study), 8–12 weeks old, and weighing between 250 and 300 g were used for the experiment. The rats were placed in a polypropylene cage with six rats per cage in conventional environmental conditions (25 ± 3°C and 12 h natural light–dark cycle). All animals had unlimited regular access to food and water. The animals were acclimatized in the laboratory environment for 10 days prior to the study. The study was conducted in compliance with the National Center for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) as per Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. All animals were fasted for 18 h before the experiment as per OECD (Organisation for Economic Co‐operation and Development) guidelines (OECD, 1996); induction of anesthesia was carried out by using mild diethyl ether and blood was collected. Anesthesia was induced by delivering 3.20% minimum alveolar concentration (MAC50) value for diethyl ether by using a large glass‐covered chamber (Flecknell, 2015; Miranda et al., 2011). All experimental animals were treated in accordance with the standard guidelines for the “care and use of animals in laboratories” published by the National Institutes of Health (NIH, 2010) and Institutional Review Committee approval number (Ref. No. 7‐077‐078) was acquired from Pokhara University, Nepal, prior to the commencement of in vivo research.
2.4. Preparation of plant extracts
Cold maceration was used to extract 300 g of bark of J. regia powder using absolute ethanol as the solvent. Whatman grade 1 filter paper and clean muslin cloth were used to filter the resultant extract. The liquid extract was evaporated in a rotatory evaporator (Heidolph, Germany) at 250–175 mbar pressure, 90 rpm (revolutions per minute), 40°C water bath temperature, and 5°C chilling temperature, until the solvent was completely evaporated. The concentrated filtrate was kept in a glass vial in vacuum desiccators for complete drying and further use.
2.5. Qualitative phytochemical analysis
Standardized tests were used to identify the presence of plant secondary metabolites in the extract (Bhatnagar et al., 2012; Vishnoi, 2000).
2.6. Quantitative phytochemical analysis
2.6.1. Total phenolic content
Folin–Ciocalteu method was used to determine the overall phenolic content of the ethanolic extract of J. regia (Singleton & Rossi, 1965). In short, 5 mL of distilled water, 1 mL of the Folin–Ciocalteu reagent, and 1 mL of 1 mg/mL plant extract were combined. After 5 minutes, 1 mL of sodium carbonate (10% w/v) was added and shaken. Using a Cary 60 UV–Visible Spectrophotometer (Agilent, United States), the absorbance was observed at 725 nm after it had been left to stand for 60 min. Gallic acid (GA) (25–500 mg/L) served as the reference to quantify the overall content of the phenolic compounds, and the findings were represented as gallic acid equivalents (GAE) in milligrams for every dry weight of the extract in grams.
2.6.2. Total flavonoid content
Total flavonoid content was determined according to the method explained by Zhishen et al. The flavonoid content of J. regia extract was quantified by colorimetric method using aluminum chloride (Zhinshen et al., 1999). Shortly, 4 mL of distilled water was mixed with 1 mL of 1 mg/mL of the plant extract. Then, 0.3 mL of 5% solution of sodium nitrite was added to the mixture followed by the addition of 0.3 mL of 20% aluminum chloride after 5 min, and the mixture was left to stand for 6 min. After that, one molar sodium hydroxide (2 mL) was added. The absorbance was measured against a blank at 510 nm by using a spectrophotometer. Quercetin was used as the standard. Per gram of dry weight of the extract's total flavonoid content was represented as milligram of quercetin equivalent.
2.7. Antioxidant activity
The scavenging of 2,2‐diphenyl‐1‐picrylhydrazyl (DPPH) free radicals, nitric oxide (NO) radicals, and hydrogen peroxide (H2O2) were used to measure the antioxidant activity of J. regia extract.
2.7.1. DPPH scavenging activity
The technique mentioned by Mensor et al. was used to assess the DPPH free radicals neutralizing activity of the extract of J. regia (Mensor et al., 2001). Briefly, 2 mL of J. regia bark ethanolic extract (0.1–100 μg/mL) was combined with a solution of 2 mL DPPH (60 μM). The combination was permitted to react completely for half an hour at ambient temperature in dark. By using an ultraviolet (UV)–visible spectrophotometer, the deterioration of the purple hue of the J. regia extract was determined at 518 nm. As a positive control, ascorbic acid was dissolved in ethanol to create a stock solution with the same concentration. The test solution without the sample served as a negative control. The proportion of radicals scavenging capacity for DPPH was determined using the formula:
Whereas, C abs and T abs are control and J. regia ethanolic extract absorbance, respectively.
After plotting the inhibition percentage versus extract concentration, the antioxidant activity of the extract was determined by using a graph and represented in relation to half‐maximal inhibitory concentration (IC50).
2.7.2. Nitric oxide radicals scavenging activity
The nitric oxide (NO) radicals scavenging test outlined by Rao was employed to determine the free radicals scavenging activity of J. regia (Rao, 1997). For this, 1 mL of sodium nitroprusside solution and 1 mL of the extract in a range of concentrations (0.1–100 μg/mL) were combined and kept at 29°C for 2.5 h. After incubation, the reaction mixture received 2 mL Griess reagent, and the optical density was observed using an ultraviolet (UV)–visible spectrophotometer at 548 nm. The proportion of nitric oxide radicals' inhibition by the curcumin standard and the antioxidant activity of the plant extract were estimated as follows:
Where C abs and T abs are control and J. regia ethanolic extract absorbance, respectively.
2.7.3. Hydrogen peroxide (H2O2 ) scavenging activity
Using the Ruch and Co. approach, the potential of the extract to scavenge hydrogen peroxide was evaluated (Ruch et al., 1989). Shortly, 1 mL of extract (100 μg/mL) and 0.6 mL of 40 mM hydrogen peroxide made with 0.1 M of phosphate buffer (pH 7.4) were mixed. After 10 minutes of incubation, the entire volume was filled with buffer up to 3 mL and the optical density was measured at 230 nm using a ultraviolet (UV)–visible spectrophotometer using a buffer as blank. Similar to the reaction mixture, the control lacked the test sample. The following equation was used to compute the H2O2 scavenging activity:
where C abs and T abs are control and J. regia ethanolic extract absorbance, respectively.
2.8. Acute toxicity test
To investigate the oral toxicity of J. regia extract, OECD (Organisation for Economic Co‐operation & Development) No. 423 criteria were followed (OECD, 1996). Prior to and for 3–4 h after the extract was administered, all rats underwent overnight fasting with free access to water. For this, female nonpregnant and nulliparous healthy rats were used as per OECD guidelines. The extract was delivered orally as one dose of 2000, 4000, and 5000 mg per kg of body mass in three individual rats. The treated rats were monitored for 4 h with half‐hour intervals, and then for 2 weeks with a day's interval for the intake of food and water, death, and general toxicity manifestations, such as diarrhea, lethargy, tremor, reduced weight, and paralysis. The J. regia extract is considered to have a high degree of safety if no animal death was noted in a study with one rat, meaning that its fatal dose (LD50) is larger than 5000 mg/kg.
2.9. Grouping and dosing of experimental animals
Six groups of six rats (healthy male) each were used by randomly assigning the rats to the following treatments: normal control group (distilled water at 10 mL/kg), the toxic control group (distilled water at 10 mL/kg), standard test (silymarin at 100 mg/kg), and J regia extract at 125, 250, and 500 mg/kg each day orally for seven days, after which a single dose of 50% CCl4 dissolved in olive oil at a dose of (1.5 mL/kg, i.p.) was administered on the eighth day for all groups, except normal control group. The normal control group was treated with 1.5 mg/kg i.p. olive oil only. The precise amount of plant extract to be utilized for evaluating its hepatoprotective activity was determined using an oral acute toxicity test. The J. regia extract, silymarin, and control substances were all delivered orally through oral gavage tubes at a volume of 1 mL per 100 g. J. regia extract was dissolved in distilled water, which was used in both oral acute toxicity and hepatoprotective studies.
2.10. Hepatoprotective activity
The procedure utilized to test hepatoprotective activity was modified from that employed before by Sintayehu et al. (2012). Each rat had to undergo an 18‐hour fast before weighting. As stated earlier, the experimental animals were split up into six separate groups and dosed accordingly.
Rats were weighed the following nine days, and blood was extracted from the retro‐orbital plexus while they were lightly sedated with ether. Aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), direct bilirubin (DBI), total bilirubin (TBI), total serum protein (TP), and the serum albumin levels were among the blood biochemical markers that were measured as part of the liver damage test. For the liver blood test, blood samples from each rat were collected in sterile gel tubes. These tubes were then spun in a centrifuge to release the liver biomarker, which was then analyzed by a clinical chemistry analyzer (BAS‐100 TS, Labomed Inc., USA). The rats were euthanized by using diethyl ether overdose (5% MAC50 value of diethyl ether in a closed glass chamber) followed by cervical decapitation (Flecknell, 2015; Miranda et al., 2011). The absolute and relative weights (organ‐to‐body weight ratio) of the liver were recorded.
The percentage of hepatoprotection of the J. regia extract was calculated for the evaluation of hepatoprotective activity by the following equation;
where a = mean value of the hepatotoxin (CCl4) produced marker. b = mean value of hepatotoxin plus J. regia extract produced marker. c = the average value that the vehicle control produces (olive oil).
2.11. Histopathological analysis
The livers from all rats were removed and thoroughly washed using ice‐cold 0.9% normal saline and maintained in a sterile sample container in 10% buffered formalin at least for 24 h. Following that, repeated ethanol dehydration (70%–100%), xylene clearing, and paraffin wax embedding were performed on the liver samples. Hematoxylin and eosin were used to stain the liver tissue slices of 5 μm, which were then examined under a microscope for histological findings (Pearse, 1968). The liver histopathology images were captured at 10x original magnification.
2.12. Molecular docking
Various published papers confirmed the presence of variety of active constituents in Juglans regia bark (Jabli et al., 2017; Kale et al., 2012; Lamichhane et al., 2016). To this, we have accessed the Indian Medicinal Plants, Phytochemistry and Therapeutics 2.0 (IMPPAT 2.0) web server (https://cb.imsc.res.in/imppat/) (Vivek‐Ananth et al., 2023). From this web server, we have downloaded 16 chemical constituents (Figure 1) in three‐dimensional (3D) ‘sdf’ file format. The chirality and stereochemistry of the ligand was optimized. Merck molecular force field (MMFF94) was employed to minimize the local minima by inbuilt extension of AutoDock Vina 1.5.7 Ubuntu 20.04.6 platform. The number of torsion angles was set as default on MGLTools (Molecular Graphics Laboratory). The ‘sdf’ format of all ligands was converted into ‘pdbqt’ for molecular docking (Gote et al., 2023).
FIGURE 1.
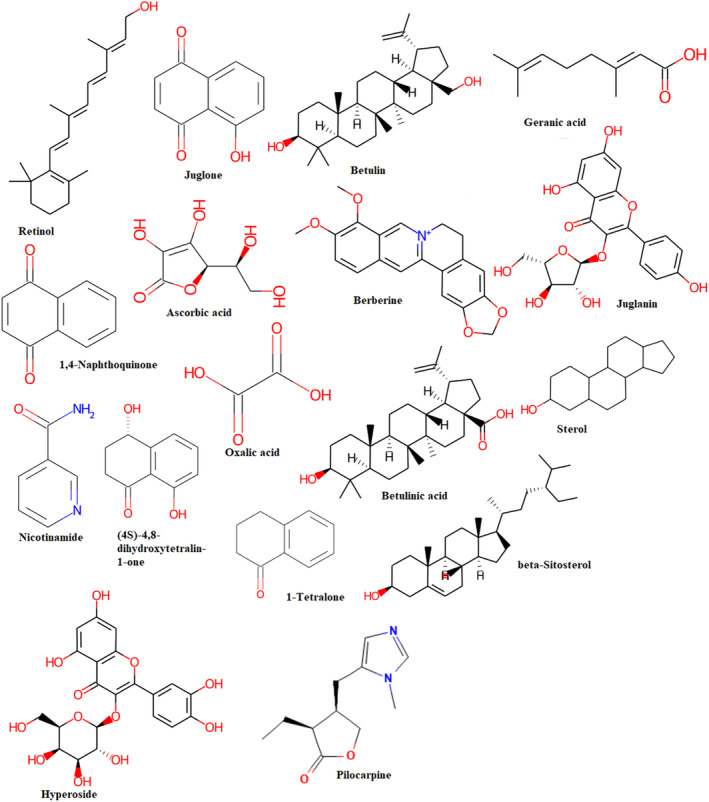
Two‐dimensional (2D) structure of selective 16 phytochemicals of Juglans regia bark and one co‐crystal ligand (Pilocarpine).
Cytochrome P450 2E1 (CYP450 2E1) enzyme (PDBID:3T3Z) of 2.3 Å X‐ray crystal structure was retrieved from the Protein Data Bank (PDB) (DeVore et al., 2012). CYP450 2E1 activates the CCl4 to induce the reactive species which may damage the liver. The structural coordinate was validated by the analysis of Ramachandran plot. Using the AutoDock 1.5.7 program, polar hydrogen and Kollman charge were added to the protein. One of the most important steps in molecular docking is the purification and processing of proteins. Therefore, the BIOVIA Discovery Studio Visualizer 2021 platform carefully removed complicated cofactors, water molecules, and heteroatom from the proteins (Thapa, Biradar, Banerjee, & Karati, 2023). The ‘pdb’ file of protein was converted into ‘pdbqt’ for docking by Open Babel software (O'Boyle et al., 2011).
The Lamarckian Genetic Algorithm of Auto Dock Vina version 1.5.7 software was used to execute molecular docking on the Linux Ubuntu 20.04.6 platform (Trott & Olson, 2009). The protein without any native ligands underwent energy minimization and the amino acids were assigned as AD4 type after calculating the missing atoms (Thapa, Nargund, Biradar, Banerjee, & Karati, 2023). The grid box around the native ligand was created (Figure 2) and the dimension file was generated from the BIOVIA Discovery Studio Visualizer 2021. Then, the binding pocket was selected.
FIGURE 2.
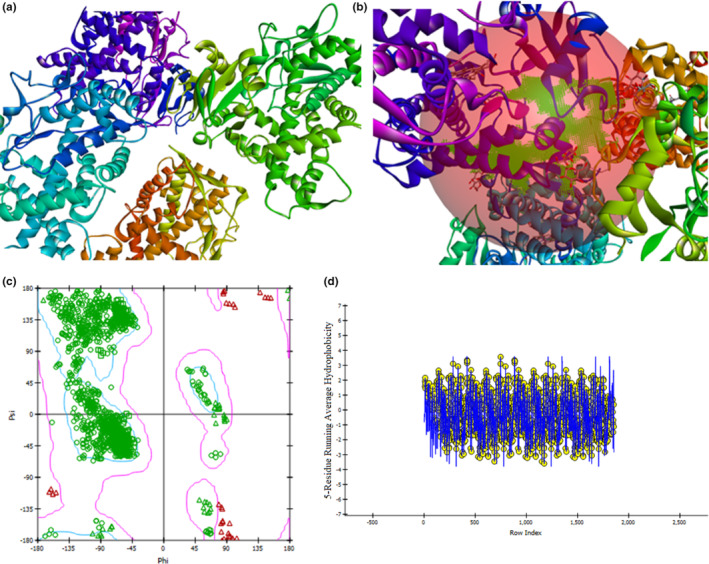
Three‐dimensional (3D) structure of CYP450 2E1 (PDBID: 3T3Z) (a), a grid box determination (b), Ramachandran plot (c), and Hydrophobic plot (d).
Ligands with a molecular weight above 500 Daltons were excluded from consideration for docking. The ligands and protein's “pdbqt” files were uploaded onto the system. The values of the center grid box were 50.700, −2.661, and 25.917 for X‐center, Y‐center, and Z‐center, respectively. The grid spacing and size of the box were established as 1 Å and 126 units, respectively. The value of 8 was assigned to exhaustiveness for all proteins in each docking computation. A docking command, namely the “Perl vina_linux” script, was executed to carry out the molecular docking process. The docking results were visualized using BIOVIA Discovery Visualizer 2021 version. The pose with the best docking score was selected and rendered for representation (Thapa, Nargund, & Biradar, 2023). The docking results were validated by calculating the Root Mean Square Deviation (RMSD) value by using PyMol software.
2.13. Retrospective searching for main components of J. regia
The authentic research articles were downloaded from the Scopus‐indexed journal by using “GC‐MS (Gas chromatography‐Mass spectroscopy) of Juglans regia” as prompt. Around eight relevant articles were selected and the main common chemical component analyzed from the articles (Al‐Rawi et al., 2021; Jabli et al., 2017; Kale et al., 2012). The ligands selected for molecular docking were validated by the GC–MS (gas chromatography–mass spectroscopy) result from previously published articles and the data stored in IMPPAT 2.0 web server (https://cb.imsc.res.in/imppat/) (Vivek‐Ananth et al., 2023) and COCONUT (Collection of Open Natural Products) (https://coconut.naturalproducts.net/) web server (Sorokina et al., 2021).
2.14. Statistical analysis
The findings are presented as the average (mean) and standard error of mean (SEM). SPSS version 16 software was used for statistical analysis. Analysis of variance (ANOVA) (one‐way) and the post hoc (Tukey test) test were carried out for statistical analysis. p values <.05 and <.001 were used to define statistically significant differences.
3. RESULTS AND DISCUSSION
In the present communication, phytochemical analysis, total phenolic and flavonoid content estimation, in vitro antioxidant activity, and in vivo hepatoprotective activity of ethanolic extract of the bark of J. regia with in silico study were evaluated. Phytochemicals are of high interest because of their substantial applications. Medicinal plants are considered as drug storehouses for traditional medicine systems, allopathic medicine systems, folk medicine, food supplements, intermediate products of pharmaceuticals, nutraceuticals, and chemical entities for various synthetic modern medicines (Ncube et al., 2008).
The liver has a requisite function in life due to its role in metabolic and detoxification actions (Muriel & Rivera‐Espinoza, 2008). When the liver is subjected to various endogenous and xenobiotic agents, numerous intermediate and finished compounds are produced. These agents are the principal causes of hepatocellular necrosis and finally disease of the liver (Lee, 2003; Wei et al., 2024). To confirm the protection of hepatocellular damage and maintain a healthy liver, the current existing treatment attends to symptomatic treatment and hepatic transplant in severe hepatic disease (Lampertico et al., 2017). Yet, there is no other better therapeutic candidate for increasing the detoxification capacity of the liver. In this regard, testing and utilization of natural‐based hepatocurative agents are significantly soaring. Since it would be very crucial to exhibit how well the plant extracts function to cut off the liver injury caused by xenobiotics. CCl4 is a commonly used hepatotoxic substance in order to assess the ability of the plant extract to protect the liver (Arshad et al., 2024; Pritchard & Apte, 2015). The human scenario is closely simulated by in vivo experimental rodent models, which also make it simple to measure biochemical and histopathological characteristics (Arshad et al., 2024; De et al., 2017). In order to evaluate the in vivo liver‐protective efficacy of the ethanolic extract of the bark of J. regia, the CCl4‐induced hepatotoxicity in rats was used as the experimental model in this investigation. Both humans and laboratory animals can develop centrizonal hemorrhagic hepatic necrosis as a result of CCl4 (De et al., 2017). In this research, the silymarin was used as the standard hepatoprotective agent, which is also used as a standard positive control in most extensively researched plant extracts in preventing the liver injury experimental models (Dawada et al., 2012).
3.1. In vitro study
3.1.1. Extraction yield, nature of the extract, and phytochemical screening
The extraction yield percentage of the ethanolic extract of the barks of J. regia was 9.87% with dark chocolate‐colored solid nature extract. Qualitative screening of phytochemicals from the extract of J. regia indicated the occurrence of alkaloids, carbohydrates, phenolics, flavonoids, saponins, and terpenoids. However, glycosides and amino acids were absent in the extract.
3.1.2. Overall content of phenolics and flavonoids
Calibration curve of gallic acid and quercetin which were taken as standard at seven different concentrations 25 to 500 mg/L was drawn, as shown in Figure 3. On the basis of the standard regression line for gallic acid (y = 0.006x + 0.122; R 2 = .992) and quercetin (y = 0.000x − 0.005; R 2 = .993), the equivalent total phenolic content and total flavonoid content were calculated. J. regia extract showed 496.67 ± 1.02 milligram GE/g dry extract weight and 2028.02 ± 0.19 milligram QE/g dry extract weight of phenol and flavonoid content, respectively (Figure 3).
FIGURE 3.
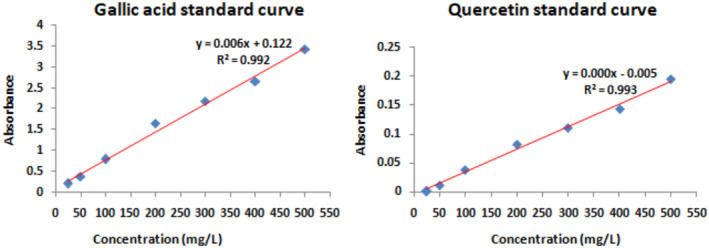
Gallic acid and quercetin standard calibration curve for calculation of total phenolic and falvonoid contents.
In accordance with prior research, J. regia ethanolic extract includes active phytoconstituents such as polyphenols, alkaloids, flavonoids, terpenoids, carbohydrates, and amino acids that may have hepatoprotective properties alone or in combination (Doss, 2009). The ethanolic extract of the barks of J. regia displayed significant levels of total phenolic and flavonoid contents. These results are in line with the results of past studies that showed the hepatoprotective action of these phytochemicals (Alghazeer et al., 2018).
3.1.3. Antioxidant activity
Ethanolic extract of the bark of J. regia showed the highest DPPH free radicals scavenging activity, i.e. 94.71 ± 0.33 percentage of radicals scavenged with IC50 value (2.31 μg/mL) at the concentration of 100 μg/mL compared to ascorbic acid. In contrast, moderate and lower percentage of radicals scavenging capacity was found for the scavenging of NO and H2O2 radicals, respectively (Table 1).
TABLE 1.
Percentage of DPPH, NO, and H2O2 radical acts of scavenging of J. regia extract at various concentrations.
| S. No. | Antioxidant assay method | Test sample and standard | Test concentration | IC50 value (μg/mL) | |||
|---|---|---|---|---|---|---|---|
| 0.1 μg/mL | 1 μg/mL | 10 μg/mL | 100 μg/mL | ||||
| 1. | DPPH free radical scavenging | J. regia extract | 58.20 ± 2.59 | 67.86 ± 0.73 | 88.97 ± 4.72 | 94.71 ± 0.33 | 2.31 |
| Standard (ascorbic acid) | 56.83 ± 2.86 | 63.15 ± 5.4 | 95.16 ± 1.97 | 97.83 ± 0.06 | 2.44 | ||
| 2. | NO free radical scavenging | J. regia extract | 26.98 ± 7.13 | 29.78 ± 6.97 | 37.28 ± 3.59 | 48.85 ± 1.45 | 35.66 |
| Standard (curcumin) | 27.09 ± 4.11 | 29.59 ± 4.64 | 33.35 ± 3.45 | 42.22 ± 1.93 | 79.60 | ||
| 3. | H2O2 radical scavenging | J. regia extract | – | – | – | 28.95 ± 4.51 | – |
| Standard (ascorbic acid) | – | – | – | 87.99 ± 0.2 | – | ||
Note: Average ± SEM (standard error of mean) (n = 3) is used to express all data.
A single antioxidant assay method is not sufficient for the assessment of the antioxidant potential of plant extracts. Since different harmful radicals scavenging assays use different experimental techniques and have different assay principles. For instance, this study employed the DPPH free radical assay, NO radicals scavenging assay, and the hydrogen peroxide assay technique. Both phenol and flavonoid compounds show medicinal activity such as antibacterial, antiviral, anti‐inflammatory, anticancer, antiallergic, and hepatoprotective activities, making them capable of scavenging singlet oxygen and other free radicals (Bravo, 1998; Montoro et al., 2005). Our findings, which were in line with the earlier discovery, revealed that high phenolic and flavonoid contents are associated with high levels of harmful radicals scavenging potential when assessed by adopting the DPPH free radicals test technique (Sahreen et al., 2011). However, no association was found in the case of scavenging hydrogen peroxide radicals. This may be because, depending on the chemical makeup, the antioxidant activity of the phenolic compounds varies greatly (Satué‐Gracia et al., 1997). In addition, some chemical constituents, such as sugar or ascorbic acid present in the extract, also interfere with the radicals scavenging activity (Singleton & Rossi, 1965).
As reported by Trifunschi et al., plant extracts showed hepatoprotective activity mainly because of their capacity to scavenge free radicals (Trifunschi et al., 2015). As CCl4‐induced toxicity generates inflammatory mediators such as monocytes, neutrophils, interleukin‐6 (IL‐6), and tumor necrosis factor‐alpha, plants exhibit anti‐inflammatory action for hepatoprotection (Yoshioka et al., 2017). The decrease in the serum liver indicators further suggests that flavonoids may protect against CCl4‐induced cell membrane instability and damage. It is in agreement with the outcome of the study conducted by Tarahovsky et al. (2014). Alkaloids, saponins, and flavonoids, among other plant secondary metabolites, have hepatoprotective effects by acting as anti‐inflammatory agents. This result is consistent with prior research that has demonstrated the anti‐inflammatory properties of phytoconstituents (Huang et al., 2017).
3.1.4. Molecular docking
In this molecular docking study, 16 phytochemicals were evaluated against the CYP450 2E1 enzyme using computational methods. Molecular docking allows us to predict the binding affinity and interactions between small molecules (the phytochemicals) and the target protein (CYP450 2E1) to understand their potential as inhibitors. Cytochrome P450 2E1 enzyme (PDBID:3T3Z) contains a native ligand which is Pilocarpine. As regards the structure, the Pilocarpine is a heterocyclic imidazole derivative. This substance is derived from plants belonging to the genus Pilocarpus. Pilocarpus microphyllus is the exclusive source of Pilocarpine, and its commercial manufacture is solely obtained from the leaves of this plant (Hancock, 2007). Since the native ligand is a plant‐based imidazole derivative, it has some structural similarity to 16 ligands selected by us. Therefore, 3T3Z was chosen as protein/target for the docking study and the results were compared with those of the native ligand Pilocarpine.
Leu197, Leu171, Ala157, Pro319, Ile236, Ala108, Val108, Ala240, Leu45, and Phe46 were the most common amino acids that participated in the interaction (Supplementary Materials, Figures S1). The binding energies observed ranged from −4.3 to −9.2 kcal/mol (Table 4), indicating varying degrees of affinity between the phytochemicals and the enzyme. Lower binding energies suggest stronger binding interactions between the compounds and the enzyme. Two compounds that were particularly noteworthy were Betulinic acid and Beta‐sitosterol, which showed significant binding energies of −9.1 and −9.2 kcal/mol, respectively (Figure 4). Furthermore, Betulin and Sterol also showed significant binding energies of −8.9 and −8.2 kcal/mol. Interestingly, 1‐tetralone and hyperoside exhibited a similar binding energy of −8.0 kcal/mol. These values suggest a strong affinity of these compounds for the active site of the CYP450 2E1 enzyme, potentially indicating their efficacy as inhibitors. All the 16 ligands showed RMSD value less than 2 Å (Table 4).
TABLE 4.
Binding energy, amino acid involved in interaction, and RMSD value of 16 ligand molecules.
| SN | Name of compounds | Binding energy (kcal/mol) | Amino acids | RMSD value (Å) |
|---|---|---|---|---|
| 1. | Retinol | −7.2 | Leu197, Leu171, Ala157 | 1.388 |
| 2. | Juglone | −6.0 | Tyr349, Lys324 | 0.996 |
| 3. | Betulin | −8.9 | Ala240, Pro466 | 0.926 |
| 4. | Geranic acid | −6.3 | Leu48, Glu49, Leu50, Phe B:46, Phe D:46, Leu D:45, Leu B:45, Ile42 | 1.359 |
| 5. | Juglanin | −7.8 | Lys461, His226, Pro462, Leu460, Pro319, Ala105, Ala108 | 1.004 |
| 6. | Berberine | −7.2 | Lys55, Pro54, Phe360 | 1.038 |
| 7. | Ascorbic acid | −5.6 | Arg233, His226 | 0.584 |
| 8. | 1,4‐Naphthoquinone | −5.8 | Ile469, Val464 | 1.030 |
| 9. | Nicotinamide | −5.8 | Leu48, Leu B:45, Leu B:46, Leu D:45, Leu D:46 | 1.013 |
| 10. | Oxalic acid | −4.3 | Arg126, Arg435, Arg100 | 0.379 |
| 11. | (4S)‐4,8‐dihydroxy‐tetralin‐1‐one | −7.1 | Leu45, Leu48, PheD: 46, PheB:46 | 1.072 |
| 12. | Betulinic acid | −9.1 | Gly43, Gly63, Ile41 | 1.706 |
| 13. | Sterol | −8.2 | Leu45, PheB:46, PheD:46 | 1.045 |
| 14. | 1‐Tetralone | −8.0 | Leu45, Phe46 | 0.871 |
| 15. | Beta‐sitosterol | −9.2 | Pro319, Ile236, Ala108, Val108, Ala240 | 1.108 |
| 16. | Hyperoside | −8.0 | Pro462, Pro319, Ile236, Ala108, Pro104 | 1.080 |
| 17. | Pilocarpine | −5.5 | Try398, Pro408 | – |
FIGURE 4.
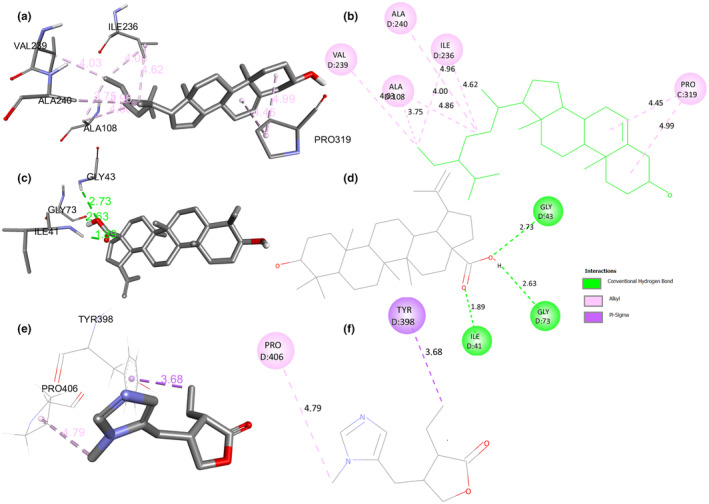
Two‐dimensional and three‐dimensional (2D and 3D) molecular docking interaction of Beta‐Sitosterol (a, b), Betulinic acid (c, d), and Pilocarpine (e, f) with CYP450 2E1 (PDBID: 3T3Z).
In comparison to the native ligand Pilocarpine, which had a binding energy of −5.5 kcal/mol, both Beta‐sitosterol and Betulinic acid had considerably stronger binding affinity. This suggests that they have the potential to be more effective compounds in their interaction with CYP450 2E1. Additionally, all the phytochemicals showed the stronger affinity than the co‐crystal native ligand (Pilocarpine) against the enzyme, except oxalic acid (−4.3 kcal/mol). The precise interactions observed during the molecular docking approach offer additional understanding of the binding mechanisms of these drugs. Beta‐sitosterol exhibited six pi‐alkyl interactions, indicating that its binding to the enzyme involves hydrophobic interactions. Conversely, Betulinic acid displayed three typical hydrogen bonds, suggesting a distinct mechanism of interaction characterized by specific hydrogen bonding with the active site residues of the enzyme. Because Betulinic acid exhibits hydrogen bonding, it may be a superior phytoconstituent for future research to consider as a potential therapeutic candidate, as hydrogen bonding is crucial to the molecular docking process that establishes the ligand–receptor‐binding mechanism (Chen et al., 2016). CYP450 2E1, a hepatic enzyme, metabolizes the hepatotoxic agent carbon tetrachloride (CCl4) in rat models. CYP450 2E1 activates CCl4, generating highly reactive radicals that induce oxidative stress, leading to lipid peroxidation, protein, and DNA damage in hepatocytes. Elevated CYP450 2E1 expression intensifies reactive oxygen species (ROS) production, exacerbating oxidative stress in the liver. Consequently, cellular damage, inflammation, and hepatocyte necrosis occur. Modulating CYP450 2E1 activity is explored as a potential strategy to mitigate CCl4‐induced liver damage (Khan et al., 2012; Shaban et al., 2023; Xu et al., 2017). The dried bark of J. regia contains bioactive polyphenols (NirmlaDevi et al., 2011). However, a number of compounds, including Beta‐sitosterol, ascorbic acid, juglone, folic acid, gallic acid, regiolone, Betulinic acid, and quercetin‐3‐α‐L‐arabinoside, show hepatoprotective activity, which have been isolated from the J. regia bark (Zakavi et al., 2013). A similar result was expressed by our in silico study. Beta‐sitosterol (−9.2 kcal/mol) and Betulinic acid (−9.1 kcal/mol) return the strong affinity toward the CYP450 2E1 enzyme. It is noteworthy to mention that both the complexes have strong stability, which was determined by the RMSD value (less than 2 Å).
We found some interesting information about the binding modalities in the interactions between Betulinic acid and the CYP450 2E1 enzyme. The binding profile of Betulinic acid was highly significant, since it formed three hydrogen bonds with essential amino acid residues located in the active region of the enzyme. More precisely, these interactions took place with Gly43 at a distance of 2.73 Å, Gly73 at a distance of 2.63 Å, and Ile41 at a very close distance of 1.89 Å (Figure 4). The carboxylic acid group (‐COOH) of Betulinic acid plays a crucial role in creating these hydrogen bonds, as seen by its involvement in all three interactions. The results of this study clarify the specific mechanism by which Betulinic acid binds to CYP450 2E1 and demonstrate its capacity to block the activity of enzyme. These findings provide an important information for future experimental investigations and the development of pharmaceuticals that target CYP450 2E1.
These results emphasize the potential of Betulinic acid and Beta‐sitosterol as interesting candidates for further experimental validation and possible development as inhibitors of the CYP450 2E1 enzyme. Beta‐sitosterol has the potential to be an excellent choice for development as a therapeutic candidate due to its ability to inhibit through hydrophobic interactions. On the other hand, Betulinic acid inhibits through conventional hydrogen interactions. This is owing to their substantial binding affinities and unique ways of interacting with the target enzyme.
3.1.5. Main components of J. regia reported from database and articles
To validate the presence of different phytoconstituents, we have investigated the published articles and different plant databases like COCONUT and IMPPAT 2.0. We observed different secondary metabolites recorded in the database, which we then verified by comparing with published spectral data. We only mention the reported phytoconstituents having GC–MS data in Table 5 (some fatty acids or long‐chain hydrocarbons, which are not pharmaceutically important, are excluded from the list of ligands). Different spectral studies revealed the presence of variety of aromatic and heterocyclic components in J. regia bark, which may help in the hepatoprotective activity. The prominent components of J. regia bark include sterol, steric acid, oleic acid, n‐heptadecanoic acid, palmitic acid, n‐octadecane, etc (Table 5).
TABLE 5.
Gas chromatography–mass spectroscopy (GC–MS) profiling of active component of J. regia (Al‐Rawi et al., 2021; Kale et al., 2012).
| SN | Main component of J. regia |
|---|---|
| 1. | Campesterol |
| 2. | Stigmasterol |
| 3. | Citric acid |
| 4. | 17‐Octadecynoic acid |
| 5. | Linoleic acid ethyl ester |
| 6. | 9,12‐Octadecadienoic acid |
| 7. | 13‐Octadecenoic acid |
| 8. | Stearic acid |
| 9. | Lactic acid |
| 10. | Ethanimidic acid |
| 11. | Oleic acid |
| 12. | Palmitic acid |
| 13. | n‐Heptadecanoic acid |
| 14. | n‐Octadecane |
| 15. | Beta‐sitosterol |
| 16. | Betulinic acid |
3.2. In vivo study
3.2.1. Oral acute toxicity study
Oral administration of the ethanol extract of J. regia bark did not exhibit any alterations in eating amount, and behavioral changes including changes in movement, activity, hair texture, and pupil size. At a dosage of 5000 mg/kg, no morbidity or mortality was seen. The fatal dose (LD50) of the J. regia extract is categorized as Globally Harmonized System (GHS) Category 5 since its estimated concentration is more than 5000 mg/kg, which is confirmed and tested by/as per the OECD oral acute toxicity test. Since J. regia extract up to 5000 mg/kg is safe for oral administration to rat.
3.2.2. Body and liver weight of the experimental animals
Table 2 lists the starting and ending body weight, liver weight, and the relative liver weight of the six experimental groups that were sacrificed after the first eight days of the research. Over the 8‐day study period of the experiment, there were no discernible changes observed in the general conditions of the animals in either group. Comparing the CCl4‐treated group with the usual control group, there was a reduction in body weight. In contrast to the normal control, the toxic control, and standard test groups, the rats treated with ethanolic extract of J. regia bark at dosages of 250 and 500 mg/kg revealed significantly lower liver weight (p < .05).
TABLE 2.
Experimental animals’ body weight, liver weight, and weight gain.
| Parameters | Experimental group | |||||
|---|---|---|---|---|---|---|
| Normal control group (DW; 10 mL/kg) | Toxic control group (DW; 10 mL/kg) | Standard group (Silymarin; 100 mg/kg) | J. regia extract (500 mg/kg) | J. regia extract (250 mg/kg) | J. regia extract (125 mg/kg) | |
| Initial body weight (g) | 276.50 ± 22.39 | 241.84 ± 13.80 | 231.84 ± 6.64 | 285.67 ± 28.65 | 260.50 ± 21.39 | 258.34 ± 19.74 |
| Final body weight (g) | 300.16 ± 22.38 | 260.67 ± 17.11 | 250.16 ± 10.31 | 290.84 ± 29.27 | 265.67 ± 23.87 | 270.50 ± 19.87 |
| Weight gain (g) | 23.67 ± 1.20 | 18.84 ± 2.54 | 18.84 ± 4.10 | 5.16 ± 1.97a–c | 5.16 ± 3.26a–c | 12.16 ± 3.74 |
| Liver weight (g) | 10.62 ± 1.12 | 11.40 ± 0.78 | 10.84 ± 0.93 | 12.79 ± 1.36 | 10.20 ± 1.33 | 11.51 ± 1.52 |
| Relative liver weight | 3.49 ± 0.14 | 4.44 ± 0.38 | 4.31 ± 0.25 | 4.55 ± 0.64 | 3.79 ± 0.23 | 4.18 ± 0.38 |
Note: The results depict the average and standard error of mean (SEM) of six rats. Administered vehicle: DW (distilled water), olive oil, and CCl4 in 1:1 ratio at a dose of 1.5 mL/kg was administered via i.p. route on eighth day with all groups, except the normal control group. Relative liver weight = (Liver weight/Body weight) × 100. a–c p < .05 significantly different from the normal, toxic, and standard control group, respectively.
Rats treated with only CCl4 showed increase in the liver weight due to the emergence to the liver infiltration, vacuolization, and inflammation (Table 2, Figure 5). Gain to the liver weight is a sign of hepatic damage and may be brought on by the retention of water in the cytoplasm of hepatocytes, which causes the liver cells to expand and ultimately result in an increase in the total liver weight (Mahmood et al., 2014; Mulla et al., 2009).
FIGURE 5.
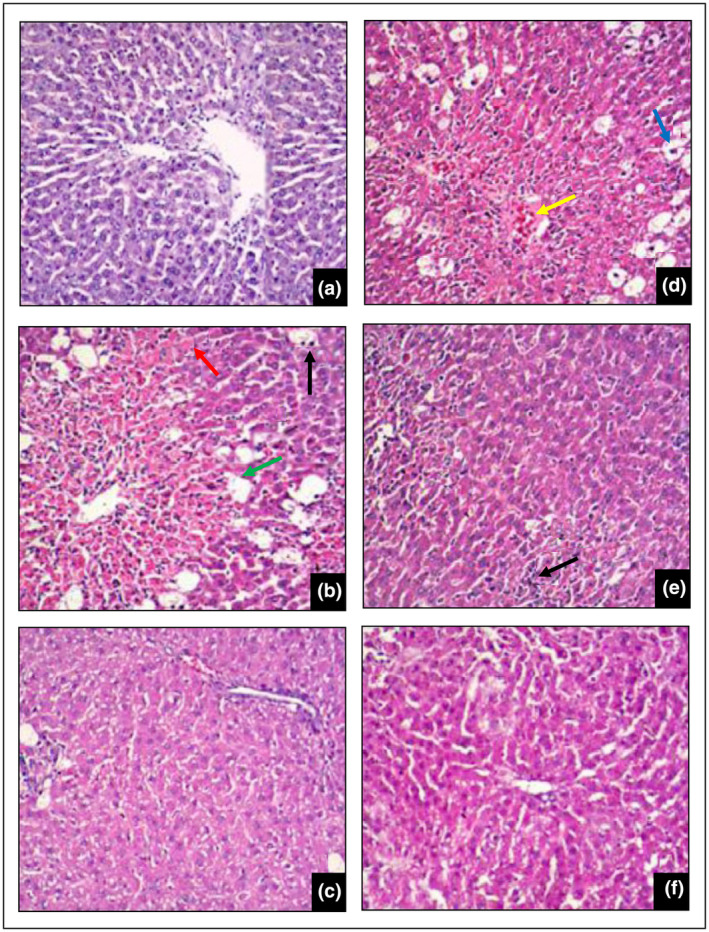
Effect of J. regia bark ethanolic extract on rats’ liver histology following CCl4‐induced hepatic damage. (a) Normal group; (b) Toxic group (CCl4 only); (c) CCl4 + 100 mg per kg silymarin; (d) CCl4 + 125 mg per kg J. regia extract; (e) CCl4 + 250 mg per kg J. regia extract; and (f) CCl4 + 500 mg per kg J. regia extract. (×100), n = 6. (green arrow line): degeneration of ballooning significantly; (blue arrow line): fatty alterations; (red arrow line): necrosis of hepatic cells; (black arrow line): infiltration of inflammatory cells; (yellow arrow line): conspicuous congestion.
3.2.3. Impact of the ethanolic extract of the bark of J. regia on the serum level of liver biomarker
The results of oral administration of extracts from J. regia bark on the different liver biomarker levels in the serum of rats with the injured liver are depicted in Table 3 and Figure 6. The extract of J. regia at 250 and 500 mg/kg diminished the serum levels of ALT, ALP, DBI, and TBI of rats with hepatic damage highly significantly (p < .001). Similarly, J. regia extract (250 and 500 mg/kg) increased the serum albumin levels of rats with significant liver injury (p < .001) compared to the toxic control group. The extract of J. regia 125 mg per kg also lowered the serum levels of ALT, ALP, TBI and increased the serum albumin levels of rats with the liver injury with significant variation (p < .005). Dosage of 100 mg per kg silymarin as the standard also had highly significant (p < .001) differences from the toxic control group for reducing the serum ALT, ALP, direct bilirubin (DBI), and total bilirubin (TBI) levels and increasing the serum albumin levels.
TABLE 3.
Effect of the J. regia extract on serum biochemical parameters for the liver injury tests.
| Parameters | Experimental group | |||||
|---|---|---|---|---|---|---|
| Normal control (10 mL/kg DW) | Toxic control (10 mL/kg DW) | Standard test (Silymarin 100 mg/kg) | J. regia extract (500 mg/kg) | J.regia extract (250 mg/kg) | J. regia extract (125 mg/kg) | |
| AST | 131.02 ± 2.75 | 372.45 ± 3.74 | 196.72 ± 2.09 (72.78) | 223.52 ± 3.48 (61.68) | 269.96 ± 3.51 (42.45) | 320.75 ± 5.31 (21.41) |
| ALT | 27.4 ± 1.13 | 115.87 ± 9.25 b | 33.58 ± 1.13 d (93.01) | 51.19 ± 0.94 d (73.1) | 66.99 ± 1.71 d (55.25) | 93.99 ± 1.07 c (17.94) |
| ALP | 134.01 ± 1.48 | 344.98 ± 15.90 b | 176.79 ± 2.88 d (79.72) | 226.90 ± 2.66 d (55.97) | 251.73 ± 1.70 d (44.2) | 281.15 ± 0.93 d (30.25) |
| TP | 8.51 ± 0.07 | 7.25 ± 0.07 | 8.18 ± 0.01 (73.8) | 8.06 ± 0.02 (64.28) | 8.01 ± 0.01 (60.31) | 7.76 ± 0.04 (40.47) |
| DBI | 0.21 ± 0.02 | 0.96 ± 0.003 b | 0.63 ± 0.01 d (44) | 0.74 ± 0.01 d (29.34) | 0.86 ± 0.001 d (13.34) | 0.91 ± 0.08 (6.67) |
| TBI | 0.38 ± 0.007 | 0.80 ± 0.03 b | 0.46 ± 0.003 d (80.95) | 0.54 ± 0.01 d (61.9) | 0.59 ± 0.01 d (50) | 0.65 ± 0.01 d (35.71) |
| Albumin | 3.84 ± 0.14 | 0.46 ± 0.003 a | 3.42 ± 0.20 d (87.57) | 3.14 ± 0.07 d (79.28) | 2.89 ± 0.02 d (71.89) | 2.69 ± 0.03 c (65.97) |
Note: The results represent the mean and standard error of mean (SEM) of six rats. Numbers in parentheses indicate % hepatoprotection.
Abbreviation: DW, distilled water.
p < .05 substantially different from the normal group (given DW only).
p < .001 considerable variation with the normal group (given DW only).
p < .05 substantially different from the toxic group (group was given DW and CCl4).
p < .001 considerably different from the toxic group (group was given DW and CCl4).
FIGURE 6.
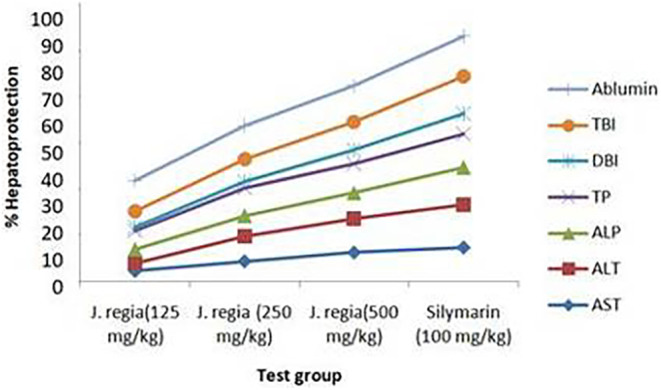
Percentage of hepatoprotection of J. regia extract at different doses.
The liver biomarker levels in the serum, such as ALT, ALP, DBI, and TBI, were substantially different (p < .001) in the toxic control group in contrast with the normal group. In contrast to the normal group, the albumin levels in the toxic group were substantially different (p < .05). J. regia extract showed high percentage of hepatoprotection against CCl4‐induced elevated levels of AST, ALT, ALP, TBI, and DBI, and decreased TP and serum albumin level in liver as a dose‐dependent manner (high at 500 mg/kg), as shown in both Table 3 and Figure 6.
Carbon tetrachloride (CCl4)‐induced hepatotoxicity elevates the levels to the liver enzymes, namely, total bilirubin, alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase, which are released in the blood during liver injury. The disruption of polyribosomes on the endoplasmic reticulum, which lowers protein production, also results in decreased levels of total protein and the serum albumin (Alqasoumi & Abdel‐Kader, 2012). In our research, treatment with the J. regia bark extract at doses (500, 250, and 125 mg/kg) resulted in a significant reduction of the serum enzymes such as ALT, ALP, DBI, and TBI induced by the CCl4. Despite this, J. regia bark extract also helped to increase the serum albumin and protein levels caused by CCl4.
The liver blood test of rats treated with CCl4 showed that the treatment with ethanolic extract of J. regia bark suppressed the serum ALT and ALP enzyme levels, validating the plasma membrane stability and healing of the CCl4‐induced hepatocyte damage. This is consistent with earlier research conducted on other plant extracts (Gutiérrez & Solís, 2009; Zahira et al., 2023). Similarly, the decrease in the serum levels of DBI and TBI by J. regia bark extract in the CCl4‐treated group is an indication of stabilization of biliary dysfunction and improvement of metabolic function of the liver. The extract reversed the unfavorable effects of CCl4 on total protein and the serum albumin, indicating an increase in the functional state of the hepatic cell to generate proteins. These results were concurring with the previous studies (Shrivastava & Bhambar, 2017).
3.2.4. Histopathological studies
Figure 5 depicts the histological characteristics of the rat liver from the normal control, the toxic control, positive control, and experimental groups. Histological observation of Figure 5d–f further supports the ability of the extract of J. regia to restore the cellular integrity in a dose‐dependent manner, hence confirming its hepatoprotective effect. With significant fatty alterations, ballooning degeneration, necrosis, and inflammatory cell attack, the toxic control group (CCl4 alone) demonstrated a clear breakdown of the hepatic cell architecture. The dose of 125 mg/kg of the extract with CCl4 provided less hepatoprotection with fatty alterations and noticeable congestion. However, the dose of 250 mg/kg of the extract with CCl4 showed less liver damage with noticeable hepatic inflammation. Administration of J. regia ethanolic extract at a dose of 500 mg/kg showed a marked curative effect in damaged hepatic cell architecture by CCl4.
The liver histology from normal control‐treated groups showed a normal hepatic lobule, hepatocyte cell structure, and hepatic architecture, while the toxic control‐treated group exhibited severe damage to the liver section, cellular necrosis, fatty changes, ballooning degeneration, fibrosis, and the liver inflammation in some areas. This finding was concurring with that of a previous study (Fahmy, 2016). The damage of hepatic cell architecture induced by CCl4 was prevented by J. regia extract in a dose‐dependent manner, which is comparable to that of the silymarin‐treated group.
4. CONCLUSION
The extract from J. regia bark exhibited strong antioxidant activity by effectively scavenging DPPH radicals, which can be attributed to its high levels of flavonoids and phenolic content. The hepatoprotective effects of J. regia extracts were demonstrated by a significant reduction in ALT, ALP, DBI, and TBI levels, as well as a significant increase in serum albumin levels compared to the toxic control group. These findings were further supported by the liver histopathological study. Our study provides compelling evidence that administering a 500 mg/kg dose of ethanolic extract from the bark of J. regia can effectively reduce liver damage in rats with CCl4‐induced liver toxicity. This shows that the extract has potential as a hepatoprotective agent. Furthermore, the results of molecular docking support the hepatoprotective effect of J. regia by inhibiting the CYP450 2E1 enzyme. This inhibition is attributed to the high affinity of Beta‐sitosterol (−9.2 kcal/mol) and Betulinic acid (−9.1 kcal/mol) against CYP450 2E1. The study suggests that conducting a preclinical investigation on J. regia is strongly advised in order to produce a powerful hepatoprotective drug from this plant. Subsequent studies can evaluate the influence of preventive measures on other organs.
AUTHOR CONTRIBUTIONS
Bipindra Pandey: Conceptualization (lead); data curation (lead); formal analysis (lead); investigation (lead); methodology (lead); resources (lead); software (equal); visualization (lead); writing – original draft (lead). Shankar Thapa: Formal analysis (equal); software (equal); visualization (equal). Atisammodavardhana Kaundinnyayana: Investigation (equal); resources (equal); supervision (supporting); validation (equal); writing – review and editing (equal). Sushil Panta: Investigation (lead); methodology (equal); project administration (lead); resources (lead); supervision (lead); validation (equal); writing – review and editing (supporting).
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare for this study.
ETHICAL CONSIDERATION
All experimental animals were handled according to the widely accepted Guide for the Care and Use of Laboratory Animals for handling and using animals released by the National Institutes of Health (NIH, 2011). Prior to starting the animal experiments, Institutional Review Committee approval (Ref. No. 7‐077‐078) was obtained from Pokhara University, Pokhara, Nepal.
PRESENTATION AT A MEETING
Presented at Digital NAFLD Summit 2021 organized by the European Association for the Study of the Liver (EASL), Home of Hepatology, Official registration agency Congrex, Switzerland, September, 2021.
Supporting information
Data S1.
ACKNOWLEDGMENTS
The authors express gratitude to Pokhara University for providing a postgraduate full scholarship and research facilities.
Pandey, B. , Thapa, S. , Kaundinnyayana, A. , & Panta, S. (2024). Hepatoprotective effects of Juglans regia on carbon tetrachloride‐induced hepatotoxicity: In silico/in vivo approach. Food Science & Nutrition, 12, 6482–6497. 10.1002/fsn3.4288
DATA AVAILABILITY STATEMENT
All data and materials for this study will be provided upon request to the corresponding author. Supplementary file link.
REFERENCES
- Alghazeer, R. , Elgahmasi, S. , Elnfati, A. H. , Elhensheri, M. , Al‐Griw, M. A. , Awayn, N. , & El‐Nami, M. (2018). Antioxidant activity and hepatoprotective potential of flavonoids from Arbutus pavarii against CCl4 induced hepatic damage. Biotechnology Journal International, 21, 1–12. [Google Scholar]
- Al‐Nadaf, A. , Hussein, R. , & Awadallah, A. (2024). Juglans regia L. fruit pellicle extract‐based bioreduction of silver nanoparticles: Structural features and in vivo therapeutic effects against ethanol‐induced peptic ulcers. Journal of Pharmacy & Pharmacognosy Research, 12(2), 193–203. [Google Scholar]
- Alqasoumi, S. I. , & Abdel‐Kader, M. S. (2012). Screening of some traditionally used plants for their hepatoprotective effect. INTECH Open Access Publisher. [Google Scholar]
- Al‐Rawi, R. , Bashir, Y. , Mustafa, A. , Omar, M. , Al‐Rawi, N. , Saeed, M. , Uthman, A. , & Al‐Rawi, N. H. (2021). Teeth whitening and antibacterial effects of Juglans regia bark: A preliminary study. International Journal of Dentistry, 2021, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin, K. A. , & Nagy, M. A. (2009). Effect of Carnitine and herbal mixture extract on obesity induced by high fat diet in rats. Diabetology and Metabolic Syndrome, 1(1), 17. 10.1186/1758-5996-1-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad, M. , Kousar, S. , Din, A. , Afzaal, M. , Faisal, M. N. , Sharif, M. K. , Rasheed, H. , Saeed, F. , Akram, N. , Ahmed, F. , & Khan, M. R. (2024). Hepatoprotective efficacy of quinoa seed extract against CCl4‐induced acute liver toxicity in rat model. Food Science & Nutrition, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asha, K. , Sapana, S. , Sucheta, G. , Kavita, M. , Nirmala, D. , & Jyoti, S. (2010). Elements from stem bark of orchard tree‐Juglans regia . International Journal of ChemTech Research, 2(1), 548–550. [Google Scholar]
- Aydın, S. , Gökçe, Z. , & Yılmaz, Ö. (2015). The effects of Juglans regia L. (walnut) extract on certain biochemical paramaters and in the prevention of tissue damage in brain, kidney, and liver in CCl4 applied Wistar rats. Turkish Journal of Biochemistry, 40(3), 241–250. [Google Scholar]
- Bhatnagar, S. , Sahoo, S. , Mohapatra, A. K. , & Bahera, D. R. (2012). Phytochemical analysis, antioxidant and cytotoxic activity of medicinal plant Combretum roxburghi (Family: Combretaceae). International Journal of Drug Development & Research, 4(2), 193–202. [Google Scholar]
- Bravo, L. (1998). Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutrition Reviews, 56(11), 317–333. [DOI] [PubMed] [Google Scholar]
- Chen, D. , Oezguen, N. , Urvil, P. , Ferguson, C. , Dann, S. M. , & Savidge, T. C. (2016). Regulation of protein‐ligand binding affinity by hydrogen bond pairing. Science Advances, 2(3), e1501240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, E. B. , Patwardhan, M. , Raheja, R. , Alpers, D. H. , Andrade, R. J. , Avigan, M. I. , Lewis, J. H. , Rockey, D. C. , Chui, F. , Iacob, A. M. , Linardi, C. C. , Regev, A. , Shick, J. , & Isabel Lucena, M. (2024). Drug‐induced liver injury in the elderly: Consensus statements and recommendations from the IQ‐DILI initiative. Drug Safety, 47(4), 301–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawada, S. , Zade, V. , Dabhadkar, D. , & Pare, S. (2012). Hepatoprotective activity of alcoholic leaf extract of Alianthus exelssa root against carbon tetrachloride toxicity induced in albino rat Rattus rattus (Wistar). International Journal of PharmTech Research, 4(2), 609–615. [Google Scholar]
- De, S. , Suresh, R. , Babu, A. M. S. S. , & Aneela, S. (2017). In‐vivo hepatoprotective activity of methanolic extracts of Sphaeranthus amaranthoides and Oldenlandia umbellate . Pharmacognosy Journal, 9(1), 98–101. [Google Scholar]
- Devarbhavi, H. , Asrani, S. K. , Arab, J. P. , Nartey, Y. A. , Pose, E. , & Kamath, P. S. (2023). Global burden of liver disease: 2023 update. Journal of Hepatology, 79, 516–537. [DOI] [PubMed] [Google Scholar]
- DeVore, N. M. , Meneely, K. M. , Bart, A. G. , Stephens, E. S. , Battaile, K. P. , & Scott, E. E. (2012). Structural comparison of cytochromes P450 2A6, 2A13, and 2E1 with pilocarpine. The FEBS Journal, 279(9), 1621–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doss, A. (2009). Preliminary phytochemical screening of some Indian medicinal plants. Ancient Science of Life, 29(2), 12–16. [PMC free article] [PubMed] [Google Scholar]
- El‐Beltagi, H. S. , Rageb, M. , El‐Saber, M. M. , El‐Masry, R. A. , Ramadan, K. M. , Kandeel, M. , Alhajri, A. S. , & Osman, A. (2024). Green synthesis, characterization, and hepatoprotective effect of zinc oxide nanoparticles from Moringa oleifera leaves in CCl4‐treated albino rats. Heliyon, 10(9), e30627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy, N. (2016). Protective effect of Terminalia muelleri against carbon tetrachloride‐induced hepato and nephro‐toxicity in mice and characterization of its bioactive constituents. Pharmaceutical Biology, 54(2), 303–313. [DOI] [PubMed] [Google Scholar]
- Flecknell, P. (2015). Laboratory animal anaesthesia. Academic press. [Google Scholar]
- Gote, S. , Thapa, S. , Dubey, S. , Nargund, S. L. , & Biradar, M. S. (2023). Computational investigation of quinazoline derivatives as Keap1 inhibitors for Alzheimer's disease. Informatics in Medicine Unlocked, 41, 101334. [Google Scholar]
- Gutiérrez, R. , & Solís, R. (2009). Hepatoprotective and inhibition of oxidative stress in liver of Prostechea michuacana. Records of Natural Products, 3(1), 46. [Google Scholar]
- Hancock, J. (2007). Pilocarpine. In xPharm: The comprehensive pharmacology reference, (pp. 1–7). Elsevier. [Google Scholar]
- Huang, W. , Wang, Y. , Jiang, X. , Sun, Y. , Zhao, Z. , & Li, S. (2017). Protective effect of flavonoids from Ziziphus jujuba cv. Jinsixiaozao against acetaminophen‐induced liver injury by inhibiting oxidative stress and inflammation in mice. Molecules, 22(10), 1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabli, M. , Sebeia, N. , Boulares, M. , & Faidi, K. (2017). Chemical analysis of the characteristics of Tunisian Juglans regia L. fractions: Antibacterial potential, gas chromatography–mass spectroscopy and a full investigation of their dyeing properties. Industrial Crops and Products, 108, 690–699. [Google Scholar]
- Kale, A. , Gadkari, T. , Devare, S. , Deshpande, N. , & Salvekar, J. (2012). GC‐MS study of stem bark extract of Juglans regia L. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 3(1), 740–743. [Google Scholar]
- Khan, R. A. , Khan, M. R. , & Sahreen, S. (2012). CCl4‐induced hepatotoxicity: Protective effect of rutin on p53, CYP2E1 and the antioxidative status in rat. BMC Complementary and Alternative Medicine, 12(1), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourkoumpetis, T. , & Sood, G. (2019). Pathogenesis of alcoholic liver disease: An update [Review]. Clinics in Liver Disease, 23(1), 71–80. 10.1016/j.cld.2018.09.006 [DOI] [PubMed] [Google Scholar]
- Lamichhane, S. , Hussain, A. , Paudel, M. , & Jha, R. (2016). Phytochemical, antimicrobial and GC‐MS analysis of Junglans regia Linn. Chemical Science Review and Letters, 5(20), 147–156. [Google Scholar]
- Lampertico, P. , Agarwal, K. , Berg, T. , Buti, M. , Janssen, H. L. , Papatheodoridis, G. , Zoulim, F. , & Tacke, F. (2017). EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. Journal of Hepatology, 67(2), 370–398. [DOI] [PubMed] [Google Scholar]
- Lee, W. M. (2003). Drug‐induced hepatotoxicity. New England Journal of Medicine, 349(5), 474–485. [DOI] [PubMed] [Google Scholar]
- Mahmood, N. , Mamat, S. , & Kamisan, F. (2014). Amelioration of paracetamol‐induced hepatotoxicity in rat by the administration of methanol extract of Muntingia calabura L. leaves. BioMed Research International, 1–10. 10.1155/2014/695678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqbool, M. , Rasool, S. , Dar, M. A. , Bashir, R. , & Khan, M. (2019). Hepatotoxicity and hepatoprotective agents: A mini review. PharmaTutor, 7(9), 34–40. [Google Scholar]
- Mensor, L. L. , Menezes, F. S. , Leitao, G. G. , Reis, A. S. , dos Santos, T. C. , Coube, C. S. , & Leitao, S. G. (2001). Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method [Research support, non‐U.S. Gov't]. Phytotherapy Research, 15(2), 127–130. [DOI] [PubMed] [Google Scholar]
- Miranda, E. G. d. , Nascimento, V. P. d. , Waisberg, D. R. , Sousa, M. W. G. d. , Lima, M. F. M. B. , Silva, D. d. S. , & Waisberg, J. (2011). Inhalation anesthesia equipment for rats with provision of simultaneous anesthetic and oxygen. Acta Cirúrgica Brasileira, 26, 140–143. [DOI] [PubMed] [Google Scholar]
- Montoro, P. , Braca, A. , Pizza, C. , & De Tommasi, N. (2005). Structure–antioxidant activity relationships of flavonoids isolated from different plant species. Food Chemistry, 92(2), 349–355. [Google Scholar]
- Mulla, W. A. , Salunkhe, V. R. , & Bhise, S. B. (2009). Hepatoprotective activity of hydroalcoholic extract of leaves of Alocasia indica (Linn.). [PubMed]
- Muriel, P. , & Rivera‐Espinoza, Y. (2008). Beneficial drugs for liver diseases. Journal of Applied Toxicology: An International Journal, 28(2), 93–103. [DOI] [PubMed] [Google Scholar]
- National Research Council . (2010). Division on Earth, Life Studies, Institute for Laboratory Animal Research, Committee for the Update of the Guide for the Care, & Use of Laboratory Animals, Eight Edition (pp. 27–246). National Academic Press. [Google Scholar]
- Ncube, N. , Afolayan, A. , & Okoh, A. (2008). Assessment techniques of antimicrobial properties of natural compounds of plant origin: Current methods and future trends. African Journal of Biotechnology, 7(12), 1797–1806. [Google Scholar]
- NirmlaDevi, T. , Apraj, V. , Bhagwat, A. , Mallya, R. , Sawant, L. , & Pandita, N. (2011). Pharmacognostic and phytochemical investigation of Juglans regia Linn. bark. Pharmacognosy Journal, 3(25), 39–43. [Google Scholar]
- O'Boyle, N. M. , Banck, M. , James, C. A. , Morley, C. , Vandermeersch, T. , & Hutchison, G. R. (2011). Open Babel: An open chemical toolbox. Journal of Cheminformatics, 3(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD . (1996). OECD Guidelines for the Testing of Chemicals No. 423: Acute oral toxicity—Acute toxic class method. OECD. [Google Scholar]
- Omotayo, M. A. , Ogundare, O. C. , Oluwafunmilayo, A. , & Longe, S. A. (2015). Hepatoprotective effect of Mangifera‐indica stem bark extracts on paracetamol‐induced oxidative stress in albino rats. European Scientific Journal, 11(24), 299–309. [Google Scholar]
- Pandey, B. , Baral, R. , Kaundinnyayana, A. , & Panta, S. (2023). Promising hepatoprotective agents from the natural sources: A study of scientific evidence. Egyptian Liver Journal, 13(1), 14. [Google Scholar]
- Pandey, K. H. , Khadka, P. , Thapa, S. K. , Baral, P. , Pandit, A. , & Panta, S. (2013). Analysis of anthelmintic activity of Crateva unilocularis Buch.‐Ham. leaf. World Journal of Pharmacy and Pharmaceutical Sciences, 2(6), 4443–4448. [Google Scholar]
- Pearse, A. (1968). Histochemistry (Vol. 1, 3rd ed., pp. 20–100). Churchill Livingstone. [Google Scholar]
- Pritchard, M. T. , & Apte, U. (2015). Models to study liver regeneration. In Liver regeneration (pp. 15–40). Elsevier. [Google Scholar]
- Rao, M. (1997). Nitric oxide scavenging by curcuminoids. Journal of Pharmacy and Pharmacology, 49(1), 105–107. [DOI] [PubMed] [Google Scholar]
- Real, M. , Barnhill, M. S. , Higley, C. , Rosenberg, J. , & Lewis, J. H. (2019). Drug‐induced liver injury: Highlights of the recent literature. Drug Safety, 42, 365–387. [DOI] [PubMed] [Google Scholar]
- Ruch, R. J. , Cheng, S. , & Klaunig, J. E. (1989). Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis, 10, 1003–1008. [DOI] [PubMed] [Google Scholar]
- Sahreen, S. , Khan, M. R. , & Khan, R. A. (2011). Phenolic compounds and antioxidant activities of Rumex hastatus D. Don. Leaves. Journal of Medicinal Plants Research, 5(13), 2755–2765. [Google Scholar]
- Satué‐Gracia, M. T. , Heinonen, M. , & Frankel, E. N. (1997). Anthocyanins as antioxidants on human low‐density lipoprotein and lecithin−liposome systems. Journal of Agricultural and Food Chemistry, 45(9), 3362–3367. [Google Scholar]
- Shaban, N. Z. , Awad, O. M. , Fouad, G. M. , Hafez, A. M. , Abdul‐Aziz, A. A. , & El‐Kot, S. M. (2023). Prophylactic and curative effects of Carica papaya Linn. pulp extract against carbon tetrachloride‐induced hepatotoxicity in male rats. Environmental Science and Pollution Research, 30(10), 27815–27832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava, P. , & Bhambar, R. S. (2017). Hepatoprotective activity of Bridelia retusa leaves against paracetamol‐induced liver damage in Swiss albino mice. Drug Invention Today, 9(1), 9. [Google Scholar]
- Singleton, V. L. , & Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic‐phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16(3), 144–158. [Google Scholar]
- Sintayehu, B. , Bucar, F. , Veeresham, C. , & Asres, K. (2012). Hepatoprotective and free radical scavenging activities of extracts and a major compound isolated from the leaves of Cineraria abyssinica Sch. Bip. exA. Rich. Pharmacognosy Journal, 4(29), 40–46. [Google Scholar]
- Song, X. , Hou, K. , Zhou, H. , Yang, J. , Cao, T. , & Zhang, J. (2024). Liver organoids and their application in liver cancer research. Regenerative Therapy, 25, 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokina, M. , Merseburger, P. , Rajan, K. , Yirik, M. A. , & Steinbeck, C. (2021). COCONUT online: Collection of open natural products database. Journal of Cheminformatics, 13(1), 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souid, A. , Giambastiani, L. , Castagna, A. , Santin, M. , Vivarelli, F. , Canistro, D. , Morosini, C. , Paolini, M. , Franchi, P. , Lucarini, M. , Raffaelli, A. , Giorgetti, L. , Ranieri, A. , Longo, V. , Pozzo, L. , & Vornoli, A. (2024). Assessment of the antioxidant and hypolipidemic properties of Salicornia europaea for the prevention of TAFLD in rats. Antioxidants, 13(5), 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarahovsky, Y. S. , Kim, Y. A. , Yagolnik, E. A. , & Muzafarov, E. N. (2014). Flavonoid–membrane interactions: Involvement of flavonoid–metal complexes in raft signaling. Biochimica et Biophysica Acta‐Biomembranes, 1838(5), 1235–1246. [DOI] [PubMed] [Google Scholar]
- Thapa, S. , Biradar, M. S. , Banerjee, J. , & Karati, D. (2023). In‐silico approach for predicting the inhibitory effect of home remedies on severe acute respiratory syndrome Coronavirus‐2. Makara Journal of Science, 27(3), 5. [Google Scholar]
- Thapa, S. , Nargund, S. L. , & Biradar, M. S. (2023). Molecular design and in‐silico analysis of trisubstituted benzimidazole derivatives as Ftsz inhibitor. Journal of Chemistry, 2023, 9. [Google Scholar]
- Thapa, S. , Nargund, S. L. , Biradar, M. S. , Banerjee, J. , & Karati, D. (2023). In‐silico investigation and drug likeliness studies of benzimidazole congeners: The new face of innovation. Informatics in Medicine Unlocked, 38, 101213. [Google Scholar]
- Trifunschi, S. I. , Munteanu, M. F. F. , Ardelean, D. G. , Orodan, M. , Osser, G. M. , & Gligor, R. I. (2015). Flavonoids and polyphenols content and antioxidant activity of Ficus carica L. extracts from Romania. Zbornik Matice srpske za prirodne nauke, 128, 57–65. [Google Scholar]
- Trott, O. , & Olson, A. (2009). Software news and update AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function. Journal of Computational Chemistry, 31, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugwu, C. E. , & Suru, S. M. (2021). Medicinal plants with hepatoprotective potentials against carbon tetrachloride‐induced toxicity: A review. Egyptian Liver Journal, 11(1), 1–26. [Google Scholar]
- Vishnoi, N. K. (2000). Advanced practical organic chemistry. Vikash Publication House Private Limited. [Google Scholar]
- Vivek‐Ananth, R. , Mohanraj, K. , Sahoo, A. K. , & Samal, A. (2023). IMPPAT 2.0: An enhanced and expanded phytochemical atlas of Indian medicinal plants. ACS Omega, 8(9), 8827–8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, J. , Wang, S. , Huang, J. , Zhou, X. , Qian, Z. , Wu, T. , Fan, Q. , Liang, Y. , & Cui, G. (2024). Network medicine‐based analysis of the hepatoprotective effects of Amomum villosum Lour. on alcoholic liver disease in rats. Food Science & Nutrition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. , Ma, H. Y. , Liang, S. , Sun, M. , Karin, G. , Koyama, Y. , Hu, R. , Quehenberger, O. , Davidson, N. O. , Dennis, E. A. , Kisseleva, T. , & Brenner, D. A. (2017). The role of human cytochrome P450 2E1 in liver inflammation and fibrosis. Hepatology Communications, 1(10), 1043–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, M. , Huo, Y. , Yin, S. , & Hu, H. (2018). Mechanisms of acetaminophen‐induced liver injury and its implications for therapeutic interventions [Review]. Redox Biology, 17, 274–283. 10.1016/j.redox.2018.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka, H. , Usuda, H. , Fujii, H. , & Nonogaki, T. (2017). Sasa veitchii extracts suppress acetaminophen‐induced hepatotoxicity in mice. Environmental Health and Preventive Medicine, 22(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younossi, Z. M. , Wong, G. , Anstee, Q. M. , & Henry, L. (2023). The global burden of liver disease. Clinical Gastroenterology and Hepatology, 21, 1978–1991. [DOI] [PubMed] [Google Scholar]
- Zahira, A. , Sultana, S. , Rasul, A. , Sultana, T. , & Hassan, M. (2023). Hepatoprotective effects of almond shells against carbon tetrachloride induced liver injury in albino rats. Saudi Journal of Biological Sciences, 30(11), 103811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakavi, F. , Golpasand Hagh, L. , Daraeighadikolaei, A. , Farajzadeh Sheikh, A. , Daraeighadikolaei, A. , & Leilavi Shooshtari, Z. (2013). Antibacterial effect of Juglans regia bark against oral pathologic bacteria. International Journal of Dentistry, 2013, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhinshen, J. , Mengcheng, T. , & Jianming, W. (1999). The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry, 64, 555–559. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
All data and materials for this study will be provided upon request to the corresponding author. Supplementary file link.


