Abstract
Prior studies raise questions about whether persistent postconcussive symptoms (PCS) are differentiable from mental health sequelae of traumatic brain injury (TBI). To investigate whether PCS represented a distinct symptom domain, we evaluated the structure of post-concussive and psychological symptoms using data from The Army STARRS Pre/Post Deployment Study, a panel survey of three U.S. Army Brigade Combat Teams that deployed to Afghanistan. Data from 1229 participants who sustained probable TBI during deployment completed ratings of past-30-day post-concussive, posttraumatic stress, and depressive symptoms three months after their return. Exploratory factor analysis (EFA; n = 300) and confirmatory factor analysis (CFA; n = 929) of symptom ratings were performed in independent subsamples. EFA suggested a model with 3 correlated factors resembling PCS, posttraumatic stress, and depression. CFA confirmed adequate fit of the 3-factor model (CFI = .964, RMSEA = .073 [.070, .075]), contingent upon allowing theoretically defensible cross-loadings. Bifactor CFA indicated that variance in all symptoms was explained by a general factor (λ = .36–.93), but also provided evidence of domain factors defined by (a) reexperiencing/hyperarousal, (b) cognitive/somatic symptoms, and (c) depressed mood/anhedonia. Soldiers with more severe TBI had higher cognitive/somatic scores, whereas soldiers with more deployment stress had higher general and reexperiencing/hyperarousal scores. Thus, variance in PCS is attributable to both a specific cognitive/somatic symptom factor and a general factor that also explains variance in posttraumatic stress and depression. Measurement of specific domains representing cognitive/somatic symptoms, reexperiencing/hyperarousal, and depressed mood/anhedonia may help clarify the relative severity of PCS, posttraumatic stress, and depression among individuals with recent TBI.
Keywords: bifactor modeling, construct validity, postconcussive syndrome, posttraumatic stress disorder, TBI symptoms
There is considerable interest in the relationship between persistent postconcussive symptoms (PCS) and mental disorders in individuals who have sustained traumatic brain injury (TBI). TBI is considered a hallmark injury for service members returning from Iraq and Afghanistan because of the propensity of blast injuries occurring from improvised explosive devices (Stein & McAllister, 2009). Prevalence estimates of TBI among service members who have deployed in support of Operations Enduring Freedom, Iraqi Freedom, and New Dawn have ranged between 9% and 28%, with differences likely attributable to variation in sample characteristics and TBI assessment procedures (Lindquist, Love, & Elbogen, 2017; Schwab et al., 2017; Stein et al., 2015; Terrio et al., 2009).
Postconcussive Symptoms Following TBI
The symptoms collectively referred to as PCS include fatigue, sleep problems, headaches, dizziness/balance problems, light and noise sensitivity, difficulty concentrating, memory deficits, irritability, and other mood/behavior changes (American Psychiatric Association, 2000; Binder, 1986; Bryant & Harvey, 1999; McCrea, 2008; World Health Organization, 1992). These diverse symptoms are commonly organized into three general symptom domains: cognitive, somatic, and emotional (Herrmann et al., 2009; Laborey et al., 2014; Potter, Leigh, Wade, & Fleminger, 2006). Symptoms typically resolve within three months of injury (Alves, Macciocchi, & Barth, 1993); however, 6% to 15% of individuals who sustain TBI continue to experience PCS for a year or longer (Ryan & Warden, 2003; Stein & McAllister, 2009). Persistent PCS is associated with poor outcomes, including mental disorders, cognitive impairment, neurological deficiencies, and lower life satisfaction (Bryant & Harvey, 1999; Kirkham et al., 2016; Kleffelgaard et al., 2017; Stålnacke, Björnstig, Karlsson, & Sojka, 2005).
Relationships Among PCS and Mental Health Factors
Recent investigations have raised concerns about the specificity of PCS to TBI exposure and unclear boundaries of PCS with other conditions that may arise from the same event, such as posttraumatic stress disorder (PTSD; Hoge et al., 2008; Lagarde et al., 2014; Stein & McAllister, 2009). Symptoms of PTSD include (a) intrusive thoughts, nightmares, flashbacks, emotional distress and physical reactivity after exposure to traumatic reminders, (b) avoidance of trauma-related stimuli, (c) negative thoughts or feelings that have significantly worsened as a result of trauma exposure, and (d) trauma-related arousal and reactivity such as irritability, aggression, risky behavior, hypervigilance, heightened startle reactions, difficulty concentrating, and trouble sleeping (American Psychiatric Association, 2013). Several PTSD symptoms (i.e., difficulty concentrating, sleep problems, fatigue, irritability) directly overlap with PCS (Stein & McAllister, 2009).
Among military service members returning from Iraq and Afghanistan, the prevalence of PTSD has been reported upward of 16%, which is likely an underestimate given barriers to diagnosis and treatment (Sareen et al., 2007). Many of these PTSD diagnoses co-occur with TBI; for example, a study by Hoge et al. (2008) found that among U.S. Army soldiers returning from Iraq, 44% of those who reported mild TBI with loss of consciousness also met criteria for PTSD. Moreover, a prospective study found that deployment-acquired TBI was associated with increased risk of developing PTSD following return from deployment (Stein et al., 2015).
Most investigations have conceptualized PTSD and persistent PCS as distinct but associated phenomena; however, some raise questions about their discriminant validity. For instance, among military service members, the relationship between mild TBI and PCS was nonsignificant after controlling for symptoms of PTSD and depression (Belanger, Kretzmer, Vanderploeg, & French, 2010; Hoge et al., 2008; Schneiderman, Braver, & Kang, 2008). In a study of emergency department patients with TBI and extracranial injuries, PCS was common in both subgroups (Lagarde et al., 2014). TBI was a significant predictor of PTSD symptoms—but not PCS—at 3 months postinjury. Additionally, a correspondence analysis showed similar behavior of PCS and the hyperarousal symptoms of PTSD, suggesting that PCS and PTSD items did not measure dissociable symptom domains (Lagarde et al., 2014).
PCS also co-occurs with other mental health problems, such as depression. Shared characteristics of PCS and major depressive episodes include sleep problems, fatigue, and difficulty concentrating; all of which are commonly reported by soldiers following deployment (Wilk, Herrell, Wynn, Riviere, & Hoge, 2012). Evidence suggests that soldiers who sustain deployment-acquired TBI are at increased risk of postdeployment major depressive episode (Stein et al., 2015) and that predeployment psychological distress is a risk factor for postdeployment PCS (Stein et al., 2016). Moreover, depressive symptoms are strongly associated with PCS among patients with TBI (Herrmann et al., 2009).
Understanding the source(s) of symptoms following TBI is complicated not only by the overlap between PCS and mental health problems, but also by high comorbidity of mental disorders such as PTSD and depression. Many studies have investigated relationships among the symptoms of PTSD and depressive disorders in trauma-exposed individuals, with most indicating that PTSD and depression are distinct but related domains (e.g., Blanchard, Buckley, Hickling, & Taylor, 1998; Gros, Simms, & Acierno, 2010; Post, Feeny, Zoellner, & Connell, 2016). However, some provide evidence of a “general stress reaction” that encompasses both PTSD and depressive symptoms (Au, Dickstein, Comer, Salters-Pedneault, & Litz, 2013; Dekel, Solomon, Horesh, & Ein-Dor, 2014; Elhai et al., 2011; see also Watson, 2005), indicating that the boundary between PTSD and depression may be indistinct among trauma-exposed individuals.
Although a few prior studies have examined relationships among PCS, PTSD, and depression (Morissette et al., 2011; Segev et al., 2016), we are unaware of any that investigated the latent structure of postconcussive, posttraumatic stress, and depressive symptoms in the wake of TBI. Another issue that has yet to be considered is the potential contribution of broader traits (e.g., general distress/negative affectivity; Goldberg, Krueger, Andrews, & Hobbs, 2009; Thomas, 2012; Watson, 2005) to variance in both PCS and symptoms of mental disorders.
The Current Study
Both the complexity of events surrounding TBI (i.e., insult to the brain that often occurs in conjunction with stress exposure) and the definitional overlap among common TBI sequalae (e.g., PCS, PTSD, depression) pose challenges for clinicians who aim to clarify causes of persistent symptoms and relative contributions of different symptom domains to the patient’s dysfunction. Questions about the validity of PCS, particularly its distinctiveness from mental disorders, further complicate diagnosis and treatment planning. An analysis of the structure of symptoms following TBI may clarify whether PCS comprises a symptom domain that is distinct from posttraumatic stress and depression, and elucidate how to best measure severity of specific symptom domains among individuals who present with more than one (or all) of these problems.
With latent variable analysis, measurement models can be tested to evaluate whether patterns of relationships among observed variables are consistent with a hypothesized structure (e.g., distinct latent dimensions representing PCS, posttraumatic stress, and depression), and how individual differences in one latent dimension relate to individual differences in other latent dimensions (e.g., Strauss & Smith, 2009). Subsequently, factor scores can be examined in relation to external correlates, to determine whether expected associations are present. Where symptom dimensions are strongly associated, higher-order and bifactor analyses can be used to assess whether superordinate factors contribute to relationships among individual symptoms and lower-order/domain factors (e.g., Thomas, 2012).
In the current study, exploratory factor analysis (EFA) and confirmatory factor analysis (CFA) were performed to assess whether indicators of PCS defined a distinct latent dimension, and whether measurement models that distinguished PCS from posttraumatic stress and depression provided good fit for the symptom-level data. Bifactor CFA models were subsequently fit to examine the extent to which variance in individual symptoms was explained by a general factor versus domain factors reflecting specific symptom dimensions (e.g., PCS, posttraumatic stress, and/or depression). Lastly, we derived factor scores from the final models and tested for significant differences by TBI severity (very mild vs. mild vs. more-than-mild) and deployment stress exposure (high vs. low). We expected that soldiers with high deployment stress exposure would score higher on factors representing posttraumatic stress and other predominantly affective dimensions, whereas soldiers with greater TBI severity would score higher on factor(s) defined by cognitive and somatic symptoms that are prominent in PCS.
Method
Sample and Data Source(s)
Details regarding the design and conduct of the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS) are available elsewhere (Kessler, Colpe, et al., 2013; Ursano et al., 2014). The Pre/Post Deployment Study (PPDS) population consisted of soldiers in three Brigade Combat Teams (BCTs), whose members deployed to Afghanistan 1–2 months after administration of the baseline PPDS survey. The average length of deployment for the BCTs was approximately 10 months. Participants gave their informed, written consent to participate in the PPDS baseline and follow-up surveys. All procedures were approved by the Human Subjects Committees of all collaborating organizations.
Baseline (T0) PPDS respondents completed a computerized, self-administered questionnaire (SAQ) that collected information about sociodemographic characteristics, health (including TBI history), lifetime mental disorders, and various risk and resilience factors. Follow-up SAQs were administered approximately 1 month (T1), 3 months (T2), and 9 months (T3) after the BCTs returned from deployment. The T1 SAQ was a brief assessment of experiences that occurred during deployment (e.g., deployment stressors, TBI). The T2 and T3 SAQs were more extensive, assessing recent experiences, 30-day mental disorders, and 30-day somatic and cognitive symptoms including those that characterize PCS.
A total of 9,949 soldiers were present for duty in the three BCTs during baseline (T0). Among the soldiers present, 8,558 (86.0%) completed the T0 survey and provided consent to link their survey responses to their Army/Department of Defense administrative records. Of those, 7,742 subsequently deployed to Afghanistan. Because this study focused on sequelae of recent TBI, the analysis was conducted using data from respondents who reported deployment-acquired TBI at T1 (N = 1,310) and completed key sections of the survey at T2 (i.e., those assessing PCS, posttraumatic stress, and depression), resulting in a final sample N = 1,229. The 81 TBI-exposed soldiers who could not be included in the analysis because of absence of T2 data did not differ from those included in terms of age, sex, race, ethnicity, education, marital status, or history of TBI prior to the index deployment (ps > .10).
Measures
TBI.
Probable deployment-acquired TBI was assessed via self-report in the T1 survey using questions developed specifically for Army STARRS. An initial probe, How many times during your recent deployment did you have a head, neck, or blast injury that. . . , was followed by questions about alteration or loss of consciousness (LOC) and lapse of memory that occurred. Following prior PPDS studies (Stein et al., 2015, 2016), we characterized each respondent as having had: (a) No TBI (excluded from the analysis); (b) probable “very mild” TBI (alteration but no LOC [“didn’t knock you out but caused you to be dazed or see stars”] and no lapse of memory); (c) probable “mild TBI” (LOC [“knocked you out”] for less than 30 min and/or lapse of memory for less than 30 min); or (d) probable “more-than-mild TBI” (LOC for 30 min or more or lapse in memory lasting 30 min or more). Using these criteria, TBIs classified as very mild or mild would match up with most standard and widely used definitions of mild TBI (e.g., American Congress of Rehabilitation Medicine, 1993). The majority of TBIs classified as more-than-mild would fall into a higher category (i.e., moderate or severe) of clinical severity.
The T0 survey included analogous assessment of lifetime TBIs that occurred prior to the index deployment. To increase confidence in the accuracy of reports of these injuries (many of which were from childhood/adolescence), we focused solely on predeployment TBIs for which the respondent endorsed LOC. Frequency of any lifetime TBI with LOC (of any duration) and number of lifetime TBIs with LOC (0, 1, or 2 or more) are included in the sample descriptive statistics.
Deployment stress severity.
The T1 survey used items adapted from the Deployment Risk and Resilience Inventory (King, King, Vogt, Knight, & Samper, 2006; Vogt, Proctor, King, King, & Vasterling, 2008) and the Joint Mental Health Advisory Team 7 survey (Joint Mental Health Advisory Team 7, 2011) to assess the frequency of stressful deployment experiences (e.g., “During your deployment, how many times did you . . . fire rounds at the enemy or take enemy fire? . . . have members of your unit who were seriously wounded or killed?”). Responses to these items were discretized and summed to create a deployment stress score (theoretical range = 0 to 16; for details, see Campbell-Sills et al., 2018). Based on preliminary analysis of the functional form of the association of total deployment stress scores with a clinically salient criterion (incidence of PTSD or MDE at T2), scores <6 were considered low-to-moderate deployment stress and scores >6 were considered high deployment stress. The clinically significant distinction between low-to-moderate deployment stress and high deployment stress was supported by Anderson et al.’s (2019) finding that, among soldiers with no lifetime history of PTSD prior to the index deployment, high deployment stress was prospectively associated with more than triple the odds of past-month PTSD at 3 or 9 months postdeployment (AOR = 3.52, 95% CI [2.94 –4.21]), relative to low-to-moderate deployment stress.
Postdeployment PCS.
Past 30-day somatic and cognitive symptoms were assessed in a T2 survey section that inquired generally about health problems without any reference to TBI or other injuries or events. Included in the current analysis were 10 items reflecting an array of symptoms that may follow TBI: balance problems or dizziness; sensitivity to noise; sensitivity to light; memory problems; irritability; difficulty concentrating; headaches; feeling tired out or being easily fatigued; tinnitus; and sleep problems. Respondents rated each symptom on a 5-point frequency scale that ranged from None of the time (0) through All or almost all of the time (4).
A previous report described derivation of a postconcussive symptoms scale (PCS-8; Stein et al., 2016) that included eight of the 10 items considered in our analysis. Criterion validity of the scale was supported by findings that PPDS respondents with deployment-acquired TBI scored substantially higher on the PCS-8 than soldiers without deployment-acquired TBI, and soldiers with mild TBI scored higher than those with very mild TBI (soldiers with more-than-mild TBI were excluded from the study; Stein et al., 2016). The PCS-8 also demonstrated good internal consistency (Cronbach’s alpha = .88). Sleep problems were not included in the PCS-8 because of a concern that these were less specific to PCS and could indicate other conditions that are common after deployment (PTSD, depression, primary insomnia). However, because our study directly evaluates overlap among PCS and other conditions, sleep disturbance was included as an indicator of PCS. We also included an item assessing tinnitus, given evidence that this somatic symptom also may follow TBI (e.g., Yurgil et al., 2016). The internal consistency of the expanded PCS scale used here was good (Cronbach’s alpha = .89).
Postdeployment posttraumatic stress symptoms.
The Stressful Experiences section of the PPDS T2 survey included a 30-day Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM–IV) and DSM–5 PTSD symptom assessment based on items from the civilian PTSD Checklist for DSM–IV (PCL-C; Weathers, Litz, Herman, Huska, & Keane, 1993) and the PTSD Checklist for DSM–5 (PCL-5; Weathers et al., 2013). Each item was rated on a scale from 0 (not at all) to4 (extremely). A detailed explanation of PPDS survey assessment of posttraumatic stress symptoms can be found in Rosellini et al. (2015) and the full survey section (along with other survey sections that contained items used in the current analysis) can be viewed at https://starrsls.org/. Here we considered any T2 survey item that corresponded to the DSM–5 criteria for PTSD; these were represented by a combination of PCL-C and PCL-5 items.
Postdeployment depressive symptoms.
T2 respondents completed an adapted version of the Composite International Diagnostic Interview Screening Scales (Kessler & Ustün, 2004). The required items for the depression module assessed depressed mood, feeling discouraged, loss of interest or pleasure, and feelings of worthlessness during the past 30 days. Frequency of these symptoms was rated from 0 (none of the time) to 4 (all or almost all of the time).
Statistical Method
The T2 survey items that assessed postconcussive, posttraumatic stress, and depressive symptoms were considered for inclusion in the factor analyses. Item content was first reviewed with the aim of identifying redundant items, as inclusion of near-identical indicators could yield spurious findings (e.g., factors defined by two to three redundant items). Three similarly worded items were found in the Your Health and Stressful Experiences sections of the survey: these assessed irritability, difficulty concentrating, and sleep problems. Other similarly worded items were found in the Depression and Stressful Experiences sections: these assessed loss of interest and trouble experiencing positive feelings. The instructions for the Stressful Experiences section of the survey directed respondents to rate reactions to stressful experiences (“Highly stressful experiences can cause a number of reactions. How much were you bothered by any of these reactions in the past 30 days because of any highly stressful experience that ever happened to you?”), whereas the Health Problems and Depression sections did not link the queried symptoms to specific events. We selected the irritability, difficulty concentrating, sleep problems, and loss of interest/pleasure items from the Health Problems and Depression survey sections in lieu of the similarly worded items from the Stressful Experiences section, because we wanted indicators to reflect overall severity of symptoms (irrespective of whether the respondent felt they were reactions to a stressful experience). For example, we wanted the indicator of sleep problems to reflect overall severity of sleep problems in the preceding 30 days, not just the severity of sleep problems that the respondent thought were related to a past stressful experience. This resulted in the elimination of five items from the Stressful Experiences survey section: loss of interest in activities you used to enjoy; trouble experiencing positive feelings; irritable behavior, angry outbursts, or acting aggressively; having difficulty concentrating; and trouble falling or staying asleep.
Data from the remaining 29 items were analyzed using Mplus Version 7. WLSMV estimation, which defines item responses as ordered categorical, was the preferred method over MLR with categorical variables because of easier computational burden and the availability of global fit indices for model evaluation.
Approximately one third (n = 300) of cases were randomly chosen for an EFA with WLSMV estimation and geomin rotation. Eigenvalues, scree plots, and global fit indices were examined to evaluate one- to six-factor models. We considered a combination of global fit indices that included the comparative fit index (CFI), Tucker-Lewis Index (TLI), root mean square error of approximation (RMSEA) and its 90% confidence interval, and standardized root-mean-square residual (SRMR). Fit indices were evaluated in relation to recommended thresholds for good fit, with CFI and TLI close to or above .95; RMSEA close to or below .06; SRMR close to or below .08 (Hu & Bentler, 1999); other guidelines suggest RMSEA values up to .08 reflect adequate fit (MacCallum, Browne, & Sugawara, 1996). We also inspected pattern matrices to determine whether each indicator exhibited a salient loading (e.g., λ ≥ .30) on at least one factor (Costello & Osborne, 2005). We expected some salient cross-loadings, given the definitional overlap among PCS, PTSD, and depression. Thus, retention of specific cross-loadings was based on whether the cross-loadings were conceptually reasonable (e.g., sleep disturbance could plausibly be related to PCS, posttraumatic stress, or depression).
Model structures derived from EFA were subsequently evaluated in CFA of data from the remaining sample (n = 929). First-order CFA models were specified and overall fit was evaluated using CFI, TLI, and RMSEA. The weighted root-mean-square residual (WRMR) also is reported (SRMR was not available for the WLSMV-estimated CFA models). In addition, modification indices were reviewed to identify localized points-of-strain in model fit. If a modification index was extremely elevated, and the corresponding path was theoretically meaningful, we tried freeing the path in question and reevaluating fit.
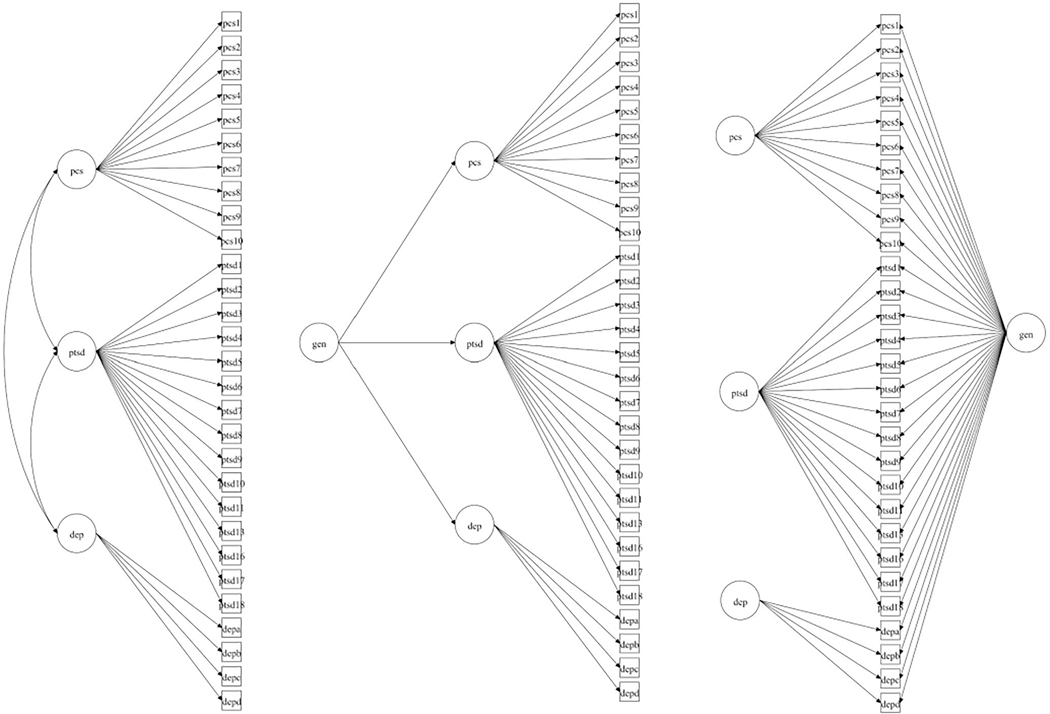
After finalizing the first-order CFA model, we performed a second-order CFA to explore whether a global factor might explain covariance of the first-order factors and substantial variance in the indicators. To accomplish this, the magnitude of the second-order factor loadings (i.e., loadings of the first-order factors on a high-order factor) and the relationship of the second-order factor to the indicators were examined. If the second-order CFA results suggested a viable global factor, we planned to conduct bifactor CFA, which first accounts for item variance explained by a common general factor, and then estimates the degree to which residual item variance is explained by domain factors that are orthogonal to the general factor. Figure 1 shows a comparison of the first-order, higher-order, and bifactor CFA models.
Figure 1.
Comparison of model structures (left = single-order; middle = second-order; right = bifactor).
Lastly, given sufficient evidence of a viable bifactor model, omega coefficients were used to determine the internal consistency of multidimensional, composite factors. Omegas are calculated for both the general factor and each domain factor and can be interpreted similarly to Cronbach’s alpha (Rodriguez, Reise, & Haviland, 2016). An OmegaH score is a modified Omega score representing the percent of variance in total scores attributed to individual differences on the general factor; for domain factors, OmegaH score represents the percentage of variance in a subscale score explained by a domain factor, after partitioning out variance in that subscale score explained by the general factor (Rodriguez et al., 2016). Factor determinancy scores are the correlations between calculated factor scores and the factors themselves, with a correlation >.90 indicating strong representation of the latent factor (Gorsuch, 1983).
Results
Descriptive Analyses
The sociodemographic, Army service, and injury-related characteristics of the sample are reported in Table 1. Approximately 42.5% of the sample reported a history of TBI with LOC prior to the index deployment. With respect to the severity of TBI(s) that occurred during the index deployment, 65% of the sample reported very mild TBI (alteration of consciousness only), 27% reported mild TBI (LOC and/or posttraumatic amnesia for <30 min), and 7% reported more-than-mild TBI (LOC and/or posttraumatic amnesia for ≥30 min).
Table 1.
Sample Characteristics (N = 1229)
| Variable | n (%) or M (SD) |
|---|---|
|
| |
| Age at T0 | 24.99 (5.28) |
| Gender | |
| Female | 30 (2.4%) |
| Male | 1188 (96.7%) |
| Missing/Unobtainable | 11 (0.9%) |
| Ethnicity | |
| Non-Hispanic | 1042 (84.8%) |
| Hispanic | 183 (14.9%) |
| Missing/Unobtainable | 4 (0.3%) |
| Race | |
| Caucasian | 928 (75.5%) |
| Black | 108 (8.8%) |
| Native American/Alaskan Native | 8 (0.7%) |
| Asian | 38 (3.1%) |
| Other/Multiracial | 147 (12.0%) |
| Marital status | |
| Married | 642 (52.2%) |
| Never Married | 439 (35.7%) |
| Divorced | 63 (5.1%) |
| Separated | 74 (6.0%) |
| Missing/Unobtainable | 11 (0.9%) |
| Education level | |
| GED or equivalent | 103 (8.4%) |
| High school diploma | 512 (41.7%) |
| Some post-HS education | 320 (26.0%) |
| Post-HS education with degree/cert. | 88 (7.2%) |
| Associate’s | 97 (7.9%) |
| Bachelor’s | 91 (7.4%) |
| Graduate/Professional | 15 (1.2%) |
| Missing/Unobtainable | 3 (0.2%) |
| Any TBI prior to index deploymenta | |
| Predeployment TBI | 559 (45.5%) |
| No predeployment TBI | 670 (54.5%) |
| Number of TBIs prior to index deploymenta | |
| 0 | 670 (54.5%) |
| 1 | 214 (14.4%) |
| 2+ | 345 (28.1%) |
| Deployment-acquired TBI Severity | |
| Very mild | 808 (65.7%) |
| Mild | 333 (27.1%) |
| Mod/Severe | 88 (7.2%) |
Note. GED = General Equivalency Diploma; HS = high school; TBI = traumatic brain injury. The sample comprised Army STARRS respondents who deployed to Afghanistan, reported an injury consistent with TBI on the PPDS survey, and completed the PPDS T2 survey sections that assessed posttraumatic stress, depression, and postconcussive symptoms.
For TBIs that occurred prior to the index deployment, only injuries with loss of consciousness (of any duration) were counted.
Exploratory Factor Analysis
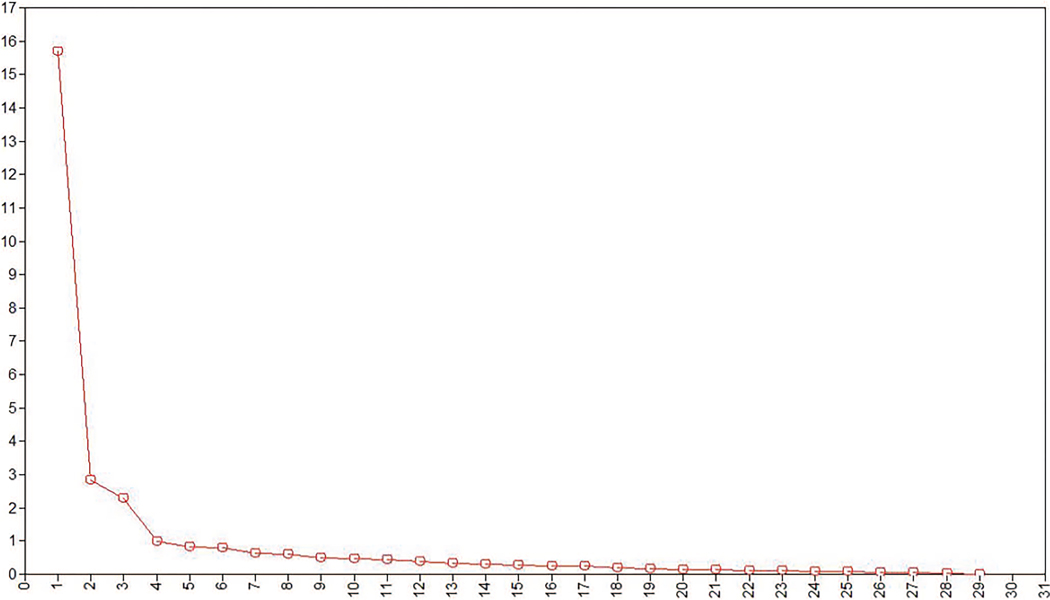
Table 2 shows the Spearman’s Rho correlations of all items included in EFA, and Table 3 shows fit statistics for EFA models with 1 to 5 factors. Though global fit improved following addition of each factor, evidence from the scree plot (see Figure 2), eigen-values (Table 4, bottom row), and pattern matrices suggested the three-factor solution to be optimal (CFI = .964, RMSEA = .074 [.068–.080]). The four-factor solution, though viable (eigen-value = .989), was rejected given that the constituent items of the last factor primarily loaded on other factors.
Table 2.
Spearman’s Rho Matrix of Items From PCS, Posttraumatic Stress, and Depression Measures (N = 1229)
| Item | M | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 13 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||||||||||||||||
| 1. PCS1 | 1.51 | 1.40 | 1 | ||||||||||||||||||||||||||||
| 2. PCS2 | 1.27 | 1.32 | .463 | 1 | |||||||||||||||||||||||||||
| 3. PCS3 | 1.07 | 1.24 | .350 | .626 | 1 | ||||||||||||||||||||||||||
| 4. PCS4 | 1.64 | 1.31 | .327 | .415 | .420 | 1 | |||||||||||||||||||||||||
| 5. PCS5 | 1.95 | 1.23 | .317 | .371 | .359 | .548 | 1 | ||||||||||||||||||||||||
| 6. PCS6 | 1.70 | 1.26 | .333 | .451 | .432 | .726 | .659 | 1 | |||||||||||||||||||||||
| 7. PCS7 | 2.31 | 1.34 | .305 | .363 | .375 | .463 | .517 | .538 | 1 | ||||||||||||||||||||||
| 8. PCS8 | 1.47 | 1.25 | .318 | .427 | .476 | .485 | .426 | .507 | .409 | 1 | |||||||||||||||||||||
| 9. PCS9 | 0.95 | 1.11 | .353 | .470 | .532 | .485 | .411 | .488 | .366 | .475 | 1 | ||||||||||||||||||||
| 10. PCS10 | 2.04 | 1.17 | .269 | .356 | .370 | .483 | .527 | .583 | .548 | .418 | .411 | 1 | |||||||||||||||||||
| 1. DEP1 | 0.88 | 1.07 | .123 | .244 | .219 | .300 | .408 | .373 | .332 | .204 | .272 | .407 | 1 | ||||||||||||||||||
| 2. DEP2 | 0.91 | 1.13 | .111 | .235 | .221 | .307 | .418 | .388 | .321 | .204 | .268 | .388 | .789 | 1 | |||||||||||||||||
| 3. DEP3 | 0.85 | 1.13 | .142 | .244 | .280 | .327 | .411 | .403 | .331 | .245 | .302 | .440 | .699 | .716 | 1 | ||||||||||||||||
| 4. DEP4 | 0.54 | 0.98 | .080 | .189 | .191 | .222 | .308 | .322 | .257 | .154 | .223 | .342 | .647 | .663 | .624 | 1 | |||||||||||||||
| 1. PTS1 | 0.82 | 1.06 | .273 | .337 | .255 | .321 | .374 | .358 | .381 | .268 | .271 | .282 | .364 | .329 | .326 | .284 | 1 | ||||||||||||||
| 2. PTS2 | 0.74 | 1.05 | .256 | .317 | .252 | .310 | .365 | .348 | .389 | .286 | .264 | .268 | .305 | .274 | .285 | .229 | .796 | 1 | |||||||||||||
| 3. PTS3 | 0.51 | 0.92 | .267 | .335 | .289 | .357 | .391 | .395 | .376 | .320 | .313 | .285 | .311 | .275 | .317 | .272 | .665 | .660 | 1 | ||||||||||||
| 4. PTS4 | 0.66 | 0.98 | .254 | .311 | .267 | .325 | .400 | .378 | .351 | .280 | .274 | .304 | .379 | .359 | .345 | .316 | .700 | .623 | .650 | 1 | |||||||||||
| 5. PTS5 | 0.62 | 0.98 | .285 | .349 | .302 | .346 | .397 | .386 | .371 | .294 | .294 | .313 | .331 | .336 | .332 | .307 | .644 | .620 | .676 | .677 | 1 | ||||||||||
| 6. PTS6 | 0.66 | 1.05 | .194 | .307 | .261 | .266 | .316 | .306 | .325 | .228 | .272 | .291 | .356 | .345 | .338 | .304 | .624 | .583 | .546 | .614 | .580 | 1 | |||||||||
| 7. PTS7 | 0.48 | 0.93 | .201 | .335 | .286 | .290 | .321 | .338 | .325 | .257 | .290 | .286 | .345 | .347 | .342 | .327 | .586 | .558 | .575 | .619 | .598 | .735 | 1 | ||||||||
| 8. PTS8 | 0.43 | 0.85 | .204 | .315 | .299 | .374 | .330 | .353 | .305 | .305 | .294 | .285 | .314 | .305 | .321 | .251 | .502 | .486 | .498 | .505 | .522 | .588 | .593 | 1 | |||||||
| 9. PTS9 | 0.40 | 0.89 | .170 | .262 | .237 | .289 | .326 | .312 | .274 | .211 | .249 | .297 | .419 | .427 | .391 | .399 | .417 | .360 | .418 | .432 | .447 | .463 | .490 | .471 | 1 | ||||||
| 10. PTS10 | 0.34 | 0.82 | .159 | .229 | .206 | .258 | .291 | .295 | .278 | .216 | .220 | .240 | .355 | .337 | .281 | .341 | .500 | .432 | .445 | .489 | .462 | .525 | .537 | .489 | .616 | 1 | |||||
| 11. PTS11 | 0.42 | 0.87 | .206 | .287 | .276 | .327 | .391 | .356 | .330 | .264 | .288 | .302 | .437 | .432 | .363 | .387 | .530 | .497 | .514 | .554 | .540 | .558 | .574 | .491 | .663 | .694 | 1 | ||||
| 13. PTS13 | 0.59 | 0.96 | .165 | .283 | .262 | .325 | .406 | .385 | .335 | .231 | .293 | .349 | .509 | .514 | .487 | .432 | .499 | .454 | .478 | .534 | .508 | .542 | .524 | .460 | .584 | .516 | .634 | 1 | |||
| 16. PTS16 | 0.31 | 0.75 | .195 | .277 | .271 | .298 | .339 | .323 | .286 | .268 | .271 | .281 | .278 | .289 | .296 | .285 | .461 | .444 | .476 | .483 | .497 | .440 | .460 | .418 | .459 | .454 | .473 | .437 | 1 | ||
| 17. PTS17 | 1.04 | 1.21 | .260 | .353 | .312 | .336 | .368 | .368 | .381 | .271 | .294 | .305 | .272 | .261 | .263 | .217 | .542 | .535 | .545 | .568 | .596 | .474 | .476 | .460 | .390 | .365 | .457 | .465 | .447 | 1 | |
| 18. PTS18 | 0.97 | 1.15 | .287 | .390 | .306 | .357 | .390 | .405 | .367 | .300 | .300 | .313 | .284 | .294 | .288 | .238 | .579 | .557 | .549 | .553 | .630 | .483 | .492 | .442 | .368 | .364 | .460 | .466 | .447 | .757 | 1 |
Note. PCS = postconcussive symptoms; DEP = depression; PTS = posttraumatic stress. See Table 4 for item descriptions. PTSD Checklist items 12, 14, 15, 19, 20 are omitted because of overlapping content with PCS and depression items.
Table 3.
Fit Indices for WLSMV-Estimated EFA Models (n = 300)
| Model name | CFI | TLI | RMSEA | 90% RMSEA | SRMR | Factor correlationsa |
|---|---|---|---|---|---|---|
|
| ||||||
| 1-factor | .864 | .853 | .133 | .128–.138 | .138 | |
| 2-factor | .915 | .901 | .110 | .104–.115 | .092 | R1,2 = .517 |
| 3-factor | .964 | .955 | .074 | .068–.080 | .046 | R1 × 2 = .432, R1 × 3 = .144, R2 × 3 = .559 |
| 4-factor | .973 | .963 | .067 | .060–.073 | .040 |
R1 × 2 = .504, R1 × 3 = .344, R1 × 4 = .287, R2 × 3 = .560, R2 × 4 = .309, R3 × 4 = .143 |
| 5-factor | .984 | .975 | .055 | .047–.062 | .033 | R1 × 2 = .593, R1 × 3 = .317, R1 × 4 = −.003, |
|
R1 × 5 = .214, R2 × 3 = .539, R2 × 4 = .066, R2 × 5 = .221, R3 × 4 = −.127, R3 × 5 = .133, R4 × 5 = .008 |
||||||
Note. CFI = comparative fit index; TLI = Tucker-Lewis Index; RMSEA = root mean square error of approximation; SRMR = root-mean-square residual. Boldface indicates the model that was accepted as the final EFA model. See Table 4 for denoted factors.
Figure 2.
Eigenvalue plot for WLSMV-estimated EFA (n = 300). See the online article for the color version of this figure.
Table 4.
WLSMV-Estimated, Goemin-Rotated EFA Loadings (n = 300)
| 5-factor |
4-factor |
3-factor |
2-factor |
1-factor |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | Description | 1.PCS | 2.PTS | 3.DEP | 4.Int | 5.HA | 1.PCS | 2.PTS | 3.DEP | 4.HA | 1.PCS | 2.PTS | 3.DEP | 1.Dis | 2.PCS | |
|
| ||||||||||||||||
| PCS1 | Ringing in ears | .511 | .051 | −.049 | .265 | .078 | .450 | −.005 | −.052 | .319 | .466 | .228 | −.081 | .066 | .521 | .437 |
| PCS2 | Noise sensitivity | .764 | .039 | .002 | .246 | .033 | .689 | −.001 | −.005 | .305 | .686 | .228 | .016 | .124 | .723 | .663 |
| PCS3 | Light sensitivity | .842 | .039 | −.084 | .206 | −.080 | .764 | .000 | −.090 | .261 | .747 | .196 | −.032 | .035 | .810 | .663 |
| PCS4 | Memory problems | .700 | .099 | .028 | −.252 | .053 | .719 | .169 | .075 | −.154 | .660 | .024 | .318 | .255 | .594 | .684 |
| PCS5 | Irritability | .568 | .032 | .360 | −.033 | .143 | .548 | .043 | .398 | .047 | .524 | .083 | .486 | .509 | .365 | .738 |
| PCS6 | Difficulty concentrating | .767 | .000 | .216 | −.197 | .069 | .770 | .083 | .247 | −.133 | .720 | −.035 | .472 | .368 | .578 | .778 |
| PCS7 | Sleep problems | .626 | −.072 | .296 | .080 | −.097 | .554 | −.070 | .289 | .086 | .534 | .009 | .356 | .312 | .414 | .591 |
| PCS8 | Headaches | .750 | .070 | −.204 | −.041 | .051 | .734 | .087 | −.170 | .055 | .682 | .115 | −.012 | −.006 | .728 | .545 |
| PCS9 | Balance/dizziness | .753 | .003 | .028 | .170 | −.079 | .676 | −.013 | .013 | .206 | .658 | .144 | .071 | .108 | .671 | .617 |
| PCS10 | Tiredness/fatigue | .612 | −.103 | .450 | −.047 | −.130 | .549 | −.060 | .445 | −.053 | .515 | −.082 | .562 | .479 | .289 | .652 |
| DEP1 | Sad/depressed | −.022 | .083 | .911 | .068 | .048 | −.068 | .043 | .939 | .053 | −.035 | .067 | .913 | 1.098 | −.520 | .883 |
| DEP2 | Discouraged | .028 | .049 | .929 | .022 | .061 | −.013 | .019 | .965 | .018 | .009 | .020 | .962 | 1.125 | −.519 | .917 |
| DEP3 | No interest/pleasure | .142 | .033 | .815 | −.033 | −.011 | .100 | .005 | .850 | −.010 | .107 | .005 | .863 | .978 | −.358 | .779 |
| DEP4 | Feeling down/worthless | −.042 | .183 | .777 | −.002 | −.119 | −.085 | .158 | .783 | −.021 | −.069 | .133 | .773 | .953 | −.428 | .703 |
| PTS1 | Repeated memories | −.043 | .847 | .033 | .372 | .016 | −.092 | .719 | −.006 | .480 | −.019 | 1.019 | −.183 | .690 | .295 | .866 |
| PTS2 | Repeated dreams | −.002 | .793 | −.007 | .371 | .020 | −.056 | .671 | −.047 | .479 | .007 | .983 | −.226 | .636 | .299 | .819 |
| PTS3 | Reliving experience | .130 | .777 | .016 | .227 | −.004 | .097 | .657 | .019 | .359 | .129 | .864 | −.056 | .675 | .314 | .865 |
| PTS4 | Upset by reminders | .021 | .748 | .157 | .148 | .028 | .008 | .628 | .183 | .282 | .042 | .780 | .115 | .760 | .162 | .843 |
| PTS5 | Physical reactions | .049 | .795 | .027 | .240 | .031 | .024 | .656 | .040 | .381 | .068 | .881 | −.062 | .689 | .265 | .846 |
| PTS6 | Avoiding mem/thoughts | .015 | .882 | .044 | .061 | −.344 | −.009 | .903 | −.054 | .048 | −.099 | .895 | .020 | .794 | .094 | .834 |
| PTS7 | Avoiding reminders | −.038 | 1.003 | −.019 | .015 | −.345 | −.046 | 1.003 | −.096 | .033 | −.149 | .982 | −.009 | .842 | .089 | .880 |
| PTS8 | Trouble remembering | .093 | .899 | −.085 | −.172 | −.250 | .125 | .876 | −.075 | −.076 | −.001 | .793 | .079 | .747 | .133 | .813 |
| PTS9 | Strong neg beliefs | .033 | .735 | .175 | −.261 | −.013 | .088 | .673 | .266 | −.080 | .006 | .611 | .352 | .840 | .008 | .824 |
| PTS10 | Blaming someone | −.185 | 1.027 | −.023 | −.258 | −.039 | −.082 | .927 | .075 | −.085 | −.171 | .835 | .167 | .851 | −.003 | .828 |
| PTS11 | Strong neg feelings | −.032 | .846 | .076 | −.241 | .048 | .047 | .769 | .176 | −.057 | −.032 | .707 | .263 | .831 | .038 | .832 |
| PTS13 | Distant or cut off | .022 | .633 | .327 | −.104 | .043 | .050 | .548 | .397 | .054 | .034 | .556 | .416 | .858 | −.017 | .823 |
| PTS16 | Too many risks | .079 | .571 | .107 | −.181 | .174 | .128 | .444 | .244 | .086 | .113 | .482 | .263 | .640 | .106 | .686 |
| PTS17 | Superalert | .034 | .756 | −.014 | .021 | .315 | .052 | .442 | .215 | .474 | .106 | .793 | .028 | .694 | .232 | .826 |
| PTS18 | Jumpy/easily startled | .028 | .733 | −.014 | .049 | .428 | .059 | .413 | .226 | .508 | .118 | .795 | .022 | .688 | .249 | .830 |
| Eigenvalue | .817 | .989 | 2.281 | 2.826 | 15.699 | |||||||||||
Note. Boldface indicates salient factor loadings (>.30). PCS = postconcussive symptoms; PTS = posttraumatic stress symptoms; DEP = depression; Int = intrusions; HA = hyperarousal; Dis = distress. PTSD Checklist items 12, 14, 15, 19, and 20 omitted because of overlap with PCS and depression items.
Table 4 shows the pattern matrices for the one- to five-factor EFA solutions. The pattern matrix for the three-factor solution shows latent dimensions resembling PCS, Posttraumatic Stress, and Depression in that all items from the PCS measure loaded on the first factor (loadings = 0.45–.77), all PCL items loaded on the second factor (loadings = 0.41–1.00), and all items from the depression measure loaded on the third factor (loadings = 0.77–0.96). As expected, some of the nonspecific symptoms (i.e., irritability, difficulty concentrating, sleep problems, tiredness/fatigue, strong negative beliefs, and feeling distant or cut off) demonstrated salient cross-loadings (loadings = −.35–0.56).
Confirmatory Factor Analyses
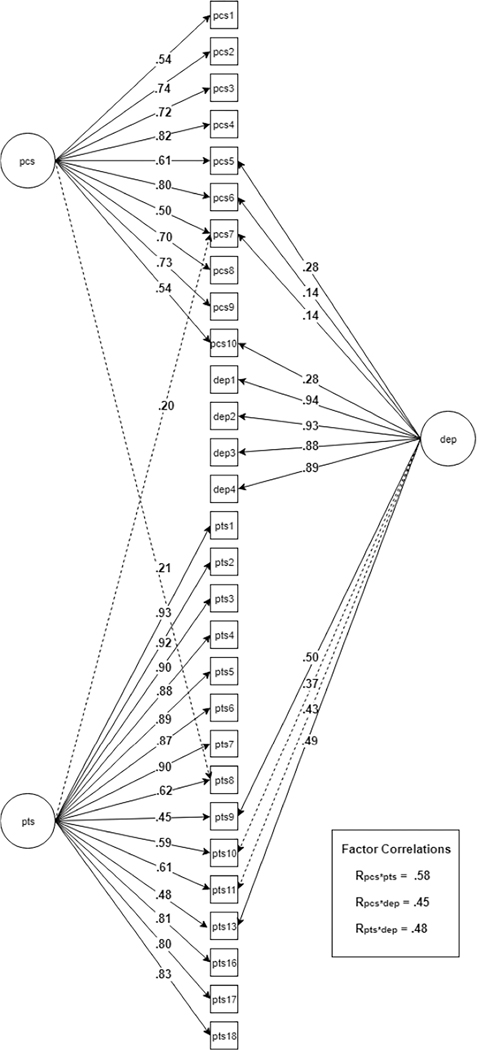
Prior to confirming the three-factor EFA solution in the independent subsample, we tested two simpler three-factor CFA models in which each indicator was allowed to load on only one latent factor. The first was a three-factor model in which each item was allowed to load only on a factor representing its original scale (e.g., items from the PCL were allowed to load on only one factor; see Table 5, 3-Factor Questionnaire-Based). The second was a three-factor model in which each item was allowed to load only on the factor for which it displayed a primary loading in EFA (Table 5, 3-Factor EFA Primary Loadings). These models did not fit the data well, with modification indices suggesting multiple points of strain. Next, we specified a three-factor model that, in addition to the EFA primary loadings, allowed cross-loadings that were evident in EFA (Table 5, “3-Factor EFA Primary Loadings and Cross-Loadings). Model fit markedly improved, with the model demonstrating adequate fit (CFI = .955, RMSEA = .080 [.077, .083]). However, several substantially elevated modification indices suggested that allowing additional cross-loadings would improve model fit. After confirming that these were conceptually defensible, we allowed four additional cross-loadings to be freely estimated: blaming yourself/someone else (PTS10) and strong negative beliefs (PTS11) on the factor defined by depression items; sleep problems (PCS7) on the factor defined by posttraumatic stress items; and trouble remembering (PTS8) on the factor defined by PCS items (Table 5, 3-Factor EFA Primary Loadings and Cross-Loadings, Plus MI-Based Cross-Loadings). Model fit improved when these paths were freed (CFI = .964, RMSEA = .073 [.070, .075]). Figure 3 shows a representation of this final CFA model including the PCS, posttraumatic stress, and depression factors.
Table 5.
Fit Indices for WLSMV-Estimated CFA Models (n = 929)
| Model | Model name | CFI | TLI | RMSEA | 90% RMSEA | WRMR |
|---|---|---|---|---|---|---|
|
| ||||||
| 1 | 3-factor, questionnaire-based | .945 | .940 | .088 | .085–.091 | 2.39 |
| 2 | 3-factor, EFA primary loadings | .934 | .928 | .096 | .094–.099 | 2.63 |
| 3 | 3-factor, EFA primary loadings and cross-loadings | .955 | .950 | .080 | .077–.083 | 2.11 |
| 4 | 3-factor EFA primary loadings and cross-loadings, plus MI-based cross-loadings | .964 | .959 | .073 | .070–.075 | 1.86 |
| 5 | Second-order 3-factor EFA primary loadings and cross-loadings, plus MI-based cross-loadings | “ | ” | |||
| 6 | Bifactor model with questionnaire-based domain factors | .957 | .950 | .080 | .077–.083 | 1.88 |
| 7 | Bifactor model with EFA primary loadings and cross-loadings | .967 | .960 | .072 | .069–.075 | 1.63 |
| 8 | Bifactor model with EFA primary loadings and cross-loadings, plus all CFA-based cross-loadings shown in Fig. 8 | No convergence (iterations = 10,000) | ||||
Note. CFI = comparative fit index; TLI = Tucker-Lewis Index; RMSEA = root mean square error of approximation; WRMR = weighted root-mean-square residual. Boldface indicates models that were accepted as the final first-order CFA and bifactor CFA models.
Figure 3.
Final first-order CFA model with all cross-loadings (dashed arrows indicate MI-based cross-loadings; factor correlations not depicted in diagram).
The significant factor intercorrelations (Rs = .45–.58) observed in the first-order CFA model suggested that a higher-order or general factor might explain substantial variance in the indicators. Although a second-order CFA model would yield the same fit as the first-order CFA model with three correlated factors, we fit it to gather preliminary evidence for a general factor, by examining the magnitude of the second-order factor loadings and the relationship of the second-order factor to the indicators. As expected, the three lower-order factors loaded strongly on the second-order factor (.74, .79, and .61, respectively), suggesting that a higher-order factor explained large proportions of variance in each of the lower-order domains. All indicators had salient loadings on the second-order factor (λs > .35).
Given evidence of a superordinate factor underlying the three symptom domains, we began fitting bifactor CFA models. As a first step, we tested a bifactor CFA model in which each item was permitted to load only on the general factor and the domain factor representing its original scale (Table 5, Bifactor Model With Questionnaire-Based Domain Factors). This model showed adequate fit (CFI = .957, RMSEA = .080 [.077, .083]). We next ran a bifactor model containing the four cross-loadings observed in EFA and confirmed in CFA (Table 5, Bifactor Model With EFA Primary Loadings and Cross-Loadings). Model fit was improved relative to the first bifactor model (CFA = .967, RMSEA = .072 [.069, .075]). We next tried including the additional cross-loadings that were specified in our final CFA model (based on modification indices); however, that model would not converge, indicating underidentification (Table 5, Bifactor Model With EFA Primary Loadings and Cross-Loadings, Plus CFA-Based Cross-Loadings). We therefore accepted the Bifactor Model With EFA Primary Loadings and Cross-Loadings as the final bifactor model. Based on the pattern and magnitude of item-factor loadings, we labeled the domain factors Re-experiencing/Hyperarousal, Depressed Mood/Anhedonia, and Cognitive/Somatic Symptoms. Loadings for this model can be seen in Table 6. All items displayed salient loadings on the general factor. Similarly, all items drawn from the PCS and depression measures exhibited salient loadings on the Cognitive/Somatic and Depressed Mood/Anhedonia domain factors, respectively. Many items from the PCL did not exhibit salient loadings on any domain factor, being largely explained by the general factor. However, several items assessing reexperiencing and hyperarousal defined a distinct domain factor.
Table 6.
Standardized Loadings for Final Bifactor Model (Model 7)
| Item | Item description | Gen | Cognitive/sensory | Re-exp/Hyperarousal | Dep mood/Anhedonia |
|---|---|---|---|---|---|
|
| |||||
| PCS1 | Tinnitus | .360 | .389 | — | — |
| PCS2 | Noise sensitivity | .497 | .533 | — | — |
| PCS3 | Light sensitivity | .450 | .589 | — | — |
| PCS4 | Memory problems | .528 | .647 | — | — |
| PCS5 | Irritability | .568 | .451 | — | .259 |
| PCS6 | Difficulty concentrating | .568 | .651 | — | .220 |
| PCS7 | Sleep problems | .545 | .421 | .192 | .203 |
| PCS8 | Headaches | .467 | .506 | — | — |
| PCS9 | Balance problems/dizziness | .483 | .547 | — | — |
| PCS10 | Tiredness/fatigue | .456 | .483 | — | .349 |
| DEP1 | Feeling sad/depressed | .544 | — | — | .763 |
| DEP2 | Feeling discouraged | .529 | — | — | .780 |
| DEP3 | Loss of interest/pleasure | .543 | — | — | .680 |
| DEP4 | Feeling worthless | .518 | — | — | .724 |
| PTS1 | Repeated memories | .817 | — | .474 | — |
| PTS2 | Repeated dreams | .783 | — | .527 | — |
| PTS3 | Reliving experience/flashbacks | .815 | — | .393 | — |
| PTS4 | Feeling upset by reminders | .834 | — | .281 | — |
| PTS5 | Physical reactions to reminders | .829 | — | .310 | — |
| PTS6 | Avoiding memories | .860 | — | .108 | — |
| PTS7 | Avoiding reminders | .897 | — | .112 | — |
| PTS8 | Trouble remembering event | .773 | — | .064 | — |
| PTS9 | Strong negative beliefs | .809 | — | —.264 | .211 |
| PTS10 | Blaming oneself/others | .861 | — | —.180 | — |
| PTS11 | Strong negative feelings | .935 | — | —.178 | — |
| PTS13 | Feeling distant/cut-off | .800 | — | —.106 | .273 |
| PTS16 | Excessive risk-taking | .793 | — | .142 | — |
| PTS17 | Superalert/on guard | .684 | — | .484 | — |
| PTS18 | Jumpy/easily startled | .710 | — | .505 | — |
Note. PCS = postconcussive symptoms; PTS = posttraumatic stress symptoms; DEP = depression; Gen = general factor; Re-exp/Hyperarousal = re-experiencing/hyperarousal items; Dep mood/Anhedonia = depressed mood/anhedonia symptoms.
Omega values for the general factor and for the Cognitive/Somatic, Reexperiencing/Hyperarousal, and Depressed Mood/Anhedonia domain factors were .98, .92, .98, and .96, respectively. These values indicate excellent internal consistency of the general factor and each domain factor. OmegaH and OmegaHS values were .86, .48, .03, and .33, respectively. These values suggest strong influence of the general factor on item variance; although the Cognitive/Somatic (and to a lesser extent, Depressed Mood/Anhedonia) factors explained substantive variance in those domains, beyond that explained by the general factor. In contrast, the Re-Experiencing/Hyperarousal factor was essentially subsumed by the general factor. Factor determinacy scores (.93–.99) indicated that factor score estimates were valid representations of the defined factors.
Relative Contributions of General and Domain Factors to Specific Symptoms
Loadings from our final bifactor model were squared to observe variance explained by the general factor as well as contributing domain factors. Percentages of variance explained can be seen in Table 7. The general factor explained the majority of variance in many items drawn from the PCL, particularly feeling strong negative emotions, avoiding reminders and memories of stressful experiences, blaming oneself or someone else for stressful experiences, and feeling upset and having physical reactions to reminders of stressful experiences. The general factor also explained substantial amounts of variance in symptoms such as sleep problems and irritability, which feature in clinical descriptions of all three conditions of interest (PCS, posttraumatic stress, and depression). A number of items also had substantive variance explained by the domain factors. As suggested by the corresponding factor labels, domain factors explained variance in reexperiencing and hyperarousal; cognitive (difficulty concentrating, memory problems) and somatic (balance/dizziness, light sensitivity, noise sensitivity, headaches) symptoms; and depressed mood, anhedonia, hopelessness, and worthlessness.
Table 7.
Variance Explained by Final Bifactor Model (Model 7)
| Item | Item description | %VE general factor | %VE cognitive/sensory | %VE Re-exp/Hyperarousal | %VE Dep mood/Anhedonia | %VE total |
|---|---|---|---|---|---|---|
|
| ||||||
| PTS11 | Strong negative feelings | 86 | — | 5 | — | 91 |
| PTS7 | Avoiding reminders | 81 | — | 0 | — | 82 |
| PTS6 | Avoiding memories | 75 | — | 0 | — | 75 |
| PTS10 | Blaming oneself/others | 73 | — | 5 | — | 78 |
| PTS4 | Feeling upset by reminders | 72 | — | 6 | — | 77 |
| PTS5 | Physical reactions | 71 | — | 7 | — | 78 |
| PTS1 | Repeated memories | 70 | — | 19 | — | 89 |
| PTS3 | Reliving/flashbacks | 69 | — | 12 | — | 82 |
| PTS2 | Repeated dreams | 65 | — | 24 | — | 89 |
| PTS16 | Excessive risk-taking | 64 | — | 1 | — | 65 |
| PTS9 | Strong negative beliefs | 63 | — | 9 | 5 | 77 |
| PTS13 | Feeling distant/cut-off | 63 | — | 2 | 8 | 73 |
| PTS8 | Trouble remembering event | 60 | — | 0 | — | 60 |
| PTS18 | Jumpy/easily startled | 53 | — | 23 | — | 76 |
| PTS17 | Superalert/on guard | 49 | — | 21 | — | 70 |
| PCS7 | Sleep problems | 35 | 15 | — | 3 | 53 |
| PCS6 | Difficulty concentrating | 32 | 43 | — | 5 | 80 |
| PCS5 | Irritability | 32 | 21 | — | 7 | 59 |
| DEP1 | Feeling sad or depressed | 29 | — | — | 59 | 88 |
| DEP3 | Loss of interest/pleasure | 29 | — | — | 47 | 76 |
| DEP2 | Feeling discouraged | 27 | — | — | 61 | 89 |
| PCS4 | Memory problems | 27 | 42 | — | — | 70 |
| DEP4 | Feeling worthless | 26 | — | — | 53 | 79 |
| PCS2 | Noise sensitivity | 24 | 29 | — | — | 53 |
| PCS9 | Balance/dizziness | 23 | 30 | — | — | 53 |
| PCS8 | Headaches | 22 | 26 | — | — | 47 |
| PCS10 | Tiredness/fatigue | 21 | 23 | — | 12 | 56 |
| PCS3 | Light sensitivity | 20 | 35 | — | — | 55 |
| PCS1 | Tinnitus | 13 | 15 | — | — | 28 |
Note. PCS = postconcussive symptoms; PTS = posttraumatic stress symptoms; DEP = depression; %VE = percentage of variance explained; Re-exp/Hyperarousal = re-experiencing/hyperarousal symptoms; Dep mood/Anhedonia = depressed mood/anhedonia symptoms.
Factor scores were computed for the final first-order CFA and bifactor CFA models (see Table 8), and examined in relation to deployment stress exposure (high or low-to-moderate) and deployment-acquired TBI severity (very mild, mild, or more-than-mild). Soldiers with high deployment stress exposure had significantly greater PCS, Posttraumatic Stress, and Depression factor scores from the first-order CFA model. With respect to bifactor scores, soldiers with high deployment stress had significantly higher general factor scores (MΔ = .17 [.02], p < .001) and Reexperiencing/Hyperarousal scores (MΔ = .05 [.02], p = .013).
Table 8.
First-Order Factor Scores and Bifactor Scores by TBI Severity and Level of Deployment Stress
| TBI severity |
Stress exposure |
||||||
|---|---|---|---|---|---|---|---|
| Factor score | Very mild (n = 610) | Mild (n = 260) | More than mild (n = 58) | p | Low (n = 410) | High (n = 519) | p |
|
| |||||||
| First-order CFA model | |||||||
| PCS | −.08 (.47) | .17 (.49) | .10 (55) | <.001 | −.10 (.48) | .08 (.50) | <.001 |
| Posttraumatic stress | −.03 (.77) | .26 (.85) | .12 (.84) | <.001 | −.18 (.71) | .26 (.82) | <.001 |
| Depression | .05 (.79) | .11 (.83) | .02 (.81) | .522 | −.05 (.79) | .16 (.80) | <.001 |
| Bifactor CFA model | |||||||
| General | .00 (.30) | .10 (.32) | .06 (.32) | <.001 | −.06 (.27) | .11 (.31) | <.001 |
| Cognitive/Somatic | −.07 (.34) | .08 (.33) | .05 (.44) | <.001 | −.03 (.34) | −.02 (.35) | .419 |
| Re-experiencing/Hyperarousal | −.02 (.28) | .04 (.33) | −.01 (.33) | .067 | −.03 (.27) | .02 (.31) | .013 |
| Depressed mood/Anhedonia | .06 (.59) | −.04 (.63) | −.07 (.70) | .043 a | .05 (.62) | .00 (.61) | .265 |
Note. PCS = postconcussive symptoms; TBI = traumatic brain injury; CFA = confirmatory factor analysis. Boldface indicates significant differences within the overall model.
No significant paired comparisons following multiple-comparison correction.
Using scores from the first-order CFA model, soldiers with very mild TBI severity had significantly lower PCS scores than those with mild TBI (MΔ = −.25 [.04], p < .001) and more-than-mild TBI (MΔ = −.18 [.07], p = .025); they also had significantly lower Posttraumatic Stress scores than those with mild TBI (MΔ = −.29 [.06], p < .001). With regard to bifactor scores, soldiers with very mild TBI had lower Cognitive/Somatic factor scores compared with those with mild (MΔ = −.15 [.03], p < .001) and more-than-mild TBI (MΔ = −.12 [.05], p = .042).
Discussion
Military service members with deployment-acquired TBI may experience a variety of symptoms in the months following injury. It can be difficult for clinicians to ascertain the relative contributions of TBI and mental disorders to the persistent symptoms experienced by some members of this population, which complicates diagnosis and treatment planning. The purpose of this study was to evaluate the structure of symptoms following deployment-acquired TBI, with special focus on whether symptoms known as PCS are distinct from mental health problems such as posttraumatic stress or depression. To address this question, we conducted factor analyses of postconcussive, posttraumatic stress, and depressive symptoms reported by TBI-exposed soldiers approximately three months after their return from deployment. To our knowledge, this represents the first investigation to evaluate the structure of symptoms from all three domains (PCS, posttraumatic stress, and depression) among individuals with recent TBI.
Across EFA and CFA models, we observed evidence of a latent PCS dimension that was distinct from posttraumatic stress and depression. Yet our results also pointed to substantial overlap of the three symptom domains. The EFA and first-order CFA results highlighted the substantial correlations among the PCS, Posttraumatic Stress, and Depression factors as well as the variable specificity of symptoms that define these domains (i.e., several symptoms loaded on more than one latent factor). Moreover, in bifactor CFA, we found that a general factor explained substantial proportions of variance in all symptoms represented on the measures of PCS, posttraumatic stress, and depression. Notably, items with the strongest loadings on the general factor assessed negative feelings, beliefs, and action tendencies (i.e., avoidance). Although prior evidence suggests that general distress/negative affectivity is a strong determinant of symptoms across diagnostic categories (including stress-related and depressive disorders; Brown, Chorpita, & Barlow, 1998; Goldberg et al., 2009; Thomas, 2012; Watson, 2005), we cannot necessarily conclude that the general factor reflects this characteristic. Because all latent variable indicators derived from self-report measures, it is also possible that the general factor reflects symptom reporting style (i.e., tendency to minimize or amplify symptoms on self-report measures; see also Limitations below).
In addition to showing that variance in all indicators was explained by a general factor, our bifactor CFA revealed that remaining item variance was explained by clinically interpretable domain factors. A Cognitive/Somatic factor was primarily defined by difficulty concentrating, memory problems, light sensitivity, dizziness/balance problems, noise sensitivity, and headaches; symptoms that align with clinical descriptions of PCS (American Psychiatric Association, 2000; World Health Organization, 1992), A second domain factor was defined by reexperiencing (recurrent dreams, memories, and flashbacks) and hyperarousal (exaggerated startle; hypervigilance), which are prominent and relatively specific features of posttraumatic stress (Brown & McNiff, 2009; Holowka, Marx, Kaloupek, & Keane, 2012). Notably, the second domain factor did not explain substantive variance in symptoms from the other DSM–5 PTSD symptom clusters (avoidance and negative alterations in cognition and mood). Symptoms from those clusters, which are associated not only with posttraumatic stress but also with more general anxious apprehension (Barlow, 2002) and depression (e.g., Beck, 2008; Tull, Gratz, Salters, & Roemer, 2004), were largely explained by the general factor.
A third domain factor was defined by items assessing depressed mood, anhedonia, hopelessness, and worthlessness. These symptoms are cardinal features of unipolar depression. Contrasting with the results of the EFA and single-order CFA, very little variance in nonspecific symptoms was explained by the Depressed Mood/Anhedonia factor. Given prior evidence of unclear boundaries between PTSD and depression (Au et al., 2013; Dekel et al., 2014; Elhai et al., 2011), the distinctiveness of the Depressed Mood/Anhedonia and Reexperiencing/Hyperarousal factors is noteworthy; suggesting that measurement of these specific domains could help clarify relative severity of posttraumatic stress and depression among service members with deployment-acquired TBI.
We assessed the reliability of our latent factors using omega coefficients, which gauge internal consistency in the context of a multidimensional structure. The general factor was highly internally consistent and explained the majority of variance in the items. The domain factors were also internally consistent, and the Cognitive/Somatic and Depressed Mood/Anhedonia factors explained substantive variance in their constituent symptoms over and above that explained by the general factor. However, the Re-Experiencing/Hyperarousal domain factor was poorly differentiated from the general factor, suggesting that this specific symptom domain is difficult to distinguish from shared variance in symptom reporting within this population.
Additional information about the validity of the factors was obtained through examination of factor scores in relation to deployment stress exposure and TBI severity. Results generally supported the construct validity of the bifactor scores, in that soldiers with high deployment stress exposure exhibited significantly higher General Distress and Reexperiencing/Hyperarousal factor scores, whereas soldiers with more severe TBI (mild or more-than-mild) displayed higher Cognitive/Somatic factor scores than those with very mild TBI. Scores from the first-order factor model performed somewhat worse in terms of discriminating expected effects of deployment stress exposure versus TBI severity. Soldiers with high deployment stress exposure had elevated scores on all three first-order factors (PCS, Posttraumatic Stress, and Depression); and those with mild TBI scored significantly higher than those with very mild TBI on both PCS and Posttraumatic Stress. Thus, the bifactor scores may have improved specificity relative to the scores from the first-order factor model (Thomas, 2012).
Limitations
Several limitations affect the implications of these findings. The sample predominantly comprised male Army soldiers who sustained TBI during combat deployment, which limits the generalizability of results to other populations and injury circumstances. Additionally, the EFA and CFAs were conducted using subsamples taken from the same study sample. Replication of these results in independent samples of individuals with TBI is necessary before firm conclusions can be drawn.
Another limitation is that data were collected via self-report and are thus vulnerable to response bias and invalid reporting. As noted above, an implication of the reliance on self-report is that the general factor may reflect response style (e.g., overall tendency to minimize vs. magnify symptoms) as opposed or in addition to a more clinically meaningful trait such general distress/negative affectivity. In the absence of corroborating information from other modalities (e.g., clinician interviews) or response validity measures, we cannot rule out the possibility that the general factor reflects symptom reporting style. Tendencies to minimize versus amplify symptoms also might influence respondents’ reporting of deployment stress exposure and TBI severity, which could conceivably lead to inflated correlations between symptom severity and severity of TBI/stress exposure.
On the other hand, concerns about the validity of PPDS data are mitigated to an extent by the confidential nature of the surveys and thorough informed consent process, which may guard against underreporting, and by results of the Army STARRS clinical reappraisal study, which validated diagnoses based on the PTSD and depression survey items against blinded structured clinical interviews (Kessler, Heeringa, et al., 2013; Kessler, Santiago, et al., 2013). An attempt to corroborate deployment-acquired TBIs reported on the PPDS T1 survey using Army/Department of Defense administrative data revealed poor concordance (Stein et al., 2015), although this does not invalidate the self-reported injuries given that mild TBIs—which comprise the large majority of TBIs sustained in this sample—are often medically undiagnosed (Peskind, Brody, Cernak, McKee, & Ruff, 2013; Kristman et al., 2014) or underreported (Chase & Nevin, 2015). We acknowledge that interview-based assessment of TBI might have yielded more valid data, given that it would have provided opportunities for follow-up questions and other clarification (e.g., Corrigan & Bogner, 2007). Although interview-based assessment was not feasible in PPDS, future studies should attempt to corroborate our findings among individuals whose TBI diagnoses were based on structured or other clinical interviews.
A previously validated measure of PCS was not included in the PPDS surveys, although results of a prior study (Stein et al., 2016) supported the internal consistency and criterion validity of a measure that included nearly all of the PCS items included in our analysis. Because the PCL items were not administered in the standard manner (i.e., by simply administering the full PCL-C or PCL-5), the psychometric properties of the original PCL-C and PCL-5 may not have been retained. Finally, although we had a rationale for excluding five PCL items (see Statistical Methods), we tested the robustness of our findings by repeating the EFA, first-order CFA, and bifactor CFA after reinserting those PCL items into the analyses. Results were highly consistent with the reported findings, with EFA and first-order CFA supporting a model with three correlated factors resembling PCS, posttraumatic stress, and depression, and bifactor CFA indicating a strong general factor that explained variance in all items, as well as domain factors reflecting cognitive/sensory symptoms, reexperiencing/hyperarousal, and depressed mood/anhedonia. These results reinforced the main study findings, in that the structure of the EFA, first-order CFA, and bifactor CFA symptom domains was comparable when the excluded items were reintroduced.
Conclusions
Findings of this study suggest that PCS can be distinguished from posttraumatic stress, depression, and general distress/symptom reporting among soldiers with recent TBI. Variance in PCS was attributable to both a specific cognitive/somatic symptom factor and a general factor that also explained variance in posttraumatic stress and depression. Measurement of specific domains defined by cognitive/somatic symptoms, reexperiencing/hyperarousal, and depressed mood/anhedonia may help clarify the relative severity of PCS, posttraumatic stress, and depression among individuals with recent TBI. Future research should attempt to replicate these findings and to extend them by investigating associations of unique domain factor profiles with injury characteristics and long-term outcomes of TBI.
Public Significance Statement.
Research on symptoms following traumatic brain injury (TBI) shows strong overlap between postconcussive symptoms (PCS) and mental disorders like posttraumatic stress disorder and depression. We analyzed data from >1,000 soldiers with recent TBI and found that differences in PCS severity are attributable to (a) a general factor that also contributes to differences in posttraumatic stress and depression and (b) a specific factor that is characterized by cognitive and somatic symptoms.
Acknowledgments
The Army STARRS Team consists of Co-Principal Investigators: Robert J. Ursano, MD (Uniformed Services University of the Health Sciences) and Murray B. Stein, MD, MPH (University of California San Diego and VA San Diego Healthcare System); Site Principal Investigators: Steven Heeringa, PhD (University of Michigan), James Wagner, PhD (University of Michigan), and Ronald C. Kessler, PhD (Harvard Medical School). Army liaison/consultant: Kenneth Cox, MD, MPH (US Army Public Health Center); and other team members: Pablo A. Aliaga, MA (Uniformed Services University of the Health Sciences); COL David M. Benedek, MD (Uniformed Services University of the Health Sciences); Laura Campbell-Sills, PhD (University of California San Diego); Carol S. Fullerton, PhD (Uniformed Services University of the Health Sciences); Nancy Gebler, MA (University of Michigan); Robert K. Gifford, PhD (Uniformed Services University of the Health Sciences); Meredith House, BA (University of Michigan); Paul E. Hurwitz, MPH (Uniformed Services University of the Health Sciences); Sonia Jain, PhD (University of California San Diego); Tzu-Cheg Kao, PhD (Uniformed Services University of the Health Sciences); Lisa Lewandowski-Romps, PhD (University of Michigan); Holly Herberman Mash, PhD (Uniformed Services University of the Health Sciences); James E. McCarroll, PhD, MPH (Uniformed Services University of the Health Sciences); James A. Naifeh, PhD (Uniformed Services University of the Health Sciences); Tsz Hin Hinz Ng, MPH (Uniformed Services University of the Health Sciences); Matthew K. Nock, PhD (Harvard University); Nancy A. Sampson, BA (Harvard Medical School); CDR Patcho Santiago, MD, MPH (Uniformed Services University of the Health Sciences); LTC Gary H. Wynn, MD (Uniformed Services University of the Health Sciences); and Alan M. Zaslavsky, PhD (Harvard Medical School). Army STARRS was sponsored by the Department of the Army and funded under cooperative agreement U01MH087981 (2009-2015) with the U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Mental Health (NIH/NIMH). Subsequently, STARRS-LS was sponsored and funded by the Department of Defense (USUHS Grant HU0001-15-2-0004). The contents are solely the responsibility of the authors and do not necessarily represent the views of the Department of Health and Human Services, NIMH, the Department of the Army, the Department of Veterans Affairs, or the epartment of Defense. Murray B. Stein has in the past three years been a consultant for Actelion, Aptinyx, Bionomics, Dart Neuroscience, Healthcare Management Technologies, Janssen, Oxeia Biopharmaceuticals, Pfizer, and Resilience Therapeutics. In the past 3 years, Ronald C. Kessler received support for his epidemiological studies from Sanofi Aventis; was a consultant for Johnson & Johnson Wellness and Prevention, Sage Pharmaceuticals, Shire, Takeda; and served on an advisory board for the Johnson & Johnson Services, Inc., Lake Nona Life Project. Ronald C. Kessler is a co-owner of DataStat, Inc., a market research firm that carries out healthcare research. The remaining authors have no disclosures.
Contributor Information
Stephanie Agtarap, Department of Psychiatry, University of California San Diego.
Laura Campbell-Sills, Department of Psychiatry, University of California San Diego.
Michael L. Thomas, Department of Psychology, Colorado State University
Ronald C. Kessler, Department of Health Care Policy, Harvard Medical School
Robert J. Ursano, Center for the Study of Traumatic Stress, Department of Psychiatry, Uniformed Services University of the Health Sciences
Murray B. Stein, Department of Psychiatry and Department of Family Medicine and Public Health, University of California San Diego, and VA San Diego Healthcare System, San Diego, California.
References
- Alves W, Macciocchi SN, & Barth JT (1993). Postconcussive symptoms after uncomplicated mild head injury. The Journal of Head Trauma Rehabilitation, 8, 48–59. 10.1097/00001199-199309000-00007 [DOI] [Google Scholar]
- American Congress of Rehabilitation Medicine. (1993). ACRM brain injury definition: The definition of mild brain injury. The Journal of Head Trauma Rehabilitation, 8, 86–87. 10.1097/00001199-199309000-00010 [DOI] [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed., text revision). Washington, DC: Author. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Anderson L, Campbell-Sills L, Ursano RJ, Kessler RC, Sun X, Heeringa SG, . . . Jain S. (2019). Prospective associations of perceived unit cohesion with postdeployment mental health outcomes. Depression and Anxiety, 36, 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au TM, Dickstein BD, Comer JS, Salters-Pedneault K, & Litz BT (2013). Co-occurring posttraumatic stress and depression symptoms after sexual assault: A latent profile analysis. Journal of Affective Disorders, 149, 209–216. 10.1016/j.jad.2013.01.026 [DOI] [PubMed] [Google Scholar]
- Barlow DH (2002). Anxiety and its disorders. New York, NY: Guilford Press. [Google Scholar]
- Beck AT (2008). The evolution of the cognitive model of depression and its neurobiological correlates. The American Journal of Psychiatry, 165, 969–977. 10.1176/appi.ajp.2008.08050721 [DOI] [PubMed] [Google Scholar]
- Belanger HG, Kretzmer T, Vanderploeg RD, & French LM (2010). Symptom complaints following combat-related traumatic brain injury: Relationship to traumatic brain injury severity and posttraumatic stress disorder. Journal of the International Neuropsychological Society, 16, 194–199. 10.1017/S1355617709990841 [DOI] [PubMed] [Google Scholar]
- Binder LM (1986). Persisting symptoms after mild head injury: A review of the postconcussive syndrome. Journal of Clinical and Experimental Neuropsychology, 8, 323–346. 10.1080/01688638608401325 [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Buckley TC, Hickling EJ, & Taylor AE (1998). Posttraumatic stress disorder and comorbid major depression: Is the correlation an illusion? Journal of Anxiety Disorders, 12, 21–37. 10.1016/S0887-6185(97)00047-9 [DOI] [PubMed] [Google Scholar]
- Brown TA, Chorpita BF, & Barlow DH (1998). Structural relationships among dimensions of the DSM–IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. Journal of Abnormal Psychology, 107, 179–192. 10.1037/0021-843X.107.2.179 [DOI] [PubMed] [Google Scholar]
- Brown TA, & McNiff J. (2009). Specificity of autonomic arousal to DSM–IV panic disorder and posttraumatic stress disorder. Behaviour Research and Therapy, 47, 487–493. 10.1016/j.brat.2009.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA, & Harvey AG (1999). Postconcussive symptoms and posttraumatic stress disorder after mild traumatic brain injury. Journal of Nervous and Mental Disease, 187, 302–305. 10.1097/00005053-199905000-00006 [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Ursano RJ, Kessler RC, Sun X, Heeringa SG, Nock MK, . . . Stein MB (2018). Prospective risk factors for post-deployment heavy drinking and alcohol or substance use disorder among U.S. Army soldiers. Psychological Medicine, 48, 1624–1633. 10.1017/S0033291717003105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase RP, & Nevin RL (2015). Population estimates of undocumented incident traumatic brain injuries among combat-deployed U.S. military personnel. The Journal of Head Trauma Rehabilitation, 30, E57–E64. 10.1097/HTR.0000000000000061 [DOI] [PubMed] [Google Scholar]
- Corrigan JD, & Bogner J. (2007). Initial reliability and validity of the Ohio State University TBI identification method. The Journal of Head Trauma Rehabilitation, 22, 318–329. 10.1097/01.HTR.0000300227.67748.77 [DOI] [PubMed] [Google Scholar]
- Costello AB, & Osborne JW (2005). Best practices in exploratory factor analysis: Four recommendations for getting the most from your analysis. Practical Assessment, Research & Evaluation, 10, 1–9. [Google Scholar]
- Dekel S, Solomon Z, Horesh D, & Ein-Dor T. (2014). Posttraumatic stress disorder and depressive symptoms: Joined or independent sequelae of trauma? Journal of Psychiatric Research, 54, 64–69. 10.1016/j.jpsychires.2014.03.003 [DOI] [PubMed] [Google Scholar]
- Elhai JD, de Francisco Carvalho L, Miguel FK, Palmieri PA, Primi R, & Christopher Frueh B. (2011). Testing whether posttraumatic stress disorder and major depressive disorder are similar or unique constructs. Journal of Anxiety Disorders, 25, 404–410. 10.1016/j.janxdis.2010.11.003 [DOI] [PubMed] [Google Scholar]
- Goldberg DP, Krueger RF, Andrews G, & Hobbs MJ (2009). Emotional disorders: Cluster 4 of the proposed meta-structure for DSM–5 and ICD-11. Psychological Medicine, 39, 2043–2059. 10.1017/S0033291709990298 [DOI] [PubMed] [Google Scholar]
- Gorsuch RL (1983). Factor analysis (2nd ed.). Hillsdale, NJ: LEA. [Google Scholar]
- Gros DF, Simms LJ, & Acierno R. (2010). Specificity of posttraumatic stress disorder symptoms: An investigation of comorbidity between posttraumatic stress disorder symptoms and depression in treatment-seeking veterans. Journal of Nervous and Mental Disease, 198, 885–890. 10.1097/NMD.0b013e3181fe7410 [DOI] [PubMed] [Google Scholar]
- Herrmann N, Rapoport MJ, Rajaram RD, Chan F, Kiss A, Ma AK, . . . Lanctôt KL (2009). Factor analysis of the Rivermead Post-Concussion Symptoms Questionnaire in mild-to-moderate traumatic brain injury patients. The Journal of Neuropsychiatry and Clinical Neurosciences, 21, 181–188. 10.1176/jnp.2009.21.2.181 [DOI] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, & Castro CA (2008). Mild traumatic brain injury in U.S. Soldiers returning from Iraq. The New England Journal of Medicine, 358, 453–463. 10.1056/NEJMoa072972 [DOI] [PubMed] [Google Scholar]
- Holowka DW, Marx BP, Kaloupek DG, & Keane TM (2012). PTSD symptoms among male Vietnam veterans: Prevalence and associations with diagnostic status. Psychological Trauma: Theory, Research, Practice, and Policy, 4, 285–292. 10.1037/a0023267 [DOI] [Google Scholar]
- Hu LT, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling, 6, 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- Joint Mental Health Advisory Team 7. (2011). Operation Enduring Freedom 2010 Afghanistan: Report. Washington, DC: Office of the Surgeon General United States Army Medical Command and Office of the Command Surgeon HQ, CENTCOM, and the Office of the Command Surgeon United States Forces Afghanistan (USFOR-A). Retrieved from https://armymedicine.health.mil/Reports [Google Scholar]
- Kessler RC, Colpe LJ, Fullerton CS, Gebler N, Naifeh JA, Nock MK, . . . Heeringa SG. (2013). Design of the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). International Journal of Methods in Psychiatric Research, 22, 267–275. 10.1002/mpr.1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Heeringa SG, Colpe LJ, Fullerton CS, Gebler N, Hwang I, . . . Ursano RJ (2013). Response bias, weighting adjustments, and design effects in the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). International Journal of Methods in Psychiatric Research, 22, 288–302. 10.1002/mpr.1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Santiago PN, Colpe LJ, Dempsey CL, First MB, Heeringa SG, . . . Ursano RJ (2013). Clinical reappraisal of the Composite International Diagnostic Interview Screening Scales (CIDI-SC) in the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). International Journal of Methods in Psychiatric Research, 22, 303–321. 10.1002/mpr.1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, & Ustün TB (2004). The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). International Journal of Methods in Psychiatric Research, 13, 93–121. 10.1002/mpr.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LA, King DW, Vogt DS, Knight J, & Samper RE (2006). Deployment Risk and Resilience Inventory: A collection of measures for studying deployment-related experiences of military personnel and veterans. Military Psychology, 18, 89–120. 10.1207/s15327876mp1802_1 [DOI] [Google Scholar]
- Kirkham B, Johnson P, Hunt C, Gladstone J, Michalak A, Masanic C, . . . Ouchterlony D. (2016). Persistence of chronic headache, fatigue and depression in patients who are symptomatic 6+ months following a mild or moderate traumatic brain injury at study entry. Brain Injury, 30, 681–682. [Google Scholar]
- Kleffelgaard I, Langhammer B, Hellstrom T, Sandhaug M, Tamber AL, & Soberg HL (2017). Dizziness-related disability following mild-moderate traumatic brain injury. Brain Injury, 31, 1436–1444. 10.1080/02699052.2017.1377348 [DOI] [PubMed] [Google Scholar]
- Kristman VL, Borg J, Godbolt AK, Salmi LR, Cancelliere C, Carroll LJ, . . . Cassidy JD. (2014). Methodological issues and research recommendations for prognosis after mild traumatic brain injury: Results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Archives of Physical Medicine and Rehabilitation, 95, S265–S277. 10.1016/j.apmr.2013.04.026 [DOI] [PubMed] [Google Scholar]
- Laborey M, Masson F, Ribéreau-Gayon R, Zongo D, Salmi LR, & Lagarde E. (2014). Specificity of postconcussion symptoms at 3 months after mild traumatic brain injury: Results from a comparative cohort study. The Journal of Head Trauma Rehabilitation, 29, E28–E36. 10.1097/HTR.0b013e318280f896 [DOI] [PubMed] [Google Scholar]
- Lagarde E, Salmi LR, Holm LW, Contrand B, Masson F, Ribéreau-Gayon R,... Cassidy JD (2014). Association of symptoms following mild traumatic brain injury with posttraumatic stress disorder vs. postconcussion syndrome. Journal of the American Medical Association Psychiatry, 71, 1032–1040. 10.1001/jamapsychiatry.2014.666 [DOI] [PubMed] [Google Scholar]
- Lindquist LK, Love HC, & Elbogen EB (2017). Traumatic brain injury in Iraq and Afghanistan veterans: New results from a National Random Sample Study. The Journal of Neuropsychiatry and Clinical Neurosciences, 29, 254–259. 10.1176/appi.neuropsych.16050100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum RC, Browne MW, & Sugawara HM (1996). Power analysis and determination of sample size for covariance structure modeling. Psychological Methods, 1, 130–149. 10.1037/1082-989X.1.2.130 [DOI] [Google Scholar]
- McCrea M. (2008). Mild traumatic brain injury and postconcussion syndrome: The new evidence base for diagnosis and treatment. Oxford, UK: Oxford University Press. [Google Scholar]
- Morissette SB, Woodward M, Kimbrel NA, Meyer EC, Kruse MI, Dolan S, & Gulliver SB (2011). Deployment-related TBI, persistent postconcussive symptoms, PTSD, and depression in OEF/OIF veterans. Rehabilitation Psychology, 56, 340–350. 10.1037/a0025462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskind ER, Brody D, Cernak I, McKee A, & Ruff RL (2013). Military- and sports-related mild traumatic brain injury: Clinical presentation, management, and long-term consequences. The Journal of Clinical Psychiatry, 74, 180–188. 10.4088/JCP.12011co1c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post LM, Feeny NC, Zoellner LA, & Connell AM (2016). Post-traumatic stress disorder and depression co-occurrence: Structural relations among disorder constructs and trait and symptom dimensions. Psychology and Psychotherapy: Theory, Research and Practice, 89, 418–434. 10.1111/papt.12087 [DOI] [PubMed] [Google Scholar]
- Potter S, Leigh E, Wade D, & Fleminger S. (2006). The Rivermead Post Concussion Symptoms Questionnaire: A confirmatory factor analysis. Journal of Neurology, 253, 1603–1614. 10.1007/s00415-006-0275-z [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Reise SP, & Haviland MG (2016). Evaluating bifactor models: Calculating and interpreting statistical indices. Psychological Methods, 21, 137–150. 10.1037/met0000045 [DOI] [PubMed] [Google Scholar]
- Rosellini AJ, Heeringa SG, Stein MB, Ursano RJ, Chiu WT, Colpe LJ, . . . Kessler RC. (2015). Lifetime prevalence of DSM–IV mental disorders among new soldiers in the U.S. Army: Results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). Depression and Anxiety, 32, 13–24. 10.1002/da.22316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan LM, & Warden DL (2003). Post concussion syndrome. International Review of Psychiatry, 15, 310–316. 10.1080/09540260310001606692 [DOI] [PubMed] [Google Scholar]
- Sareen J, Cox BJ, Afifi TO, Stein MB, Belik SL, Meadows G, & Asmundson GJ (2007). Combat and peacekeeping operations in relation to prevalence of mental disorders and perceived need for mental health care: Findings from a large representative sample of military personnel. Archives of General Psychiatry, 64, 843–852. 10.1001/archpsyc.64.7.843 [DOI] [PubMed] [Google Scholar]
- Schneiderman AI, Braver ER, & Kang HK (2008). Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: Persistent postconcussive symptoms and posttraumatic stress disorder. American Journal of Epidemiology, 167, 1446–1452. 10.1093/aje/kwn068 [DOI] [PubMed] [Google Scholar]
- Schwab K, Terrio HP, Brenner LA, Pazdan RM, McMillan HP, MacDonald M, . . . Scher AI (2017). Epidemiology and prognosis of mild traumatic brain injury in returning soldiers: A cohort study. Neurology, 88, 1571–1579. 10.1212/WNL.0000000000003839 [DOI] [PubMed] [Google Scholar]
- Segev S, Shorer M, Rassovsky Y, Pilowsky Peleg T, Apter A, & Fennig S. (2016). The contribution of posttraumatic stress disorder and mild traumatic brain injury to persistent post concussive symptoms following motor vehicle accidents. Neuropsychology, 30, 800–810. 10.1037/neu0000299 [DOI] [PubMed] [Google Scholar]
- Stålnacke BM, Björnstig U, Karlsson K, & Sojka P. (2005). One-year follow-up of mild traumatic brain injury: Post-concussion symptoms, disabilities and life satisfaction in relation to serum levels of S-100B and neurone-specific enolase in acute phase. Journal of Rehabilitation Medicine, 37, 300–305. 10.1080/16501970510032910 [DOI] [PubMed] [Google Scholar]
- Stein MB, Kessler RC, Heeringa SG, Jain S, Campbell-Sills L, Colpe LJ, . . . the Army STARRS collaborators. (2015). Prospective longitudinal evaluation of the effect of deployment-acquired traumatic brain injury on posttraumatic stress and related disorders: Results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS). The American Journal of Psychiatry, 172, 1101–1111. 10.1176/appi.ajp.2015.14121572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, & McAllister TW (2009). Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. The American Journal of Psychiatry, 166, 768–776. 10.1176/appi.ajp.2009.08101604 [DOI] [PubMed] [Google Scholar]
- Stein MB, Ursano RJ, Campbell-Sills L, Colpe LJ, Fullerton CS, Heeringa SG, . . . Kessler RC. (2016). Prognostic indicators of persistent post-concussive symptoms after deployment-related mild traumatic brain injury: A prospective longitudinal study in U.S. Army soldiers. Journal of Neurotrauma, 33, 2125–2132. 10.1089/neu.2015.4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss ME, & Smith GT (2009). Construct validity: Advances in theory and methodology. Annual Review of Clinical Psychology, 5, 1–25. 10.1146/annurev.clinpsy.032408.153639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrio H, Brenner LA, Ivins BJ, Cho JM, Helmick K, Schwab K, . . . Warden D. (2009). Traumatic brain injury screening: Preliminary findings in a U.S. army brigade combat team. The Journal of Head Trauma Rehabilitation, 24, 14–23. 10.1097/HTR.0b013e31819581d8 [DOI] [PubMed] [Google Scholar]
- Thomas ML (2012). Rewards of bridging the divide between measurement and clinical theory: Demonstration of a bifactor model for the Brief Symptom Inventory. Psychological Assessment, 24, 101–113. 10.1037/a0024712 [DOI] [PubMed] [Google Scholar]
- Tull MT, Gratz KL, Salters K, & Roemer L. (2004). The role of experiential avoidance in posttraumatic stress symptoms and symptoms of depression, anxiety, and somatization. Journal of Nervous and Mental Disease, 192, 754–761. 10.1097/01.nmd.0000144694.30121.89 [DOI] [PubMed] [Google Scholar]
- Ursano RJ, Colpe LJ, Heeringa SG, Kessler RC, Schoenbaum M, & Stein MB, & the Army STARRS collaborators. (2014). The Army study to assess risk and resilience in servicemembers (Army STARRS). Psychiatry: Interpersonal and Biological Processes, 77, 107–119. 10.1521/psyc.2014.77.2.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt DS, Proctor SP, King DW, King LA, & Vasterling JJ (2008). Validation of scales from the Deployment Risk and Resilience Inventory in a sample of Operation Iraqi Freedom veterans. Assessment, 15, 391–403. 10.1177/1073191108316030 [DOI] [PubMed] [Google Scholar]
- Watson D. (2005). Rethinking the mood and anxiety disorders: A quantitative hierarchical model for DSM–5. Journal of Abnormal Psychology, 114, 522–536. 10.1037/0021-843X.114.4.522 [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Herman DS, Huska JA, & Keane TM (1993, October). The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. Paper presented at the 9th Annual Conference of the ISTSS, San Antonio, TX. [Google Scholar]
- Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, & Schnurr PP (2013). The PTSD checklist for DSM–5 (PCL-5). Washington, DC: National Center for PTSD. [Google Scholar]
- Wilk JE, Herrell RK, Wynn GH, Riviere LA, & Hoge CW (2012). Mild traumatic brain injury (concussion), posttraumatic stress disorder, and depression in U.S. soldiers involved in combat deployments: Association with postdeployment symptoms. Psychosomatic Medicine, 74, 249–257. 10.1097/PSY.0b013e318244c604 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (1992). The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. Geneva, Switzerland: World Health Organization. [Google Scholar]
- Yurgil KA, Clifford RE, Risbrough VB, Geyer MA, Huang M, Barkauskas DA, . . . the MRS Team. (2016). Prospective associations between traumatic brain injury and postdeployment tinnitus in active-duty marines. The Journal of Head Trauma Rehabilitation, 31, 30–39. 10.1097/HTR.0000000000000117 [DOI] [PubMed] [Google Scholar]