Abstract
Nephrotic syndrome (NS) in pregnancy has been associated with poor fetal outcomes. Focal segmental glomerulosclerosis (FSGS) is one of the common causes of NS and can be primary or secondary. However, there are few case reports of FSGS diagnosed in the peripartum period and the approaches to management. We report the case of a 27-year-old gravida 2 para 1 Caucasian woman diagnosed with NS at 22 weeks of gestation. Her serum creatinine was 46 µmol/L (0.48 mg/dL), serum albumin 14 g/L (1.4 g/dL) and 24-h urinary protein 9.79 g/day with no haematuria. Serology was negative for lupus, phospholipase A2 receptor antibody, hepatitis and HIV. Paraprotein screening was also negative. The patient declined a renal biopsy. Differential diagnoses at this stage included minimal change disease and FSGS. Six weeks after commencing empirical treatment with high-dose oral prednisolone, there was no response; hence, tacrolimus was initiated. Due to concern for maternal and fetal well-being, the decision was made to deliver via Caesarean section at 31 weeks, given worsening proteinuria (23.18 g/24 h). A live male infant was delivered weighing 1625 g. Renal biopsy at 4 weeks post-partum was consistent with primary FSGS. This case highlights the strategies we utilised to manage a gravid patient presenting with nephrotic syndrome at 22 weeks gestation, where diagnosis could only be confirmed on renal biopsy in the postpartum period.
Keywords: Nephrology, obstetrics/gynaecology, focal segmental glomerulosclerosis (FSGS), nephrotic syndrome, obstetric medicine, glomerular disorder, histopathology
Introduction
Focal segmental glomerulosclerosis (FSGS) is a hyper-filtrative glomerular disorder that can cause nephrotic syndrome (NS). FSGS accounted for 42% of biopsied cases in 26 pregnancies with NS. 1 FSGS is a histopathological pattern of glomerular injury and is heterogeneous in the mechanisms of disease.2–5 Various circulating factors are thought to be implicated in the development of idiopathic or primary FSGS, including soluble urokinase plasminogen activator receptor (suPAR) 3 and a soluble form of calcium/calmodulin-dependent serine/threonine kinase (CASK), 6 In contrast, secondary FSGS occurs as a maladaptive response to podocyte injury from drugs, infections, obesity, reduced renal mass and certain genetic mutations. 5 FSGS relating to genetic mutations or genetic syndromes is often poorly responsive to immunosuppression.
Renal protein excretion is physiologically increased in pregnancy due to increased glomerular filtration rate and increases in renal blood flow and cardiac output. A daily protein excretion of up to 300 mg is normal in pregnancy. 7 NS, which refers to a urinary protein excretion of 3.5 g or more over 24 h in conjunction with oedema, hypoalbuminaemia and hyperlipidaemia, is historically associated with poor fetal outcomes.8,9
Case
We describe the case of a 27-year-old Caucasian female patient, gravida 2 para 1, referred to our renal service at 22 weeks gestation with a nephrotic range proteinuria of 9.79 g over 24 h, normal serum creatinine of 46 µmol/L (0.48 mg/dL), serum albumin of 14 g/L (1.4 g/dL) and no haematuria or leukocyturia on urine microscopy. She had no background medical history or family history of kidney disease. Her first pregnancy 3 years prior was uncomplicated. The patient weighed 168 kg with a body mass index of 54.9 kg/m2. She had been started on aspirin 100 mg daily from 13 weeks gestation for low levels of pregnancy-associated plasma protein A. She was a lifelong non-smoker.
Physical examination revealed lower limb oedema and was otherwise unremarkable. Blood pressure was 130/82 mmHg with a regular pulse rate of 93 beats per minute. A 24-h blood pressure profile was subsequently performed and revealed normotensive readings.
Viral and infective serology were negative, as was a renal vasculitic and glomerulonephritis screen. Serum and urine electrophoresis (EPG) and immunofixation electrophoresis (IEPG) did not reveal a monoclonal paraprotein. C3 and C4 complement levels were not reduced. Glycated haemoglobin (HbA1c) and random serum glucose level were within normal ranges at 5.1% and 5.4 mmol/L (97.3 mg/dL), respectively. Renal ultrasound showed no structural abnormalities.
The patient was offered a renal biopsy at 23 weeks, however, declined the procedure. Pre-eclampsia was considered to be unlikely based on clinical grounds. Differential diagnoses at this stage included minimal change disease (MCD) and FSGS. Secondary FSGS in the context of morbid obesity and hyper-filtration was a possibility; however, it rarely presents with nephrotic syndrome and the absence of proteinuria in the patient’s first pregnancy 2 years prior made primary or genetic FSGS more likely. Prednisolone 60 mg daily was commenced empirically and tacrolimus (at a dose of 1 mg twice a day) 5 weeks later at 28 weeks and 5 days gestation as there was no reduction in proteinuria. The patient was therapeutically anti-coagulated on low molecular weight heparin (enoxaparin), started on methyldopa at 28 weeks gestation for hypertension, and subsequently insulin for gestational diabetes. Fetal growth and umbilical arterial Doppler readings were satisfactory on serial ultrasounds. Serum levels of creatinine, urate and liver transaminases as well as platelet count remained within normal range until delivery (Table 1).
Table 1.
The patient’s biochemistry including serum creatinine, albumin, AST, ALT and urate, as well as platelet count and 24-h urinary protein excretion from 22 weeks of gestation to 16 months postpartum.
| 22 weeks gestation | 26 weeks gestation | 28 weeks gestation | 29 weeks gestation | 30 weeks gestation | 31 weeks gestation | 4 weeks postpartum | 3 months postpartum | 5 months postpartum | 7 months postpartum | 14 months postpartum | 16 months postpartum | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum creatinine (µmol/L) | 46 | 41 | 43 | 46 | 44 | 46 | 61 | 90 | 100 | 150 | 250 | 345 |
| Serum creatinine (mg/dL) | 0.52 | 0.46 | 0.49 | 0.52 | 0.50 | 0.52 | 0.69 | 1.02 | 1.13 | 1.70 | 2.83 | 3.90 |
| Serum albumin (g/L) | 14 | 21 | 18 | 17 | 15 | 14 | 10 | 18 | 18 | 20 | 22 | 22 |
| Serum albumin (g/dL) | 1.4 | 2.1 | 1.8 | 1.7 | 1.5 | 1.4 | 1.0 | 1.8 | 1.8 | 2.0 | 2.2 | 2.2 |
| 24-h urinary protein (g) | 9.79 | 5.78 | 17.27 | 12.74 | 17.48 | 23.18 | 20.12 | 14.56 | 27.32 | 24.72 | 14.30 | – |
| Spot urine albumin-creatinine ratio (mg/mmol) | – | – | – | – | – | – | – | 1311.6 | 1285.4 | 1332.2 | 1194.4 | 1409.7 |
| AST/ALT (U/L) | 14/12 | 6/13 | 9/15 | 13/38 | 12/37 | 9/13 | 17/13 | 21/9 | 15/8 | 16/11 | 13/8 | 12/6 |
| Platelets (×109/L) | 280 | 283 | 320 | 304 | 278 | 282 | 374 | 472 | 510 | 627 | 523 | 396 |
| Serum urate (mmol/L) | 0.23 | 0.18 | 0.21 | 0.24 | 0.25 | 0.23 | 0.26 | – | – | – | – | – |
ALT, alanine transaminase; AST, aspartate transaminase.
The decision was made to deliver via elective Caesarean section at 31 weeks due to concerns over the deleterious effects of increasing proteinuria (23.18 g/24 h) on maternal and fetal well-being. A live male infant was delivered weighing 1625 g with APGAR 10 scores of 9, 9 and 9 at 1, 5 and 10 min, respectively. Postpartum the patient elected not to breastfeed and was started on enalapril for hypertension and frusemide for fluid overload. A kidney biopsy performed 4 weeks postpartum was consistent with FSGS-not otherwise specified (NOS) (Figure 1). Immunofluorescence staining was not suggestive of immune-complex, immunoglobulin or complement-mediated glomerular disease. Electron microscopy findings of 100% podocyte effacement was suggestive of primary FSGS 2 (Figure 2). Genetic testing returned negative. She was continued on tacrolimus 1 mg twice a day and at 2 months postpartum mycophenolate mofetil (MMF) 1000 mg twice a day was added given lack of reduction of proteinuria. Unfortunately, the patient experienced recurrent infections in the subsequent year due to immunosuppression and she never achieved remission. At 16 months postpartum, she had progressed to end-stage renal failure. Her immunosuppression was ceased, and she was referred for pre-dialysis education.
Figure 1.
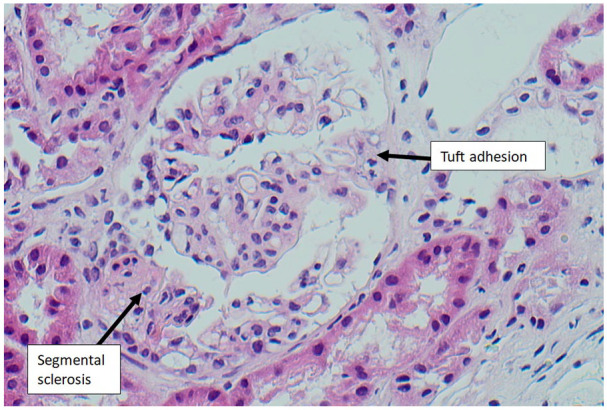
Light microscopy (haematoxylin and eosin stain, 200× magnification) of glomerulus showing features of FSGS-NOS, with segmental sclerotic lesion and adhesion to the overlying Bowman’s capsule (tuft adhesion).
FSGS-NOS, focal segmental glomerulosclerosis-not otherwise specified.
Figure 2.
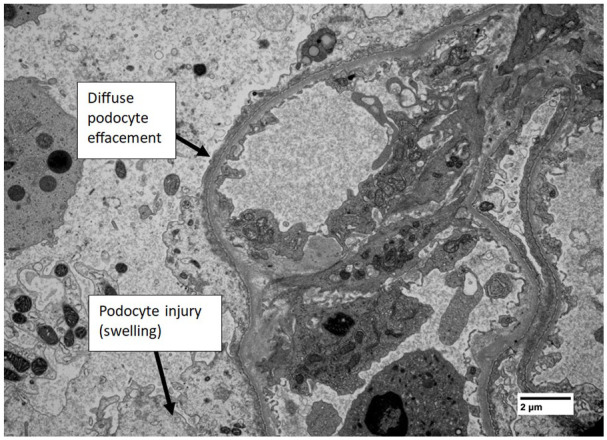
Electron microscopy (5000× magnification, uranyl acetate and lead citrate stains) showing severe effacement and fusion of foot processes to an extent of 100% of the loops, in keeping with primary FSGS. There is evidence of podocyte injury (swelling). There are no significant electron dense deposits in non-sclerosed segments and no fibrils or deposition disease seen. There is no evidence of thrombotic microangiopathy.
FSGS, focal segmental glomerulosclerosis.
Discussion
FSGS that develops de novo in pregnancy has been postulated by some as a progression of endotheliosis, which can occur as a result of gestation-related hyperfiltrative stress. 11 Pre-eclampsia should always be a consideration when evaluating the new onset of proteinuria and hypertension in patients after 20 weeks of gestation. 12 Our patient remained normotensive without requiring anti-hypertensive medication until 28 weeks gestation, and this made an initial diagnosis of pre-eclampsia unlikely. However, in more diagnostically ambiguous cases, a measurement of the ratio of soluble fms-like tyrosine kinase-1 (sFlt-1) to placental growth factor (PlGF) is useful in distinguishing pre-eclampsia from other causes of NS. 13 We instituted high-dose corticosteroid therapy on the presumption that the patient had a primary glomerular disease. MCD is usually steroid-responsive, with remission rates of 80%–90% in the non-pregnant adult population 14 while corticosteroids are traditionally used in combination with calcineurin inhibitors (CNIs) or alkylating agents in the treatment of membranous nephropathy. 15 High-dose corticosteroids are the mainstay of treatment in FSGS, with steroid-resistant disease declared following persistence of NS after 4–6 months of treatment.16,17 Recommended regimens usually consist of oral prednisolone at a dosage of 0.5–2.0 mg/kg/day (minimum 60 mg/day) for 2–6 months, followed by a slow taper over the next 1–4 months,18–20 although alternate-day regimens have also been trialled to minimize cumulative steroid exposure.21,22
Steroid-sparing agents used to treat FSGS include CNIs, rituximab, and cytotoxic drugs such as MMF, chlorambucil and cyclophosphamide.20,23,24 Out of these, CNIs are considered relatively safe for use in pregnancy. 25 Data from the paediatric population may suggest a more favourable side-effect profile with tacrolimus when compared with ciclosporin in terms of reduced nephrotoxicity, cholesterol levels and cosmetic side effects, as well as significantly greater rates of relapse in those treated with the latter. 26 The use of rituximab in pregnancy where other treatment options exist is not recommended given the risk of neonatal B-cell depletion and immunosuppression. 27
Our patient had many of the poor prognostic markers for renal survival in FSGS, which includes nephrotic range proteinuria, hypoalbuminaemia, an estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2, histopathological findings of interstitial fibrosis and tubular atrophy as well as interstitial inflammation and at diagnosis, and failure to achieve remission. 28 These findings however were reported as part of a study whose FSGS cohort was mostly male, Black and under 40. The same study reported an overall renal survival of 86% at 5 years, 75% at 19 years and 55% at 15 years. Interestingly, immunosuppression was not associated with better renal survival in the FSGS cohort. The median time to remission on a high-dose prednisone regimen has been cited as 4–6 months in one review, with overall remission rates of 47%–66%. 29 A retrospective review by Stirling and cohort 30 found that patients who achieved remission had a 94% rate of 5-year survival off dialysis, compared with a 53% rate in those who did not. Some studies have examined the utility of the fractional excretion of immunoglobulin G (IgG) to predict responsiveness to steroid therapy, with conflicting results.31,32 Given the relative paucity of reported cases of FSGS in pregnancy, it is difficult to comment on whether the physiological changes relating to the gravid state affect the disease trajectory in this population or response to immunosuppressive treatment.
Conclusion
We described the case of a 27-year-old woman whose NS was detected at 22 weeks of gestation and postpartum was found to have primary FSGS. Some of the management issues we encountered included establishing a provisional diagnosis of glomerulonephritis during her pregnancy without renal biopsy, and the use of immunosuppression in a gravid patient. Our patient failed to achieve remission despite immunosuppression post-partum and progressed to end-stage renal failure requiring dialysis quite rapidly. Future management issues for this patient include monitoring for recurrence of FSGS and the effects of additive immunosuppression should she proceed to renal transplantation.
Acknowledgments
None.
Footnotes
Author contributions: All authors contributed to the writing, reviewing, editing and approval for publication of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Ethical approval: Not applicable (for case report).
Guarantor: Lucy Qiu-Yun Wang (main author).
Patient consent: Written informed consent has been received from the patient for the publication of this case report.
Trial registration: Not applicable.
ORCID iD: Lucy Wang  https://orcid.org/0000-0002-9916-2733
https://orcid.org/0000-0002-9916-2733
References
- 1. De Castro I, Easterling TR, Bansal N, et al. Nephrotic syndrome in pregnancy poses risks with both maternal and fetal complications. Kidney Int 2017; 91: 1464–1472. [DOI] [PubMed] [Google Scholar]
- 2. De Vriese AS, Sethi S, Nath KA, et al. Differentiating primary, genetic, and secondary FSGS in adults: a clinicopathologic approach. J Am Soc Nephrol 2018; 29(3): 759–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wei C, Trachtman H, Li J, et al. Circulating suPAR in two cohorts of primary FSGS. J Am Soc Nephrol 2012; 23(12): 2051–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang J-J, Ma X-X, Hao L, et al. A systematic review and meta-analysis of outcomes of pregnancy in CKD and CKD outcomes in pregnancy. Clin J Am Soc Nephrol 2015; 10(11): 1964–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fogo AB. Causes and pathogenesis of focal segmental glomerulosclerosis. Nat Rev Nephrol 2015; 11(2): 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang X, Herr F, Vernochet A, et al. CASK, the soluble glomerular permeability factor, is secreted by macrophages in patients with recurrent focal and segmental glomerulo-sclerosis. Front Immunol 2020; 11: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheung KL, Lafayette RA. Renal physiology of pregnancy. Adv Chronic Kidney Dis. 20(3): 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siligato R, Gembillo G, Cernaro V, et al. Maternal and fetal outcomes of pregnancy in nephrotic syndrome due to primary glomerulonephritis. Front Med 2020; 7: 563094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yao T, Yao H, Wang H. Diagnosis and treatment of nephrotic syndrome during pregnancy. Chin Med J 1996; 109(6): 471–473. [PubMed] [Google Scholar]
- 10. Apgar V, Holaday DA, James S, et al. Evaluation of the newborn infant - second report. JAMA 1958; 168(15): 1985–1988. [DOI] [PubMed] [Google Scholar]
- 11. Guillén OAO, Silva RIV, Gonzalez BM, et al. Collapsing lesions and focal segmental glomerulosclerosis in pregnancy: a report of 3 cases. Am J Kidney Dis 2019; 74(6): 837–843. [DOI] [PubMed] [Google Scholar]
- 12. Stillman IE, Karumanchi SA. The glomerular injury of preeclampsia. Am Soc Nephrol 2007; 18(8): 2281–2284. [DOI] [PubMed] [Google Scholar]
- 13. Suzuki T, Ichikawa D, Nakata M, et al. Nephrotic syndrome due to preeclampsia before 20 weeks of gestation: a case report. BMC Nephrol 2020; 21(1): 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vivarelli M, Massella L, Ruggiero B, et al. Minimal change disease. Clin J Am Soc Nephrol 2017; 12(2): 332–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scolari F, Alberici F, Mescia F, et al. Therapies for membranous nephropathy: a tale from the old and new millennia. Front Immunol 2022; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rood IM, Bavinck A, Lipska-Ziętkiewicz BS, et al. Later response to corticosteroids in adults with primary focal segmental glomerular sclerosis is associated with favorable outcomes. Kidney Int Rep 2021; 7(1): 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cattran DC, Rao P. Long-term outcome in children and adults with classic focal segmental glomerulosclerosis. Am J Kidney Dis 1998; 32(1): 72–79. [DOI] [PubMed] [Google Scholar]
- 18. Burgess E. Management of focal segmental glomerulosclerosis: evidence-based recommendations. Kidney Int Suppl 1999; 70: S26–S32. [DOI] [PubMed] [Google Scholar]
- 19. Glassock RJ. Therapy of idiopathic nephrotic syndrome in adults. A conservative or aggressive therapeutic approach? Am J Nephrol 1993; 13(5): 422–428. [DOI] [PubMed] [Google Scholar]
- 20. Braun N, Schmutzler F, Lange C, et al. Immunosuppressive treatment for focal segmental glomerulosclerosis in adults. Cochrane Database Syst Rev 2008; 3: CD003233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nagai R, Cattran DC, Pei Y. Steroid therapy and prognosis of focal segmental glomerulosclerosis in the elderly. Clin Nephrol 1994; 42(1): 18–21. [PubMed] [Google Scholar]
- 22. Bolton WK, Atuk NO, Sturgill BC, et al. Therapy of the idiopathic nephrotic syndrome with alternate day steroids. Am J Med 1977; 62(1): 60–70. [DOI] [PubMed] [Google Scholar]
- 23. Ponticelli C, Rizzoni G, Edefonti A, et al. A randomized trial of cyclosporine in steroid-resistant idiopathic nephrotic syndrome. Kidney Int 1993; 43(6): 1377–1384. [DOI] [PubMed] [Google Scholar]
- 24. Banfi G, Moriggi M, Sabadini E, et al. The impact of prolonged immunosuppression on the outcome of idiopathic focal-segmental glomerulosclerosis with nephrotic syndrome in adults. A collaborative retrospective study. Clin Nephrol 1991; 36(2): 53–59. [PubMed] [Google Scholar]
- 25. Hladunewich MA, Bramham K, Jim B, et al. Managing glomerular disease in pregnancy. Nephrol Dial Transplant 2017; 32: i48–i56. [DOI] [PubMed] [Google Scholar]
- 26. Choudhry S, Bagga A, Hari P, et al. Efficacy and safety of tacrolimus versus cyclosporine in children with steroid-resistant nephrotic syndrome: a randomized controlled trial. Am J Kidney Dis 2009; 53(5): 760–769. [DOI] [PubMed] [Google Scholar]
- 27. Wiles K, Chappell L, Clark K, et al. Clinical practice guideline on pregnancy and renal disease. BMC Nephrol 2019; 20: 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Forster BM, Nee R, Little DJ, et al. Focal segmental glomerulosclerosis, risk factors for end stage kidney disease, and response to immunosuppression. Kidney360 2020; 2(1): 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Korbet SM. Treatment of primary FSGS in adults. J Am Soc Nephrol 2012; 23(11): 1769–1776. [DOI] [PubMed] [Google Scholar]
- 30. Stirling CM, Mathieson P, Boulton-Jones JM, et al. Treatment and outcome of adult patients with primary focal segmental glomerulosclerosis in five UK renal units. QJM 2005; 98(6): 443–449. [DOI] [PubMed] [Google Scholar]
- 31. Bazzi C, Petrini C, Rizza V, et al. Fractional excretion of IgG predicts renal outcome and response to therapy in primary focal segmental glomerulosclerosis: a pilot study. Am J Kidney Dis 2003; 41(2): 328–335. [DOI] [PubMed] [Google Scholar]
- 32. Deegens JK, Wetzels JF. Fractional excretion of high- and low-molecular weight proteins and outcome in primary focal segmental glomerulosclerosis. Clin Nephrol 2007; 68(4): 201–208. [DOI] [PubMed] [Google Scholar]


