Abstract
Military Veterans have a higher risk of incident atherosclerotic cardiovascular disease (ASCVD) than the general population and are often clinically complex. We studied the changes in cardiovascular risk factors with a lifestyle intervention in this population. We retrospectively analyzed data from 67 participants (mean age 69.2 (SD 7.9) years; 97% male) with atherosclerotic heart disease and/or type 2 diabetes in a 15-week, multiple health behavior change (MHBC) intervention implemented in a Veterans Affairs (VA) Behavioral Medicine Clinic. The intervention promoted a whole foods, plant-based (WFPB) diet, physical activity, and cognitive-behavioral stress management. We assessed cardiometabolic risk factors at baseline, 1 month into the intervention, and at 15 weeks (post-treatment). Among intervention completers (n = 67), we observed statistically significant improvements in waist circumference (−2.8 inches, P = .03), systolic blood pressure (−7.9 mmHg, P = .03), LDL cholesterol (−11.27 mg/dL, P = .04), fasting glucose (−15.10 mg/dL, P = .03), and hemoglobin A1c (−0.55%, P = .017) at post-treatment. Participants with type 2 diabetes (n = 34) achieved improvements in hemoglobin A1c (−0.80%, P = .007), systolic blood pressure (−10.98 mmHg, P = .01), and diastolic blood pressure (−6.65 mmHg, P = .03) at post-treatment. Medication usage did not significantly change. Veterans who completed the MHBC intervention achieved significant improvements in cardiometabolic risk in a routine VA clinical practice setting.
Keywords: cardiovascular risk reduction, military veterans, multiple health behavior change intervention, plant-based diet, lifestyle medicine
“Veterans who completed the HDRP achieved statistically significant improvements in waist circumference, systolic blood pressure, LDL-C, fasting glucose, and hemoglobin A1c from baseline to post-treatment.”
Introduction
Heart disease continues to be the leading cause of death in the United States. 1 With the continued trend of increasing atherosclerotic cardiovascular disease (ASCVD), the American Heart Association (AHA) is putting more focus on prevention-related interventions, particularly for vulnerable populations. 2 Alarmingly, military Veterans have a higher risk of incident heart disease and cardiovascular events than the general population, with estimates suggesting Veterans develop heart disease at about twice the rate of non-veterans.3-5 Veterans with ASCVD are often clinically complex and may require multifaceted secondary prevention programs.6,7
Cardiac rehabilitation (CR) represents the primary lifestyle modification intervention for Veterans with incident heart disease. 8 CR primarily emphasizes medically supervised exercise with home-based physical activity as the core component, together with education (e.g., hearty healthy diet, managing stress, tobacco cessation, and medications) and use of motivational interviewing to promote behavioral change.9,10 In general, center-based and home-based CR have been shown to reduce mortality and cardiometabolic risk factors among veteran and non-veteran populations.11-14 Difficulties with patient enrollment and engagement continue to limit its overall impact. 15 CR also has limited impact on the larger population living with ASCVD, as only a portion of this population meet CR eligibility (i.e., within 1 year after myocardial infarction, acute coronary syndrome, chronic stable angina, percutaneous cardiac intervention, or coronary artery bypass surgery). Secondary prevention programs for ASCVD may be effective for patients who are outside of the CR eligibility window, although it is unclear how such an intervention could be most optimally designed.
While focusing on one specific behavior change might simplify the secondary prevention intervention and improve its feasibility, some research suggests that attempting multiple health behavior changes (MHBC) simultaneously is associated with greater behavior change. 16 Researchers have begun to demonstrate the benefits of intensifying CR by adding a stress management component, with initial research suggesting that such an approach can reduce cardiac mortality by approximately 50% compared with standard CR at 5-year follow-up. 17 This is not surprising, as stress management training for heart disease patients on its own shows promise, with prior research demonstrating improvements in physiological measures of heart health, including mental stress and exercise-induced myocardial ischemia, flow-mediated dilation, wall motion abnormalities, baroreceptor sensitivity, and heart rate variability.18,19 Considering the higher prevalence of posttraumatic stress disorder (PTSD) among Veterans20,21 and the association of PTSD and heart disease,22-24 enhancing stress management might be especially important to optimize cardiometabolic risk reduction within secondary prevention interventions for Veterans with ASCVD.
There may be benefits to making dietary modification intervention a more central component of CR and other secondary ASCVD prevention programs. Diet is one of the most important modifiable risk factors for heart disease, as it is associated with overweight/obesity, diabetes, hypertension, and metabolic syndrome.25-27 In the United States and globally, poor diet now contributes to a greater risk of death and disability-adjusted life-years than tobacco and high blood pressure.28,29 Programs that have included a whole foods, plant-based (WFPB) diet (whole, unprocessed, plant-based foods including grains, legumes, vegetables, fruits, nuts, seeds) have demonstrated promising results in two small studies with patients with heart disease. A small nonrandomized clinical trial of a WFPB diet only intervention resulted in regression of atherosclerotic plaques in 73% of participants, with arrested progression in the other 27%, after 5 years. 30 Remarkably, these participants also had no adverse cardiovascular events after 12 years. 31 In a small randomized controlled trial (RCT), Ornish and colleagues 32 studied the effect of an intervention that combined a WFPB diet and stress management component, and they found 91% of participants experienced reduced angina after only 24 days.
Ornish and colleagues subsequently expanded his heart disease intervention to include a WFPB diet, exercise, stress management, and group support components, and studied its efficacy (i.e., Lifestyle Heart Trial)33,34 and effectiveness in 3 large clinical trials (i.e., Multicenter Lifestyle Demonstration Project, 35 Multisite Cardiac Lifestyle Intervention Program, 36 and Highmark Blue Cross/Blue Shield Study 37 ). The Ornish program has also been shown to be effective and adaptable for a military population,38,39 and altogether demonstrating high rates of adherence and improved cardiometabolic risk profiles.33-39 These studies demonstrate the benefits of MHBC interventions for atherosclerotic heart disease that include a rigorous WFPB dietary component. These studies also tested interventions that were time-intensive (e.g., 8 hours in clinic per week), which might be less feasible for many patients. It is unclear whether similar clinical improvement can be obtained through a less time-intensive approach.
The Heart Disease Reversal Program (HDRP) at the Sacramento Veterans Affairs (VA) Medical Center was developed to create a streamlined, MHBC intervention modeled after the Ornish and other similar heart disease reversal programs, offered within routine clinical practice. 40 HDRP fits in the category of intensive therapeutic lifestyle change (ITLC) treatment programs. 41 The HDRP has served a broad range of Veterans with ASCVD (e.g., <1-year post cardiac event/eligible for cardiac rehabilitation, and those >1-year post-event) and type 2 diabetes, with varying levels of functional ability.
The current paper evaluates the outcomes of the 15-week HDRP on cardiometabolic risk factors in a population of US Veterans. We hypothesized that Veterans who completed the HDRP would have a more favorable cardiometabolic risk profile post-intervention compared to baseline.
Methods
Recruitment
The study population included patients who received health care at the VA Medical Center in Sacramento, California, USA. All patients were Veterans of the United States military. This retrospective chart review was approved by the local Institutional Review Board of the VA Northern California Health Care System (#18-12-00828). Participant informed consent was not required for this study.
Participants in the HDRP intervention were contacted through mailed invitation letters with a one-page fact sheet describing the program and also referred to the HDRP by Cardiology, Primary Care and Mental Health providers. Patients were screened by telephone for key inclusion/exclusion criteria. Inclusion criteria were a diagnosis of atherosclerotic heart disease or type 2 diabetes. Exclusion criteria were a history of bariatric surgery, stage 4 or 5 chronic kidney disease, dementia, or being high risk for disruptive behavior or suicide. There was no maximum age, and exercise capacity was not used as exclusion criteria. After approval by telephone screening, patients were invited to attend the HDRP seminar. All patients were medically cleared by a physician to participate in the HDRP. After the seminar, patients decided whether they would participate in the intervention. For this research study, we received a waiver for informed consent, as collected data were only obtained from medical chart review.
Program Design
The HDRP was an adaptation of interdisciplinary lifestyle interventions which have been shown to induce regression of atherosclerotic blockages.33,34,42 The 15-week HDRP was offered in a Behavioral Medicine Clinic at the Sacramento VA Medical Center. 40 Participants underwent medication reconciliation with a clinical pharmacist at baseline, and medications were adjusted as needed by each patient’s primary care physician, cardiologist and/or HDRP consulting physician. Program staff included a clinical health psychologist (program director), registered dietitian nutritionist, clinical pharmacist, and a consulting physician. Patients completed the program in cohorts of 6-10 patients, and spouses/partners were also encouraged to attend all sessions. All intervention appointments in our study were conducted in-person.
HDRP intervention design was primarily based on Social Cognitive Theory, 43 the Health Belief Model, 44 and the Transactional Model of Stress and Coping. 45 The intervention consisted of one seminar where Veterans decided whether to enroll in HDRP, 3 individual sessions with a clinical health psychologist, and 12 interdisciplinary group sessions. The program began with a 120-minute seminar summarizing the scientific background and rationale for the HDRP, basic guidelines of the WFPB diet, physical activity, and stress management components, with the aim of increasing perceived severity of ASCVD and benefits of lifestyle change. Participants who enrolled in HDRP then had 3 weeks to prepare before the group sessions began, allowing time to gradually reduce animal food intake, increase plant food intake, prepare their kitchen, seek social support (i.e., find a health buddy), and mentally prepare to fully engage in the intervention. Next, patients had their first of 3 (60 minute) individual sessions with a clinical health psychologist at baseline (1 week prior to the first group session), then at 1-month (the week after the fourth group session), and post-treatment (the week after the 12th group session). Motivational Interviewing was used to share objective assessment results (i.e., “cardiometabolic profile”) and promote engagement and behavior change. The individual sessions served to promote maximum initial motivation, goal-setting, and individualization of the program.
Twelve 90-minute weekly group sessions formed the primary vehicle for the delivery of the 3 components of the intervention. We chose 12 weeks of group sessions to mirror the duration of the group sessions of the Ornish program, yet we reduced the group frequency to once-weekly instead of twice weekly, and group session duration was 90 total minutes per week instead of 8 hours weekly as found in the Ornish program. 37 The intervention dose consisted of 21 total hours over 15 weeks (in comparison to 72 hours for the Ornish program). This number of sessions/duration also matched the VA MOVE weight management group program at our facility and nationally. 46 Furthermore, 12 weekly 90-minute group sessions also matched the structure of evidence-based group psychotherapies commonly offered by VA psychologists (e.g., CBT for Depression Group), making it fit in the scheduling grid and ultimately more feasible within routine practice.
Group sessions were co-facilitated by a psychologist, dietitian, and pharmacist, and session topics for each HDRP component are summarized in Table 1. Similar to other heart disease reversal interventions, all participants were required to adopt the WFPB diet, as quickly as possible (i.e., beginning with group session 1, “go all in”).33,47 As noted, discussion topics included education on cardiometabolic disease processes of atherosclerosis and insulin resistance, and the mechanisms of promoting disease regression. Nutrition education topics included how to achieve this ultra low-fat (∼10% of daily kilocalories)/high carbohydrate (∼80% of daily kilocalories) version of the WFPB diet, 47 importance of eating a variety of plant foods daily, practical matters related to the macronutrients (protein, carbohydrates, and fat), exposure to culturally diverse recipes, minimizing alcohol use, and cooking instruction (e.g., how to sauté with water and without oil, using a chef’s knife, steaming technique).
Table 1.
Group Intervention Components of the Heart Disease Reversal Program.
| Session number | Nutrition | Exercise | Stress management |
|---|---|---|---|
| 1 | Orientation to HDRP | Self-monitor physical activity | None |
| Intro to nutrition component | |||
| No-oil sauté method | |||
| Culturally based flavor combinations | |||
| 2 | Diet troubleshooting | Intro to exercise component | Coping with food cravings |
| Steaming technique | Proper walking technique | Mindful eating | |
| 3 | Protein | Exercise for stress management | Intro to stress management component |
| Batch cooking | Exercise troubleshooting | Diaphragmatic breathing | |
| Cooking dried legumes | |||
| 4 | Carbohydrates and diabetes | Exercise for blood sugar management | Awareness of thinking |
| Cooking whole grains | “The Hook” metaphor; progressive muscle relaxation | ||
| 5 | Managing social situations and eating out | Safe exercise progression | Challenging negative thinking |
| Dr Greger’s daily dozen | Exercise troubleshooting | Gratitude journal | |
| Postural relaxation | |||
| 6 | Fat: Types, omega 3’s, problems with low-carb/high-fat diets | Lifestyle activity | Problem- vs Emotion-Focused coping |
| Safe exercise progression | Guided beach imagery | ||
| Exercise troubleshooting | |||
| 7 | Dietary methods for boosting nitric oxide | Exercise and artery function | Acceptance and softening |
| Cooking with herbs | Clarifying values | ||
| Mindfulness meditation | |||
| 8 | Circadian rhythm and optimizing weight loss | Safe exercise progression | Activity scheduling |
| Exercise troubleshooting | Savoring | ||
| Guided forest imagery | |||
| 9 | Optimizing brain health | Exercise, cognition, and brain health | Social support |
| 5 acts of kindness | |||
| Lovingkindness meditation | |||
| 10 | Iron, calcium, and iodine | Exercise and bone health | Assertive communication |
| Healthy bones | Anger awareness | ||
| Food synergy and antioxidants | Autogenic relaxation | ||
| 11 | Other reasons for eating plant-based (environment, food contaminantion) | Optimum amount of exercise | Optimizing sleep |
| Light meditation | |||
| 12 | Final group session; summary review, planning for the future, maintaining gains, program feedback | ||
The physical activity component was primarily guided by social cognitive theory and it focused on promoting the adoption of a moderate-intensity walking program, increasing everyday lifestyle activity, and reducing prolonged sitting. 48 No specific standard amount or type of physical activity was universally required of all participants; rather, participants were encouraged to walk, or pursue whatever activity plan their physician medically cleared them to do. Participants were provided education on the biopsychosocial benefits of physical activity and reducing sedentary behavior in an effort to enhance outcome expectancies and expectations. Education also included the basics of starting a walking program safely (e.g., medical clearance, awareness of cardiac symptoms and when to cease activity and seek emergency medical care, achieving a moderate-intensity level, importance of good shoes and appropriate clothing). Strategies also included the use of self-monitoring to establish a baseline level of activity and quantify safe and gradual levels of progression throughout the intervention. Self-efficacy for physical activity was promoted by establishing small realistic activity goals to engage in activities that participants enjoyed and were intrinsically motivated to engage in, providing instruction on proper walking posture and technique, and assistance with problem-solving to overcome barriers to activity. Finding ways to be more active in everyday life (e.g., interrupting prolonged sitting, taking the long way to walk to places, parking far away) were also encouraged.
The stress management component primarily consisted of cognitive-behavioral stress management (CBSM) training, which has been effective with various medical populations including those with heart disease, prostate cancer, breast cancer, and HIV/AIDS.18,49–52 Rather than being limited to the practice of various relaxation or meditation techniques, CBSM is a psychotherapy that includes many methods that are grounded in the Transactional Model of Stress and Coping 45 and facilitate a range of aims: building stress awareness, teaching stress/anxiety reduction techniques, modifying faulty cognitive appraisals, building problem- and emotion-focused coping skills, practicing acceptance, reducing social isolation, reducing risk behavior, and enhance treatment adherence. 53 The HDRP stress management component also integrated positive psychology interventions in ways that were conceptually coherent and enhanced the core elements of CBSM (e.g., gratitude journal as an additional way to promote adaptive, helpful, and undistorted thinking; 5 acts of kindness as another method for increasing social connection).54,55 Lastly, the stress management component included behavioral sleep management strategies.
Cardiovascular Disease (CVD) Risk Profile
Participants’ height, weight, and waist circumference were measured by study staff or other VA providers at baseline (measures taken prior to the first group session, but no earlier than 3 months before), 1-month (measures taken during the week between group sessions 3 and 4), and post-treatment which corresponds to 15 weeks after the first individual session appointment at baseline (measures obtained during the week prior to the 12th and final group session, or as late as the final individual session). Patient height previously recorded in the medical chart was used for the purpose of calculating Body Mass Index (BMI) in this study. All patients completed blood tests for fasting glucose, hemoglobin A1C, fasting lipid panel (total cholesterol [TC], low density lipoprotein-cholesterol [LDL-C], high density lipoprotein-cholesterol [HDL-C], triglycerides [TG]) and high sensitivity C-reactive protein (CRP) at baseline, 1-month, and post-treatment. Lipids were measured by colorimetric absorbance spectroscopy via an enzymatic assay. hs-CRP was measured by infrared absorption spectroscopy via immunoassay.
Study Design
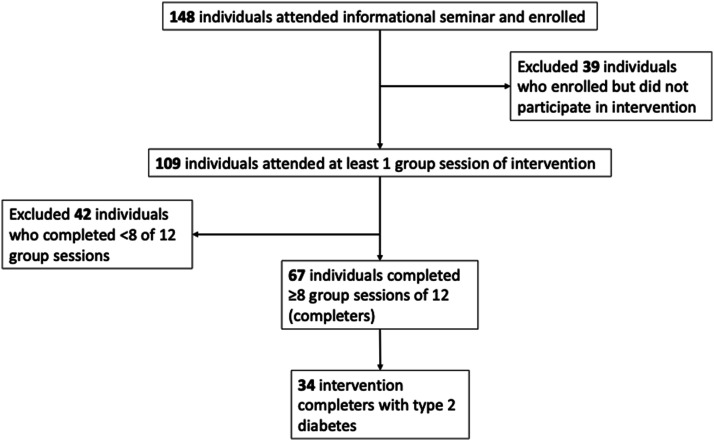
For all patients who attended the HDRP seminar between October, 2016 and January 2020, demographic data and medical histories were obtained through chart review. Researchers accessed medical records to gather data up to 1 year prior to starting the HDRP, at baseline, one month and post-treatment. Completion of the HDRP was defined as attending at least 70% of HDRP group sessions (i.e., at least 8 of 12 group sessions). The 70% session attendance benchmark was chosen as a clear, quantitative benchmark of a sufficient intervention “dose” and to help judge the feasibility of our intervention. 56 Non-completers were defined as participants who did not attend at least 70% of group sessions (including both those Veterans who decided to cease their participation early and those who remained engaged in the intervention but attended less than 8 sessions) and they were excluded from the final analysis (Figure 1). Researchers had no contact with patients at the time of the retrospective analysis.
Figure 1.
Study flowchart of participant inclusion.
Statistical Analysis
Data analysis was completed using SAS 9.4. Descriptive statistics were used to summarize the baseline characteristics and demographics of Veterans who participated in the HDRP. Average change from baseline to 1-month and baseline to post-treatment were calculated for each cardiometabolic risk factor. The PROC TTEST procedure was used to perform 2-tailed, paired t-tests for the cardiometabolic risk factors. A bootstrapping procedure with a sample of 10 000 with a randomized seed was performed due to the small sample size of the study. The analysis was completed for all intervention completers, and on the subset of participants who had type 2 diabetes (with or without ASCVD). P-values of <.05 were considered statistically significant.
Results
Among all participants (N = 109), 50% completed 10-12 group sessions (out of 12 total group sessions), 13% completed 7-9 sessions, and 17% completed 4-6 sessions. Twenty percent of participants completed 3 or fewer sessions.
Altogether, the intervention non-completers and completers were very similar in terms of age (68 years and 69.2 years, respectively), BMI (31.9 and 31.5, respectively), and total service connection disability rating (52% and 49%, respectively). A smaller proportion of non-completers had their spouse/partner attend intervention sessions with them (21%) compared to completers (33%). In terms of medical conditions, the greatest discrepancy between non-completers and completers was in chronic pain diagnosis (non-completers with 50%, completers with 42%). There were also more non-completers with CHF (33% vs 28%) and COPD (14% vs 12%), compared to completers. Among the psychiatric disorders, more non-completers had a depressive disorder (38% vs 34%), PTSD (40% vs 37%), and anxiety disorder (12% vs 9%), compared to completers. Some health conditions were more common among the completers, including type 2 diabetes (51% vs 45%), adjustment disorder (30% vs 24%), bipolar disorder (9% vs 5%), and atrial fibrillation (19% vs 17%).
Baseline characteristics of the Veteran participants who completed the HDRP are presented in Table 2. The sample of HDRP completers predominately consisted of older adults with a mean age of 69 years, were mostly White (70%), not Hispanic or Latino (95%), and male (97%). Three-quarters of the veterans were married. The majority of veterans who participated were referred by Cardiology, as 91% of sample were diagnosed with atherosclerotic heart disease. More than one-third had a history of myocardial infarction (35%) and percutaneous cardiac intervention (PCI) (42%). About one-quarter of the participants had a history of coronary artery bypass graft (CABG) surgery. Half of the sample was diagnosed with type 2 diabetes. Over half of the sample had been diagnosed with a psychiatric disorder, with posttraumatic stress disorder (37%) and a depressive disorder (34%) being the most common. Almost half of the Veterans experienced chronic pain (42%). Approximately 80% of the sample was taking cholesterol-lowering medication. The average participant was taking more than 2 hypertension medications at baseline. Among the participants with type 2 diabetes, about a quarter were on insulin, averaging 37 units of daily insulin. About one-quarter had a 100% VA service connection rating (the highest rating of disability severity in the VA schedule of ratings) and about one-quarter were not service-connected at all (did not receive VA disability for medical or psychiatric conditions) or rated at 0% (lowest possible rating of VA disability severity).
Table 2.
Baseline Characteristics of Veteran Participants Who Completed HDRP (n = 67).
| Variable | All completers (n = 67) | Completers with type 2 diabetes (n = 34) |
|---|---|---|
| Mean (± SD) or n (%) | Mean (± SD) or n (%) | |
| Demographics | ||
| Age (years) | 69.22 (7.87) | 70.32 (6.72) |
| Gender | ||
| Male | 65 (97.01%) | 33 (97.06%) |
| Female | 2 (2.99%) | 1 (2.94%) |
| Transgender | 0 (0%) | 0 (0%) |
| Race | ||
| White | 47 (70.15%) | 21 (61.76%) |
| Black or African-American | 8 (11.94%) | 5 (14.71%) |
| American Indian or Alaska Native | 5 (7.46%) | 4 (11.76%) |
| Asian | 3 (4.48%) | 3 (8.82%) |
| Native Hawaiian or other Pacific Islander | 1 (1.49%) | 1 (2.94%) |
| Did not answer (unknown) | 3 (4.48%) | 3 (9.09%) |
| Ethnicity (Hispanic/Latino) | 3 (4.48%) | 1 (2.94%) |
| Marital status | ||
| Married | 51 (76.12%) | 26 (76.47%) |
| Divorced | 7 (10.45%) | 2 (5.88%) |
| Unmarried | 4 (5.97%) | 3 (8.82%) |
| Widowed | 2 (2.99%) | 2 (5.88%) |
| Did not answer (unknown) | 3 (4.48%) | 1 (2.94%) |
| Anthropometrics and vitals | ||
| BMI (kg/m2) | 31.51 (5.79) | 32.22 (6.09) |
| Systolic blood pressure (mmHg) | 132.9 (22.00) | 135.5 (21.24) |
| Diastolic blood pressure (mmHg) | 71.31 (13.68) | 71.33 (13.94) |
| Pulse (beats per minute) | 65.07 (11.00) | 67.21 (11.58) |
| Waist circumference (inches) | 44.83 (5.86) | 45.48 (6.06) |
| Medical history | ||
| Diagnosis of atherosclerotic heart disease | 61 (91.04%) | 31 (91.18%) |
| Past MI | 24 (35.82%) | 13 (38.24%) |
| Past PCI | 28 (41.79%) | 12 (38.24%) |
| Past CABG | 16 (23.88%) | 12 (35.29%) |
| Past CVA | 5 (7.46%) | 4 (11.76%) |
| Past TIA | 6 (8.96%) | 6 (17.65%) |
| Diagnosis of type 2 diabetes mellitus | 34 (50.75%) | 34 (100%) |
| Diagnosis of hypertension | 60 (89.55%) | 33 (97.06%) |
| Diagnosis of hyperlipidemia | 62 (92.54%) | 33 (97.06%) |
| Diagnosis of PVD | 6 (8.96%) | 5 (14.71%) |
| Diagnosis of CHF | 19 (28.36%) | 11 (32.35%) |
| Diagnosis of atrial fibrillation | 13 (19.40%) | 5 (14.71%) |
| Anticoagulation medication | 13 (19.4%) | 6 (17.65%) |
| Past traumatic brain injury | 3 (4.48%) | 1 (2.94%) |
| Psychiatric disorder (participants with at least one) | 39 (58.21%) | 20 (58.82%) |
| Diagnosis of depressive disorder | 23 (34.33%) | 15 (44.12%) |
| Diagnosis of PTSD | 25 (37.31%) | 12 (35.29%) |
| Diagnosis of anxiety disorder | 6 (8.96%) | 4 (11.76%) |
| Diagnosis of adjustment disorder | 2 (2.99%) | 1 (2.94%) |
| Diagnosis of bipolar disorder | 6 (8.96%) | 2 (5.88%) |
| Diagnosis of chronic pain | 28 (41.79%) | 15 (44.12%) |
| Diagnosis of COPD | 8 (11.94%) | 7 (20.59%) |
| Family history of dementia | 3 (4.48%) | 2 (5.88%) |
| Medications | ||
| Taking cholesterol-lowering medication (yes) | 53 (79.1%) | 29 (85.29%) |
| Total number of hypertension medications | 2.19 (SD = 1.28) | 2.59 (SD = 1.16) |
| Taking insulin (yes) | 9 (13.43%) | 9 (26.47%) |
| Taking short-acting insulin (Aspart) (yes) | 5 (7.46%) | 5 (14.71%) |
| Total daily long-acting insulin dose in insulin-requiring patient | 37.08 (35.99) | 37.08 (35.99) |
| Total number of diabetes medications in patient with diabetes | 1.44 (1.13) | 1.44 (1.13) |
| Service connection (%) | ||
| Total SC-0% | 19 (28.36%) | 8 (23.53%) |
| Total SC-100% | 17 (25.37%) | 10 (29.41%) |
| SC for atherosclerotic heart disease | 17 (14.93%) | 8 (23.53%) |
| SC for hypertensive vascular disease | 5 (7.46%) | 1 (2.94%) |
| SC for coronary artery bypass | 4 (5.97%) | 3 (8.82%) |
| SC for MI | 2 (2.99%) | 1 (2.94%) |
| SC for diabetes mellitus | 10 (14.93%) | 10 (29.41%) |
Abbreviations: BMI, body mass index, CABG, coronary artery bypass graft, CHF, congestive heart failure, COPD, chronic obstructive pulmonary disease, CVA, cerebrovascular accident, MI, myocardial infarction, PCI, percutaneous cardiac intervention, PTSD, post-traumatic stress disorder, PVD, peripheral vascular disease, SC, service-connected disability, TIA, transient ischemic attack.
Several improvements in cardiometabolic risk factors were observed among the entire sample of intervention completers (Table 3). At the 1-month follow-up, TC significantly decreased by 18.1 mg/dL and LDL-C decreased by 15 mg/dL. Improvement in diastolic blood pressure was statistically significant as well (−5.2 mmHg). Systolic blood pressure also decreased (−8.2 mmHg) and approached statistical significance (P = .0536).
Table 3.
Change in Cardiometabolic Risk Factors of all Completers of HDRP (n = 67).
| Risk factor | Baseline mean (SD) | 1-month mean (SD) | Difference at 1 month (P-value) | Post-treatment mean (SD) | Difference at post treatment (P-value) |
|---|---|---|---|---|---|
| Body weight (lbs) | 218.4 (42.37) | 213.2 (42.72) | −5.26 (0.52) | 208.3 (40.82) | −10.08 (0.17) |
| BMI (kg/m2) | 31.51 (5.79) | 30.8 (5.53) | −0.70 (0.52) | 30.52 (6.72) | −0.98 (0.38) |
| Waist circumference (inches) | 44.83 (5.86) | 44.25 (5.78) | −0.58 (0.67) | 42.06 (5.38) | −2.76* (0.03) |
| Systolic blood pressure (mmHg) | 132.9 (22.00) | 124.70 (17.58) | −8.19 (0.0536) | 125.0 (17.27) | −7.88* (0.03) |
| Diastolic blood pressure (mmHg) | 71.31 (13.68) | 66.08 (10.93) | −5.23* (0.047) | 66.69 (11.09) | −4.62 (0.13) |
| Pulse (beats per minute) | 65.07 (11.00) | 61.00 (12.08) | −4.07 (0.13) | 62.96 (10.92) | −2.11 (0.34) |
| TC (mg/dl) | 134.1 (39.23) | 116.00 (28.96) | −18.08** (0.008) | 122.4 (34.71) | −11.67 (0.09) |
| LDL-C (mg/dl) | 70.39 (32.75) | 55.44 (26.02) | −14.95* (0.01) | 59.12 (26.57) | −11.27* (0.04) |
| HDL-C (mg/dl) | 39.35 (10.86) | 37.34 (13.76) | −2.01 (0.39) | 38.48 (11.13) | −0.87 (0.66) |
| Triglycerides (mg/dl) | 126.8 (83.78) | 126.7 (73.32) | −0.12 (0.99) | 117.8 (84.80) | −9.00 (0.56) |
| Fasting glucose (mg/dl) | 127.8 (41.04) | 120.1 (41.86) | −7.75 (0.34) | 112.7 (38.12) | −15.10* (0.03) |
| Hemoglobin A1C (%) | 6.75 (1.43) | 6.36 (1.21) | −0.39 (0.21) | 6.20 (1.06) | −0.55* (0.017) |
| CRP (mg/L) | 2.61 (2.72) | 1.74 (2.05) | −0.86 (0.10) | 1.88 (2.30) | −0.73 (0.13) |
Note. TC, total cholesterol; LDL-C, low density lipoprotein-cholesterol; HDL-C, high density lipoprotein-cholesterol; CRP, C-Reactive Protein, *P < 0.05, **P < 0.01.
At end of treatment, the intervention completers lost an average of 10 lbs., amounting to 4.6% of their baseline body weight, although it was not statistically significant. They experienced a significant decrease in waist circumference of 2.76 inches. Systolic blood pressure also significantly improved (−7.9 mmHg). Furthermore, glycemic control improved on both measures, with fasting glucose decreasing by 15 mg/dL and hemoglobin A1c decreasing by 0.55%. There was a significant decrease in LDL-C of 11 mg/dL, but HDL-C and triglycerides were essentially unchanged at 1-month and post-treatment.
The subsample of intervention completers who had type 2 diabetes mellitus (n = 34) demonstrated even greater improvements post-treatment (Table 4). Statistically significant improvements were observed in hemoglobin A1c (−0.80%), systolic blood pressure (−11 mmHg), and diastolic blood pressure (−6.6 mmHg). This group experienced a 3.4-inch decrease in waist circumference, although this finding was only approaching statistical significance (P = .052).
Table 4.
Change in Cardiometabolic Risk Factors in Participants With Type 2 Diabetes Who Completed HDRP (n = 34).
| Risk factor | Baseline mean (SD) | 1-month mean (SD) | Difference at 1 month (P-value) | Post-treatment mean (SD) | Difference at post treatment (P-value) |
|---|---|---|---|---|---|
| Body weight (lbs.) | 218.0 (40.60) | 210.5 (39.25) | −7.44 (0.49) | 206.5 (37.42) | −11.44 (0.24) |
| BMI (kg/m2) | 32.22 (6.09) | 31.23 (5.56) | −0.99 (0.53) | 31.32 (7.74) | −0.91 (0.60) |
| Waist circumference (inches) | 45.48 (6.06) | 44.5 (5.90) | −0.98 (0.60) | 42.08 (5.33) | −3.40 (0.052) |
| Systolic blood pressure (mmHg) | 135.5 (21.24) | 130.0 (18.92) | −5.48 (0.36) | 124.5 (12.44) | −10.98* (0.01) |
| Diastolic blood pressure (mmHg) | 71.33 (13.94) | 64.94 (11.06) | −6.39 (0.09) | 64.69 (10.40) | −6.65* (0.03) |
| Pulse (beats per minute) | 67.21 (11.58) | 64.55 (14.58) | −2.66 (0.55) | 63.78 (10.80 | −3.42 (0.28) |
| TC (mg/dL) | 122.3 (33.36) | 108.9 (27.87) | −13.44 (0.11) | 114.9 (33.45) | −7.45 (0.38) |
| LDL-C (mg/dL) | 59.87 (27.11) | 46.92 (26.14) | −12.95 (0.07) | 50.07 (20.68) | −9.80 (0.12) |
| HDL-C (mg/dL) | 36.94 (10.10) | 36.61 (16.74) | −0.32 (0.92) | 38.45 (13.21) | 1.52 (0.61) |
| Triglycerides (mg/dL) | 130.6 (69.45) | 143.2 (74.24) | 12.61 (0.51) | 128.6 (101.7) | −1.94 (0.93) |
| Fasting glucose (mg/dL) | 147.1 (46.71) | 141.4 (49.99) | −5.75 (0.66) | 129.2 (46.56) | −17.88 (0.12) |
| Hemoglobin A1C (%) | 7.64 (1.30) | 7.22 (1.26) | −0.42 (0.31) | 6.84 (1.03) | −0.80** (0.007) |
| CRP (mg/L) | 2.77 (2.88) | 1.62 (1.79) | −1.14 (0.13) | 1.82 (2.14) | −0.95 (0.16) |
Note. TC, total cholesterol, LDL-C, low density lipoprotein-cholesterol, HDL-C, high density lipoprotein-cholesterol, CRP, C-reactive protein, *P < 0.05, **P < 0.01.
There were no statistically significant changes in number of medications used for cholesterol, hypertension, and diabetes mellitus (data not shown).
Discussion
This study provides preliminary support that a MHBC intervention that promoted a WFPB diet, physical activity, and cognitive-behavioral stress management, may be a feasible, acceptable, and effective approach for cardiometabolic risk reduction in a sample of older Veterans with atherosclerotic heart disease and multiple comorbidities. The HDRP intervention is consistent with the American College of Lifestyle Medicine’s (ACLM) 6 lifestyle pillars, as it promotes a plant-based diet, physical activity, stress management, restorative sleep, social connection, and avoidance of risky substances. 57 The intervention also aligns with lifestyle habits and principles found among the longest-lived populations (i.e., Blue Zones). 58 Veterans who completed the HDRP achieved statistically significant improvements in waist circumference, systolic blood pressure, LDL-C, fasting glucose, and hemoglobin A1c from baseline to post-treatment. Veterans with type 2 diabetes mellitus had significant improvements in systolic and diastolic blood pressure, as well as hemoglobin A1c.
Among all intervention completers, improvements in diastolic blood pressure, total cholesterol, and LDL-C were greater at 1-month follow-up than at post-treatment. This finding may be explained by the way that the 1-month follow-up assessment was framed for the participants. Participants were encouraged to make substantial lifestyle changes with the benefit of receiving feedback on their lab tests after only 4 weeks, as opposed to waiting 15 weeks until the end of the program. This was done to encourage participants who were skeptical of their ability to do the intervention diet (including avoiding all animal foods), to try their best and “go all-in” (physical activity and stress management strategies were gradually incorporated). This strategy was drawn from other heart disease and diabetes reversal programs30,32,59 that found, in particular, that rapid adoption of the diet led to both rapid improvements in the most concerning problems (i.e., angina chest pain and high blood sugars, with weight loss) and facilitated more rapid adjustment in participants’ food preferences (i.e., greater pleasure from plant-based meals), which together supported long-term lifestyle maintenance. This strategy may have increased the number of Veterans who enrolled in the intervention who had concerns about the feasibility and approached it as a 1-month experiment, rather than firmly committing to completing the full intervention. The result may have been more participants enrolling in the HDRP at our initial seminar, yet contributed to non-completion of the intervention at post-treatment.
The question they would answer when receiving their 1-month follow-up results was, “Can I do this, and does it work?” The remaining two-thirds of the HDRP program was framed as the period when they would learn how to maintain the plant-based way of eating and integration of physical activity and stress management strategies. This phase would help the participants answer the question, “Can I enjoy this lifestyle and maintain it for the long-run?” Participants began to explore different recipes, flavors, and explored ordering plant-based meals in restaurants. This likely led to participants’ relaxing some of the nutritional guidelines. While an attenuation in outcomes from the 1-month follow-up to post-treatment may appear detrimental, this exploration and engagement in diverse culinary learning opportunities, along with efforts toward mastery of the greater challenges posed by multiple health behavior change, might be necessary for supporting long-term maintenance.
Since participants were introduced to the physical activity component beginning at group session 2, and the stress management component at session 3, it is likely that dietary change was the predominant contributor to 1-month reductions in cardiometabolic risk. Nevertheless, participants actively integrated multiple health behavior guidelines to increase physical activity and engage in routine stress management practices for the overwhelming majority of intervention sessions. While we lack adherence data, this suggests the reduced cardiometabolic risk observed in our study may have been facilitated through a combination of diet, physical activity, and stress management behaviors. Previous heart disease reversal intervention research supported the utility of simultaneous, multiple intervention components. Data from the Ornish Multisite Cardiac Lifestyle Intervention Program suggested independent, additive, and interactive effects of stress management, diet, exercise, and group support intervention components on ASCVD risk reduction. 60
Our study’s results align with previous dietary studies. The dietary component of the intervention was identified as cardioprotective in a recent state-of-the-art review that described top dietary strategies for ASCVD risk reduction. 61 Two recent randomized controlled clinical trials studying a healthy vegan diet suggest potential advantages of a strictly plant-based diet. A randomized cross-over controlled clinical trial found that an “ad libitum” (eat to fullness) vegan diet resulted in significantly greater weight loss among overweight adults than an ad libitum Mediterranean diet using PREDIMED protocol dietary guidelines.62,63 This suggests that when promoting healthy eating without restricting the volume of food consumed or prescribing specific calorie deficits, a strictly plant-based diet may have a weight loss advantage. Furthermore, a recent study of healthy identical twin adults found that a healthy vegan diet resulted in greater reductions in LDL-C, fasting insulin level, and body weight, compared to a healthy omnivorous diet. 64
While not statistically significant in our study, Veterans with and without type 2 diabetes lost approximately 5% of their body weight by the end of treatment, which is clinically significant. This amount of body weight loss has been shown to be associated with improved glycemic control, blood pressure, and lipids. 65 Waist circumference improved in our study and is considered the best routine measure of visceral fat (i.e., central obesity). Interventions such as a WFPB diet, 66 moderate-intensity physical activity, 67 and stress management training68,69 may all help reduce visceral fat via a variety of mechanisms.
Glycemic control improved among all our intervention completers, and to a larger degree among those with type 2 diabetes. While recommended in the VA/DoD clinical practice guidelines for management of type 2 diabetes, it is interesting that our dietary approach (i.e., a diet that is approximately 80% carbohydrate) is substantially different from another more common dietary recommendation for diabetes management, which is to significantly reduce carbohydrate consumption (e.g., 13%–50% of total daily caloric intake from carbohydrates). 70 Our results are consistent with clinical trials showing that a healthy vegan diet can be a successful approach in patients with diabetes mellitus.59,71 Multiple mechanisms may explain the advantages of a strict plant-based diet in promoting glycemic control, including reduction in intracellular lipid in muscle and the liver, improved beta cell function, increased incretin secretion by the gut, and greater weight loss.59,71,72 Physical activity also has a significant effect on glycemic control. 73 VA/DoD clinical practice guidelines for type 2 diabetes also recommend mindfulness-based stress reduction for patients with diabetes distress. 70 Improved general stress management skills could improve glycemic control via multiple mechanisms, including better diabetes self-management, reductions in emotional eating, reduced stress-related preference for high-caloric, low-fiber foods, and decreased cortisol levels.74,75
Our intervention included a moderate-intensity walking program aimed at achieving the recommended amount of aerobic exercise in clinical practice and national guidelines.76,77 Walking programs have been shown to be effective for secondary cardiovascular prevention, with an optimal risk/benefit ratio. 78 While our intervention only included walking (i.e., aerobic exercise), lifestyle activity, and reduced sitting, research supports the benefits of resistance training as well. 79 Physical activity also improves stress management, likely through shared and differing mechanisms than cognitive-behavioral stress management training. 19
Including stress management training in the HDRP intervention is supported by previous research. The preponderance of research data suggest that psychological factors may be causally linked with pathophysiological and behavioral factors driving ASCVD and that psychosocial interventions can have a beneficial effect on cardiovascular health. 80 Additional support for including stress management is drawn from the Lifestyle Heart Trial (randomized controlled trial of the Ornish Program For Reversing Heart Disease), where analyses demonstrated that improvement in stress management was associated with atherosclerosis regression (i.e., reduction in percent diameter stenosis) at 5-year follow-up, even after controlling for improvements in diet. 81
Approximately 60% of our study sample had a history of at least one psychiatric disorder, suggesting that inclusion of psychotherapeutic strategies might be of particular importance in this Veteran population. Furthermore, use of a more robust, theoretically grounded stress management component, delivered by a psychologist, may be critical to facilitate improvement in core coping skills. The stress management component included in the intervention aimed to foster the development of skills that are foundational to the management of not only stress, but also for the treatment of PTSD, depressive disorders, and anxiety disorders (e.g., cognitive restructuring for depression, anxiety, PTSD, pleasant activity scheduling for depression and to reduce avoidance found in anxiety and PTSD, stimulus control and sleep restriction strategies for insomnia). Psychologists’ contributions to such MHBC lifestyle interventions also include enhanced pre-treatment screening of participants’ psychiatric status and skillfully managing challenging group dynamics, as they are highly trained in group facilitation.
Contrary to our expectations, Veterans in our study did not experience a reduction in hs-CRP level, despite the intervention focusing on diet, physical activity, and stress, all of which are associated with inflammation and hs-CRP.82-84 This is in contrast to a recent RCT found that a healthy vegan diet significantly decreased inflammation when compared to the AHA diet, measured by hs-CRP. 82 It is possible that we had Veterans in our study who had other common inflammatory health conditions (e.g., inflammatory bowel disease, rheumatoid arthritis, which are known to elevate hs-CRP). 85 If true, then this could have obscured any positive effect of the HDRP on chronic inflammation related to unhealthy lifestyle habits. Since the broad range of inflammatory diseases were not part of our exclusionary criteria or data collection, we were unable to determine if these health conditions impacted our intervention’s effect on hs-CRP levels.
The primary strength of our study is that it is the first to provide preliminary data on the effectiveness of a MHBC lifestyle intervention including a WFPB dietary component on cardiometabolic risk factors among a population of community-dwelling, older US Veterans primarily with atherosclerotic heart disease. Our study aligned with the results of a pilot lifestyle medicine intervention for post-stroke Veterans, though our study had a larger sample size. 86 Our retrospective study of a clinical intervention offered within routine clinical practice suggests that our approach may be feasible and acceptable to many older Veterans with ASCVD or type 2 diabetes treated within VA outpatient settings. HDRP was successfully implemented in a Behavioral Medicine Clinic, within the Mental Health Service, utilizing staff time and resources that are available at most VA health care facilities and without external funding. The intervention also aligns with the VA’s national Whole Health Initiative, which aims to facilitate a cultural transformation across VA health care, including through expanded availability of healthy lifestyle interventions for Veterans. 87
Limitations of our study include the small sample size and the absence of a control group. Additionally, we also did not collect dietary or activity adherence data, so we could not determine the degree of behavioral changes made by participants. Furthermore, our study sample consisted almost entirely of men, thus limiting the generalizability of our results to other genders. Additionally, there is the potential for survivorship bias in this study. While we do not have data on who exactly dropped out due to finding the intervention unacceptable or not feasible (as opposed to those who remained engaged in the intervention yet attended 7 or fewer of 12 group sessions and were thus considered “non-completers”), or their specific reasons for doing so, our non-completer rate was 39% and higher than desired.
It may be that our streamlined version of the Ornish program was too abbreviated to provide enough support to enable optimal engagement for some participants. The intervention might be improved through additional individual appointments with program staff for those who are having more difficulty. Also, as noted earlier, we may have enrolled uncommitted participants with reservations about the program but who were willing to do a 1-month experiment or trial, subsequently leading to an increased number of non-completers. While our study non-completers and completers were similar in terms of age, BMI, and health conditions, non-completers did have slightly higher rates of chronic pain, CHF, depressive disorders, PTSD, and anxiety disorders. Furthermore, a smaller proportion of non-completers had their spouse/partner attend the intervention with them, suggesting strong social support (including within the home) may facilitate intervention engagement and adherence, while potentially reducing barriers (e.g., family disagreement about dietary guidelines and recipes). Worsened physical health has been found to increase the risk of premature drop out in other lifestyle intervention studies. 88 We also did not capture all aspects of disease severity and disability, and so we cannot rule out their role in facilitating intervention non-completion. For example, we included the presence of medical/psychiatric disorders per their medical record, yet not whether those participants had already had effective medical/psychiatric treatment and/or established stability, and so non-completers may have indeed been affected by poorly managed health conditions. Our study population may benefit from the use of telehealth options (e.g., video, or hybrid video/in-person appointments) which would reduce the impact of common barriers (e.g., eliminate traveling when experiencing physical pain flare-ups, lack of reliable transportation, challenges fitting in multiple medical appointments each week, avoidance of groups due to PTSD or anxiety).
Completion rate might also have been affected by the intervention guidelines. In terms of nutrition, some participants may have preferred a more flexible diet (i.e., plant-predominant diet) than the intervention diet (i.e., ultra low fat/high carbohydrate WFPB diet, most similar to Esselstyn approach). Also, the intervention included a wide range of intervention components and variety of strategies and goals. This may have led to some participants finding the some of the intervention content to not be as relevant to them. For example, due to variability in prevalence of psychiatric disorders, and the fact that we did not require all participants to report higher levels of stress and a need for stress management to enroll in the intervention, it may be that the stress management component may not have been needed or desired by all participants. This could be addressed through greater efforts to individualize the program and ensuring that stress management strategies included have a strong rationale in the absence of distress (e.g., emphasizing inoculation against future acute stress, positive psychology strategies, meditation, engagement with one’s faith community and spiritual practices such as prayer which can have stress management effects).
Lastly, contrary to other heart disease reversal interventions, our intervention did not begin with a retreat. An initial time-intensive, immersive introduction may facilitate building of critical start-up skills, bonding between participants and staff, and softening the stress of adjusting to intensive lifestyle changes. More research is needed to clarify the most effective ways to increase participant completion of the intervention.
Conclusion
This retrospective study of a MHBC lifestyle intervention that promoted a WFPB diet, physical activity, and cognitive-behavioral stress management found significant improvements in cardiometabolic risk factors in a routine practice within an outpatient VA behavioral medicine clinic. This novel VA intervention of the present study is promising and should be further tested in prospective studies using larger sample sizes of Veterans. Future studies should be of longer duration and include the collection of detailed dietary, physical activity, and stress data, including intervention adherence data. Health care systems that provide intensive lifestyle interventions such as HDRP can facilitate positive patient outcomes and support the efforts of clinicians who do not have the skill-set or time to provide high intensity lifestyle programs for the treatment and possible reversal of ASCVD and type 2 diabetes. 89
Acknowledgments
The authors wish to express their gratitude to the health care providers (in addition to the authors) who assisted with the delivery of the intervention: Lisa Wagaman, RDN, Janelle Embree, MS, RDN, CDE, Carrie Caputo, RDN, Alysa Kubota, PharmD, Cynthia Spann, PharmD, Sarah Ali, PharmD, Karen Soong, PharmD, June Taylor, MSN, RN, CNL, and Saul Schaefer, MD, FACC, FAHA.
Footnotes
Author Contributions: Themis Yiaslas: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data Curation, Writing—Original Draft, Writing—Review and Editing, Visualization, Project administration. Tara Rogers-Soeder: Investigation, Data Curation, Writing—Original Draft, Writing—Review and Editing. Gregory Ono: Investigation, Data Curation, data collection, data management, delivery of intervention. Rachel Kitazono: Writing—Original Draft, Writing—Review and Editing. Ajay Sood: Formal analysis, Writing—Review and Editing, Supervision, Project administration.
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Data Availability Statement
Data described in this manuscript will be made available upon request.*
ORCID iD
Themis A. Yiaslas https://orcid.org/0000-0003-3858-2598
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation. 2023;147(8):e93-e621. doi: 10.1161/CIR.0000000000001123. [Erratum in: Circulation. 2023 Feb 21;147(8):e622] [Erratum in: Circulation. 2023 Jul 25;148(4):e4]. [DOI] [PubMed] [Google Scholar]
- 2.Angell SY, McConnell MV, Anderson CAM, et al. The American Heart Association 2030 impact goal: a presidential advisory from the American Heart Association. Circulation. 2020;141(9):e120-e138. doi: 10.1161/CIR.0000000000000758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinojosa R. Cardiovascular disease among United States military veterans: evidence of a waning healthy soldier effect using the National Health Interview Survey. Chron Illness. 2020;16(1):55-68. doi: 10.1177/1742395318785237. Epub June 25, 2018. [DOI] [PubMed] [Google Scholar]
- 4.Hinojosa R. Sex, age, race/ethnicity, veteran status and the likelihood of reporting cardiovascular conditions in the National Health Interview Survey. J Cardiovasc Nurs. 2019;34(3):215-221. doi: 10.1097/JCN.0000000000000561 [DOI] [PubMed] [Google Scholar]
- 5.Assari S. Veterans and risk of heart disease in the United States: a cohort with 20 years of follow up. Int J Prev Med. 2014;5(6):703-709. [PMC free article] [PubMed] [Google Scholar]
- 6.Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? Arch Intern Med. 2000;160:3252-3257. [DOI] [PubMed] [Google Scholar]
- 7.Maddox TM, Plomondon ME, Petrich M, et al. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs clinical assessment, reporting, and tracking program). Am J Cardiol. 2014;114:1750e-1757. doi: 10.1016/j.amjcard.2014.08.045 [DOI] [PubMed] [Google Scholar]
- 8.Writing Committee Members. Thomas RJ, King M, et al. AACVPR/ACCF/AHA 2010 update: performance measures on cardiac rehabilitation for referral to cardiac rehabilitation/secondary prevention services: a report of the American Association of Cardiovascular and Pulmonary Rehabilitation and the American College of Cardiology Foundation/American Heart Association task force on performance measures (writing committee to develop clinical performance measures for cardiac rehabilitation). Circulation. 2010;122(13):1342-1350. doi: 10.1161/CIR.0b013e3181f5185b [DOI] [PubMed] [Google Scholar]
- 9.AACVPR. Guidelines for Cardiac Rehabilitation Programs. 6th ed. Champaign, IL: Human Kinetics; 2021. [Google Scholar]
- 10.Krishnamurthi N, Schopfer DW, Shen H, Rohrbach G, Elnaggar A, Whooley MA. Association of home-based cardiac rehabilitation with lower mortality in patients with cardiovascular disease: results from the Veterans Health Administration Healthy Heart Program. J Am Heart Assoc. 2023;12(5):e025856. doi: 10.1161/JAHA.122.025856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dibben GO, Faulkner J, Oldridge N, et al. Exercise-based cardiac rehabilitation for coronary heart disease: a meta-analysis. Eur Heart J. 2023;44(6):452-469. doi: 10.1093/eurheartj/ehac747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnamurthi N, Schopfer DW, Shen H, Whooley MA. Association of cardiac rehabilitation with survival among US Veterans. JAMA Netw Open. 2020;3(3):e201396. doi: 10.1001/jamanetworkopen.2020.1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson L, Oldridge N, Thompson DR, et al. Exercise-based cardiac rehabilitation for coronary heart disease: cochrane Systematic Review and Meta-Analysis. J Am Coll Cardiol. 2016;67(1):1-12. doi: 10.1016/j.jacc.2015.10.044 [DOI] [PubMed] [Google Scholar]
- 14.Jafri SH, Imran TF, Medbury E, et al. Cardiovascular outcomes of patients referred to home based cardiac rehabilitation. Heart Lung. 2022;52:1-7. doi: 10.1016/j.hrtlng.2021.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritchey MD, Maresh S, McNeely J, et al. Tracking cardiac rehabilitation participation and completion among medicare beneficiaries to inform the efforts of a national initiative. Circ Cardiovasc Qual Outcomes. 2020;13(1):e005902. doi: 10.1161/CIRCOUTCOMES.119.005902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva CC, Presseau J, van Allen Z, et al. Effectiveness of interventions for changing more than one behavior at a time to manage chronic conditions: a systematic review and meta-analysis. Ann Behav Med. 2024;58(6):432-444. doi: 10.1093/abm/kaae021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blumenthal JA, Sherwood A, Smith PJ, et al. Enhancing cardiac rehabilitation with stress management training: a randomized, clinical efficacy trial. Circulation. 2016;133(14):1341-1350. doi: 10.1161/CIRCULATIONAHA.115.018926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blumenthal JA, Babyak M, Wei J, et al. Usefulness of psychosocial treatment of mental stress-induced myocardial ischemia in men. Am J Cardiol. 2002;89(2):164-168. doi: 10.1016/s0002-9149(01)02194-4 [DOI] [PubMed] [Google Scholar]
- 19.Blumenthal JA, Sherwood A, Babyak MA, et al. Effects of exercise and stress management training on markers of cardiovascular risk in patients with ischemic heart disease: a randomized controlled trial. JAMA. 2005;293(13):1626-1634. doi: 10.1001/jama.293.13.1626 [DOI] [PubMed] [Google Scholar]
- 20.Goldstein RB, Smith SM, Chou SP, et al. The epidemiology of DSM-5 posttraumatic stress disorder in the United States: results from the national epidemiologic survey on alcohol and related conditions-III. Soc Psychiatr Psychiatr Epidemiol. 2016;51(8):1137-1148. doi: 10.1007/s00127-016-1208-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marmar CR, Schlenger W, Henn-Haase C, et al. Course of posttraumatic stress disorder 40 years after the vietnam war: findings from the National Vietnam Veterans Longitudinal study. JAMA Psychiatr. 2015;72(9):875-881. doi: 10.1001/jamapsychiatry.2015.0803 [DOI] [PubMed] [Google Scholar]
- 22.Ahmadi N, Hajsadeghi F, Mirshkarlo HB, Budoff M, Yehuda R, Ebrahimi R. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol. 2011;108(1):29-33. doi: 10.1016/j.amjcard.2011.02.340 [DOI] [PubMed] [Google Scholar]
- 23.Beristianos MH, Yaffe K, Cohen B, Byers AL. PTSD and risk of incident cardiovascular disease in aging Veterans. Am J Geriatr Psychiatr. 2016;24(3):192-200. doi: 10.1016/j.jagp.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 24.Wolf EJ, Schnurr PP. PTSD-related cardiovascular disease and accelerated cellular aging. Psychiatr Ann. 2016;46:527-532. doi: 10.3928/00485713-20160729-01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straznicky NE, Lambert EA, Lambert GW, Masuo K, Esler MD, Nestel PJ. Effects of dietary weight loss on sympathetic activity and cardiac risk factors associated with the metabolic syndrome. J Clin Endocrinol Metab. 2005;90(11):5998-6005. doi: 10.1210/jc.2005-0961 [DOI] [PubMed] [Google Scholar]
- 26.Malik S, Wong ND, Franklin SS, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245-1250. doi: 10.1161/01.CIR.0000140677.20606.0E [DOI] [PubMed] [Google Scholar]
- 27.Grundy SM. What is the contribution of obesity to the metabolic syndrome? Endocrinol Metab Clin N Am. 2004;33:267-282. doi: 10.1016/j.ecl.2004.03.001 [DOI] [PubMed] [Google Scholar]
- 28.US Burden of Disease Collaborators. Mokdad AH, Ballestros K, et al. The state of US health, 1990-2016 burden of diseases, injuries, and risk factors among US states. JAMA. 2018;319(14):1444-1472. doi: 10.1001/jama.2018.0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray CJL. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393:1958-1972. https://www.thelancet.com/pdfs/journals/lancet/PIIS0140-6736(19)30041-8.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esselstyn CB, Ellis SG, Medendorp SV, Crowe TD. A strategy to arrest and reverse coronary artery disease: a 5-year longitudinal study of a single physician’s practice. J Fam Pract. 1995;41(6):560-568. [PubMed] [Google Scholar]
- 31.Esselstyn CB, Jr. Updating a 12-year experience with arrest and reversal therapy for coronary heart disease (an overdue requiem for palliative cardiology). Am J Cardiol. 1999;84(3):339-341. doi: 10.1016/s0002-9149(99)00290-8 [DOI] [PubMed] [Google Scholar]
- 32.Ornish D, Scherwitz LW, Doody RS, et al. Effects of stress management training and dietary changes in treating ischemic heart disease. JAMA. 1983;249(1):54-59. [PubMed] [Google Scholar]
- 33.Ornish DM, Brown SE, Scherwitz LW, et al. Can lifestyle changes reverse coronary atherosclerosis? The lifestyle heart trial. Lancet. 1990;336:129-133. doi: 10.1016/0140-6736(90)91656-u [DOI] [PubMed] [Google Scholar]
- 34.Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease: five-year follow-up of the Lifestyle Heart Trial. JAMA. 1998;280(23):2001-2007. doi: 10.1001/jama.280.23.2001 [DOI] [PubMed] [Google Scholar]
- 35.Koertge J, Weidner G, Elliott-Eller M, et al. Improvement in medical risk factors and quality of life in women and men with coronary artery disease in the multicenter lifestyle demonstration project. Am J Cardiol. 2003;91(11):1316-1322. doi: 10.1016/s0002-9149(03)00320-5 [DOI] [PubMed] [Google Scholar]
- 36.Frattaroli J, Weidner G, Merritt-Worden TA, Frenda S, Ornish D. Angina pectoris and atherosclerotic risk factors in the multisite cardiac lifestyle intervention program. Am J Cardiol. 2008;101(7):911-918. doi: 10.1016/j.amjcard.2007.11.039 [DOI] [PubMed] [Google Scholar]
- 37.Silberman A, Banthia R, Estay IS, et al. The effectiveness and efficacy of an intensive cardiac rehabilitation program in 24 sites. Am J Health Promot. 2010;24(4):260-266. doi: 10.4278/ajhp.24.4.arb [DOI] [PubMed] [Google Scholar]
- 38.Marshall D, Elaine W, Vernalis M. The effect of a one-year lifestyle intervention program on carotid intima media thickness. Mil Med. 2011;176(7):798-804. doi: 10.7205/milmed-d-10-00447 [DOI] [PubMed] [Google Scholar]
- 39.Marshall DA, Walizer EM, Vernalis MN. Achievement of heart health characteristics through participation in an intensive lifestyle change program (coronary artery disease reversal study). J Cardiopulm Rehabil Prev. 2009;29(2):84-94. doi: 10.1097/HCR.0b013e31819a00b2 [DOI] [PubMed] [Google Scholar]
- 40.Yiaslas TA, Sood A, Ono G, et al. The design and implementation of a heart disease reversal program in the Veterans Health Administration: before and during the COVID-19 pandemic. Fed Pract. 2020;37(12):558-565. doi: 10.12788/fp.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly JH. Chapter 87: high intensity therapeutic lifestyle change. In: Rippe J, ed. Lifestyle Medicine. 3rd ed. Boca Raton, FL: CRC Press; 2019:1019-1032. [Google Scholar]
- 42.Schaefer S, Hussein H, Gershony GR, Rutledge JC, Kappagoda CT. Regression of severe atherosclerotic plaque in patients with mild elevation of LDL cholesterol. J Invest Med. 1997;45(9):536-541. [PubMed] [Google Scholar]
- 43.Bandura A. Social Foundations of Thought and Action: A Social Cognitive Theory. Hoboken, NJ: Prentice-Hall; 1986. [Google Scholar]
- 44.Janz NK, Becker MH. The health belief model: a decade later. Health Educ Q. 1984;11:1-47. doi: 10.1177/109019818401100101 [DOI] [PubMed] [Google Scholar]
- 45.Lazarus R, Folkman S. Stress, Appraisal, and Coping. Berlin, Germany: Springer; 1984. [Google Scholar]
- 46.Dahn JR, Fitzpatrick SL, Llabre MM, et al. Weight management for veterans: examining change in weight before and after MOVE. Obesity. 2011;19(5):977-981. doi: 10.1038/oby.2010.273 [DOI] [PubMed] [Google Scholar]
- 47.Esselstyn CB, Jr, Gendy G, Doyle J, Golubic M, Roizen MF. A way to reverse CAD? J Fam Pract. 2014;63(7):356-364b. [PubMed] [Google Scholar]
- 48.Baruth M, Wilcox S. Behavioral theories and strategies for promoting exercise. In: Riebe D, ed. ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Alphen aan den Rijn, Netherlands: Wolters Kluwer; 2018:377-404. [Google Scholar]
- 49.Lukens C, Turkoglu D, Burg MM. Stress management with cardiac patients. In: Dornelas A, ed. Stress Proof the Heart. Berlin, Germany: Springer; 2012:199-221. [Google Scholar]
- 50.Penedo FJ, Molton I, Dahn JR, et al. A randomized clinical trial of group-based cognitive-behavioral stress management in localized prostate cancer: development of stress management skills improves quality of life and benefit finding. Ann Behav Med. 2006;31(3):261-270. doi: 10.1207/s15324796abm3103_8 [DOI] [PubMed] [Google Scholar]
- 51.Antoni MH, Lechner SC, Kazi A, et al. How stress management improves quality of life after treatment for breast cancer. J Consult Clin Psychol. 2006;74(6):1143-1152. doi: 10.1037/0022-006X.74.6.1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antoni MH. Stress management effects on psychological, endocrinological, and immune functioning in men with HIV infection: empirical support for a psychoneuroimmunological model. Stress. 2003;6(3):173-188. doi: 10.1080/1025389031000156727 [DOI] [PubMed] [Google Scholar]
- 53.Penedo FJ, Antoni MH, Schneiderman N. Cognitive-Behavioral Stress Management for Prostate Cancer Recovery: Facilitator Guide. Oxford, UK: Oxford University Press; 2008. [Google Scholar]
- 54.Seligman MEP. Authentic Happiness. Louisville, KY: Atria; 2002. [Google Scholar]
- 55.Lyubomirsky S. The How of Happiness. London, UK: Penguin Books; 2007. [Google Scholar]
- 56.Teresi JA, Yu X, Stewart AL, Hays RD. Guidelines for designing and evaluating feasibility pilot studies. Med Care. 2022;60(1):95-103. doi: 10.1097/MLR.0000000000001664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phillips EM, Frates EP, Park DJ. Lifestyle medicine. Phys Med Rehabil Clin. 2020;31(4):515-526. doi: 10.1016/j.pmr.2020.07.006 [DOI] [PubMed] [Google Scholar]
- 58.Kreouzi M, Theodorakis N, Constantinou C. Lessons learned from Blue Zones, lifestyle medicine pillars and beyond: an update on the contributions of behavior and genetics to wellbeing and longevity. Am J Lifestyle Med. 2022. doi: 10.1177/15598276221118494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29(8):1777-1783. doi: 10.2337/dc06-0606 [DOI] [PubMed] [Google Scholar]
- 60.Daubenmier JJ, Weidner G, Sumner MD, et al. The contribution of changes in diet, exercise, and stress management to changes in coronary risk in women and men in the multisite cardiac lifestyle intervention program. Ann Behav Med. 2007;33(1):57-68. doi: 10.1207/s15324796abm3301_7 [DOI] [PubMed] [Google Scholar]
- 61.Sikand G, Severson T. Top 10 dietary strategies for atherosclerotic cardiovascular risk reduction [published correction appears in Am J Prev Cardiol. 2021 Mar 22;6:100174]. Am J Prev Cardiol. 2020;4:100106. doi: 10.1016/j.ajpc.2020.100106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barnard ND, Alwarith J, Rembert E, et al. A Mediterranean diet and low-fat vegan diet to improve body weight and cardiometabolic risk factors: a randomized, cross-over trial. J Am Nutraceutical Assoc. 2022;41(2):127-139. doi: 10.1080/07315724.2020.1869625 [DOI] [PubMed] [Google Scholar]
- 63.Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378(25):e34. doi: 10.1056/NEJMoa1800389 [DOI] [PubMed] [Google Scholar]
- 64.Landry MJ, Ward CP, Cunanan KM, et al. Cardiometabolic effects of omnivorous vs vegan diets in identical twins: a randomized clinical trial. JAMA Netw Open. 2023;6(11):e2344457. doi: 10.1001/jamanetworkopen.2023.44457. [Erratum in JAMA Netw Open. 2023 Dec 1;6(12):e2344457. doi: 10.1001/jamanetworkopen.2023.50422]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Franz MJ, Boucher JL, Rutten-Ramos S, Van Wormer JJ. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. J Acad Nutr Diet. 2015;115(9):1447-1463. doi: 10.1016/j.jand.2015.02.031 [DOI] [PubMed] [Google Scholar]
- 66.Kahleova H, Tura A, Hill M, Holubkov R, Barnard ND. A plant-based dietary intervention improves beta-cell function and insulin resistance in overweight adults: a 16-week randomized clinical trial. Nutrients. 2018;10(2):189. doi: 10.3390/nu10020189. Published Feb 9, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waters DL, Aguirre L, Gurney B, et al. Effect of aerobic or resistance exercise, or both, on intermuscular and visceral fat and physical and metabolic function in older adults with obesity while dieting. J Gerontol A Biol Sci Med Sci. 2022;77(1):131-139. doi: 10.1093/gerona/glab111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marniemi J, Kronholm E, Aunola S, et al. Visceral fat and psychosocial stress in identical twins discordant for obesity. J Intern Med. 2002;251(1):35-43. doi: 10.1046/j.1365-2796.2002.00921.x [DOI] [PubMed] [Google Scholar]
- 69.Drapeau V, Therrien F, Richard D, Tremblay A. Is visceral obesity a physiological adaptation to stress? Panminerva Med. 2003;45(3):189-195. [PubMed] [Google Scholar]
- 70.VA/DoD Clinical Practice Guideline . Management of type 2 diabetes mellitus. https://www.healthquality.va.gov/guidelines/CD/diabetes/VADoD-Diabetes-CPG_Final_508.pdf; 2023. Accessed May 7, 2024.
- 71.Kahleova H, Matoulek M, Malinska H, et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with type 2 diabetes. Diabet Med. 2011;28:549-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kahleova H, Petersen KF, Shulman GI, et al. Effect of a low-fat vegan diet on body weight, insulin sensitivity, postprandial metabolism, and intramyocellular and hepatocellular lipid levels in overweight adults: a randomized clinical trial. JAMA Netw Open. 2020;3(11):e2025454. doi: 10.1001/jamanetworkopen.2020.25454. [published correction appears in JAMA Netw Open. 2021;4(1):e2035088] [published correction appears in JAMA Netw Open. 2021 Feb 1;4(2):e210550] [published correction appears in JAMA Netw Open. 2021 May 3;4(5):e2115510]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shah SZA, Karam JA, Zeb A, et al. Movement is improvement: the therapeutic effects of exercise and general physical activity on glycemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Diabetes Ther. 2021;12(3):707-732. doi: 10.1007/s13300-021-01005-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zamani-Alavijeh F, Araban M, Koohestani HR, Karimy M. The effectiveness of stress management training on blood glucose control in patients with type 2 diabetes. Diabetol Metab Syndrome. 2018;10:39. doi: 10.1186/s13098-018-0342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eshete A, Mohammed S, Deresse T, Kifleyohans T, Assefa Y. Association of stress management behavior and diabetic self-care practice among diabetes type II patients in North Shoa Zone: a cross-sectional study. BMC Health Serv Res. 2023;23(1):767. doi: 10.1186/s12913-023-09752-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.U.S. Department of Health and Human Services . Physical activity guidelines for Americans. 2021. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf. Updated August 24. Accessed 7 May, 2024.
- 77.Writing Committee Members. Virani SS, Newby LK, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with Chronic Coronary disease: a report of the American heart association/American College of cardiology joint committee on clinical practice guidelines. J Am Coll Cardiol. 2023;82(9):833-955. doi: 10.1016/j.jacc.2023.04.003. [published correction appears in J Am Coll Cardiol. 2023 Oct 31;82(18):1808]. [DOI] [PubMed] [Google Scholar]
- 78.Murtagh EM, Murphy MH, Boone-Heinonen J. Walking: the first steps in cardiovascular disease prevention. Curr Opin Cardiol. 2010;25(5):490-496. doi: 10.1097/HCO.0b013e32833ce972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Acosta-Manzano P, Rodriguez-Ayllon M, Acosta FM, Niederseer D, Niebauer J. Beyond general resistance training. Hypertrophy versus muscular endurance training as therapeutic interventions in adults with type 2 diabetes mellitus: a systematic review and meta-analysis. Obes Rev. 2020;21(6):e13007. doi: 10.1111/obr.13007 [DOI] [PubMed] [Google Scholar]
- 80.Levine GN, Cohen BE, Commodore-Mensah Y, et al. Psychological health, well-being, and the mind-heart-body connection: a scientific statement from the American Heart Association. Circulation. 2021;143(10):e763-e783. doi: 10.1161/CIR.0000000000000947 [DOI] [PubMed] [Google Scholar]
- 81.Pischke CR, Scherwitz L, Weidner G, Ornish D. Long-term effects of lifestyle changes on well-being and cardiac variables among coronary heart disease patients. Health Psychol. 2008;27(5):584-592. doi: 10.1037/0278-6133.27.5.584 [DOI] [PubMed] [Google Scholar]
- 82.Shah B, Newman JD, Woolf K, et al. Anti-inflammatory effects of a vegan diet versus the American Heart Association-recommended diet in coronary artery disease trial. J Am Heart Assoc. 2018;7(23):e011367. doi: 10.1161/JAHA.118.011367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van 't Klooster CC, van der Graaf Y, Ridker PM, et al. The relation between healthy lifestyle changes and decrease in systemic inflammation in patients with stable cardiovascular disease. Atherosclerosis. 2020;301:37-43. doi: 10.1016/j.atherosclerosis.2020.03.022 [DOI] [PubMed] [Google Scholar]
- 84.Johnson TV, Abbasi A, Master VA. Systematic review of the evidence of a relationship between chronic psychosocial stress and C-reactive protein. Mol Diagn Ther. 2013;17(3):147-164. doi: 10.1007/s40291-013-0026-7 [DOI] [PubMed] [Google Scholar]
- 85.Plebani M. Why C-reactive protein is one of the most requested tests in clinical laboratories? Clin Chem Lab Med. 2023;61(9):1540-1545. doi: 10.1515/cclm-2023-0086 [DOI] [PubMed] [Google Scholar]
- 86.Krauss J, Frates E, Parekh M, Chan J, Kiratli BJ, Myers J. Comprehensive lifestyle medicine program improves fitness, function, and blood pressure in poststroke Veteran cohort: a pilot study. Am J Lifestyle Med. 2022;16(6):765-771. doi: 10.1177/1559827620988659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.US Department of Veterans Affairs . Whole Health. https://www.va.gov/WHOLEHEALTH/index.asp. Updated June 27, 2024. Accessed July 1, 2024. [Google Scholar]
- 88.Krachler B, Söderholm A, Ekman F, et al. Intensive lifestyle intervention for cardiometabolic prevention implemented in healthcare: higher risk predicts premature dropout. Am J Lifestyle Med. 2024. doi: 10.1177/15598276241259961 [DOI] [Google Scholar]
- 89.Frates B, Kelley J. Lifestyle medicine: intensity of intervention versus intensity of patient response. Am J Lifestyle Med. 2023;17(3):371-373. doi: 10.1177/15598276231158094 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data described in this manuscript will be made available upon request.*