Abstract
Background
Scrub typhus is an acute febrile infectious disease highly prevalent in the Asia Pacific region, often referred to as the “tsutsugamushi triangle.” This mite-borne rickettsial zoonosis is caused by Orientia tsutsugamushi, an intracellular Gram-negative organism that primarily targets endothelial cells. The resulting vasculitis leads to multisystem involvement. In terms of neurological manifestations, meningoencephalitis is the most common presentation of scrub typhus. Other frequent neurological manifestations include cranial nerve paresis, transverse myelitis, and polyneuropathy. Status epilepticus, while reported, is a rare presenting feature of this infection. Although scrub typhus has been documented to present as limbic encephalitis, there have been no previous descriptions in the literature of neuroradiological patterns affecting the basal ganglia or extra-limbic cortices in this condition.
Case Report
We report a case of a 23-year-old previously healthy woman who presented with scrub typhus meningoencephalitis. The condition manifested as encephalitis with involvement of the basal ganglia and extra-limbic cortices. She presented with generalized convulsive status epilepticus, which was complicated by cortical multifocal myoclonus.
Discussion
Scrub typhus can be a significant diagnostic challenge, potentially presenting with generalized convulsive status epilepticus and mimicking both clinical and radiological features of arboviral encephalitides, such as those caused by West Nile and Japanese encephalitis viruses. Furthermore, as demonstrated in this case, its radiological presentation can resemble that of autoimmune encephalitis. Given that scrub typhus is amenable to treatment with antibiotics, such as doxycycline and azithromycin, which do not increase seizure risk, it should be considered in the differential diagnosis for patients presenting with seizures or encephalitis, especially in endemic areas.
Keywords: cortical multifocal myoclonus, meningoencephalitis, scrub typhus, status epilepticus
Introduction
Scrub typhus, an acute febrile infectious disease highly prevalent in the Asia Pacific region (tsutsugamushi triangle), is a mite-born rickettsial zoonosis caused by Orientia tsutsugamushi. 1 This intracellular Gram-negative organism targets endothelial cells, causing vasculitis and leading to multisystem involvement. 1
Meningoencephalitis is the most common neurological manifestation of scrub typhus. However, other neurological manifestations include cranial nerve paresis, transverse myelitis, and polyneuropathy (including Guillain-Barré syndrome).1,2 Further rarer neurological manifestations include limbic encephalitis, 3 cerebral venous sinus thrombosis, 4 diaphragmatic myoclonus, 5 pure alexia as part of disconnection syndromes due to transient splenial lesion, 6 posterior reversible encephalopathy syndrome, 7 Opalski syndrome, 7 parkinsonism, cerebellitis, 7 isolated opsoclonus and opsoclonus myoclonus (with or without ataxia),7,8 myositis, polyradiculoneuropathy with cranial neuropathy, 7 acute transient behavioral changes, 7 and fibromyalgia. Status epilepticus has been rarely reported as a presenting manifestation of scrub typhus. 9 Specifically, a case series reported that 10 out of 66 cases (15.2%) developed status epilepticus, but all had normal brain magnetic resonance imaging (MRI) findings and good outcomes. 9
Although scrub typhus can manifest as limbic encephalitis, 3 there are no previous descriptions of neuroradiological patterns suggestive of autoimmune encephalitis affecting basal ganglia (caused by anti-CV2, anti-D2, and/or anti-NMDA receptor antibodies) or extra-limbic cortices (caused by anti-NMDAr, anti-voltage-gated calcium channel, anti-GABA-A, and/or anti-GluR3). 10
We report a patient with scrub typhus meningoencephalitis that manifested as autoimmune encephalitis with basal ganglia and extra-limbic cortices involvement. The patient presented with generalized convulsive status epilepticus complicated by cortical multifocal myoclonus, a combination not previously reported in this infectious disease.
Case Report
A 23-year-old previously healthy woman from rural West Bengal, India, was brought to the emergency department in an unconscious state. She had a history of abrupt onset multiple bilateral tonic-clonic seizures for the last 6 hours, between which there was no recovery of consciousness. Additionally, she had experienced abrupt onset, intermittent, high-grade fever for the last 5 days associated with headache, neck stiffness, nausea, and vomiting.
On rapid neurological examination, the patient presented with altered consciousness, pyrexia, and bilateral tonic-clonic seizures occurring in clusters. Given the clinical picture consistent with generalized convulsive status epilepticus, 11 immediate treatment was initiated. The patient received intravenous lorazepam (4 mg IV bolus immediately followed by an additional 4 mg IV after 5 minutes), a loading dose of phenytoin (600 mg IV in 0.9% normal saline), and a loading dose of levetiracetam (1000 mg IV). Vital signs were within normal parameters, except for an elevated core body temperature (40.1°C). Positive meningeal signs were elicited, including nuchal rigidity and positive Kernig's and Brudzinski's signs. A thorough systemic examination revealed no evidence of rash, eschar, lymphadenopathy, hepatosplenomegaly, or jaundice. Fundoscopic examination demonstrated bilateral grade 3 papilledema (according to the modified Frisen scale). Notable clinical findings included a right-side tongue bite and spontaneous bowel and bladder incontinence. The patient was subsequently transferred to the intensive care unit. Empiric antimicrobial therapy was initiated, comprising intravenous ceftriaxone, vancomycin, and acyclovir. Concurrently, a regimen of 4 antiseizure medications (phenytoin, levetiracetam, lacosamide, and clobazam) was administered. This comprehensive approach resulted in complete cessation of seizure activity..
Hematological analysis revealed lymphocytic leukocytosis accompanied by an elevated erythrocyte sedimentation rate. Renal function tests, thyroid function panel, serum glucose levels, and electrolyte concentrations were within reference ranges. Hepatic function tests demonstrated mild transaminitis. A comprehensive serological panel was performed, yielding negative results for the following: hepatitis B and C, human immunodeficiency virus (1, 2), dengue virus, Plasmodium species, Salmonella typhi (typhoid), and leptospira species. Additionally, serological tests for other neurotropic viruses relevant to the clinical presentation were negative.
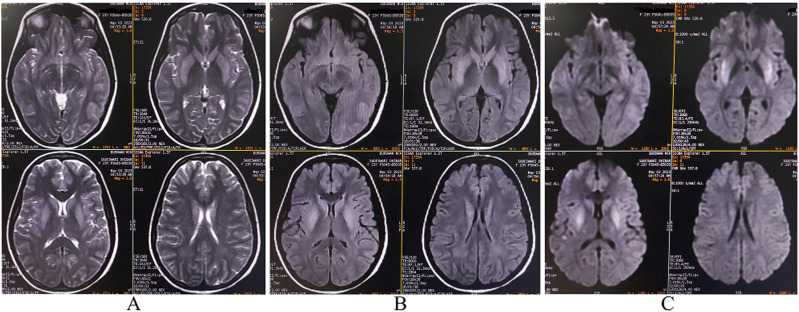
MRI of the brain with gadolinium contrast revealed bilateral symmetrical hyperintense lesions on T2-weighted imaging, T2-fluid-attenuated inversion recovery imaging, and diffusion-weighted imaging. These lesions appeared iso-to-hypointense on apparent diffusion coefficient mapping and were localized to the bilateral basal ganglia, left parieto-temporal cortex, and bilateral basi-frontal cortex. No mass effect was observed. Meningeal involvement was evident, characterized by thickening and enhancement along the bilateral sulci, basal cisterns, and superior cerebellar cisterns. The overall imaging features were consistent with meningoencephalitis. (Figure 1).
Figure 1.
Magnetic resonance imaging of the brain demonstrates bilateral symmetrical hyperintense lesions on T2-weighted imaging (A), fluid-attenuated inversion recovery imaging (B), and diffusion-weighted imaging (C). These lesions are localized to the the bilateral basal ganglia, left parieto-temporal cortex, and bilateral basi-frontal cortex.
Cerebrospinal fluid (CSF) analysis revealed an elevated opening pressure of 30 cm H2O, a cell count of 64/μL (100% lymphocytes), elevated protein levels at 84 mg/dL, glucose levels of 103.8 mg/dL, and adenosine deaminase levels of 1.3 mg/dL. CSF polymerase chain reaction (CSF) assays were negative for several neurotropic viruses, including, Japanese encephalitis, West Nile virus, Zika virus, Nipah virus, rabies virus, herpes simplex virus, and human immunodeficiency virus. Additionally, CSF culture and PCR were negative for Mycobacterium tuberculosis.
Upon further review of the patient’s history, it was noted that the onset of febrile illness was accompanied by an acute change in mental status. This manifested as behavioral problems, including aggressiveness, use of profane language, and self-injurious actions. These, fneuropsychiatric symptoms developed abruptly in conjunction with the initial presentation of fever.
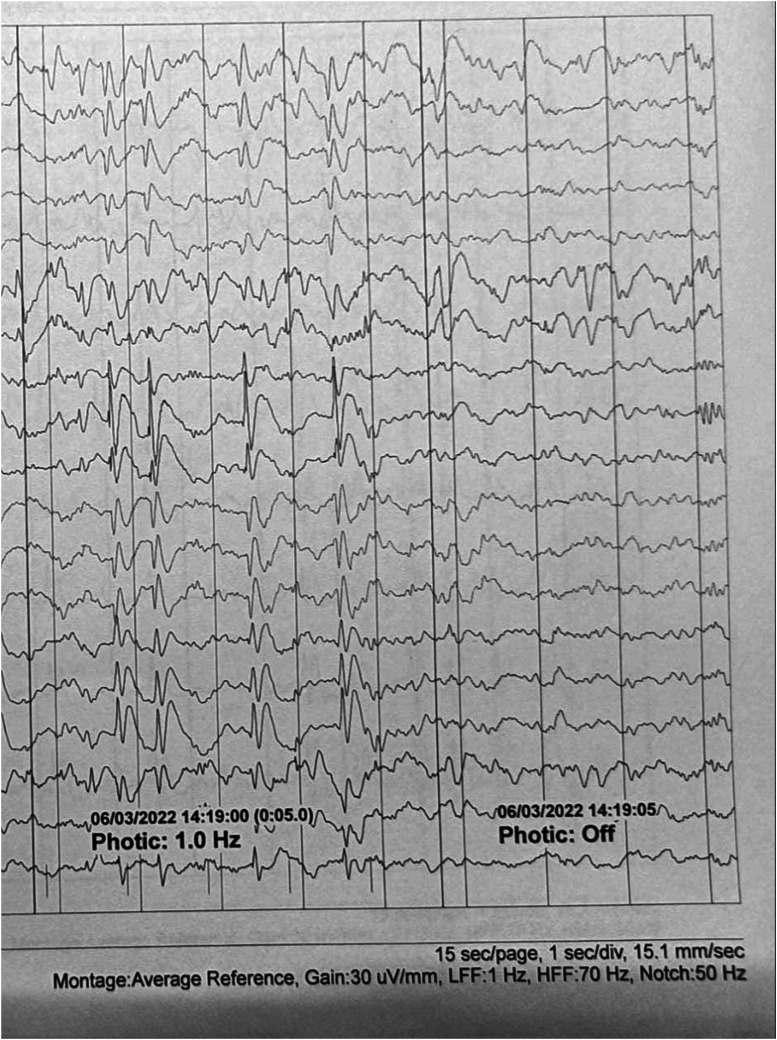
Following the initial 24-hour period, the patient's seizures subsided, but she remained febrile and unresponsive. She subsequently developed myoclonic jerks affecting the tongue, lips, and left upper and lower extremities, consistent with cortical multifocal myoclonus. This clinical diagnosis was confirmed by electroencephalography (EEG), which demonstrated generalized polyspike-and-wave discharges occurring at a frequency of 0.5-1 Hz. These discharges were time-locked with the observed clinical manifestations, meeting the criteria for electroclinical seizures. However, the EEG pattern did not evolve into status epilepticus (Figure 2).
Figure 2.
Electroencephalogram during wakefulness demonstrates paroxysmal generalized 0.5-1 Hz spike-and-wave and polyspike-and-wave discharges of approximately 3-4 seconds duration and up to 600 μV of amplitude with maximum electronegativity in both frontocentral regions (F3-C3>F7-T3>T5/F4-C4>F8-T4>T6). This response is replicated during intermittent photic stimulation at low frequencies (1 Hz). These discharges are time-locked with involuntary and arrhythmic movements involving the tongue, lips, and left upper and lower extremities.. Montage type: average; Recording speed: 30 mm/s; Sensitivity (also known as [aka] “gain”): 30 μV/mm; High-frequency filter: 70 Hz; Low-frequency filter: 1 Hz; Notch filter: 50 Hz cutoff frequency. The interval between 2 continuous vertical lines is equivalent to 1 second.
The differential diagnosis encompassed several possibilities. Viral encephalitis, with particular consideration given to arboviral etiologies, was a primary concern. Autoimmune encephalitis, potentially unmasked by the current illness, was also considered based on the constellation of symptoms including febrile illness, behavioral abnormalities, status epilepticus, presentation resembling febrile infection-related epilepsy syndrome (FIRES), and atypical movement disorders. Additionally, Wilson's disease was evaluated as a potential underlying condition. Serum and CSF samples were analyzed for autoimmune and paraneoplastic encephalitis panels, with negative results. Furthermore, serum ceruloplasmin levels and both serum and urinary copper concentrations were within normal limits, effectively ruling out Wilson's disease.
Serum and CSF were tested for Orientia tsutsugamushi IgM using enzyme immunoassay, yielding positive results. These findings were further corroborated by immunochromatography and Weil-Felix tests. Blood and CSF cultures were negative for other microbial pathogens. Based on these results, a definitive diagnosis of scrub typhus meningoencephalitis was established. Consequently, acyclovir and vancomycin were discontinued, and oral doxycycline therapy was initiated via nasogastric tube. The patient's clinical status began to improve within 48 hours, as evidenced by gradual return of consciousness and ability to follow commands. Defervescence occurred after 4 days of doxycycline administration, with no recurrence of seizures. Following 14 days of antibiotic treatment, the patient demonstrated complete orientation to time and place, remained seizure-free, and experienced resolution of myoclonus. At the 3 months follow-up evaluation, the patient exhibited no residual neurological deficits, was maintained on levetiracetam monotherapy (750 mg twice daily), and demonstrated complete resolution of the previously noted MRI abnormalities.
Discussion
While a case of scrub typhus presenting as limbic autoimmune encephalitis has been previously documented, 3 this report represents the first instance demonstrating radiological evidence of basal ganglia and extra-limbic cortical involvement, manifesting clinically with generalized convulsive status epilepticus and subsequent development of myoclonus.
Orientia tsutsugamushi invades the endothelial and macrophage-monocyte systems in the periphery, subsequently gaining access to the central nervous system via hematogenous dissemination. 1 The host response to this neuroinvasion results in the activation of specific transcription factors: nuclear factor-kappa B (NF-κB) in macrophages, and both NF-κB and activator protein 1 (AP-1) in endothelial cells. This activation cascade leads to the expression of various chemokine genes. In macrophages, this includes macrophage inflammatory protein 1α/β (MIP-1α/β), MIP-2, and monocyte chemoattractant protein-1 (MCP-1). Endothelial cells produce MCP-1, interleukin-8 (IL-8), and regulated upon activation, normal T cell expressed and secreted (RANTES/CCL5). 1 These inflammatory processes culminate in a range of neuropathological changes, including parenchymal inflammation, leptomeningeal infiltration, perivasculitis, infarction, demyelination, and formation of typhus nodules, which collectively contribute to the observed neurological manifestations. The resulting clinical features may arise from direct damage to specific anatomic areas, leading to altered functioning of those regions, or they may be immune-mediated. 1
Myoclonus is a hyperkinetic movement disorder characterized by sudden, brief, involuntary movements involving a single or group of muscles. 12 It can be classified according to etiology into primary or secondary to an underlying disorder.5,12,13 By distribution, myoclonus is classified as focal, multifocal, or generalized, and by provoking factors as spontaneous and reflex. 12 The anatomical origin can be subdivided into cortical, subcortical, spinal, and peripheral myoclonus. 12 Our patient developed cortical myoclonus, which mainly affects the hands and face and is predominantly triggered by tactile stimuli. 12 Differentiating myoclonic jerks from other hyperkinetic movement disorders may be challenging. EEG can be particularly valuable for diagnosing epileptic (cortical) myoclonus (as in our patient), typically manifesting as generalized spike/polyspike-and-wave complexes. 14
In the differential diagnosis, autoimmune encephalitis or a para-infectious autoinflammatory syndrome such as FIRES should be considered. FIRES typically presents with prior febrile infection usually occurring between 24 hours and 2 weeks before the abrupt onset of super-refractory status epilepticus. However, an exhaustive evaluation for infectious causes remains crucial, particularly to exclude pathogens endemic to the region. The etiology of FIRES remains elusive, although growing evidence suggests a heterogeneous origin resulting in fulminant non-antibody-mediated neuroinflammation. 15 Similar clinico-radiological presentations can be observed in arthropod-borne viral encephalitides, such as as those caused by West Nile and Japanese encephalitis virus. However, the clinical course in this case would be atypical for autoimmune encephalitides such as those associated with anti-CV2, anti-NMDA receptor, anti-voltage-gated calcium channel, and anti-GABA-A receptor antibodies. These autoimmune conditions typically present with a subacute onset, evolving over weeks to months.10,16
In conclusion, scrub typhus can present as a significant diagnostic challenge, potentially manifesting with generalized convulsive status epilepticus. Its clinical and radiological features may mimic those of arthropod-borne viruses such as West Nile and Japanese Encephalitis viruses. 17 or even autoimmune encephalitis, as demonstrated in this case. Given that scrub typhus is amenable to treatment with antibiotics that do not increase seizure risk, such as doxycycline and azithromycin, 18 it warrants consideration in the differential diagnosis of patients presenting with seizures or encephalitis, particularly in endemic regions. EEG can play a crucial role in guiding clinical decision-making and subsequent management strategies. 14 Finally, there is a need for more comprehensive, prospective studies to elucidate the evolution and prognosis of this newly recognized radiological and phenotypic neurological manifestation of scrub typhus.
Footnotes
Author Contributions: All authors contributed significantly to the creation of this manuscript; each fulfilled the criterion as established by the ICMJE.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Julián Benito-León disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health, Bethesda, MD, USA (NINDS #R01 NS39422), the European Commission (grant ICT-2011-287739, NeuroTREMOR), the Ministry of Economy and Competitiveness (grant RTC-2015-3967-1, NetMD–platform for the tracking of movement disorder), the Spanish Health Research Agency (grant FIS PI12/01602 and grant FIS PI16/00451) and The Recovery, Transformation and Resilience Plan at the Ministry of Science and Innovation (grant TED2021-130174B-C33, NETremor)
Ethics Statement
Informed Consent
Written informed consent was obtained from the patient for the publication of this case report and accompanying images.
ORCID iDs
Ritwik Ghosh https://orcid.org/0000-0002-8192-0807
Julian Benito-León https://orcid.org/0000-0002-1769-4809
Moisés León-Ruiz https://orcid.org/0000-0002-1766-4183
References
- 1.Rana A, Mahajan SK, Sharma A, Sharma S, Verma BS, Sharma A. Neurological manifestations of scrub typhus in adults. Trop Doct 2017;47(1):22-25. doi: 10.1177/0049475516636543 [DOI] [PubMed] [Google Scholar]
- 2.Ghosh R, Biswas S, Mandal A. et al. Scrub typhus presenting as unilateral abducens nerve palsy. Neuro Ophthalmol. 2021;46(2):99-103. doi: 10.1080/01658107.2021.1909073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biswas S, Ghosh R, Roy D. et al. Scrub typhus masquerading as limbic encephalitis. Neurohospitalist. 2022;12(1):105-110. doi: 10.1177/19418744211016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biswas U, Ghosh R, Chakraborty A, et al. Cerebral venous sinus thrombosis following scrub typhus infection: a case report and a review of the literature. Med Res Arch 2022;10(10):10.18103/mra.v10i10.3196. doi: 10.18103/mra.v10i10.3196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosh R, León-Ruiz M, Bandyopadhyay S, Roy D, Benito-León J. Scrub typhus presenting as diaphragmatic myoclonus. Neurol Sci 2022;43(6):4023-4024. doi: 10.1007/s10072-022-06021-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosh R, Dubey S, Roy D, Benito-León J. Pure alexia as a presenting manifestation of scrub typhus. Neurologia. 2023;38(4):307-309. doi: 10.1016/j.nrleng.2022.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh R, Mandal A, León-Ruiz M. et al. Rare neurological and neuropsychiatric manifestations of scrub typhus: a case series of 10 cases. Neurologia. 2022. 28(22):S2173-5808. doi: 10.1016/j.nrleng.2022.07.001. Epub ahead of print. PMID: 35907627. [DOI] [PubMed] [Google Scholar]
- 8.Ralph R, Prabhakar AT, Sathyendra S, Carey R, Jude J, Varghese GM. Scrub typhus-associated opsoclonus: clinical course and longitudinal outcomes in an indian cohort. Ann Indian Acad Neurol. 2019;22(2):153-158. doi: 10.4103/aian.AIAN_198_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalita J, Mani VE, Bhoi SK, Misra UK. Status epilepticus in scrub typhus. Epilepsia. 2016;57(7):e125-e128. doi: 10.1111/epi.13412. [DOI] [PubMed] [Google Scholar]
- 10.Patel A, Meng Y, Najjar A, Lado F, Najjar S. Autoimmune encephalitis: a physician’s guide to the clinical spectrum diagnosis and management. Brain Sci. 2022;12(9):1130. doi: 10.3390/brainsci12091130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trinka E, Cock H, Hesdorffer D. et al. A definition and classification of status epilepticus--report of the ILAE task force on classification of status epilepticus. Epilepsia. 2015;56(10):1515-1523. doi: 10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- 12.Kojovic M, Cordivari C, Bhatia K. Myoclonic disorders: a practical approach for diagnosis and treatment. Ther Adv Neurol Disord. 2011;4(1):47-62. doi: 10.1177/1756285610395653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh R, Maity A, Biswas U, Das S, Benito-León J. Lance-Adams syndrome: an unusual complication of snakebite envenomation. Toxicon 2022;209:50-55. doi: 10.1016/j.toxicon.2022.02.008 [DOI] [PubMed] [Google Scholar]
- 14.Abu-Hegazy M, Elmoungi A, Eltantawi E, Esmael A. Electrophysiological characteristics and anatomical differentiation of epileptic and non-epileptic myoclonus. Egypt J Neurol Psychiatry Neurosurg 2021;57:124. doi: 10.1186/s41983-021-00374-5 [DOI] [Google Scholar]
- 15.Specchio N, Wirrell EC, Scheffer IE. et al. International league against epilepsy classification and definition of epilepsy syndromes with onset in childhood: position paper by the ILAE task force on nosology and definitions. Epilepsia. 2022;63(6):1398-1442. doi: 10.1111/epi.17241. [DOI] [PubMed] [Google Scholar]
- 16.Graus F, Titulaer MJ, Balu R. et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mangat R, Louie T. Arbovirus Encephalitides. Treasure Island (FL): StatPearls Publishing; 2024. https://www.ncbi.nlm.nih.gov/books/NBK560866/ [PubMed] [Google Scholar]
- 18.Wanleenuwat P, Suntharampillai N, Iwanowski P. Antibiotic-induced epileptic seizures: mechanisms of action and clinical considerations. Seizure 2020;81:167-174. doi: 10.1016/j.seizure.2020.08.012 [DOI] [PubMed] [Google Scholar]