Abstract
Introduction
Rapid correction of hyponatremia can result in osmotic demyelination syndrome (ODS). Sheehan’s syndrome, a rare pituitary disorder caused by severe postpartum hemorrhage, is a potential cause of chronic hyponatremia. This case report describes a rare progression of extrapontine myelinolysis to central pontine myelinolysis, ultimately leading to ODS, following the correction of chronic hyponatremia associated with Sheehan’s syndrome. Notably, this event occurred a decade after the initial postpartum hemorrhage due to placenta previa.
Case Report
A 40-year-old woman from rural West Bengal, India, presented in a comatose state after five years of progressively worsening symptoms, including fatigue, gastrointestinal disturbances, cold intolerance, hair loss, and severe apathy, which had been misdiagnosed as psychogenic and treated with selective serotonin reuptake inhibitors. Two days before her admission to our hospital, she was diagnosed with a lower respiratory tract infection, dehydration, and severe hyponatremia (118 mEq/L) at a local private healthcare facility. Despite treatment with 3% sodium chloride and intravenous antibiotics, her condition deteriorated, prompting her transfer. At the time of hospitalization, the patient was diagnosed with chronic hyponatremia and hypopituitarism consistent with Sheehan’s syndrome. This condition was attributed to a severe postpartum hemorrhage that occurred a decade prior, resulting from placenta previa. Initial MRI revealed extrapontine myelinolysis, and the correction of her “compensated” hyponatremia was identified as the cause of her neurological decline. Follow-up MRIs at 7 and 14 weeks confirmed the development of cavitating ODS.
Discussion
This case highlights several key points: First, even a relatively gradual correction of hyponatremia can precipitate ODS, especially in patients with chronic conditions like Sheehan’s syndrome. Second, it underscores the importance of meticulous management of chronic hyponatremia to prevent severe neurological outcomes. Third, it illustrates the diagnostic challenges of differentiating Sheehan’s syndrome from primary psychiatric disorders, particularly in low-resource settings where the syndrome remains prevalent. The case also emphasizes the need for awareness among healthcare providers about the potential for severe complications arising from even minor corrections in serum sodium levels in such patients.
Keywords: extrapontine myelinolysis, central pontine myelinolysis, sheehan’s syndrome, chronic hyponatremia, postpartum hemorrhage, placenta previa, cavitating osmotic demyelination syndrome, rapid electrolyte correction, panhypopituitarism, neurological complications, postpartum endocrinopathy
Introduction
Hyponatremia is the most common electrolyte disorder, occurring in up to 25% of hospitalized patients. 1 It is classified as either acute (lasting less than 48 hours) or chronic (persisting for more than 48 hours). 1 Osmotic demyelination syndrome (ODS) encompasses both central pontine myelinolysis (CPM) and extrapontine myelinolysis (EPM). It typically occurs following rapid and significant shifts in osmolarity, most commonly due to the rapid correction of hyponatremia from any cause.1–3 Advanced neuroimaging techniques have expanded the ability to identify a broader spectrum of cases, though ODS remains a rare condition. 2 ODS is characterized by a range of movement disorders, including tremors, ataxia, mutism, parkinsonism, dystonia, catatonia, and, in rare cases, jaw clonus.2,4
Sheehan’s syndrome, also known as postpartum hypopituitarism, is a life-threatening endocrine emergency caused by ischemic pituitary necrosis. While rare in developed countries, it remains a significant cause of hypopituitarism in developing countries like India, primarily due to postpartum hemorrhage (PPH).5-7 Symptoms typically become evident years after delivery, although in rare cases, they can develop acutely. 5 Diagnosis is based on clinical manifestations combined with a history of PPH, with hormone levels and/or stimulation tests used to confirm clinical suspicion. Hormone replacement therapy is the only available management option. 5
We report a case of cavitating ODS, initially presenting as EPM and later progressing to CPM, caused by the rapid correction of chronic hyponatremia related to Sheehan’s syndrome. This occurred one decade after an episode of PPH due to placenta previa.
Case Report
A 40-year-old female from rural West Bengal, India, was admitted to a local private healthcare facility on December 19, 2023, with complaints of fever, cough, and disorientation for the last three days. She was clinically diagnosed with a lower respiratory tract infection associated with dehydration and severe hyponatremia, (with an initial serum sodium level of 118 mEq/L recorded at the time of admission). She was treated with 3% sodium chloride (NaCl) and intravenous antibiotics (amoxicillin-clavulanate), along with other supportive measures. Within 24 hours of receiving 3% NaCl, her sodium levels increased from 118 to 124 mEq/L. However, she experienced a single episode of brief hypoglycemia (capillary blood glucose: 47 mg/dL), which was promptly managed at the healthcare facility.
Due to further deterioration in her condition, she was referred to our hospital. Upon arrival at the emergency department on the morning of December 21, 2023, she was found to be in a deep comatose state (E1/V1/M1), with pupils sluggishly but equally reactive to light and bilaterally mute plantar responses. Her vital signs were recorded as blood pressure 86/50 mmHg, pulse 68/min, capillary blood glucose 64 mg/dL, and respiratory rate 12/min. Physical examination revealed the loss of both axillary and pubic hair, along with madarosis.
The complete blood cell count revealed normocytic normochromic anemia (hemoglobin 9.0 g/dL) with lymphocytosis (total leukocyte count 8,700/mm³, lymphocytes 42%) and eosinophilia (eosinophils 10%), along with an elevated erythrocyte sedimentation rate (40 mm/hr). Her HbA1c was 4.3%, and hepatic function tests were within normal limits. Renal function tests indicated mild prerenal azotemia (FeNa <1; blood urea nitrogen/creatinine ratio >20). Thyroid function tests revealed central hypothyroidism (Table 1).
Table 1.
Laboratory Parameters of the Patient.
| Plasma Hormone | Patient Value | Reference range |
|---|---|---|
| TSH | 2.84 mIU/ml | 0.4-4.2 |
| FT4 | 0.6 ng/dl | 0.8-2 |
| FT3 | 0.35 ng/ml | 1.4-4.2 |
| 8 AM CORTISOL | 2 μg/dl | 3.7-19.4 |
| FSH | 9.40 mIU/ml | 35-151 (post-menopausal) |
| LH | 4.40 mIU/ml | 8.2-40.8 (post-menopausal) |
| Prolactin | 1 ng/dl | 1.5-18.5 (post-menopausal) |
| IGF 1 | 96 ng/ml | 103-258 (40-49, age group) |
| 8 AM ACTH | 4 pg/ml | 10-60 |
Despite receiving 3% NaCl therapy at the previous healthcare facility, her serum sodium remained low at 124 mEq/L, and her potassium level was mildly elevated at 5.0 mEq/L. Serum calcium was also mildly increased, likely due to dehydration. She required continuous glucose infusion to maintain euglycemia. Arterial blood gas analysis indicated mild metabolic acidosis. Suspecting an Addisonian crisis, she was administered intravenous fluids along with a high dose of intravenous hydrocortisone (100 mg every eight hours). Levothyroxine (25 mcg/day) was initiated two days later.
Upon revisiting the patient’s history, it was revealed that she had experienced excessive vaginal bleeding due to delayed placental expulsion caused by placenta previa during her last childbirth ten years ago. She received three units of packed red blood cells following the delivery. Subsequently, she was unable to lactate, remained amenorrheic, and faced difficulties conceiving. However, she never sought gynecological evaluation for these issues.
Over the past five years, the patient reported experiencing progressive generalized fatigue, decreased work capacity, abdominal pain, nausea, vomiting, diarrhea, anorexia, dizziness, cold intolerance, and a gradual loss of axillary and pubic hair. These symptoms worsened significantly over the last month, leading to extreme fatigue even at rest, excessive sleepiness, generalized slowness, a profoundly low mood, loss of interest in daily activities, severely reduced appetite, apathy, markedly diminished communicative abilities, and intermittent headaches. Despite these issues, her symptoms were consistently managed as “psychogenic/somatic,” and she was prescribed various selective serotonin reuptake inhibitors (SSRIs) on multiple occasions over nearly four years.
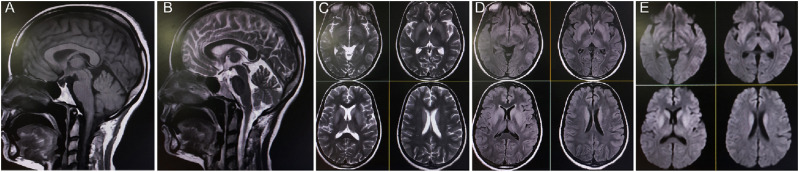
Notably, her previous medical records indicated multiple blood reports confirming persistent hyponatremia, with sodium levels ranging from 123 to 132 mEq/L, for at least the past three years. In the three months prior to admission, she had been prescribed oral tolvaptan (15 mg/day for five days, once each month) by local physicians to manage hyponatremia when her blood sodium concentration dropped below 125 mEq/L. The prolonged use of several high-dose SSRIs for treating refractory depressive symptoms may have contributed to the development of chronic hyponatremia. However, pituitary hormonal panels revealed panhypopituitarism (Table 1). Brain magnetic resonance imaging (Figures 1 and 2) conducted on December 24, 2023, showed an empty sella turcica and symmetrical hyperintensity in the bilateral caudate and putamen nuclei, along with the globus pallidus, consistent with EPM.
Figure 1.
Sagittal T1-weighted (A) and T2-weighted (B) images reveal an empty sella turcica. Axial T2-weighted (C), T2-fluid-attenuated inversion recovery (D), and diffusion-weighted images (E) demonstrate symmetrical hyperintensity involving the bilateral caudate and putamen nuclei, as well as the globus pallidus, consistent with extra-pontine myelinolysis.
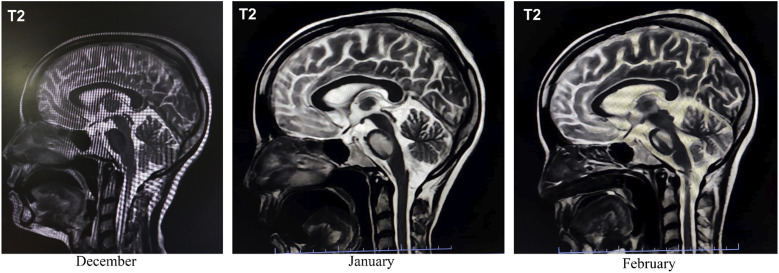
Figure 2.
Serial sagittal T2-weighted MRI scans demonstrate the temporal evolution of the condition over three months. The December scan shows normal morphology with no evident lesions. By January, hyperintense signal changes within the pons become apparent, indicating the early stage of central pontine myelinolysis. The February scan reveals further progression of the pontine hyperintensity, consistent with ongoing osmotic demyelination. These images collectively document the dynamic nature of central pontine myelinolysis.
The clinical history and laboratory values pointed to chronic hyponatremia related to Sheehan’s syndrome. This condition was attributed to a severe PPH that occurred a decade prior, resulting from placenta previa. The non-rapid correction of her chronic and “compensated” hyponatremia, which disrupted the internal cerebral osmotic milieu, was identified as the critical factor leading to her severe neurological condition. This disruption was a consequence of the previously unrecognized Sheehan’s syndrome.
Following the administration of intravenous fluids and hydrocortisone, the patient’s resistant hypotension, azotemia, hyponatremia, and recurrent spontaneous hypoglycemia improved rapidly, along with a marked improvement in her consciousness. However, repeated neurological examinations revealed features of parkinsonism, including a hypokinetic-rigid-mute state with bilateral upper limb dystonic posturing. After six weeks of admission, she was discharged in a hemodynamically stable condition with the following medications: oral trihexyphenidyl (3 mg/day), clonazepam (1 mg/day), levodopa/carbidopa 100/25 mg (four times/day), hydrocortisone (15 mg/day), levothyroxine (50 mcg/day), and pramipexole (1 mg/day). Growth hormone and gonadal hormone replacement therapy were considered but could not be initiated due to infrastructural and financial constraints.
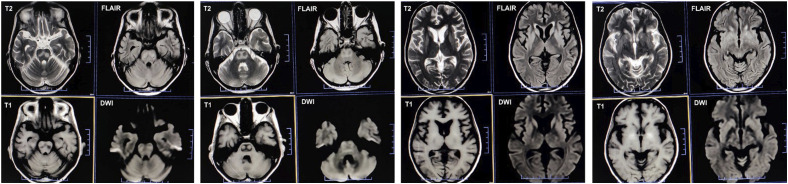
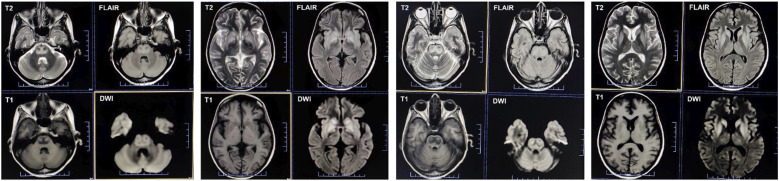
At the seven-week follow-up, a repeat brain MRI (Figures 2 and 3) revealed further subacute changes in the central pons, along with the previously noted extrapontine alterations, reinforcing the radiological diagnosis of ODS. Over subsequent monthly follow-ups, her neurological symptoms progressively improved. By the 14-week follow-up post-admission, she was able to comprehend and execute all commands. Although her speech remained monotonous, hypophonic, and dysarthric, she required only minimal assistance to walk. An additional brain MRI (Figures 2 and 4) showed mild resolution of the earlier changes. The hydrocortisone dosage was reduced to 10 mg/day, and she was prescribed oral supplements of calcium, vitamin D, bisphosphonate, and potassium.
Figure 3.
Seven-week follow-up axial MRI sequences depict the progression of osmotic demyelination syndrome. T2-weighted images (first row, first and third columns) and fluid-attenuated inversion recovery images (first row, second and fourth columns) show evolving hyperintense lesions in the central pons, resembling “goggles,” indicative of cavitating central pontine myelinolysis. T1-weighted images (second row, first and third columns) reveal characteristic hypointense areas corresponding to the same pontine region. Diffusion-weighted imaging (second row, second and fourth columns) demonstrates restricted diffusion within these lesions, consistent with the cytotoxic edema associated with acute demyelination.
Figure 4.
Fourteen-week follow-up axial MRI sequences illustrate the progression of osmotic demyelination syndrome. T2-weighted and fluid-attenuated inversion recovery images (first row) display a mild reduction in the intensity of the pontine lesions, suggesting partial resolution. The corresponding T1-weighted images (second row, first and third columns) show persistent hypointensity, while diffusion-weighted imaging sequences (second row, second and fourth columns) indicate diminished restricted diffusion, correlating with the evolving stage of myelin repair and the resolution of edema.
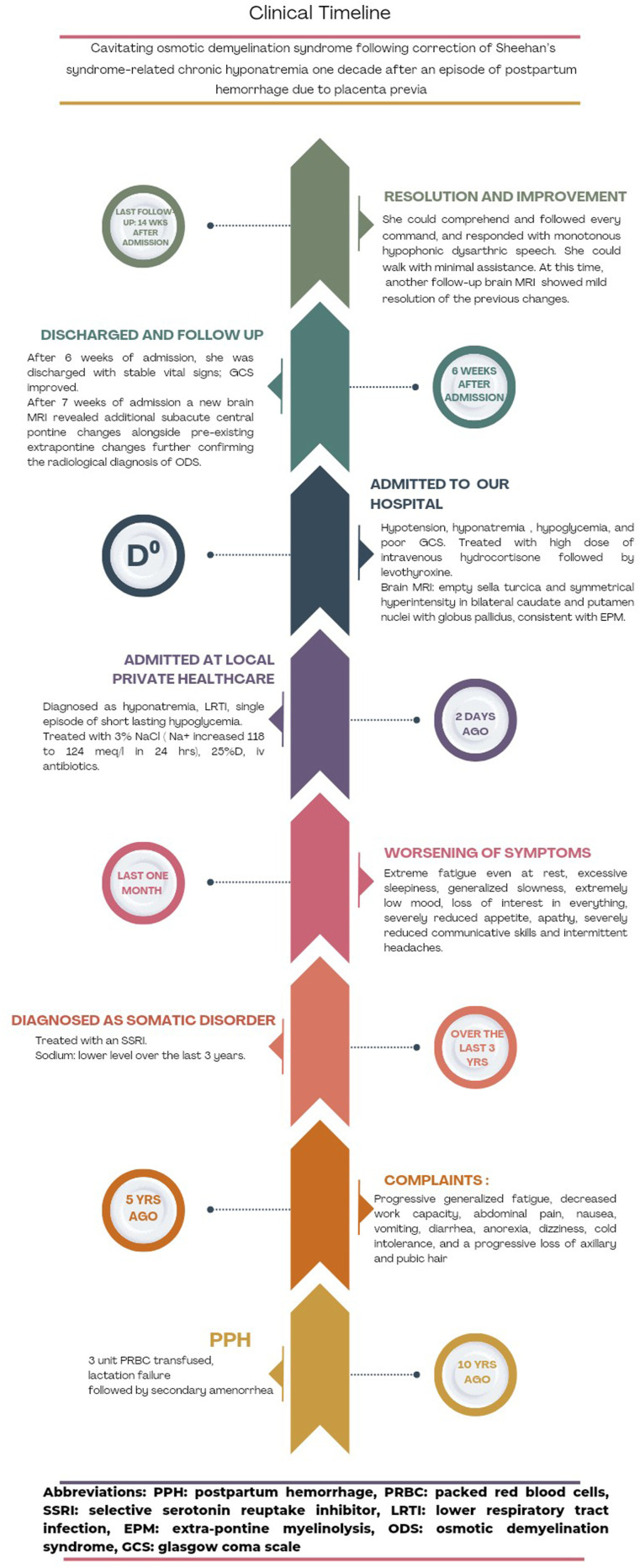
The patient’s clinical timeline is depicted in Figure 5.
Figure 5.
Patient’s clinical timeline.
Discussion
To our knowledge, this is the first reported case of EPM caused by the relatively rapid correction of chronic hyponatremia related to Sheehan’s syndrome, occurring a decade after an episode of PPH due to placenta previa. In the most similar reported case, 2 Sheehan’s syndrome led to repeated episodes of hyponatremia over two years, with the PPH not associated with placenta previa, unlike in our case. Placenta previa, a condition in which the placenta partially or completely covers the cervix during pregnancy, is a significant risk factor for PPH and can result in considerable morbidity and mortality for both the mother and the neonate. 8
Hypoglycemic encephalopathy was considered among the differential diagnoses. The patient had only a single episode of hypoglycemia, which was quickly identified and promptly addressed. Additionally, she was under continuous monitoring throughout her hospitalization, making an undetected episode of prolonged asymptomatic hypoglycemia unlikely. Nonetheless, subsequent first and second follow-up MRIs revealed further evidence of EPM and CPM.
Regarding the pathophysiological mechanisms of Sheehan’s syndrome, PPH leads to impaired blood supply to the pituitary gland, which becomes enlarged during pregnancy. Predisposing factors include a small sella turcica, vasospasms induced by PPH, and/or thrombosis associated with pregnancy or coagulation disorders. Additionally, autoimmunity may play a role in the progressive deterioration of pituitary function.5,6
The clinical presentation of Sheehan’s syndrome can mimic other conditions. Symptoms arise from the decrease or absence of one or more pituitary hormones and can range from failure to lactate and nonspecific symptoms like fatigue to severe adrenal crises. Due to the arrangement of hormone-secreting cells relative to the vasculature, the secretion of growth hormone and prolactin is most commonly affected, followed by follicle-stimulating hormone and luteinizing hormone. In cases of severe pituitary necrosis, the secretion of thyroid-stimulating hormone and adrenocorticotropic hormone is also impaired. 5
Hyponatremia can be a presenting manifestation of Sheehan’s syndrome. 2 Hyponatremia associated with Sheehan’s syndrome can be chronic or may appear in the early postpartum period. 2 Antidiuretic hormone (ADH) plays a role in the pathogenic mechanism of hyponatremia in Sheehan's syndrome. However, the cause of ADH secretion in hyponatremia associated with hypopituitarism is primarily related to adrenocortical insufficiency. 2 The glucocorticoid deficit acts as a physiological, rather than osmotic, stimulus for ADH secretion. 2 Glucocorticoid substitution is the mainstay treatment for hyponatremia associated with hypopituitarism, as it enhances the renal excretion of solute-free water. 2
The chronicity of hyponatremia, along with the magnitude and speed of its correction, are the most critical risk factors for the development of ODS during hyponatremia correction. 1 Neuroimaging, typically performed 2-3 days after the correction of hyponatremia, often reveals radiological signs of myelin loss in various brain regions, including but not limited to the pons. 1 Extrapontine lesions are at least as common as pontine lesions, which are rarely isolated. 1 Chronic hyponatremic encephalopathy is generally less dramatic than its acute counterpart and is characterized by symptoms such as malaise, nausea, gait disturbances, attention deficits, and mild confusion. 1
From a neurological perspective, patients with serum sodium levels above 128-130 mEq/L are considered to be at a reduced risk for brain complications during the correction of hyponatremia. 1 Several agents are effective in treating chronic hyponatremia, depending on the underlying cause, including urea, vaptans, fluid restriction, and salt tablets. 1 Recent reports indicate that up to half of patients with ODS can experience significant recovery. 1
This case is significant for several reasons. First, it demonstrates that even a relatively gradual correction of hyponatremia can lead to ODS. Second, it underscores the critical importance of meticulous management when correcting chronic hyponatremia. Third, it highlights that chronic hyponatremia, particularly in the context of panhypopituitarism (as seen in Sheehan’s syndrome), is often long-standing and well-tolerated due to the brain’s adaptive mechanisms that mitigate the effects of chronic hyponatremia and hypo-osmolarity. However, as this case illustrates, even minor acute increases in serum sodium levels can precipitate ODS.
Furthermore, chronic panhypopituitarism (including Sheehan’s syndrome) can masquerade as a primary psychiatric illness, which can easily create a diagnostic dilemma and lead to misdiagnosis and therapeutic delay.9-11 The prescription of antidepressants, particularly SSRIs and serotonin-norepinephrine reuptake inhibitors, can exacerbate pre-existing hyponatremia. 12
It is crucial to emphasize the distinctive radiological feature observed in our case, known as the pontine “goggles” appearance, which strongly suggests cavitation. 13 This unique pattern warrants further evaluation in larger patient cohorts to validate its diagnostic utility, as cavitation can also occur in conditions such as pontine infarction or severe demyelination.
The authors recommend that a history of early (premature) menopause and lactation failure following a delivery complicated by PPH should always be considered when treating middle-aged female patients with apathy syndrome who present with recurrent hyponatremia and spontaneous hypoglycemic episodes. This is particularly important in rural Indian settings, where Sheehan’s syndrome remains prevalent.
Strict vigilance, accurate differentiation of pituitary disease-associated apathy syndrome from depressive syndrome, 11 timely diagnosis, and appropriate management of Sheehan’s syndrome-related hyponatremia are essential to avoid diagnostic delays and improve patient outcomes. 7
Acknowledgements
Dr. J. Benito-León is supported by the National Institutes of Health, Bethesda, MD, USA (NINDS #R01 NS39422), the European Commission (grant ICT-2011- 287739, NeuroTREMOR), the Ministry of Economy and Competitiveness (grant RTC-2015-3967-1, NetMD—platform for the tracking of movement disorder), the Spanish Health Research Fund (grant FIS PI12/01602 and grant FIS PI16/00451) and The Recovery, Transformation, and Resilience Plan at the Ministry of Science and Innovation (grant TED2021-130174B-C33, NETremor).
Footnotes
Author Contributions: All authors contributed significantly to the creation of this manuscript; each fulfilled the criterion established by the ICMJE.
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Julián Benito-León is supported by the National Institutes of Health, Bethesda, MD, USA (NINDS #R01 NS39422), the European Commission (grant ICT-2011- 287739, NeuroTREMOR), the Ministry of Economy and Competitiveness (grant RTC-2015-3967-1, NetMD—platform for the tracking of movement disorder), the Spanish Health Research Fund (grant FIS PI12/01602 and grant FIS PI16/00451) and The Recovery, Transformation, and Resilience Plan at the Ministry of Science and Innovation (grant TED2021-130174B-C33, NETremor).
Disclosures: Dr Alamgir Shaikh (alamraju6950@gmail.com) reports no relevant disclosures. Dr Moisés León-Ruiz (pistolpete271285@hotmail.com) reports no relevant disclosures. Dr Ritwik Ghosh (ritwikmed2014@gmail.com) reports no relevant disclosures. Dr Manoj Soren (dr.msmanoj@gmail.com) reports no relevant disclosures. Dr Bilwatosh Mukhopadhyay (dr.bilwatosh@gmail.com) reports no relevant disclosures. Dr Shyamal Kanti Pal (drshyamalkantipal1973@gmail.com) reports no relevant disclosures. Dr Julián Benito-León (jbenitol67@gmail.com) reports no relevant disclosures.
Ethical Statement
Informed Consent
Written informed consent was obtained from the patient participating in the study (consent for research).
ORCID iDs
Ritwik Ghosh https://orcid.org/0000-0002-8192-0807
Julian Benito-Leon https://orcid.org/0000-0002-1769-4809
Moisés León Ruiz https://orcid.org/0000-0002-1766-4183
References
- 1.GankamKengne F. Adaptation of the brain to hyponatremia and its clinical implications. J Clin Med. 2023;12:1714. doi: 10.3390/jcm12051714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siddiqui FM, Siddiqui MR, Hoque A, Shujon SR, Hossain A. Selective extrapontine myelinolysis in osmotic demyelination syndrome in a case of previously undiagnosed Sheehan’s syndrome with recurrent hyponatraemia - a rare association. J Med. 2011;12:77-80. [Google Scholar]
- 3.Ghosh R, Ray A, Roy D, Das S, Dubey S, Benito-León J. Parkinsonism with akinetic mutism following osmotic demyelination syndrome in a SARS-CoV-2 infected elderly diabetic woman: a case report. Neurologia. 2022;37:706-708. doi: 10.1016/j.nrleng.2021.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosh R, Roy D, Dubey S, Lahiri D, Chatterjee S, Finsterer J. Jaw clonus in neuromyelitis optica spectrum disorder with subsequent osmotic demyelination syndrome. J Fam Med Prim Care. 2020;9:1209-1211. doi: 10.4103/jfmpc.jfmpc_1117_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karaca Z, Laway BA, Dokmetas HS, Atmaca H, Kelestimur F. Sheehan syndrome. Nat Rev Dis Prim. 2016;2:16092. doi: 10.1038/nrdp.2016.92 [DOI] [PubMed] [Google Scholar]
- 6.Ghosh R, León-Ruiz M, Roy D, et al. Panhypopituitarism and central diabetes insipidus almost three decades after russell's viper envenomation: a remarkable case report and literature review. Med Res Arch. 2022;10: 3195. doi: 10.18103/mra.v10i10.3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siddiqui SS, Dominic N, Kumar S, et al. A challenging diagnosis of sheehan's syndrome in non-obstetric critical care and emergency settings: a case series of five patients with varied presentations. J Crit Care Med (TarguMures). 2022;8:214-222. doi: 10.2478/jccm-2022-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson Bagga FM, Sze A. Placenta previa. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK539818/ [PubMed] [Google Scholar]
- 9.Jaramillo AM, Gonzalez R. Psychiatric and neurocognitive manifestations of Sheehan syndrome: a case report. Prim Care Companion CNS Disord. 2017;19: 1601996. doi: 10.4088/PCC.16l01996 [DOI] [PubMed] [Google Scholar]
- 10.Tıkır B, Göka E, Aydemir MÇ, Gürkan Ş. Psikotik bozukluk ve. Psychotic disorder and sheehan’s syndrome: etiology or comorbidity? a case report. Türk Psikiyatri Derg. 2015;26:142-145. [PubMed] [Google Scholar]
- 11.Weitzner MA, Kanfer S, Booth-Jones M. Apathy and pituitary disease: it has nothing to do with depression. J Neuropsychiatry Clin Neurosci. 2005;17:159-166. doi: 10.1176/jnp.17.2.159 [DOI] [PubMed] [Google Scholar]
- 12.Zhao Z, Zhao F, Jin P, et al. SSRI/SNRI -induced hyponatremia: a case series of 26 patients in a single institution from 2018 to 2020. Psychiatr Q. 2023;94:113-125. doi: 10.1007/s11126-023-10018-x [DOI] [PubMed] [Google Scholar]
- 13.Garg D, Agarwal A, Garg A. Cavitating osmotic demyelination with an unusual pontine appearance. Ann Indian Acad Neurol. 2023;26:1040-1042. doi: 10.4103/aian.aian_732_23 [DOI] [PMC free article] [PubMed] [Google Scholar]