Abstract
Dercas nina Mell 1913 (Pieridae) is a little-studied butterfly species endemic to China that flies primarily in the forest canopy. Genome skimming by Illumina sequencing allowed assembly of 146,702 reads for complete 1471.3-fold mean coverage of the circular 15,264 bp mitogenome from D. nina consisting of 82.1% AT nucleotides. A gene order typical of butterflies was recovered consisting of 13 protein-coding genes, 22 tRNAs, two rRNAs, and a predicted control region. The Dercas nina COX1 open reading frame begins with atypical start codon CGA. Six protein-coding genes (COX1, COX2, ND2, ND3, ND4, ND5) with single-nucleotide (T) stop codons, and two protein-coding genes (ATP6, ATP8) with two-nucleotide (TA) stop codons encoded in the DNA were inferred to be completed by adenine nucleotides from the Poly-A tail of the mRNA. Bayesian’s phylogenetic reconstruction places the D. nina and D. lycorias mitogenomes as sister clades. Dercas mitogenomes were sister to those from genus Colias in the monophyletic subfamily Coliadinae. The mitogenome phylogeny is consistent with previous molecular phylogenetic hypotheses based on other markers, but differs somewhat from a morphology-based hypothesis that suggested that Dercas was more closely related to genus Gonepteryx. This may falsify the hypothesis or may instead reflect mitochondrial-nuclear phylogenetic discordance.
Keywords: Illumina sequencing, mitogenomics, genome skimming
1. Introduction
The pierid butterfly genus Dercas Doubleday [1847] (Insecta: Lepidoptera: Pieridae: Coliadinae) is currently thought to be comprised of five species found in south and southeast Asia (Schulze and Fiedler 1997). These butterflies fly primarily high in the forest canopy and consequently, many aspects of their biology are not well-studied, but they do visit the ground to take up water and nutrients from damp soils (Schulze and Fiedler 1997; Schulze et al. 2001). Dercas lycorias adults are known pollinators of Hedychium coccineum (Zingiberaceae) flowers (Gao et al. 2012). Dercas larvae feed in the forest canopy on the leaves of the woody vines in genus Dalbergia (Fabaceae). This larval feeding pattern has been suggested as a synapomorphy for the genus Dercas, separating it from the related and morphologically similar genus, Gonepteryx (Schulze and Fiedler 1997; Bozano et al. 2016). Dercas nina Mell 1913 is a mostly yellow butterfly found primarily in the middle latitudes of China. The Chinese common name of this species is 橙翅方粉蝶 (Liu 2019), which in English translates to ‘orange-winged pierid butterfly’. In keeping with the Chinese name, we propose ‘orange-winged sulphur butterfly’ as the English common name for D. nina. Here, we report the complete mitochondrial genome sequence of D. nina assembled from Illumina sequence libraries.
This mitogenome was assembled through a course-based inquiry exercise (Marcus et al. 2010), conducted by the undergraduate students making up the Living Prairie Mitogenomics Consortium, which assembles previously undocumented arthropod mitogenomes for improved DNA-based species identification and phylogenetics (Living Prairie Mitogenomics Consortium 2017, 2018, 2019, 2022; Marcus 2018; Ajibola et al. 2019; Aguila et al. 2021). Student participants assembled and annotated the mitogenome sequence for presentation here. This strategy for sequencing and annotation increases knowledge of mitogenome structure and evolution, while simultaneously training junior scientists in the techniques required for this work.
Worldwide, there are approximately 19,500 species of butterflies (Kawahara et al. 2023), and many species have few genomic resources available. Producing these resources for understudied species and training personnel to manipulate and analyze genomic resources are the main goals of the Living Prairie Mitogenomics Consortium. The D. nina mitogenome shows many features typical of butterflies, making it a good exemplar for students learning to assemble and annotate these sequences.
2. Materials and methods
2.1. Sample collection and preservation
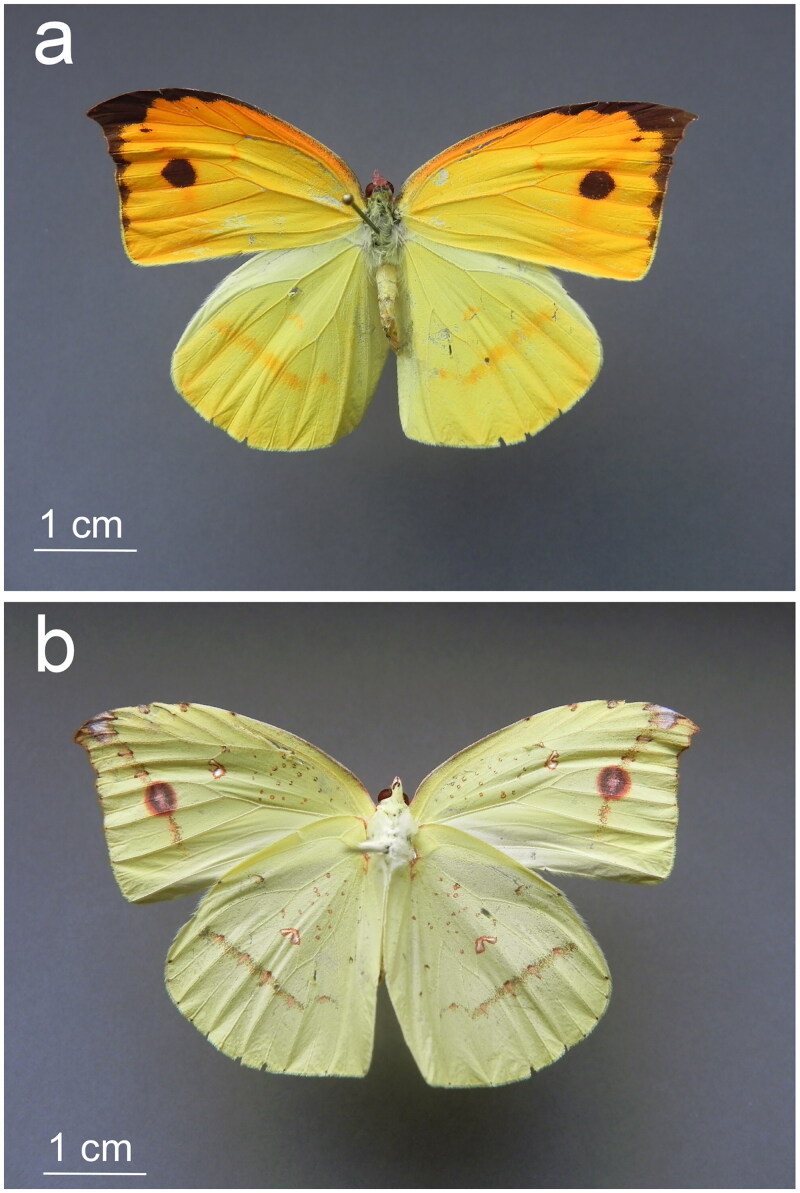
A specimen of D. nina (lab code DN2020.1) was collected in Guilin, Guangxi Zhuan Autonomous Region, China (GPS 25.2819 N, 110.2863 E) in July 2020. All animal sample collection protocols complied with the current laws of China. The specimen (Figure 1) was distinguished from congeners and identified using the original species description with accompanying key to the genus (Mell 1913) on the basis of morphological characteristics including: pointed apex of the forewing, dorsal forewings suffused with orange-red pigment, black costal edge dusted with yellowish scales basally, ventral forewings have a brown-red ‘seam line’ running from the forewing apex to vein M3, and a bright yellow dorsal hindwing with an orange-reddish tinged ‘seam line’ bending at M3. The seam lines correspond to the distal bands of the central symmetry system of the nymphalid ground plan (Nijhout 1991). The specimen was deposited in the Wallis Roughley Museum of Entomology, University of Manitoba (http://www.wallisroughley.ca/, Jason Gibbs, Jason.Gibbs@umanitoba.ca) as voucher number WRME0507742.
Figure 1.
Photographs of the (a) dorsal and (b) ventral aspects of the D. nina specimen sampled for DNA in this study (photographed by Jeffrey M. Marcus). A neutral 18% grey card was used for the image backdrop. A 1 cm scale bar is included for each image.
2.2. DNA sequencing and genome assembly
A leg was removed from the specimen and total genomic DNA was prepared using a DNeasy Blood and Tissue kit (Qiagen, Düsseldorf, Germany) following the standard animal tissue extraction protocol with the following modifications as previously described (McCullagh and Marcus 2015): First, tissue was ground up in 180 μL of tissue lysis buffer ATL (Qiagen, Hilden, Germany) using a mortar and pestle; next, 20 μL of protein kinase K (Qiagen, Hilden, Germany, 600 mU/mL) was added to the mixture and then incubated in a 55 °C water bath for 1 h. The remainder of the purification steps were conducted exactly as described by the Qiagen protocol. Upon protocol completion, extracted and resuspended DNA was evaluated for yield and quality on a NanoDrop 2000 spectrophotometer (1.9 ng DNA/μL; Thermo Scientific, Wilmington, DE) and a Qubit 2.0 fluorometer (1.6142 ng DNA/μL; Life Technologies, Carlsbad, CA). DNA was stored in Eppendorf tubes (Eppendorf, Hamburg, Germany) at −20 °C until required (Peters and Marcus 2017).
The DNA sample was sheared by sonication with an S220 Focused-Ultrasonicator (Covaris, Woburn, MA). The shotgun sequencing library was prepared using NEBNext Ultra II DNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA) and later sequenced by Illumina NovaSeq6000 equipped with an S4 PE150 flow cell and paired end reagent kit (San Diego, CA) (Marcus 2018)
The mitogenome assembly was created using Geneious Prime 2023.1 software (Biomatters, Auckland, New Zealand) (Kearse et al. 2012) which assembled the sequence library against a Dercas lycorias mitogenome (GenBank OR263671) (Wei et al. 2022) reference sequence without filtering.
2.3. Annotation and analysis
Mitochondrial genes were initially identified within Geneious Prime by aligning the D. nina mitogenome assembly to the annotated D. lycorias reference mitogenome and transferring homologous annotations to the newly assembled sequence. All gene positions were verified by comparisons between the newly assembled and reference mitogenomes using the ‘Align Two Sequences blastn’ option within GenBank BLAST+ 2.15.0 (Camacho et al. 2009). Additionally, the structure and location of all tRNAs were verified using ARWEN v.1.2 (Laslett and Canbäck 2008). The structure of the 16S rRNA was modeled using RNAfold as implemented in the ViennaRNA Package 2.0 (Lorenz et al. 2011). Geneious Prime was used for manual adjustments of gene annotations for start/stop codons and Proksee (Grant et al. 2023) was used to create the circular mitogenome map.
Phylogenetic analysis included the complete mitogenome of D. nina, along with the sole previously published Dercas mitogenome available from GenBank (from D. lycorias, OR263671; Wei et al. 2022), and a representative mitogenome from one species from each of the 14 other pierid genera with a previously reported complete mitogenome. To avoid making assumptions about the sister taxon to the Pieridae, we also included mitogenomes from 19 species representing the major clades of the six other butterfly families (Hedylidae, Hesperiidae, Lycaenidae, Nymphalidae, Papilionidae, and Riodinidae) (Table 1) (McCullagh et al. 2020). Three of these species in family Papilionidae were used as the outgroup to root the phylogenetic tree. Mitogenome sequences were aligned in CLUSTAL Omega (Sievers et al. 2011) and analyzed using Bayesian inference with the GTR + I + G model (model selected using jModeltest 2.1.1; Darriba et al. 2012) in MrBayes version 3.2.7a (Ronquist and Huelsenbeck 2003; Ronquist et al. 2012). Bayesian’s phylogenetic analysis included two runs consisting of three hot chains and one cold chain for 10 million iterations with sampling every 1000 generations.
Table 1.
List of 35 butterfly species, GenBank accession numbers, specimen origin, family, and reference for sequences used in reconstruction of phylogenetic trees (Figure 3).
| Species name | GenBank accession number | Specimen origin | Family | Reference |
|---|---|---|---|---|
| Acraea zetes | KT371361 | UK | Nymphalidae | Timmermans, Lees, et al. (2016) |
| Ancema ctesia | ON710999 | China | Lycaenidae | Liu C (unpublished) |
| Anthocharis mandschurica | MT499329 | China | Pieridae: Pierinae | Zhou et al. (2020) |
| Apodemia mormo | KJ647171 | N America | Riodinidae | Kim and Kim (2016) |
| Aporia hastata | OP373108 | China | Pieridae: Pierinae | Jia et al. (2023) |
| Apostictopterus fuliginosus | MH985707 | China | Hesperiidae | Han et al. (2018) |
| Appias lalage | MF576060 | China | Pieridae: Pierinae | Zhang et al. (2019) |
| Baltia butleri | MH380204 | China | Pieridae: Pierinae | Nie et al. (2018) |
| Carterocephalus silvicola | KJ629163 | Korea | Hesperiidae | Kim et al. (2014) |
| Cepora nadina | OP779722 | China | Pieridae: Pierinae | Wei et al. (2022) |
| Coenonympha tullia | KM592972 | China | Nymphalidae | Timmermans, Viberg, et al. (2016) |
| Colias erate | KP715146 | China | Pieridae: Coliadinae | Wu et al. (2016) |
| Curetis acuta | MZ196213 | China | Lycaenidae | Weng Q (unpublished) |
| Delias pasithoe | MK252291 | China | Pieridae: Pierinae | Wang J (unpublished) |
| Dercas lycorias | OR263671 | China | Pieridae: Coliadinae | Wei et al. (2022) |
| Dercas nina | OR797085 | China | Pieridae: Coliadinae | This study |
| Dichorragia nesimachus | KF590541 | Taiwan | Nymphalidae | Wu et al. (2014) |
| Dodona eugenes | MT890732 | China | Riodinidae | Wei et al. (2021) |
| Gonepteryx amintha | OP526832 | China | Pieridae: Coliadinae | Zhao Z (unpublished) |
| Hamadryas epinome | KM378244 | Peru | Nymphalidae | Cally et al. (2016) |
| Ixias pyrene | OP779726 | China | Pieridae: Pierinae | Wei et al. (2022) |
| Junonia lemonias | KP941756 | China | Nymphalidae | McCullagh and Marcus (2015) |
| Limenitis sydyi | KY593939 | Taiwan | Nymphalidae | Chen et al. (2018) |
| Macrosoma conifera | MT852025 | Costa Rica | Hedylidae | McCullagh et al. (2020) |
| Pachliopta aristolochiae | KU950357 | China | Papilionidae | Li X, Xin T and Xia B (unpublished) |
| Papilio demoleus | KR024009 | China | Papilionidae | Niu et al. (2016) |
| Pareronia anais | OP779723 | China | Pieridae: Pierinae | Wei et al. (2022) |
| Parnassius apollo | KF746065 | China | Papilionidae | Wang et al. (2015) |
| Pieris napi | MT576638 | China | Pieridae: Pierinae | Yu et al. (2020) |
| Polyommatus amorata | ON411620 | China | Lycaenidae | Chen WT (unpublished) |
| Polyura arja | KF590540 | China | Nymphalidae | Wu et al. (2014) |
| Pontia daplidice | MH380207 | China | Pieridae: Pierinae | Nie et al. (2018) |
| Prioneris thestylis | OP779724 | China | Pieridae: Pierinae | Wei et al. (2022) |
| Talbotia naganum | MH380205 | China | Pieridae: Pierinae | Nie et al. (2018) |
| Zemeros flegyas | MK521434 | China | Riodinidae | Shi et al. (2020) |
Convergence was determined using an effective sample size (ESS) estimation (which must exceed 625) as implemented in Convenience (Fabreti and Höhna 2022), and the first 2.5 million generations were discarded as burn-in. The resulting analysis resulted in an average deviation of split frequencies of 0.000348 and a mean estimated marginal likelihood of −193600.98.
3. Results
Two paired sequence libraries of 161,604,768 reads of 150 bp each (GenBank SRA SRR26257187) were created for D. nina. Two slightly different circular mitochondrial genome variants were assembled from 146,702 reads from these libraries which differ by only three SNPs, two 1-bp indels, and one 8-bp indel in a short region of the 16S rRNA. The phasing of this variation was possible because all of the observed polymorphisms occur within a span of no more than 101 bp and are detectable linked together within individual reads of the sequence library. RNAfold modeling of 16S structure shows that this variation occurs as length variation of a single helix and size-variation in the associated terminal loop within domain II of this rRNA.
The more common mitogenome variant 1 was 15,254 bp long and lacked nucleotides at all of the indel sites. The somewhat rarer mitogenome variant 2 was 15,264 bp and had additional nucleotides present in all three indel locations. The mitochondrial genome was reported to GenBank as accession OR797085, as the consensus of these two variants with the locations of the SNPs indicated by assigning degenerate nucleotide code symbols, while the positions of the indels are indicated by N’s in the overall consensus sequence. An alignment of the two variant 16S rRNA sequences with the consensus is provided in Supplementary Figure 1, while each of these 16S rRNA sequences is provided in FASTA format in Supplementary Figure 2. Depictions of the predicted structures of each of the variants are included in Supplementary Figure 3. The assembled consensus sequence was composed of 146,702 reads with nucleotide composition: 40.2% A, 10.6% C, 7.3% G, and 41.9% T. Consensus assembly mitogenome sequence coverage was 100% with a mean depth of coverage of 1471.3-fold (minimum 698-fold, maximum 2215-fold, Supplementary Figure 4).
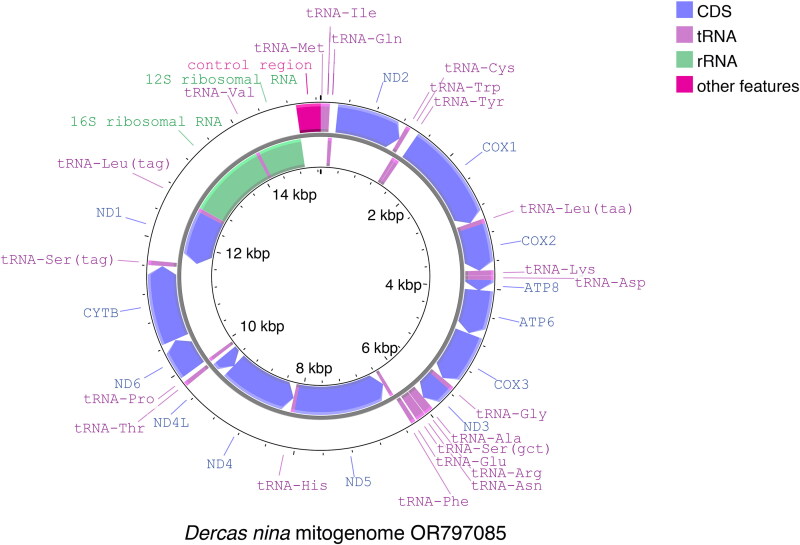
The gene composition and order of the D. nina mitogenome (Figure 2) is identical to that of most butterfly mitogenomes. The D. nina protein coding gene start codons include: ATG (ATP6, COX2, COX3, CYTB, ND1, ND4, ND4L), ATT (ATP8, ND2, ND3, ND5, ND6), and CGA, an atypical COX1 start codon also found in COX1 in many other insects (Liao et al. 2010). Six protein-coding genes (COX1, COX2, ND2, ND3, ND4, ND5) with predicted single-nucleotide (T) stop codons, and two protein-coding genes (ATP6, ATP8) with predicted two-nucleotide (TA) stop codons may be completed by post-transcriptional addition of 3′ A residues from the Poly-A tail. The predicted control region and mitochondrial rRNAs are typical for Lepidoptera, while the tRNAs have typical cloverleaf secondary structures except for trn-Ser(act) where the dihydrouridine arm has been replaced with a loop. A putative 7 bp lepidopteran mitochondrial transcription terminator (mtTERM) binding site (ATACTAA) was detected between tRNA-Ser(tga) and ND1.
Figure 2.
Circular mitochondrial genome feature map of D. nina created using Proksee software (Grant et al. 2023). Protein-coding genes are labeling in blue, tRNAs are labeled in purple, rRNAs are labeled in green, and the predicted control region is labeled in pink.
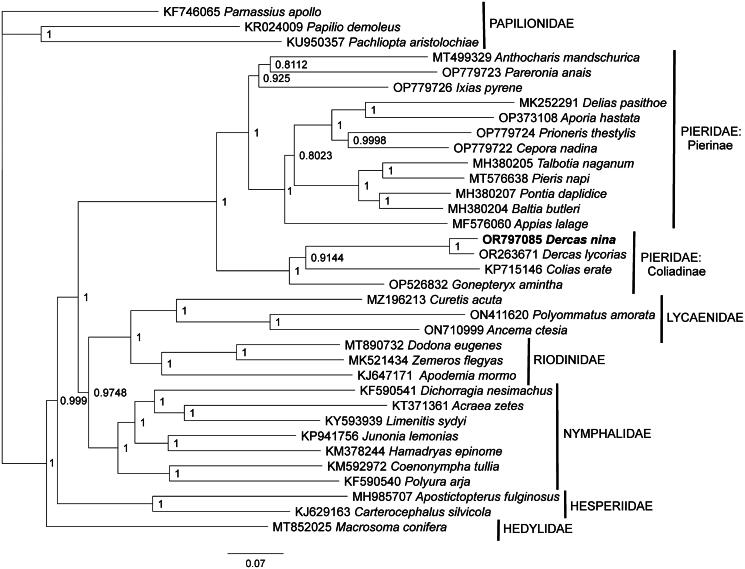
Phylogenetic analysis (Figure 3) placed the D. nina mitogenome as sister to that of D. lycorias. Dercas mitogenomes were found as sister to Colias mitogenomes, with a Gonepteryx mitogenome as the outgroup within pierid subfamily Coliadinae. The availability of complete mitogenomes from both D. nina and D. lycorias provides an opportunity to make a variety of sequence comparisons between these congeners. The complete mitogenomes (OR797085 and OR263671) show an overall 96.17% sequence identity. Comparing just the complete COX1 coding sequences from these accessions, which is often used for phylogenetic analysis, shows a 97.00% sequence identity. Focusing on just the DNA barcode region of COX1, which is often used for specimen identification, there is between 96.80% and 97.54% sequence identity between the barcode region of D. nina (OR797085) and five barcode sequences from D. lycorias (GenBank: OR263671, OR965399, ON436245; BOLD: VNMB2893-24, VNMB2894-24).
Figure 3.
Bayesian’s inference phylogeny (GTR + I + G model, average deviation of split frequencies = 0.000348, mean estimated marginal likelihood = −193600.98) of the Dercas nina mitogenome, 15 additional mitogenomes from family Pieridae, and 19 species from six other butterfly families (Table 1). Three species in family Papilionidae were used as the phylogenetic outgroup to root the tree. The tree was produced by 10 million iterations in MrBayes with sampling every 1000 generations. At each node, the Bayesian posterior probability values determined by MrBayes are given. The scale bar depicts an average number of 0.7 substitutions per site per unit length.
4. Discussion and conclusions
The D. nina mitogenome contains many structural features that make it similar to the mitogenomes of many other Lepidoptera, including a conserved gene arrangement (Park et al. 2016), a trn-Ser(AGN) where the dihydrouridine arm has been replaced with a loop (McCullagh and Marcus 2015), and the presence of a canonical 7 bp lepidopteran mtTERM binding site (ATACTAA) (Cameron and Whiting 2008; Gong et al. 2012) between tRNA-Ser(TCR) and ND1. The two 16S rRNA variant genotypes detected in the mitogenome assembly for D. nina differ only slightly and do not disrupt either the fine-scale or the overall structure of the resulting rRNAs, so we anticipate that both variants encode functional gene products.
The similarity in the levels of sequence identity between D. nina and D. lycorias complete mitogenomes, complete COX1 coding sequences, and COX1 barcode regions is consistent with prior observations in other taxa (Peters and Marcus 2017). This suggests that the amount of sequence identity in the COX1 DNA barcode region between two species might be useful as a predictor of the degree of sequence identity between their entire mitogenomes.
Phylogenetic analysis found genus Dercas mitogenomes as monophyletic, as might be predicted based on taxonomy. Contrary to the morphology-based predictions of Schulze and Fiedler (1997), Dercas mitogenomes were not found to be sister to the mitogenome from Gonepteryx, but rather Dercas mitogenomes were sister to Colias mitogenomes, with Gonepteryx as an outgroup, which is more consistent with some previous molecular phylogenetic analyses based on other molecular markers (Ding and Zhang 2017; Wei et al. 2022). Whether this finding should be interpreted as an experimental artifact attributable to limited representation of mitogenomes from genera in the subfamily Coliadinae in the phylogenetic analysis, as evidence falsifying the Dercas-Gonepteryx sister clade hypothesis, or whether it reflects mitochondrial-nuclear phylogenetic discordance within the Coliadinae should be determined through additional investigations.
Supplementary Material
Acknowledgements
We would like to thank Genome Quebec for assistance with library preparation and sequencing.
Funding Statement
This work received support from NSERC under Grant [RGPIN-2022-05016] and from the University of Manitoba under the University Research Grants Program.
Author contributions
All authors have made substantial contributions to this manuscript. Every author analyzed and interpreted the data presented here independently and conducted a literature review as individual graded course assignments before the work of all authors was brought together for synthesis and resolution of any discrepancies or omissions by the class. JMM conceived, designed, and supervised the experimental manipulations and created the initial draft of the manuscript based on the experimental findings, analyses, and interpretations of all of the authors. All authors participated in critically reviewing, revising, and proofreading the manuscript prior to submission as course assignments. JMM supervised revisions to the manuscript and all authors have read and agreed to the published version of the manuscript and agree to be accountable for all aspects of the work so as to ensure that any questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethical approval
All applicable international, national, and/or institutional guidelines for the importation, care, and use of animals were strictly followed. All animal sample collection protocols complied with the current laws of China. Canadian Council on Animal Care (CCAC) and University of Manitoba guidelines do not regulate research or procedures involving insects, so this work is exempt from CCAC regulations.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov/ under the accession no. OR797085. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA1023248, SRR26257187, and SAMN37648387, respectively.
References
- Aguila CP, Aikens RM, Ateliey PK, Buhr HM, Castro MG, Chua RJ, Dayal N, Deane HN, Dennehy B, Esenbekova M, et al. 2021. The complete mitochondrial genome of the Indian leafwing butterfly Kallima paralekta (Insecta: Lepidoptera: Nymphalidae). Mitochondrial DNA B Resour. 6(1):274–277. doi: 10.1080/23802359.2020.1862000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajibola S, Arya V, Barker EN, Biggar KT, Bohemier DM, Braga JN, Buchel JL, Bui V, Burtniak JM, Dueck CE, et al. 2019. The complete mitochondrial genome of the brown pansy butterfly, Junonia stygia (Aurivillius, 1894), (Insecta: Lepidoptera: Nymphalidae). Mitochondrial DNA B Resour. 5(1):41–43. doi: 10.1080/23802359.2019.1693921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozano GC, Coutsis JG, Herman P, Allegrucci G, Cesaroni D, Sbordoni V.. 2016. Guide to the Butterflies of the Palearctic Region. Pieridae Part III. Subfamily Coliadinae. Tribes Rhodocerini, Euremini, Coliadini genus Catopsilia, and Subfamily Dismorphiinae. Milano, Italy: Omnes Artes. [Google Scholar]
- Cally S, Lhuillier E, Iribar A, Garzón-Orduña I, Coissac E, Murienne J.. 2016. Shotgun assembly of the complete mitochondrial genome of the neotropical cracker butterfly Hamadryas epinome. Mitochondrial DNA A DNA Mapp Seq Anal. 27(3):1864–1866. doi: 10.3109/19401736.2014.971262. [DOI] [PubMed] [Google Scholar]
- Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL.. 2009. BLAST+: architecture and applications. BMC Bioinformatics. 10(1):421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron SL, Whiting MF.. 2008. The complete mitochondrial genome of the tobacco hornworm, Manduca sexta, (Insecta: Lepidoptera: Sphingidae), and an examination of mitochondrial gene variability within butterflies and moths. Gene. 408(1–2):112–123. doi: 10.1016/j.gene.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Chen Y-C, Wang C-T, Lees DC, Wu L-W.. 2018. Higher DNA insert fragment sizes improve mitogenomic assemblies from metagenomic pyrosequencing datasets: an example using Limenitidinae butterflies (Lepidoptera, Nymphalidae). Mitochondrial DNA A DNA Mapp Seq Anal. 29(6):840–845. doi: 10.1080/24701394.2017.1373106. [DOI] [PubMed] [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D.. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 9(8):772–772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C, Zhang Y.. 2017. Phylogenetic relationships of Pieridae (Lepidoptera: Papilionoidea) in China based on seven gene fragments. Entomol Sci. 20(1):15–23. doi: 10.1111/ens.12214. [DOI] [Google Scholar]
- Fabreti LG, Höhna S.. 2022. Convergence assessment for Bayesian phylogenetic analysis using MCMC simulation. Methods Ecol Evol. 13(1):77–90. doi: 10.1111/2041-210X.13727. [DOI] [Google Scholar]
- Gao J, Sheng C, Yang S.. 2012. Adaptive significance of mass-flowering in Hedychium coccineum (Zingiberaceae). Biodivers Sci. 20:376–385. doi: 10.3724/SP.J.1003.2012.10034. [DOI] [Google Scholar]
- Gong Y-j, Shi B-c, Kang Z-j, Zhang F, Wei S-j. 2012. The complete mitochondrial genome of the oriental fruit moth Grapholita molesta (Busck) (Lepidoptera: Tortricidae). Mol Biol Rep. 39(3):2893–2900. doi: 10.1007/s11033-011-1049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JR, Enns E, Marinier E, Mandal A, Herman EK, Chen C-y, Graham M, Van Domselaar G, Stothard P.. 2023. Proksee: in-depth characterization and visualization of bacterial genomes. Nucleic Acids Res. 51(W1):W484–W492. doi: 10.1093/nar/gkad326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Huang Z, Tang J, Chiba H, Fan X.. 2018. The complete mitochondrial genomes of two skipper genera (Lepidoptera: Hesperiidae) and their associated phylogenetic analysis. Sci Rep. 8(1):15762. doi: 10.1038/s41598-018-34107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y-Q, Zhang X, Hu S-J.. 2023. Complete mitochondrial genome of the little-known regional endemic Aporia hastata (Oberthür, 1892) (Lepidoptera: Pieridae). Mitochondrial DNA B Resour. 8(5):589–592. doi: 10.1080/23802359.2023.2213353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara AY, Storer C, Carvalho APS, Plotkin DM, Condamine FL, Braga MP, Ellis EA, St Laurent RA, Li X, Barve V, et al. 2023. A global phylogeny of butterflies reveals their evolutionary history, ancestral hosts and biogeographic origins. Nat Ecol Evol. 7(6):903–913. doi: 10.1038/s41559-023-02041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 28(12):1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Kim I.. 2016. Complete mitochondrial genome of the Mormon metalmark butterfly, Apodemia mormo (Lepidoptera: Riodinidae). Mitochondrial DNA A DNA Mapp Seq Anal. 27(2):789–791. doi: 10.3109/19401736.2014.915539. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Wang AR, Park JS, Kim I.. 2014. Complete mitochondrial genomes of five skippers (Lepidoptera: Hesperiidae) and phylogenetic reconstruction of Lepidoptera. Gene. 549(1):97–112. doi: 10.1016/j.gene.2014.07.052. [DOI] [PubMed] [Google Scholar]
- Laslett D, Canbäck B.. 2008. ARWEN: a program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics. 24(2):172–175. doi: 10.1093/bioinformatics/btm573. [DOI] [PubMed] [Google Scholar]
- Liao F, Wang L, Wu S, Li Y-P, Zhao L, Huang G-M, Niu C-J, Liu Y-Q, Li M-G.. 2010. The complete mitochondrial genome of the fall webworm, Hyphantria cunea (Lepidoptera: Arctiidae). Int J Biol Sci. 6(2):172–186. doi: 10.7150/ijbs.6.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G. 2019. Chinese and foreign butterflies appreciation 中外蝴蝶鉴赏. Beijing, China: China Scientific Book Services. [Google Scholar]
- Living Prairie Mitogenomics Consortium . 2017. The complete mitochondrial genome of the lesser aspen webworm moth Meroptera pravella (Insecta: Lepidoptera: Pyralidae). Mitochondrial DNA B Resour. 2(1):344–346. doi: 10.1080/23802359.2017.1334525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Living Prairie Mitogenomics Consortium . 2018. The complete mitochondrial genome of the giant casemaker caddisfly Phryganea cinerea (Insecta: Trichoptera: Phryganeidae). Mitochondrial DNA B Resour. 3(1):375–377. doi: 10.1080/23802359.2018.1450686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Living Prairie Mitogenomics Consortium . 2019. The complete mitochondrial genome of the North American pale summer sedge caddisfly Limnephilus hyalinus (Insecta: Trichoptera: Limnephilidae). Mitochondrial DNA B Resour. 4:413–415. doi: 10.1080/23802359.2018.1547158. [DOI] [Google Scholar]
- Living Prairie Mitogenomics Consortium . 2022. The complete mitochondrial genome of the smudged eighty-eight butterfly Diaethria gabaza eupepla (Salvin & Godman, 1868) (Insecta: Lepidoptera: Nymphalidae). Mitochondrial DNA B Resour. 7:673–675. doi: 10.1080/23802359.2022.2065220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz R, Bernhart SH, Höner zu Siederdissen C, Tafer H, Flamm C, Stadler PF, Hofacker IL.. 2011. ViennaRNA Package 2.0. Algorithms Mol Biol. 6(1):26. doi: 10.1186/1748-7188-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JM, Hughes TM, McElroy DM, Wyatt RE.. 2010. Engaging first year undergraduates in hands-on research experiences: the upper green river barcode of life project. J Coll Sci Teach. 39:39–45. [Google Scholar]
- Marcus JM. 2018. Our love–hate relationship with DNA barcodes, the Y2K problem, and the search for next generation barcodes. AIMS Genet. 5(1):1–23. doi: 10.3934/genet.2018.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh BS, Alexiuk MR, Payment JE, Hamilton RV, Lalonde MML, Marcus JM.. 2020. It’s a moth! It’s a butterfly! It’s the complete mitochondrial genome of the American moth-butterfly Macrosoma conifera (Warren, 1897) (Insecta: Lepidoptera: Hedylidae)! Mitochondrial DNA B Resour. 5(3):3633–3635. doi: 10.1080/23802359.2020.1831991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh BS, Marcus JM.. 2015. The complete mitochondrional genome of Lemon Pansy, Junonia lemonias (Lepidoptera: Nymphalidae: Nymphalinae). J Asia Pac Entomol. 18(4):749–755. doi: 10.1016/j.aspen.2015.08.006. [DOI] [Google Scholar]
- Mell R. 1913. Die Gattung Dercas Dbl. Int Entomol Zeitsch. 7:193–194. [Google Scholar]
- Nie L, Wang Y, Huang D, Tao R, Su C, Hao J, Zhu C.. 2018. Mitochondrial genomes of four pierid butterfly species (Lepidoptera: Pieridae) with assessments about Pieridae phylogeny upon multiple mitogenomic datasets. Zool Syst. 43:387–409. doi: 10.11865/zs.201834. [DOI] [Google Scholar]
- Nijhout HF. 1991. The development and evolution of butterfly wing patterns. Washington: Smithsonian Institution Press. [Google Scholar]
- Niu FF, Zhu L, Wang S, Wei SJ.. 2016. The mitochondrial genome of the multicolored Asian lady beetle Harmonia axyridis (Pallas) and a phylogenetic analysis of the Polyphaga (Insecta: Coleoptera). Mitochondrial DNA A DNA Mapp Seq Anal. 27(4):2725–2727. doi: 10.3109/19401736.2015.1046165. [DOI] [PubMed] [Google Scholar]
- Park JS, Kim MJ, Jeong SY, Kim SS, Kim I.. 2016. Complete mitochondrial genomes of two gelechioids, Mesophleps albilinella and Dichomeris ustalella (Lepidoptera: Gelechiidae), with a description of gene rearrangement in Lepidoptera. Curr Genet. 62(4):809–826. doi: 10.1007/s00294-016-0585-3. [DOI] [PubMed] [Google Scholar]
- Peters MJ, Marcus JM.. 2017. Taxonomy as a hypothesis: testing the status of the Bermuda buckeye butterfly Junonia coenia bergi (Lepidoptera: Nymphalidae). Syst Entomol. 42(1):288–300. doi: 10.1111/syen.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP.. 2003. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 19(12):1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP.. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61(3):539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze CH, Fiedler K.. 1997. Notes on the biology of Dercas gobrias (Hewitson, 1864) (Lepidoptera: Pieridae, Coliadinae). Trans Lepid Soc Japan. 48:25–30. doi: 10.18984/LEPID.48.1_25. [DOI] [Google Scholar]
- Schulze CH, Linsenmair KE, Fiedler K.. 2001. Understorey versus canopy: patterns of vertical stratification and diversity among Lepidoptera in a Bornean Rain Forest. Plant Ecol. 153(1–2):133–152. doi: 10.1023/A:1017589711553. [DOI] [Google Scholar]
- Shi Q-h, Sun G, Fang Y, Zhang L-h, Zhang J-c.. 2020. The complete mitochondrial genome of Punchinello butterfly, Zemeros flegyas (Lepidoptera: Riodinidae) and its phylogenetic implications. Mitochondrial DNA B. 5(2):1567–1569. doi: 10.1080/23802359.2020.1742604. [DOI] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, et al. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 7(1):539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans MJTN, Lees DC, Thompson MJ, Sáfián S, Brattström O.. 2016a. Mitogenomics of ‘Old World Acraea’ butterflies reveals a highly divergent ‘Bematistes’. Mol Phylogenet Evol. 97:233–241. doi: 10.1016/j.ympev.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Timmermans MJTN, Viberg C, Martin G, Hopkins K, Vogler AP.. 2016b. Rapid assembly of taxonomically validated mitochondrial genomes from historical insect collections. Biol J Linn Soc. 117(1):83–95. doi: 10.1111/bij.12552. [DOI] [Google Scholar]
- Wang Y-L, Chen Y-H, Xia C-C, Xia X-Q, Tao R-S, Hao J-S.. 2015. The complete mitochondrial genome of the Common Red Apollo, Parnassius epaphus (Lepidoptera: Papilionidae: Parnassiinae). J Asia Pac Entomol. 18(2):239–248. doi: 10.1016/j.aspen.2015.02.002. [DOI] [Google Scholar]
- Wei F, Huang W, Fang L, He B, Zhao Y, Zhang Y, Shu Z, Su C, Hao J.. 2022. Spatio-temporal evolutionary patterns of the Pieridae butterflies (Lepidoptera: Papilionoidea) inferred from mitogenomic data. Genes. 14(1):72. doi: 10.3390/genes14010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z-X, Sun G, Shiu J-Y, Fang Y, Shi Q-H.. 2021. The complete mitochondrial genome sequence of Dodona eugenes (Lepidoptera: Riodinidae). Mitochondrial DNA B Resour. 6(3):816–818. doi: 10.1080/23802359.2021.1884014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L-W, Lin L-H, Lees D, Hsu Y-F.. 2014. Mitogenomic sequences effectively recover relationships within brush-footed butterflies (Lepidoptera: Nymphalidae). BMC Genomics. 15(1):468. doi: 10.1186/1471-2164-15-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Fang J, Li W, Han D, Wang H, Zhang B.. 2016. The complete mitochondrial genome of Colias erate (Lepidoptera: Pieridae). Mitochondrial DNA A DNA Mapp Seq Anal. 27(6):4209–4210. doi: 10.3109/19401736.2015.1022743. [DOI] [PubMed] [Google Scholar]
- Yu H, Shi M-R, Xu J.. 2020. The complete mitochondrial genome of the Pieris napi (Lepidoptera: Pieridae) and its phylogenetic implication. Mitochondrial DNA B Resour. 5(3):3035–3036. doi: 10.1080/23802359.2020.1797565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Yin J, Ma P, Li T, Cao T, Zhong Y.. 2019. The complete mitochondrial genomes of Aporia crataegi, Gonepteryx rhamni, and Appias remedios (Lepidoptera, Pieridae) and phylogenetic relationship of other Pieridae species. Int J Biol Macromol. 129:1069–1080. doi: 10.1016/j.ijbiomac.2019.02.124. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zhang C, Wang S, Liu Y, Wang N, Liang B.. 2020. A mitogenomic phylogeny of pierid butterflies and complete mitochondrial genome of the yellow tip Anthocharis scolymus (Lepidoptera: Pieridae). Mitochondrial DNA B Resour. 5(3):2587–2589. doi: 10.1080/23802359.2020.1781578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genome sequence data that support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov/ under the accession no. OR797085. The associated BioProject, SRA, and Bio-Sample numbers are PRJNA1023248, SRR26257187, and SAMN37648387, respectively.